Dendritic Cells Produce CXCL13 and Participate in the Development of Murine Small Intestine Lymphoid Tissues (original) (raw)
Abstract
In the adult intestine, luminal microbiota induce cryptopatches to transform into isolated lymphoid follicles (ILFs), which subsequently act as sites for the generation of IgA responses. The events leading to this conversion are incompletely understood. Dendritic cells (DCs) are components of cryptopatches (CPs) and ILFs and were therefore evaluated in this process. We observed that the adult murine intestine contains clusters of DCs restricted to the CP/ILF continuum. A numerical and cell associative hierarchy in the adult intestine and a chronologic hierarchy in the neonatal intestine demonstrated that these clusters form after the coalescence of CD90+ cells to form CPs and before the influx of B220+ B lymphocytes to form ILFs. Cluster formation was dependent on lymphotoxin and the lymphotoxin β receptor and independent of lymphocytes. The ILF DC population was distinguished from that of the lamina propria by the absence of CD4+CD11c+ cells and an increased proportion of CD11c+B220+ cells. The formation of clusters was not limited by DC numbers but was induced by luminal microbiota. Moreover, in the absence of the chemokine CXCL13, CP transformation into ILF was arrested. Furthermore, ILF DCs express CXCL13, and depletion of DCs resulted in regression of ILFs and disorganization of CPs. These results reveal DC participation in ILF transformation and maintenance and suggest that in part this may be due to CXCL13 production by these cells.
The mucosal immune system is charged with the task of protecting a vast environmentally exposed surface from potential pathogens. To accomplish this feat, the mucosal immune system uses a variety of strategies using aspects of both the innate and adaptive immune system; principal among these in the intestine is the production and secretion of IgA.1 Solitary intestinal lymphoid tissues (SILTs) are recently appreciated participants in this process and can function as sites for the generation of T cell-dependent and T cell-independent IgA.2,3 SILT encompasses a spectrum ranging from nascent lymphoid tissues, or cryptopatches (CPs), to their more developed descendants containing lymphocytes, isolated lymphoid follicles (ILFs).4 CP development and their subsequent progression to ILFs recapitulate secondary lymphoid tissue development; however, unlike secondary lymphoid tissues, which are fully formed from birth, CPs and ILFs have a plasticity that allows the transition of CPs into ILFs, and their subsequent regression back to CPs, throughout life in response to changes in local stimuli including changes in the luminal microbiota.5,6,7,8,9 ILFs, but not CP, contribute to mucosal protection by acting as sites for the initiation IgA responses, which can subsequently alter the intestinal flora to return to the homeostatic state.5,9 Accordingly, understanding how CPs transform into ILFs is central to understanding how the mucosal immune system functions to manage the luminal microbiota and protect from potential pathogens.
Our understanding of the transitioning of CP into ILFs is an evolving area. CPs are groups of lineage marker (lin)−,c-kit+,CD90+ cells clustered at the base of villi, whereas their descendents, ILFs, are more developed and organized lymphoid tissues containing lymphocytes.4,10,11,12 The lin−c-kit+ CP cells share many properties with fetal lymphoid tissue inducer (LTi) cells, including cell surface molecule expression and a requirement for the transcription factor RORγt in their development.12,13,14 Like fetal LTi cells, these CP cells mediate the early steps of lymphoid tissue genesis by delivering a lymphotoxin (LT) signal to LT β receptor (LTβR) expressing stromal cells resulting in a self-sustaining cluster, or CP.2,12 Studies suggest that unlike ILFs, the numbers of CPs remain relatively constant in response to changes in microbiota.5,8 Although it is clear that CPs can transition into ILFs, that this process is driven by local stimuli, and that ILFs can contribute to mucosal immunity by acting as sites for IgA production, the cellular events and molecular pathways relevant for this transition are relatively unexplored. Cell type-specific signals have been identified to play a role in ILF development.15,16,17,18 However, these signals specific for ILF development are delivered by B lymphocytes or expressed by B lymphocytes, which define the presence of ILFs and are intimately involved in the process of IgA production. Therefore, it is difficult to assess a role for these signals in ILF transitioning as opposed to other aspects of ILF function. To this end, we investigated a role for dendritic cells (DCs) in ILF development. DCs have been observed to be a component of both CPs and ILFs, and therefore, understanding the role of DC in this process could provide insight into the steps linking CPs to ILFs.
Here we identify the presence of DC clusters distributed throughout the adult murine intestine. In normal animals, these DC clusters occur exclusively as part of the continuum of CPs and ILFs and are less numerous than CPs and more numerous than ILFs. Studies of the neonatal intestine revealed that the formation of these clusters occurs after the coalescence of CD90+c-kit+ cells, to form CPs, and before the influx of B220+ cells, to form ILFs. Moreover, studies of genetically manipulated animals revealed that the formation of these DC clusters is dependent on the LT and LTβR signals required for CP development and independent of the presence of lymphocytes. We observed that DC cluster formation was not limited by DC numbers, and recruitment of DCs to the developing CP/ILF to form clusters was driven by changes in the luminal microbiota. Consistent with an active role for DCs in this process, we observed that deletion of DCs results in the loss of ILFs and the disorganization of CPs. Recent studies demonstrated CXCR5, the receptor for the chemokine CXCL13, plays a role in recruiting B cells to developing ILFs.18 We found that ILF DCs are sources of CXCL13. Taken together, these findings demonstrate that DCs provide a link between CPs and ILFs and that the transitioning of CPs into ILFs occurs in part due to the recruitment of DCs, which produce CXCL13.
Materials and Methods
Mice and Treatments
Mice used for this study were housed in a specific-pathogen free facility and fed routine chow diet. Animal procedures and protocols were performed in accordance with the institutional review board at Washington University School of Medicine (St. Louis, MO). C57BL/6 mice, RAG-deficient mice on the C57BL/6 background, transgenic mice expressing the diphtheria toxin (DT) receptor under the control of the CD11c promoter (CD11c-DTR), and CXCL13-deficient mice were purchased from The Jackson Laboratory (Bar Harbor, ME). LTα-deficient mice, a gift from Dr. D. Chaplin (University of Alabama Birmingham, Birmingham, AL) were bred onto the C57BL/6 background for >10 generations before use in experiments. LTβR-deficient mice on the C57BL/6 background were a gift from Dr. K. Pfeffer (Technical University of Munich, Munich, Germany).
LTβR-Ig production and treatment was performed as described previously.19 Progeny receiving LTβR-Ig in utero was analyzed at ≥12 weeks of age.
PEGylated recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) was produced as described previously.20 Recombinant murine Fms-like tyrosine kinase 3 ligand (Flt3L) fused to the FC region of human IgG1 (Flt3L-Ig) was purified via protein G affinity chromatography from supernatants generated by a Flt3L-Ig-producing J558L cell line (generous gift from Dr. M. Colonna, Washington University). The activity of the recombinant Flt3L-Ig was confirmed by its ability to differentiate bone marrow derived DCs as described previously.21 Seven- to 10-week-old C57BL/6 mice were injected i.p. daily with 5 μg of recombinant PEGylated murine GM-CSF, PBS, 20 μg of recombinant murine Flt3L-Ig, or 100 μg of human IgG (Bayer, Elkhart, IN). After 14 days, mice were sacrificed, and intestines were analyzed.
Gnotobiotic C57BL/6 mice were provided by Digestive Disease Research Core Center Murine Models Facility at Washington University School of Medicine. Gnotobiotic mice were conventionalized by exposure to cecal contents from conventionally housed C57BL/6 mice as described previously.7 At the time of sacrifice, cecal cultures from gnotobiotic mice were obtained to confirm the absence of bacterial flora.
