Intakes of (n-3) Fatty Acids and Fatty Fish Are Not Associated with Cognitive Performance and 6-Year Cognitive Change in Men Participating in the Veterans Affairs Normative Aging Study (original) (raw)
Abstract
High intake of fish and (n-3) PUFA may protect against age-related cognitive decline. However, results are inconsistent and limited data exist regarding changes in multiple cognitive functions over a longer period of time. In this study, we assessed the association between fatty fish intake as well as (n-3) PUFA intake with cognitive performance and cognitive change over 6 y in 1025 elderly men. Participants were from the Veterans Affairs Normative Aging Study. Cognitive function was assessed with a battery of cognitive tests focusing on factors representing memory/language, speed, and visuospatial/attention. Dietary intakes were assessed with a validated FFQ. We used general linear models to assess cross-sectional associations and mixed models to assess the associations over time. Models were adjusted for age, education, BMI, smoking, diabetes, and intake of alcohol, saturated fat, vitamin C, and vitamin E. The mean age of participating men was 68 y at baseline. Median fish consumption ranged from 0.2 to 4.2 servings/wk across quartiles. Cross-sectional analyses showed no association between fatty fish or (n-3) PUFA intake and cognitive performance. Longitudinal analyses, over 6 y of follow-up, also did not show any significant associations between fatty fish or (n-3) PUFA intake and cognitive change. In this population of elderly men, intake of neither fatty fish nor (n-3) PUFA was associated with cognitive performance.
Introduction
Age-related cognitive impairment is considered to be a strong risk factor for the development of dementia (1). There has been increasing scientific interest in the hypothesis that fish consumption, particularly fatty fish, and the intake of the marine (n-3) PUFA, eicosapentaenoic acid [EPA;7 20:5(n-3)] and docosahexaenoic acid [22:6(n-3)] might play a protective role against age-related cognitive decline and dementia. Cognitive performance has been examined in relation to dietary intake of (n-3) PUFA and fish (2–5) or (n-3) PUFA concentrations in the blood in several cross-sectional studies (6,7). Results have been inconsistent, showing either that high intake or high concentrations of (n-3) PUFA in the blood were associated with better cognitive performance (2,3,5,6) or no association (4,7).
Longitudinal studies examining the association between fish or (n-3) PUFA intake and cognitive performance have also yielded contradictory results. In some studies, high fish and/or (n-3) PUFA intake was protective against 5- or 6-y cognitive decline (4,8), whereas no association was found in 3-y cognitive decline in the Zutphen Elderly Study (3). Studies using (n-3) PUFA concentrations in the blood have generally shown higher concentrations are associated with lower risk of cognitive decline (9,10). Cognitive function in older adults declines differentially in specific cognitive domains (11). If (n-3) PUFA are associated with cognitive functioning, they may affect specific cognitive domains differently, as different mechanisms could underlie each domain. However, data on these effects are limited, because most studies have used only a global measure of cognitive performance. We know of only 3 studies that have addressed the association between (n-3) PUFA and cognitive performance in different domains. Two showed a reduced risk of impaired speed with higher intake of fatty fish or (n-3) PUFA (2) or with higher plasma (n-3) PUFA proportions over 3 y (12) and 1 showed that higher proportions of (n-3) PUFA reduced the risk of decline in verbal fluency (10). This prospective cohort study of elderly men was based on longitudinal data with 3 measurements over a 6-y follow-up period. We examined the association between fatty fish intake as well as (n-3) PUFA intake with changes in multiple factors of cognitive functioning.
Participants and Methods
Participants
The Normative Aging Study (NAS), a longitudinal study of aging established by the Veterans Administration (now Department of Veterans Affairs), started in 1963 by recruiting men in the Boston area who were originally free of heart disease or other major health problems. The study cohort initially consisted of 2280 community-dwelling men who were between 21 and 81 y of age (mean 42 y) on entry during 1963–1970. Participating men return every 3–5 y for a health examination, at which time they complete several questionnaires. Dietary intake data have been collected since 1987. Since 1993, a battery of cognitive tests was added to these visits and cognitive performance was assessed in 3 cycles, ∼3 y apart. Relations of B vitamin and homocysteine (Hcy) concentrations with cognitive performance (13) and cognitive decline (14) in a subgroup of this NAS population have been previously reported. The Institutional Review Boards of both the Boston Veterans Affairs Medical Center and Tufts New England Medical Center approved the study and all participants gave written informed consent.
Cognitive measures
The battery of cognitive tests was designed to be appropriate for an aging population, including tests specifically chosen to assess cognitive status and changes in adults with various pathologic conditions such as Alzheimer's Disease [Consortium to Establish a Registry for Alzheimer's Disease (CERAD) (15)]. Some tests from the Neurobehavioral Evaluation System (NES2) were also included (16). Selected tests focus on language, speed, attention, memory, and spatial copying. The vocabulary, Boston naming, continuous performance, and pattern memory tests were administered only once, the other tests repeatedly. The Mini-Mental State Examination (MMSE) was used as a global measure of cognitive function (17).
Memory tests
Word list memory test (adapted from CERAD).
Ten words were presented on a computer screen consecutively, for 2 s each, and participants were then asked to recall these words. Three consecutive trials were administered and the score was the sum of words remembered (maximum score 30). After an intervening spatial copying task, participants were asked again to recall the memorized words (delayed recall, maximum score 10).
Backward digit span test (Wechsler Adult Intelligence Scale-Revised).
Participants were read a list of digits and asked to recall these in backward sequence. The score was the total span of digits recalled correctly in backward order, with a maximum of 8 (18).
