TGF-β1 dampens the susceptibility of dendritic cells to environmental stimulation, leading to the requirement for danger signals for activation (original) (raw)
Abstract
In contrast to its favourable effects on Langerhans cell (LC) differentiation, transforming growth factor (TGF)-β1 has been reported to prevent dendritic cells from maturing in response to tumour necrosis factor (TNF)-α, interleukin (IL)-1β, or lipopolysaccharide (LPS). We first characterized the effects of TGF-β1 on dendritic cell function by testing the response of TGF-β1-treated monocyte-derived dendritic cells (MoDCs) to maturation stimuli that LCs receive in the epidermis, namely, haptens, ATP and ultraviolet (UV). TGF-β1 treatment, which augmented E-cadherin and down-regulated dendritic cell-specific ICAM3-grabbing non-integrin on MoDCs, significantly suppressed their CD86 expression and hapten-induced expression of IL-1β and TNF-α mRNA and protein. As TGF-β1-treated MoDCs lacked Langerin expression, we demonstrated the suppressive effects of TGF-β1 on haematopoietic progenitor cell-derived dendritic cells expressing both CD1a and Langerin. These suppressive effects of TGF-β1 increased with the duration of treatment. Furthermore, TGF-β1-treated MoDCs became resistant to apoptosis/necrosis induced by high hapten, ATP or UV doses. This was mainly attributable to dampened activation of p38 mitogen-activated protein kinase (MAPK) in TGF-β1-treated MoDCs. Notably, although ATP or hapten alone could only induce CD86 expression weakly and could not augment the allogeneic T-cell stimulatory function of TGF-β1-treated MoDCs, ATP and hapten synergized to stimulate these phenotypic and functional changes. Similarly, 2,4-dinitro, 1-chlorobenzene (DNCB) augmented the maturation of TGF-β1-treated MoDCs upon co-culture with a keratinocyte cell line, in which ATP released by the hapten-stimulated keratinocytes synergized with the hapten to induce their maturation. These data may suggest that TGF-β1 protects LCs from being overactivated by harmless environmental stimulation, while maintaining their ability to become activated in response to danger signals released by keratinocytes.
Keywords: antigen-presenting cells, apoptosis, costimulatory molecules, dendritic cells, Langerhans cells
Introduction
Various lines of evidence show that transforming growth factor (TGF)-β1 plays significant roles in the biology of dendritic cells (DCs), particularly Langerhans cells (LCs). The development of LCs both in vitro and in vivo has been shown to be completely dependent on TGF-β1. TGF-β1-deficient mice lack LCs in their epidermis, whereas other DC populations appear normal.1 In humans, the development of LCs from haematopoietic progenitor cells (HPCs) in serum-free culture requires TGF-β1.2–4 Moreover, Caux et al.5 have demonstrated that the differentiation of CD1a+ precursors from HPCs into LCs is dependent on endogenously produced TGF-β1, while the addition of exogenous TGF-β1 polarizes the differentiation of CD14+ DC precursors into LCs. We have also reported that the induction of differentiation of LCs from HPCs is absolutely dependent on exogenous TGF-β1 in culture containing macrophage colony-stimulating factor (M-CSF), but not in culture containing granulocyte–macrophage colony-stimulating factor (GM-CSF).6 However, Geissmann et al.7 also reported that peripheral blood monocytes cultured in the presence of TGF-β1, GM-CSF and interleukin (IL)-4 differentiated into LC-like cells, as demonstrated by their phenotypic and functional characteristics.
In contrast to its favourable effects on LC differentiation, TGF-β1 prevents DCs from maturing in response to tumour necrosis factor (TNF)-α, IL-1β or lipopolysaccharide (LPS), although it does not block the CD40 ligand (CD40L)-induced up-regulation of costimulatory and major histocompatibility complex (MHC) molecules and IL-12 production.8 In addition, TGF-β1 increases the expression of chemokine (C-C motif) receptor-1 (CCR-1), -3, -5 and -6 and chemokine (C-X-C motif) receptor-4 (CXCR-4) on monocyte-derived LC-like cells and blocks the TNF-α-induced up-regulation of CCR-7.9 Thus, while TGF-β1 seems to skew the differentiation of haematopoietic precursors towards LCs, it simultaneously hampers the final maturation of these cells. Recently, Fogel-Petrovic et al. have also confirmed the inhibitory effect of TGF-β1 on DC maturation.10
In this study, to further characterize the effects of TGF-β1 on LC function, we compared the response of TGF-β1-treated monocyte-derived DCs (MoDCs) with those of MoDCs exposed to various maturation stimuli that LCs are assumed to receive in the epidermis, namely, haptens, ultraviolet light B (UVB) and ATP, which is a danger signal that is released by damaged keratinocytes.11 We found that TGF-β1-treated MoDCs were less sensitive in the induction of maturation as well as apoptosis induced by these stimuli, mainly as a result of the suppressed activation of p38 mitogen-activated protein kinase (MAPK). As TGF-β1-treated MoDCs expressed E-cadherin, down-regulated dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN), but lacked Langerin expression, we confirmed the effects of TGF-β1 on DC maturation using CD34+ HPC-derived DCs that expressed both CD1a and Langerin. In contrast, we demonstrated that ATP could synergistically activate TGF-β1-treated MoDCs with haptens. Finally, in a co-culture with TGF-β1-treated MoDCs and a keratinocyte cell line, we succeeded in demonstrating that 2,4-dinitro, 1-chlorobenzene (DNCB), a representative hapten, augments the maturation of TGF-β1-treated MoDCs through synergy between its direct effect on TGF-β1-treated MoDCs and the purinergic signal via ATP released by hapten-stimulated keratinocytes.
Materials and methods
Media and reagents
The medium used for the culture of MoDCs was RPMI-1640 supplemented with 25 mm HEPES buffer (Sigma Chemical Co., St Louis, MO), 2 mm l-glutamine, 1 mm sodium pyruvate, 1% penicillin, streptomycin and fungizone antibiotic solution (Sigma), and 10% fetal calf serum (Bioserum, Canterbury, Victoria, Australia) (complete medium). The medium used for the culture of HPCs was X-VIVO 15, a serum-free medium (Bio Whittaker, Walkersville, MD). The buffer used to purify CD14+ monocytes or CD34+ HPCs from peripheral blood mononuclear cells (PBMC) was phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin (containing less than 1 ng/mg detectable endotoxin) (Sigma) and 5 mm ethylenediaminetetraacetic acid (EDTA) [magnetic antibody cell sorting (MACS) buffer]. NiCl2 and DNCB (Wako Pure Chemicals, Osaka, Japan) were used to stimulate the MoDCs. The endotoxin content of the final dilution used was < 30 pg/ml, as determined by the Limulus amebocyte lysate assay (Seikagaku Co Inc., Tokyo, Japan). We used fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated anti-CD86 antibody (Ab), PE-conjugated anti-CD80 Ab, PE-conjugated anti-DC-SIGN Ab (PharMingen, San Diego, CA), FITC-conjugated anti-CD1a Ab (Dako A/S Inc., Glostrop, Denmark), PE-conjugated anti-CD1a Ab, PE-conjugated anti-CD83 Ab (Beckman Coulter Company, Marseille, France), PE-conjugated anti-E-cadherin Ab (R&D Systems, Minneapolis, MN) and FITC- or PE-conjugated isotype-matched mouse control Abs [immunoglobulin G1 (IgG1) and IgG2a] (PharMingen) for immunostaining. MACS colloidal supermagnetic microbeads conjugated with anti-human CD14 monoclonal Ab (CD14 microbeads) were purchased from Miltenyi Biotec Inc. (Sunnyvale, CA). Recombinant human GM-CSF, IL-4, and TGF-β1 from Biosource (Camarillo, CA) and IL-1β, stem cell factor (SCF) and Flt3 ligand (Flt3L) from PeproTech EC Ltd (London, UK) were used. NiCl2 was solubilized in distilled water, while DNCB was solubilized in dimethyl sulphoxide (DMSO) at a concentration of 1 m. The final concentration of DMSO was always less than 0·1% and cultures of MoDCs with 0·1% DMSO were also examined as a control. This study was approved by the ethics committee of Tohoku University Graduate School of Medicine, Sendai, Japan, and all subjects gave informed consent before the examinations.
