Sex Steroid Hormones in Older Men: Longitudinal Associations with 4.5-Year Change in Hip Bone Mineral Density—The Osteoporotic Fractures in Men Study (original) (raw)
Abstract
Context: There is limited information on the association between sex hormones and bone loss in older men.
Objective: Our objective was to determine the longitudinal association between sex steroid hormones and bone mineral density (BMD).
Design and Setting: We conducted a prospective study of 5995 men aged at least 65 yr old at six U.S. clinical centers.
Participants: Sex steroid hormones were measured in a random sample of 1602 men. After exclusions, 1238 men were included in cross-sectional analyses and 969 in longitudinal analyses. Baseline sex hormones were measured using liquid chromatography-mass spectrometry. Bioavailable (Bio) estradiol (BioE2) and testosterone (BioT) were calculated from mass action equations. SHBG was measured using chemiluminescent substrate.
Main Outcome Measures: BMD of the total hip, measured at baseline and once or twice afterward over 4.6 yr of follow-up, was evaluated.
Results: The annualized percent change in hip BMD increased with decreasing BioE2 (P trend = 0.03). Men with the lowest BioE2 (<39.7 pmol/liter) compared with the highest BioE2 (≥66.0 pmol/liter) experienced 38% faster rate of BMD loss (P < 0.05). There was no association between BioT and hip BMD loss. Men with lowest BioE2, lowest BioT, and highest SHBG experienced a 3-fold faster rate of BMD loss compared with men with higher levels (P = 0.02). A threshold effect of SHBG was observed; the rate of hip BMD loss increased in men with SHBG of 49–60 nm.
Conclusions: Low BioE2 and high SHBG levels were associated with lower BMD and faster hip BMD loss. The combination of low BioE2, low BioT, and high SHBG was associated with significantly faster rates of BMD loss.
Low bioavailable estradiol and high sex hormone binding globulin are independently associated with lower bone mineral density (BMD) and faster rates of BMD loss.
Population studies of older men have reported positive correlations between total estradiol, in particular, bioavailable estradiol (BioE2) and bone mineral density (BMD) (1,2,3,4,5). Testosterone has been shown to be correlated with BMD in some (4,6,7) but not all studies (8,9,10). Low total and BioE2 concentrations have also been linked with faster rates of bone loss in older men (11,12,13,14). There was no association between total or bioavailable testosterone (BioT) concentrations and change in BMD (12). Similarly, the rate of increase in BMD in younger men (age 22–39) was correlated with total and BioE2 but not with total or BioT (11). A threshold level for BioE2 below which aging men begin to lose bone was suggested to be approximately 40 pmol/liter (11pg/ml) (11,15).
The combination of low BioE2, low BioT, and high SHBG has been associated with a 3-fold increased risk of fractures in men enrolled in the Osteoporotic Fractures in Men Study (MrOS) (16). High SHBG may be linked to an increased fracture risk because the concentrations of free and bioavailable hormones will be lower. High SHBG may also directly influence fracture via receptor-mediated mechanisms or the interaction of sex steroid hormones with cellular receptors (17,18,19).
To further explore the cross-sectional and longitudinal association of sex steroid hormones with areal BMD and rates of BMD loss, we analyzed data from a random sample of men enrolled in MrOS.
Subjects and Methods
Study population
The MrOS enrolled 5995 participants as previously described (20,21). Eligible participants were at least 65 yr of age and ambulatory and had not had bilateral hip replacement surgery. The Institutional Review Board approved the study protocol, and written informed consent was obtained.
Analytic sample
A random sample of 1602 men was chosen for these analyses. We excluded men reporting osteoporosis medication, oral corticosteroids, and hormone therapy at baseline, leaving a sample of 1340 men (84%). Of these, 1238 had complete sex hormone data and formed the analytical sample for the cross-sectional analyses. A total of 1234 (77%) of the men in the random sample had repeat BMD. Of these, 969 (78%) were not taking any of the exclusion medications and formed the analytical sample for the longitudinal analyses.
Sex steroid measurements
At baseline, fasting morning blood was collected; serum was stored at −70 C. A combined gas chromatographic negative ionization tandem mass spectrometry and liquid chromatographic electrospray tandem mass spectrometry bioanalytical method was used to measure testosterone, estradiol, and estrone (Taylor Technology, Princeton NJ). The ranges of detection for estradiol were 0.625–80 pg/ml, for estrone were 1.56–200 pg/ml, and for testosterone were 2.5–320 ng/dl. Duplicate aliquots were assayed and averaged. The intraassay and interassay coefficients of variation (CV), respectively, were 2.5 and 6% for testosterone, 6.4 and 10.1% for estradiol, and 5.2 and 12.9% for estrone. SHBG concentration was determined on an Immulite analyzer with chemiluminescent substrate (Diagnostic Products Corp., Los Angeles, CA). The standard curve ranged from 0.2–180 nmol/liter/I with intraassay CV of 4.6% and interassay CV of 5.8%. Calculation of the free and bioavailable fractions of testosterone and estradiol used the methods described by Södergård et al. (22).
Measurement of BMD, bone mineral content (BMC), and total hip area
BMD (grams per square centimeter), BMC, and area of the total hip were measured using dual-energy x-ray absorptiometry (DXA) (QDR 4500 W; Hologic Inc., Bedford, MA.). Centralized quality control procedures, certification of DXA operators, and standardized procedures for scanning were used to ensure reproducibility of DXA measurements. At baseline, hip phantoms were circulated to all clinical sites. The inter-scanner CV was 0.9%. To adjust for inter-clinic differences, statistical models include indicator variables for the individual scanners. Each clinic scanned a spine and hip phantom throughout the study to monitor longitudinal changes in measures of bone density and content, and correction factors were applied to participant data as appropriate. The precision of DXA scans of the hip was 1–2% (23).
Total hip BMD was measured at baseline and at a follow-up visit for an ancillary sleep study (an average of 3.4 yr after baseline) and/or second visit for the main cohort (an average of 4.6 yr after baseline). Men were included in the longitudinal analyses if they had a total hip BMD measurement at the sleep study visit, visit 2, or both.
Other measures
Race/ethnicity, smoking, alcohol consumption, medical and fracture history, self-reported health status, and height at age 25 were determined by questionnaire at baseline. The Physical Activity Score for the Elderly (PASE) (24) was used to assess physical activity level. Height (centimeters) was measured on Harpenden stadiometers and weight (kilograms) on standard balance beam or digital scales, with participants wearing light clothing without shoes. Body mass index (BMI) was calculated as kilograms per square meter. Weight change was calculated by subtracting baseline weight from the follow-up weight. Grip strength was measured by a hand-held Jamar (Sammons Preston Rolyan, Bolingbrook, IL) dynamometer in both right and left arms. The average grip strength was used in the analyses. Information on calcium intake was obtained using the modified Block Food Frequency questionnaire (25).
Statistical analyses
We compared the characteristics of the men across quintiles of BioE2 using either analyses of variance or Kruskal-Wallis tests (continuous variables) or χ2 (categorical variables). We calculated simple Pearson correlations and partial correlations adjusting for age and weight between the sex hormone and skeletal measures. We used least-square means to examine the age, race, clinic, and weight-adjusted mean total hip BMD, BMC, and area across quintiles of the sex hormones. Linear regression models were used to compare each quintile of the sex hormone to the reference quintile (quintile 5 for estradiol and quintile 1 for testosterone and SHBG). The BioE2 models were further adjusted for estrone to test whether BioE2 effects were independent of estrone.
Longitudinal statistical methods
Random-effects regression models were used to examine the association between baseline sex hormone levels and changes in BMD, BMC, and area. Random-effects models account for between-subject variation and within-subject correlations between repeated bone measurements. Model coefficients were estimated using the restricted maximum likelihood method. Time was modeled as a continuous covariate, measured as the number of years from the baseline to follow-up bone measurements. A quadratic term for time was also considered to account for a nonlinear time trend; in all models, the quadratic time terms were not significant, so time was modeled linearly. Fixed effects included age, race, clinical center, weight at baseline, and weight change since baseline. Change in bone is reported as annualized percent change. The mean change for each quintile of the sex hormone measures was compared with the mean for the fifth quintile, and the P value for trend across the quintiles was determined.
The mixed-model estimates were used to estimate BMD at each year of follow-up by quintile of baseline BioE2, BioT, and SHBG and plotted to visualize changes over time.
We used restricted cubic splines to determine whether the relationship between BMD loss and BioE2, BioT, or SHBG was nonlinear and whether a threshold existed (26,27). We placed knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles of centered BioE2, BioT, and SHBG and calculated three cubic terms for each participant. To fit the spline and determine whether the sex hormones were associated with BMD loss, interactions of each of the cubic spline predictors with time as well as the individual cubic spline terms were entered in mixed models. The −2 log likelihood (−2LL) for this model was compared with the −2LL for the model without the interactions between the spline terms and time. Differences in −2LL were compared against a χ2 with three degrees of freedom to determine whether the spline fit was significantly better than the linear fit. If the difference in the −2LL was significant, the spline curve for BMD loss vs. the sex hormone was plotted to determine whether it suggested a threshold for BMD loss.
Mixed models were also used to evaluate combinations of BioE2, BioT, and SHBG on annualized rates of BMD loss. We dichotomized BioE2 and BioT at the lowest quintile and SHBG at the highest quintile and examined the annualized rate of hip BMD loss in eight mutually exclusive categories.
All analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Results
Men were on average 73.5 yr; 90% were Caucasian, and 85% reported good to excellent health (Table 1). Self-reported prevalence of medical conditions was generally low except for hypertension and osteoarthritis. There was no difference in age or race across BioE2 quintiles. Body weight and BMI were higher with higher BioE2. Despite the low prevalence of current smoking, fewer men with the highest BioE2 reported smoking. Men with higher BioE2 had better grip strength, but the absolute difference was small. There was no difference in height, height loss since age 25, health status, physical activity, alcohol consumption, calcium intake, or fracture history across quintiles of BioE2. Men with higher BioE2 were more likely to report hypertension, but there was little difference in other self-reported medical conditions. Further adjustment for body weight had little effect on these results.
Table 1.
Baseline characteristics in overall cohort and across quintiles of BioE2
| Characteristics | Baseline BioE2 | P value | |||||
|---|---|---|---|---|---|---|---|
| Overall (n = 1238) | Quintile 1 (n = 247) | Quintile 2 (n = 248) | Quintile 3 (n = 247) | Quintile 4 (n = 248) | Quintile 5 (n = 248) | ||
| Age (yr), mean ± sd | 73.5 ± 5.8 | 74.1 ± 5.9 | 73.4 ± 5.8 | 73.6 ± 6.0 | 73.2 ± 5.6 | 73.3 ± 5.6 | 0.40 |
| Race/ethnicity, n (%) | |||||||
| Caucasian | 1117 (90.2) | 225 (91.1) | 223 (89.9) | 223 (90.3) | 222 (89.5) | 224 (90.3) | 0.76 |
| African-American | 46 (3.7) | 8 (3.2) | 8 (3.2) | 10 (4.1) | 7 (2.8) | 13 (5.2) | |
| Asian/Hispanic/Other | 75 (6.1) | 14 (5.7) | 17 (6.9) | 14 (5.7) | 19 (7.7) | 11 (4.4) | |
| Weight (kg), mean ± sd | 83.8 ± 13.0 | 81.6 ± 13.0 | 83.9 ± 12.4 | 82.8 ± 12.7 | 84.2 ± 13.6 | 86.4 ± 13.1 | 0.0009 |
| Height (cm), mean ± sd | 174.4 ± 6.9 | 174.4 ± 7.2 | 174.7 ± 6.7 | 174.2 ± 7.5 | 174.7 ± 6.7 | 174.2 ± 6.6 | 0.80 |
| BMI (kg/m2), mean ± sd | 27.5 ± 3.7 | 26.8 ± 3.8 | 27.4 ± 3.5 | 27.3 ± 3.4 | 27.5 ± 3.8 | 28.5 ± 4.0 | <0.0001 |
| Height loss since age 25 (cm), mean ± sd | 3.6 ± 3.0 | 3.8 ± 3.6 | 3.8 ± 3.4 | 3.6 ± 2.7 | 3.5 ± 2.2 | 3.5 ± 2.8 | 0.69 |
| Excellent, good self-reported health, n (%) | 1058 (85.5) | 207 (83.8) | 212 (85.8) | 206 (83.4) | 215 (86.7) | 218 (87.9) | 0.57 |
| PASE score, mean ± sd | 147.4 ± 68.3 | 144.7 ± 69.1 | 151.9 ± 69.1 | 145.5 ± 71.2 | 152.5 ± 71.1 | 142.1 ± 60.3 | 0.34 |
| Smoking status, n (%) | 0.009 | ||||||
| Never | 450 (36.4) | 76 (30.8) | 94 (37.9) | 73 (29.6) | 98 (39.5) | 109 (44.0) | |
| Past | 735 (59.4) | 155 (62.7) | 141 (56.9) | 164 (66.4) | 143 (57.7) | 132 (53.2) | |
| Current | 53 (4.3) | 16 (6.5) | 13 (5.2) | 10 (4.1) | 7 (2.8) | 7 (2.8) | |
| Alcohol (no. drinks/wk), mean ± sd | 4.6 ± 7.3 | 3.9 ± 6.8 | 4.2 ± 6.3 | 5.6 ± 8.7 | 4.8 ± 7.5 | 4.6 ± 7.0 | 0.12 |
| Dietary calcium intake (mg/d), mean ± sd | 794.5 ± 385.7 | 799.9 ± 386.7 | 786.7 ± 397.8 | 780.0 ± 370.7 | 804.9 ± 370.0 | 801.0 ± 404.6 | 0.95 |
| History of any fracture after age 50, n (%) | 270 (21.9) | 62 (25.1) | 57 (23.1) | 47 (19.1) | 57 (23.2) | 47 (19.0) | 0.38 |
| Maternal history of fracture, n (%) | 264 (21.3) | 53 (21.5) | 57 (23.0) | 55 (22.3) | 56 (22.6) | 43 (17.3) | 0.54 |
| Average grip strength (kg), mean ± sd | 38.8 ± 7.7 | 37.2 ± 7.7 | 39.4 ± 7.8 | 38.9 ± 7.3 | 39.4 ± 7.9 | 39.0 ± 7.9 | 0.009 |
| History of COPD, n (%) | 135 (10.9) | 28 (11.3) | 35 (14.1) | 21 (8.5) | 25 (10.1) | 26 (10.5) | 0.36 |
| History of diabetes, n (%) | 198 (16.0) | 40 (16.3) | 37 (14.9) | 34 (13.8) | 38 (15.4) | 49 (19.8) | 0.44 |
| History of kidney stones, n (%) | 169 (13.7) | 25 (10.1) | 36 (14.5) | 32 (13.0) | 42 (16.9) | 34 (13.7) | 0.27 |
| History of hypertension, n (%) | 551 (44.5) | 93 (37.7) | 106 (42.7) | 123 (49.8) | 100 (40.3) | 129 (52.0) | 0.004 |
| History of prostate cancer, n (%) | 67 (5.4) | 12 (4.9) | 12 (4.8) | 20 (8.1) | 11 (4.4) | 12 (4.8) | 0.35 |
| History of osteoarthritis, n (%) | 247 (20.0) | 42 (17.0) | 47 (19.0) | 51 (20.7) | 53 (21.4) | 54 (21.8) | 0.66 |
| Total estradiol (pg/ml), mean ± sd | 22.8 ± 7.4 | 14.3 ± 3.4 | 18.9 ± 3.0 | 22.4 ± 3.3 | 25.8 ± 4.3 | 32.5 ± 5.8 | <0.0001 |
| Total estradiol (pmol/liter), mean ± sd | 83.6 ± 27.3 | 52.3 ± 12.6 | 69.4 ± 11.1 | 82.1 ± 12.0 | 94.6 ± 15.7 | 119.4 ± 21.4 | <0.0001 |
| BioE2 (pg/ml), mean ± sd | 14.6 ± 4.6 | 8.8 ± 1.8 | 12.0 ± 0.7 | 14.2 ± 0.6 | 16.4 ± 0.7 | 21.3 ± 3.4 | <0.0001 |
| BioE2 (pmol/liter), mean ± sd | 53.4 ± 16.8 | 32.4 ± 6.6 | 44.2 ± 2.4 | 52.0 ± 2.3 | 60.2 ± 2.7 | 78.2 ± 12.5 | <0.0001 |
| Estrone (pg/ml), mean ± sd | 35.5 ± 12.3 | 23.7 ± 7.9 | 29.0 ± 8.1 | 33.6 ± 10.1 | 37.5 ± 10.7 | 43.6 ± 13.2 | <0.0001 |
| Estrone (pmol/liter), mean ± sd | 123.9 ± 45.4 | 87.6 ± 29.1 | 107.3 ± 30.1 | 124.4 ± 37.3 | 138.6 ± 39.5 | 161.4 ± 48.9 | <0.0001 |
| Total testosterone (ng/dl), mean ± sd | 409.3 ± 155.9 | 351.6 ± 133.7 | 391.9 ± 150.8 | 419.5 ± 142.7 | 431.6 ± 172.6 | 451.9 ± 158.1 | <0.0001 |
| Total testosterone (nmol/liter), mean ± sd | 14.2 ± 5.4 | 12.2 ± 4.6 | 13.6 ± 5.2 | 14.5 ± 5.0 | 15.0 ± 6.0 | 15.7 ± 5.5 | <0.0001 |
| BioT (ng/dl), mean ± sd | 206.4 ± 62.5 | 169.5 ± 53.9 | 196.2 ± 55.5 | 210.8 ± 54.5 | 217.5 ± 60.1 | 237.9 ± 66.4 | <0.0001 |
| BioT (nmol/liter), mean ± sd | 7.2 ± 2.2 | 5.9 ± 1.9 | 6.8 ± 1.9 | 7.3 ± 1.9 | 7.6 ± 2.1 | 8.3 ± 2.3 | <0.0001 |
| SHBG (nmol/liter) mean ± sd | 49.4 ± 19.3 | 53.0 ± 20.1 | 49.0 ± 19.7 | 49.9 ± 19.2 | 49.2 ± 20.9 | 46.0 ± 15.5 | 0.002 |
Total estradiol (r = 0.89) and estrone (r = 0.60) were positively correlated with BioE2. Total testosterone (r = 0.22) and BioT (r = 0.39) were modestly positively correlated with BioE2. SHBG was weakly inversely correlated with BioE2 (r = −0.14) and positively correlated with BioT (r = 0.22).
Cross-sectional results
The estrogen measures were all positively correlated and SHBG, negatively correlated with BMD with little association with testosterone after adjusting for age and weight (Table 2). The sex hormone-BMC correlation was weaker. Estradiol was negatively correlated and testosterone and SHBG, positively correlated with bone area. Examination of the correlations between the skeletal outcomes and BioE2 stratified at our median value (<52 pmol/liter) did not reveal any important differences in the correlations (data not shown).
Table 2.
Correlation between sex hormones and total hip BMD, BMC, and area
| Total hip BMD | Total hip BMC | Total hip area | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Age and weight adjusted | Unadjusted | Age and weight adjusted | Unadjusted | Age and weight adjusted | |
| Total estradiol | 0.08b | 0.09b | 0.04 | 0.04 | −0.04 | −0.05 |
| BioE2 | 0.17d | 0.14d | 0.11c | 0.06a | −0.06a | −0.09b |
| Estrone | 0.09b | 0.10c | 0.04 | 0.04 | −0.07a | −0.08b |
| Total testosterone | −0.16d | −0.06a | −0.12d | 0.01 | 0.002 | 0.11d |
| BioT | −0.07a | 0.002 | −0.06a | 0.07a | −0.005 | 0.11d |
| SHBG | −0.20d | −0.11d | −0.15d | −0.05 | 0.03 | 0.08b |
Total hip BMD was higher with increasing total estradiol, BioE2, and estrone but lower with higher SHBG (Table 3). Total hip BMD was 5.8% higher in men in quintile 5 vs. 1 of BioE2 and 4.5% higher in quintile 1 vs. 5 of SHBG. There was no association between testosterone and BMD. Total hip BMC was higher with greater estradiol concentrations, but the magnitude of the association was weaker than observed for BMD. The mean total hip bone area was 2.5% lower in men with the highest BioE2 compared with the lowest. In contrast, mean total hip bone area was 2.4% higher in men with the highest BioT compared with the lowest. Further adjustment of all of the estradiol models with testosterone or SHBG levels tended to strengthen these associations (data not shown). Additional adjustment of the BioE2 models for estrone had little effect on our results (data not shown).
Table 3.
Adjusted mean baseline total hip BMD, BMC, and area by quintile of sex hormone measures
| Quintile | P value for trend | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Total estradiol | ||||||
| BMD (g/cm2) | 0.940c | 0.956a | 0.965 | 0.959 | 0.979 | 0.002 |
| BMC (g) | 41.26 | 41.71 | 42.39 | 41.37 | 42.25 | 0.22 |
| Area (cm2) | 43.86a | 43.56 | 43.89a | 43.14 | 43.16 | 0.02 |
| BioE2 | ||||||
| BMD (g/cm2) | 0.933d | 0.954b | 0.955b | 0.970 | 0.987 | <0.0001 |
| BMC (g) | 41.18a | 41.69 | 41.51 | 42.25 | 42.37 | 0.03 |
| Area (cm2) | 44.08b | 43.62 | 43.39 | 43.54 | 42.97 | 0.004 |
| Estrone | ||||||
| BMD (g/cm2) | 0.943c | 0.944c | 0.969 | 0.954b | 0.986 | 0.0002 |
| BMC (g) | 41.60 | 41.42a | 42.17 | 41.08b | 42.71 | 0.15 |
| Area (cm2) | 44.04a | 43.84 | 43.43 | 43.03 | 43.30 | 0.004 |
| Total testosterone | ||||||
| BMD (g/cm2) | 0.962 | 0.971 | 0.959 | 0.961 | 0.946 | 0.11 |
| BMC (g) | 41.30 | 41.76 | 41.93 | 42.28 | 41.73 | 0.33 |
| Area (cm2) | 42.88 | 43.01 | 43.72a | 43.95b | 44.05b | 0.0001 |
| BioT | ||||||
| BMD (g/cm2) | 0.957 | 0.959 | 0.956 | 0.973 | 0.954 | 0.76 |
| BMC (g) | 41.24 | 41.43 | 41.60 | 42.56a | 42.16b | 0.03 |
| Area (cm2) | 43.11 | 43.15 | 43.43 | 43.76 | 44.16 | 0.001 |
| SHBG | ||||||
| BMD (g/cm2) | 0.974 | 0.963 | 0.957 | 0.973 | 0.932c | 0.007 |
| BMC (g) | 41.53 | 42.25 | 41.39 | 42.61 | 41.21 | 0.85 |
| Area (cm2) | 42.61 | 43.87c | 43.24 | 43.77b | 44.10d | 0.0004 |
Longitudinal results
The annualized rates of change in total hip BMD, BMC, and area across quintiles of sex steroid hormones are summarized in Table 4. For total hip BMD, the annualized rate of BMD loss increased with decreasing BioE2 (P trend = 0.03). Men with lowest compared with the highest BioE2 experienced a 38% faster rate of bone loss, and the difference between quintile 1 and 5 was statistically significant (P = 0.05). There was no association between total estradiol, estrone, total testosterone, or BioT and rate of hip BMD loss. The annualized change in BMD was more than 2-fold faster in men with the highest compared with the lowest SHBG (P = 0.003), and BMD loss increased with greater SHBG (P trend = 0.002).
Table 4.
Adjusted mean total hip BMD loss, BMC loss, and area gain by quintile of baseline sex hormone measures
| Sex hormone measure | Total hip BMD loss | Total hip BMC loss | Total hip area gain | |||
|---|---|---|---|---|---|---|
| Loss (%/yr) | P value | Loss (%/yr) | P value | Gain (%/yr) | P value | |
| Total estradiol quintiles | 0.43 | 0.04 | 0.01 | |||
| 1 (<61.3 pmol/liter) | −0.380 | 0.96 | −0.295 | 0.25 | 0.129 | 0.06 |
| 2 (61.3–74.2 pmol/liter) | −0.421 | 0.60 | −0.334 | 0.12 | 0.121 | 0.04 |
| 3 (74.2–87.0 pmol/liter) | −0.267 | 0.18 | −0.140 | 0.83 | 0.188 | 0.32 |
| 4 (87.0–104.6 pmol/liter) | −0.269 | 0.18 | −0.088 | 0.47 | 0.223 | 0.64 |
| 5 (≥104.6 pmol/liter) | −0.369 | Reference | −0.164 | Reference | 0.254 | Reference |
| BioE2 quintiles | 0.03 | 0.009 | 0.14 | |||
| 1 (<39.7 pmol/liter) | −0.472 | 0.05 | −0.371 | 0.02 | 0.134 | 0.11 |
| 2 (39.7–48.1 pmol/liter) | −0.338 | 0.67 | −0.199 | 0.37 | 0.189 | 0.43 |
| 3 (48.1–56.0 pmol/liter) | −0.374 | 0.37 | −0.276 | 0.10 | 0.158 | 0.20 |
| 4 (56.0–66.0 pmol/liter) | −0.235 | 0.45 | −0.081 | 0.85 | 0.190 | 0.44 |
| 5 (≥66.0 pmol/liter) | −0.293 | Reference | −0.101 | Reference | 0.241 | Reference |
| Estrone quintiles | 0.43 | 0.02 | 0.007 | |||
| 1 (<86.2 pmol/liter) | −0.386 | 0.58 | −0.270 | 0.08 | 0.156 | 0.04 |
| 2 (86.2–106.5 pmol/liter) | −0.361 | 0.81 | −0.319 | 0.03 | 0.101 | 0.003 |
| 3 (106.5–127.6 pmol/liter) | −0.322 | 0.93 | −0.209 | 0.20 | 0.150 | 0.03 |
| 4 (127.6–159.1 pmol/liter) | −0.300 | 0.69 | −0.136 | 0.58 | 0.225 | 0.29 |
| 5 (≥159.1 pmol/liter) | −0.324 | Reference | −0.071 | Reference | 0.290 | Reference |
| Total testosterone quintiles | 0.73 | 0.66 | 0.11 | |||
| 1 (<9.81nmol/liter) | −0.338 | 0.90 | −0.227 | 0.57 | 0.152 | 0.26 |
| 2 (9.81–12.17 nmol/liter) | −0.319 | 0.90 | −0.209 | 0.70 | 0.157 | 0.28 |
| 3 (12.17–14.70 nmol/liter) | −0.298 | 0.67 | −0.191 | 0.81 | 0.149 | 0.25 |
| 4 (14.70–18.17 nmol/liter) | −0.405 | 0.38 | −0.222 | 0.59 | 0.234 | 0.82 |
| 5 (≥18.17 nmol/liter) | −0.336 | Reference | −0.165 | Reference | 0.220 | Reference |
| BioT quintiles | 0.17 | 0.21 | 0.45 | |||
| 1 (<5.43 nmol/liter) | −0.427 | 0.07 | −0.347 | 0.04 | 0.123 | 0.12 |
| 2 (5.43–6.56 nmol/liter) | −0.330 | 0.50 | −0.145 | 0.82 | 0.234 | 0.89 |
| 3 (6.56–7.47 nmol/liter) | −0.306 | 0.69 | −0.169 | 0.64 | 0.175 | 0.43 |
| 4 (7.47–8.78 nmol/liter) | −0.371 | 0.21 | −0.262 | 0.16 | 0.149 | 0.23 |
| 5 (≥8.78 nmol/liter) | −0.275 | Reference | −0.117 | Reference | 0.221 | Reference |
| SHBG quintiles | 0.002 | 0.06 | 0.24 | |||
| 1 (<33.3 nmol/liter) | −0.249 | 0.003 | −0.110 | 0.04 | 0.182 | 0.43 |
| 2 (33.3–42.6 nmol/liter) | −0.318 | 0.03 | −0.250 | 0.49 | 0.112 | 0.07 |
| 3 (42.6–50.9 nmol/liter) | −0.220 | 0.001 | −0.049 | 0.01 | 0.218 | 0.84 |
| 4 (50.9–62.9 nmol/liter) | −0.422 | 0.36 | −0.288 | 0.71 | 0.184 | 0.49 |
| 5 (≥62.9 nmol/liter) | −0.516 | Reference | −0.335 | Reference | 0.226 | Reference |
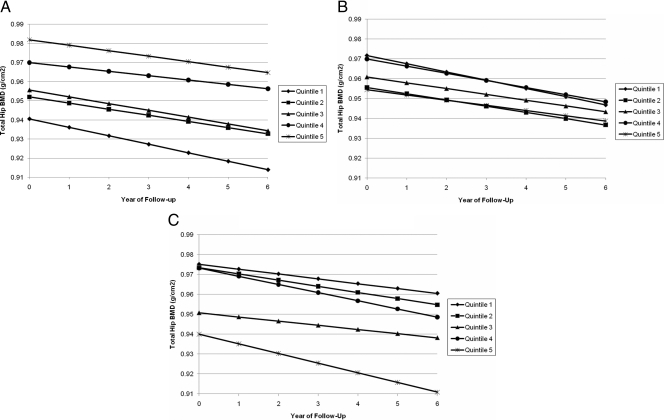
Changes in BMD across quintiles of BioE2, BioT, and SHBG are graphically shown in Fig. 1, A–C. As shown in Fig. 1A, total hip BMD was highest in men with the highest BioE2, but over time, total hip BMD declined in men within each quintile. There was some suggestion that the decline over time was greatest in men with the lowest BioE2 shown by the steeper slope for quintile 1. For BioT (Fig. 1B), total hip BMD declined over time in all men, irrespective of their BioT (P trend = 0.17). There was virtually no difference in the slope across the BioT quintiles, indicating that BMD loss was similar for all quintiles. The strongest association between sex hormone and annualized rate of change in BMD was observed for SHBG (Fig. 1C). In general, BMD loss increased as SHBG levels increased, shown by the steeper lines for the higher quintiles (P trend = 0.002).
Figure 1.
A, Change in total hip BMD by quintile of baseline bioavailable estradiol. Results have been adjusted for age, race, clinic, baseline weight, and weight change (P trend = 0.03). B, Change in total hip BMD by quintile of baseline bioavailable testosterone. Results have been adjusted for age, race, clinic, baseline weight, and weight change (P trend = 0.17). C, Change in total hip BMD by quintile of baseline SHBG. Results have been adjusted for age, race, clinic, baseline weight, and weight change.
The annualized rate of loss in total hip BMC was lower with higher levels of total estradiol, BioE2 and estrone (P trend all <0.05) (Table 4). Men with the lowest BioE2 experienced an annualized rate of loss in BMC that was more than three times faster than men with the highest BioE2. There was no association between total testosterone and BioT and total hip BMC loss. The annualized rate of total hip BMC loss was three times faster in men with the highest SHBG compared with the lowest SHBG (P = 0.04), with loss generally increasing with greater SHBG level (P trend = 0.06).
Total hip bone area generally increased over time, and the increase tended to be numerically higher in men with the greatest total estradiol, BioE2, and estrone, but the association was not significant for BioE2 (P trend = 0.14). There was no association between total testosterone, BioT, or SHBG and changes in bone area. Further adjustment of the estradiol models with testosterone, SHBG, or estrone had no effect (data not shown).
None of the three-way interactions (sex hormone × age × time and sex hormone × BMI × time) were statistically significant, suggesting that the association between sex steroid hormones and BMD loss did not differ by age or BMI (data not shown).
Threshold levels
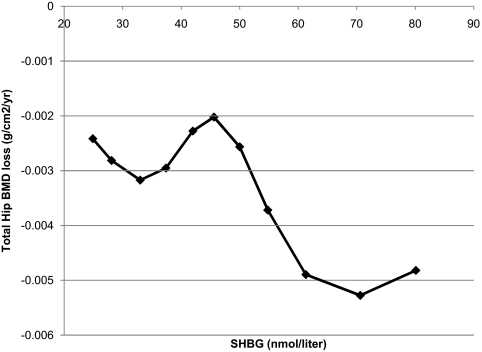
The cubic spline curves showed that the spline fit was not better than the linear fit for BioE2 or BioT. However, the spline fit was significantly better than the linear model for SHBG. As shown in Fig. 2, the rate of BMD loss was similar from the minimum value up to the mean SHBG (49 nm). Then the rate of BMD loss increased dramatically in men with SHBG of 49 nm to about the 80th percentile (60 nm).
Figure 2.
Spline fit of change in total hip BMD vs. baseline SHBG. Results have been adjusted for age, race, clinic, baseline weight, and weight change.
Combination effects of estradiol, testosterone, and SHBG on bone loss
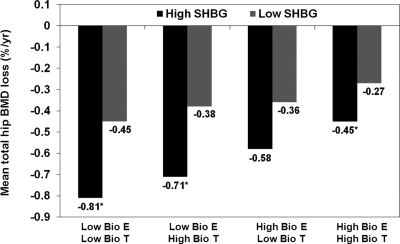
When the combined effects of sex steroids and SHBG were examined, men with the lowest SHBG experienced slower rates of BMD loss compared with men with higher SHBG, independent of circulating BioE2 and BioT (Fig. 3). Men with the lowest BioE2, lowest BioT, and highest SHBG experienced a 3-fold faster rate of bone loss.
Figure 3.
Combined effect of BioE2, BioT, and SHBG on total hip BMD loss. Results have been adjusted for age, race, clinic, baseline weight, and weight change. *, P < 0.05 vs. the referent group (high BioE2, high BioT, and low SHBG). The number of men in each category is as follows: 1) low BioE2, low BioT, high SHBG, n = 15; 2) low BioE2, low BioT, low SHBG, n = 43; 3) low BioE2, high BioT, high SHBG, n = 24; 4) low BioE2, high BioT, low SHBG, n = 95; 5) high BioE2, low BioT, high SHBG, n = 9; 6) high BioE2, low BioT, low SHBG, n = 107; 7) high BioE2, high BioT, high SHBG, n = 131; and 8) high BioE2, High BioT, low SHBG, n = 545.
Discussion
In this population-based study of sex steroid hormones, we found cross-sectional associations between total hip BMD and both total and BioE2, whereby men with the highest estradiol had about 6% higher BMD than men with the lowest estradiol. These associations were independent of age, race, and body weight and are consistent with previous research (1,2,3,4,5). Bioavailable estradiol concentrations were positively related to hip BMC. Total testosterone levels were unrelated to hip BMD, but hip bone area tended to be lower across increasing estradiol quintiles but higher with greater testosterone levels. Men with the highest SHBG had significantly lower BMD and larger bone area. There was no association of SHBG with BMC.
The positive cross-sectional association between circulating testosterone and bone area is consistent with data from rats that demonstrate that testosterone enhances periosteal apposition (28). Testosterone was also shown to be a positive predictor of cortical bone size in a study of about 1800 young Swedish men (29). Other studies have reported no association (30,31) or a negative association (32) between testosterone and bone size. The observation that testosterone was associated with higher bone area was not, however, supported by the longitudinal results where there was no association between testosterone and changes in bone area. It is possible that sex steroids track through life and during growth, higher testosterone levels are associated with larger bones, consistent with the cross-sectional results. But with aging, bone size changes appear to be more positively related to BioE2.
We found that the rate of annualized loss of hip BMD and BMC decreased with greater BioE2 levels, but there was no association between testosterone and annualized rates of BMD loss. Men with the highest SHBG experienced 2-fold and 3-fold faster rates of BMD and BMC loss, respectively, compared with men with the lowest SHBG. Total hip bone area tended to increase over time, but there was little evidence that this increase was related to sex steroid hormones or SHBG. Our results are consistent with previous studies showing an association between circulating estradiol, but not testosterone, with rates of bone loss (5,11,12,13,14). Khosla et al. (15) have suggested a threshold of bioavailable estradiol at approximately 40 pmol/liter. In the present study, men with the lowest (<39.7 pmol/liter) BioE2 lost significantly greater BMD and BMC in comparison with men with the highest BioE2. This quintile cutoff was very close to the published threshold. Above this level, the rate of BMD loss did not differ across quintiles 2–5. However, the spline analyses did not identify a significant threshold, perhaps because we measured areal BMD, a mix of cortical and trabecular BMD, and this threshold was most significant for cortical volumetric BMD (32). Our results are also consistent with a previous paper from MrOS Sweden (33) and MrOS U.S. (16) showing a threshold of estradiol for fracture risk, although the threshold level was slightly lower than that described for BMD. Other studies support a threshold level for BioE2 (12,34,35). Taken together, our results are consistent with the observation that a threshold for estradiol exists in older men, below which estradiol is associated with lower BMD, faster rates of bone loss, and increased risk of fractures.
We found a significant threshold for SHBG at the mean level of 49.4 nmol/liter. Men with SHBG above 49.4 nmol/liter, up to 60 nm, experienced significantly faster rates of BMD loss. This threshold is consistent with the MrOS Sweden where men with SHBG in the highest quartile (>52.5 nmol/liter) had an increased risk of fractures. In MrOS U.S., a threshold for SHBG and fracture risk was also identified, although the threshold was higher at more than 59.1 nmol/liter. The results suggest a nonlinear relationship between SHBG and rates of bone loss and fracture. A metaanalysis of all of these studies would be helpful in defining the threshold for BioE2 and SHBG.
We previously reported in MrOS that the proportion of men with total testosterone deficiency (testosterone <200 ng/dl, 6.9 nmol/liter) and estradiol deficiency (estradiol <10 pg/ml, 36.7 pmol/liter) was greater in men with hip BMD T-score below −2.5 (36). We also showed that the odds of rapid hip bone loss (annualized rate of hip bone loss >3% per year) was 3-fold higher in men who were testosterone deficient and 2-fold higher among men who were estrogen deficient. These results were based on a smaller sample of men with testosterone and estradiol deficiency. Because of concerns about the specificity of RIA, particularly at low estradiol levels, we repeated the sex steroid hormone measures using state of the art mass spectrometry with chromatography.
Previous analyses from MrOS suggested that the strongest association between sex steroids, SHBG, and fracture were in analyses when the combination of all three measures were considered (16). We found similar results for rates of BMD loss. Men who had the lowest BioE2, the lowest BioT, and the highest SHBG experienced an annualized rate of bone loss that was three times faster than men with high BioE2, high BioT, and low SHBG. These results suggest that each hormone plays a role in maintaining BMD longitudinally. Delineating each hormone’s role in maintaining BMD is complex because the bioavailable measures were derived from mass action equations that include SHBG. Nevertheless, future research should consider the role of each individual hormone and their interactions on maintaining BMD.
Estrone is a biologically weaker estrogen than estradiol but circulates at greater concentrations. Estrone was moderately correlated with BioE2, but the relative importance of estrone is unknown in comparison with BioE2. We found similar results between estrone and BioE2 and baseline BMD. Longitudinally, BioE2 was strongly linked with BMD loss, but estrone was not. The associations of BioE2 with BMD and BMD loss were independent of estrone, suggesting that BioE2 is the predominant estrogen in older men.
This study has a number of strengths. It is one of the largest to examine the association between sex steroids and rates of bone loss in community-dwelling men. Bone loss was measured over an average of 4.6 yr. Sex steroid hormones were measured using gas chromatography-mass spectrometry, the most sensitive method, which minimizes the inaccuracy at low concentrations. We adjusted for important covariates in our models and tested for thresholds using cubic spline analyses. Finally, we were one of the first to consider the effects of combinations of hormones and rates of BMD loss.
This study has several limitations. The cohort was primarily Caucasians; associations may differ in other ethnic groups. We did not have repeated measures of sex hormones and SHBG so cannot correlate changes in sex steroids with changes in BMD. Bone area using DXA scans is a crude measure of bone size, and in MrOS, the measure with the most in-person variability was bone area. Other imaging modalities, such as quantitative computed tomography, should be used to better understand changes in bone size. Finally, we adjusted for many covariates, but as in any observational study, there could be residual confounding.
In summary, men with low BioE2 had the lowest hip BMD and BMC and experienced the fastest rate of hip BMD loss. Testosterone levels alone were unrelated to BMD or changes in BMD. Our results are consistent for a threshold level of both BioE2 and SHBG for rates of bone loss. Finally, men with the combination of low BioE2, low BioT, and high SHBG experienced significantly faster rate of BMD loss.
Footnotes
The MrOS is supported by National Institutes of Health (NIH) funding. The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research provide support under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study, “Outcomes of Sleep Disorders in Older Men,” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
Disclosure Summary: J.A.C. has received research support from Novartis Pharmaceuticals. E.B.-C. has received grant support and/or consulting fees from Amgen, Eli Lilly and Co., Merck & Co., Inc., and Pfizer Pharmaceuticals. D.C.B., E.S.O., K.E.E., H.A.F., L.M., B.C.T., and S.K.E. report no conflicts.
First Published Online June 16, 2010
Abbreviations: BMC, Bone mineral content; BMD, bone mineral density; BMI, body mass index; BioE2, bioavailable estradiol; BioT, bioavailable testosterone; CV, coefficient of variation; DXA, dual-energy x-ray absorptiometry; −2LL, −2 log likelihood; MrOS, Osteoporotic Fractures in Men Study.
References
- Khosla S, Melton 3rd LJ, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL 1998 Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83:2266–2274 [DOI] [PubMed] [Google Scholar]
- Center JR, Nguyen TV, Sambrook PN, Eisman JA 1999 Hormonal and biochemical parameters in the determination of osteoporosis in elderly men. J Clin Endocrinol Metab 84:3626–3635 [DOI] [PubMed] [Google Scholar]
- Szulc P, Hofbauer LC, Heufelder AE, Roth S, Delmas PD 2001 Osteoprotegerin serum levels in men: correlation with age, estrogen, and testosterone status. J Clin Endocrinol Metab 86:3162–3165 [DOI] [PubMed] [Google Scholar]
- van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW 2000 Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab 85:3276–3282 [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Nesi B, Pratelli L, Cucinotta D, Bastagli L, Cavalli G 2000 Body composition, sex steroids, IGF-1, and bone mineral status in aging men. J Gerontol A Biol Sci Med Sci 55:M516–M521 [DOI] [PubMed] [Google Scholar]
- Kelly PJ, Pocock NA, Sambrook PN, Eisman JA 1990 Dietary calcium, sex hormones, and bone mineral density in men. BMJ 300:1361–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S, Khaw KT, Cassidy A, Compston JE 1993 Sex hormones and bone mineral density in elderly men. Bone Miner 20:133–140 [DOI] [PubMed] [Google Scholar]
- Paller CJ, Shiels MS, Rohrmann S, Basaria S, Rifai N, Nelson W, Platz EA, Dobs A 2009 Relationship of sex steroid hormones with bone mineral density (BMD) in a nationally representative sample of men. Clin Endocrinol (Oxf) 70:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchuk NO, van Schoor NM, Pluijm SM, Smit JH, de Ronde W, Lips P 2007 The association of sex hormone levels with quantitative ultrasound, bone mineral density, bone turnover and osteoporotic fractures in older men and women. Clin Endocrinol (Oxf) 67:295–303 [DOI] [PubMed] [Google Scholar]
- Araujo AB, Travison TG, Leder BZ, McKinlay JB 2008 Correlations between serum testosterone, estradiol, and sex hormone-binding globulin and bone mineral density in a diverse sample of men. J Clin Endocrinol Metab 93:2135–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Melton 3rd LJ, Atkinson EJ, O'Fallon WM 2001 Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab 86:3555–3561 [DOI] [PubMed] [Google Scholar]
- Gennari L, Merlotti D, Martini G, Gonnelli S, Franci B, Campagna S, Lucani B, Dal Canto N, Valenti R, Gennari C, Nuti R 2003 Longitudinal association between sex hormone levels, bone loss, and bone turnover in elderly men. J Clin Endocrinol Metab 88:5327–5333 [DOI] [PubMed] [Google Scholar]
- Van Pottelbergh I, Goemaere S, Kaufman JM 2003 Bioavailable estradiol and an aromatase gene polymorphism are determinants of bone mineral density changes in men over 70 years of age. J Clin Endocrinol Metab 88:3075–3081 [DOI] [PubMed] [Google Scholar]
- Bjørnerem A, Emaus N, Berntsen GK, Joakimsen RM, Fønnebø V, Wilsgaard T, Oian P, Seeman E, Straume B 2007 Circulating sex steroids, sex hormone-binding globulin, and longitudinal changes in forearm bone mineral density in postmenopausal women and men: the Tromso study. Calcif Tissue Int 81:65–72 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton 3rd LJ, