Disease Progression in Hemodynamically Stable Patients Presenting to the Emergency Department With Sepsis (original) (raw)
. Author manuscript; available in PMC: 2015 Jan 5.
Abstract
Background
Aggressive diagnosis and treatment of patients presenting to the emergency department (ED) with septic shock has been shown to reduce mortality. To enhance the ability to intervene in patients with lesser illness severity, a better understanding of the natural history of the early progression from simple infection to more severe illness is needed.
Objectives
The objectives were to 1) describe the clinical presentation of ED sepsis, including types of infection and causative microorganisms, and 2) determine the incidence, patient characteristics, and mortality associated with early progression to septic shock among ED patients with infection.
Methods
This was a multicenter study of adult ED patients with sepsis but no evidence of shock. Multivariable logistic regression was used to identify patient factors for early progression to shock and its association with 30-day mortality.
Results
Of 472 patients not in shock at ED presentation (systolic blood pressure > 90 mm Hg and lactate < 4 mmol / L), 84 (17.8%) progressed to shock within 72 hours. Independent factors associated with early progression to shock included older age, female sex, hyperthermia, anemia, comorbid lung disease, and vascular access device infection. Early progression to shock (vs. no progression) was associated with higher 30-day mortality (13.1% vs. 3.1%, odds ratio [OR] = 4.72, 95% confidence interval [CI] = 2.01 to 11.1; p ≤ 0.001). Among 379 patients with uncomplicated sepsis (i.e., no evidence of shock or any end-organ dysfunction), 86 (22.7%) progressed to severe sepsis or shock within 72 hours of hospital admission.
Conclusions
A significant portion of ED patients with less severe sepsis progress to severe sepsis or shock within 72 hours. Additional diagnostic approaches are needed to risk stratify and more effectively treat ED patients with sepsis.
Keywords: sepsis, outcomes, septic shock, progression, biomarkers
The emergency department (ED) is a common location for the initial evaluation and management of patients with sepsis. Infection-related conditions account for over 10 million ED visits in the United States annually, sepsis causes an estimated 750,000 deaths per year; and it is the 10th leading cause of death overall.1–3 However, the initial evaluation of patients with suspected infection in the ED is complicated by 1) the lack of specificity of systemic inflammatory response syndrome (SIRS) criteria for infection;4,5 2) the heterogeneity of clinical manifestations, including clinical signs and symptoms, site of infection, comorbid conditions, and etiologic microorganisms;6,7 and 3) the challenge in rapidly identifying patients most likely to progress to severe illness or death, especially among patients who are not severely ill at initial evaluation.
Patients who are identified as high risk (persistent hypotension and / or lactate levels greater than 4 mmol /L) who present to the ED are candidates for early protocolized intervention, which includes addressing the infectious source and aggressive resuscitation. Early goal-directed therapy (EGDT) initiated in the ED has been shown to reduce mortality, especially in patients with hemodynamic compromise.8,9 However, risk assessment and optimal management for patients with suspected infection without shock at initial presentation to the ED are less clear. There is a critical need for rapid, accurate, and early identification of patients at risk for disease progression and hemodynamic compromise given the availability of time-sensitive treatment regimens such as EGDT and recombinant activated protein C.8,10 Yet, the overall incidence and adverse outcomes of early clinical progression in patients with sepsis in the ED have not been well studied.
The objective of this prospective, multicenter cohort study of patients presenting to the ED with sepsis was to 1) describe the clinical presentation of sepsis, including types of infection and causative microorganisms; 2) to determine the incidence and mortality associated with early progression to septic shock among ED patients with infection; and 3) to evaluate patient characteristics associated with early progression to septic shock.
METHODS
Study Design
This was a secondary analysis of data collected for a larger study: the Community Acquired Pneumonia & Sepsis Outcome Diagnostics (CAPSOD) study, which was a prospective, multicenter National Institutes of Health–sponsored study to develop novel diagnostic and prognostic tests for severe sepsis and community-acquired pneumonia in the ED (ClinicalTrials.gov identifier NCT00258869). This study was approved by the institutional review board at each institution, and written, informed consent was obtained from all study participants or their legal designates.
Study Setting and Population
Patient enrollment was conducted at three EDs: Duke University Medical Center in Durham, North Carolina (annual census 65,000 patients); the Veterans Affairs Medical Center in Durham, North Carolina (annual census 40,000 patients); and the Henry Ford Hospital in Detroit, Michigan (annual census 95,000 patients). Subjects were screened primarily during daytime weekday hours in the ED between 2005 and 2007. Patients were eligible if they had a known or suspected infection and if they exhibited two or more SIRS criteria.5 Patients were excluded if they had an imminently terminal comorbid condition or advanced AIDS (CD4 count < 50 / μL), were being treated with an antibiotic, or were participating in an ongoing clinical trial.