Generation of MoDCs from peripheral blood monocytes and their treatment with TGF-β1
PBMC were isolated from heparinized fresh leucocyte-enriched buffy coats from different donors using Lymphoprep (Nycomed Pharma As, Oslo, Norway). After several washes with PBS, 1 × 108 PBMC were treated with 150 μl of CD14 microbeads in 600 μl of MACS buffer at 4° for 30 min. After washing with MACS buffer, the microbead-coated cells were separated by using the magnetic cell separator MACS (Miltenyi Biotec) according to the manufacturer’s protocol. Before culturing, we determined the percentage of CD14+ cells in these preparations by flow cytometry and only used cell specimens containing more than 98% CD14+ cells in further experiments.
The CD14+ monocytes (2 × 106 cells/ml) were then cultured in complete medium containing 100 ng/ml each of GM-CSF and IL-4 for 6 days. One half of the culture medium was changed on days 3 and 6. To examine the effects of TGF-β1 on MoDCs, we either added various concentrations of TGF-β1 to the culture from the beginning of the culture or added the optimal concentration of TGF-β1 at different time intervals after the initiation of the culture. To obtain TGF-β1-treated MoDCs, CD14+ monocytes (2 × 106 cells/ml) were cultured in complete medium containing 100 ng/ml each of GM-CSF, IL-4 and TGF-β1 for 6 days.
Stimulation of TGF-β1-treated or untreated MoDCs with haptens, ATP or UVB
On the 6th or 7th day, TGF-β1-treated or untreated MoDCs were treated with different concentrations of ATP or the representative haptens NiCl2 and DNCB. UVB treatment was performed by exposing the cells, which were floating in a 60-mm Petri dish in 2 ml of PBS containing 1% fetal calf serum (FCS), to various doses of UVB using FL20SE lamps (Toshiba Light and Technology, Tokyo, Japan) that emitted wavelengths of 275–400 nm with an emission peak at 315 nm (mostly within UVB) and had an intensity of 2·5 W/m2 of the target area.
In some experiments, the MoDCs were stimulated with different concentrations of ATP combined with 300 μm NiCl2, 10 μm DNCB or 30 ng/ml TNF-α.
Co-culture of TGF-β1-treated MoDCs with the keratinocyte cell line HaCaT
TGF-β1-MoDCs were co-cultured with HaCaT12 for 12 hr, and then treated or not treated with the ATP antagonist suramin (Calbiochem, San Diego, CA) for 1 hr and stimulated with DNCB for 48 hr. CD86 expression on the TGF-β1-treated MoDCs was then examined by flow cytometry.
Isolation of CD34+ HPCs and cell culture
Umbilical cord blood samples were obtained according to institutional guidelines. Cells bearing CD34 antigen were isolated from mononuclear cell fractions through positive selection as described by Jaksits et al.13 Briefly, mononuclear cells were prepared by discontinuous density gradient centrifugation using Lymphoprep™ (Nycomed Pharma As, Oslo, Norway). CD34+ HPCs were isolated from these mononuclear cells using a Direct CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec GmBH, Bergisch Gladbach, Germany) and MiniMACS separation columns according to the manufacturer’s protocol.
CD34+ HPCs (1 × 104 cells/ml/well) were cultured in X-VIVO 15 medium in 24-well tissue culture plates supplemented with 100 ng/ml M-CSF, in conjunction with a combination of 20 ng/ml SCF, 100 ng/ml Flt3L, 2·5 ng/ml TNF-α and various concentrations of TGF-β1 according to the methods described by Mollah et al.6 Cultures were kept for 1 week without adding or changing the medium or cytokines. After culturing, cells were collected from the cultures by gentle pipetting to harvest DC clusters. Clusters were further purified by layering on top of 6 ml of 7·5% bovine serum albumin (BSA; Sigma) in 15-ml tubes, and after 30 min on ice, single cells in suspension were removed by aspirating the BSA columns until 3·5 ml remained. Clusters were concentrated by centrifugation at 300 g, resuspended in growth media, and used as cluster-purified HPC-derived DCs.
Induction of maturation
Cluster-purified HPC-derived DCs (HPC-DCs) were stimulated with NiCl2 or ATP to induce maturation in X-VIVO 15 containing 100 ng/ml M-CSF, 20 ng/ml SCF, 100 ng/ml Flt3L and 2·5 ng/ml TNF-α with different concentrations of TGF-β1. These clusters were cultured for 48 hr. After stimulation, the recovered cells were analysed by flow cytometry for the expression of CD86.
Flow cytometry
TGF-β1-treated or untreated MoDCs or HPC-derived DCs were stained with a combination of FITC-conjugated anti-CD1a Ab, anti-CD86 Ab, or isotype-matched mouse control Ab and PE-conjugated anti-CD1a Ab, anti-CD80 Ab, anti-CD83 Ab, anti-CD86 Ab, anti-DC-SIGN Ab, anti-E-cadherin Ab, or isotype-matched mouse control Ab (10 μg/ml). After washing with buffer (PBS plus 1% FCS and 0·02% NaN3), the cells were analysed by FACSan® using cellquest software (Becton Dickinson, San Jose, CA). Dead cells, visualized by staining with 0·5 μg/ml propidium iodide (PI) solution, were excluded. To compare the intracytoplasmic expression of Langerin protein, we permeabilized cells using Cytofix/Cytoperm solution (BD PharMingen).14
Detection of apoptotic and necrotic cells
TGF-β1-treated or untreated MoDCs were washed with PBS 12 hr after hapten, ATP or UVB treatment, and stained for Annexin V–FITC (Clontech Laboratories, Inc., Palo Alto, CA) according to the manufacturer’s protocol. After adding 0·5 mg/ml PI solution, apoptotic cells (Annexin V-positive and PI-negative cells) and necrotic cells (PI-positive cells) were then quantified by flow cytometry.15
Quantification of mRNA expression by real-time polymerase chain reaction (PCR)
MoDCs cultured with different concentrations of TGF-β1 were treated for 6 hr with various concentrations of haptens or ATP. RNA was then extracted using the guanidinium thiocyanate method as described by the manufacturer (Isogen; Nippon Gene Inc., Toyama, Japan) and first-strand cDNA was synthesized in RNase-free conditions using the TaKaRa RNA PCR kit (AMV) (Takara Biochemicals, Osaka, Japan) as described in the manufacturer’s protocol. Quantitative, fluorescent PCR was performed using the TaqMan system (ABI 7700; PE Applied Biosystems, Foster City, CA). The primers and probes used in these studies have been described previously.16 PCRs were performed in triplicate in 30-μl total reaction volumes with 66 nm TaqMan probe, 400 nm forward primers, 400 nm reverse primers, and 2 × TaqMan universal PCR Master (PE Applied Biosystems). Thermal cycling was performed in the ABI Prism 7700 detection system (PE Applied Biosystems) as follows: first, 2 min at 50° to deplete contaminating RNA, and then a 10-min denaturation at 95°, followed by 40 cycles of 95° for 15 seconds and 60° for 1 min. The GAPDH or cytokine cDNA levels generated from the cellular RNA were calculated using standard curves that were generated with bona fide human GAPDH or cytokine cDNAs and that showed a linear relationship between the number of cycles required to exceed the threshold and the number of copies of cDNA added.17 The triplicate data for the ratio of cytokine mRNA/GAPDH mRNA of MoDCs or TGF-β1-treated MoDCs were calculated by dividing each cytokine mRNA level by the mean of triplicate data for the GAPDH mRNA level.