Seven- to 10-week-old CD11c-DTR mice or nontransgenic littermates were injected i.p. with 4 ng/g body weight of DT (Sigma-Aldrich, St. Louis, MO) on day 0 and with 2 ng/g body weight of DT 24 hours later on day 1. Groups of mice were analyzed on day 1 for the presence of intestinal DCs by flow cytometry and whole mounts, and on day 2 for the presence of B220+ clusters and CD90+ clusters.
Whole Mounts and Immunohistochemistry
Whole mounts of the adult small intestine were performed as described previously.7 To evaluate the neonatal intestine by whole mount, the intestine was mounted with the serosal side facing up, and standard immunohistochemistry was performed. Tissues were examined under a dissecting microscope at ×65 and were mounted on glass slides as 4-cm segments and examined under an upright light or fluorescent microscope at 100 to 400X magnification. Well-developed lymphoid tissues falling on the antimesenteric border of neonatal small intestine were considered PPs and, consequently, excluded from the analysis.
For immunohistochemistry, intestines were embedded in optimal cutting temperature compound (Sakura Finetek, Torrence, CA), frozen, and serial 8-μm sections were cut at an axis perpendicular to the villi for the evaluation of CD11c+ clusters. Staining of frozen sections was performed as described previously.16 Pseudo-colored black-and-white images from fluorescent microscopy were obtained with an Axioskop 2 microscope using Axiovision software (Carl Zeiss MicroImaging, Thornwood, NY).
To detect CXCL13 in tissues, mice were perfused with cold PBS, followed by 4% paraformaldehyde. Intestines were removed and incubated in a solution of 4% paraformaldehyde and 2% sucrose for 30 minutes, embedded in optimal cutting temperature compound, and frozen, and serial 8-μm sections of intestine were obtained. To detect CXCL13 in single cells, ILF cellular populations were isolated as previously described16 and adhered to ImmunoSelect adhesion slides (MoBiTec, Göttingen, Germany), and immunohistochemistry was performed. CXCL13 was detected with a polyclonal rabbit anti-mouse CXCL13 antibody (eBioscience, San Diego, CA) using biotinylated goat anti-rabbit secondary antibody and streptavidin-conjugated cyanine 3 (Jackson ImmunoResearch Laboratories, West Grove, PA).
Flow Cytometric Analysis
ILF cellular populations were isolated from C57BL/6 mice receiving LTβR-Ig in utero, and lamina propria (LP) cellular populations, not including PP, were isolated as described previously.16 To release DCs, ILF cellular populations were incubated with 20 U/ml collagenase type VIII (Sigma-Aldrich) in RPMI 1640 medium (BioWhittaker, Walkersville, MD) containing 2 mmol/L Glutamax I (l-alanyl-l-glutamine; Invitrogen Life Technologies, Carlsbad, CA), 1 mmol/L sodium pyruvate, 5% calf serum (HyClone, Logan, UT), 0.05 mmol/L 2-mercaptoethanol, nonessential amino acids (Invitrogen Life Technologies), and sodium bicarbonate (Invitrogen Life Technologies) at 37°C with shaking for 15 minutes. ILF cellular populations were incubated with HBSS (BioWhittaker) containing 5 mmol/L EDTA at 37°C with shaking for 10 minutes and washed with PBS before staining for flow cytometry. ILF cellular populations from multiple mice were pooled for analysis. LP cellular populations from individual mice were isolated as previously described19 and analyzed. Forward scatter, side scatter, and the incorporation of 7-aminoactinomycin D were used to gate out dead cells. Remaining events were analyzed in four channels using a FACScan cytometer (BD Biosciences, San Jose, CA) retrofitted with a second laser. Data acquisition was performed using CellQuest (BD Biosciences) and Rainbow (Cytek, Fremont, CA) software. Data analysis was performed on a Macintosh G4 computer running FlowJo software (Tree Star, Ashland, OR). Antibodies used for analysis included anti-mouse CD11c (BD Biosciences), anti-mouse CD4, anti-mouse CD8α, anti-mouse CD11b, anti-mouse B220, and appropriate isotype controls (all from eBioscience).
Real-Time PCR
ILF cellular populations were isolated from C57BL/6 mice treated with LTβR-Ig in utero as described above. CD11c+ and CD11c− cells were isolated using anti-CD11c microbeads (Miltenyi Biotec, Auburn, CA) following the manufacturers recommendation, RNA was isolated treated with DNase and transcribed into cDNA. The following primers were used: 18s, forward, 5′-CGGCTACCACATCCAAGGAA-3′, and reverse, 5′-GCTGGAATTACCGCGGCT-3′; and CXCL13, forward, 5′-CAGAATGAGGCTCAGCACAGC-3′, and reverse 5′-CAGAATACCGTGGCCTGGAG-3′. To construct a standard, RNA was isolated from the spleen of C57BL/6 mice, transcribed into cDNA, and used to generate a PCR product that was cloned using the pCRII-TOPO vector and cloning kit (Invitrogen Life Technologies) per the manufacturer’s instructions.
Statistical Analysis
Data analysis using a Student’s _t_-test and a one-way analysis of variance with a Tukey’s posttest was performed using GraphPad Prism (GraphPad Software, San Diego, CA).
Results
The Adult Murine Small Intestine Contains Discreet Clusters of DCs That Are Components of the of CP/ILF Spectrum
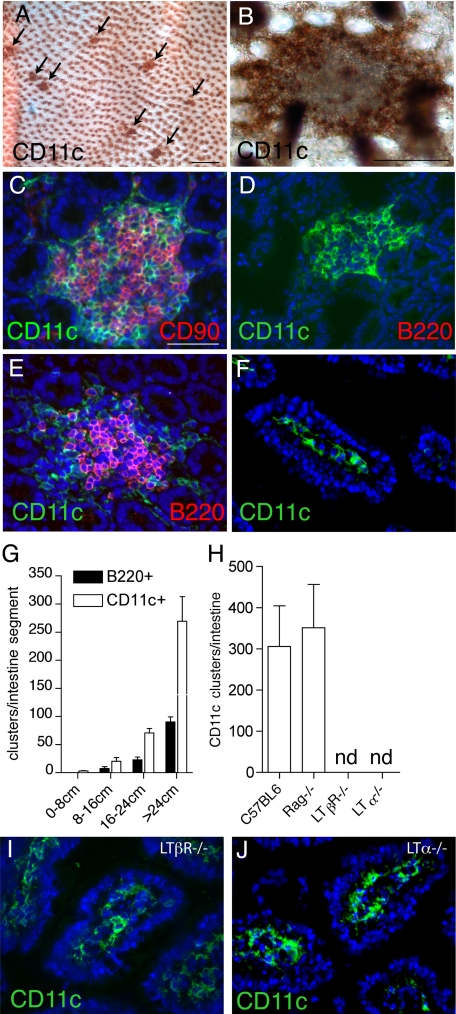
To evaluate the regional distribution and macroscopic and microscopic organization of intestinal DCs, we examined intestinal whole mounts stained with anti-CD11c, a widely used marker to identify murine DCs.22 Whole mounts of the small intestine revealed discreet clusters of DCs located at the base of the villi (Figure 1, A and B). To evaluate the association of these CD11c+ cellular clusters with components of the CP/ILF spectrum, we performed immunhistochemistry to colocalize the CD11c+ clusters with the CD90+ clusters of CP cells and the B220+ clusters identifying ILFs. Multicolor immunohistochemistry revealed that all of the CD11c+ cellular clusters colocalized with CD90+ cellular clusters (Figure 1C). The CD90+ clusters were also c-kit+ (data not shown). B220+ cells were found to be absent from some CD11c+ cellular clusters and present in others (Figure 1, D–E). Consistent with the above observations implying that the CD11c+ clusters could identify an intermediate stage of CPs developing into ILFs, CD11c+ clusters were more numerous than B220+ clusters but shared a similar distribution within the small intestine (Figure 1G). Moreover, we observed that the formation of the CD11c+ clusters was unaffected in RAG−/− mice and therefore independent of B lymphocytes (Figure 1H), which enter SILTs late in the process of CPs transforming into ILFs, and play important roles in the organization of ILFs.15 CD11c+ cluster formation was dependent on LT and the LTβR, which are required for the coalescence of the CD90+ cells to form CP23 (Figure 1H). Notably, although CD11c+ clusters were absent, we observed a prominent CD11c+ cellular population in the LP in LTα−/− and LTβR−/− mice (Figure 1 I and J), which resembled that seen in C57BL/6 mice (Figure 1F), thus demonstrating differential requirements for the presence of CD11c+ cells in the LP and the development of CD11c+ clusters.