Pattern memory (NES2).
One pattern was presented on the computer screen, which was followed after a brief interval by 3 similar patterns, from which participants were asked to identify the original pattern. The scores were the number of correct responses (maximum 25) and mean response latency for correct decisions.
Language tests
Verbal fluency (CERAD).
Participants were asked to name as many animals as possible within 1 min.
Boston Naming Test-short form (CERAD).
Participants were asked to identify 15 line-drawn objects by name (maximum score 15).
Vocabulary (Wechsler Adult Intelligence Scale-Revised).
Participants were asked to define words of increasing difficulty, which were scored according to quality of definition (maximum score 70).
Tests of perceptual speed and attention
Pattern comparison (NES2).
Participants were asked to choose the odd pattern from 3 similar patterns displayed on a computer monitor. The scores were the number of correct responses (maximum 25) and the mean response latency for correct decisions.
Continuous performance test (NES2).
Participants were asked to press a button when they saw a large letter “S,” but no other letter, on a computer monitor. The score was the mean response latency for items in the best 2 of 6 trials (10 target items in each trial). Best trials were defined as trials on which no or minimal errors were made and for which the mean response latencies were the fastest (19).
Spatial copying task-constructional praxis (CERAD).
Participants were asked to copy a circle, crossed rectangles, a vertical diamond, and a cube. These figures are augmented by the tilted triangles, 8-dot circle, horizontal diamond, and tapered box (from the Developmental Test of Visual-Motor Integration) (20) and the overlapping pentagons from the MMSE (17). The accuracy of the copied figures was scored by trained staff using criteria from the CERAD and Developmental Test of Visual-Motor Integration. The resulting score was the total number of figures drawn correctly (maximum score 9). A second score was weighted by the degree of difficulty of the figure, resulting in a maximum score of 26.
Dietary assessment
The participant's average frequency of consumption during the previous year was estimated with the 126-item semiquantitative Willett FFQ (21,22). This questionnaire requests participants to record the number of times they consume each of the food items using 7 response categories ranging from rarely/never to ≥2/d. The form was mailed to the participants before their examination visit and checked for completeness at the examination. Forms were processed through a nutrient database at the Channing Laboratory at Harvard University to obtain estimates of usual daily nutrient intake. Four fish items were included: dark-meat fish [e.g. bluefish, mackerel, salmon, sardines, or swordfish; 1.37 g of (n-3) fatty acids per portion]; canned tuna (0.69 g); other fish (0.17 g); and shrimp, lobster, or scallops (0.46 g). We calculated average daily fish intake by summing these 4 fish items and we calculated fatty fish intake by summing the frequencies of tuna and dark-meat fish.
Other measurements
Information on education level, smoking history, and medical history was obtained by questionnaire. Height and weight were measured to calculate BMI (kg/m2). Fasting plasma samples were drawn at the VA field site and stored at −80°C. These samples were then taken to the Nutrition Evaluation Laboratory at Tufts University Human Nutrition Research Center on Aging for analysis. Total Hcy in plasma was measured using an adaptation of a previously described method (23). Plasma folate and vitamin B-12 concentrations were measured by radio assay with the use of a commercially available kit from Bio-Rad.
Statistical analysis
Differences in baseline characteristics among different categories of fatty fish consumers and (n-3) PUFA intake were compared using ANOVA or Kruskal-Wallis for continuous variables and chi-square for categorical variables. For the cognitive measures, we used Tukey's post hoc test to examine differences between quartiles. For privacy reasons, participants aged >89 y (n = 1 for cross-sectional analysis and n = 4 for the longitudinal analysis) were recoded to 89 y. For the cross-sectional analysis, principal components analysis (SAS PROC FACTOR procedure) with varimax rotation was used to create composite scores for separate dimensions of cognitive function while retaining as much of the individual test's variance as possible. This analysis included 451 men in total (who had baseline scores on all included tests) and resulted in 3 factors: 1) a memory/language factor; 2) a visuospatial/attention factor; and 3) a speed factor.
We used general linear models to investigate the association between fatty fish or (n-3) PUFA intake (independent) and cognitive performance (dependent) at baseline. To test for linear trend, the quartile median value for dietary (n-3) PUFA or fatty fish was assigned to each participant in that quartile. To investigate the association of fatty fish and (n-3) PUFA intake on 6-y cognitive change, a repeated mixed coefficients model (SAS PROC MIXED procedure) was used. This procedure takes into account the intraindividual correlation of repeated measurements and does not exclude participants with incomplete data at follow-up. Logarithmic transformation was applied to all dietary measures before including them in the regression models to improve linearity. We calculated energy adjusted intake for all dietary measures with the residual method (24) and created quartiles for fatty fish intake as well as (n-3) PUFA intake. Fatty fish consumption and intake of (n-3) PUFA were entered as class variables into the model and the outcome variables, i.e. baseline cognitive functioning and 6-y cognitive change, were treated as continuous variables. Users of cod liver oil (n = 29) and fish oil capsules (n = 11) were excluded from the analysis.
Models were adjusted for age, educational level (<12, 12, 13–15, ≥16 y), BMI (kg/m2), smoking (current, past, or never), diabetes (yes/no), alcohol intake (g/d), saturated fat intake (g/d), vitamin C intake (mg/d), and vitamin E intake (mg/d). These confounders were included based on previously published associations as well as associations with the exposure and outcome (change in the _β_ coefficient ≥10%) in the current data set. We checked for interaction between fish intake, as well as (n-3) PUFA intake, with age, education, BMI, and diabetes as categorical variables and no significant interactions were observed (_P_ > 0.05).
We also analyzed models additionally adjusted for physical activity, plasma folate, Hcy, and vitamin B-12. However, because including these variables did not affect results, they were removed from the final models. All statistical analyses were carried out using SAS (version 9.1; SAS Institute). Two-sided _P_-values ≤ 0.05 were considered significant.
Results
Baseline characteristics.
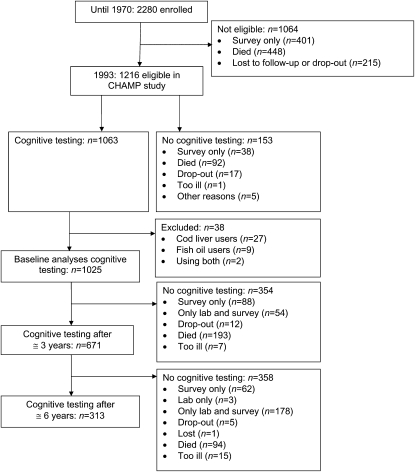
Of the 2280 men originally enrolled, 1216 were still participating in the on-site examination portion of the study in 1993 when the cognitive measures were initiated. Of these, 87% (1063) completed baseline cognitive testing. See Figure 1 for the flow of participants. Of these, 671 completed a second battery at 3 y and 313 at 6 y. Reasons for loss to follow-up were mainly due to death or movement from the area. Men who moved continued participation by mail survey but did not continue the in-person exam, including the cognitive testing. Due to the high participant burden, not all men completed all tests. A shorter battery was offered to those refusing the full battery. Individual test completion ranged from 487 to 982 and when combined to create factors, this resulted in a sample size of 451 with complete measures for all included tests.
FIGURE 1 .
Participant flow in the study from initiation to cognitive measures.
The mean age of completing participants at baseline cognitive measurement was 68 y. Total mean fish consumption was 2.4 servings/wk of total fish and 1.3 servings/wk of fatty fish. Mean total (n-3) PUFA intake was 0.28 g/d. Median fatty fish consumption ranged from 0.2 to 2.8 portions/wk across quartiles. Men in the higher quartiles of (n-3) PUFA consumption had more years of education and were less likely to be smokers than those in the lower quartiles. Men with low (n-3) PUFA intake had higher intakes of total energy and saturated fat and lower intakes of alcohol and vitamins C and E (Table 1). Unadjusted baseline cognitive scores for word list memory tests (P = 0.01 and 0.03), the Boston naming test (P = 0.01), and continuous performance test differed (P = 0.01) among quartiles of fatty fish consumers (Table 2). Unadjusted baseline scores across the quartiles of (n-3) PUFA intake did not differ significantly for any of the cognitive tests (data not shown).
TABLE 1.
Baseline characteristics of 1025 men in the NAS by (n-3) PUFA intake1
| Energy-adjusted quartiles of (n-3) PUFA intake, g/d, median (range ) | |||||
|---|---|---|---|---|---|
| 1 0.10 (0.06–0.13) | 2 0.19 (0.17–0.21) | 3 0.29 (0.26–0.31) | 4 0.45 (0.40–0.55) | P2 | |
| n | 256 | 256 | 257 | 256 | |
| Age, y | 68.9 ± 7.33 | 67.5 ± 7.1 | 67.8 ± 7.3 | 68.5 ± 7.3 | 0.12 |
| Education, n (%) | 0.05 | ||||
| <12 y | 25 (10) | 22 (9) | 19 (7) | 16 (6) | |
| 12 y | 87 (34) | 80 (31) | 72 (28) | 51 (20) | |
| 13–15 y | 62 (24) | 61 (24) | 59 (23) | 81 (32) | |
| ≥16 y | 82 (32) | 93 (36) | 107 (42) | 108 (42) | |
| BMI, kg/m2 | 27.8 ± 4.1 | 27.9 ± 3.7 | 27.9 ± 3.6 | 27.7 ± 3.9 | 0.92 |
| Smoking status, n (%) | 0.01 | ||||
| Current | 26 (10) | 17 (7) | 16 (6) | 5 (2) | |
| Former | 153 (60) | 148 (58) | 160 (62) | 159 (62) | |
| Never | 76 (30) | 91 (36) | 81 (32) | 92 (36) | |
| Alcohol, g/d | 4.9 (0.5–15.8)4 | 7.8 (2.5–18.2) | 8.0 (3.0–17.8) | 7.5 (2.7–15.1) | 0.33 |
| Physical activity score5 | 3 (2–6) | 3 (2–5) | 2 (1–4) | 2 (1–3) | 0.0007 |
| Diabetes, n (%) | 38 (15) | 40 (16) | 37 (15) | 51 (20) | 0.32 |
| MMSE score | 28 (27–29) | 28 (27–29) | 28 (27–29) | 28 (27–29) | 0.66 |
| Plasma folate, nmol/L | 21 (14–30) | 21 (13–32) | 20 (12–29) | 21 (14–32) | 0.66 |
| Plasma vitamin B-12, pmol/L | 309 (241–394) | 289 (225–378) | 330 (243–409) | 347 (286–451) | 0.0018 |
| Plasma Hcy, μmol/L | 10 (9–12) | 10 (9–12) | 10 (8–12) | 10 (9–12) | 0.33 |
| Total energy intake, kJ/d | 8667 (6883–10668) | 7436 (5581–8939) | 7875 (6263–9362) | 8127 (6464–9692) | <0.0001 |
| Saturated fat intake,6g/d | 23.7 (19.6–27.8) | 22.2 (19.2–26.0) | 21.2 (17.6–24.3) | 19.7 (16.1–22.6) | <0.0001 |
| Vitamin C intake,6mg/d | 168 (121–261) | 183 (126–277) | 190 (136–311) | 219 (155–305) | 0.0042 |
| Vitamin E intake,6mg/d | 12.4 (1.2–37.5) | 17.6 (8.5–39.1) | 18.6 (5.8–48.3) | 19.7 (7.6–153) | 0.13 |
| Total fish intake,67servings/wk | 0.8 (0.4–1.1) | 1.8 (1.5–2.1) | 2.3 (1.9–2.8) | 4.1 (3.2–5.1) | <0.0001 |
| Fatty fish intake,67servings/wk | 0.4 (0.1–0.7) | 0.8 (0.5–1.1) | 1.3 (1.0–1.5) | 1.9 (1.5–3.6) | <0.0001 |
TABLE 2.