Detection of cytokine production by enzyme-linked immunosorbent assay (ELISA)
MoDCs cultured in the presence of different concentrations of TGF-β1 were treated with various concentrations of haptens or ATP for 48 hr, after which their culture supernatants were subjected to ELISAs measuring their production of IL-1β and TNF-α. For this, ELISA kits [from R&D Systems for IL-1β and from Endogen, Inc. (Woburn, MA) for TNF-α] that used 96-well microtitre plates were employed according to the manufacturers’ instructions. The IL-1β and TNF-α levels were calculated using standard curves obtained with recombinant IL-1β (from 3·9 to 250 pg/ml) and recombinant TNF-α (from 0 to 400 pg/ml).
T-cell proliferation assay
Day 6 MoDCs or TGF-β1-treated MoDCs were stimulated with NiCl2 (300 mm), ATP (300 mm) or both NiCl2 and ATP. After 48 hr, they were irradiated (3000 rad) and cultured with T cells in triplicate. In a mixed lymphocyte reaction (MLR), graded numbers of MoDCs or TGF-β1-treated MoDCs were cultured with 105 allogeneic T cells purified from PBMC from healthy volunteers using Pan T Cell Isolation Kit II (Miltenyi Biotec) in 96-well flat-bottom culture plates. After 5 days in the MLR assay, cells were pulsed for the last 16 hr with [3H]thymidine (1·0 μCi/well) (Amersham Bioscience, Little Chalfont, UK). Radioactive incorporation was measured by standard liquid scintillation counting and results were presented as the mean ± standard deviation obtained from triplicate cultures.
Immunoblotting
TGF-β1-treated or untreated MoDCs were cultured for 30 min with or without 1 mm NiCl2, 30 μm DNCB or 1 mm ATP, or treated with graded doses of UVB, and then washed twice in cold PBS and resuspended in 100 μl of lysis buffer [1% Nonidet P-40, 20 mm Tris-HCl, pH 8·0, 137 μm NaCl, 10% glycerol, 2 mm EDTA, 10 μl/ml leupeptin, 10 μg/ml aprotinin, 1 mm phenylmethylsulphonyl fluoride (PMSF) and 1 mm sodium orthovandate]. The nuclei and insoluble cell debris were removed by centrifugation at 4° for 10 min at 14 000 g and the postnuclear extracts were suspended in 2 × sodium dodecyl sulphate (SDS) sample buffer [313 mm Tris-HCl, pH 6·8, 10% SDS, 2-mercaptoethanol (2-ME), 50% glycerol and 0·01% bromophenol blue] and heated at 95° for 3 min. The protein samples were then fractionated by 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). The non-specific Ab-binding sites on the membranes were blocked with 1% BSA and 0·01% Tween 20 in saline (10 mm Tris-HCl, pH 7·4, and 100 mm NaCl) for 20 min at 37°. Immunoblotting of phosphorylated or non-phosphorylated p42/44 extracellular signal-regulated kinases (ERKs), stress-activated protein kinases/ c-JUN N-terminal kinase (SAPK/JNK), p38 MAPK or protein kinase B (Akt) was then performed using relevant immunoblotting kits purchased from Cell Signaling Technology, Inc. (Danvers, MA) according to the manufacturer’s instructions. The blots were visualized by enhanced chemiluminescence. To ensure that there were similar amounts of MAPKs in each sample, the same membranes were stripped, re-probed with monoclonal antibodies (mAbs) to p42/44 ERKs, SAPK/JNK, p38 MAPK or Akt, and developed with HRP-conjugated secondary Abs by enhanced chemiluminescence.
Statistical analysis
The statistical significance of differences in the cytokine mRNA/GAPDH mRNA ratio between TGF-β1-treated and untreated MoDCs was analysed using the unpaired Student _t-_test. To compare data from different donors, we calculated the per cent suppression of the cytokine mRNA/GAPDH mRNA ratio as follows:
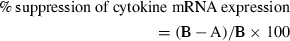
where A is the cytokine mRNA/GAPDH mRNA ratio for TGF-β1-treated MoDCs stimulated with haptens or ATP, and B is the cytokine mRNA/GAPDH mRNA ratio for MoDCs stimulated with haptens or ATP. The statistical significance of differences in per cent suppression of cytokine mRNA expression between MoDCs and TGF-β1-treated MoDCs was evaluated using the paired _t-_test. We also calculated the per cent suppression of cytokine production as follows:
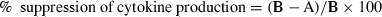
where A is the cytokine produced by TGF-β1-treated MoDCs stimulated with haptens or ATP, and B is the cytokine produced by MoDCs stimulated with haptens or ATP. The statistical significance of the difference in the production of cytokine between MoDCs and TGF-β1-treated MoDCs was analysed using the paired _t-_test. The statistical significance of differences between [3H]thymidine incorporation by T cells stimulated with MoDCs and that of T cells treated with TGF-β1-treated MoDCs was analysed using the unpaired Student _t-_test. In multiple comparisons, we applied a one-way analysis of variance (ANOVA).
Results
TGF-β1 treatment of MoDCs suppresses their activation by various maturation stimuli
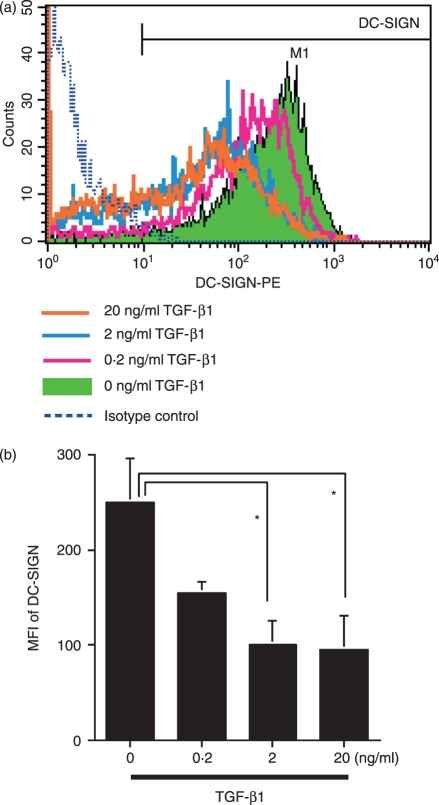
It has been demonstrated that TGF-β1-treated MoDCs had an increased level of E-cadherin, contained Birbeck granules7,8 and down-regulated DC-SIGN expression,18 while they lacked Langerin expression.19 As shown in Fig. 1, we confirmed these previous observations, demonstrating the dose-dependent down-regulation of DC-SIGN expression by MoDCs following treatment with TGF-β1. However, there was no increase in cell surface or intracytoplasmic Langerin expression under the same conditions (data not shown). These data suggested that TGF-β1 drives the differentiation of CD14+ monocytes from dermal DCs to LC-like DCs, as initially reported by Geissmann et al.,7 although they lack certain hallmarks that identify LCs in vivo.
Figure 1.
Dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN) expression by monocyte-derived dendritic cells (MoDCs) is suppressed by transforming growth factor (TGF)-β1 treatment. CD14+ monocytes were cultured in complete medium containing 100 ng/ml each of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 and different concentrations of TGF-β1. After 6 days of culture, DC-SIGN expression was analysed by flow cytometry. (a) Representative data from three independent experiments; (b) the summarized data. The mean fluorescence intensity (MFI) ± standard error of the mean from three independent experiments is shown. *Means P < 0·05 by paired _t-_test. PE, phycoerythrin.
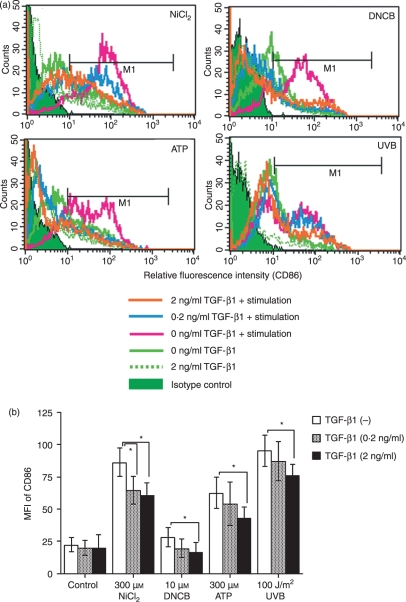
We then examined the effect of TGF-β1 on the subsequent maturation of MoDCs that had been induced by treatment with hapten,15,20 ATP21–23 or UVB. MoDCs that had been generated by GM-CSF and IL-4 treatment for 6 days in the presence or absence of different concentrations of TGF-β1 were cultured for 48 hr with 300 μm ATP, 100 J/m2 UVB, or the haptens NiCl2 (300 μm) and DNCB (10 μm) in the presence of the same concentration of TGF-β1 as used for the induction of MoDCs. Flow cytometric analysis of the cells revealed that TGF-β1 dose-dependently suppressed the ability of haptens, ATP or UVB to increase CD86 expression (Fig. 2a and b). Unfortunately, however, as these stimuli did not significantly augment CD80 or CD83 expression by MoDCs, we could not evaluate the effects of TGF-β1 on the expression of these markers (data not shown).
