Ginseng Compounds: An Update on Their Molecular Mechanisms and Medical Applications (original) (raw)
. Author manuscript; available in PMC: 2010 Aug 25.
Published in final edited form as: Curr Vasc Pharmacol. 2009 Jul;7(3):293–302. doi: 10.2174/157016109788340767
Abstract
Ginseng is one of the most widely used herbal medicines and is reported to have a wide range of therapeutic and pharmacological applications. Ginsenosides, the major pharmacologically active ingredients of ginseng, appear to be responsible for most of the activities of ginseng including vasorelaxation, antioxidation, anti-inflammation and anti-cancer. Approximately 40 ginsenoside compounds have been identified. Researchers are now focused on using purified individual ginsenoside to reveal the specific mechanism of functions of ginseng instead of using whole ginseng root extracts. Each ginsenoside may have different effects in pharmacology and mechanisms due to their different chemical structures. Among them the most commonly studied ginsenosides are Rb1, Rg1, Rg3, Re, Rd and Rh1. The molecular mechanisms and medical applications of ginsenosides have attracted much attention and hundreds of papers have been published in the last few years. The general purpose of this update is to provide current information on recently described effects of ginsenosides on antioxidation, vascular system, signal transduction pathways and interaction with receptors. Their therapeutic applications in animal models and humans as well as the pharmacokinetics and toxicity of ginsenosides are also discussed in this review. This review concludes with some thoughts for future directions in the further development of ginseng compounds as effective therapeutic agents.
Keywords: Ginsenoside, antioxidant, structure, eNOS, receptor, signal transduction pathway, therapeutic application, pharmacokinetics, toxicity
I. INTRODUCTION
Ginseng is a perennial herb of the Araliaceae family, species in the genus Panax, and a highly valued medicinal plant in the Far East that has gained popularity in the West during the past decade [1, 2]. The name ginseng comes from the Chinese words “Jen Sheng”, meaning “man-herb”, because of the humanoid shape of the root or rhizome of the plant, which is part of the plant most commonly consumed. The name Panax means “all healing,” which describes the traditional belief that ginseng has properties to heal all aspects of the body. The most common ginsengs are Asian ginseng (Panax ginseng C. A. Meyer) and American ginseng (Panax quinquefolium L.). Panax ginseng cultivated in China, Japan, Korea and Russia has been used as a medicinal plant in China for thousands of years [1]. Panax quinquefolium L., grown in the United States and Canada and been used by Native Americans for hundreds of years [3], is a more popular herbal and nutritional supplement used throughout the world [2, 4]. Ginseng and its constituents, ginsenosides, are thought to possess antineoplastic, antistress and antioxidant effects.
Ginseng is one of the most frequently purchased herbs in the US due to its potential as a chemopreventive agent or adjuvant treatment [5]. In 2002, a national survey of men and women in the US estimated that 4–5% of those aged 45–64 years used ginseng [6]. Two Canadian surveys found that 17–32% of patients with cardiovascular disease reported use of herbs and 6% of those using herbs reported ginseng use [7, 8]. Many H–V infected patients on antiretroviral therapy also take herbal medicines or natural health products. One survey found that 67% of HIV-infected patients on antiretroviral therapy were also taking a natural health product [9]. It was reported to be the 10th most used complementary and alternative medicine in HIV infected patients, used by 34% of those studied [10].
Ginseng is reported to have a wide range of therapeutic and pharmacological uses [11–14]. Researchers are now focused on using purified individual ginsenoside to reveal the mechanism of functions of ginseng instead of using whole ginseng root [11–16]. This may avoid discrepancies as previously reviewed [11, 12, 14]. Each ginsenoside may have different effect in pharmacology and mechanisms due to their different structures. Approximately 40 ginsenoside compounds have been identified, and the separation and analysis methods of ginsenosides are well reviewed [17]. Ginsenosides appear to be responsible for most of the activities of ginseng including vasorelaxation, antioxidation, anti-inflammation and anti-cancer. Among them the most commonly studied ginsenosides are Rb1, Rg1, Rg3, Re, and Rd. A detailed review about effects of ginsenosides Rb1 and Rg1 on anti-amnestic and anti-aging and the mechanism of action was published [16]. Nah et al. also reviewed the studies of effects of ginsenosides on the central nervous system and the peripheral nervous system [18]. A few years ago we reviewed the history of ginseng and the molecular mechanisms and cardiovascular clinical applications of ginseng root [15]. The molecular mechanisms and medical applications of ginsenosides have attracted much attention and hundreds of papers have been published in the last few years. Thereby, it is timely to update recent research progresses of ginsenosides as antioxidants, ligands of receptors and of medical effects on the cardiovascular, immune and neurological systems, signal transduction pathways, and clinical applications as well as pharmacokinetics and toxicity issues.
II. CHEMICAL STRUCTURES AND CLASSIFICATIONS
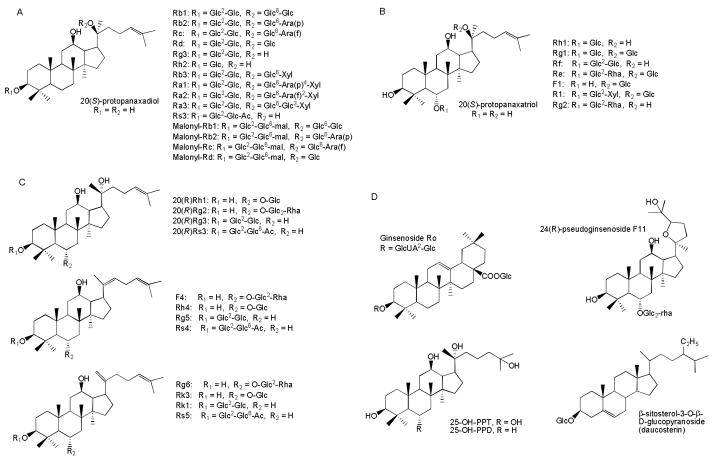
Accumulating evidence suggests that ginsenosides, also called ginseng saponins, are the major pharmacologically active ingredients of ginseng. The ginseng root contains 2–3% ginsenosides of which Rg1, Rc, Rd, Re, Rb1, Rb2, and Rb0 are quantitatively the most important. American ginseng has a higher content of ginsenosides than other ginseng species such as Asian ginseng (Panax ginseng) [19]. Ginsenosides have a 4-ring, steroid-like structure with sugar moieties attached, and, thus far, more than 40 different ginsenosides have been identified and isolated from the root of P. ginseng [16, 20]. Each ginsenoside has at least 2 (carbon-3 and -20) or 3 (carbon-3, -6 and -20) hydroxyl groups, which are free or bound to monomeric, dimeric, or trimeric sugars. Ginsenosides also exist as stereoisomers depending on the position of hydroxyl group on carbon-20. Based on their chemical structures, ginsenosides are generally divided into 2 groups: protopanaxadiols (PD) and protopanaxatriols (PT). The sugar moities in the PD group attach to 3-position of dammarane-type triterpine including Rb1, Rb2, Rc, Rd, Rg3, Rh2, and Rh3 (Fig. 1A), whereas the sugar moities in the PT group attach to 6-position of dammarane-type triterpine including Re, Rf, Rg1, Rg2, and Rh1 (Fig. 1B) [21]. The pseudoginsenoside F11 belongs to PT group although the carbon chain at 20-position is replaced by a tetrahydrofuran ring (Fig. 1C). Several new ginsenosides such as 25-OH-PPD and 25-OH–PPT were recently isolated from ginseng fruit and 25-OH-PPD shows a strong preventive effect to cancer cells (Fig. 1D) [22–24]. Four malonyl derivatives of ginsenosides Rb1, Rb2, Rc and Rd have also been reported (Fig 1C) [25]. The malonyl derivatives and ginsenosides Ro are also called “acidic” ginsenosides while the others are named “neutral” ginsenosides [17].
Fig. 1.
Structure of selected ginsenosides. A. protopanaxadiols (PD). B. protopanaxatriols (PT). C. derivatives of PD and PT. D. new ginsenosides. Glc, β-D-glucose; Rha, α-L-rhamnose; Ara(p), αL-arabinose(pyranose); Ara(f), α-L-arabinose(furanose); Xyl, β-D-xylose; GlcUA, β-D-glucuronic acid; mal, malonyl; Ac, acetyl.