Baseline cognitive measures of aging men across energy-adjusted quartiles of fatty fish intake12
| Energy-adjusted quartiles of fatty fish intake, servings/wk,3 median (range) | ||||||
|---|---|---|---|---|---|---|
| n | 1 0.21 (−0.02–0.38) | 2 0.80 (0.66–0.90) | 3 1.25 (1.11–1.38) | 4 2.79 (1.76–3.61) | P | |
| Memory | ||||||
| Word list memory, total 3 trials | 871 | 18.5 ± 4.0a (16–21) | 19.6 ± 3.5b (17–22) | 19.1 ± 3.6 (17–21) | 18.5 ± 3.9a (16–21) | 0.01 |
| Word list delayed recall | 869 | 6.1 ± 2.0a (5–7) | 6.6 ± 1.8b (5–8) | 6.5 ± 1.8 (5–8) | 6.3 ± 2.0 (5–8) | 0.03 |
| Backward digit span, total span | 766 | 5.1 ± 2.2 (3–7) | 5.2 ± 2.3 (4–7) | 5.0 ± 2.4 (3–7) | 4.8 ± 2.3 (3–6) | 0.31 |
| Pattern memory, total correct | 488 | 18.6 ± 3.0 (17–21) | 19.2 ± 3.2 (18–21) | 19.5 ± 3.3 (18–22) | 19.4 ± 2.6 (18–21) | 0.12 |
| Language | ||||||
| Verbal fluency, total correct, | 873 | 18.2 ± 5.2 (15–22) | 19.0 ± 4.7 (15–22) | 19.1 ± 4.9 (16–22) | 18.7 ± 5.0 (15–22) | 0.26 |
| Boston naming test, total correct | 488 | 19.1 ± 1.1 (19,20) | 19.3 ± 0.9b (19,20) | 19.3 ± 0.9b (19,20) | 18.9 ± 1.5a (18–20) | 0.01 |
| Vocabulary, total correct | 489 | 50.5 ± 8.8 (45–57) | 51.5 ± 9.5 (46–59) | 51.6 ± 10.5 (47–59) | 49.5 ± 10.1 (43–57) | 0.28 |
| Speed | ||||||
| Pattern memory, mean response time correct trials, s | 488 | 6.1 ± 1.7 (5.0–6.8) | 5.8 ± 1.4 (4.7–6.7) | 5.6 ± 1.4 (4.8–6.5) | 5.7 ± 1.4 (4.5–6.6) | 0.06 |
| Pattern comparison test, mean response time correct trials, s | 880 | 5.8 ± 1.7 (4.6–6.7) | 5.7 ± 1.6 (4.6–6.4) | 5.5 ± 1.5 (4.5–6.3) | 5.7 ± 1.5 (4.7–6.5) | 0.33 |
| Continuous performance test, mean response time 2 best trials | 487 | 354 ± 58b (315–373) | 333 ± 43a (309–354) | 338 ± 52 (304–361) | 349 ± 59 (306–380) | 0.01 |
| Visuospatial | ||||||
| Pattern comparison test, total correct | 880 | 23.8 ± 1.6 (23–25) | 24.0 ± 1.7 (24,25) | 24.0 ± 1.3 (24,25) | 23.9 ± 1.7 (24,25) | 0.42 |
| Sum of drawings 1–9 | 982 | 5.5 ± 1.7 (4–7) | 5.6 ± 1.8 (4–7) | 5.7 ± 1.8 (5–7) | 5.7 ± 1.7 (5–7) | 0.63 |
| Sum of weighted drawings 1–9 | 982 | 14.6 ± 5.6 (10–19) | 14.8 ± 5.6 (11–19) | 15.3 ± 5.9 (12–19) | 15.1 ± 5.5 (12–19) | 0.53 |
Cognitive performance (cross-sectional analysis).
After adjusting for age and education, as well as other potential confounding variables, there were no significant protective associations between quartiles of fatty fish or (n-3) PUFA intake and cognitive dimensions, as measured by the factor scores. Rather, and in contrast to our hypothesis, performance tended to be impaired on the cognitive factor memory/language with greater intake of fatty fish (P = 0.09) or (n-3) PUFA (P = 0.17) (Table 3). However, examination of the main contributing cognitive test for each of the cognitive factors as the dependent variable did not show this (Table 4).
TABLE 3.
Baseline cognitive factors for 451 elderly men in the NAS, by (n-3) PUFA and fatty fish intake1
| Energy-adjusted quartiles | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | _P_-trend | |
| median (range) | |||||
| (n-3) PUFA intake, g/d | 0.10 (0.06–0.13) | 0.19 (0.17–0.21) | 0.29 (0.26–0.31) | 0.45 (0.40–0.55) | |
| Memory/language2 | |||||
| Model 13 | 0.05 (−0.13, 0.23) | 0.02 (−0.16, 0.19) | 0.01 (−0.16, 0.18) | −0.07 (−0.25, 0.10) | 0.35 |
| Model 24 | 0.08 (−0.10, 0.27) | 0.02 (−0.16, 0.19) | 0.02 (−0.16, 0.19) | −0.12 (−0.30, 0.06) | 0.17 |
| Visuospatial/attention5 | |||||
| Model 13 | −0.04 (−0.22, 0.14) | 0.10 (−0.08, 0.27) | −0.05 (−0.22, 0.12) | −0.01 (−0.18, 0.17) | 0.98 |
| Model 24 | −0.03 (−0.21, 0.16) | 0.11 (−0.06, 0.28) | −0.06 (−0.23, 0.11) | −0.02 (−0.20, 0.16) | 0.76 |
| Speed6 | |||||
| Model 13 | 0.00 (−0.18, 0.18) | −0.03 (−0.21, 0.14) | 0.09 (−0.08, 0.26) | −0.07 (−0.24, 0.11) | 0.83 |
| Model 24 | −0.01 (−0.19, 0.17) | −0.04 (−0.21, 0.14) | 0.08 (−0.09, 0.25) | −0.04 (−0.22, 0.14) | 0.90 |
| Fatty fish intake,5 servings/wk | 0.21 (−0.02–0.38) | 0.80 (0.66–0.90) | 1.25 (1.11–1.38) | 2.79 (1.76–3.61) | |
| Memory/language2 | |||||
| Model 13 | 0.02 (−0.16, 0.20) | 0.18 (0.01, 0.35) | 0.05 (−0.13, 0.22) | −0.23 (−0.40, −0.07) | 0.17 |
| Model 24 | 0.05 (−0.13, 0.24) | 0.21 (0.04, 0.38) | 0.02 (−0.15, 0.20) | −0.27 (−0.44, −0.10) | 0.09 |
| Visuospatial/attention6 | |||||
| Model 13 | 0.02 (−0.16, 0.21) | −0.02 (−0.19, 0.16) | 0.02 (−0.16, 0.20) | −0.02 (−0.20, 0.15) | 0.75 |
| Model 24 | 0.07 (−0.12, 0.26) | 0.00 (−0.17, 0.18) | 0.01 (−0.17, 0.18) | −0.07 (−0.24, 0.10) | 0.33 |
| Speed7 | |||||
| Model 13 | 0.14 (−0.04, 0.32) | −0.01 (−0.18, 0.16) | −0.10 (−0.27, 0.08) | −0.03 (−0.19, 0.14) | 0.10 |
| Model 24 | 0.13 (−0.05, 0.32) | −0.02 (−0.19, 0.16) | −0.09 (−0.27, 0.08) | −0.01 (−0.18, 0.16) | 0.16 |
TABLE 4.
Representative baseline tests/cognitive factor for men in the NAS by (n-3) PUFA and fatty fish intake1
| Energy-adjusted quartiles | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | _P_-trend | |
| median (range) | |||||
| (n-3) PUFA intake, g/d | 0.10 (0.06–0.13) | 0.19 (0.17–0.21) | 0.29 (0.26–0.31) | 0.45 (0.40–0.55) | |
| Word list total 3 trials | |||||
| Model 12, n = 871 | 18.9 (18.4, 19.3) | 19.3 (18.8, 19.7) | 18.9 (18.5, 19.4) | 18.7 (18.3, 19.2) | 0.61 |
| Model 23, n = 848 | 19.0 (18.5, 19.5) | 19.3 (18.8, 19.7) | 18.9 (18.4, 19.4) | 18.8 (18.3, 19.2) | 0.45 |
| Spatial copying, sum of drawings | |||||
| Model 12, n = 982 | 5.7 (5.4, 5.9) | 5.6 (5.4, 5.9) | 5.6 (5.4, 5.8) | 5.6 (5.4, 5.8) | 0.58 |
| Model 23, n = 960 | 5.7 (5.5, 5.9) | 5.6 (5.4, 5.9) | 5.5 (5.3, 5.8) | 5.5 (5.3, 5.8) | 0.21 |
| Pattern comparison test, mean response time | |||||
| Model 12, n = 880 | 5.7 (5.5, 5.9) | 5.6 (5.4, 5.8) | 5.8 (5.6, 6.0) | 5.5 (5.4, 5.7) | 0.75 |
| Model 23, n = 853 | 5.6 (5.4, 5.8) | 5.6 (5.4, 5.8) | 5.8 (5.6, 6.0) | 5.5 (5.4, 5.7) | 0.97 |
| Fatty fish intake,4servings/wk | 0.21 (−0.02–0.38) | 0.80 (0.66–0.90) | 1.25 (1.11–1.38) | 2.79 (1.76–3.61) | |
| Word list total 3 trials | |||||
| Model 12, n = 871 | 18.8 (18.3, 19.3) | 19.6 (19.1, 20.1) | 18.8 (18.4, 19.3) | 18.5 (18.1, 19.0) | 0.73 |
| Model 23, n = 848 | 18.9 (18.4, 19.4) | 19.7 (19.2, 20.2) | 18.8 (18.3, 19.3) | 18.6 (18.1, 19.0) | 0.66 |
| Spatial copying, sum of drawings | |||||
| Model 12, n = 982 | 5.6 (5.4, 5.8) | 5.6 (5.4, 5.8) | 5.7 (5.5, 5.9) | 5.6 (5.4, 5.8) | 0.99 |
| Model 23, n = 960 | 5.6 (5.4, 5.9) | 5.6 (5.4, 5.9) | 5.6 (5.4, 5.8) | 5.5 (5.3, 5.8) | 0.60 |
| Pattern comparison test, mean response time | |||||
| Model 12, n = 880 | 5.7 (5.5, 5.9) | 5.7 (5.5, 5.9) | 5.7 (5.5, 5.8) | 5.6 (5.4, 5.8) | 0.53 |
| Model 23, n = 853 | 5.7 (5.5, 5.9) | 5.6 (5.4, 5.8) | 5.7 (5.5, 5.9) | 5.6 (5.4, 5.8) | 0.67 |
Change in cognitive performance over 6 y (prospective analysis).
Of the 1025 men included in the cross-sectional analysis, 671 returned for follow-up measurements after 3 y and 313 returned after 6 y. The 313 men with complete follow-up were 2 y younger than those who did not return for the follow-up measurements but did not differ significantly on any other variable examined here. Cognitive tests did not differ across quartiles of fish or (n-3) PUFA intakes (Table 5). As in the cross-sectional analysis, performance on the cognitive factor memory/language tended to be impaired with greater intake of fatty fish (P = 0.09) or (n-3) PUFA (P = 0.17). Repeating these analyses with only the participants who had complete follow-up over the 6 y did not show different results.
TABLE 5.
6-y cognitive change for 1025 men in the NAS by (n-3) PUFA and fatty fish intake1
| Energy-adjusted quartiles | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | P | |
| median (range) | |||||
| (n-3) PUFA intake, g/d | 0.10 (0.06–0.13) | 0.19 (0.17–0.21) | 0.29 (0.26–0.31) | 0.45 (0.40–0.55) | |
| Word list total 3 trials | |||||
| Model 12 | 19.0 (18.6, 19.3) | 18.9 (18.5, 19.2) | 19.3 (19.0, 19.7) | 18.9 (18.5, 19.2) | 0.09 |
| Model 23 | 19.0 (18.6, 19.4) | 19.0 (18.6, 19.3) | 19.3 (19.0, 19.7) | 18.9 (18.5, 19.3) | 0.17 |
| Spatial copying, sum of drawings | |||||
| Model 12 | 5.8 (5.6, 6.0) | 5.9 (5.7, 6.1) | 5.9 (5.8, 6.1) | 5.9 (5.7, 6.1) | 0.70 |
| Model 23 | 5.8 (5.6, 6.0) | 5.9 (5.7, 6.1) | 5.9 (5.8, 6.1) | 5.8 (5.7, 6.0) | 0.70 |
| Pattern comparison test, mean response time | |||||
| Model 12 | 5.8 (5.6, 5.9) | 5.6 (5.5, 5.8) | 5.6 (5.5, 5.8) | 5.6 (5.4, 5.7) | 0.21 |
| Model 23 | 5.7 (5.6, 5.9) | 5.6 (5.5, 5.7) | 5.6 (5.5, 5.8) | 5.6 (5.4, 5.7) | 0.32 |
| Fatty fish intake,4servings/wk | 0.21 (−0.02–0.38) | 0.80 (0.66–0.90) | 1.25 (1.11–1.38) | 2.79 (1.76–3.61) | |
| Word list total 3 trials | |||||
| Model 12 | 19.0 (18.6, 19.3) | 19.2 (18.9, 19.6) | 19.1 (18.8, 19.5) | 18.7 (18.4, 19.1) | 0.09 |
| Model 23 | 19.0 (18.7, 19.4) | 19.3 (18.9, 19.6) | 19.2 (18.8, 19.5) | 18.7 (18.4, 19.1) | 0.08 |
| Spatial copying, sum of drawings | |||||
| Model 12 | 5.8 (5.6, 6.0) | 5.8 (5.7, 6.0) | 6.0 (5.8, 6.1) | 5.9 (5.7, 6.1) | 0.54 |
| Model 23 | 5.8 (5.7, 6.0) | 5.8 (5.7, 6.0) | 5.9 (5.8, 6.1) | 5.8 (5.7, 6.0) | 0.70 |
| Pattern comparison test, mean response time | |||||
| Model 12 | 5.7 (5.6, 5.9) | 5.8 (5.6, 5.9) | 5.5 (5.4, 5.7) | 5.6 (5.5, 5.8) | 0.08 |
| Model 23 | 5.7 (5.5, 5.8) | 5.7 (5.6, 5.8) | 5.5 (5.4, 5.7) | 5.6 (5.5, 5.8) | 0.18 |
Discussion
The present study is one of the few prospective cohort studies to examine prospective associations between fish and (n-3) PUFA intake with cognitive change in multiple cognitive factors. Our findings in this sample of aging men do not support the hypothesis that higher fish/(n-3) PUFA intake is associated with better cognitive function or with less cognitive decline on any of the cognitive tests.
Strengths of the current study include the ability to assess cognitive performance not only cross-sectionally but also prospectively with multiple repeated measurements. Furthermore, we used an extensive battery of cognitive tests appropriate for an aging population. This is an important advantage over the use of only general cognitive tests such as the MMSE, which was originally designed as a screening tool and not as a measure of change (17). To limit the number of outcome variables, we defined 3 cognitive factors that accounted for most of the variance observed. However, because not all cognitive tests were performed in all participants, this also decreased the number of participants for each cognitive factor compared with the number of participants that performed some of the individual cognitive tests. Therefore, we repeated our analyses with the cognitive test that contributed most to each cognitive factor. However, despite the fact that this increased the number of participants, these analyses did not show different results.