Figure 2.
Stimulation of monocyte-derived dendritic cells (MoDCs) with haptens, ATP or ultraviolet B (UVB) does not augment CD86 expression when transforming growth factor (TGF)-β1 is present. CD14+ monocytes were cultured in complete medium containing 100 ng/ml each of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 and different concentrations of TGF-β1. After 6 days of culture, they were stimulated for 48 hr with the indicated concentrations of NiCl2, 2,4-dinitro, 1-chlorobenzene (DNCB) and ATP and the optimal dose of UVB and then analysed for their expression of CD86 by flow cytometry. Representative data from six independent experiments are shown in (a) and the data are summarized in (b). Mean fluorescence intensity (MFI) ± standard error of the mean from six independent experiments is shown. *Means P < 0·05 by paired _t-_test.
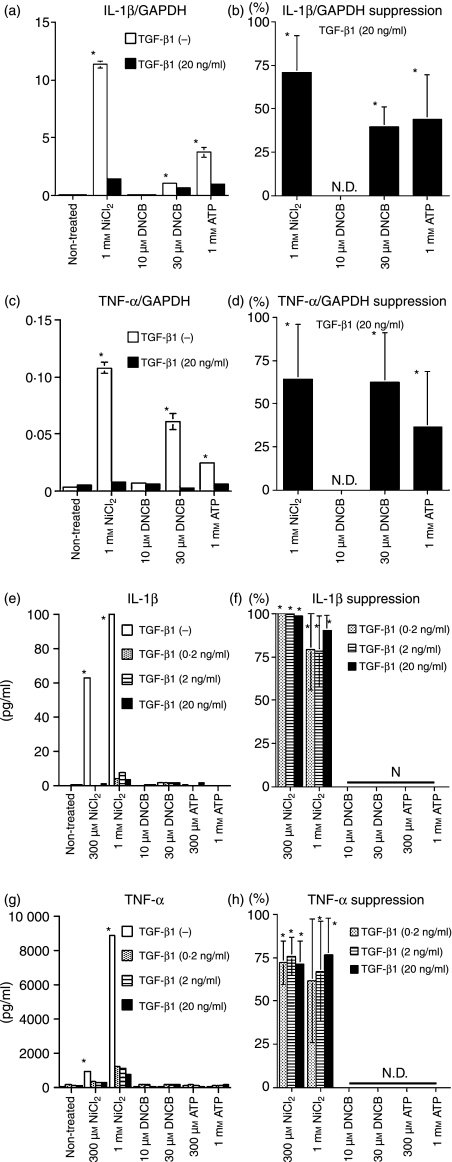
We next examined the effect of TGF-β1 on the cytokine mRNA and protein response of MoDCs to haptens and ATP. We found that, while stimulation with 1 mm NiCl2, 30 μm DNCB and 1 mm ATP induced the MoDCs to produce IL-1β mRNA, TGF-β1 treatment significantly suppressed this response (Fig. 3a and b). Similarly, TGF-β1 treatment significantly suppressed the increase in TNF-α mRNA expression by MoDCs treated with 1 mm NiCl2, 30 μm DNCB and 1 mm ATP (Fig. 3c and d). In addition, ELISA revealed that stimulation with 300 μm or 1 mm NiCl2 elicited IL-1β and TNF-α protein expression in the MoDCs, and that co-treatment with TGF-β1 suppressed this response (Fig. 3e, f, g, and h). Notably, the increase in TNF-α mRNA expression by MoDCs stimulated with DNCB and ATP was not matched by an increase in the IL-1β or TNF-α protein level.
Figure 3.
Stimulation of monocyte-derived dendritic cells (MoDCs) with haptens, ATP or ultraviolet B (UVB) does not augment cytokine mRNA and protein production when transforming growth factor (TGF)-β1 is present. CD14+ monocytes were cultured in complete medium containing 100 ng/ml each of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 and different concentrations of TGF-β1. After 6 days of culture, they were stimulated with the indicated concentrations of NiCl2, 2,4-dinitro, 1-chlorobenzene (DNCB) and ATP. After 6 hr of culture, the IL-1β GAPDH and tumour necrosis factor (TNF)-α/GAPDH mRNA ratios were determined by real-time polymerase chain reaction (PCR). Representative data from three independent experiments are shown in (a) and (c). Results are presented as the mean ± standard deviation obtained from triplicate cultures. *Means P < 0·05 by unpaired Student’s _t-_test. The data are summarized in (b) and (d). The mean per cent suppression ± standard error of the mean (SEM) for three independent experiments is shown. *P<0·05 by paired _t-_test. After 48 hr of culture, the concentrations of IL-1β and TNF-α in the culture supernatants were examined by enzyme-linked immunosorbent assay (ELISA). Representative data from three independent experiments are shown in (e) and (g). Results are presented as the mean ± standard deviation obtained from triplicate cultures. *Means P < 0·05 by unpaired Student’s _t-_test. The data from these experiments are summarized in (f) and (h). The mean per cent suppression ± SEM for three independent experiments is shown. *P<0·05 by paired _t-_test. ND, not detected.
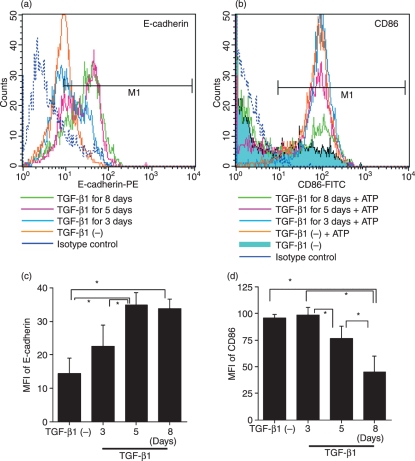
We speculated that these suppressive effects of TGF-β1 on MoDC maturation may be attributable to one of two possibilities: either TGF-β1 suppresses the signal transduction of MoDCs that is triggered by the maturation stimuli, or it promotes the further differentiation of the cells into LCs or LC-like cells, which are characterized by their resistance to various maturation stimuli. To discriminate between these possibilities, we established monocyte cultures containing GM-CSF and IL-4 and added TGF-β1 at varying time-points after initiating the culture. After 6 days of culture, we examined the E-cadherin expression of the MoDCs and then tested whether 48 hr of ATP stimulation augmented their CD86 expression. The E-cadherin expression of the cells increased the longer they had been co-cultured with TGF-β1 (Fig. 4a and c). Similarly, the ability of ATP to stimulate MoDC CD86 expression decreased the longer the cells had been co-cultured with TGF-β1 (Fig. 4b and d). These data suggest that TGF-β1 induces the development of LC-like cells that are resistant to various maturation stimuli.
Figure 4.
The ability of ATP to stimulate monocyte-derived dendritic cell (MoDC) CD86 expression decreases the longer the cells have been co-cultured with transforming growth factor (TGF)-β1. Monocytes were cultured with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 and 20 ng/ml TGF-β1 was added at different time intervals after the initiation of the culture. After 6 days of culture, the E-cadherin expression of some of the cells was examined by flow cytometry (a). The remaining cells were stimulated with 300 μm ATP for 2 days and their CD86 expression was determined by flow cytometry (b). Representative data from three different experiments are shown. Summarized data from three different experiments for the change in relative fluorescence intensity are shown in (c) and (d). The mean fluorescence intensity ± standard error of the mean from three independent experiments is shown. *Means P<0·05 by paired _t-_test. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
The effects of TGF-β1 on CD34+ HPC-derived DCs
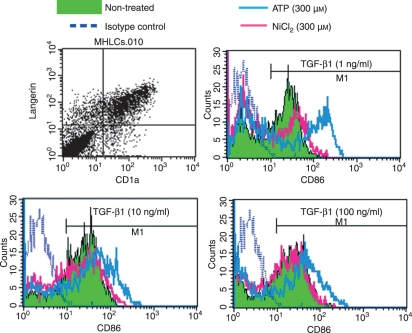
As the TGF-β1-treated MoDCs used in this study unfortunately did not express Langerin, one of the hallmarks of epidermal LCs, it remained unclear whether this scenario could be extrapolated to epidermal LCs. Therefore, it was essential to examine whether LCs were truly hyporesponsive to the environmental stimuli that we examined in this study. However, it is not easy to isolate LCs from the skin while excluding ATP effects because the procedure for dissociating epidermal cells from the skin potentially gives rise to stressing of keratinocytes, which is thought to induce the release of ATP from the keratinocytes. Therefore, in this study, we examined whether TGF-β1 affects the maturation of Langerin+ DCs induced by NiCl2 or ATP using CD34+ HPC-derived DCs. HPC-derived DCs obtained from the culture containing more than 1 ng/ml of TGF-β1 expressed both CD1a and Langerin (Fig. 5a). When HPC-DCs obtained from the culture containing 1 ng/ml or more of TGF-β1 were stimulated with NiCl2, they could not augment CD86 expression (Fig. 5b–d). In contrast, ATP significantly augmented CD86 expression by HPC-DCs obtained from the culture containing 1 ng/ml of TGF-β1 (Fig. 5b). When we increased the concentration of TGF-β1, HPC-derived DCs attenuated their augmentation of CD86 expression induced by ATP depending on TGF-β1 concentration (Fig. 5c and d). These data suggest that TGF-β1 also suppresses the maturation of LCs induced by hapten or ATP.
Figure 5.
Transforming growth factor (TGF)-β1 suppresses CD86 expression by Langerin+ haematopoietic progenitor cell (HPC)-derived dendritic cells (DCs) stimulated with ATP or NiCl2. CD34+ HPCs were cultured in X-VIVO 15 medium in 24-well tissue culture plates supplemented with 100 ng/ml macrophage colony-stimulating factor (GM-CSF), in conjunction with a combination of stem cell factor (SCF), Flt3 ligand (Flt3L), tumour necrosis factor (TNF)-α, and various concentrations of TGF-β1 for 10–14 days. Cell surface and intracytoplasmic Langerin expression by CD34+ HPC-derived DCs cultured in the presence of 1 ng/ml of TGF-β1 was examined by flow cytometry (a). The CD86 expression of HPC-derived DCs cultured in the presence of 1 ng/ml (b), 10 ng/ml (c), and 100 ng/ml (d) of TGF-β1 was examined 48 hr after stimulation with 300 μm ATP or 300 μm NiCl2. Representative data from three different experiments are shown.
TGF-β1-treated MoDCs are resistant to the apoptosis and necrosis induced by high concentrations of maturation stimuli
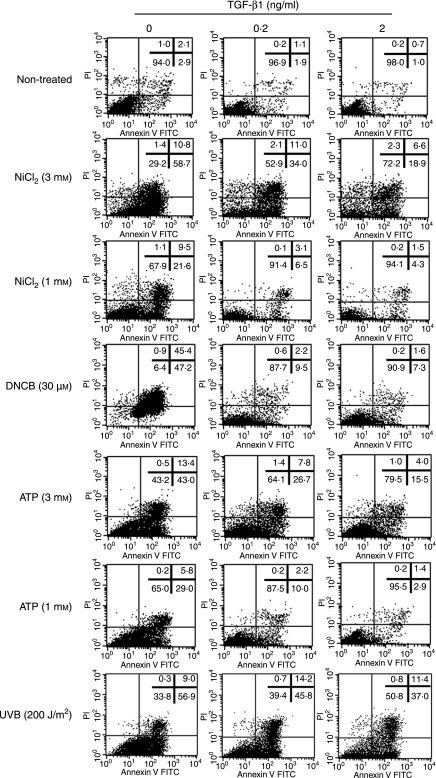
We have reported that the treatment of MoDCs with 1–3 mm NiCl2 induces their apoptosis, while treatment with more than 30 μm DNCB induces necrosis but not apoptosis.15 We have also shown that ≥ 50 J/m2 UVB induces MoDC apoptosis,23 and Coutinho-Silva et al.24 and Nihei et al.25 have reported that ≥ 100 μm ATP induces DC apoptosis. Therefore, we next examined the effect of TGF-β1 on the apoptosis/necrosis of MoDCs that is induced by the high doses of these stimuli. MoDCs or TGF-β1-treated MoDCs were stimulated for 12 hr with 1 or 3 mm NiCl2 or 300 μm ATP or 30 μm DNCB and then stained for Annexin V, which allows apoptotic cells to be discriminated from necrotic cells. MoDCs treated with TGF-β1 were resistant to both the apoptosis induced by NiCl2, ATP and UVB and the necrosis induced by DNCB (Fig. 6). Interestingly, while DNCB stimulation induced the untreated MoDCs to show necrosis, the TGF-β1-treated MoDCs showed apoptosis rather than necrosis in response to this stimulus.
Figure 6.
Transforming growth factor (TGF)-β1-treated monocyte-derived dendritic cells (MoDCs) are resistant to the apoptosis or necrosis that is induced by high concentrations of haptens or ATP. CD14+ monocytes were cultured with granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-4 and different concentrations of TGF-β1 for 6 days and then stimulated with 1 or 3 mm NiCl2, 30 μm 2,4-dinitro, 1-chlorobenzene (DNCB), 1 or 3 mm ATP, or 200 J/m2 of UVB. After 12 hr of culture, the apoptotic cells (Annexin V-positive and PI-negative cells) and necrotic cells (PI-positive cells) were then quantified by flow cytometry by double staining with fluorescein isothiocyanate (FITC)-Annexin V and propidium iodide. The data represent three different experiments.
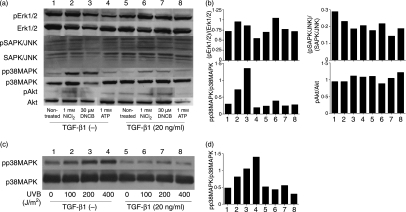
TGF-β1 preferentially suppresses the activation of p38 MAPK
To clarify the mechanism by which TGF-β1-treated MoDCs become hyporesponsive to hapten, ATP and UVB stimulation, we examined the phosphorylation of three MAPKs, namely, p38, ERK1/2 and SAPK/JNK, and the PI-3 kinase Akt. The stimulation of untreated MoDCs with NiCl2, DNCB, ATP or UVB significantly augmented the phosphorylation of p38 MAPK only (Fig. 7a and b). This augmentation of p38 phosphorylation was markedly diminished if the cells had been treated with TGF-β1 before being cultured with the maturation stimuli. We also found that all samples, regardless of whether they had been treated with TGF-β1 and/or activated with maturation stimuli, had equivalent levels of non-phosphorylated MAPKs or Akt (Fig. 7a and b).
Figure 7.
Transforming growth factor (TGF)-β1 treatment of monocyte-derived dendritic cells (MoDCs) suppresses the phosphorylation of p38 mitogen-activated protein kinases (MAPK) induced by haptens, ATP or ultraviolet B (UVB). CD14+ monocytes were cultured with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 with or without 20 ng/ml TGF-β1 for 6 days and then stimulated with the indicated NiCl2, 2,4-dinitro, 1-chlorobenzene (DNCB) and ATP concentrations (a and b) or the indicated UVB dose (c and d). After 30 min, the levels of phosphorylated and unphosphorylated p42/44 extracellular signal-regulated kinases (ERKs), stress-activated protein kinases/ c-JUN N-terminal kinase (SAPK/JNK) p38 MAPK and protein kinase B (Akt) were examined using immunoblotting kits (a and c). These data were also analysed by densitometry (b and d). Representative data from three different experiments are shown.
