VLDL hydrolysis by LPL activates PPAR-α through generation of unbound fatty acids (original) (raw)
Abstract
Recent evidence suggests that lipoproteins serve as circulating reservoirs of peroxisomal proliferator activated receptor (PPAR) ligands that are accessible through lipolysis. The present study was conducted to determine the biochemical basis of PPAR-α activation by lipolysis products and their contribution to PPAR-α function in vivo. PPAR-α activation was measured in bovine aortic endothelial cells following treatment with human plasma, VLDL lipolysis products, or oleic acid. While plasma failed to activate PPAR-α, oleic acid performed similarly to VLDL lipolysis products. Therefore, fatty acids are likely to be the PPAR-α ligands generated by VLDL lipolysis. Indeed, unbound fatty acid concentration determined PPAR-α activation regardless of fatty acid source, with PPAR-α activation occurring only at unbound fatty acid concentrations that are unachievable under physiological conditions without lipase action. In mice, a synthetic lipase inhibitor (poloxamer-407) attenuated fasting-induced changes in expression of PPAR-α target genes. Apolipoprotein CIII (apoCIII), an endogenous inhibitor of lipoprotein and hepatic lipase, regulated access to the lipoprotein pool of PPAR-α ligands, because addition of exogenous apoCIII inhibited, and removal of endogenous apoCIII potentiated, lipolytic PPAR-α activation. These data suggest that the PPAR-α response is generated by unbound fatty acids released locally by lipase activity and not by circulating plasma fatty acids.
Keywords: peroxisome proliferator activated receptor, triglyceride-rich lipoprotein, lipoprotein lipase, apolipoprotein CIII, nonesterified fatty acid, very low-density lipoprotein
The peroxisomal proliferator activated receptor (PPAR) family of nuclear hormone receptors functions as transcriptional nodal points in the regulation of energy metabolism and inflammation (1). Three PPAR isotypes have been identified: PPAR-α and PPAR-δ, which stimulate fatty acid oxidation and share many target genes, but still differ in tissue distribution and functional effects, and PPAR-γ, which activates lipid storage and adipogenesis. In vitro analyses demonstrate that PPARs are activated by high concentrations of fatty acids and their derivatives, although the physiological context and relevance of these effects have remained unclear (2, 3). Together, these data have led to the view of PPARs as lipid sensors; despite this, the upstream factors determining generation and delivery of the lipid ligands are poorly understood.
Intracellular fatty acids can be generated by three main sources: de novo lipogenesis, enzymatic hydrolysis, or uptake from extracellular sources. Plasma NEFA circulate bound to albumin or can be generated locally by vascular lipases acting on lipoproteins. Lipolytic processing of lipoproteins appears to be an important source of PPAR-α and -δ ligands as reported by us and others (4–6). In vitro, LPL-mediated lipolysis of VLDL stimulates PPAR-α and PPAR-δ activity and downstream responses, whereas similar quantities of NEFA from plasma fail to recapitulate these effects (4–6). Lipolysis of HDL by endothelial lipase also generates PPAR-α ligands (7). In mouse models, transgenic LPL overexpression and treatment with a pharmacological LPL activator, NO-1886, indicate that LPL lipolytic products are sufficient to promote PPAR-α-dependent peroxisomal proliferation and induction of PPAR-α target genes; likewise, cardiac-specific LPL-deficient animals have reduced expression of PPAR-α target genes (6, 8, 9). Together these data reveal that specific lipoproteins serve as circulating pools of PPAR-α and δ ligands.
The unexplained observation that VLDL lipolysis products activate PPAR-α whereas NEFA from plasma do not remains a key unresolved issue in understanding how fatty acids and their handling influence cellular responses. Differences in chemical composition could potentially account for the effective activation of PPAR-α by triglyceride-rich lipoprotein lipolysis metabolites as opposed to plasma NEFA. For example, VLDL contains retinoids, phospholipids, and other nonfatty acid compounds that may play a role in activating PPAR-α. Alternatively, the source and delivery of the fatty acid may alter its cellular metabolism and signaling properties. For example, arachidonic acid released by phospholipase A2-mediated cleavage of phospholipids is preferentially channeled to eicosanoid biosynthesis. Therefore, it is possible that differences in chemistry or delivery of lipolysis products underlie their preferential activation of PPAR-α. Moreover, the activity of LPL is under the control of multiple additional inputs, including the action of apolipoprotein CIII (apoCIII), an endogenous LPL inhibitor. Whether regulators of LPL activity such as apoCIII modulate PPAR activity remains unknown. In the current study, we assessed the biochemical basis of lipolytic PPAR-α activation, the role of apoCIII in determining PPAR-α activity, and the evidence for their contribution to PPAR-α function in vivo.
MATERIALS AND METHODS
Cell culture, PPAR-α reporter, and fatty acid uptake
Bovine aortic endothelial cells (BAEC) were transfected with a PPAR-α transactivation assay as previously described (6). Briefly, BAEC were grown in Dulbecco's Modified Eagle's Medium (Invitrogen) supplemented with 10% fetal bovine serum (Omega Scientific), penicillin/streptomycin (Invitrogen), and l-glutamine (Invitrogen). Cells were seeded in 24-well plates and transfected as previously described using Fugene HD transfection reagent (Roche Diagnostics) (6). Twenty-four hours after transfection, cells were treated as indicated for 18 h. Cells were harvested and luciferase and β-galactosidase activity were measured by addition of substrate, luciferin (BD Biosciences) and chlorophenol red β-galactoside (Sigma), respectively, with quantification of resulting photoemission or absorbance. PPAR-α activation was calculated as the ratio of luciferase activity to β-galactosidase activity. Cell culture medium was analyzed for NEFA content using an enzymatic colorimetric kit (Wako). The equilibrium concentration of unbound oleate was calculated as described by Spector et al. (10). To assess fatty acid uptake, BAEC were incubated with 90 μM unlabeled oleic acid spiked with 9,10-3H oleic acid (1 µCi/well; 33.4 Ci/mmol; Perkin Elmer) or 10 μg/ml VLDL labeled, as previously described, with 3H trioleate (Perkin Elmer) as indicated for 20 min (11). Cells were washed with ice-cold PBS and lysed with 0.1 M NaOH. Cell lysate was analyzed for protein concentration and cell-associated CPM. All experiments were performed in triplicate.