III. GINSENOSIDES AND ANTIOXIDATION
Reactive oxygen species (ROS) have been shown to play a key role in atherosclerotic plaque formation and to be involved in various vascular injuries. Extensive studies have been conducted on the protective effects of ginseng against free radical damage on the vascular endothelium. American ginseng has also been reported to have antioxidant activity in vitro [26]. American ginseng administration increased the activity of the antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPX) in rats [27]. Zhong et al. examined cellular structures of free radical damage on myocardial cells induced by xanthine [28]. They measured free radicals with an electron spin resonance technique and discovered certain ginsenosides (Rb1, Rb2, Rb3, Rc, Re, Rg1, Rg2, and Rh1) counteracting the action of free radicals induced by xanthine. In an animal model, Chen et al. [29] showed that ginsenosides protected against myocardial reperfusion injury with a concomitant increase in 6-keto-Prostaglandin F1a and a decrease in lipid peroxidation, and also protected the rabbit pulmonary and aortic endothelium against electrolysis-induced free radical damage. Additionally, Gillis showed the protective effects of ginsenosides on an injured rabbit pulmonary endothelium induced by a variant of ROS [12]. Ginseng prevented manifestations of ROS injury by promoting the release of nitric oxide (NO). We demonstrate that the endothelial dysfunction induced by homocysteine and HIV protease inhibitors was effectively blocked by Rb1 and other ginsenosides [30, 31] and these results proved that Rb1 and other ginsenosides fully blocked ROS production. Ginsenoside Re has shown antioxidant effects in cardiomyocytes [32], and neuroprotective effects on amyloid and serum free medium induced cellular damage [33]. Ginsenoside Rd can enhance astrocyte differentiation from neural stem cells [34]. Ginsenosides have proved to exert protective effects that are attributed to their antioxidant ability through increasing internal antioxidant enzymes and acting as a free-radical scavenger [32, 35–37].
The relationship between the structure of ginsenoside and its antioxidative or prooxidative activity has been studied in free radical-induced hemolysis of human erythrocytes by Liu et al. [38–41]. It was found that the individual ginsenoside (20(S)-protopanaxadiol or 20(S)-protopanaxatriol) behaves as an antioxidant if a glucose is attached to the 20-position of the triterpene dammarane, such as Re, Rd, and R1, but as a prooxidant if there are no sugar moieties attached to the 20-position of the ginsenoside such as Rg3, Rh2 and Rg2. If a glucose attached to the 6-position instead of 20-position sugar moieties, however, the ginsenoside still act as an antioxidant, that is Rh1. Liu et al. demonstrated that the positions of sugar moieties make the protective activities complicated. On the other hand, the electron paramagnetic resonance (EPR) study of hydroxyl radical-scavenging of ginsenosides found that several ginsenosides showed strong hydroxyl radical scavenging activity and among them 20(S)-Rg3 showed the strongest activity [42–44]. It is not in line to the hemolysis study where the 20(S)-Rg3 act as a prooxidant which means Rg3 did not protect the radical-induced hemolysis or scavenge the radical. Therefore, there are still many unknown factors to be further investigated.
IV. EFFECTS ON THE eNOS SYSTEM
Recently, we have demonstrated that highly active antiretroviral therapy (HAART) drugs may cause vasomotor dysfunction, endothelial nitric oxide synthase (eNOS) downregulation and oxidative stress of the porcine arteries and human endothelial cells, while ginsenosides Rb1, Rc and Re can effectively block these detrimental effects of HAART in vitro [30, 45]. Recent studies from us and others indicated that the eNOS system and ROS may play a crucial role in HAART-associated side effects. Ginseng compounds have a potential to be developed for this purpose because of their history of therapeutic applications and recent discoveries of the molecular actions [30, 45]. For example, ginsenoside Rb1 can effectively block homocysteine-induced endothelial dysfunction and superoxide anion production as well as eNOS downregulation in porcine coronary arteries [30]. Ginsenoside Rb1 also has protective effects on oxidized low-density lipoprotein (oxLDL)-injuring human vascular endothelial cells [46].
Ginsenosides have been shown to stimulate NO production in several systems. Yu et al. examined the purified ginsenoside Rb1 inducing NO production in human aortic endothelial cells [47]. Leung et al. found that Rg1 increased the phosphorylation of glucocorticoid receptor (GR), phosphatidylinositol-3 kinase (PI3K), Akt/PKB and eNOS leading to increase NO production in human umbilical vein endothelial cells (HUVECs) [48]. Kang et al. investigated the relaxation mechanism of ginsenoside Rg3 using isolated canine corpus cavernosum [49]. These results indicate that the mechanism responsible for the relaxation by ginsenoside Rg3 is not by stimulating eNOS for the canine corporal smooth muscle relaxation, but by increasing cyclic nucleotide levels through phosphodiesterases (PDE) inhibition [49]. Furukawa et al. provided compelling evidence that ginsenoside Re activates eNOS to release NO, resulting in activation of the slowly activating delayed rectifier K+ current [50]. Ginsenoside Rg1 enhances NO production and the expression of eNOS mRNA in TNF-α-stimulated HUVECs. Ginsenoside Rg1 regulates the expression of many genes in endothelial cells and protected endothelial cells from TNF-α-induced activation. Microarray analysis has provided with valuable insights into the atheroprotective mechanism by gingsenoside Rg1 [51]. Further studies on the functional roles of these genes in TNF-α-induced activation are warranted.
V. SIGNAL TRANSDUCTION PATHWAYS
Although ginsenosides have been widely used as pharmacological agents for a long time, only a few reports have demonstrated their effects on signal transduction pathways in recent years [52, 53]. Ginsenosides are responsible for their effects on the central nervous system and the peripheral nervous system through the regulation of various types of ion channels, such as voltage-dependent and ligand-gated ion channels, in neuronal and heterologously expressed cells. For example, Xue et al. observed that ginsenosides Rg1 and Rb1 played a major role on the modulation of neurotransmission, where Rb1 promotes neurotransmitter release by increasing the phosphorylation of synapsins via the PKA pathway, while the Rg1 has no relation with the phosphorylation of synapsins [53]. Nah et al. reviewed studies of effects of ginsenosides on the central nervous system [18].
Ginsenosides also play a major inhibitory effect on signal transduction pathways. Ginsenoside Rg1 can block C-Jun N-terminal kinase (JNK) signaling cascade through the protective effect of Rg1 against the phosphorylation of JNK [54]. Ginsenoside Rh2 and compound K showed a significant inhibitory effect on TNF-α-induced expression of intercellular adhesion molecule-1 in human astroglial cells by suppressing TNF-α-induced phosphorylation of IκBα kinase and the subsequent phosphorylation and degradation of IκBα [55]. Additionally, the same treatment inhibited TNF-α-induced phosphorylation of MKK4 and the subsequent activation of the JNK-AP-1 pathway.
Ginsenosides are involved in ion channel regulation. Ginsenoside 20(S)- but not 20(R)-Rg3 and carbohydrate portion of Rg3 play important roles in rat brain NaV1.2 channel regulations, which inhibits voltage-dependent brain Na+ channel activity expressed in Xenopus laevis oocytes [56]. A recent study by the same group found that reduction of double bond in aliphatic side of Rg3 cause an enhancement or loss of brain Na+ channel current inhibitions. These results provide evidence that the aliphatic side chain of Rg3 is involved in Na+ channel regulation and that the enhancement or loss on Na+ channel current inhibitions by Rg3 depends on chemical structures of the aliphatic side chain of Rg3 [57]. Jiang et al. [58, 59] examined the antihypertrophic effect of ginsenoside Rb1-induced by prostaglandin F2α (PGF2α) in vitro and investigated the possible mechanisms involved in the calcineurin (CaN) signal transduction pathway. Their data imply that Rb1 attenuates cardiac hypertrophy, and the underlying mechanism may be involved in the inhibition of the Ca2+-CaN signal transduction pathway [58, 59].
VI. INTERACTION WITH POTENTIAL RECEPTORS
Most natural products can act as full agonist to activate the receptor and result in a maximal biological response. Ligand-induced changes in receptors result in physiological changes which constitute the biological activity of the ligands. Ginsenosides were demonstrated to exert beneficial effects on the cardiovascular system, in which ginsenoside-Re was reported to stimulate vasodilation and angiogenesis in vivo [60, 61]. Angiogenesis is a fundamental process in both physiological and pathological conditions. Therapeutic angiogenesis is now drawing more attention as a treatment of chronic wound or gastric ulcer as well as ischemic tissues [62, 63].
Ginsenosides have been used as ligands for receptors, and their activities and mechanisms were investigated. Ginsenosides Re, Rg1 and Rb1 were demonstrated being functional ligands of glucocorticoid receptor (GR) [48, 64–66], and androgen receptor [47]. They acted as agonists and induced rapid ion influx and NO production in endothelial cells as mentioned in the eNOS section above [48, 50, 64, 65]. For example, Rg1 can indeed serve as an agonist ligand for GR and the activated GR then induces a rapid NO production from eNOS via the non-transcriptional PI3K/Akt pathway [48]. Re acts as a specific agonist for the nongenomic pathway of sex steroid receptors, and NO released from activated eNOS underlies cardiac K+ channel activation and protection against ischemia-reperfusion injury [50]. Ginsenoside Re releases NO via a membrane sex steroid receptors, resulting in K(Ca) channel activation in vascular smooth muscle cells, promoting vasodilation and preventing severe arterial contraction [66].