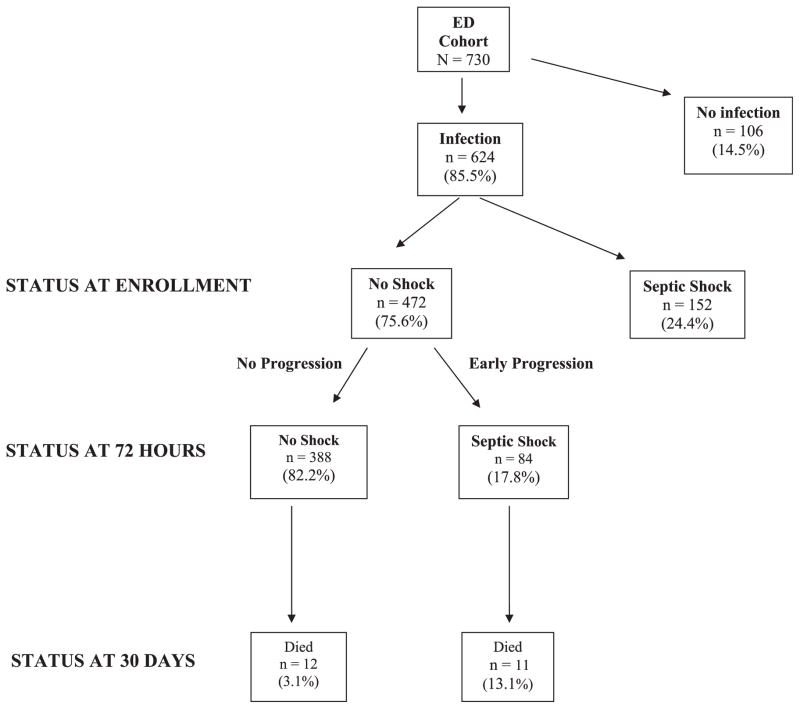
A total of 730 patients over 18 years of age were enrolled. Because the primary objective of this analysis was to determine the incidence of progression to shock among patients not initially in shock at the time of evaluation in the ED (i.e., patients not generally considered candidates for protocolized resuscitation), patients who were hypotensive despite fluid resuscitation or who had a lactate level of >4 mmol /L were not included in the analysis (n = 152). An additional 106 patients were later determined not to have an infection and were also excluded from the analysis. The final data set used for analysis contained 472 patients with confirmed infection who were not in shock at the time of enrollment in the ED (Figure 1).
Figure 1.
Study cohort. The study cohort at enrollment and subsequent patient outcomes at 72 hours and 30 days.
Study Protocol
After informed consent was obtained, patients or their representative were asked by trained research assistants to complete a standardized questionnaire including demographics and symptoms. A microbiologic evaluation included two sets of blood cultures, urine culture, pneumococcal urinary antigen test, and cultures of other sites as clinically indicated. Other baseline measurements included a complete blood count, blood chemistries, urinalysis, and chest radiographs. Trained study coordinators at each site recorded vital signs and laboratory and imaging results from the initial ED encounter and at 24-hour intervals for up to 72 hours or until death. After 30 days, in-hospital mortality and microbiologic culture results were determined from the patients’ medical records. All data were collected in electronic case report forms with decision support logic and stored in a HIPAA-compliant database (Prosanos Inc., Harrisburg PA).
Adjudication of Infections and Patient Outcomes
Adjudication of all patient records with respect to infection status was conducted at least 30 days after hospital discharge. Determination of infection status and patient outcome was made by a study physician, board certified in emergency medicine (SWG), after review of all study data and patient medical records. Infection status was categorized as follows: 1) infection and causative organism identified; 2) infection, but causative organism not identified; or 3) infection unlikely. Causative organisms were classified as 1) at least one positive blood culture for Staphylococcus aureus, Gram-negative bacteria, Candida albicans, or Streptococcus pneumoniae; 2) two or more positive blood cultures for another single organism; 3) a positive culture from another sterile source (e.g., cerebrospinal, joint); or 4) a positive urinary pneumococcal antigen with a clinical picture compatible with pneumonia.
For patients not clearly meeting the above criteria for infection by the primary adjudicator, the medical records were reviewed by a second investigator who was board certified in internal medicine and infectious diseases (CWW). A third individual with specialty training in internal medicine and infectious diseases (ELT) performed an independent adjudication of a sample of 10% of the patient records. Agreement on the infection classification between this individual and the primary adjudicator was high (κ = 0.82), exceeding the 0.80 threshold considered “almost perfect agreement.”11
Study Definitions
Patients were categorized as having uncomplicated sepsis, severe sepsis, or septic shock at the time of study enrollment and at each subsequent 24-hour interval during their hospitalizations. Uncomplicated sepsis was defined as sepsis without evidence of shock or end-organ dysfunction. Severe sepsis was defined as two or more SIRS criteria with evidence of end-organ dysfunction (including metabolic [lactate > 1.5 times upper limit of normal or arterial pH < 7.30], hematologic [platelet count < 80 × 103], pulmonary [intubation or PaO2 / FiO2 < 250], renal [urine output < 0.5 mL / kg / hr despite adequate fluid resuscitation], or cardiac [mean arterial pressure (MAP) < 65 mm Hg or systolic blood pressure (sBP) < 90 mm Hg despite adequate fluid resuscitation]).12,13 Septic shock was defined as tissue hypoperfusion, including hypotension (sBP < 90 mm Hg or MAP < 65 mm Hg) persisting despite initial fluid challenge or a blood lactate concentration equal to or greater than 4 mmol / L.12,13 Early progression to septic shock was defined as development of shock within the first 72 hours after enrollment among patients who had no evidence of shock upon initial evaluation in the ED.
Data Analysis
Baseline patient demographics, clinical signs and symptoms, infection classification, and outcomes were compiled. Median values with interquartile ranges (IQRs) were used to describe continuous variables, and numbers with percentages were reported for categorical variables.