Lipoprotein isolation and characterization
All plasma samples were obtained by the Cholesterol Research Center at Children's Hospital Oakland Research Institute. Blood was collected from healthy subjects after an overnight fast. The protocol was approved by the Institutional Review Board of Children's Hospital and Research Center Oakland, and informed consent was obtained from all volunteers. An aliquot of plasma was added to an immunoaffinity column prepared from purified goat anti-human apoCIII sera (International Immunology Corp.) covalently linked to Affigel-10 (BioRad Laboratories) following the manufacturer's instructions. Following overnight incubation at 4°C, the unbound fraction, depleted of apoCIII-containing particles, was collected and concentrated. VLDLs were isolated from plasma or the unbound immunoaffinity column fraction by ultracentrifugation (d<1.006, 40,000 rpm, 24 h). ApoB was measured by immunoturbidimetric assay (Bacton Assay Systems and Express 550 Plus analyzer). ApoCIII was measured in triplicate by sandwich-style enzyme-linked immunosorbent assay with purchased goat anti-human apoCIII (International Immunology Corp.). Triglyceride content was quantified by enzymatic end point assay (Sigma).
In vivo experiments
Male C57BL6 mice were fasted for 2 h prior to treatment and divided into three groups (n = 5 per group). Group 1 (Fed) were injected with saline and returned to ad libidum feeding. Group 2 (Fasted) were injected with saline and fasted for 24 h. Group 3 (Fasted + P-407) were injected with poloxamer-407 (P-407; 500 mg/kg, ip) and fasted for 24 h. Animals were euthanized, and all harvested tissues and plasma samples were stored at −80°. The treatment protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri-Kansas City. RNA was isolated from homogenized tissue using the QIAGEN RNAeasy kit (Qiagen). Relative quantitative PCR was performed on the ABI7900 system using SYBR green master mix in triplicate (Applied Biosystems). All genes were normalized to an endogenous control gene (gusb). The primers used were: 70-kDa peroxisomal membrane protein (pmp70): 5′-TGTTCAGGACTGGATGGATG-3′ (forward), 5′-TGGCAAACTGGGGTTTATG -3′ (reverse); cd36: 5′-GCTTGCAAATCCAAGAATG-3′(forward), 5′- CGGCTTTACCAAAGATGTAGC-3′ (reverse); pparα: 5′-CCTGAACATCGAGTGTCGAA-3′(forward), 5′- CAGCTCCGATCACACTTGTC-3′ (reverse); malonyl-CoA dehydrogenase (mlycd): 5′-CTCGGGACCTTCCTCATAAA-3′(forward), 5′- ATAGGCGACAGGCTTGAAAA-3′ (reverse); carnitine palmitoyl-transferase 1a (cpt1a): 5′-ACGGAGTCCTGCAACTTTGT-3′(forward), 5′-GTACAGGTGCTGGTGCTTTTC-3′ (reverse); medium chain acyl-CoA dehydrogenase (mcad): 5′-GCCCAGAGAGCTCTAGACGA-3′(forward), 5′-GTTCAACCTTCATCGCCATT-3′ (reverse); carnitine palmitoyl-transferase 1b (cpt1b): 5′-CCAGATCTGCATGTTTGACC-3′(forward), 5′-TGCTGGAGATGTGGAAGAA-3′ (reverse); acyl-CoA oxidase (acox): 5′-CATGCGGATTAATGAGAGCA-3′(forward), 5′- TCCGACATTCTTCGATACCA-3′ (reverse); lipoprotein lipase (lpl): 5′-CAAGAGAAGCAGCAAGAT-3′(forward), 5′-CACTGTGCCGTACAGAGAA-3′ (reverse); and β-glucuronidase: 5′-CATGAGAGTGGTGTTGAG GATCA-3′ (forward), 5′-CCCATTCACCCACACAACTG-3′ (reverse).
Statistical analysis
One-way ANOVA with post hoc analysis (Tukey's honestly significant difference) was used to test for differences in treatment effects. Paired two-tailed _t_-tests were used to analyze differences in PPAR-α activation between lipoprotein fractions isolated from the same donor. All analyses were performed using JMP version 7.0 (SAS institute Inc.). Data are presented as mean ± SE.
RESULTS
Albumin inhibits PPAR-α activation by VLDL lipolytic products
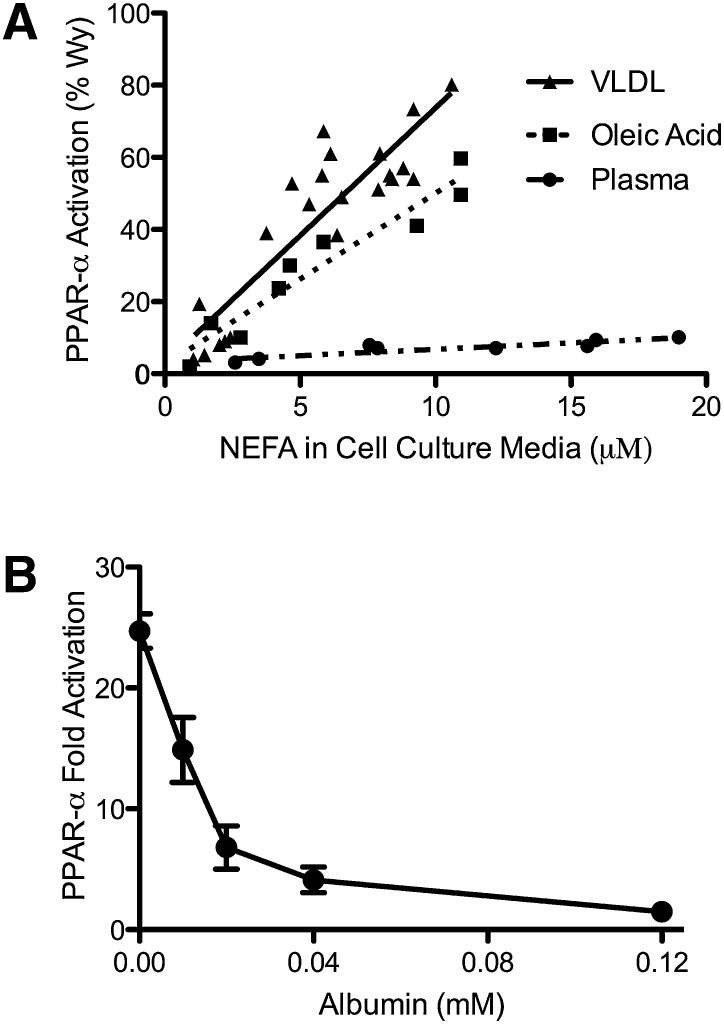
To test if differences in chemistry explain the disparity in PPAR-α activation observed with plasma and lipolysis products, the ability of LPL/VLDL, oleic acid, and plasma to stimulate PPAR-α was evaluated on a molar basis in BAEC. Oleic acid activated PPAR-α similarly to VLDL lipolytic products, while plasma failed to activate PPAR-α despite the presence of fatty acids (Fig. 1A). Given that oleic acid is the major fatty acid in plasma, the ability of VLDL to activate PPAR-α in preference to plasma likely results from differential delivery rather than chemistry. To test if LPL-/VLDL-mediated PPAR-α activation depends on the generation of unbound fatty acids, BAEC were incubated with LPL/VLDL and increasing concentrations of albumin. Albumin in combination with LPL/VLDL decreased PPAR-α activation in a concentration-dependent fashion with half-maximal inhibition at 0.014 mM albumin (Fig. 1B). PPAR-α activation by a synthetic ligand, WY14643, was not altered by addition of 0.015 mM albumin (WY14643: 33.85 ±.78 vs. WY14643 + albumin: 33.00 ± .57-fold; P = 0.51).
Fig. 1.
VLDL-derived fatty acids serve as potent PPAR-α ligands due to efficient delivery. BAEC were transfected with the PPAR α-LBD-GAL4 reporter system, as described in “Methods,” treated for 18 h, and cell lysate assayed for luciferase and β-galoctosidase activity. A: BAEC were exposed to various concentrations of LPL (1, 3, or 10 units/ml) and VLDL (1, 3, 10, or 30 μg/ml), oleic acid (0, 5, 10, or 20 μM at an unbound oleic acid concentration of 2450 nM), or plasma (0–5% v/v). This produced a range of NEFA concentrations, as measured in the cell culture media at the end of treatment. For oleic acid, a linear relationship exists between oleic acid added and NEFA concentration in the cell culture media (data not shown), such that ∼60% of NEFA added remains in the media following incubation with cells. PPAR-α activity is presented as percentage of activation by 10 µM Wy14643, a synthetic PPAR-α ligand. B: Transfected BAEC were incubated with VLDL (10 µg protein/ml) and LPL (10 units/ml) and increasing concentrations of albumin in triplicate. PPAR-α activity is expressed as fold activation over control.
Unbound fatty acid concentration determines fatty acid uptake and PPAR-α activation
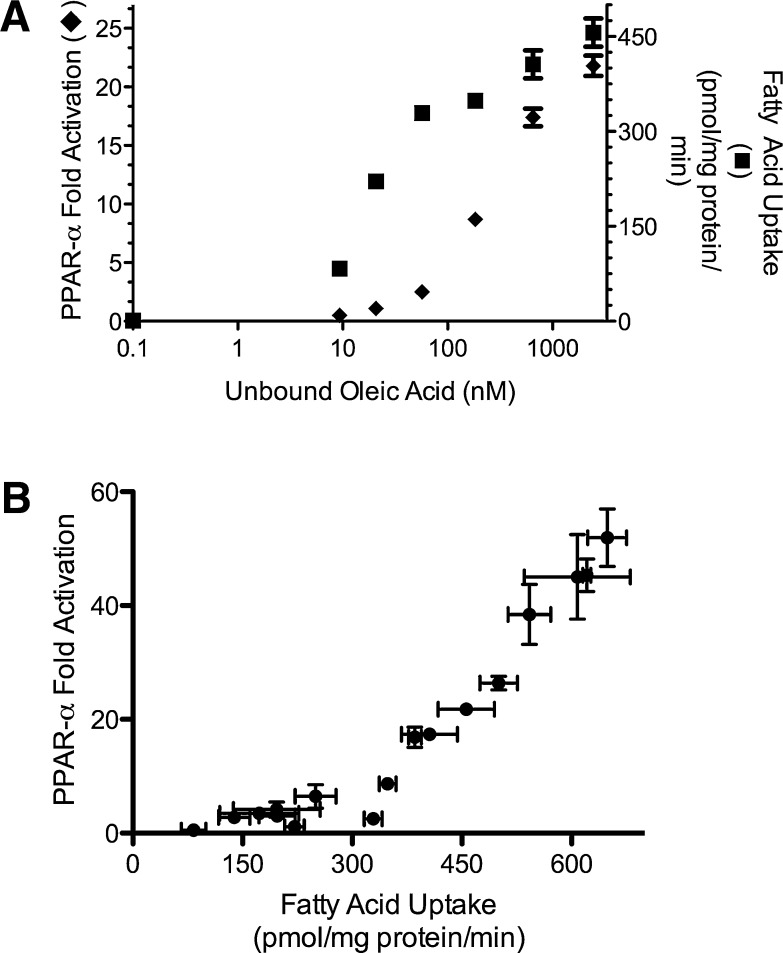
Whereas at low oleic acid levels (as in Fig. 1A), fatty acid uptake is related to total concentration, at physiological NEFA levels, the unbound fatty acid concentration becomes the major determinant of uptake (12). To determine the relationship between fatty acid uptake and PPAR-α activation, fatty acid uptake and PPAR-α reporter activity were measured in parallel in BAEC treated with varying concentrations of unbound oleic acid generated by varying the albumin content added to 90 μM oleic acid. As expected, both fatty acid uptake and PPAR-α activation increased with unbound oleic acid concentration (Fig. 2A); however, the two variables displayed markedly different kinetics, with half-maximal values achieved at 21 nM for fatty acid uptake and 286 nM for PPAR-α activation. Further experiments with varied unbound oleic acid concentrations revealed that fatty acid uptake above 300 pmol/mg protein/min displayed a linear relationship with PPAR-α activation (Fig. 2B). Interestingly, fatty acid uptake above 300 pmol/mg protein/min was unachievable at physiological unbound fatty acid concentrations (∼6–30 nM), explaining the failure of plasma NEFA to activate PPAR-α (12, 13). Conversely, VLDL (10 μg/ml) treated with LPL (10 units/ml) generated fatty acid uptake (463 ± 2 pmol/mg protein/min) sufficient to activate PPAR-α.