Rhule et al. investigated the potential for notoginseng extracts to modulate Toll-like receptor (TLR) ligand-induced activation of cultured dendritic cells (DC2.4) and found that ginsenoside Rg1 and Rb1 effectively inhibited lipopolysaccharide-stimulated cytokine production [67]. Dendritic cells (DC2.4) play a central role in the regulation of both inflammation and adaptive immunity. Lee et al. demonstrated that ginsenoside Rg3 inhibited non-competitively 5-hydroxytryptamine 3A subunit receptor (5-HT3A) channel activity on extracellular side of the cell through interactions with residues V291, F292, and I295 in the channel gating region of TM2 [68–70].
Panax ginseng may inhibit tumor growth by affecting both cancer cells and their blood supply. Researchers have found so far that 3 purified ginsenosides are capable of affecting neovascularization and angiogenesis-related properties of endothelial cells. For example, ginsenoside Rb1 can potently inhibit angiogenesis in vivo and in vitro which is a crucial step in tumor growth and metastasis [71]. Its mechanism was that Rb1 suppressed the formation of endothelial tube-like structures through modulation of pigment epithelium-derived factor via estrogen receptor-β (ER_β_) [71, 72]. Rg1 was found to be a phytoestrogen that exerted estrogen-like activity even without direct interaction with oestrogen receptor (ER) in human breast cancer (MCF-7) cells via phosphorylation of AF-1 domain in the absence of receptor binding [73]. These actions of ginsenosides may have potential value in anti-cancer and anti-angiogenesis therapy although some discrepancy still remained in these studies [72].
VII. THERAPEUTIC APPLICATIONS IN ANIMAL MODELS
In animal models, ginseng is able to decrease platelet aggregation [15, 74, 75]. This inhibitory action may be mediated by raising platelet cAMP levels, decreasing production and release of thromboxane A2, and inhibiting prostacyclin (PGI2) production. The retardation of aortic atherosclerotic plaque formation was observed in the rabbit model after eight weeks of feeding ginseng orally [76]. Ginsenosides Rd and Rb were able to attenuate oxidative damage [77, 78], while Re to possess significant anti-hyperglycemic actions and to normalize effectively the impaired oxidative stress in the kidney and eye of the diabetic rats [79]. The preventive effect of ginsenosides on angioplasty-induced neointimal formation was seen in a rat model [80]. Animal studies suggested that ginsenoside Rb1 increased glucose uptake into sheep erythrocytes in a dose dependent manner, while another ginsenoside Rb2 increased the activity of the rate-limiting glycolytic enzymes that affect insulin secretion and modulate glucose disposal [81, 82].
Ginsenosides Rg1 and Rb1 enhance glutamate release in rat cerebrocortical nerve terminals [83]. They have also been shown to have beneficial effects on the central nervous system, especially cognitive function like learning and memory [14, 84]. It has been demonstrated, for example, that ginsenoside Rg1 or Rb1 administration is able to increase the performance in different animal models of learning/memory, such as passive avoidance and Morris water maze tasks [85–87].
In animal model studies, ginsenosides have shown protective or inhibitory effects on some diseases or reactions [77, 78, 83, 88–96]. Ginsenoside Rb1 can prevent the ischemic brain damage or ischemic injury to spiral ganglion cells [78, 90, 91, 93], possibly by acting as a neurotropic factor-like agent and by scavenging free radicals, which are overproduced in situ during and after brain ischemia [78]. The results suggest that gisenosides may be useful for the treatment of neurodegenerative diseases such as Parkinson disease and Alzheimer disease [93]. Rg1 has a protective effect on glutamate-induced lung injury in mice, indicating its clinical application in some lung diseases associated with glutamate toxicity [97]. Orally administered Rd has an immunological adjuvant activity and elicits a Th1 and Th2 immune response by regulating production and gene expression of Th1 cytokines and Th2 cytokines [96]. Ginsenosides potently inhibited the passive cutaneous anaphylaxis (PCA) reaction induced by IgE [95]. These ginsenosides also significantly reduced mRNA expression levels of cyclooxygenase (COX)-2, interleukin (IL)-1β, TNF-α and interferon-γ induced by oxazolone applied to mouse ears [95]. In a rat model with vascular dementia, ginsenoside Rg2 protects memory impairment via anti-apoptosis [89]. The capacity for ginsenoside Rg2 to modulate the expression of apoptotic related proteins suggests that ginsenoside Rg2 may represent a potential treatment strategy for vascular dementia or other ischemic insults [89].
VIII. CLINICAL APPLICATIONS
Natural products and/or their synthetically developed active components have been used in medicine to prevent and treat a variety of disorders. Ginseng is one of the most commonly used natural products with a number of pharmacological effects including immunomodulatory, anti-inflammatory and anti-tumor activities. For example, clinical studies on the effects of ginseng supplements showed that ginseng, added to conventional treatment of diabetes, significantly improved glycemic control by lowering postprandial glycemia without precipitating preprandial hypoglycemia in type II diabetics [98]; treating impotent men with erectile dysfunction (ED) with Korean Red Ginseng (KRG) can effectively improve male ED [99]. Clinically, ginseng has been frequently used in combination with chemotherapy to reduce the side effects of anti-cancer drugs [100]. Furthermore, clinical trials have demonstrated certain therapeutic benefits of ginseng in treating hypertension, attenuating atherosclerotic processes, and improving cardiac function [14, 101–104]. Detail information about clinical studies of ginseng root can be obtained from several reviews [12, 13, 83, 98, 105].
Individual ginsenoside, however, due to incomplete pharmacokinetic parameters and unknown toxicities [106–109], has not been reported for the clinical study so far. There are only few pharmacokinetic studies about ginseng or ginsenosides in humans [110, 111]. Many studies about purified ginsenosides have been devoted to the investigation of their beneficial effects on the pharmacological activities by using animal models and cultured cells. In animal studies, ginsenosides had many protective activities as discussed above, which can be potentially used to treat human diseases. Ginsenosides Rg3 and Rh2 have been reported to have a cell-growth suppressive effect on various cancer cells [112, 113]. Ginsenoside 25-OH-PPD had significant, dose-dependent effects on apoptosis, proliferation, and cell cycle progression [114] and showed preventive effect to cancer cells [22, 23]. Ginsinosides Rh2 and Rb1 have also shown activity in reducing ischemic brain injury in rats after oral administration [78, 90, 91, 93, 115].
Researchers have studied safe dosage of ginsenosides used on animals. For example, a low dose (10 μM or 11.09 μg/ml) of Rb1 has significant preventive effect on HUVEC proliferation and superoxide anion production in vitro and found that Rb1 completely blocked the effect of homocysteine on endothelial cells [30, 46, 116]. Orally administered ginsenoside Re, Rg1, or Rg3 of only 25 mg/kg of the compounds in the Tg2576 mouse model results in a significant reduction the amount of Alzheimer’s Aβ peptide detected in the brains of these animals at 18 h post-drug administration [117]. Although results from animal teratogenicity study may not reflect the circumstances in humans, we should be careful with using ginsenosides [108]
IX. PHARMACOKINETICS AND TOXICITY ISSUES
The investigations of the pharmacokinetics and bioavailability of ginsenosides can link data from pharmacological assays to clinical effects and also help in designing rational dosage regimens. The analytical methods for determining ginsenosides have been achieved using thin layer chromatography (TLC), enzyme immunoassay (EIA), high performance liquid chromatography with ultraviolet detection (HPLC–UV), high performance liquid chromatography with fluorescence detection (HPLC–FLD), liquid chromatography–evaporative light-scattering detection (LC–ELSD), and LC MS and liquid chromatography-tandem mass spectrometry (LC–MS/MS) [17, 118]. Among these methods, HPLC–MS and MS/MS techniques provide excellent methods for the simultaneous quantification of multiple ginsenosides in animal plasma and are successfully applied to the pharmacokinetic study of a multiple-constituent natural medicine even at a low dose There are a few reports on LC/MS analysis of ginsenosides extracted from biological samples [114, 118–124].
The only pharmacokinetics studies of ginsenosides in human are reported by Cui et al. [110, 111], which showed that ginsenosides are present in urine after oral ingestion. About 1.2% of the dose was recovered in 5 days. Further investigations are necessary to evaluate the pharmacokinetics and placental transfer of ginsenosides in humans.