Figure 1.
Intestinal DCs form discreet clusters that are part of the CP/ILF continuum. Using anti-CD11c-stained whole mounts (brown staining, A and B) and traditional immunohistochemistry (green staining, C–F, I, and J), we evaluated the regional location, organization, and cellular associations intestinal DCs in wild-type (A–G) and genetically manipulated mice on the C57BL/6 background (H–J). We observed that the intestine contained multiple clusters of DCs (arrows, A), and these clusters were located at the base of villi (en face view of whole mount at higher power, B). All of the DC clusters we observed were associated with CD90+ cells (C), consistent with their localization to CP. The DC clusters contained variable numbers of B220+ cells; some containing no B220+ cells (D) and others colocalizing with B220+ clusters (E). DC clusters were more numerous than B220+ clusters but had an identical regional distribution (G). Like the formation of ILFs and CPs, the formation of DC clusters was dependent on LT and the LTβR but independent of the presence of T or B lymphocytes (H). However unlike the development of DC clusters, the presence of LP DCs was not dependent on LTα or LTβR (I and J). n = 3 or more animals from each group for data in G and H. nd = none detected. Scales bars in panels A = 500 μm and in panels B and C = 100 μm.
CD11c+ Clusters Develop after CD90+ Clusters and before B220+ Clusters in the Neonatal Intestine
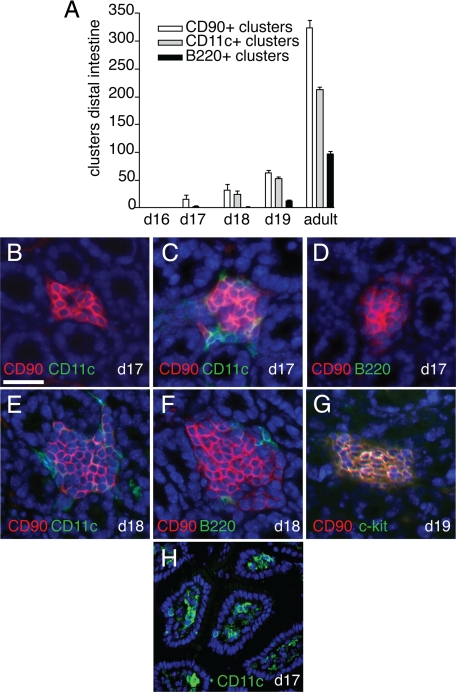
In contrast to secondary lymphoid tissues, CPs and ILFs do not form during embryogenesis but develop in the neonatal period, with CPs being first observed 14 to 17 days after birth11 and ILFs developing later, ∼25 days after birth in the C57BL/6 strain.10 To evaluate the timing of CD11c+ cluster development and its relationship to CP and ILF development in the neonatal period, we evaluated intestines from neonatal C57BL/6 mice and compared the numbers of CD90+, CD11c+, and B220+ clusters and determined the colocalization of these cell types using multicolor immunohistochemistry. We observed that the clusters in the neonatal intestine were first observed and more prominent in the distal intestine and therefore limited our analysis to the distal one-third of the neonatal intestine. We did not find CD90+, CD11c+, or B220+ cellular clusters corresponding to components of the CP/ILF spectrum before day 17 of life. However, we observed that CPs, as defined by clusters of CD90+c-kit+ cells, first appeared on day 17 of life, and a few of these clusters contained CD11c+ cells, but none contained B220+ cells (Figure 2, A–D). Accordingly, we observed rare CD11c+ clusters and no B220+ clusters on day 17 of life (Figure 2A). The B220+ clusters first appeared on day 18 of life and were infrequent (Figure 2A). We observed that on day 18 of life, some CD90+ clusters contained a few B220+ cells and that most contained CD11c+ cells (Figure 2, A, E, and F). In no instance did we observe CD90+ clusters containing B220+ cells and lacking CD11c+ cells. By day 19 of neonatal life, the entire complement of cellular clusters comprising the known CP/ILF spectrum was present and in proportions approximately equivalent to that seen in the adult intestine (Figure 2A). CD90+ clusters were also c-kit+ (Figure 2, F and G). Although CD11c+ clusters were rare, LP CD11c+ cellular populations were prominent on neonatal day 17 (Figure 2H), further demonstrating differences between the presence of CD11c+ cells in the LP and the development of CD11c+ clusters. These observations suggest an order of entry of cell types into the developing ILFs in the neonatal period with DCs preceding B lymphocytes and are consistent with the above findings in the adult intestine.
Figure 2.
CD11c+ clusters develop after CD90+ clusters and before B220+ clusters in the neonatal intestine. CPs and ILFs first appear in the neonatal period. To examine the chronology of cellular cluster formation in SILT development, we evaluated the presence of CD90+, CD11c+, or B220+ clusters in the intestine of C57BL/6 neonatal mice. None of these cellular clusters was present before day 17 of life (A). CD90+ clusters were present on day 17 of life, a few of these clusters contained CD11c+ cells, none of these clusters contained B220+ cells (A–D). On day 18 of life, CD11c+ clusters were present and were nearly as numerous as the CD90+ clusters (A). In each instance the CD11c+ clusters were associated with CD90+ clusters, and a few clusters contained B220+ cells (E and F). The full complement of cellular clusters was present by day 19 of life and in proportions that approximate that seen in the adult intestine (A). All CD90+ clusters were also c-kit+ (G). Although DC clusters were rare, LP DC populations were readily apparent on day 17 of neonatal life (H). n = 3 or more mice for each time point and type of cellular cluster in examined in A. Scale bar = 50 μm.