Fig. 2.
Fatty acid uptake determines PPAR-α activation. A: In parallel experiments, BAECs were treated with oleic acid (90 μM) and varying concentrations of albumin, and PPAR-α activation and fatty acid uptake were determined as described in Methods. B: Varying total (0–180 μM) and unbound oleic acid concentrations (0–2,450 nM) were used to generate a range of fatty acid uptake. Fatty acid uptake above 300 pmol/mg protein/min displayed a strong linear relationship with PPAR-α activation (_r_2 = 0.98; P < 0.05).
Lipase inhibition prevents fasting-induced increases in PPAR-α target genes in vivo
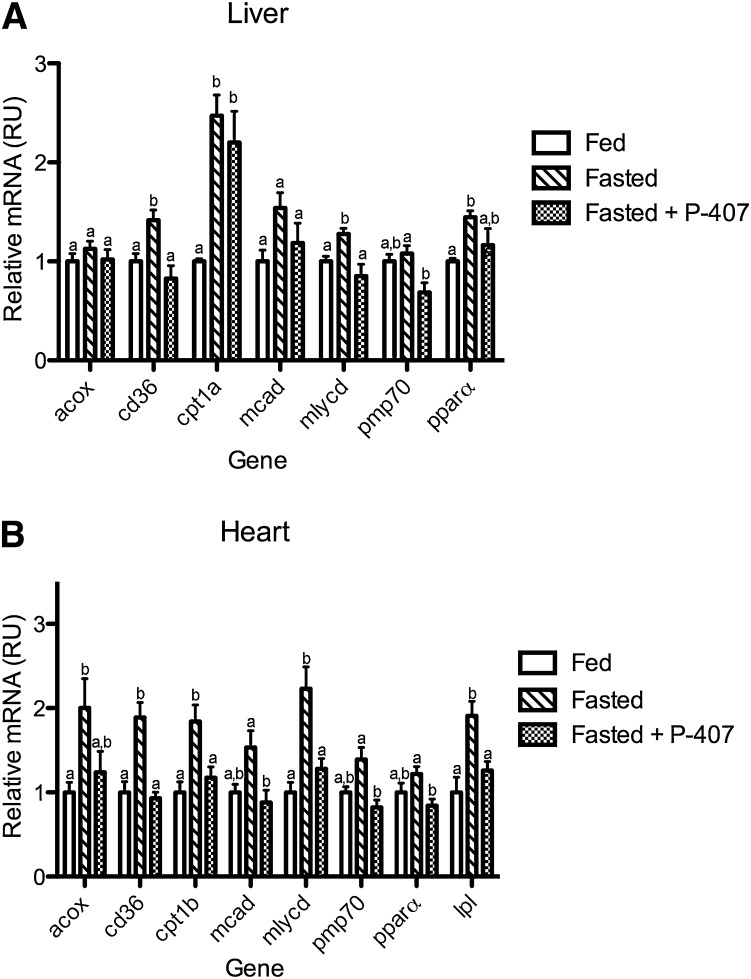
To test the contribution of lipase action to the in vivo generation of PPAR-α ligands, the transcriptional response of PPAR-α target genes to fasting was quantified in mice treated with a lipase inhibitor, the nonionic detergent P-407, or vehicle control at the outset of a 24 h fast. Fasting decreased triglyceride concentrations (75 ± 14 vs. 40 ± 6 mg/dl; P < 0.05), whereas P-407 caused severe hypertri-glyceridemia (3,578 ± 798 mg/dl).
In addition, fasting increased hepatic and cardiac expression of a PPAR-α target gene cassette, including pparα, cd36, acox, mlycd, mcad, pmp70, cpt1a, and cpt1b (Fig. 3A, B). In mice treated with P-407, hepatic transcriptional changes in response to fasting were absent for mlycd and cd36, diminished for pmp70, and unchanged for mcad, pparα, acox, and cpt1b (Fig. 3A). P-407 treatment prevented the fasting response for all PPAR-α target genes tested in the heart (Fig. 3B).
Fig. 3.
P-407 inhibits the transcriptional response to fasting in vivo. Following an initial 2 h fast, 9-week-old male C57Bl6 mice were treated with saline or P-407 (500 mg/kg, i.p.) and fasted for an additional 24 h. A group of saline-injected animals was fed ad libitum for the 24 h period. Abundance of mRNA for PPAR-α target genes was determined by RT-PCR and normalized to a control gene (gusb) in the liver (A) and heart (B). Groups not sharing a common superscript letter are significantly different (P < 0.05).