Generally, ginsenosides are very poorly absorbed following oral administration in vivo [114, 121]. Li et al. studied the pharmacokinetic of the oral administration of ginseng powder in rats and found that the absorption of ginsenosides was quick, but the maximum concentration of R1, Rg1, Rd, Re and Rb1 in rat plasma was from 1.5 to 6.4 μg/ml [122, 123]. The absolute bioavailability of Panax notoginsenoside R1, ginsenside Rg1, Rd, Re and Rb1 were of 9.29%, 6.06%, 2.36%, 7.06% and 1.18%, respectively. Wang et al. reported the absolute bioavailability of Rd in dogs was 0.26% [121]. It was reported the absolute bioavailability of ginsenoside Rg3 in rats was 2.63% [125] or undetectable in oral dosing samples [126]. Xu et al. reported the oral bioavailability of Rg1 was 18.4% in rats [127] and Li et al. reported the absolute bioavailability was 15.62% for Rg1, 0.28% for Rb1 and 0.34% for Rd [128]. Paek et al. reported the absolute bioavailability was 35.0% for a ginseng saponin metabolite compound K at the 20 mg/kg dose [129].
Several newly identified ginsenosides, such as 25-OH-PPD, 20(S)-25-methoxyl-dammarane-3β, 12β, 20-triol (25-OCH3-PPD) [22–24], had significant, dose-dependent effects on apoptosis, proliferation, and cell cycle progression. 25-OH-PPD, its IC50 values for most cell lines were in the range of 10–60 μM, demonstrating a 5–15 fold greater growth inhibition than Rg3 [114]. The absolute bioavailability of 25-OH-PPD is 64.8±14.3% (range 44.1–75.9%) which is the highest among the reports in ginseng compounds, and it is very beneficial to the drug with anti-tumor activity in clinical applications in the future. Pharmacokinetic studies of selected ginsenosides in rats, dogs or human plasmas are provided in Table 1.
Table 1.
Pharmacokinetic studies of selected ginsenosides in rats, dogs or human plasmas
| Ginsinosides | Animal model | Method | Dosage | Absolute bioavailability | Ref. |
|---|---|---|---|---|---|
| 25-OH-PPD | rat plasma, oral | HPLC/MS | 10 mg/kg | 64.8% | [114] |
| Rh2 | Rat, oral, in vivo | LC/MS, ESI/MS | 100 mg/kg | 0.25% | [119] |
| Rh1, Rg1 | Rat plasma, i.v., or i.g. | LC/MS | 100 mg/kg | 1.33% Rg1 | [118] |
| 20(R)-, 20(S)-Rg2 | Rat plasma, i.v., | HPLC | 25 mg/kg | - | [120] |
| Rd | Human plasma, in vivo | LC/ESI/MS | 10 mg/kg | - | [124] |
| R1, Rg1, Rd, Re, | Rat plasma | HPLC/ESI/MS | 10 mg/kg Rb1 | 9.29%, 6.06%, 2.36%, 7.06% and 1.18% | [122, 123] |
| Rd | Dog plasma, i.v., oral | LC/MS | 2 mg/kg (oral)0.2 mg/kg (i.v) | 0.26% | [121] |
| Rg1 | Rat, oral, in vivo, in vitro | HPLC | 50 mg/kg | 1.52–6.60% | [130] |
| Rg3 | Rat plasma | LC/ESI/MS | 50 mg/kg | 2.63% | [125, 126] |
| multiple | Rat plasma | LC/ESI/MS | 300 mg/kg | - | [123] |
| Rg1, Rb1 | Rat plasma | HPLC | 50 mg/kg | 18.4% (Rg1)4.35% (Rb1) | [127] |
| compound K | Rat | LC/MS | 20 mg/kg | 35.0% | [129] |
The reasons of the poor bioavailability of ginsenosides include that these compounds may be destroyed in the gastrointestinal tract, metabolized by intestinal microflora and excreted from bile or urine [130]. On the other hand, low membrane permeability may be a more important factor in determining the extent of absorption [130]. The higher absolute bioavailability is found in the rats and it could be hypothesized that 25-OH-PPD possesses deglycosylated mother aglycone structure, lower molecular weight, higher hydrophobility than those of ginsenoside Rg3. Thus, 25-OH-PPD is well absorbed by the digestive tract [114].
These ginsenosides 25-OH-PPD, 20(S)-25-methoxyl-dammarane-3β, 12β, 20-triol (25-OCH3-PPD) also have low toxicity to non-cancer cells and no observable host toxicity in animals either alone or in combination with conventional therapies [22–24]. These compounds may have potential as novel prostate cancer therapeutic agents [22–24]. Furthermore, ginsenoside Rg3 in mouse model studies has a preventive effect on DNA damage and cell death induced by cyclophosphamide [131] and has an inhibitory effect on genotoxicity, chemical and histological changes induced by ethylenediaminetetraacetic acid (EDTA), which is widely used in food [132]. The dosage used in these studies was 20 mg/kg and it was found that ginsenoside Rg3 alone did not induce any genotoxicity in mouse peripheral lymphocyte cells and bone marrow cells [131].
However, ginseng is commonly used by pregnant women and the most common reason for consumption is ‘good for pregnancy and fetus. Data concerning the potential beneficial and adverse effects of ginseng during pregnancy is sparse. As discussed in the clinical applications, individual ginsenoside has not been used on the patients due to its unknown toxicity. Researchers have investigated the toxicity of ginsenosides Rb1, Re, Rc, and Rh2 recently using a whole mouse embryo culture model and in intestinal Caco-2 cells [106–109]. Chan et al. reported that ginsenosides Rb1 and Re were embryotoxic. Rb1 or Re induced a strong embryotoxic effect at a concentration of 50 μg/ml. However, Rc did not demonstrate any adverse effect towards developing rat embryos at the same concentration as of Re. It seems that Re induced a severe developmental delay with significant reduction in morphological scores of all systems assessed, rather than a teratogenic effect on a particular organ system. Using the same model, Liu et al. found that ginsenoside Rb1 at 50 mg/ml affected allantois, flexion, branchial arch, and limb buds [108]. At this concentration, the embryonic crown-rump length, head length, and somite number were also reduced significantly compared to the control group [108]. Although results from animal tests may not reflect the true complexion in humans and the potential mechanism of developmental toxicity of ginsenosides remains unclear, these results suggest that ginseng compounds should be used with caution by pregnant women until more human data are available [107, 108].
X. SUMMARY AND FUTURE DIRECTIONS
Ginseng is believed to be the most traditional medical herb contains many active constituent ginsenosides. It has extensive pharmacological effects and specific mechanisms of action in Chinese herb medicine. Ginsenosides can inhibit ROS production, stimulate NO production, increase immune function, enhance central nervous system function, and prevent cardiovascular or other diseases. Animal studies indicate that ginsenosides have different activities in both physiological and pathologic conditions. How these effects relate to the ginsenoside structures are not yet fully elucidated. Future research involving each ginsenoside should include the mechanisms of action, specificity, structure and function relationship, detailed pharmacokinetics and toxicity studies, and therapeutic studies in both animal models and human trials.
Acknowledgments
This work is partially supported by research grants from the National Institutes of Health (Qizhi Yao: DE15543 and AT003094; and Changyi Chen: HL72716, EB-002436, and HL083471) and by the Michael E. DeBakey Department of Surgery, Baylor College of Medicine, Houston, Texas.