The Willett FFQ includes questions on different fish items and has been validated in several studies (21,25,26). In a validation study with a random sample of 127 men aged 45–70 y living in the Boston area, these 4 items on seafood intake were shown to be reproducible and useful measures of seafood intake. Correlations between 2 administrations of the questionnaire 1 y apart ranged from 0.48 (fish) to 0.67 (shellfish) (26). The correlation between fish intake as reported on the questionnaire with fish intake assessed with 2 1-wk dietary records was 0.61 (25). Although plasma, erythrocyte, or phospholipid proportions of (n-3) PUFA are often considered more objective measures of (n-3) PUFA intake (27), these values were not available in the present study. However, it has been shown that these biochemical measures may provide only limited information about absolute values of (n-3) PUFA intake, because they may vary for participants with similar dietary intake due to other participant characteristics (28). Welch et al. (29) found that only ∼25% of the variation in plasma (n-3) PUFA was explained by fish and fish oil consumption. Furthermore, because they only reflect intake over the past week or 2, they may be less indicative of long-term exposure than responses to a FFQ for the past year (27).
Because the number of individuals participating in the consecutive cognitive test series of our study decreased substantially (from 1025 to 671 after 3 y to 313 after 6 y of follow-up), bias due to incomplete follow-up may have influenced our results. However, except for the fact that participants with complete follow-up were 2 y younger, they did not differ from the participants with incomplete follow-up. An under- or overestimation of the effect is unlikely, because fish intake and cognitive scores were also comparable between completers and noncompleters.
The trend for the impaired, although not significant, memory/language factor with greater fish or (n-3) PUFA intake was unexpected and has not to our knowledge been shown before. We have no clear explanation for this result. Also, when we performed the same analysis with only the cognitive test that contributed most to each factor, we did not observe this trend despite the larger sample size. Because we made multiple comparisons, it may also have been a chance finding. Our cross-sectional results are in agreement with those of 2 other studies, which also observed no significant associations (3,4). They are also in agreement with 2 studies that evaluated (n-3) concentrations in the blood and found no association with cognitive function (7,12). However, other studies have found positive associations with (n-3) PUFA concentrations and cognitive function (6) and with fish or (n-3) PUFA intake and global cognitive function and speed (2).
The null results of our longitudinal analyses are consistent with the first publication from the Zutphen study data, which also showed no clear association between fish consumption and 3-y cognitive decline (3). However, 5-y cognitive decline was later inversely associated with EPA- docosahexaenoic acid intake in that study population (4). The Chicago Health and Aging Project showed that fish consumption, but not intake of (n-3) PUFA, was associated with less cognitive decline (8). A few studies have shown that a higher (n-3) PUFA (9) or EPA concentration in blood (7) is associated with less cognitive decline. Dullemeijer et al. (12) observed an association between (n-3) PUFA plasma concentrations and sensorimotor and complex speed but did not observe associations for memory, information-processing speed, or verbal fluency. Beydoun et al. (10) observed that lower concentrations of plasma (n-3) fatty acids were associated with greater decline in verbal fluency but not in memory or psychomotor speed. The findings of these few studies assessing different cognitive domains are not consistent. More prospective studies, as well as intervention studies investigating the association between (n-3) PUFA and domain-specific measures, are needed.
Our study population consumed an average of 2.4 servings of total fish per week, which is relatively high compared with other study populations in The Netherlands and the United States, where average fish intake was once per week or less (3,8,30), but lower than in a Norwegian population, where intake was 5 servings/wk (5). Our study population consisted of men only. The Zutphen study also included only men, but they observed a protective effect of a higher fish and (n-3) PUFA intake on 5-y cognitive decline (4). Relative to that study, the age of our men was 7 y younger, 68 compared with 75 y, and the MMSE was 1.3 points higher. The participants of the Chicago Health and Aging project, where fish consumption was also associated with slower cognitive decline, were 74 y, also several years older than in our study (8). It is, therefore, possible that a protective effect of fish intake may be more apparent with advancing age and cognitive decline. On the other hand, there is increasing concern about contaminants, such as mercury and dioxins, in certain types of fish, although a review performed in 2006 showed that the benefits of moderate (1–2 servings/wk) fish consumption exceed the potential risks, at least for cardiovascular and neurological outcomes (31). Although we cannot assess this exposure directly, it remains possible that the benefits of the (n-3) PUFA intake among the relatively high fish consumers in this study may have been attenuated by negative effects of contaminants. Further investigation in this area is needed.
In conclusion, higher intake of fish or (n-3) PUFA was not associated with better cognitive performance at baseline or with lower 6-y cognitive decline in any of the cognitive tests in this population of aging men. Studies with longer observation of changes in specific cognitive domains are needed to clarify the current conflicting results observed in the literature.
Acknowledgments
K.L.T. and O.R. designed the analysis; A.S. and E.K.K. provided data and input on the parent study; O.R. analyzed the data and wrote the first draft; J.M.G. and L.C.G.P.M.d.G. provided critical review. K.L.T. had primary responsibility for final content. All authors read and approved the final manuscript.
1
Supported in part by the USDA, Agricultural Research Service, agreement 58-1950-7-707, and by the organization “Jan Brouwer Fonds.” The Cognition and Health in Aging Men Project is supported by the Clinical Science Research and Development Service of the US Department of Veterans Affairs and by the NIH (grants R01-AA08941, R01-AG13006, R01-AG14345, R01-AG18436, 5-P42-ES05947, and R01-ES05257). The VA Normative Aging Study is supported by the Cooperative Studies Program/ERIC, U.S. Department of Veterans Affairs, and is a research component of the Massachusetts Veterans Epidemiology Research and Information Center. The views expressed in this paper are those of the authors and do not necessarily represent the views of the U.S. Department of Veterans Affairs.
2
Author disclosures: O. van de Rest, A. Spiro III, E. Krall-Kaye, J. M. Geleijnse, L. C. P. G. M. de Groot, and K. L. Tucker, no conflicts of interest.
7
Abbreviations used: CERAD, Consortium to Establish a Registry for Alzheimer's Disease; EPA, eicosapentaenoic acid; Hcy, homocysteine; MMSE, Mini-Mental State Examination; NAS, Normative Aging Study; NES2, Neurobehavioral Evaluation System.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–57. [DOI] [PubMed] [Google Scholar]
- 2.Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62:275–80. [DOI] [PubMed] [Google Scholar]
- 3.Kalmijn S, Feskens EJ, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol. 1997;145:33–41. [DOI] [PubMed] [Google Scholar]
- 4.van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr. 2007;85:1142–7. [DOI] [PubMed] [Google Scholar]
- 5.Nurk E, Drevon CA, Refsum H, Solvoll K, Vollset SE, Nygard O, Nygaard HA, Engedal K, Tell GS, et al. Cognitive performance among the elderly and dietary fish intake: the Hordaland Health Study. Am J Clin Nutr. 2007;86:1470–8. [DOI] [PubMed] [Google Scholar]
- 6.Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer's disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–12. [DOI] [PubMed] [Google Scholar]
- 7.Laurin D, Verreault R, Lindsay J, Dewailly E, Holub BJ. Omega-3 fatty acids and risk of cognitive impairment and dementia. J Alzheimers Dis. 2003;5:315–22. [DOI] [PubMed] [Google Scholar]
- 8.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62:1849–53 [DOI] [PubMed]
- 9.Heude B, Ducimetiere P, Berr C. Cognitive decline and fatty acid composition of erythrocyte membranes-The EVA Study. Am J Clin Nutr. 2003;77:803–8. [DOI] [PubMed] [Google Scholar]
- 10.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2007;85:1103–11. [DOI] [PubMed] [Google Scholar]
- 11.van Boxtel MP, Buntinx F, Houx PJ, Metsemakers JF, Knottnerus A, Jolles J. The relation between morbidity and cognitive performance in a normal aging population. J Gerontol A Biol Sci Med Sci. 1998;53:M147–54. [DOI] [PubMed] [Google Scholar]
- 12.Dullemeijer C, Durga J, Brouwer IA, van de Rest O, Kok FJ, Brummer RJ, van Boxtel MP, Verhoef P. N-3 fatty acid proportions in plasma and cognitive performance in older adults. Am J Clin Nutr. 2007;86:1479–85. [DOI] [PubMed] [Google Scholar]
- 13.Riggs KM, Spiro A III, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am J Clin Nutr. 1996;63:306–14. [DOI] [PubMed] [Google Scholar]
- 14.Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A III. High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82:627–35. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–65. [DOI] [PubMed] [Google Scholar]
- 16.Letz R. NES2 user's manual (version 4.4). Winchester (MA): Neurobehavioral Systems, Inc.; 1991.
- 17.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 18.Wechsler D. Manual for the Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981.
- 19.Eisen EA, Letz RA, Wegman DH, Baker EL Jr, Pothier L. Defining measurement precision for effort dependent tests: the case of three neurobehavioural tests. Int J Epidemiol. 1988;17:920–6. [DOI] [PubMed] [Google Scholar]
- 20.Keery BE. The developmental test of visual visual-motor integration manual. Revised ed. Cleveland: Modern Curriculum Press; 1989.
- 21.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. [DOI] [PubMed] [Google Scholar]
- 22.Willett WC, Reynolds RD, Cottrell-Hoehner S, Sampson L, Browne ML. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc. 1987;87:43–7. [PubMed] [Google Scholar]
- 23.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52. [DOI] [PubMed] [Google Scholar]
- 24.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 25.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 26.Hunter DJ, Rimm EB, Sacks FM, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol. 1992;135:418–27. [DOI] [PubMed] [Google Scholar]
- 27.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38:2012–22. [PubMed] [Google Scholar]
- 28.Ma J, Folsom AR, Shahar E, Eckfeldt JH. Plasma fatty acid composition as an indicator of habitual dietary fat intake in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Am J Clin Nutr. 1995;62:564–71. [DOI] [PubMed] [Google Scholar]
- 29.Welch AA, Bingham SA, Ive J, Friesen MD, Wareham NJ, Riboli E, Khaw KT. Dietary fish intake and plasma phospholipid n-3 polyunsaturated fatty acid concentrations in men and women in the European Prospective Investigation into Cancer-Norfolk United Kingdom cohort. Am J Clin Nutr. 2006;84:1330–9. [DOI] [PubMed] [Google Scholar]
- 30.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–82. [DOI] [PubMed] [Google Scholar]
- 31.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–99. [DOI] [PubMed] [Google Scholar]
- 32.Willett W, Stampfer MJ. Implications of total energy intake for epidemiologic analyses. In: Willett W, editor. Nutritional epidemiology. New York: Oxford University Press; 1998. p. 273–301.