Meta-Analysis: Treatment of Attention-Deficit/Hyperactivity Disorder in Children With Comorbid Tic Disorders (original) (raw)
. Author manuscript; available in PMC: 2014 Mar 5.
Published in final edited form as: J Am Acad Child Adolesc Psychiatry. 2009 Sep;48(9):884–893. doi: 10.1097/CHI.0b013e3181b26e9f
Abstract
Objective
The Food and Drug Administration currently requires the package inserts of most psychostimulant medications to list the presence of a tic disorder as a contraindication to their use. Approximately half of children with Tourette’s syndrome experience comorbid attention-deficit/hyperactivity disorder (ADHD). We sought to determine the relative efficacy of different medications in treating ADHD and tic symptoms in children with both Tourette’s syndrome and ADHD.
Method
We conducted a PubMed search to identify all double-blind, randomized, placebo-controlled trials examining the efficacy of medications in the treatment of ADHD in the children with comorbid tics. We used a random effects meta-analysis with standardized mean difference as our primary outcome to estimate the effect size of pharmaceutical agents in the treatment of ADHD symptoms and tics.
Results
Our meta-analysis included nine studies involving 477 subjects. We assessed the efficacy of six medications—dextroamphetamine, methylphenidate, alpha-2 agonists (clonidine and guanfacine), desipramine, atomoxetine, and deprenyl. Methylphenidate, alpha-2 agonists, desipramine, and atomoxetine demonstrated efficacy in improving ADHD symptoms in children with comorbid tics. Alpha-2 agonists and atomoxetine significantly improved comorbid tic symptoms. Although there was evidence that supratherapeutic doses of dextroamphetamine worsens tics, there was no evidence that methylphenidate worsened tic severity in the short term.
Conclusions
Methylphenidate seems to offer the greatest and most immediate improvement of ADHD symptoms and does not seem to worsen tic symptoms. Alpha-2 agonists offer the best combined improvement in both tic and ADHD symptoms. Atomoxetine and desipramine offer additional evidence-based treatments of ADHD in children with comorbid tics. Supratherapeutic doses of dextroamphetamine should be avoided.
Keywords: tic disorders, attention-deficit/hyperactivity disorder, methylphenidate, α2 adrenergic agonists, meta-analysis
The Food and Drug Administration (FDA) currently requires the package inserts of most psychostimulant medications to list the presence of a tic disorder or a family history of Tourette’s syndrome as a contraindication to their use.1 At least half of the children with Tourette’s syndrome experience comorbid attention-deficit/hyperactivity disorder (ADHD).2,3 When ADHD is present in children with tics, the ADHD typically causes greater impairment in academic performance and social relationships than the tics themselves.4,5
Psychostimulant medications are currently the first line of treatment for children with ADHD.6 Psychostimulants have demonstrated superior short-term efficacy to both nonpsychostimulant medications and psychosocial treatment for ADHD in head-to-head clinical trials and in meta-analyses.7–10 In fact, roughly 70% of children with ADHD respond to a single stimulant when tried.11
The use of psychostimulants in the treatment of ADHD in children with comorbid tics remains controversial. In 1983, manufacturers of psychostimulants began listing the presence of tic disorders or a family history of Tourette’s syndrome as a contraindication to their use. This warning was issued based on a large collection of case reports and case series published beginning in 1963.12,13 These case reports associated the emergence of de novo tics and exacerbation of existing tic symptoms with methylphenidate, dextroamphetamine, and pemoline. A particularly influential case series of 15 children, who developed tics while taking psychostimulants, helped lead the FDA to require listing contraindications to psychostimulant medications.14
Biological plausibility further lends support to the possibility that psychostimulants can cause or exacerbate tics.15 Psychostimulants act by increasing dopamine concentration at the synaptic cleft. It has been hypothesized that the pathogenesis of tics is associated with increased dopamine activity in the basal ganglia.16 Antipsychotics, the most effective pharmacological agent used in the treatment of tics, act as dopamine antagonists.17,18
On the other hand, several lines of evidence suggest that the association between psychostimulants and tics may be a result of confounding. Approximately 20% of children with ADHD develop a chronic tic disorder.18 When tics and ADHD co-occur, symptoms of ADHD typically precede the onset of tic symptoms by 2 to 3 years; thus, a proportion of children diagnosed initially with ADHD may have undiagnosed tic symptoms.18 Based on these data, one would expect a substantial proportion of children diagnosed with ADHD to develop tics regardless of treatment. This observation limits the ability of case reports or observational data to establish a causal association between psychostimulant use and tics.
The warnings and scientific debate surrounding the potential of psychostimulants to exacerbate tics creates clinical uncertainty for practitioners treating ADHD in children with comorbid tics. Several nonpsychostimulant medications for ADHD, such as atomoxetine, α2 adrenergic agonists (including guanfacine and clonidine), and tricyclic antidepressants (such as desipramine), have been studied in children with ADHD and tics over recent years. The goals of this meta-analysis are to examine double-blind, randomized, placebo-controlled studies of children with ADHD and comorbid tics to determine whether psychostimulants (methylphenidate and amphetamine derivatives) exacerbate tic symptoms and to determine the relative efficacy of different ADHD medications (methylphenidate-derived stimulants, amphetamine-derived stimulants, and alpha-2 agonists and tricyclic antidepressants) in the treatment of ADHD and tics in children with both conditions.
METHOD
Search Strategy for Identification of Studies
Two reviewers searched PubMed for relevant studies using the search “attention-deficit disorder with hyperactivity (MeSH) or ADHD or ADDH or hyperactive* or hyperkin* or “attentiondeficit*” or ‘brain dysfunction”’ and “tic disorders (MeSH) or Tourette* or tic.” The search was further limited to randomized clinical trials and meta-analyses. The references of included articles, as well as review articles and meta-analyses in this area, were searched for citations of further relevant published and unpublished research.