LIST OF ABBREVIATIONS
COX
cyclooxygenase
CaN
calcineurin
DC2.4
dendritic cells
eNOS
endothelial nitric oxide synthase
EPR
electron paramagnetic resonance
GPX
glutathione peroxidase
GR
glucocorticoid receptor
HAART
highly active antiretroviral therapy
Hcy
homocysteine
HPLC
high performance liquid chromatography
HUVEC
human umbilical vein endothelial cell
IC50
half maximal (50%) inhibitory concentration (IC) of a substance
JNK
C-Jun N-terminal kinase
LC–MS/MS
liquid chromatography-tadem mass spectrometry
NO
nitric oxide
oxLDL
oxidized low density lipoprotein
PD
protopanaxadiols
PDE
phosphodiesterases
PGF2α
prostaglandin F2α
PI3K
phosphatidylinositol-3 kinase
PKB
protein kinase B
PT
protopanaxatriols
ROS
reactive oxygen species
SOD
superoxide dismutase
TLR
toll-like receptor
TNF-α
tumor necrosis factor-alpha
References
- 1.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–74. [PubMed] [Google Scholar]
- 2.Bames AS, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults. Adv Data. 2004;343:1–19. [PubMed] [Google Scholar]
- 3.Borchers AT, Keen CL, Stern JS, Gershwin ME. Inflammation and Native American medicine: the role of botanicals. Am J Clin Nutr. 2000;72:339–47. doi: 10.1093/ajcn/72.2.339. [DOI] [PubMed] [Google Scholar]
- 4.Harkey MR, Henderson GL, Gershwin ME, Stern JS, Hackman RM. Variability in commercial ginseng products: an analysis of 25 preparations. Am J Clin Nutr. 2001;73:1101–6. doi: 10.1093/ajcn/73.6.1101. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal M. Herb sales down in mainstream market, up in natural food stores. Herbalgram. 2002 summer;55:60. [Google Scholar]
- 6.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. Jama. 2002;287:337–44. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 7.Pharand C, Ackman ML, Jackevicius CA, Paradiso-Hardy FL, Pearson GJ. Use of OTC and herbal products in patients with cardiovascular disease. Ann Pharmacother. 2003;37:899–904. doi: 10.1345/aph.1C163. [DOI] [PubMed] [Google Scholar]
- 8.Wood MJ, Stewart RL, Merry H, Johnstone DE, Cox JL. Use of complementary and alternative medical therapies in patients with cardiovascular disease. Am Heart J. 2003;145:806–12. doi: 10.1016/S0002-8703(03)00084-X. [DOI] [PubMed] [Google Scholar]
- 9.Gore-Felton C, Vosvick M, Power R, et al. Alternative therapies: a common practice among men and women living with HIV. J Assoc Nurses AIDS Care. 2003;14:17–27. doi: 10.1177/1055329003014003002. [DOI] [PubMed] [Google Scholar]
- 10.Standish LJ, Greene KB, Bain S, et al. Alternative medicine use in HIV-positive men and women: demographics, utilization patterns and health status. AIDS Care. 2001;13:197–208. doi: 10.1080/095401201300059759. [DOI] [PubMed] [Google Scholar]
- 11.Buettner C, Yeh GY, Phillips RS, Mittleman MA, Kaptchuk TJ. Systematic review of the effects of ginseng on cardiovascular risk factors. Ann Pharmacother. 2006;40:83–95. doi: 10.1345/aph.1G216. [DOI] [PubMed] [Google Scholar]
- 12.Gillis CN. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 13.Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137:183S–5S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 14.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–93. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q, Chen CJ. Molecular mechanisms and clinical applications of ginseng root for cardiovascular disease. Med Sci Monit. 2004;10:RA187–92. [PubMed] [Google Scholar]
- 16.Cheng Y, Shen LH, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–9. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 17.Fuzzati N. Analysis methods of ginsenosides. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:119–33. doi: 10.1016/j.jchromb.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Nah SY, Kim DH, Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007;13:381–404. doi: 10.1111/j.1527-3458.2007.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dharmananda S. The nature of ginseng: traditional use, modern research, and the question of dosage. HerbalGram. 2002;54:34–51. [Google Scholar]
- 20.Nah SY. Ginseng: Recent advances and trends. Korean J Ginseng Sci. 1997;21:1–12. [Google Scholar]
- 21.Seo JY, Lee JH, Kim NW, et al. Effect of a fermented ginseng extract, BST204, on the expression of cyclooxygenase-2 in murine macrophages. Int Immunopharmacol. 2005;5:929–36. doi: 10.1016/j.intimp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Zhao Y, Rayburn ER, Hill DL, Wang H, Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. 2007;59:589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Rayburn ER, Hao M, et al. Experimental therapy of prostate cancer with novel natural product anti-cancer ginsenosides. Prostate. 2008;68:809–19. doi: 10.1002/pros.20742. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Wang H, Rayburn ER, Zhao Y, Hill DL, Zhang R. 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol, a novel natural product for prostate cancer therapy: activity in vitro and in vivo and mechanisms of action. Br J Cancer. 2008;98:792–802. doi: 10.1038/sj.bjc.6604227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitagawa I, Taniyama T, Yoshikawa M, Ikennishi Y, Nakagawa Y. Chem Pharm Bull. 1989;37:2961. [Google Scholar]
- 26.Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000;203:1–10. doi: 10.1023/a:1007078414639. [DOI] [PubMed] [Google Scholar]
- 27.Fu Y, Ji LL. Chronic ginseng consumption attenuates age-associated oxidative stress in rats. J Nutr. 2003;133:3603–9. doi: 10.1093/jn/133.11.3603. [DOI] [PubMed] [Google Scholar]
- 28.Zhong G, Jiang Y. Calcium channel blockage and anti-free-radical actions of ginsenosides. Chin Med J (Engl) 1997;110:28–9. [PubMed] [Google Scholar]
- 29.Chen X. Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin Exp Pharmacol Physiol. 1996;23:728–32. doi: 10.1111/j.1440-1681.1996.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhou W, Chai H, Lin PH, Lumsden AB, Yao Q, Chen C. Ginsenoside Rb1 blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2005;41:861–8. doi: 10.1016/j.jvs.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Chai H, Yao Q, Chen C. Molecular mechanisms of HIV protease inhibitor-induced endothelial dysfunction. J Acquir Immune Defic Syndr. 2007;44:493–9. doi: 10.1097/QAI.0b013e3180322542. [DOI] [PubMed] [Google Scholar]
- 32.Xie JT, Shao ZH, Vanden Hoek TL, et al. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol. 2006;532:201–7. doi: 10.1016/j.ejphar.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Ji ZN, Dong TT, Ye WC, Choi RC, Lo CK, Tsim KW. Ginsenoside Re attenuate beta-amyloid and serum-free induced neurotoxicity in PC12 cells. J Ethnopharmacol. 2006;107:48–52. doi: 10.1016/j.jep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Shi Q, Hao Q, Bouissac J, Lu Y, Tian S, Luu B. Ginsenoside-Rd from Panax notoginseng enhances astrocyte differentiation from neural stem cells. Life Sci. 2005;76:983–95. doi: 10.1016/j.lfs.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 35.Deng HL, Zhang JT. Anti-lipid peroxilative effect of ginsenoside Rb1 and Rg1. Chin Med J (Engl) 1991;104:395–8. [PubMed] [Google Scholar]
- 36.Lim JH, Wen TC, Matsuda S, et al. Protection of ischemic hippocampal neurons by ginsenoside Rb1, a main ingredient of ginseng root. Neurosci Res. 1997;28:191–200. doi: 10.1016/s0168-0102(97)00041-2. [DOI] [PubMed] [Google Scholar]
- 37.Tian J, Fu F, Geng M, et al. Neuroprotective effect of 20(S)-ginsenoside Rg3 on cerebral ischemia in rats. Neurosci Lett. 2005;374:92–7. doi: 10.1016/j.neulet.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z, Li Z, Liu X. Effect of ginsenoside Re on cardiomyocyte apoptosis and expression of Bcl-2/Bax gene after ischemia and reperfusion in rats. J Huazhong Univ Sci Technolog Med Sci. 2002;22:305–9. doi: 10.1007/BF02896771. [DOI] [PubMed] [Google Scholar]