TGF-β1-treated MoDCs are activated by combined stimulation with haptens and ATP
We have reported that murine LCs up-regulate their expression of class II MHC antigen and their antigen-presenting function after hapten is painted on the skin, whereas chemicals that simply irritate the skin rather than sensitize animals do not have this effect.26,27 Supporting this is the observation that several haptens induce the maturation of purified MoDCs.20,28–34 Unexpectedly, however, we have here shown that TGF-β1-treated MoDCs, which are reported to share several of the functional characteristics of epidermal LCs,7,28 are hyporesponsive to haptens.
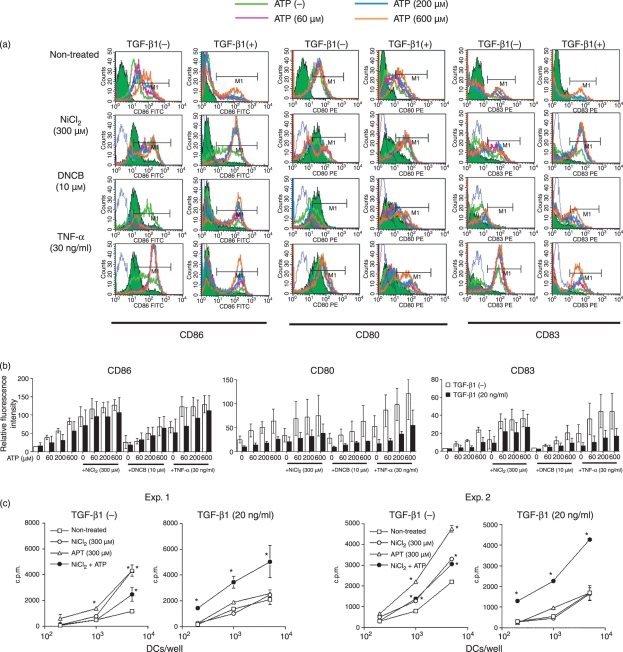
Therefore, we speculated that the full activation of LCs by various maturation stimuli requires an additional signal. Recently, Mizumoto et al.35 demonstrated that keratinocytes treated with haptens release ATP, which can induce the maturation of MoDCs. We then wondered whether simultaneous stimulation with a hapten together with the well-known danger signal ATP would provide the necessary signals needed for the complete activation of TGF-β1-treated MoDCs. To investigate this, we stimulated TGF-β1-treated and -untreated MoDCs with ATP together with NiCl2 or DNCB. As a positive control, we also tested the effect of simultaneous stimulation with ATP and TNF-α. As reported by Schnurr et al.,21 ATP stimulated the expression of CD86 by MoDCs synergistically with TNF-α, regardless of whether the cells had been treated with TGF-β1 (Fig. 8a and b). Similarly, the ATP plus hapten combination also induced a similar synergistic effect if the MoDCs had been treated with TGF-β1 before stimulation. Unexpectedly, the untreated MoDCs showed apoptosis or necrosis when stimulated with ATP plus hapten (data not shown). The expression of CD80 or CD83 by MoDCs was not significantly augmented by stimulation with either hapten or TNF-α alone or in combination with ATP.
Figure 8.
ATP and haptens synergistically augment the CD86 expression of monocyte-derived dendritic cells (MoDCs) and their antigen-presenting function only when the MoDCs have been treated with transforming growth factor (TGF)-β1. CD14+ monocytes were cultured with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 with or without 20 ng/ml TGF-β1 for 6 days and then stimulated for 48 hr with graded concentrations of ATP along with the indicated concentrations of NiCl2, 2,4-dinitro, 1-chlorobenzene (DNCB) or tumour necrosis factor (TNF)-α. The CD86, CD80 and CD83 expression of the cells was examined by flow cytometry. Representative data from four independent experiments are shown in (a). The data are summarized in (b). The mean fluorescence intensity ± standard error of the mean (SEM) from three independent experiments is shown. In the mixed lymphocyte reaction (MLR), graded numbers of MoDCs or TGF-β1-treated MoDCs stimulated with NiCl2 (300 μm) or ATP (300 μm) or both NiCl2 and ATP together were cultured with 105 allogeneic T cells in 96-well flat-bottom culture plates. After 5 days in the MLR assay, the cells were pulse-labelled for the last 16 hr with [3H]thymidine. Results are presented as the mean ± standard deviation obtained from triplicate cultures in (c). *Means P < 0·05 by unpaired Student’s _t-_test. c.p.m., counts per minute; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Next, we examined differences in allogeneic T-cell stimulatory function between MoDCs and TGF-β1-treated MoDCs. Interestingly, the allogeneic T-cell stimulatory function of MoDCs treated with ATP or NiCl2 significantly increased, while the combination did not further augment this function. In contrast, the allogeneic T-cell stimulatory function of TGF-β1-treated MoDCs was not increased only by stimulation with either NiCl2 or ATP, while the combination significantly augmented this function (Fig. 8c). These data support the notion that the TGF-β1-treated MoDCs may need a danger signal as well as a hapten to become fully mature.
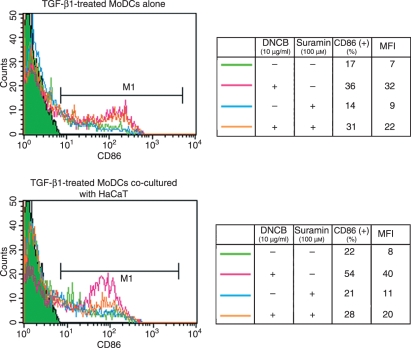
DNCB-induced activation of TGF-β1-treated MoDCs is augmented by ATP released by co-cultured keratinocytes
To further test the notion proposed above that TGF-β1-treated MoDCs may need a danger signal as well as another maturational signal to become fully mature, we generated TGF-β1-treated MoDCs and cultured them with DNCB alone or in co-culture with the keratinocyte cell line HaCaT. To investigate the role of the ATP emanating from the DNCB-stimulated keratinocytes, we added suramin, a non-specific P2 receptor antagonist,36 to some of the cultures. When the TGF-β1-treated MoDCs were cultured on their own, DNCB augmented their CD86 expression somewhat (Fig. 8). Suramin had little effect on this. Significantly, co-culture with HaCaT markedly enhanced this effect of DNCB on TGF-β1-treated MoDC maturation, and this effect was strongly antagonized by suramin (Fig. 9). This supports the notion that TGF-β1-treated MoDCs need a danger signal such as ATP released by keratinocytes as well as a hapten before they can become mature.
Figure 9.
Co-culture with keratinocytes markedly augments the 2,4-dinitro, 1-chlorobenzene (DNCB)-induced CD86 expression of transforming growth factor (TGF)-β1-treated monocyte-derived dendritic cells (MoDCs). TGF-β1-treated MoDCs were co-cultured with the keratinocyte cell line HaCaT for 12 hr and then treated or not treated with the ATP antagonist suramin for 1 hr before culture with DNCB for 48 hr. The CD86 expression of the TGF-β1-treated MoDCs was then examined by flow cytometry. The data represent two independent experiments. MFI, mean fluorescence intensity.
Discussion
Since the first observation by Geissmann et al.7 that demonstrated the effects of TGF-β1 on MoDCs, evidence has accumulated suggesting that TGF-β1 drives the differentiation of CD14+ monocytes from dermal DCs to LC-like DCs, although they are not identical to epidermal LCs.18,19,28 In this study, we first confirmed these previous observations.
Next, we showed that the treatment of MoDCs with TGF-β1 resulted in the inhibition of their hapten-, ATP- or UVB-induced maturation in a TGF-β1 dose-dependent manner. These suppressive effects of TGF-β1 on MoDCs are consistent with the observations that have been previously reported by Geissmann et al.7 and Fogel-Petrovic et al.,10 although they examined its effects on MoDCs stimulated with stimulants different from ours. As TGF-β1-treated MoDCs are not identical to epidermal LCs, our observation demonstrating the suppressive effects of TGF-β1 on MoDCs cannot be extrapolated to LCs. Therefore, we used HPC-derived DCs that expressed both CD1a and Langerin. Even in these HPC-derived DCs, TGF-β1 significantly attenuated their maturation induced by NiCl2 and ATP.