Selection of Studies
The titles and abstracts of studies obtained by this search strategy were scrutinized by two reviewers to determine if they were potentially eligible for inclusion in this review. Eligibility for the study was based on scrutiny of the full articles for the following inclusion criteria: they were randomized, double-blind, placebo-controlled, clinical trials comparing a medication with placebo; and participants included were children and adolescents younger than 18 years diagnosed with ADHD or hyperkinetic disorder and a tic disorder by explicit criteria (i.e., DSM or International Statistical Classification of Diseases, 10th Revision, criteria). Studies were excluded if the primary study population did not include patients with both ADHD and tic disorders and the medication of interest was given for less than 1 week.
Outcome Measures
Our primary outcome measures were mean change in rating scales examining tic and ADHD severity. Acceptable clinical scales for rating of tic severity (in order of preference) were the Yale Global Tic Severity Scale total tic score or global score, Shapiro Tourette Syndrome Severity Scale, Hopkins Motor/Vocal Tic Scale, Global Tic Severity Scale or tic counts based on standardized video recordings.19–22 Acceptable clinical scales for rating of ADHD severity (in order of preference) were the ADHD Rating Scale, Conner’s Abbreviated Questionnaire for Teachers or Parents, Inattention/Overactivity With Aggression Conner’s Teacher’s Rating Scale, or other validated rating scales for ADHD.23–27 As a secondary outcome, we examined the relative efficacy of medications on the inattention and hyperactivity/impulsivity symptoms of ADHD separately. Results for all analyses were stratified by type of medication used. Medication categories were divided into methylphenidate derivatives, amphetamine derivatives, atomoxetine, alpha-2 agonists, tricyclic antidepressants, and deprenyl. We also qualitatively examined side effects reported in each of these trials.
Choice of Summary Statistic
For our primary outcomes, mean improvement in tic and ADHD severity was measured as standardized mean difference and was pooled for overall meta-analysis. Standardized mean difference was favored over weighted mean difference as the primary outcome because rating scales differed between the included studies.
Meta-analytic Procedure
For the inclusion of crossover trials along with traditional parallel group trials in our meta-analysis, two different methods were used. These methods were derived from the standard accepted methodology for incorporating crossover trials into meta-analyses in scientific literature.28,29 When individual subject data were available for baseline and each crossover period, we used the available data to compute the actual measurements of treatment effect needed for this study—mean difference and SD. If individual subject data were not available from the original manuscript, then the reported subject number, mean difference in treatments, and p value or T statistic was used to retrieve the SD of paired observations. There was no evidence of carryover effects in crossover trials. For further information on how this information was computed, please refer to our previous article.30 Crossover and parallel group studies were then incorporated into a single meta-analysis using the generic inverse variation method of RevMan 4.2.8.31 A random effects model was chosen for the meta-analysis because there was considerable heterogeneity between the studies.
Assessment of Publication Bias
Relevant from all the included trials were entered into a funnel plot (trial effect size [ES] plotted against sample size) to detect any publication bias.32
Assessment of Heterogeneity
Heterogeneity of treatment response was assessed from the forest plot of weighted mean differences and relative risk for individual studies. Statistical estimates of heterogeneity were performed using the I2 heterogeneity statistic in RevMan.31 Because the I2 test has low power to detect heterogeneity in meta-analysis when there are few trials with small sample size, the threshold for statistical significance was set at p < .1. This threshold for significance using the I2 test is conventional in meta-analysis.31 When heterogeneity was present between trials, difference in duration of trial length, dosage, and formulation of medication was examined.
RESULTS
Included Studies
We included nine studies involving 477 subjects (177 in crossover studies) in our analysis.33–41 Four studies, involving 191 subjects (122 in crossover studies), compared methylphenidate-derivatives with placebo.33–35,38 Three studies, involving 134 subjects (34 in crossover studies), compared α-agonist medications with placebo. 36,38,39 Two studies, involving 75 subjects (34 in crossover studies), compared desipramine with placebo. 39,40 One study, involving 148 subjects, compared atomoxetine with placebo and a crossover study of 15 children compared deprenyl with placebo.37 Table 1 depicts the characteristics of included studies in this meta-analysis. In some cases, studies did not contribute data to all outcomes. These instances are indicated in the text. Authors were contacted for additional data, but in each case, too much time had elapsed between the original studies and the data request (in some cases as long as 15 years) for authors to be able to supply meaningful data on the cohorts.
TABLE 1.
Characteristics of Included Studies
| Author | Year | N | AgeRange, y | Sex(% Male) | Design | Length ofTreatment, wk | MPH | AMP | α2 Agonist | DMI | ATOM | DEP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gadow et al.34 | 1992 | 11 | 6–12 | 91 | Crossover | 2 | X | |||||
| Singer et al.39 | 1995 | 34 | 7–14 | 91 | Crossover | 6 | X | X | ||||
| Feigin et al.41 | 1996 | 15 | 7–16 | 88 | Crossover | 8 | X | |||||
| Castellanos et al.33 | 1997 | 20 | 6–13 | 100 | Crossover | 3 | X | X | ||||
| Scahill et al.36 | 2001 | 34 | 6–14 | 91 | Parallel | 8 | X | |||||
| TSSG38 | 2002 | 103 | 7–14 | 89 | Parallel | 16, CLON; 12, MPH | X | X | ||||
| Spencer et al.40 | 2002 | 41 | 5–17 | 83 | Parallel | 6 | X | |||||
| Allen et al.37 | 2005 | 148 | 7–17.5 | 89 | Parallel | 18 | X | |||||
| Gadow et al.35 | 2007 | 71 | 6–12 | 80 | Crossover | 2 | X |
Two articles were excluded from use because they contained redundant data with other trials included in the meta-analysis.42,43 We found one additional study evaluating the efficacy of lofexidine (an alpha-2 agonist) compared with placebo that we also excluded from all analyses.44 This article was retracted from publication because of plagiarism of a previous article in this area, and an investigation by the journal raised suspicion about the legitimacy of the study.45
Methylphenidate Derivatives
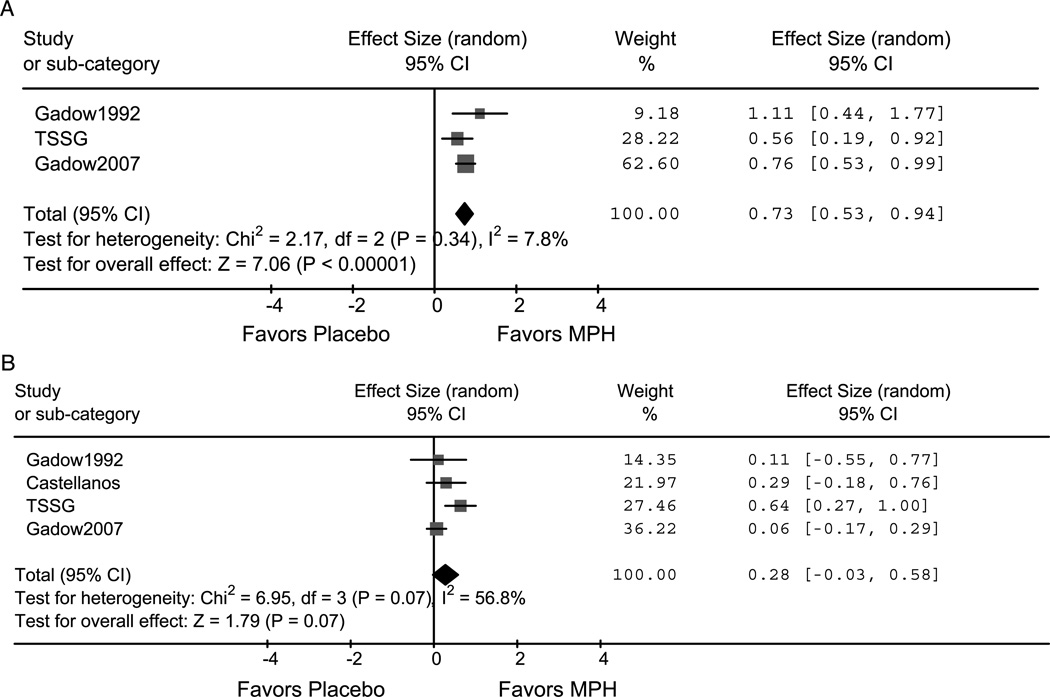
Methylphenidate demonstrated superior efficacy when compared with placebo in the treatment of ADHD in children with comorbid tics (ES = 0.73; 95% confidence interval [CI] 0.53–0.94; z = 7.1, p < .001). Additionally, methylphenidate was effective in treating both the inattention (ES = 0.41; 95% CI 0.21–0.62; z = 3.9, p = < .001) and hyperactivity/impulsive symptoms (ES = 0.82; 95% CI 0.43–1.21; z = 4.2, p = < .001) of ADHD. Methylphenidate also improved tic symptoms at trend levels (ES = 0.28; 95% CI −0.03 to 0.58; z = 1.8, p = .07). There was a high degree of heterogeneity in the trials examining the effects of methylphenidate on tics (I2 = 57%, p = .07). The Tourette Syndrome Study Group Study, which was of significantly longer duration than the other three studies, showed a greater ES.33–35,38 Although there were differences in the dosing of methylphenidate (1.2 mg/kg b.i.d. compared with 0.5 mg/kg b.i.d.), these differences did not seem to influence tic severity. Figures 1A, B depict the forest plots of the effects of methylphenidate on ADHD and tic severity. Adverse events with methylphenidate treatment were not well described in any of these four studies.
Fig. 1.
A and B, Methylphenidate effect on ADHD and tic severity. Forrest plots of methylphenidate’s effect on ADHD (A) and tic severity (B). Methylphenidate significantly improved ADHD severity compared with placebo and improved tic symptoms at trend levels (Gadow et al.34,35 and Castellanos et al.33). MPH = methylphenidate derivatives; TSSG = Tourette Syndrome Study Group.
Amphetamine Derivatives
One crossover study involving 12 boys with ADHD and Tourette’s syndrome examined changes in tic severity with dexamphetamine treatment of ADHD.33 This study used an escalating dose of dexamphetamine from 7.5 to 22.5 mg per dose b.i.d. The highest dose of dexamphetamine, 1.28 mg/kg per day, is above the suggested dose range of this agent. Tic severity was significantly increased with dexamphetamine treatment (ES = −0.59; 95% CI −1.06 to −0.13; z = 2.5, p = .01). When lower doses of dexamphetamine (0.82 mg/kg per day) were used instead, six of these same subjects showed no significant change in tic severity with treatment, and the majority of the subjects experienced reductions in tic severity. No measurements regarding effects on ADHD severity were available from this study. Insomnia and decreased appetite were noted to be common side effects of dexamphetamine treatment in this trial.33
Alpha-2 Agonists
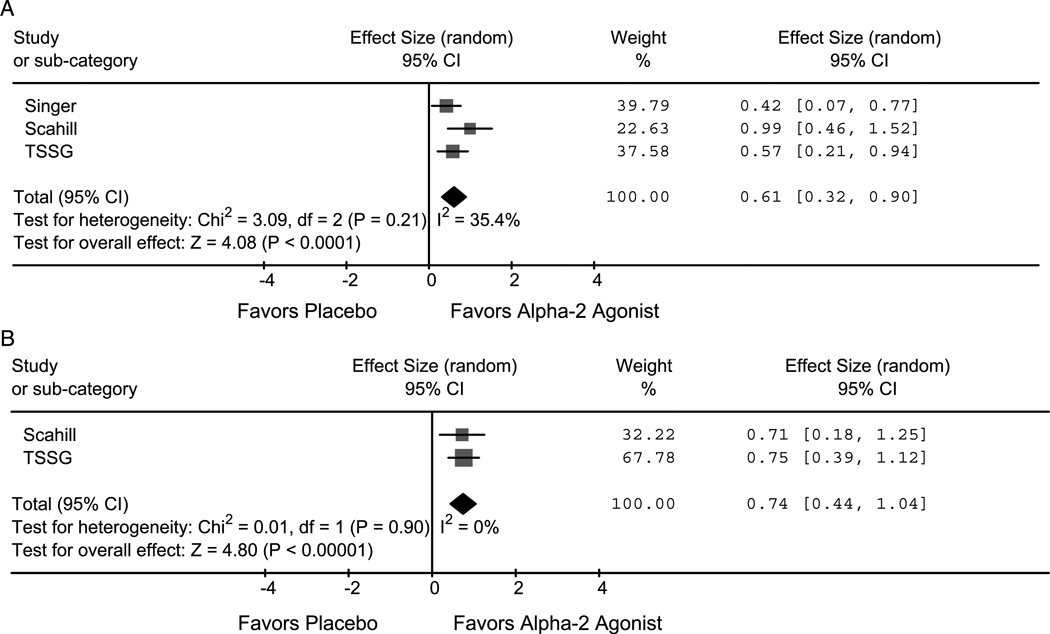
Two studies evaluating clonidine38,39 and one study examining the efficacy of guanfacine contributed to this analysis.36 Alpha-2 agonists significantly improved both tic (ES = 0.74; 95% CI 0.44–1.04; z = 4.8, p < .001) and ADHD (ES = 0.61; 95% CI 0.32−0.90; z = 4.1, p < .001) severity. Figures 2A, B depict forest plots of the effects of alpha-2 agonists on ADHD and tic severity. Alpha-2 agonists also significantly improved hyperactivity/ impulsive (ES = 0.75; 95% CI 0.22−1.27; z = 2.8, p = .005) symptoms of ADHD. Alpha-2 agonist effects on inattention symptoms of ADHD demonstrated a large ES (0.76; 95% CI −0.33 to 1.86; z = 1.4, p = .17) but did not reach the threshold of significance because of a large degree of heterogeneity between the two studies (I2 = 91%, p = .001). Differences between the two studies that could have contributed to this heterogeneity include that the studies used different alpha-2 agonists (clonidine versus guanfacine), that the trials were of different duration (8 weeks versus 16 weeks), and that there were potential differences in techniques used to assess time spent on “on-task behaviors.”Alpha-2 agonists were associated with a statistically significant increased rate of sedation (guanfacine = 41%, clonidine = 48%) compared with placebo (6%) in these trials.36,38 With continued treatment, this sedation often dissipated.36
Fig. 2.
A and B, Alpha-2 agonists effect on ADHD and tic severity. Forrest plots of alpha-2 agonists’ effect on ADHD (A) and tic severity (B). Alpha-2 agonists significantly improved both ADHD and tic severity compared with placebo (Singer et al.39 and Scahill et al.36). TSSG = Tourette Syndrome Study Group.
Desipramine
Two studies examined the efficacy of desipramine compared with placebo in the treatment of ADHD and comorbid tics.39,40 One study was a crossover study comparing 6-week cycles of clonidine and desipramine with placebo.39 The other was a 6-week parallel group study of 41 children that compared desipramine with placebo. Only the parallel group study contributed data on tic severity to this analysis.40 Desipramine demonstrated significant improvement of ADHD symptoms (ES = 0.80; 95% CI 0.02–1.57; z = 2.0, p = .04) and also tic symptoms at trend levels (ES = 0.44; 95% CI −0.02 to 0.91; z = 1.9, p = .06). There was significant heterogeneity in the analysis of ADHD efficacy (I2 = 86%, p = .008). This heterogeneity may be due to differences in the rating scales used (Child Behavior Checklist hyperactivity scale versus ADHD Rating Scale). The Child Behavior Checklist hyperactivity scale is rather old and has fewer questions examining ADHD symptoms and thus may be prone to a greater variance than newer scales of ADHD. Dosage and length of treatment with desipramine in these two studies were roughly equivalent. Adverse effects with desipramine treatment occurred with greater frequency than with placebo in both trials. Only one study reported on the frequencies of specific adverse events.40 In this trial, there was a statistically significant increase in diastolic blood pressure and pulse rate in children treated with desipramine compared with placebo.40
Atomoxetine
Only a single 16-week parallel-group study involving 148 children examined the efficacy of atomoxetine compared with placebo.37 Atomoxetine significantly improved both tic (ES = 0.32; 95% CI 0.09–0.56; z = 2.7, p = .007) and ADHD (ES = 0.51; 95% CI 0.27–0.74; z = 4.3, p < .001) severity compared with placebo. No data on atomoxetine effects on inattention and hyperactivity/impulsive symptoms were available. Atomoxetine was more likely to cause nausea (16%) and decreased appetite (16%) when compared with placebo (1%–3%) in this trial.
Deprenyl
Deprenyl is a type B monoamine oxidase inhibitor. Deprenyl is chemically similar to selegiline (l-deprenyl) except without the isomeric designation. A single crossover study with 15 completers of 24 children has compared deprenyl with placebo in the treatment of Tourette’s syndrome and ADHD.41 Deprenyl did not show significant improvement in treating ADHD symptoms (ES = 0.18; 95% CI −0.34 to 0.70; z = 0.7, p = .50) but did demonstrate improvement in treating tic symptoms at trend levels (ES = 0.52; 95% CI −0.01 to 1.04; z = 1.9, p = .051). No data on deprenyl’s effects on inattention and hyperactivity/ impulsive symptoms were available. Deprenyl is not currently available in the United States, although selegiline is available. Deprenyl did not demonstrate any adverse events at a statistically greater rate than placebo in this trial.
Combined Pharmacological Treatments
Only a single study examined the efficacy of a combination of pharmacological agents. This study compared the combination of clonidine and methylphenidate with placebo, as well as each medication separately. The combination treatment significantly improved both ADHD (ES = 1.09; 95% CI 0.72–1.45; z = 5.8, p < .001) and tic symptoms (ES = 0.75; 95% CI 0.38–1.12; z = 4.0, p < .001). This combination treatment also improved both the inattention (ES = 0.60; 95% CI 0.23–0.96; z = 3.2, p = .001) and hyperactivity/impulsive symptoms (ES = 1.00; 95% CI 0.64–1.37; z = 5.4, p < .001) of ADHD. Sedation (35%), commonly experienced during clonidine treatment, was diminished in children taking additional methylphenidate (21%) but still greater than placebo (6%).38
DISCUSSION
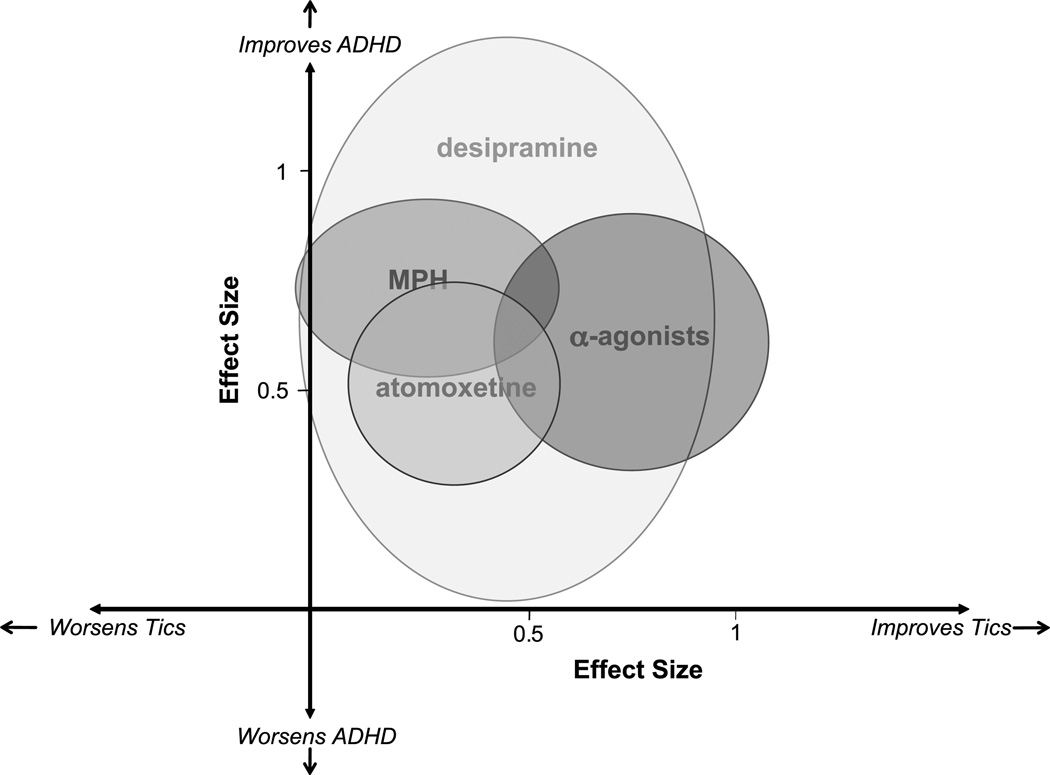
Meta-analysis of randomized placebo-controlled trials in the children with ADHD and comorbid tics demonstrated that methylphenidate, alpha-2 agonists, desipramine, and atomoxetine have shown efficacy in treating ADHD symptoms. Furthermore, none of these four medications seemed to worsen tic severity. Alpha-2 agonists and atomoxetine have demonstrated statistically significant improvement in tic symptoms with treatment, whereas both methylphenidate and desipramine demonstrated improvement in tic symptoms at trend levels with a comparable ES to atomoxetine. Figure 3 plots the ESs and their 95% CI in the treatment of tics and ADHD. In addition, preliminary data from a single study suggest that supratherapeutic doses of dextroamphetamine can worsen tics.33
Fig. 3.
Effectiveness of medications in treating ADHD and tic disorders. Bubbles represent point estimate and 95% confidence interval for medications in terms of effect size (ES) in treating ADHD and tic symptoms based on a meta-analysis of double-blind, placebo-controlled trials in children with ADHD and comorbid tic disorders. Effect size estimates in treating ADHD symptoms for pharmacological agents were methylphenidate (ES = 0.73; 95% CI 0.53–0.94), alpha-2 agonists (ES = 0.61; 95% CI 0.32–0.90), desipramine (ES = 0.80; 95% CI 0.02–0.91) and atomoxetine (ES = 0.51; 95% CI 0.27–0.74). Effect size estimates in treating tic symptoms for pharmacological agents were methylphenidate (ES = 0.28; 95% CI −0.03 to 0.58), alpha-2 agonists (ES = 0.74; 95% CI 0.44–1.04), desipramine (ES = 0.44; 95% CI −0.02 to 0.91), and atomoxetine (ES = 0.32; 95% CI 0.09–0.56). Supratherapeutic doses of dextroamphetamine modestly worsened tic symptoms (ES = −0.59; 95% CI −1.06 to−0.13) in a small crossover trial. These data are not depicted as there were no estimates for ADHD improvement in the trial. The ESs for deprenyl, a monoamine oxidase B inhibitor, that is not available in the United States and did not demonstrate efficacy in the treatment of ADHD or tics, is also not depicted in this figure. MAO-B = monoamine oxidase B; MPH = methylphenidate derivatives.
When considering which medication to choose, besides considering simply the efficacy of medications in ameliorating ADHD and tic severity, clinicians must also consider side effects and family attitudes toward the risk/benefit profile of each medication. Methylphenidate has the quickest onset of action of any of these agents and may be particularly beneficial in the children necessitating immediate improvement. On the other hand, there is an FDA warning on package inserts contraindicating its use in patients with tic disorders. Furthermore, there remain questions regarding the long-term efficacy of stimulants and concerns regarding their long-term impact on growth.46 Psychostimulants also have common side effects such as insomnia and appetite suppression. Alpha-2 agonists are fairly well tolerated in children aside from sedation. However, there remains a concern with rebound hypertension on abrupt discontinuation of these medications, (especially clonidine).47 Atomoxetine, although fairly well tolerated, does not have data concerning its long-term use equivalent to other agents considered in this algorithm. Although desipramine has demonstrated efficacy in multiple double-blind trials of ADHD, it is generally not considered a first-line agent because of cardiac arrhythmias associated with its use.48
The Tourette Syndrome Association Medical Advisory Board recently published guidelines for the assessment and pharmacotherapy of Tourette’s syndrome. 49 The recommendations regarding the treatment of ADHD in patients with comorbid tics were as follows: “Given the added disability attributable to ADHD in children and adolescents with TS, aggressive treatment of ADHD in these cases is warranted. After a review of the alternatives and the family’s preference, treatment may start with an alpha-2 agonist (guanfacine or clonidine) or stimulant medication. Combined treatment with an alpha-2 agonist and stimulant may produce better outcomes than either treatment alone.” The data from this meta-analysis support the conclusions from the medical advisory board. Data from this meta-analysis suggest additionally that concerns about tic exacerbations from methylphenidate at therapeutic doses may be overstated. Evidence from randomized placebo-controlled trials show no indication of worsening tics with treatment and, in fact, show that methylphenidate treatment may improve tics modestly. If the goal of treatment is to target both ADHD and tic symptoms, alpha-2 agonist medications may be more desirable when compared with psychostimulants.
Data from the Tourette Syndrome Study Group Study, which examined the comparative efficacy of clonidine and methylphenidate, suggested that “clonidine appeared to be most helpful for impulsivity and hyperactivity,” whereas “methylphenidate appeared to be most helpful for inattention.”38 Meta-analysis of data from all the studies examining the efficacy of alpha-2 agonists and methylphenidate did not distinguish these agents by their efficacy in treating these subtypes of symptoms in ADHD. Alpha-2 agonists demonstrated ESs of 0.76 and 0.75 in treating inattention and hyperactivity/ impulsive symptoms of ADHD, whereas the ESs for methylphenidate were 0.41 and 0.82, respectively. Alpha-2 agonists seem like a better choice of medication when targeting comorbid tic symptoms, and methylphenidate derivatives as a better choice when targeting tics is not a priority. A combination treatment of both agents may be most effective in targeting both disorders together. Concerns have been raised based on case report level data regarding the possible increased risk for cardiac adverse events and sudden death when using clonidine and methylphenidate in combination.50 These concerns have not been borne out in randomized controlled trials.51 Caution is warranted when using combination therapy in children with known cardiac defects.
In light of our findings, it is important to take note of the limitations of this meta-analysis. The ESs of medications in trials are influenced by many factors besides the efficacy of the medication. Differences in the precision of rating scales influence ES. This difference may have been particularly influential in our measures of efficacy in treating ADHD, and the inattention and hyperactivity/impulsive symptom subtypes, because multiple different rating scales and raters were used in trials. Yale Global Tic Severity Scale ratings of tic severity were used to assess tic severity in the majority of studies included in this meta-analysis, so these differences influenced our measures of tic severity much less. For several outcomes, we found significant heterogeneity between study results, suggesting that differences in trial design may have influenced ESs. Such differences in trial design include the type of rating scale and dose and duration of treatment. With a relatively small number of studies contributing to this meta-analysis, it is not possible to determine which of these hypothesized factors is contributing to the heterogeneity. Exploring this heterogeneity is often informative in better understanding situations for optimal medication efficacy. The trials in this meta-analysis include primarily male subjects (80%–100%). It is unknown how well the results of this meta-analysis generalize to girls with ADHD and comorbid tics.
Despite these limitations, our meta-analysis demonstrated the efficacy of four medications (methylphenidate, alpha-2 agonists, atomoxetine, and desipramine) in the treatment of ADHD in the children with comorbid tics. The ESs observed in the treatment of ADHD in children with comorbid tics were quite similar to those of meta-analyses of the children with ADHD alone. For instance, our estimate of the ES for methylphenidate in the treatment of ADHD in the children with comorbid tics was 0.73 (95% CI 0.53–0.94) compared with 0.78 (95% CI 0.64–0.91) in a meta-analysis involving 2,897 children with ADHD alone.52 Our estimated ES for alpha-2 agonists in this meta-analysis was 0.61 (95% CI 0.32–0.90) compared with 0.58 (95% CI 0.27–0.89) a meta-analysis involving 150 children with ADHD alone.53 Our estimated ES for atomoxetine in children with comorbid tics was 0.51 (95% CI 0.27–0.74) compared with 0.64 (95% CI 0.51–0.76) in a recent meta-analysis of 1,615 children with ADHD alone.54 These data suggest that estimates of the efficacy of medications in the children with ADHD alone may be generalizable to their efficacy in treating these same symptoms in children with ADHD and comorbid tics. Our meta-analysis also suggested that none of these medications, with the possible exception of supratherapeutic doses of amphetamine derivatives, worsened tic severity. Alpha-2 agonists significantly improved both ADHD and tic symptoms with larger combined effects than any of the other medications. Combination therapy, with both an alpha-2 agonist and a methylphenidate derivative, seems to be a particularly beneficial intervention based on results from the Tourette Syndrome Study Group trial.38
Acknowledgments
The authors acknowledge the National Institute of Mental Health support of the Yale Child Study Center Research Training Program (M.H.B. and J.F.L.), K05MH076273 ( J.F.L.), the National Institute of Health Loan Repayment Program (M.H.B.), the support of the Tourette’s Syndrome Association ( J.F.L.), the APIRE/Eli Lilly Psychiatric Research Fellowship (M.H.B.), and the APA/ NIMH PMRTP Program (A.L.W.).