- 39.Liu ZQ, Luo XY, Liu GZ, Chen YP, Wang ZC, Sun YX. In vitro study of the relationship between the structure of ginsenoside and its antioxidative or prooxidative activity in free radical induced hemolysis of human erythrocytes. J Agric Food Chem. 2003;51:2555–8. doi: 10.1021/jf026228i. [DOI] [PubMed] [Google Scholar]
- 40.Liu ZQ, Luo XY, Sun YX, Chen YP, Wang ZC. Can ginsenosides protect human erythrocytes against free-radical-induced hemolysis? Biochim Biophys Acta. 2002;1572:58–66. doi: 10.1016/s0304-4165(02)00281-7. [DOI] [PubMed] [Google Scholar]
- 41.Li GX, Liu ZQ. The protective effects of ginsenosides on human erythrocytes against hemin-induced hemolysis. Food Chem Toxicol. 2008;46:886–92. doi: 10.1016/j.fct.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Kang KS, Yokozawa T, Yamabe N, Kim HY, Park JH. ESR study on the structure and hydroxyl radical-scavenging activity relationships of ginsenosides isolated from Panax ginseng C A Meyer. Biol Pharm Bull. 2007;30:917–21. doi: 10.1248/bpb.30.917. [DOI] [PubMed] [Google Scholar]
- 43.Kang KS, Kim HY, Baek SH, Yoo HH, Park JH, Yokozawa T. Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 2007;30:724–8. doi: 10.1248/bpb.30.724. [DOI] [PubMed] [Google Scholar]
- 44.Kang KS, Yamabe N, Kim HY, Okamoto T, Sei Y, Yokozawa T. Increase in the free radical scavenging activities of American ginseng by heat processing and its safety evaluation. J Ethnopharmacol. 2007;113:225–32. doi: 10.1016/j.jep.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 45.Chai H, Zhou W, Lin P, Lumsden A, Yao Q, Chen C. Ginsenosides block HIV protease inhibitor ritonavir-induced vascular dysfunction of porcine coronary arteries. Am J Physiol Heart Circ Physiol. 2005;288:H2965–71. doi: 10.1152/ajpheart.01271.2004. [DOI] [PubMed] [Google Scholar]
- 46.He F, Guo R, Wu SL, Sun M, Li M. Protective effects of ginsenoside Rb1 on human umbilical vein endothelial cells in vitro. J Cardiovasc Pharmacol. 2007;50:314–20. doi: 10.1097/FJC.0b013e3180cab12e. [DOI] [PubMed] [Google Scholar]
- 47.Yu J, Eto M, Akishita M, Kaneko A, Ouchi Y, Okabe T. Signaling pathway of nitric oxide production induced by ginsenoside Rb1 in human aortic endothelial cells: a possible involvement of androgen receptor. Biochem Biophys Res Commun. 2007;353:764–9. doi: 10.1016/j.bbrc.2006.12.119. [DOI] [PubMed] [Google Scholar]
- 48.Leung KW, Cheng YK, Mak NK, Chan KK, Fan TP, Wong RN. Signaling pathway of ginsenoside-Rg1 leading to nitric oxide production in endothelial cells. FEBS Lett. 2006;580:3211–6. doi: 10.1016/j.febslet.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 49.Kang YJ, Sohn JT, Chang KC. Relaxation of canine corporal smooth muscle relaxation by ginsenoside saponin Rg3 is independent from eNOS activation. Life Sci. 2005;77:74–84. doi: 10.1016/j.lfs.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 50.Furukawa T, Bai CX, Kaihara A, et al. Ginsenoside Re, a main phytosterol of Panax ginseng, activates cardiac potassium channels via a nongenomic pathway of sex hormones. Mol Pharmacol. 2006;70:1916–24. doi: 10.1124/mol.106.028134. [DOI] [PubMed] [Google Scholar]
- 51.Lu JP, Ma ZC, Yang J, Huang J, Wang SR, Wang SQ. Ginsenoside Rg1-induced alterations in gene expression in TNF-alpha stimulated endothelial cells. Chin Med J (Engl) 2004;117:871–6. [PubMed] [Google Scholar]
- 52.Lee JH, Jeong SM, Lee BH, et al. Prevention of ginsenoside-induced desensitization of Ca2+-activated Cl- current by microinjection of inositol hexakisphosphate in Xenopus laevis oocytes: involvement of GRK2 and beta-arrestin I. J Biol Chem. 2004;279:9912–21. doi: 10.1074/jbc.M310824200. [DOI] [PubMed] [Google Scholar]
- 53.Xue JF, Liu ZJ, Hu JF, Chen H, Zhang JT, Chen NH. Ginsenoside Rb1 promotes neurotransmitter release by modulating phosphorylation of synapsins through a cAMP-dependent protein kinase pathway. Brain Res. 2006;1106:91–8. doi: 10.1016/j.brainres.2006.05.106. [DOI] [PubMed] [Google Scholar]
- 54.Chen XC, Zhou YC, Chen Y, Zhu YG, Fang F, Chen LM. Ginsenoside Rg1 reduces MPTP-induced substantia nigra neuron loss by suppressing oxidative stress. Acta Pharmacol Sin. 2005;26:56–62. doi: 10.1111/j.1745-7254.2005.00019.x. [DOI] [PubMed] [Google Scholar]
- 55.Choi K, Kim M, Ryu J, Choi C. Ginsenosides compound K and Rh(2) inhibit tumor necrosis factor-alpha-induced activation of the NF-kappaB and JNK pathways in human astroglial cells. Neurosci Lett. 2007;421:37–41. doi: 10.1016/j.neulet.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 56.Lee JH, Jeong SM, Kim JH, et al. Characteristics of ginsenoside Rg3-mediated brain Na+ current inhibition. Mol Pharmacol. 2005;68:1114–26. doi: 10.1124/mol.105.015115. [DOI] [PubMed] [Google Scholar]
- 57.Lee JH, Choi SH, Lee BH, et al. Modifications of aliphatic side chain of 20(S)-ginsenoside RG3 cause an enhancement or loss of brain Na+ channel current inhibitions. Biol Pharm Bull. 2008;31:480–6. doi: 10.1248/bpb.31.480. [DOI] [PubMed] [Google Scholar]
- 58.Jiang QS, Huang XN, Dai ZK, et al. Inhibitory effect of ginsenoside Rb1 on cardiac hypertrophy induced by monocrotaline in rat. J Ethnopharmacol. 2007;111:567–72. doi: 10.1016/j.jep.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Jiang QS, Huang XN, Yang GZ, Jiang XY, Zhou QX. Inhibitory effect of ginsenoside Rb1 on calcineurin signal pathway in cardiomyocyte hypertrophy induced by prostaglandin F2alpha. Acta Pharmacol Sin. 2007;28:1149–54. doi: 10.1111/j.1745-7254.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 60.Huang YC, Chen CT, Chen SC, et al. A natural compound (ginsenoside Re) isolated from Panax ginseng as a novel angiogenic agent for tissue regeneration. Pharm Res. 2005;22:636–46. doi: 10.1007/s11095-005-2500-3. [DOI] [PubMed] [Google Scholar]
- 61.Scott GI, Colligan PB, Ren BH, Ren J. Ginsenosides Rb1 and Re decrease cardiac contraction in adult rat ventricular myocytes: role of nitric oxide. Br J Pharmacol. 2001;134:1159–65. doi: 10.1038/sj.bjp.0704377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang SC. Experimental studies on action of ginseng flower saponins and ginsenoside Re in gastric ulcers in rats. Zhong Yao Tong Bao. 1985;10:43–4. [PubMed] [Google Scholar]
- 63.Fan TP, Yeh JC, Leung KW, Yue PY, Wong RN. Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci. 2006;27:297–309. doi: 10.1016/j.tips.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Leung KW, Leung FP, Huang Y, Mak NK, Wong RN. Non-genomic effects of ginsenoside-Re in endothelial cells via glucocorticoid receptor. FEBS Lett. 2007;581:2423–8. doi: 10.1016/j.febslet.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 65.Leung KW, Pon YL, Wong RN, Wong AS. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem. 2006;281:36280–8. doi: 10.1074/jbc.M606698200. [DOI] [PubMed] [Google Scholar]
- 66.Nakaya Y, Mawatari K, Takahashi A, Harada N, Hata A, Yasui S. The phytoestrogen ginsensoside Re activates potassium channels of vascular smooth muscle cells through PI3K/Akt and nitric oxide pathways. J Med Invest. 2007;54:381–4. doi: 10.2152/jmi.54.381. [DOI] [PubMed] [Google Scholar]
- 67.Rhule A, Rase B, Smith JR, Shepherd DM. Toll-like receptor ligand-induced activation of murine DC2.4 cells is attenuated by Panax notoginseng. J Ethnopharmacol. 2008;116:179–86. doi: 10.1016/j.jep.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee BH, Lee JH, Lee SM, et al. Identification of ginsenoside interaction sites in 5-HT3A receptors. Neuropharmacology. 2007;52:1139–50. doi: 10.1016/j.neuropharm.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 69.Lee BH, Jeong SM, Ha TS, et al. Ginsenosides regulate ligand-gated ion channels from the outside. Mol Cells. 2004;18:115–21. [PubMed] [Google Scholar]
- 70.Jeong SM, Lee JH, Kim JH, et al. Stereospecificity of ginsenoside Rg3 action on ion channels. Mol Cells. 2004;18:383–9. [PubMed] [Google Scholar]