The CD11c+ Cell Populations from ILFs and the LP Have Different Phenotypes
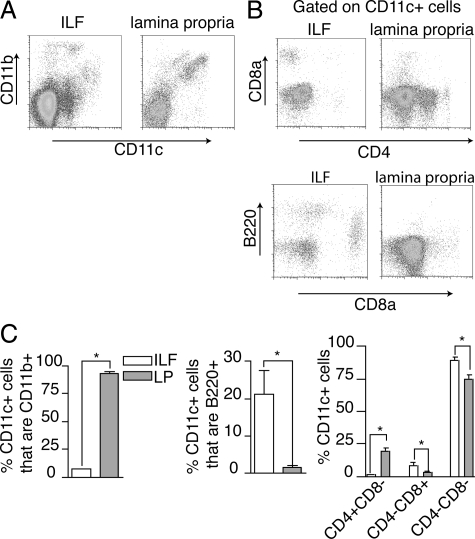
We evaluated the phenotype of the CD11c+ cells from the ILFs and compared these findings with those from DCs isolated from the diffuse LP. Because all SILTs cannot be mechanically dissociated from the LP, LP cellular populations will contain cellular components derived from SILTs. However, it is anticipated that these components will represent a minority of the LP cellular population as demonstrated by the rarity of LTi-like cells, which are significant components of SILTs, in the diffuse LP population.13 ILF CD11c+ cells were largely CD11b− and almost exclusively CD4−, whereas those from the LP were largely CD11b+ and commonly CD4+ (Figure 3). The ILF CD11c+ population contained CD8α+ cells and B220+ cells; these cell populations were less common in the LP (Figure 3, B and C). CD4 and CD8α expression have been proposed to identify subtypes of DCs within lymphoid tissues.24 Using this classification, we observed that the majority of ILF and LP CD11c+ cells did not express CD4 or CD8α; however, the LP CD11c+ cell population contained a significant proportion of CD4+ CD8α− cells, and these cells were almost completely absent from the ILFs (Figure 3C). Conversely the ILFs contained a larger population of CD11c+ cells that were CD4−CD8α+ (Figure 3C). These findings may be consistent with different origins of ILF and LP CD11c+ cells or may be consistent with ILF CD11c+ cells arising from the LP, because as anticipated, all ILF CD11c+ cell subtypes were present to some degree within the LP. However, the absence of CD4+CD11c+ cells and the enrichment in CD8α+CD11c+ cells and B220+CD11c+ cells suggest that if ILF CD11c+ cells do arise from the LP, the process of ILF development is selective for some LP CD11c+ cell types and suggests that the formation of these CD11c+ clusters does not occur from nonselective coalescence of the LP DC population.
Figure 3.
ILF and LP CD11c+ cell populations have different phenotypes. To compare the phenotype of CD11c+ cells located within the clusters to those located within the general LP, cellular populations were isolated from ILFs and LP and examined with multicolor flow cytometry. ILF CD11c+ cells were largely CD11b−, whereas in comparison those from the LP were largely CD11b+ (A and C). The ILFs contained almost no CD4+CD11c+ cells, whereas this cellular population was prominent in the LP (B and C). Conversely CD11c+B220+ and CD11c+CD8α+ cells were relatively enriched in ILFs when compared with the LP (B and C). Using CD4 and CD8α expression to classify the CD11c+ cell subtypes, we observed that CD11c+ cellular population from both the LP and ILFs was predominantly CD4−CD8α−, whereas the LP population was relatively enriched in CD4+CD8α− cells, and the ILF population was relatively enriched in CD4−CD8α+ cells (C). n = 3 or more replicates using ILF cellular populations pooled from multiple animals and LP populations for data in C. *P < 0.05.
Cell Numbers are Not Limiting in the Development of CD11c+ Clusters: The Formation of CD11c+ Clusters Can be Driven by Luminal Microbiota
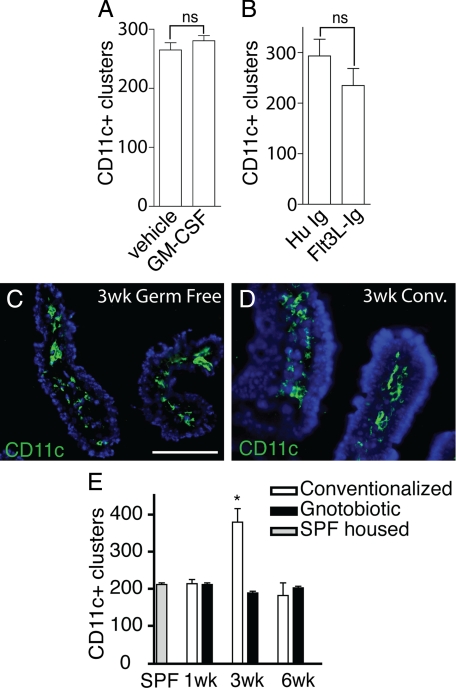
It is estimated that the murine small intestine contains 1000 CPs.11 In our experience, most CPs contain a few DCs, and a minority of CPs contain a substantial number of DCs forming a distinct cluster. This observation is consistent with the availability of DCs, as opposed to a stimulus or signal as the limiting step in forming a cluster of CD11c+ cells. GM-CSF has the capacity to differentiate a recently identified clonogenic bone marrow progenitor into DCs,25 and blood monocytes derived from this progenitor give rise small intestine LP DCs.26 Likewise, exogenous administration of Flt3L expands the intestinal DC populations27 and may preferentially expand different DC subsets than GM-CSF. We therefore examined the effect of exogenous GM-CSF or exogenous Flt3L on the numbers of DC clusters. Administration of GM-CSF or Flt3L-Ig expanded DC populations in the LP by greater than twofold (data not shown). We did not see an increase in the numbers of CD11c+ clusters in the animals administered exogenous GM-CSF or Flt3L-Ig (Figure 4, A and B). Colonization of germfree mice has been observed to increase ILF but not CP numbers.5,8 To confirm that the number of CD11c+ clusters could increase and to determine whether the numbers of CD11c+ clusters were affected by luminal microbiota, we evaluated the presence of LP CD11c+ cells and the numbers of CD11c+ clusters in gnotobiotic mice colonized with normal intestinal flora. We found that germfree mice contained an LP CD11c+ cell population similar to that seen in conventionalized mice (Figure 4, C and D). The number of CD11c+ clusters increased 3 weeks following conventionalization and subsequently returned to levels seen in germfree mice and specific pathogen-free housed mice 3 weeks later (Figure 4E). These observations indicate that the limiting event in the development of a CD11c+ cluster is unlikely cell availability, or the presence of LP CD11c+ cell populations, but is a second signal or stimulus that can be delivered by luminal microbiota. In conjunction with prior observations that the numbers of CPs do not change following conventionalization of germfree mice,5,8 these observations suggest that the recruitment of DCs is an early event in the process of CPs transforming into ILFs in response to luminal microbiota.
Figure 4.
DC numbers are not limiting for the development of CD11c+ clusters. Cluster formation can be driven by changes in luminal microbiota. To assess the availability of DCs as a limiting factor in the development of DC clusters, we treated C57BL/6 mice for 2 weeks with exogenous GM-CSF or Flt3L-Ig to increase DC numbers and then evaluated the numbers of CD11c+ clusters in the intestine. Exogenous GM-CSF or Flt3L-Ig had no effect on the number of CD11c+ clusters (A and B). To evaluate the role of luminal microbiota in CD11c+ cluster formation, the presence of LP DC populations and the number of clusters in the distal one-third of the intestine from gnotobiotic mice that remained germfree or their counterparts that were given cecal contents from conventionally housed C57BL/6 mice were examined. Three weeks following conventionalization, the presence of the LP DC population appeared similar in germfree mice and their conventionalized counterparts (C and D). The numbers of CD11c+ clusters in the mice that remained germfree throughout the experiment did not change, and this was similar to the number of clusters seen in the intestine of conventionally housed C57BL/6 mice (E). Conversely the numbers of CD11c+ clusters increased significantly 3 weeks following colonization and returned to levels seen in specific pathogen-free housed animals germ free animals 3 weeks later (E). n = 3 or more mice in each group and each time point. *P < 0.05. Scale bar = 100 μm.