To our knowledge, there have been no reports on the effects of TGF-β1 on DC apoptosis or necrosis. Therefore, we examined the effect of TGF-β1 on DC apoptosis or necrosis induced by overstimulation. As we reported previously, DNCB treatment of MoDCs did not induce any Annexin V+ propidium iodide– cells or terminal deox(d)-UTP nick end labelling (TUNEL)+ cells at any concentration, while treatment with NiCl2 induced both effects in a dose-dependent manner.15 UVB also induced Annexin V+ propidium iodide– cells.23 We found here that, compared with untreated MoDCs, TGF-β1-treated MoDCs were more resistant to these apoptosis- or necrosis-inducing effects.
Several previous studies have demonstrated the crucial role of p38 MAPK in DC maturation as well as apoptosis induction.16,23,31,33 Recently, an elegant study using a dominant active form of MAPK kinase 6, a direct upstream kinase of p38 MAPK, has demonstrated that p38 MAPK triggers the maturation of LCs.37 In this study, we demonstrated that, of the four signal transduction cascades that have been reported previously to play a crucial role in the activation of DCs,16,38,39 TGF-β1 treatment only affected the hapten/ATP/UVB-induced activation of p38 MAPK in MoDCs. Therefore, it is speculated that the effect of TGF-β1 on p38 MAPK activation by MoDCs causes the suppression of DC maturation induced by various stimuli. Interestingly, the mechanism by which TGF-β1 treatment suppresses p38 MAPK activation does not seem simply to consist of the inhibitory effect of TGF-β1 on the phosphorylation of p38 MAPK, because the E-cadherin expression and resistance to various maturation stimuli of MoDCs increased as a function of their time of co-culture with TGF-β1.
These observations may shed light on the significant differences between LCs and other myeloid DCs. Namely, LCs reside in the epidermis, the outermost layer of the skin, where they are more frequently exposed to a variety of chemical and physical stimulation in the environment than dermal DCs. While it was once believed that the only task of DCs was to induce immune responses, it is now understood that they also fulfill an important role in the maintenance of peripheral tolerance, as they can be tolerogenic for antigen presentation whilst still immature.40–42 The present study suggests that, given their frequent bombardment with many environmental stimuli, LCs have to be somewhat more difficult to activate because this would otherwise result in the inappropriate activation of potentially destructive immunological responses or their apoptosis/necrosis by harmless stimuli.
However, to fulfill their immunostimulatory roles, LCs need to be equipped with a powerful activation mechanism. This is consistent with our observation here that ATP stimulated TGF-β1-treated MoDCs synergistically with haptens. Interestingly, in this study, simultaneous stimulation of untreated MoDCs, unlike TGF-β1-treated MoDCs, with ATP and hapten induced their apoptosis or necrosis rather than increasing their maturation (data not shown). These observations suggest that LCs can utilize the signal from ATP for their phenotypic and functional maturation in conjunction with direct signals from the environment. This was supported by our observation that TGF-β1-treated MoDCs cultured with hapten showed markedly augmented CD86 expression when they were co-cultured with keratinocytes; this synergy was abrogated by an ATP antagonist, which suggests that the ATP released by the hapten-treated keratinocytes synergizes with the hapten to successfully induce MoDC maturation.
Recently, three groups of investigators independently created strains of mice in which the complete ablation of epidermal LCs can be induced spontaneously or after systemic injection of diphtheria toxin.43–46 These studies revealed that LCs are not essential for allergic contact hypersensitivity (CHS), although the three strains varied in terms of the magnitude of the CHS response that could be induced. Recently, Bennett et al.47 have shown that LCs are required for efficient sensitization in CHS, in particular, when mice are sensitized with haptens at low doses. The present study demonstrating that TGF-β1-treated MoDCs are more resistant to stimulus of maturation by haptens than TGF-β1-untreated MoDCs, which are well known to have similar phenotypic and functional characteristics to dermal DCs, suggests that, when both LCs and dermal DCs are exposed to haptens, dermal DCs are more potent than LCs in terms of antigen presentation. However, when only LCs are exposed to haptens, LCs can become activated in the presence of haptens and a danger signal, ATP. Our current studies may reflect recent in vivo studies.
Acknowledgments
This study was supported in part by the 21st COE programme of Tohoku University, by grants 19591295 and 1879077-00 from the Ministry of Education, Science and Culture of Japan, and by the New Energy and Industrial Technology Development Organization.
Glossary
Abbreviations:
ATP
adenosine tri-phosphate
DNCB
2,4-dinitro, 1-chlorobenzene
LC
Langerhans cell
MoDC
monocyte-derived dendritic cell
References
- 1.Borkowski TA, Letterio JJ, Mackall CL, Saitoh A, Wang XJ, Roop DR, Gress RE, Udey MC. A role for TGF beta1 in Langerhans cell biology. Further characterization of the epidermal Langerhans cell defect in TGF beta 1 null mice. J Clin Invest. 1997;100:575–81. doi: 10.1172/JCI119567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strobl H, Riedl E, Scheinecker C, Bello-Fernandez C, Pickl WF, Rappersberger K, Majdic O, Knapp W. TGF-beta 1 promotes in vitro development of dendritic cells from CD34+ hematopoietic progenitors. J Immunol. 1996;157:1499–507. [PubMed] [Google Scholar]
- 3.Riedl E, Strobl H, Majdic O, Knapp W. TGF-beta 1 promotes in vitro generation of dendritic cells by protecting progenitor cells from apoptosis. J Immunol. 1997;158:1591–7. [PubMed] [Google Scholar]
- 4.Strobl H, Bello-Fernandez C, Riedl E, Pickl WF, Majdic O, Lyman SD, Knapp W. Flt3 ligand in cooperation with transforming growth factor-beta1 potentiates in vitro development of langerhans-type dendritic cells and allows single-cell dendritic cell cluster formation under serum-free conditions. Blood. 1997;90:1425–34. [PubMed] [Google Scholar]
- 5.Caux C, Massacrier C, Dubois B, Valladeau J, Dezutter-Dambuyant C, Durand I, Schmitt D, Saeland S. Respective involvement of TGF-beta and IL-4 in the development of Langerhans cells and non-Langerhans dendritic cells from CD34+ progenitors. J Leukocyte Biol. 1999;66:781–91. doi: 10.1002/jlb.66.5.781. [DOI] [PubMed] [Google Scholar]
- 6.Mollah Z, Aiba S, Nakagawa S, et al. M-CSF in cooperation with transforming growth factor-b1 induces the differentiation of CD34+ hematopoietic progenitor cells into Langerhans cells under serum-free conditions without GM-CSF. J Invest Dermatol. 2003;120:256–65. doi: 10.1046/j.1523-1747.2003.12036.x. [DOI] [PubMed] [Google Scholar]
- 7.Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta 1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med. 1998;187:961–6. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geissmann F, Revy P, Regnault A, et al. TGF-beta1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol. 1999;162:4567–75. [PubMed] [Google Scholar]
- 9.Sato K, Kawasaki H, Nagayama H, Enomoto M, Morimoto C, Tadokoro K, Juji T, Takahashi TA. TGF-beta 1 reciprocally controls chemotaxis of human peripheral blood monocyte-derived dendritic cells via chemokine receptors. J Immunol. 2000;164:2285–95. doi: 10.4049/jimmunol.164.5.2285. [DOI] [PubMed] [Google Scholar]