Footnotes
Disclosure: Dr. Leckman has received funding from and provided consultation to the National Institutes of Health. The other authors report no conflicts of interest.
REFERENCES
- 1.Physicians’ Desk Reference. 61st ed. Montvale, NJ: Thomson PDR; 2007. [Google Scholar]
- 2.Robertson MM, Eapen V. Pharmacologic controversy of CNS stimulants in Gilles de la Tourette’s syndrome. Clin Neuropharmacol. 1992;15:408–425. doi: 10.1097/00002826-199210000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Khalifa N, von Knorring AL. Psychopathology in a Swedish population of school children with tic disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:1346–1353. doi: 10.1097/01.chi.0000251210.98749.83. [DOI] [PubMed] [Google Scholar]
- 4.Spencer T, Biederman J, Harding M, et al. Disentangling the overlap between Tourette’s disorder and ADHD. J Child Psychol Psychiatry. 1998;39:1037–1044. [PubMed] [Google Scholar]
- 5.Sukhodolsky DG, Scahill L, Zhang H, et al. Disruptive behavior in children with Tourette’s syndrome: association with ADHD comorbidity, tic severity, and functional impairment. J Am Acad Child Adolesc Psychiatry. 2003;42:98–105. doi: 10.1097/00004583-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Dulcan M. Practice parameters for the assessment and treatment of children, adolescents, and adults with attention-deficit/hyperactivity disorder. American Academy of Child and Adolescent Psychiatry. J Am Acad Child Adolesc Psychiatry. 1997;36(10 Suppl):85S–121S. doi: 10.1097/00004583-199710001-00007. [DOI] [PubMed] [Google Scholar]
- 7.Van der Oord S, Prins PJ, Oosterlaan J, Emmelkamp PM. Efficacy of methylphenidate, psychosocial treatments and their combination in school-aged children with ADHD: a meta-analysis. Clin Psychol Rev. 2008;28:783–800. doi: 10.1016/j.cpr.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 8.A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 9.Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed. 2006;8:4. [PMC free article] [PubMed] [Google Scholar]
- 10.Jadad AR, Boyle M, Cunningham C, Kim M, Schachar R. Treatment of attention-deficit/hyperactivity disorder. Evid Rep Technol Assess (Summ) 1999:i–viii. 1–341. [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer T, Biederman J, Wilens T, Harding M, O’Donnell D, Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35:409–432. doi: 10.1097/00004583-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Golden GS. The effect of central nervous system stimulants on Tourette syndrome. Ann Neurol. 1977;2:69–70. doi: 10.1002/ana.410020113. [DOI] [PubMed] [Google Scholar]
- 13.Denckla MB, Bemporad JR, MacKay MC. Tics following methylphenidate administration. A report of 20 cases. JAMA. 1976;235:1349–1351. [PubMed] [Google Scholar]
- 14.Lowe TL, Cohen DJ, Detlor J, Kremenitzer MW, Shaywitz BA. Stimulant medications precipitate Tourette’s syndrome. JAMA. 1982;247:1168–1169. [PubMed] [Google Scholar]
- 15.Madras BK, Miller GM, Fischman AJ. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1397–1409. doi: 10.1016/j.biopsych.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Albin RL. Neurobiology of basal ganglia and Tourette syndrome: striatal and dopamine function. Adv Neurol. 2006;99:99–106. [PubMed] [Google Scholar]
- 17.Mink JW. Basal ganglia dysfunction in Tourette’s syndrome: a new hypothesis. Pediatr Neurol. 2001;25:190–198. doi: 10.1016/s0887-8994(01)00262-4. [DOI] [PubMed] [Google Scholar]
- 18.Leckman JF. Tourette’s syndrome. Lancet. 2002;360:1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro AK, Shapiro E. Controlled study of pimozide vs. placebo in Tourette’s syndrome. J Am Acad Child Psychiatry. 1984;23:161–173. doi: 10.1097/00004583-198403000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Nolan EE, Gadow KD, Sverd J. Observations and ratings of tics in school settings. J Abnorm Child Psychol. 1994;22:579–593. doi: 10.1007/BF02168939. [DOI] [PubMed] [Google Scholar]
- 21.Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Walkup JT, Rosenberg LA, Brown J, Singer HS. The validity of instruments measuring tic severity in Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1992;31:472–477. doi: 10.1097/00004583-199205000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Achenbach TM, Edelbrock CS. Manual for the Child Behavior Checklist. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 24.Werry JS, Sprague RL, Cohen MN. Conners’ Teacher Rating Scale for use in drug studies with children–an empirical study. J Abnorm Child Psychol. 1975;3:217–229. doi: 10.1007/BF00916752. [DOI] [PubMed] [Google Scholar]
- 25.DuPaul G. Parent and teacher rating of ADHD symptoms: psychometric properties in a community-based sample. J Clin Child Psychol. 1991;20:245–253. [Google Scholar]
- 26.Pelham WE, Milich R, Murphy DA, Murphy HA. Normative data on the Iowa Conners teacher rating scale. J Clin Child Psychol. 1989;1989:259–262. [Google Scholar]
- 27.Goyette CH, Conners CK, Ulrich RF. Normative data on revised Conners Parent and Teacher Rating Scales. J Abnorm Child Psychol. 1978;6:221–236. doi: 10.1007/BF00919127. [DOI] [PubMed] [Google Scholar]
- 28.Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31:140–149. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- 29.Curtin F, Altman DG, Elbourne D. Meta-analysis combining parallel and cross-over clinical trials. I: Continuous outcomes. Stat Med. 2002;21:2131–2144. doi: 10.1002/sim.1205. [DOI] [PubMed] [Google Scholar]