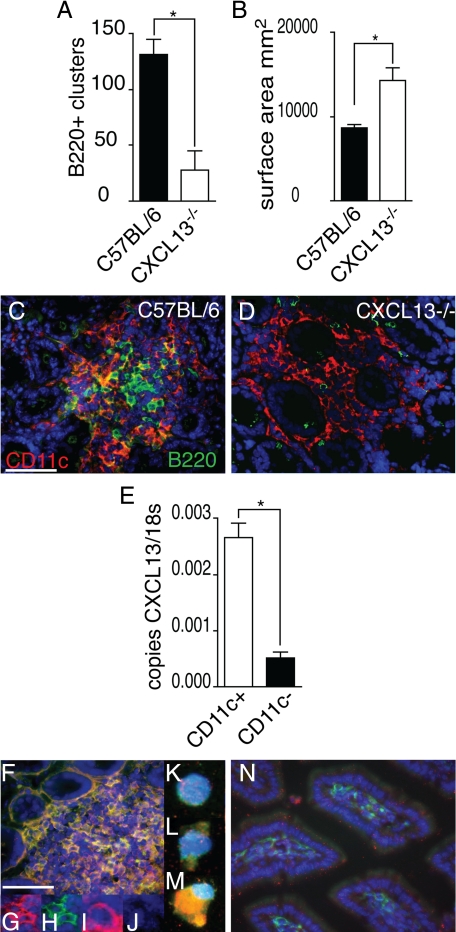
In the Absence of CXCL13, ILF Development is Arrested and Anomalous: ILF CD11c+ Cells are Sources of CXCL13
Recent studies demonstrated that B lymphocyte expression of CXCR5, the receptor for the chemokine CXCL13, is important for normal ILF development.18 Consistent with this, we found that CXCL13−/− mice had impaired ILF development as determined by decreased numbers of B220+ clusters (Figure 5A). Moreover, we observed that the B220−CD11c+ cellular clusters were significantly larger in the absence of CXCL13 (Figure 5B). In contrast to cellular clusters of similar size in wild-type mice, these large CD11c+B220− clusters in CXCL13−/− mice do not develop a central core of mononuclear cells but instead diffuse out to engulf multiple crypts (Figure 5, C and D). These findings suggest that in the absence of CXCL13, B lymphocytes are not effectively recruited into the developing ILFs, whereas DCs can be recruited and continue to accumulate. To evaluate whether CD11c+ cells were a source of CXCL13, we isolated CD11c+ and CD11c− mononuclear cells from ILFs and evaluated CXCL13 mRNA expression. We observed that ILF CD11c+ cells produce mRNA encoding CXCL13 (Figure 5E). To evaluate CXCL13 protein expression, we performed immunohistochemistry on whole small intestine and on isolated ILF cell populations. We observed that many cells in the ILFs were CXCL13+ (red staining; Figure 5F), and a significant number of these were CD11c+ (green, co-staining appears yellow; Figure 5F). Insets G and H in Figure 5 demonstrate single channels for cells staining positive for both CXCL13 (red, inset G) and CD11c (green, inset H) within the ILFs. Insets I and J on Figure 5 demonstrate single channels for a cell staining positive for CXCL13 (red, inset I) and negative for CD11c (lack of green staining, inset J). To confirm positive staining for CXCL13 on ILF single-cell populations, we isolated ILF cellular populations and performed immunohistochemistry on cells affixed to slides. We observed that some CD11c+ cells did not stain positive for CXCL13 (Figure 5, inset K and L, green staining for CD11c only), whereas other CD11c+ cells stained positive for both CD11c and CXCL13 (Figure 5, inset M, green staining for CD11c and red staining for CXCL13, costaining appears yellow). In contrast, DCs within the diffuse LP did not stain positive for CXCL13 (Figure 5N; red = CXCL13, green = CD11c). For comparison, real-time PCR analysis revealed that CXCL13 expression by isolated LP DC populations was <1/10 of that seen in ILF CD11c+ cells (data not shown). Thus, CXCL13 is important for the normal recruitment of B lymphocytes into ILFs, and DCs are a source of CXCL13, implying a potential function for CXCL13 production by DCs in ILF development.
Figure 5.
In the absence of CXCL13, ILF development is arrested and abnormal, and ILF CD11c+ cells are sources of CXCL13. Intestines from wild-type C57BL/6 mice and CXCL13−/− mice on the C57BL/6 background were evaluated for the presence of ILFs (B220+ clusters, A) and the size (B) and morphology of the CD11c+ clusters (C and D). CXCL13−/− mice were found to have significantly fewer ILFs (A), and conversely the surface area of the B220−CD11c+ cellular clusters was found to be significantly greater in CXCL13−/− mice (B). The CD11c+B220− clusters in the CXCL13−/− intestine were of a similar size to CD11c+B220+ clusters from wild-type mice but did not contain a central area filled with mononuclear cells and instead extended to engulf multiple adjacent crypts (C and D). CD11c+ and CD11c− cellular populations were isolated from ILFs from C57BL/6 mice as described in Materials and Methods, and the mRNA expression of CXCL13 was examined. CD11c+ ILF cells were found to express significantly more CXCL13 than CD11c− ILF cells (E). CXCL13 protein expression was evaluated using immunohistochemistry. CXCL13 protein (red staining, F) was detected within ILFs in the intestine of C5BL/6 mice. Many of the CXCL13-expressing cells were also CD11c+ (green staining, F; costaining appears yellow). Insets (G and H) demonstrate individual channels for cells within ILFs costaining for both CXCL13 (red, G) and CD11c (green, H). Insets (I and J) demonstrate individual channels for cells within ILFs staining for CXCL13 (red, I) and not staining for CD11c (lack of green signal, J). To confirm the results seen in whole tissues, we evaluated the protein expression of CXCL13 in isolated ILF cellular populations. ILF cells expressed CXCL13 (red signal, M) and that these cells could be CD11c+ (green signal, M; costaining appears yellow). We also observed that some CD11c+ cells (green signal, L) did not express CXCL13 (lack of red signal, L), and some cells were neither CD11c+ or CXCL13+ (lack of red and green signal, K). We did not observe CXCL13 expression by DCs located within the LP (N green = CD11c, red = CXCL13). n = 3 intestines A and B. E is representative of one of two independent experiments using pooled ILF cellular populations from three or more animals. Scale bar = 100 μm. *P < 0.05.
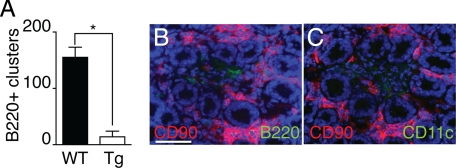
DCs Participate in the Maintenance of B Lymphocytes in ILFs
The hierarchical arrangement we observed between CD11c+ clusters and B220+ clusters in neonatal and adult mice is consistent with a role for DCs in recruiting and/or maintaining the B220+ cells within ILFs. To evaluate this possibility, we treated CD11c-DTR mice and their nontransgenic littermates with DT and evaluated them for the presence of LP DCs, CD11c+ cellular clusters, and B220+ clusters. Transgenic mice treated with DT depleted their LP DC population, whereas nontransgenic animals were unchanged (data not shown). Examination for the presence of B220+ clusters revealed significantly decreased numbers of B220+ clusters in the transgenic mice when compared with nontransgenic mice treated with DT (Figure 6A). Clusters of CD90+ cells could be easily identified in the transgenic animals following DT treatment; however, these clusters were loosely organized and contained vacant areas where B220+ and CD11c+ cells would reside (Figure 6, B and C). These disorganized clusters had a similar appearance to those seen in the CXCL13−/− mice (compare Figure 6B with Figure 5D).
Figure 6.
DCs participate in the maintenance of B lymphocytes in ILFs. To evaluate a role for CD11c+ cells in ILF maintenance CD11c-DTR transgenic mice (Tg) and wild-type C57BL/6 mice (WT) were injected with DT i.p. and evaluated for the presence of ILFs (B220+ clusters, A) and the morphology and the presence of CD90+, CD11c+, and B220+ cells in the SILT in the Tg animals (B and C). Following DT treatment, B220+ clusters were maintained in WT but were significantly decreased in the transgenic animals (A). CD90+ clusters could be easily identified in the Tg following DT treatment; however, these clusters lacked CD11c+ and B220+ cells were disorganized and engulfed the adjacent crypts (B and C). n = 3 or more animals for each condition. *P < 0.05 when compared with the WT treatment group in A. Scale bar = 100 μm.
Discussion
Our understanding of the contributions of both ends of the SILT continuum to innate and adaptive mucosal immunity continues to evolve. CPs were identified earlier than ILFs;10,11 however, their role in mucosal immunity is less established and their potential functions more diverse. The role of CPs in mucosal immunity centers around their principal hematopoietic cellular components, the lin−c-kit+ cell. Initial investigations demonstrated that CP function as sites for the extrathymic development of T lymphocytes, with the lin−c-kit+ cells being T lymphocyte progenitors.11,28,29,30,31 Subsequent studies were at odds with this function,13 and the degree to which CPs contribute to extrathymic T lymphocyte development in the euthymic condition is not agreed on. More recent studies demonstrate a potential role for the lin−c-kit+ CP cells as progenitors of an interleukin-22-producing NK-cell subset that promote epithelial repair.32,33,34,35 Whether the function of lin−c-kit+ cells as progenitors for these cell types extends throughout the CP/ILF continuum is not known. Another role for the lin−c-kit+ CP cells in mucosal immunity arose from observations linking CP and ILFs within the SILT continuum and from studies demonstrating phenotypic and functional similarities between CP cells and fetal LTi cells.12 These observations suggest that the lin−c-kit+ CPs play an integral role as initiators of lymphoid neogenesis, establishing the precursors to ILFs.2,13,14,36 ILFs contain a significant population of B lymphocytes and more developed ILFs can have germinal centers and a follicle-associated epithelium containing M cells.7,10 These and subsequent findings supported a role for ILFs as sites for the induction of IgA responses to both T lymphocyte-dependent and T lymphocyte-independent antigens.2,3,37 Thus, SILT can contribute to mucosal immunity in multiple ways and potentially at multiple levels of development; however, the function as sites of IgA induction is restricted to the more developed structures in the continuum, the ILFs. Given the prominence of IgA in the maintenance of mucosal homeostasis, how CPs transition into ILFs is an important issue in understanding mucosal immunity.
In an attempt to better understand the process of CPs transitioning into ILFs, we examined the presence and timing of DC entry into the developing SILTs. DCs have been described as a cellular component of both CPs and ILFs;10,11 therefore, the existence of DC clusters in the intestine is not unexpected. Whether DC clusters existed outside of the SILT spectrum was previously unknown, and to this end, we observed that DC clusters were restricted to the SILT continuum in the normal mouse intestine. Moreover, the numerical and cell associative hierarchy we observed in the adult and neonatal intestine coupled with the chronology of different cell types forming clusters in the neonatal intestine demonstrates that the development of DC clusters occurs at a specific point in SILT continuum after the coalescence of CD90+c-kit+ cells, and before the influx of B lymphocytes. Consistent with the DC clusters occurring exclusively in the context of SILT continuum and with their chronological positioning between the CD90+ clusters and the B220+ clusters was the absence of CD11c+ clusters in LTα−/− mice and LTβR−/− mice, which lack CPs and ILFs, and the presence of CD11c+ clusters in mice lacking lymphocytes. Notably LP DC populations were present in LTα−/− and LTβR−/− mice, demonstrating differences between the presence of DCs in the LP and the development of CD11c+ clusters.
We observed that the ILF CD11c+ cell population was heterogeneous and could be distinguished from that of the LP by the absence of CD4+CD11c+ cells, which were common in the LP, and by the enrichment in CD8α+ CD11c+ cells and B220+CD11c+ cells, which were less common in the LP. Although the phenotype of ILF DCs in comparison with LP DCs has not been previously addressed, these findings are consistent with prior observations noting differences in DC populations in different intestinal lymphoid compartments.38,39 The characteristics of the ILF CD11c+ cell population could be consistent with a selective recruitment of local DC populations into the transitioning CPs. Alternatively, expansion of pre-existing DCs could contribute to the formation of the CD11c+ clusters. Splenic DCs express the LTβR,40,41 and signaling through the LTβR expands and maintains splenic DC numbers.40,41 This parallels the requirement for LT and the LTβR we observed in the development of DC clusters. However, we do not favor this as the sole mechanism resulting in the accumulation of DCs in the developing ILFs, as lymphocytes were identified as the cellular source of the LTβR ligand responsible for maintaining the homeostasis or expansion of splenic DCs,40,41 and we observed that lymphocytes are dispensable for the development of the DC clusters.
Multiple studies demonstrated an inducible nature to ILF development, with the numbers of ILFs increasing or decreasing in response to luminal stimuli.7,8,9 Although the dynamics of this process are incompletely understood, two simple models can be envisioned. In the first, a temporally restricted stimulus starts the cascade of events resulting in the development of ILFs; this model adheres to our current understanding of the dynamics of PP development, where once initiated, development proceeds and is not dependent on exogenous stimuli for progression. In the second model, temporally or phenotypically distinct stimuli are required for development of a CP and its progression into an ILF. Our observations and recent studies support the latter model. Pabst et al8 found that conventionalization of germfree mice resulted in no change in the numbers of structures in the CP/ILF continuum but increased the number of structures progressing to become ILFs. Extending this work Brouskra et al5 found that NOD1 ligands could promote the transition of CPs into ILFs. We found that the progression of CPs containing few DCs to CPs associated with a DC cluster was not limited by DC availability but was induced by changes in the luminal microbiota. These findings are consistent with the second model where forces driving the development of CPs are temporally or physically distinct from those resulting in the progression of CPs into ILFs. Thus, CPs could represent a reservoir of preformed nascent lymphoid tissues with the capacity to develop into IgA inductive in response to appropriate stimuli.
In comparison with their role as initiators of adaptive immune responses, the role of DCs as active participants in the development of organized lymphoid tissues is relatively unexplored. Ludewig et al42 demonstrated that repeated injections of DCs expressing an immunodominant epitope of lymphocytic choriomeningitis virus glycoprotein into transgenic mice expressing lymphocytic choriomeningitis virus glycoprotein in the pancreas resulted in the formation of organized lymphoid tissue. Marinkovic et al43 found that in transgenic mice overexpressing CCL21, T lymphocytes interact with DCs to induce the formation of ectopic lymphoid tissues, and the development of these tissues was independent of LTi-like cells. These studies suggest a role for DCs in tertiary lymphoid tissue development and imply that this role may center around their function as antigen-presenting cells in T cell-dependent responses. DCs have also been observed to shape existent lymphoid tissues. DCs were found to induce endothelial cell proliferation and vascular growth in lymph nodes following immunization.44 Related studies revealed lymphatic growth in inflamed lymph nodes was dependent on B lymphocytes and that this augmented further DC migration into the inflamed lymph node.45 Somewhat in contrast to the above studies, Hashi et al46 noted CD11c+ cells enter the developing PP anlagen before mature lymphocytes, suggesting that these embryonic CD11c+ cells contribute to PP development at an early stage in a lymphocyte-independent manner. Veiga-Fernandes et al demonstrated that CD11c+ cells play an important role in PP development by delivering very early LT-dependent signals, resulting in the recruitment and coalescence of the fetal LTi cells.47 Similar to the study of Hashi et al46, we noted that CD11c+ cells accumulate after the coalescence of the c-kit+,CD90+,LTi-like CP cells. And in accord with a function for CD11c+ cells downstream of the LTi-like cells in ILF development, we found that following the loss of the CD11c+ cells, B220+ clusters are lost. Similar to the studies of Veiga-Fernandes et al, we observed that after the loss of CD11c+ cells, CD90+ clusters become disorganized, suggesting that the CD11c+ cells may have a function in CP maintenance. However, the character of the CD11c+-dependent signal differs because Veiga-Fernandes et al found that embryonic CD11c+ cells delivered a time-restricted signal during the critical window of PP development in embryogenesis, and our findings suggest that CD11c+ cells deliver a tonic signal facilitating ILF, and possibly CP, maintenance. This potential role in CP maintenance is consistent with the findings of Suzuki et al,31 which demonstrated that CD11c+ cells inhabit CPs before c-kit+ cells following irradiation and bone marrow reconstitution in athymic mice with a truncation of the common cytokine receptor γ chain.
Because of its constitutive expression in lymphoid tissues and its established role directing the normal segregation of lymphocytes and the organization of follicles, CXCL13 is often characterized as a “homeostatic” chemokine. However, several lines of evidence implicate an additional role for CXCL13 in the development of lymphoid tissues. CXCL13 and its receptor CXCR5 play important roles in the formation of intestinal lymphoid tissues documented by impaired PP development in mice deficient in either CXCL13 or CXCR5.48,49 In this context, CXCL13 produced by stromal cells functions to activate and coalesce CXCR5-expressing fetal LTi cells during embryogenesis initiating PP development, and CXCR5 expression by B lymphocytes is required for their localization to PP. Further investigations indicate that CXCL13 can participate in the de novo formation of lymphoid tissues. CXCL13 is highly expressed at the sites of new lymphoid tissue formation in a variety of chronic inflammatory conditions,50,51,52,53,54,55,56,57 and ectopic expression of CXCL13 in a transgenic mouse model resulted in the formation of new extranodal lymphoid tissues.58 In further support of a role for CXCL13 in lymphoid tissue development and consistent with our observations, CXCR5 participates in SILT development at an early stage when the LTi-like cells cluster to form CPs and at a later stage when B lymphocytes are recruited into the developing follicle.18 Therefore, multiple lines of investigation indicate that CXCL13 can play an important role in the development of a wide range of lymphoid tissues.
CXCL13 production within lymphoid tissues has largely been attributed to follicular DCs residing within germinal centers; however, consistent with our observations, several studies identified CD11c+ cells as additional sources of CXCL13. CXCL13 expression localized to structures described as peripheral dendritic elements in lymphoid tissues in the normal intestine and in aberrant lymphoid tissues formed in ulcerative colitis.51 Further investigations localized the CXCL13 expression in the aberrant lymphoid tissues to CD11c+ cells.59 Moreover, in the above study, the CXCL13 production by CD11c+ cells in intestinal lymphoid aggregates was present in small aggregates lacking a germinal center,59 further indicating that follicular DCs are unlikely the source of CXCL13 in this situation. Additional support for intestinal DCs production of CXCL13 comes from in vitro studies demonstrating CXCL13 expression by DCs following stimulation with interleukin-10 and lipopolysaccharide;60 stimuli that could be commonly encountered in the intestine. We observed that ILF DCs could express CXCL13. However, DCs were not the sole source of CXCL13 within the ILFs, because we also found CXCL13+ cells that were CD11c− within ILFs when evaluating whole tissues. The CXCL13+CD11c− cells were uncommon when we evaluated the isolated ILF cell populations, which could relate to the isolation technique, or to the preferential adherence of cell types to the slide substrate.
Our understanding of the mucosal immune system’s strategies to maintain homeostasis at a large environmentally exposed surface continues to grow. Among these are the recently appreciated diverse functions of CPs and ILFs, including their ability to generate novel lymphocyte subsets and to act as sites for the induction of T cell-dependent and T cell-independent IgA.2,3,32,33,34,35 The observations presented here add to this list by demonstrating an unappreciated role for DCs expressing CXCL13 and promoting the transition of CPs into ILFs. These observations suggest that like other cellular components of SILTs, DCs perform dual functions by shaping their microenvironment and generating immune responses.
Acknowledgments
We thank the Alvin J. Siteman Cancer Center (Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO) for the use of the High-Speed Cell Sorter Core, which provided flow cytometry services. We thank the Washington University School of Medicine Digestive Diseases Research Core Center for assistance with morphology services and gnotobiotic mice.
Footnotes
Address reprint requests to Rodney D. Newberry, M.D., 660 S. Euclid Avenue, Box 8124, St. Louis, MO 63110. E-mail: rnewberry@im.wustl.edu.
Supported by National Institutes of Health (grants DK64798 and AG028309). The Siteman Cancer Center is supported in part by a National Cancer Institute Cancer Center support grant (P30 CA91842). The Digestive Diseases Research Core Center is supported by grant P30-DK52574.
References
- Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Lorenz RG, Newberry RD. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann NY Acad Sci. 2004;1029:44–57. doi: 10.1196/annals.1309.006. [DOI] [PubMed] [Google Scholar]
- Herbrand H, Bernhardt G, Forster R, Pabst O. Dynamics and function of solitary intestinal lymphoid tissue. Crit Rev Immunol. 2008;28:1–13. doi: 10.1615/critrevimmunol.v28.i1.10. [DOI] [PubMed] [Google Scholar]
- Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- Kweon MN, Yamamoto M, Rennert PD, Park EJ, Lee AY, Chang SY, Hiroi T, Nanno M, Kiyono H. Prenatal blockage of lymphotoxin β receptor and TNF receptor p55 signaling cascade resulted in the acceleration of tissue genesis for isolated lymphoid follicles in the large intestine. J Immunol. 2005;174:4365–4372. doi: 10.4049/jimmunol.174.7.4365. [DOI] [PubMed] [Google Scholar]
- Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin β receptor, and TNF receptor I function. J Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- Pabst O, Herbrand H, Friedrichsen M, Velaga S, Dorsch M, Berhardt G, Worbs T, Macpherson AJ, Forster R. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J Immunol. 2006;177:6824–6832. doi: 10.4049/jimmunol.177.10.6824. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA. 2004;101:1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, Yamamoto H, Ishikawa H. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+IL-7R+Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat Rev Immunol. 2005;5:413–420. doi: 10.1038/nri1600. [DOI] [PubMed] [Google Scholar]
- Eberl G, Littman DR. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORγ(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- McDonald KG, McDonough JS, Newberry RD. Adaptive immune responses are dispensable for isolated lymphoid follicle formation: antigen-naive, lymphotoxin-sufficient B lymphocytes drive the formation of mature isolated lymphoid follicles. J Immunol. 2005;174:5720–5728. doi: 10.4049/jimmunol.174.9.5720. [DOI] [PubMed] [Google Scholar]
- McDonald KG, McDonough JS, Wang C, Kucharzik T, Williams IR, Newberry RD. CC Chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles. Am J Pathol. 2007;170:1229–1240. doi: 10.2353/ajpath.2007.060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, McDonough JS, McDonald KG, Huang C, Newberry RD. α4β7/MAdCAM-1 interactions play an essential role in transitioning cryptopatches into isolated lymphoid follicles and a nonessential role in cryptopatch formation. J Immunol. 2008;181:4052–4061. doi: 10.4049/jimmunol.181.6.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velaga S, Herbrand H, Friedrichsen M, Jiong T, Dorsch M, Hoffmann MW, Forster R, Pabst O. Chemokine receptor CXCR5 supports solitary intestinal lymphoid tissue formation. B cell homing, and induction of intestinal IgA responses. J Immunol. 2009;182:2610–2619. doi: 10.4049/jimmunol.0801141. [DOI] [PubMed] [Google Scholar]
- Newberry RD, McDonough JS, McDonald KG, Lorenz RG. Postgestational lymphotoxin/lymphotoxin β receptor interactions are essential for the presence of intestinal B lymphocytes. J Immunol. 2002;168:4988–4997. doi: 10.4049/jimmunol.168.10.4988. [DOI] [PubMed] [Google Scholar]
- Sainathan SK, Tu L, Bishnupuri KS, Han M, Li A, Newberry RD, McDonald KG, Crimmins DL, Houchen C, Anant S, Dieckgraefe BK. PEGylated murine granulocyte-macrophage colony-stimulating factor: production, purification, and characterization. Protein Expr Purif. 2005;44:94–103. doi: 10.1016/j.pep.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Blasius A, Vermi W, Krug A, Facchetti F, Cella M, Colonna M. A cell-surface molecule selectively expressed on murine natural interferon-producing cells that blocks secretion of interferon-α. Blood. 2004;103:4201–4206. doi: 10.1182/blood-2003-09-3108. [DOI] [PubMed] [Google Scholar]
- Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RT, Lugering A, Newell KA, Williams IR. Intestinal cryptopatch formation in mice requires lymphotoxin α and the lymphotoxin β receptor. J Immunol. 2004;173:7183–7189. doi: 10.4049/jimmunol.173.12.7183. [DOI] [PubMed] [Google Scholar]
- Wu L, Dakic A. Development of dendritic cell system. Cell Mol Immunol. 2004;1:112–118. [PubMed] [Google Scholar]
- Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney JL, Mowat AM, O'Malley JM, Williamson E, Fanger NA. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol. 1998;160:5815–5825. [PubMed] [Google Scholar]
- Oida T, Suzuki K, Nanno M, Kanamori Y, Saito H, Kubota E, Kato S, Itoh M, Kaminogawa S, Ishikawa H. Role of gut cryptopatches in early extrathymic maturation of intestinal intraepithelial T cells. J Immunol. 2000;164:3616–3626. doi: 10.4049/jimmunol.164.7.3616. [DOI] [PubMed] [Google Scholar]
- Onai N, Kitabatake M, Zhang YY, Ishikawa H, Ishikawa S, Matsushima K. Pivotal role of CCL25 (TECK)-CCR9 in the formation of gut cryptopatches and consequent appearance of intestinal intraepithelial T lymphocytes. Int Immunol. 2002;14:687–694. doi: 10.1093/intimm/dxf035. [DOI] [PubMed] [Google Scholar]
- Saito H, Kanamori Y, Takemori T, Nariuchi H, Kubota E, Takahashi-Iwanaga H, Iwanaga T, Ishikawa H. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Oida T, Hamada H, Hitotsumatsu O, Watanabe M, Hibi T, Yamamoto H, Kubota E, Kaminogawa S, Ishikawa H. Gut cryptopatches: direct evidence of extrathymic anatomical sites for intestinal T lymphopoiesis. Immunity. 2000;13:691–702. doi: 10.1016/s1074-7613(00)00068-6. [DOI] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, Di Santo JP. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- Pabst O, Herbrand H, Worbs T, Friedrichsen M, Yan S, Hoffmann MW, Korner H, Bernhardt G, Pabst R, Forster R. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol. 2005;35:98–107. doi: 10.1002/eji.200425432. [DOI] [PubMed] [Google Scholar]
- Wang C, McDonald KG, McDonough JS, Newberry RD. Murine isolated lymphoid follicles contain follicular B-lymphocytes with a mucosal phenotype. Am J Physiol Gastrointest Liver Physiol. 2006;291:G595–G604. doi: 10.1152/ajpgi.00525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, Maxwell JR, Stoklasek T, Yadav R, Williams IR, Gu X, McCormick BA, Pazos MA, Vella AT, Lefrancois L, Reinecker HC, McSorley SJ. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer’s patches. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. Intrinsic lymphotoxin-β receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 2005;22:439–450. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Wang YG, Kim KD, Wang J, Yu P, Fu YX. Stimulating lymphotoxin β receptor on the dendritic cells is critical for their homeostasis and expansion. J Immunol. 2005;175:6997–7002. doi: 10.4049/jimmunol.175.10.6997. [DOI] [PubMed] [Google Scholar]
- Ludewig B, Odermatt B, Landmann S, Hengartner H, Zinkernagel RM. Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med. 1998;188:1493–1501. doi: 10.1084/jem.188.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic T, Garin A, Yokota Y, Fu YX, Ruddle NH, Furtado GC, Lira SA. Interaction of mature CD3+CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest. 2006;116:2622–2632. doi: 10.1172/JCI28993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster B, Ekland EH, Agle LM, Chyou S, Ruggieri R, Lu TT. Regulation of lymph node vascular growth by dendritic cells. J Exp Med. 2006;203:1903–1913. doi: 10.1084/jem.20052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Hashi H, Yoshida H, Honda K, Fraser S, Kubo H, Awane M, Takabayashi A, Nakano H, Yamaoka Y, Nishikawa SI. Compartmentalization of Peyer’s patch anlagen before lymphocyte entry. J Immunol. 2001;166:3702–3709. doi: 10.4049/jimmunol.166.6.3702. [DOI] [PubMed] [Google Scholar]
- Veiga-Fernandes H, Coles MC, Foster KE, Patel A, Williams A, Natarajan D, Barlow A, Pachnis V, Kioussis D. Tyrosine kinase receptor RET is a key regulator of Peyer’s patch organogenesis, Nature. 2007;446:547–551. doi: 10.1038/nature05597. [DOI] [PubMed] [Google Scholar]
- Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Barone F, Bombardieri M, Manzo A, Blades MC, Morgan PR, Challacombe SJ, Valesini G, Pitzalis C. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjogren’s syndrome. Arthritis Rheum. 2005;52:1773–1784. doi: 10.1002/art.21062. [DOI] [PubMed] [Google Scholar]
- Carlsen HS, Baekkevold ES, Johansen FE, Haraldsen G, Brandtzaeg P. B cell attracting chemokine 1 (CXCL13) and its receptor CXCR5 are expressed in normal and aberrant gut associated lymphoid tissue. Gut. 2002;51:364–371. doi: 10.1136/gut.51.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo A, Paoletti S, Carulli M, Blades MC, Barone F, Yanni G, Fitzgerald O, Bresnihan B, Caporali R, Montecucco C, Uguccioni M, Pitzalis C. Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol. 2005;35:1347–1359. doi: 10.1002/eji.200425830. [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- Salomonsson S, Larsson P, Tengner P, Mellquist E, Hjelmstrom P, Wahren-Herlenius M. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjogren’s syndrome. Scand J Immunol. 2002;55:336–342. doi: 10.1046/j.1365-3083.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- Shi K, Hayashida K, Kaneko M, Hashimoto J, Tomita T, Lipsky PE, Yoshikawa H, Ochi T. Lymphoid chemokine B cell-attracting chemokine-1 (CXCL13) is expressed in germinal center of ectopic lymphoid follicles within the synovium of chronic arthritis patients. J Immunol. 2001;166:650–655. doi: 10.4049/jimmunol.166.1.650. [DOI] [PubMed] [Google Scholar]
- Shomer NH, Fox JG, Juedes AE, Ruddle NH, Drayton DL, Ying X, Lee J, Lesslauer W. Helicobacter-induced chronic active lymphoid aggregates have characteristics of tertiary lymphoid tissue ectopic LT α β directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase, Infect Immun. 2003;71:3572–3577. doi: 10.1128/IAI.71.6.3572-3577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura S, Braun A, Crowson C, Kurtin PJ, Cofield RH, O'Fallon WM, Goronzy JJ, Weyand CM. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167:1072–1080. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]
- Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood. 2004;104:3021–3027. doi: 10.1182/blood-2004-02-0701. [DOI] [PubMed] [Google Scholar]
- Perrier P, Martinez FO, Locati M, Bianchi G, Nebuloni M, Vago G, Bazzoni F, Sozzani S, Allavena P, Mantovani A. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. J Immunol. 2004;172:7031–7042. doi: 10.4049/jimmunol.172.11.7031. [DOI] [PubMed] [Google Scholar]