ApoCIII inhibits the lipolytic generation of PPAR-α ligands
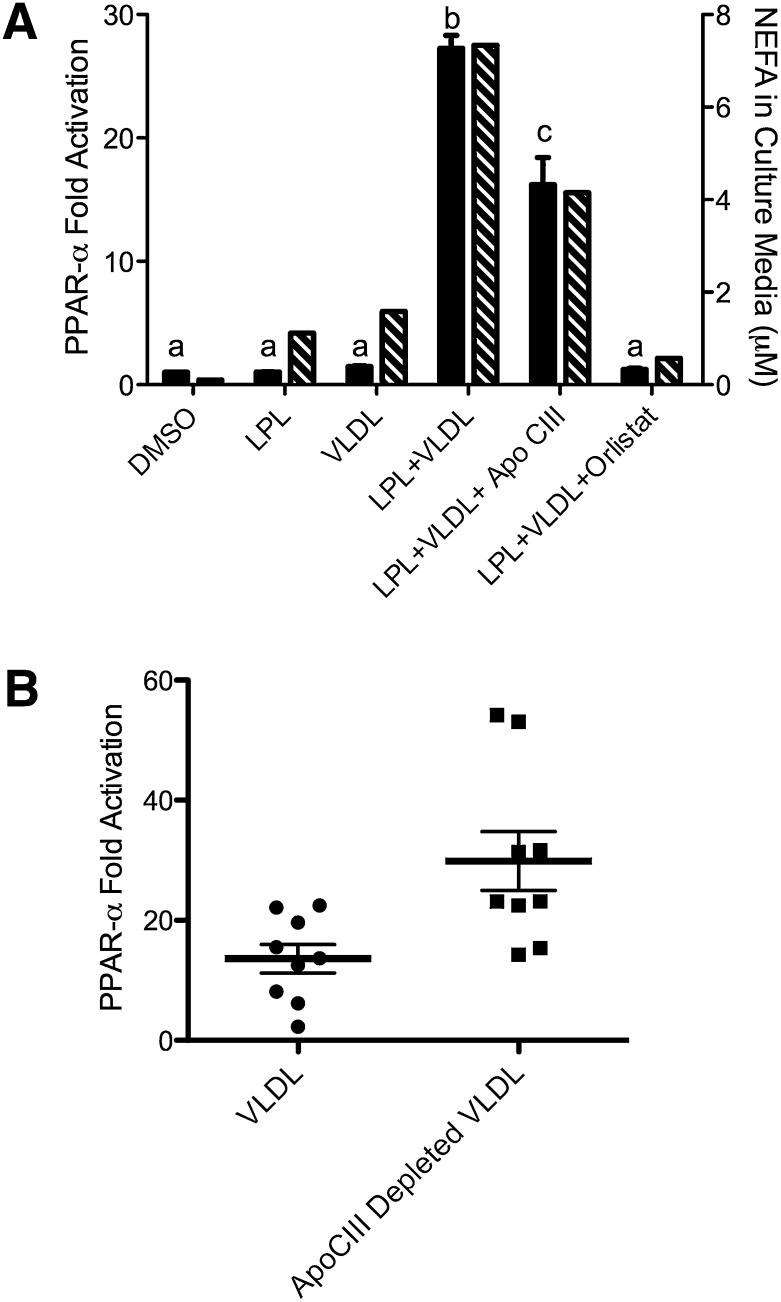
Because modifiers of lipolytic activity may also alter downstream PPAR-α activation, the role of apoCIII, a natural inhibitor of LPL, in regulating lipolytic availability of PPAR-α ligands was examined. Addition of apoCIII or orlistat, a synthetic lipase inhibitor, to VLDL decreased the resulting PPAR-α activation, concurrent with decreased release of NEFA into the cell culture media (Fig. 4A). To determine if changes in endogenous apoCIII content alter lipolytic accessibility to PPAR-α ligands, PPAR-α activation in response to VLDL particles depleted of apoCIII by immunoaffinity chromatography was compared with activation in response to total VLDL isolated from the same plasma. Immunoaffinity chromatography depleted VLDL of the majority (86.2 ± 5.8%) of apoCIII. Transfected BAEC were incubated with VLDL or apoCIII-depleted VLDL from the same donor. ApoCIII-depleted VLDL particles activated PPAR-α to a significantly greater extent than the VLDL particles derived from the same donor sample (Fig. 4B).
Fig. 4.
ApoCIII regulates access to lipoprotein-derived PPAR-α ligands. A: PPAR-α activation (solid bars) in BAEC were transfected as described in Fig. 1 and treated with LPL (10 units/ml), VLDL (10 µg protein/ml), Orlistat (10 µM), or apoCIII (3 μg/ml) as indicated for 18 h. Cell culture media was assayed for fatty acids. Groups not sharing a common superscript letter are significantly different (P < 0.05). B: BAEC were transfected as described in Fig. 1 and treated with total VLDL or apoCIII-depleted VLDL (10 μg protein/ml) and LPL (3 units/ml). The difference between the VLDL treatments was significant (P = 0.002) by paired two-tailed _t_-test.
DISCUSSION
The data presented here demonstrate the biochemical basis for the activation of PPAR-α by lipolysis of triglyceride-rich lipoproteins. On a molar basis, LPL lipolysis products activate PPAR-α similarly to oleic acid, the most abundant plasma fatty acid. Thus, more efficient delivery, rather than chemistry, likely accounts for preferential PPAR-α activation by VLDL lipolysis products. Addition of albumin to the LPL/VLDL treatment abrogates PPAR-α activation, indicating that lipolysis products activate PPAR-α by generating unbound fatty acids. Because fatty acids must be liberated from albumin prior to becoming available for tissue uptake, the unbound fatty acid concentration is a major determinant of subsequent uptake (12). Our results show that significant fatty acid uptake must occur prior to activation of PPAR-α. In fact, PPAR-α activation requires a threshold of fatty acid uptake that is unachievable at physiological plasma unbound fatty acid concentrations. This offers a simple explanation for the failure of plasma to activate PPAR-α despite the presence of ample fatty acids. Conversely, at physiological ratios of triglyceride to albumin, lipase action generates an unbound fatty acid concentration sufficient to drive fatty acid uptake beyond the PPAR-α activation threshold. This is demonstrated by the ability of plasma exposed to lipase in vivo or in vitro to activate PPAR-α (6). However, this work was done in an artificial gene reporter system and does not directly demonstrate PPAR-α ligand binding.
The biphasic relationship between fatty acid uptake and PPAR-α activation may represent different routes of fatty acid uptake. Fatty acid uptake is the sum of saturable and linear processes (14). Fatty acid uptake in the physiological range of unbound fatty acids is largely driven by the saturable process and, in the current study, failed to activate PPAR-α. The saturable process likely represents the action of fatty acid transporters, such as CD36. Intriguingly, loss of CD36 in vivo or in vitro decreases lipid uptake without altering PPAR-α activation (15, 16). Thus, plasma NEFA may fail to activate PPAR-α because they are largely taken up by CD36. However, at the higher unbound fatty acid concentrations achieved in the lipolytic microenvironment, a CD36-independent route of fatty acid uptake may become active and lead to PPAR-α activation. The nature of the alternate route of fatty acid uptake and how it may target fatty acids to the nucleus for signaling requires further study.
These data suggest that VLDL can serve as a potent source of PPAR-α ligands by generating high local concentrations of unbound fatty acids. Although tracer studies have shown that fatty acids derived from lipolysis of triglyceride-rich lipoproteins mix with plasma NEFA, fatty acid uptake is regulated by the tissue-specific activity of LPL, likely as a result of the high concentration of unbound fatty acids generated in the microenviroment surrounding active LPL (17). In fact, LPL activity has been shown to contribute a large portion of the fatty acids consumed by the heart and other tissues (18, 19).
Thus, increased lipase activity, and not the increased release of NEFA from adipose tissue, is likely to be responsible for increased PPAR-α function in the fasted state. This is supported by our finding that injection of the nonionic detergent P-407 inhibits fasting-induced increases in PPAR-α gene expression. Because P-407 inhibits lipoprotein and endothelial and hepatic lipase, the resulting alterations in PPAR-α signaling cannot be attributed to the action of a specific lipase. Although many hormonal signaling pathways are altered during the transition to the fasting state, fasting-induced increases in several genes (mcad, acox, and cpt1b) are known to be dependent on PPAR-α (20). Increased hepatic expression of mlycd, pmp70, and cd36 in response to fasting was substantially prevented by P-407 administration. The absence of an effect on cpt1a is consistent with data from studies in PPAR-α-deficient mice (20, 21). In the heart, we showed that P-407 attenuated fasting-induced increases in expression of the PPAR-α target genes that were tested. Together with the in vitro data, these findings suggest that lipase activity is necessary to activate PPAR-α. Although these data are consistent with our model, P-407 is not a specific inhibitor of lipase activity, and other effects may have confounded our results. However, P-407 does not inhibit fatty acid uptake or intracellular lipase activity and has no direct effects on PPAR-α function (22–24). Furthermore, LPL deficiency decreases expression of PPAR-α target genes and mitigates cardiac myopathy induced by PPAR-α overexpression (15). The current study supports the importance of LPL in PPAR-α activation in vivo and builds upon the genetic models to demonstrate that pharmacological inhibition of LPL limits PPAR-α activation by endogenous mechanisms.
A number of other observations support a key role of lipase activities in the generation of PPAR ligands. Lipolysis of HDL by endothelial lipase increases acox expression and inhibits leukocyte adhesion in a PPAR-α-dependent manner (7). Cardiac-specific LPL knockout animals display reduced cardiac expression of PPAR-α target genes despite increased uptake of plasma NEFA (18, 25). Conversely, mice overexpressing LPL in skeletal muscle, but not those overexpressing CD36, display peroxisomal and mitochondrial proliferation, increased oxidative fibers, and cold tolerance, hallmarks of PPAR-α and -δ activation (26, 27). Chronic exposure to heparin-releasable lipase activity also increases mitochondrial proliferation and binding of PPAR-δ to the PPAR response element in the cpt1b promoter in skeletal muscle (28). NO-1886, a pharmacological LPL stimulator, activates fatty acid oxidation genes and protects against high-fat-induced weight gain and hepatic steatosis, effects closely resembling those seen with PPAR-α activation (9). Likewise, the activity of LPL in skeletal muscle has been shown to regulate uncoupling protein 3, a PPAR-α and -δ responsive gene, independently of NEFA levels (29). Taken together these findings in animal models support the role of lipolysis products as determents of PPAR-α and -δ function irrespective of plasma NEFA levels.
Although our results focus on the delivery of PPAR-α ligands from extracellular sources, intracellular fatty acids generated by de novo lipogenesis or enzymatic hydrolysis may also activate PPAR-α. Impaired PPAR-α-dependent gene expression in hepatocyte-specific fatty acid synthase-deficient mice suggests a specific role for de novo lipogenesis products as PPAR-α ligands (30). The relevant FAS product bound to PPAR-α was recently identified as 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (31). Hepatocyte-specific knockout of another lipogenic enzyme, acetyl CoA carboxylase 1, had no effect on PPAR-α-dependent gene expression, suggesting that alternative sources of PPAR-α ligands exist (32). Accordingly, recent in vitro work has shown that genetic manipulation of access to hepatic lipid droplets alters PPAR-α activity (33). Given the potential for intracellular generation of PPAR-α ligands, our findings may be particularly relevant to cells with low rates of lipogenesis and small triglyceride stores.
The evidence for LPL-mediated PPAR-α activation suggests that other parameters that determine LPL activity might also influence PPAR-α activation. The biochemical function of apoCIII makes it a likely candidate for modulation of lipolytic PPAR-α and -δ activation. ApoCIII, an 8.8 kDa exchangeable apolipoprotein, is linked to metabolic and cardiovascular disease by strong epidemiological and genetic data, but the biology underlying these observations remains incompletely understood (34). In vitro, apoCIII inhibits LPL, hepatic lipase, heparin sulfate proteoglycan interactions, and hepatic receptor-mediated clearance (35). Recently, apoCIII has been found to have a number of proinflammatory properties, including activation of toll-like receptor 2 (36). The present findings demonstrate that apoCIII can play an additional role in PPAR-α-mediated metabolic and inflammatory functions by controlling lipolytic generation of PPAR-α ligands. Because apoCIII expression is suppressed and LPL activity is stimulated by PPAR-α, a positive feedback system may exist (37). Individuals with high apoCIII levels may have impaired generation of endogenous PPAR-α ligands and hence be particularly likely to benefit from synthetic PPAR-α ligands.
In summary, our results demonstrate that lipolysis of VLDL provides the unbound fatty acid concentration required for activation of PPAR-α by an extracellular source and that apoCIII is of importance in modulating this process.
Acknowledgments
The authors thank Myra Gloria for excellent editorial assistance.
Footnotes
Abbreviations:
apoCIII
apolipoprotein CIII
ACOX
acyl-CoA oxidase
BAEC
bovine aortic endothelial cell
CPT1
carnitine palmitoyltransferase 1
GusB
β-glucuronidase
MCAD
medium chain acyl-CoA dehydrogenase
MLYCD
malonyl-CoA dehydrogenase
PMP70
70-kDa peroxisomal membrane protein
PPAR
peroxisomal proliferator activated receptor
This work was supported by the Department of Atherosclerosis Research at the Children's Hospital Oakland Research Institute (R.M.K.) and by the National Institutes of Health Grants R0-1HL071745 and P0-1HL48743 (J.P.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Brown J. D., Plutzky J. 2007. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 115: 518–533. [DOI] [PubMed] [Google Scholar]
- 2.Kliewer S. A., Sundseth S. S., Jones S. A., Brown P. J., Wisely G. B., Koble C. S., Devchand P., Wahli W., Willson T. M., Lenhard J. M., et al. 1997. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA. 94: 4318–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forman B. M., Chen J., Evans R. M. 1997. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA. 94: 4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla A., Lee C. H., Barak Y., He W., Rosenfeld J., Liao D., Han J., Kang H., Evans R. M. 2003. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proc. Natl. Acad. Sci. USA. 100: 1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee C. H., Kang K., Mehl I. R., Nofsinger R., Alaynick W. A., Chong L. W., Rosenfeld J. M., Evans R. M. 2006. Peroxisome proliferator-activated receptor delta promotes very low-density lipoprotein-derived fatty acid catabolism in the macrophage. Proc. Natl. Acad. Sci. USA. 103: 2434–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziouzenkova O., Perrey S., Asatryan L., Hwang J., MacNaul K. L., Moller D. E., Rader D. J., Sevanian A., Zechner R., Hoefler G., et al. 2003. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase. Proc. Natl. Acad. Sci. USA. 100: 2730–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed W., Orasanu G., Nehra V., Asatryan L., Rader D. J., Ziouzenkova O., Plutzky J. 2006. High-density lipoprotein hydrolysis by endothelial lipase activates PPARalpha: a candidate mechanism for high-density lipoprotein-mediated repression of leukocyte adhesion. Circ. Res. 98: 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Augustus A. S., Buchanan J., Park T. S., Hirata K., Noh H. L., Sun J., Homma S., D'Armiento J., Abel E. D., Goldberg I. J. 2006. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J. Biol. Chem. 281: 8716–8723. [DOI] [PubMed] [Google Scholar]
- 9.Doi M., Kondo Y., Tsutsumi K. 2003. Lipoprotein lipase activator NO-1886 (ibrolipim) accelerates the mRNA expression of fatty acid oxidation-related enzymes in rat liver. Metabolism. 52: 1547–1550. [DOI] [PubMed] [Google Scholar]
- 10.Spector A. A., Fletcher J. E., Ashbrook J. D. 1971. Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry. 10: 3229–3232. [DOI] [PubMed] [Google Scholar]
- 11.Seo T., Al-Haideri M., Treskova E., Worgall T. S., Kako Y., Goldberg I. J., Deckelbaum R. J. 2000. Lipoprotein lipase-mediated selective uptake from low density lipoprotein requires cell surface proteoglycans and is independent of scavenger receptor class B type 1. J. Biol. Chem. 275: 30355–30362. [DOI] [PubMed] [Google Scholar]
- 12.Sorrentino D., Robinson R. B., Kiang C. L., Berk P. D. 1989. At physiologic albumin/oleate concentrations oleate uptake by isolated hepatocytes, cardiac myocytes, and adipocytes is a saturable function of the unbound oleate concentration. Uptake kinetics are consistent with the conventional theory. J. Clin. Invest. 84: 1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richieri G. V., Kleinfeld A. M. 1995. Unbound free fatty acid levels in human serum. J. Lipid Res. 36: 229–240. [PubMed] [Google Scholar]
- 14.Stump D. D., Nunes R. M., Sorrentino D., Isola L. M., Berk P. D. 1992. Characteristics of oleate binding to liver plasma membranes and its uptake by isolated hepatocytes. J. Hepatol. 16: 304–315. [DOI] [PubMed] [Google Scholar]
- 15.Duncan J. G., Bharadwaj K. G., Fong J. L., Mitra R., Sambandam N., Courtois M. R., Lavine K. J., Goldberg I. J., Kelly D. P. 2010. Rescue of cardiomyopathy in peroxisome proliferator-activated receptor-alpha transgenic mice by deletion of lipoprotein lipase identifies sources of cardiac lipids and peroxisome proliferator-activated receptor-alpha activators. Circulation. 121: 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J., Sambandam N., Han X., Gross R. W., Courtois M., Kovacs A., Febbraio M., Finck B. N., Kelly D. P. 2007. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ. Res. 100: 1208–1217. [DOI] [PubMed] [Google Scholar]
- 17.Teusink B., Voshol P. J., Dahlmans V. E., Rensen P. C., Pijl H., Romijn J. A., Havekes L. M. 2003. Contribution of fatty acids released from lipolysis of plasma triglycerides to total plasma fatty acid flux and tissue-specific fatty acid uptake. Diabetes. 52: 614–620. [DOI] [PubMed] [Google Scholar]
- 18.Augustus A., Yagyu H., Haemmerle G., Bensadoun A., Vikramadithyan R. K., Park S. Y., Kim J. K., Zechner R., Goldberg I. J. 2004. Cardiac-specific knock-out of lipoprotein lipase alters plasma lipoprotein triglyceride metabolism and cardiac gene expression. J. Biol. Chem. 279: 25050–25057. [DOI] [PubMed] [Google Scholar]
- 19.Voshol P. J., Rensen P. C., van Dijk K. W., Romijn J. A., Havekes L. M. 2009. Effect of plasma triglyceride metabolism on lipid storage in adipose tissue: studies using genetically engineered mouse models. Biochim. Biophys. Acta. 1791: 479–485. [DOI] [PubMed] [Google Scholar]
- 20.Leone T. C., Weinheimer C. J., Kelly D. P. 1999. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc. Natl. Acad. Sci. USA. 96: 7473–7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kersten S., Seydoux J., Peters J. M., Gonzalez F. J., Desvergne B., Wahli W. 1999. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 103: 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston T. P., Waxman D. J. 2008. The induction of atherogenic dyslipidaemia in poloxamer 407-treated mice is not mediated through PPARalpha. J. Pharm. Pharmacol. 60: 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston T. P., Palmer W. K. 1993. Mechanism of poloxamer 407-induced hypertriglyceridemia in the rat. Biochem. Pharmacol. 46: 1037–1042. [DOI] [PubMed] [Google Scholar]
- 24.Pillutla P., Hwang Y. C., Augustus A., Yokoyama M., Yagyu H., Johnston T. P., Kaneko M., Ramasamy R., Goldberg I. J. 2005. Perfusion of hearts with triglyceride-rich particles reproduces the metabolic abnormalities in lipotoxic cardiomyopathy. Am. J. Physiol. Endocrinol. Metab. 288: E1229–E1235. [DOI] [PubMed] [Google Scholar]
- 25.Augustus A. S., Kako Y., Yagyu H., Goldberg I. J. 2003. Routes of FA delivery to cardiac muscle: modulation of lipoprotein lipolysis alters uptake of TG-derived FA. Am. J. Physiol. Endocrinol. Metab. 284: E331–E339. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahimi A., Bonen A., Blinn W. D., Hajri T., Li X., Zhong K., Cameron R., Abumrad N. A. 1999. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J. Biol. Chem. 274: 26761–26766. [DOI] [PubMed] [Google Scholar]
- 27.Levak-Frank S., Radner H., Walsh A., Stollberger R., Knipping G., Hoefler G., Sattler W., Weinstock P. H., Breslow J. L., Zechner R. 1995. Muscle-specific overexpression of lipoprotein lipase causes a severe myopathy characterized by proliferation of mitochondria and peroxisomes in transgenic mice. J. Clin. Invest. 96: 976–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Roves P., Huss J. M., Han D. H., Hancock C. R., Iglesias-Gutierrez E., Chen M., Holloszy J. O. 2007. Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc. Natl. Acad. Sci. USA. 104: 10709–10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kratky D., Strauss J. G., Zechner R. 2001. Tissue-specific activity of lipoprotein lipase in skeletal muscle regulates the expression of uncoupling protein 3 in transgenic mouse models. Biochem. J. 355: 647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakravarthy M. V., Pan Z., Zhu Y., Tordjman K., Schneider J. G., Coleman T., Turk J., Semenkovich C. F. 2005. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 1: 309–322. [DOI] [PubMed] [Google Scholar]
- 31.Chakravarthy M. V., Lodhi I. J., Yin L., Malapaka R. R., Xu H. E., Turk J., Semenkovich C. F. 2009. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell. 138: 476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao J., DeMayo F. J., Li H., Abu-Elheiga L., Gu Z., Shaikenov T. E., Kordari P., Chirala S. S., Heird W. C., Wakil S. J. 2006. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc. Natl. Acad. Sci. USA. 103: 8552–8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sapiro J. M., Mashek M. T., Greenberg A. S., Mashek D. G. 2009. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J. Lipid Res. 50: 1621–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacks F. M., Alaupovic P., Moye L. A., Cole T. G., Sussex B., Stampfer M. J., Pfeffer M. A., Braunwald E. 2000. VLDL, apolipoproteins B, CIII, and E, and risk of recurrent coronary events in the Cholesterol and Recurrent Events (CARE) trial. Circulation. 102: 1886–1892. [DOI] [PubMed] [Google Scholar]
- 35.Jong M. C., Hofker M. H., Havekes L. M. 1999. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler. Thromb. Vasc. Biol. 19: 472–484. [DOI] [PubMed] [Google Scholar]
- 36.Kawakami A., Osaka M., Aikawa M., Uematsu S., Akira S., Libby P., Shimokado K., Sacks F. M., Yoshida M. 2008. Toll-like receptor 2 mediates apolipoprotein CIII-induced monocyte activation. Circ. Res. 103: 1402–1409. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Ziouzenkova O., Plutzky J. 2004. Lipolytic PPAR activation: new insights into the intersection of triglycerides and inflammation? Curr. Opin. Clin. Nutr. Metab. Care. 7: 369–375. [DOI] [PubMed] [Google Scholar]