- 10.Fogel-Petrovic M, Long JA, Misso NL, Foster PS, Bhoola KD, Thompson PJ. Physiological concentrations of transforming growth factor beta1 selectively inhibit human dendritic cell function. Int Immunopharmacol. 2007;7:1924–33. doi: 10.1016/j.intimp.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Mizumoto N, Kumamoto T, Robson SC, Sevigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–65. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 12.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaksits S, Kriehuber E, Charbonnier AS, Rappersberger K, Stingl G, Maurer D. CD34+ cell-derived CD14+ precursor cells develop into Langerhans cells in a TGF-beta 1-dependent manner. J Immunol. 1999;163:4869–77. [PubMed] [Google Scholar]
- 14.Mollah ZU, Aiba S, Manome H, Yoshino Y, Tagami H. Cord blood CD34+ cells differentiate into dermal dendritic cells in co-culture with cutaneous fibroblasts or stromal cells. J Invest Dermatol. 2002;118:450–60. doi: 10.1046/j.0022-202x.2001.01692.x. [DOI] [PubMed] [Google Scholar]
- 15.Manome H, Aiba S, Tagami H. Simple chemicals can induce maturation and apoptosis of dendritic cells. Immunology. 1999;98:481–90. doi: 10.1046/j.1365-2567.1999.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aiba S, Manome H, Nakagawa S, Mollah ZU, Mizuashi M, Ohtani T, Yoshino Y, Tagami H. p38 mitogen-activated protein kinase and extracellular signal-regulated kinases play distinct roles in the activation of dendritic cells by two representative haptens, NiCl2 and DNCB. J Invest Dermatol. 2003;120:390–8. doi: 10.1046/j.1523-1747.2003.12065.x. [DOI] [PubMed] [Google Scholar]
- 17.Anonymous . User Bulletin #2. Faster City, CA: 1997. ABI PRISM 7700 Sequence Detection System, PE Applied Biosystems. [Google Scholar]
- 18.Relloso M, Puig-Kroger A, Pello OM, et al. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-beta, and anti-inflammatory agents. J Immunol. 2002;168:2634–43. doi: 10.4049/jimmunol.168.6.2634. [DOI] [PubMed] [Google Scholar]
- 19.Bechetoille N, Andre V, Valladeau J, Perrier E, Dezutter-Dambuyant C. Mixed Langerhans cell and interstitial/dermal dendritic cell subsets emanating from monocytes in Th2-mediated inflammatory conditions respond differently to proinflammatory stimuli. J Leukocyte Biol. 2006;80:45–58. doi: 10.1189/jlb.0205109. [DOI] [PubMed] [Google Scholar]
- 20.Aiba S, Terunuma A, Manome H, Tagami H. Dendritic cells differently respond to haptens and irritants by their production of cytokines and expression of co-stimulatory molecules. Eur J Immunol. 1997;27:3031–8. doi: 10.1002/eji.1830271141. [DOI] [PubMed] [Google Scholar]
- 21.Schnurr M, Then F, Galambos P, Scholz C, Siegmund B, Endres S, Eigler A. Extracellular ATP and TNF-alpha synergize in the activation and maturation of human dendritic cells. J Immunol. 2000;165:4704–9. doi: 10.4049/jimmunol.165.8.4704. [DOI] [PubMed] [Google Scholar]
- 22.la Sala A, Ferrari D, Corinti S, Cavani A, Di Virgilio F, Girolomoni G. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J Immunol. 2001;166:1611–7. doi: 10.4049/jimmunol.166.3.1611. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa S, Ohtani T, Mizuashi M, Mollah ZU, Ito Y, Tagami H, Aiba S. p38 mitogen-activated protein kinase mediates dual role of ultraviolet B radiation in induction of maturation and apoptosis of monocyte-derived dendritic cells. J Invest Dermatol. 2004;123:361–70. doi: 10.1111/j.0022-202X.2004.23238.x. [DOI] [PubMed] [Google Scholar]
- 24.Coutinho-Silva R, Persechini PM, Bisaggio RD, et al. P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Physiol. 1999;276:C1139–47. doi: 10.1152/ajpcell.1999.276.5.C1139. [DOI] [PubMed] [Google Scholar]
- 25.Nihei OK, de Carvalho AC, Savino W, Alves LA. Pharmacologic properties of P(2Z)/P2X(7)receptor characterized in murine dendritic cells: role on the induction of apoptosis. Blood. 2000;96:996–1005. [PubMed] [Google Scholar]
- 26.Aiba S, Katz SI. Phenotypic and functional characteristics of in vivo-activated Langerhans cells. J Immunol. 1990;145:2791–6. [PubMed] [Google Scholar]
- 27.Ozawa H, Nakagawa S, Tagami H, Aiba S. Interleukin-1 beta and granulocyte-macrophage colony-stimulating factor mediate Langerhans cell maturation differently. J Invest Dermatol. 1996;106:441–5. doi: 10.1111/1523-1747.ep12343589. [DOI] [PubMed] [Google Scholar]
- 28.Aiba S, Manome H, Yoshino Y, Tagami H. In vitro treatment of human TGF-beta1-treated monocyte-derived dendritic cells with haptens can induce the phenotypic and functional changes similar to epidermal Langerhans cells in the initiation phase of allergic contact sensitivity reaction. Immunology. 2000;101:68–75. doi: 10.1046/j.1365-2567.2000.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coutant KD, de Fraissinette AB, Cordier A, Ulrich P. Modulation of the activity of human monocyte-derived dendritic cells by chemical haptens, a metal allergen, and a staphylococcal superantigen. Toxicol Sci. 1999;52:189–98. doi: 10.1093/toxsci/52.2.189. [DOI] [PubMed] [Google Scholar]
- 30.Tuschl H, Kovac R, Weber E. The expression of surface markers on dendritic cells as indicators for the sensitizing potential of chemicals. Toxicol In Vitro. 2000;14:541–9. doi: 10.1016/s0887-2333(00)00051-5. [DOI] [PubMed] [Google Scholar]
- 31.Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J Immunol. 2001;166:3837–45. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- 32.De Smedt AC, Van Den Heuvel RL, Zwi Berneman N, Schoeters GE. Modulation of phenotype, cytokine production and stimulatory function of CD34+ -derived DC by NiCl(2) and SDS. Toxicol In Vitro. 2001;15:319–25. doi: 10.1016/s0887-2333(01)00029-7. [DOI] [PubMed] [Google Scholar]
- 33.Boisleve F, Kerdine-Romer S, Rougier-Larzat N, Pallardy M. Nickel and DNCB induce CCR7 expression on human dendritic cells through different signaling pathways: role of TNF-alpha and MAPK. J Invest Dermatol. 2004;123:494–502. doi: 10.1111/j.0022-202X.2004.23229.x. [DOI] [PubMed] [Google Scholar]
- 34.Staquet MJ, Sportouch M, Jacquet C, Schmitt D, Guesnet J, Peguet-Navarro J. Moderate skin sensitizers can induce phenotypic changes on in vitro generated dendritic cells. Toxicol In Vitro. 2004;18:493–500. doi: 10.1016/j.tiv.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Mizumoto N, Mummert ME, Shalhevet D, Takashima A. Keratinocyte ATP release assay for testing skin-irritating potentials of structurally diverse chemicals. J Invest Dermatol. 2003;121:1066–72. doi: 10.1046/j.1523-1747.2003.12558.x. [DOI] [PubMed] [Google Scholar]
- 36.Jacobson KA, Mamedova L, Joshi BV, Besada P, Costanzi S. Molecular recognition at adenine nucleotide (P2) receptors in platelets. Semin Thromb Hemost. 2005;31:205–16. doi: 10.1055/s-2005-869526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorgl A, Platzer B, Taschner S, Heinz LX, Hocher B, Reisner PM, Gobel F, Strobl H. Human Langerhans-cell activation triggered in vitro by conditionally expressed MKK6 is counterregulated by the downstream effector RelB. Blood. 2007;109:185–93. doi: 10.1182/blood-2006-05-022954. [DOI] [PubMed] [Google Scholar]
- 38.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–9. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 39.Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–46. [PubMed] [Google Scholar]
- 40.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 41.Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–7. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–10. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 44.Kissenpfennig A, Henri S, Dubois B, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–54. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–76. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal Langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–20. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Bennett CL, Noordegraaf M, Martina CA, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179:6830–5. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]