A multiple variable logistic regression model was performed to identify patient characteristics associated with progression to septic shock within 72 hours of ED presentation. The dependent variable was defined as the presence of shock (yes / no) within 72 hours of initial evaluation in the ED. Predictor variables included information available to the treating emergency physician, including demographics, vital signs, and laboratory values. The adjudicated infection site and causative microorganism were also included as predictor variables. Candidate variables were screened using univariate analysis. Variables significant at p < 0.20 were selected and included in a final multivariable logistic regression model using backward selection. Additional models using forward selection and stepwise techniques were performed and yielded nearly identical results. Continuous predictor variables were explored for nonlinearity in the models after categorizing them into quartiles. There were no significant nonlinearities, so they were modeled as continuous variables. Variables significant at p < 0.05 were retained in the final multivariable models. A Kaplan-Meier survival analysis was performed to evaluate the temporal association of early progression to septic shock with 30-day mortality.
Additional analyses were performed to determine the incidence of disease progression to severe sepsis or shock within the first 72 hours among a subgroup of patients with uncomplicated sepsis (i.e., no evidence of shock or any end-organ dysfunction) at the time of enrollment. The numbers and percentages of patients with uncomplicated sepsis who progressed to severe sepsis or shock were determined. The time to occurrence of severe sepsis or shock stage among patients with uncomplicated sepsis was estimated using cumulative incidence up to 72 hours.
The association between sepsis treatment and progression to shock was evaluated based on whether a patient received appropriate antibiotic therapy within the first 24 hours of evaluation in the ED. Antimicrobial susceptibility of causative agents was used as the basis for determining appropriateness of antibiotic therapy.14,15 Therapy was considered appropriate when at least one effective drug was administered within 24 hours.16 All analyses were performed using SAS, Version 9.1.2 (SAS Institute, Cary, NC). A p value of <0.05 was considered statistically significant.
RESULTS
A total of 472 patients over 18 years of age with confirmed infection who were not in shock at the time of initial ED evaluation were enrolled (Table 1). Lung, urine, and skin were the most common adjudicated infection sites (34.3, 14.0, and 13.8%, respectively; Table 2). The most common causative microorganisms were S. aureus (26.0%), Escherichia coli (16.9%), other aerobic Gram-negative bacilli (e.g., Klebsiella spp. or Enterobacter spp.; 20.3%), and S. pneumoniae (16.9%).
Table 1.
Characteristics of 472 Patients With Sepsis but No Evidence of Shock at the Time of ED Presentation
| Variable | |
|---|---|
| Age (yr), median (IQR) | 52 (44–66) |
| Sex, n (%) | |
| Male | 248 (52.5) |
| Female | 224 (47.5) |
| Race, n (%) | |
| African American | 264 (55.9) |
| White | 186 (39.4) |
| Other | 22 (4.7) |
| Site, n (%) | |
| Duke, NC | 246 (52.1) |
| Henry Ford, MI | 195 (41.3) |
| Durham, NC | 31 (6.6) |
| Apache II score, median (IQR) | 9.0 (5.0–13.5) |
| Comorbidities, n (%) | |
| Alcohol abuse | 41 (8.7) |
| Cancer | 37 (7.8) |
| Chronic renal failure | 60 (12.7) |
| Chronic lung disease | 108 (22.9) |
| Cirrhotic liver disease | 7 (1.5) |
| Diabetes mellitus | 132 (28.0) |
| Drug use | 55 (11.7) |
| Heart failure | 41 (8.7) |
| Hemodialysis | 47 (10.0) |
| Human immunodeficiency virus | 7 (1.5) |
| Smoker | 85 (18.0) |
| Clinical variables, median (IQR) | |
| Heart rate (beats / min) | 109 (96–122) |
| Respiratory rate (breaths / min) | 20 (20–25) |
| Temperature (°C) | 38.3 (37.4–39.0) |
| Blood pressure, mean arterial, mm Hg (IQR) | 87 (78–98) |
| Laboratory values, median (IQR) | |
| Creatinine (mg / dL) | 1.1 (0.9–1.6) |
| Hematocrit (%) | 37 (33–41) |
| Platelet count (×103/μL) | 246 (179–318) |
| White blood cell count (×103/μL) | 13.2 (9.1–16.7) |
Table 2.