- 30.Bloch MH, Landeros-Weisenberger A, Dombrowski P, et al. Systematic review: pharmacological and behavioral treatment for trichotillomania. Biol Psychiatry. 2007;62:839–846. doi: 10.1016/j.biopsych.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Review Manager (RevMan) [computer program]. Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2003. [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castellanos FX, Giedd JN, Elia J, et al. Controlled stimulant treatment of ADHD and comorbid Tourette’s syndrome: effects of stimulant and dose. J Am Acad Child Adolesc Psychiatry. 1997;36:589–596. doi: 10.1097/00004583-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Gadow KD, Nolan EE, Sverd J. Methylphenidate in hyperactive boys with comorbid tic disorder: II. Short-term behavioral effects in school settings. J Am Acad Child Adolesc Psychiatry. 1992;31:462–471. doi: 10.1097/00004583-199205000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Gadow KD, Sverd J, Nolan EE, Sprafkin J, Schneider J. Immediate-release methylphenidate for ADHD in children with comorbid chronic multiple tic disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:840–848. doi: 10.1097/chi.0b013e31805c0860. [DOI] [PubMed] [Google Scholar]
- 36.Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- 37.Allen AJ, Kurlan RM, Gilbert DL, et al. Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology. 2005;65:1941–1949. doi: 10.1212/01.wnl.0000188869.58300.a7. [DOI] [PubMed] [Google Scholar]
- 38.Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58:527–536. doi: 10.1212/wnl.58.4.527. [DOI] [PubMed] [Google Scholar]
- 39.Singer HS, Brown J, Quaskey S, Rosenberg LA, Mellits ED, Denckla MB. The treatment of attention-deficit hyperactivity disorder in Tourette’s syndrome: a double-blind placebo-controlled study with clonidine and desipramine. Pediatrics. 1995;95:74–81. [PubMed] [Google Scholar]
- 40.Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2002;59:649–656. doi: 10.1001/archpsyc.59.7.649. [DOI] [PubMed] [Google Scholar]
- 41.Feigin A, Kurlan R, McDermott MP, et al. A controlled trial of deprenyl in children with Tourette’s syndrome and attention deficit hyperactivity disorder. Neurology. 1996;46:965–968. doi: 10.1212/wnl.46.4.965. [DOI] [PubMed] [Google Scholar]
- 42.Spencer TJ, Sallee FR, Gilbert DL, et al. Atomoxetine treatment of ADHD in children with comorbid Tourette syndrome. J Atten Disord. 2008;11:470–481. doi: 10.1177/1087054707306109. [DOI] [PubMed] [Google Scholar]
- 43.Gadow KD, Sverd J, Sprafkin J, Nolan EE, Ezor SN. Efficacy of methylphenidate for attention-deficit hyperactivity disorder in children with tic disorder. Arch Gen Psychiatry. 1995;52:444–455. doi: 10.1001/archpsyc.1995.03950180030005. [DOI] [PubMed] [Google Scholar]
- 44.Niederhofer H, Staffen W, Mair A. A placebo-controlled study of lofexidine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. J Psychopharmacol. 2003;17:113–119. doi: 10.1177/0269881103017001714. [DOI] [PubMed] [Google Scholar]
- 45.Bailey J. An exceptional case of plagiarism! J Psychopharmacol. 2004;18:291–292. doi: 10.1177/026988110401800301. [DOI] [PubMed] [Google Scholar]
- 46.Swanson J, Arnold LE, Kraemer H, et al. Evidence, interpretation, and qualification from multiple reports of long-term outcomes in the Multimodal Treatment study of Children With ADHD (MTA): part I: executive summary. J Atten Disord. 2008;12:4–14. doi: 10.1177/1087054708319345. [DOI] [PubMed] [Google Scholar]
- 47.Leckman JF, Ort S, Caruso KA, Anderson GM, Riddle MA, Cohen DJ. Rebound phenomena in Tourette’s syndrome after abrupt withdrawal of clonidine. Behavioral, cardiovascular, and neurochemical effects. Arch Gen Psychiatry. 1986;43:1168–1176. doi: 10.1001/archpsyc.1986.01800120054011. [DOI] [PubMed] [Google Scholar]
- 48.Biederman J, Baldessarini RJ, Wright V, Knee D, Harmatz JS, Goldblatt A. A double-blind placebo controlled study of desipramine in the treatment ADD: II. Serum drug levels and cardiovascular findings. J Am Acad Child Adolesc Psychiatry. 1989;28:903–911. doi: 10.1097/00004583-198911000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Scahill L, Erenberg G, Berlin CM, Jr, et al. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006;3:192–206. doi: 10.1016/j.nurx.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilens TE, Spencer TJ, Swanson JM, Connor DF, Cantwell D. Combining methylphenidate and clonidine: a clinically sound medication option. J Am Acad Child Adolesc Psychiatry. 1999;38:614–619. doi: 10.1097/00004583-199905000-00025. discussion 619–622. [DOI] [PubMed] [Google Scholar]
- 51.Daviss WB, Patel NC, Robb AS, et al. Clonidine for attention-deficit/hyperactivity disorder: II. ECG changes and adverse events analysis. J Am Acad Child Adolesc Psychiatry. 2008;47:189–198. doi: 10.1097/chi.0b013e31815d9ae4. [DOI] [PubMed] [Google Scholar]
- 52.Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short-acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ. 2001;165:1475–1488. [PMC free article] [PubMed] [Google Scholar]
- 53.Connor DF, Fletcher KE, Swanson JM. A meta-analysis of clonidine for symptoms of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1999;38:1551–1559. doi: 10.1097/00004583-199912000-00017. [DOI] [PubMed] [Google Scholar]
- 54.Cheng JY, Chen RY, Ko JS, Ng EM. Efficacy and safety of atomoxetine for attention-deficit/hyperactivity disorder in children and adolescents-meta-analysis and meta-regression analysis. Psychopharmacology (Berl) 2007;194:197–209. doi: 10.1007/s00213-007-0840-x. [DOI] [PubMed] [Google Scholar]