- 71.Leung KW, Cheung LW, Pon YL, et al. Ginsenoside Rb1 inhibits tube-like structure formation of endothelial cells by regulating pigment epithelium-derived factor through the oestrogen beta receptor. Br J Pharmacol. 2007;152:207–15. doi: 10.1038/sj.bjp.0707359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papapetropoulos A. A ginseng-derived oestrogen receptor beta (ERbeta) agonist, Rb1 ginsenoside, attenuates capillary morphogenesis. Br J Pharmacol. 2007;152:172–4. doi: 10.1038/sj.bjp.0707360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lau WS, Chan RY, Guo DA, Wong MS. Ginsenoside Rg1 exerts estrogen-like activities via ligand-independent activation of ERalpha pathway. J Steroid Biochem Mol Biol. 2008;108:64–71. doi: 10.1016/j.jsbmb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 74.Cui X, Sakaguchi T, Shirai Y, Hatakeyama K. Orally administered Panax ginseng extract decreases platelet adhesiveness in 66% hepatectomized rats. Am J Chin Med. 1999;27:251–6. doi: 10.1142/S0192415X99000288. [DOI] [PubMed] [Google Scholar]
- 75.Liu CX, Xiao PG. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- 76.Shi L, Fan PS, Wu L, Fang JX, Han ZX. Effects of total saponins of Panax notoginseng on increasing PGI2 in carotid artery and decreasing TXA2 in blood platelets. Zhongguo Yao Li Xue Bao. 1990;11:29–32. [PubMed] [Google Scholar]
- 77.Yokozawa T, Satoh A, Cho EJ. Ginsenoside-Rd attenuates oxidative damage related to aging in senescence-accelerated mice. J Pharm Pharmacol. 2004;56:107–13. doi: 10.1211/0022357022449. [DOI] [PubMed] [Google Scholar]
- 78.Zhang B, Matsuda S, Tanaka J, et al. Ginsenoside Rb(1) prevents image navigation disability, cortical infarction, and thalamic degeneration in rats with focal cerebral ischemia. J Stroke Cerebrovasc Dis. 1998;7:1–9. doi: 10.1016/s1052-3057(98)80015-3. [DOI] [PubMed] [Google Scholar]
- 79.Cho WC, Chung WS, Lee SK, Leung AW, Cheng CH, Yue KK. Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2006;550:173–9. doi: 10.1016/j.ejphar.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 80.Wu CH, Tsai BR, Hsieh WT, Chang GY, Mao SJ, Chang WC. The preventive effects of G115 on balloon injury-induced neointima formation in rats. Life Sci. 2001;70:669–79. doi: 10.1016/s0024-3205(01)01442-4. [DOI] [PubMed] [Google Scholar]
- 81.Hasegawa H, Matsumiya S, Uchiyama M, et al. Inhibitory effect of some triterpenoid saponins on glucose transport in tumor cells and its application to in vitro cytotoxic and antiviral activities. Planta Med. 1994;60:240–3. doi: 10.1055/s-2006-959467. [DOI] [PubMed] [Google Scholar]
- 82.Onomura M, Tsukada H, Fukuda K, et al. Effects of ginseng radix on sugar absorption in the small intestine. Am J Chin Med. 1999;27:347–54. doi: 10.1142/S0192415X99000392. [DOI] [PubMed] [Google Scholar]
- 83.Chang Y, Huang WJ, Tien LT, Wang SJ. Ginsenosides Rg1 and Rb1 enhance glutamate release through activation of protein kinase A in rat cerebrocortical nerve terminals (synaptosomes) Eur J Pharmacol. 2008;578:28–36. doi: 10.1016/j.ejphar.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 84.Radad K, Gille G, Moldzio R, Saito H, Rausch WD. Ginsenosides Rb1 and Rg1 effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004;1021:41–53. doi: 10.1016/j.brainres.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 85.Benishin CG, Lee R, Wang LC, Liu HJ. Effects of ginsenoside Rb1 on central cholinergic metabolism. Pharmacology. 1991;42:223–9. doi: 10.1159/000138801. [DOI] [PubMed] [Google Scholar]
- 86.Yamaguchi Y, Haruta K, Kobayashi H. Effects of ginsenosides on impaired performance induced in the rat by scopolamine in a radial-arm maze. Psychoneuroendocrinology. 1995;20:645–53. doi: 10.1016/0306-4530(95)00008-c. [DOI] [PubMed] [Google Scholar]
- 87.Mook-Jung I, Hong HS, Boo JH, et al. Ginsenoside Rb1 and Rg1 improve spatial learning and increase hippocampal synaptophysin level in mice. J Neurosci Res. 2001;63:509–15. doi: 10.1002/jnr.1045. [DOI] [PubMed] [Google Scholar]
- 88.Lee JH, Han Y. Ginsenoside Rg1 helps mice resist to disseminated candidiasis by Th1 type differentiation of CD4+ T cell. Int Immunopharmacol. 2006;6:1424–30. doi: 10.1016/j.intimp.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 89.Zhang G, Liu A, Zhou Y, San X, Jin T, Jin Y. Panax ginseng ginsenoside-Rg2 protects memory impairment via anti-apoptosis in a rat model with vascular dementia. J Ethnopharmacol. 2008;115:441–8. doi: 10.1016/j.jep.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 90.Fujita K, Hakuba N, Hata R, et al. Ginsenoside Rb1 protects against damage to the spiral ganglion cells after cochlear ischemia. Neurosci Lett. 2007;415:113–7. doi: 10.1016/j.neulet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 91.Yuan QL, Yang CX, Xu P, et al. Neuroprotective effects of ginsenoside Rb1 on transient cerebral ischemia in rats. Brain Res. 2007;1167:1–12. doi: 10.1016/j.brainres.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 92.Zhang HS, Wang SQ. Ginsenoside Rg1 inhibits tumor necrosis factor-alpha (TNF-alpha)-induced human arterial smooth muscle cells (HASMCs) proliferation. J Cell Biochem. 2006;98:1471–81. doi: 10.1002/jcb.20799. [DOI] [PubMed] [Google Scholar]
- 93.Sakanaka M, Zhu P, Zhang B, et al. Intravenous infusion of dihydroginsenoside Rb1 prevents compressive spinal cord injury and ischemic brain damage through upregulation of VEGF and Bcl-XL. J Neurotrauma. 2007;24:1037–54. doi: 10.1089/neu.2006.0182. [DOI] [PubMed] [Google Scholar]
- 94.Kim HA, Kim S, Chang SH, Hwang HJ, Choi YN. Anti-arthritic effect of ginsenoside Rb1 on collagen induced arthritis in mice. Int Immunopharmacol. 2007;7:1286–91. doi: 10.1016/j.intimp.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 95.Bae EA, Han MJ, Shin YW, Kim DH. Inhibitory effects of Korean red ginseng and its genuine constituents ginsenosides Rg3, Rf, and Rh2 in mouse passive cutaneous anaphylaxis reaction and contact dermatitis models. Biol Pharm Bull. 2006;29:1862–7. doi: 10.1248/bpb.29.1862. [DOI] [PubMed] [Google Scholar]
- 96.Yang Z, Chen A, Sun H, Ye Y, Fang W. Ginsenoside Rd elicits Th1 and Th2 immune responses to ovalbumin in mice. Vaccine. 2007;25:161–9. doi: 10.1016/j.vaccine.2006.05.075. [DOI] [PubMed] [Google Scholar]
- 97.Shen L, Han JZ, Li C, et al. Protective effect of ginsenoside Rg1 on glutamate-induced lung injury. Acta Pharmacol Sin. 2007;28:392–7. doi: 10.1111/j.1745-7254.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- 98.Vuksan V, Sievenpiper JL, Xu Z, et al. Konjac-Mannan and American ginsing: emerging alternative therapies for type 2 diabetes mellitus. J Am Coll Nutr. 2001;20:370S–80S. doi: 10.1080/07315724.2001.10719170. discussion 81S–83S. [DOI] [PubMed] [Google Scholar]
- 99.de Andrade E, de Mesquita AA, Claro Jde A, et al. Study of the efficacy of Korean Red Ginseng in the treatment of erectile dysfunction. Asian J Androl. 2007;9:241–4. doi: 10.1111/j.1745-7262.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- 100.Yance DR, Jr, Sagar SM. Targeting angiogenesis with integrative cancer therapies. Integr Cancer Ther. 2006;5:9–29. doi: 10.1177/1534735405285562. [DOI] [PubMed] [Google Scholar]
- 101.Nilsson M, Trehn G, Asplund K. Use of complementary and alternative medicine remedies in Sweden. A population-based longitudinal study within the northern Sweden MONICA Project. Multinational Monitoring of Trends and Determinants of Cardiovascular Disease. J Intern Med. 2001;250:225–33. doi: 10.1046/j.1365-2796.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- 102.Caron MF, Hotsko AL, Robertson S, Mandybur L, Kluger J, White CM. Electrocardiographic and hemodynamic effects of Panax ginseng. Ann Pharmacother. 2002;36:758–63. doi: 10.1345/aph.1A411. [DOI] [PubMed] [Google Scholar]
- 103.Ding DZ, Shen TK, Cui YZ. Effects of red ginseng on the congestive heart failure and its mechanism. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1995;15:325–7. [PubMed] [Google Scholar]
- 104.Sung J, Han KH, Zo JH, Park HJ, Kim CH, Oh BH. Effects of red ginseng upon vascular endothelial function in patients with essential hypertension. Am J Chin Med. 2000;28:205–16. doi: 10.1142/S0192415X00000258. [DOI] [PubMed] [Google Scholar]
- 105.Kaneko H, Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials: clinical effects of medical ginseng, korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004;95:158–62. doi: 10.1254/jphs.fmj04001x5. [DOI] [PubMed] [Google Scholar]
- 106.Chan LY, Chiu PY, Lau TK. An in-vitro study of ginsenoside Rb1-induced teratogenicity using a whole rat embryo culture model. Hum Reprod. 2003;18:2166–8. doi: 10.1093/humrep/deg401. [DOI] [PubMed] [Google Scholar]
- 107.Chan LY, Chiu PY, Lau TK. Embryotoxicity study of ginsenoside Rc and Re in in vitro rat whole embryo culture. Reprod Toxicol. 2004;19:131–4. doi: 10.1016/j.reprotox.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 108.Liu P, Xu Y, Yin H, Wang J, Chen K, Li Y. Developmental toxicity research of ginsenoside Rb1 using a whole mouse embryo culture model. Birth Defects Res B Dev Reprod Toxicol. 2005;74:207–9. doi: 10.1002/bdrb.20038. [DOI] [PubMed] [Google Scholar]
- 109.Popovich DG, Kitts DD. Mechanistic studies on protopanaxadiol, Rh2, and ginseng (Panax quinquefolius) extract induced cytotoxicity in intestinal Caco-2 cells. J Biochem Mol Toxicol. 2004;18:143–9. doi: 10.1002/jbt.20019. [DOI] [PubMed] [Google Scholar]
- 110.Cui JF, Garle M, Bjorkhem I, Eneroth P. Determination of aglycones of ginsenosides in ginseng preparations sold in Sweden and in urine samples from Swedish athletes consuming ginseng. Scand J Clin Lab Invest. 1996;56:151–60. doi: 10.3109/00365519609088602. [DOI] [PubMed] [Google Scholar]
- 111.Cui JF, Bjorkhem I, Eneroth P. Gas chromatographic-mass spectrometric determination of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol for study on human urinary excretion of ginsenosides after ingestion of ginseng preparations. J Chromatogr B Biomed Sci Appl. 1997;689:349–55. doi: 10.1016/s0378-4347(96)00304-0. [DOI] [PubMed] [Google Scholar]
- 112.Kwon HY, Kim EH, Kim SW, Kim SN, Park JD, Rhee DK. Selective toxicity of ginsenoside Rg3 on multidrug resistant cells by membrane fluidity modulation. Arch Pharm Res. 2008;31:171–7. doi: 10.1007/s12272-001-1137-y. [DOI] [PubMed] [Google Scholar]
- 113.Nakata H, Kikuchi Y, Tode T, et al. Inhibitory effects of ginsenoside Rh2 on tumor growth in nude mice bearing human ovarian cancer cells. Jpn J Cancer Res. 1998;89:733–40. doi: 10.1111/j.1349-7006.1998.tb03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang X, Zhang D, Xu J, Gu J, Zhao Y. Determination of 25-OH-PPD in rat plasma by high-performance liquid chromatography-mass spectrometry and its application in rat pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;858:65–70. doi: 10.1016/j.jchromb.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 115.Park EK, Choo MK, Oh JK, Ryu JH, Kim DH. Ginsenoside Rh2 reduces ischemic brain injury in rats. Biol Pharm Bull. 2004;27:433–6. doi: 10.1248/bpb.27.433. [DOI] [PubMed] [Google Scholar]
- 116.Ohashi R, Yan S, Mu H, et al. Effects of homocysteine and ginsenoside Rb1 on endothelial proliferation and superoxide anion production. J Surg Res. 2006;133:89–94. doi: 10.1016/j.jss.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 117.Chen F, Eckman EA, Eckman CB. Reductions in levels of the Alzheimer’s amyloid beta peptide after oral administration of ginsenosides. Faseb J. 2006;20:1269–71. doi: 10.1096/fj.05-5530fje. [DOI] [PubMed] [Google Scholar]
- 118.Sun J, Wang G, Haitang X, Hao L, Guoyu P, Tucker I. Simultaneous rapid quantification of ginsenoside Rg1 and its secondary glycoside Rh1 and aglycone protopanaxatriol in rat plasma by liquid chromatography-mass spectrometry after solid-phase extraction. J Pharm Biomed Anal. 2005;38:126–32. doi: 10.1016/j.jpba.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 119.Qian T, Cai Z, Wong RN, Jiang ZH. Liquid chromatography/mass spectrometric analysis of rat samples for in vivo metabolism and pharmacokinetic studies of ginsenoside Rh2. Rapid Commun Mass Spectrom. 2005;19:3549–54. doi: 10.1002/rcm.2232. [DOI] [PubMed] [Google Scholar]
- 120.Gui FJ, Yang XW, Li LY, Tian JM. Simultaneous enantiomer determination of 20 (R)- and 20 (S)-ginsenoside-Rg2 in rat plasma after intravenous administration using HPLC method. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;850:1–6. doi: 10.1016/j.jchromb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 121.Wang W, Wang GJ, Xie HT, et al. Determination of ginsenoside Rd in dog plasma by liquid chromatography-mass spectrometry after solid-phase extraction and its application in dog pharmacokinetics studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:8–14. doi: 10.1016/j.jchromb.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 122.Li X, Sun J, Wang G, et al. Simultaneous determination of panax notoginsenoside R1, ginsenoside Rg1, Rd, Re and Rb1 in rat plasma by HPLC/ESI/MS: platform for the pharmacokinetic evaluation of total panax notoginsenoside, a typical kind of multiple constituent traditional Chinese medicine. Biomed Chromatogr. 2007;21:735–46. doi: 10.1002/bmc.813. [DOI] [PubMed] [Google Scholar]
- 123.Li X, Wang G, Sun J, et al. Pharmacokinetic and absolute bioavailability study of total panax notoginsenoside, a typical multiple constituent traditional chinese medicine (TCM) in rats. Biol Pharm Bull. 2007;30:847–51. doi: 10.1248/bpb.30.847. [DOI] [PubMed] [Google Scholar]
- 124.Yang L, Deng Y, Xu S, Zeng X. In vivo pharmacokinetic and metabolism studies of ginsenoside Rd. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;854:77–84. doi: 10.1016/j.jchromb.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 125.Xie HT, Wang GJ, Sun JG, et al. High performance liquid chromatographic-mass spectrometric determination of ginsenoside Rg3 and its metabolites in rat plasma using solid-phase extraction for pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;818:167–73. doi: 10.1016/j.jchromb.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 126.Cai Z, Qiana Tianxiu, Wongb Ricky NS, Jiang Zhi-Hong. Liquid chromatography–electrospray ionization mass spectrometry for metabolism and pharmacokinetic studies of ginsenoside Rg3 Applications of Liquid Chromatography coupled to Mass Spectrometry in Pharmacology. 2003;492:283–93. [Google Scholar]
- 127.Xu QF, Fang XL, Chen DF. Pharmacokinetics and bioavailability of ginsenoside Rb1 and Rg1 from Panax notoginseng in rats. J Ethnopharmacol. 2003;84:187–92. doi: 10.1016/s0378-8741(02)00317-3. [DOI] [PubMed] [Google Scholar]
- 128.Li L, Sheng Y, Zhang J, Wang C, Guo D. HPLC determination of four active saponins from Panax notoginseng in rat serum and its application to pharmacokinetic studies. Biomed Chromatogr. 2004;18:849–56. doi: 10.1002/bmc.400. [DOI] [PubMed] [Google Scholar]
- 129.Paek IB, Moon Y, Kim J, et al. Pharmacokinetics of a ginseng saponin metabolite compound K in rats. Biopharm Drug Dispos. 2006;27:39–45. doi: 10.1002/bdd.481. [DOI] [PubMed] [Google Scholar]
- 130.Han M, Fang XL. Difference in oral absorption of ginsenoside Rg1 between in vitro and in vivo models. Acta Pharmacol Sin. 2006;27:499–505. doi: 10.1111/j.1745-7254.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- 131.Zhang QH, Wu CF, Duan L, Yang JY. Protective effects of ginsenoside Rg(3) against cyclophosphamide-induced DNA damage and cell apoptosis in mice. Arch Toxicol. 2008;82:117–23. doi: 10.1007/s00204-007-0224-3. [DOI] [PubMed] [Google Scholar]
- 132.Khalil WK, Ahmed KA, Park MH, Kim YT, Park HH, Abdel-Wahhab MA. The inhibitory effects of garlic and Panax ginseng extract standardized with ginsenoside Rg3 on the genotoxicity, biochemical, and histological changes induced by ethylenediaminetetraacetic acid in male rats. Arch Toxicol. 2008;82:183–95. doi: 10.1007/s00204-007-0237-y. [DOI] [PubMed] [Google Scholar]