Infection Sites, Causative Microorganisms, and Outcomes for 472 Patients With Sepsis but No Shock at the Time of ED Presentation
| Total | Shock Progression (Within First 72 Hours) | Death (Within 30 Days) | |
|---|---|---|---|
| Infection category | |||
| Infection, organism identified | 177 (37.5) | 38 (21.5) | 8 (4.5) |
| Infection, organism not identified | 295 (62.5) | 46 (15.6) | 15 (5.1) |
| Total | 472 (100) | 84 (17.8) | 23 (4.9) |
| Infection source | |||
| Bone | 13 (2.8) | 4 (30.8) | 1 (7.7) |
| Cardiac | 1 (0.2) | 0 (0) | 0 (0) |
| Catheter | 20 (4.2) | 11 (55) | 0 (0) |
| Central nervous system | 3 (0.6) | 0 (0) | 0 (0) |
| Ear, nose, and throat | 16 (3.4) | 1 (6.3) | 0 (0) |
| Gynecologic | 4 (0.8) | 0 (0) | 1 (25) |
| Intraabdominal | 47 (10) | 5 (10.6) | 1 (2.1) |
| Pulmonary | 162 (34.3) | 23 (14.2) | 13 (8.0) (11.1) |
| Skin | 65 (13.8) | 8 (12.3) | 1 (1.5) |
| Urinary tract | 66 (14) | 11 (16.7) | 1 (1.5) |
| Unknown | 75 (15.9) | 21 (28) | 5 (6.7) |
| Total | 472 (100) | 84 (17.8) | 23 (4.9) (15.5) |
| Infection causative organism* | |||
| S. auereus | 46 (26) | 8 (17.4) | 0 (0) |
| S. pneumoniae | 30 (16.9) | 4 (13.3) | 3 (10) |
| Other Gram-positive cocci | 12 (6.8) | 4 (33.3) | 2 (16.7) |
| E. coli | 30 (16.9) | 3 (10) | 0 (0) |
| Aerobic Gram-negative bacilli | 36 (20.3) | 11 (30.6) | 1 (2.8) |
| Polymicrobial | 4 (2.3) | 3 (75) | 0 (0) |
| Fungi and Candida | 3 (1.7) | 1 (33.3) | 0 (0) |
| Anaerobes | 9 (5.1) | 1 (11.1) | 0 (0) |
| Viral | 4 (2.3) | 1 (25) | 0 (0) |
| Other | 3 (1.7) | 2 (66.7) | 1 (33.3) |
| Total | 177 (100) | 38 (21.5) | 7 (4.0) |
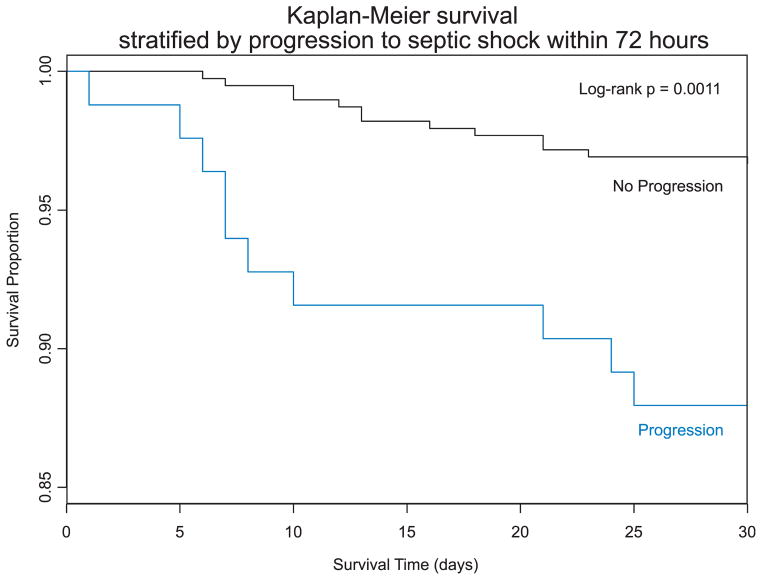
Of the 472 patients with infection who were not in shock at the time of enrollment, 84 (17.8%) progressed to septic shock within 72 hours. Factors associated with early progression to septic shock included older age, female sex, higher body temperature, anemia, comorbid lung disease, and infection associated with an indwelling vascular catheter (Table 3). Early progression to septic shock was associated with time to death (Kaplan-Meier log-rank _χ_2 = 14.4, p = 0.001; Figure 2). Early progression to shock (vs. no progression) was associated with higher 30-day mortality (13.1% vs. 3.1%, odds ratio [OR] = 4.72, 95% CI = 2.01 to 11.1; p ≤ 0.001).
Table 3.
Risk Factors for Progression to Septic Shock Within 72 Hours Among 472 ED Patients With Sepsis
| Univariate Model | Multivariate Model | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Demographics | ||||||
| Age (decade of life) | 1.16 | 1.01–1.32 | 0.030 | 1.22 | 1.05–1.42 | 0.008 |
| Sex, female | 2.62 | 1.59–4.30 | <0.001 | 2.57 | 1.50–4.40 | <0.001 |
| Race, white | 0.92 | 0.61–1.40 | 0.699 | |||
| Vital signs | ||||||
| Temperature (°C) | 1.26 | 1.02–1.55 | 0.029 | 1.34 | 1.06–1.68 | 0.013 |
| Respiratory rate (breaths / min) | 1.01 | 0.98–1.05 | 0.445 | |||
| Heart rate (beats / min) | 1.01 | 1.00–1.02 | 0.045 | |||
| Comorbidities | ||||||
| Alcohol abuse | 0.78 | 0.32–1.91 | 0.581 | |||
| Cancer | 0.70 | 0.27–1.86 | 0.480 | |||
| Cirrhotic liver disease | 1.87 | 0.36–9.80 | 0.459 | |||
| Diabetes mellitus | 1.11 | 0.66–1.87 | 0.686 | |||
| Drug abuse | 0.64 | 0.28–1.48 | 0.299 | |||
| Heart failure | 2.67 | 1.34–5.35 | 0.006 | |||
| Hemodialysis | 1.47 | 0.72–3.03 | 0.291 | |||
| Human immunodeficiency virus | 0.77 | 0.09–6.46 | 0.807 | |||
| Lung disease | 2.21 | 1.32–3.68 | 0.002 | 2.30 | 1.29–4.10 | 0.005 |
| Smoker | 0.72 | 0.37–1.39 | 0.329 | |||
| Laboratory values | ||||||
| White blood cell count (×103/μL) | 1.00 | 0.98–1.03 | 0.698 | |||
| Hematocrit (%) | 0.94 | 0.90–0.97 | 0.001 | 0.96 | 0.92–1.00 | 0.046 |
| Platelet count (×103/μL) | 1.00 | 1.00–1.00 | 0.668 | |||
| Serum lactate (mmol / L) | 1.13 | 0.75–1.70 | 0.571 | |||
| Organ dysfunction | ||||||
| Pulmonary | 1.07 | 0.50–2.31 | 0.855 | |||
| Metabolic | 0.77 | 0.09–6.46 | 0.807 | |||
| Renal | 0.58 | 0.20–1.68 | 0.312 | |||
| Infection site | 0.003 | <0.001 | ||||
| Pulmonary | 0.78 | 0.45–1.37 | 0.57 | 0.31–1.05 | ||
| Urine | 0.95 | 0.46–1.98 | 0.88 | 0.41–1.89 | ||
| Vascular catheter | 5.80 | 2.25–14.9 | 5.06 | 1.81–14.1 | ||
| Other / unknown | Reference | |||||
| Causative microorganism | 0.705 | |||||
| S. aureus | 0.99 | 0.44–2.23 | ||||
| S. pneumoniae | 0.72 | 0.24–2.15 | ||||
| Gram-negative aerobes | 1.26 | 0.66–2.43 | ||||
| Other / unknown | Reference |
Figure 2.
Kaplan-Meier survival analysis: 30-day survival stratified by early progression (72 hours) to septic shock. The figure plots 30-day survival for patients with confirmed infection who were not in shock at the time of initial ED evaluation stratified by whether they developed septic shock within 72 hours of initial ED evaluation.
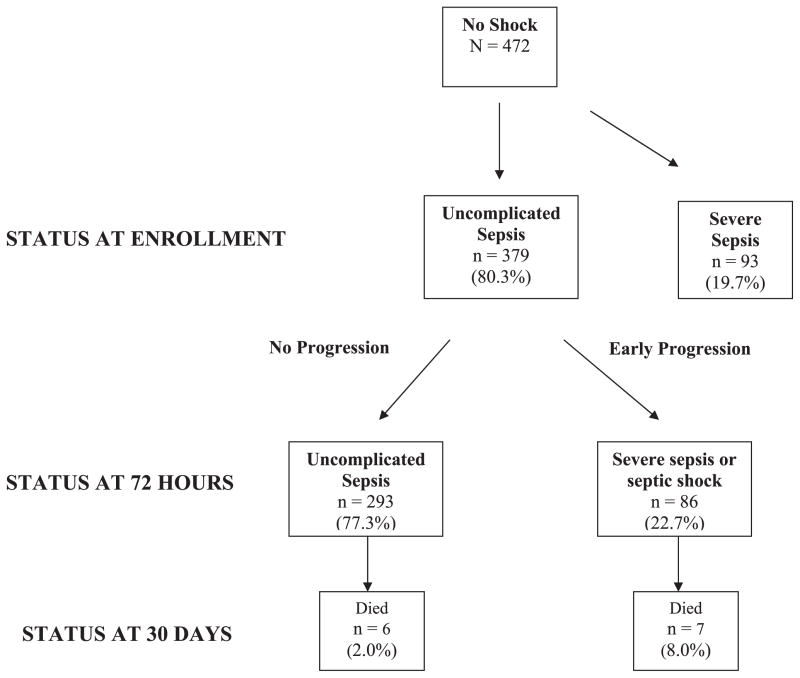
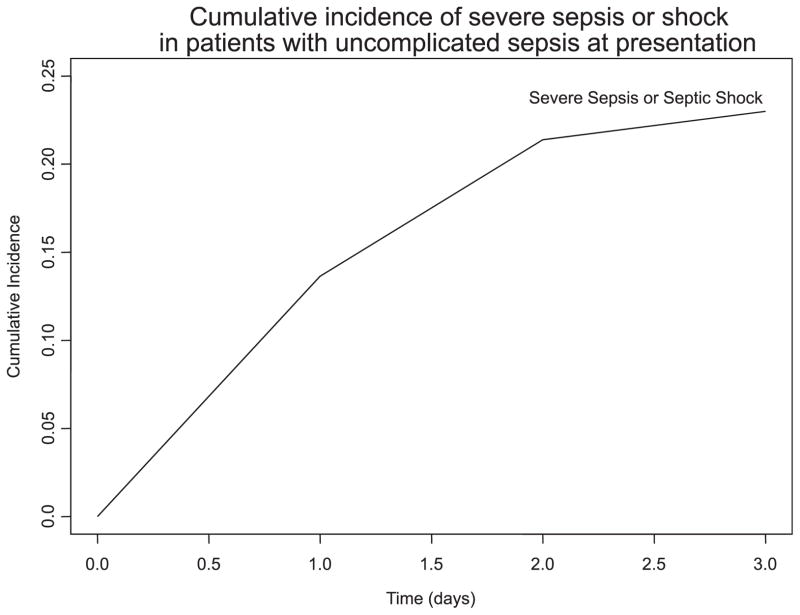
Of the 472 patients with confirmed infection not in shock at the time of enrollment, 93 had severe sepsis (Figure 3). The remaining 379 patients had no evidence of end-organ dysfunction and were categorized as having uncomplicated sepsis. Of the 379 patients with uncomplicated sepsis, 86 (22.7%) developed severe sepsis or septic shock within 72 hours (Figure 4). Progression occurred early during the hospital stay; the cumulative incidences of severe sepsis or shock at 24 and 48 hours were 13.5 and 21.4%, respectively. Among patients with uncomplicated sepsis, the cumulative incidence of death by Day 30 was 3.4%. The 30-day mortality rate in patients with early progression was 8.0% versus 2.0% in patients without early progression (χ2 = 5.72, p = 0.02).
Figure 3.
Disease progression among patients with uncomplicated sepsis. Of the 472 patients who were not in shock at the time of enrollment, 93 had evidence of end-organ dysfunction. The remaining 379 patients had no evidence of end organ dysfunction and were categorized as having uncomplicated sepsis. Of these 379 patients with uncomplicated sepsis, 86 (22.7%) developed severe sepsis or septic shock within 72 hours.
Figure 4.
Cumulative incidence of severe sepsis or septic shock among ED patients with uncomplicated sepsis (i.e., no evidence of shock or end-organ dysfunction). The figure plots 72-hour cumulative incidence of severe sepsis or septic shock among patients who had uncomplicated sepsis (i.e., no evidence of shock or end-organ dysfunction) at the time of initial ED evaluation. The cumulative incidences of severe sepsis or septic shock at 24, 28, and 72 hours were 13.5, 21.4, and 22.7%, respectively.
Of the 177 patients with definite infection and a confirmed causative microorganism, 174 had a bacterial or fungal etiology. Among these patients, 167 of 174 (96.0%) patients received appropriate antibiotics within the first 24 hours of ED presentation. The use of appropriate antibiotics was similar in patients who progressed to shock within 72 hours (37 of 38, 97.4%) and those who did not progress to shock (130 of 136, 95.8%).
DISCUSSION
Patients who are identified as high risk of sepsis (persistent hypotension and / or lactate levels of ≥4 mmol / L) who present to the ED are candidates for early protocolized intervention, which includes early antibiotic therapy, source identification and control, and aggressive resuscitation.6 The optimal diagnostic and management strategies for ED patients with less severe sepsis have not been determined, nor has its early natural history been well described. This study found that nearly one in five patients with sepsis who were not in shock upon presentation to the ED developed septic shock within 72 hours. Patient factors associated with sepsis progression included older age, female sex, anemia, comorbid lung disease, hyperthermia, and vascular access infection. Early progression to septic shock was associated with higher 30-day mortality. Among a subgroup of 379 patients with uncomplicated sepsis, 86 (22.7%) progressed to severe sepsis or septic shock by 72 hours. This progression was observed despite appropriate antibiotic therapy.
To our knowledge, this is the first study that has examined the progression to severe sepsis or shock in a multicenter cohort of patients in the ED setting. Alberti et al.17 reported that among 1,531 patients in 28 intensive care units (ICUs) with a first episode of infection on admission or during the stay, the cumulative incidences of progression to severe sepsis or shock were 20 and 24%, on Days 10 and 30, respectively. While our 30-day cumulative incidence of severe sepsis and shock (24.8%) was similar to that reported by Alberti et al. (24.0%), disease progression occurred much earlier in our ED cohort (21.4% at 48 hours) than in the cohort of Alberti et al. (<5% at 48 hours). This may reflect underlying differences in the natural history of disease progression in patients with community-acquired sepsis seen in the ED versus nosocomial sepsis seen in the ICU. Alternatively, the difference in progression rates could reflect earlier diagnosis, earlier sepsis stage, or intensity of treatment in patients already hospitalized. The high incidence of early progression to severe sepsis and shock among patients presenting to the ED highlights its time-sensitive nature, even in patients who initially do not appear critically ill.
Hyperthermia at time of presentation was strongly associated with progression to septic shock within 72 hours. Perturbations of core temperature is a common finding in sepsis, and hyperthermia is one of the SIRS criteria that define sepsis.5 Early pyrexia in sepsis is believed to reflect elevated levels of endogenous, pyrogenic mediators (acute phase reactants), together with increased metabolic rate.18,19 Temperatures higher than 38.2°C in ICU patients with sepsis has been associated with progression to severe sepsis and shock on Days 10 and 30, respectively.17 The increase in systemic oxygen demands associated with elevated temperatures exacerbate the imbalance between systemic oxygen supply and demands. This study extends the observation that pyrexia in patients without shock who present to the ED is similarly associated with an increased risk of early progression to septic shock.
Previous studies have examined the influence of race and sex on sepsis incidence and outcomes.20,21 The incidence of sepsis and sepsis-related mortality is reportedly lower in women, and several hypotheses have been proposed to explain this finding, including the role of sex hormones and sex-related gene polymorphisms associated with immune function.22 However, in our study conducted in an ED setting, women were twice as likely as men to progress to septic shock within 72 hours of presentation. Previous studies have reported sex disparities in treatment administered to patients with a variety of acute conditions, including acute myocardial infarction and patients hospitalized in ICU settings.23,24 We did not observe differences in the delivery of protocolized sepsis care or appropriate antibiotic utilization between women and men. Additional research is needed to confirm and explain sex differences in early sepsis progression.
Patients with vascular access device infections had the highest rate of progression to septic shock within 72 hours of admission (11 of 20, 55%). Catheter-related septicemia has been associated with poor outcomes and a nearly 30% attributable risk of mortality.25 Increased use of intravenous catheters for such things as maintenance dialysis or home drug delivery has resulted in significant ED utilization and costs.26 In a multicenter prospective study of 1,846 hemodialysis patients, 23% of all infection-related hospitalizations were vascular access related, accounting for one-quarter of the 871 patient study deaths.27 In light of these findings, and the significance of vascular access–related infections as a risk factor for sepsis progression in our study, more aggressive management of uncomplicated sepsis may be indicated.
Previous studies have shown that elevated serum lactate levels are associated with higher mortality in ED patients with severe sepsis and shock.28–31 In our cohort of 730 patients, elevated lactate was an independent predictor of death. The 30-day mortality rate among patients with lactate levels of >4 mmol /L was 28.6%, similar to previous reports.30,31 Yet among patients in our study who were not hypotensive and had a serum lactate concentration of <4 mmol / L, higher lactate levels did not appear to predict early progression to septic shock. Elevated serum lactate may occur in later progression from sepsis to septic shock. In addition, lactate may be elevated for a variety of reasons, including impaired clearance, depressed cellular respiration secondary to insufficient oxygen tissue delivery, impaired microcirculation, and mitochondrial dysfunction.32–36 Thus, additional metabolic biomarkers may be needed to identify high-risk sepsis patients earlier while they are in the ED.
This study has several strengths in comparison to some previous sepsis studies. First, final determination of infection status was determined by blinded study physicians based on results of systematic blood cultures and other objective diagnostic tests. As a result, 15% of SIRS patients with suspected infection initially were later determined to have a noninfectious etiology. In contrast, previous studies of sepsis progression included SIRS patients without evidence of confirmed infection. Second, this study focused on patients who were hemodynamically stable at the time of ED presentation, a subgroup that has been understudied in comparison with studies of patients with septic shock. In contrast, most previous studies of sepsis progression were conducted in hospitalized patients in the ICU and therefore involved sepsis of nosocomial origin. Finally, the present multicenter study included a diverse demographic group of patients, including African Americans, whereas previous studies of sepsis progression predominantly enrolled whites.
LIMITATIONS
This study involved three EDs and thus may not be generalizable to other ED settings with different patient characteristics, treatment patterns, and research personnel. Sepsis progression and patient outcomes are probably influenced by treatment. Thus, differences among providers and between institutions with respect to treatment may have influenced patient outcomes. Our findings, however, were consistent across the three study sites, and the low overall mortality rates may reflect systematic early detection and aggressive care for patients with severe sepsis. We did not observe any systematic differences in the use, timing, or appropriateness of antibiotic therapy or initial resuscitation.
Classification of septic shock is imperfect and complicated by the dynamic nature of the condition and challenges in obtaining precise clinical measurements. To address this issue, published definitions for septic shock were used at predetermined time points for all patients.
While screening and enrollment were available throughout all days of the week and all hours of the day at each site, most patients were enrolled during the daytime hours, given the need for prompt study specimen handling for metabolic studies. Thus, our study data may not be fully reflective of patient characteristics and ED treatment during nighttime and weekend hours.
Finally, while the overall mortality rate in our analysis cohort was only 4.9%, this is largely explained by the fact that we included a significant number of patients with uncomplicated sepsis in our analysis. The overall 30-day mortality rates in our larger patient cohort were 28.6% for patients with lactate levels of ≥4 mmol /L and 35.2% for patients with concomitant hypotension, similar to rates reported in previous ED studies.30,31
CONCLUSIONS
Approximately one of every four patients with confirmed infection who presents to the ED with uncomplicated sepsis progresses to severe sepsis or septic shock within 72 hours. Early progression to septic shock (vs. no progression) is associated with higher 30-day mortality. Better diagnostic tools are needed to identify ED patients with sepsis who are at high risk for disease progression to organ dysfunction or shock, with the hope that early intervention in this group would improve patient outcomes.
Acknowledgments
This work was supported by NIH grants AI066569 and P20RR016480, NIH contract HHSN266200400064C, and grants from Pfizer Inc. and Roche Diagnostics Inc. (2005–2010).
We thank Jukka Korpela (NIAID-DMID); Arturo Suarez (Henry Ford Hospital); Deborah Freeman, Christine Oien, and Ralph C. Corey (Duke University); Steph Reisinger and Steve Lyman (Prosanos Inc.); Brian Edmonds, Brian Grinnell, David R. Nelson, Mark D. Williams, and William L. Macias (Eli Lilly and Co.); John F. McCarthy, Alita A. Miller, and Keith R. Marotti (Pfizer Inc.); and Stacie Young (Biosite Inc.).
Footnotes
Presented at the Society for Academic Emergency Medicine, New Orleans, LA, May 2009.
This study is registered at ClinicalTrials.gov (NCT00258869).
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Incidence, cost and outcome of severe sepsis in the United States. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Alberti C, Brun-Buisson C, Burchardi H, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108–21. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- 3.McCaig L, Nawar E. Advance Data from Vital and Health Statistics. Washington DC: US Department of Health and Human Services; 2006. National Ambulatory Medical Care Survey: 2005 Emergency Department Summary; p. 372. [Google Scholar]
- 4.Jaimes F, Garcés J, Cuervo J, et al. The systemic inflammatory response syndrome (SIRS) to identify infected patients in the emergency room. Intensive Care Med. 2003;29:1368–71. doi: 10.1007/s00134-003-1874-0. [DOI] [PubMed] [Google Scholar]
- 5.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP / SCCM Consensus Conference Committee. American College of Chest Physicians / Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 6.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 7.Rangel-Frausto MS, Pittet D, Costigan M, et al. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273(2):117–23. [PubMed] [Google Scholar]
- 8.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 9.Jones AE, Brown MD, Trzeciak S, et al. The effect of a quantitative resuscitation strategy on mortality in patients with sepsis: a meta-analysis. Crit Care Med. 2008;36:2734–9. doi: 10.1097/CCM.0b013e318186f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernard GR, Vincent JL, Laterre PF, et al. Recombinant Human Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) Study Group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 11.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 12.Nguyen HB, Rivers EP, Abrahamian FM, et al. Severe sepsis and septic shock: review of the literature and emergency department management guidelines. Ann Emerg Med. 2006;48:28–54. doi: 10.1016/j.annemergmed.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Hollenberg SM, Ahrens TS, Annane D, et al. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med. 2004;32:1928–48. doi: 10.1097/01.ccm.0000139761.05492.d6. [DOI] [PubMed] [Google Scholar]
- 14.Gross PA, Barret TL, Dellinger EP, et al. Quality standards for the treatment of bacteremia. Clin Infect Dis. 1994;18:428–30. doi: 10.1093/clinids/18.3.428. [DOI] [PubMed] [Google Scholar]
- 15.Valles J, Rello J, Ochagavia A, Garnacho J, Alcala MA. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest. 2003;123:1615–24. doi: 10.1378/chest.123.5.1615. [DOI] [PubMed] [Google Scholar]
- 16.Bell DM. Promoting appropriate antimicrobial drug use: perspective from the Centers for Disease Control and Prevention. Clin Infect Dis. 2001;33:S245–50. doi: 10.1086/321857. [DOI] [PubMed] [Google Scholar]
- 17.Alberti C, Brun-Buisson C, Chevret S, et al. Systemic inflammatory response and progression to severe sepsis in critically ill infected patients. Am J Respir Crit Care Med. 2005;171:461–8. doi: 10.1164/rccm.200403-324OC. [DOI] [PubMed] [Google Scholar]
- 18.Kreymann G, Grosser S, Buggisch P, Gottschall C, Matthaei S, Greten H. Oxygen consumption and resting metabolic rate in sepsis, sepsis syndrome, and septic shock. Crit Care Med. 1993;21:1012–9. doi: 10.1097/00003246-199307000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Carré JE, Singer M. Cellular energetic metabolism in sepsis: the need for a systems approach. Biochim Biophys Acta. 2008;1777:763–71. doi: 10.1016/j.bbabio.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 20.Adrie C, Zoulay E, Francias A, et al. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest. 2007;132:1786–93. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 21.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. 2008;177:279–84. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroder J, Kahlke V, Book M, et al. Gender differences in sepsis: genetically determined? Shock. 2000;14:307–10. [PubMed] [Google Scholar]
- 23.Valentin A, Jordan B, Lang T, et al. Gender-related differences in intensive care: a multiple-center cohort study of therapeutic interventions and outcome in critically ill patients. Crit Care Med. 2003;31:1901–07. doi: 10.1097/01.CCM.0000069347.78151.50. [DOI] [PubMed] [Google Scholar]
- 24.Vaccarino V, Rathore SS, Wenger NK, et al. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 2005;353:671–82. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soufir L, Timsit JF, Mahe C, et al. Attributable morbidity and mortality of catheter-related septicemia in critically ill patients: a matched, risk-adjusted, cohort study. Infect Control Hosp Epidemiol. 1999;20:396–401. doi: 10.1086/501639. [DOI] [PubMed] [Google Scholar]
- 26.Loran MJ, McErlean M, Eaisele G, et al. The emergency department care of hemodialysis patients. Clin Nephrol. 2002;57:439–43. doi: 10.5414/cnp57439. [DOI] [PubMed] [Google Scholar]
- 27.Allon M, Depner TA, Radeva M, et al. Impact of dialysis dose and membrane on infection-related hospitalization and death: results of the HEMO study. J Am Soc Nephrol. 2003;14:1863–70. doi: 10.1097/01.asn.0000074237.78764.d1. [DOI] [PubMed] [Google Scholar]
- 28.Trzeciak S, Dellinger RP, Chansky ME, et al. Serum lactate as a predictor of mortality in patients with infection. Intensive Care Med. 2007;33:970–7. doi: 10.1007/s00134-007-0563-9. [DOI] [PubMed] [Google Scholar]
- 29.Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–7. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro NI, Howell MD, Talmor D, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–8. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Howell MD, Donnino M, Clardy P, et al. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33:1892–9. doi: 10.1007/s00134-007-0680-5. [DOI] [PubMed] [Google Scholar]
- 32.Boulos M, Astiz ME, Barua RS, et al. Impaired mitochondrial function induced by serum from septic shock patients is attenuated by inhabitation of nitric oxide synthase and poly(ADP-ribose) synthase. Crit Care Med. 2003;31:353–8. doi: 10.1097/01.CCM.0000050074.82486.B2. [DOI] [PubMed] [Google Scholar]
- 33.Trzeciak S, Dellinger RP, Parrillo JE, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2006;48:88–98. doi: 10.1016/j.annemergmed.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 34.Fink MP. Bench-to-bedside review: cytopathic hypoxia. Crit Care. 2002;6:491–9. doi: 10.1186/cc1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cairns CB. Rude unhinging of the machinery of life: metabolic approaches to hemorrhagic shock. Curr Opin Crit Care. 2001;7:437–43. doi: 10.1097/00075198-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro NI, Trzeciak S, Hollander J, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Crit Care Med. 2009;37:96–104. doi: 10.1097/CCM.0b013e318192fd9d. [DOI] [PubMed] [Google Scholar]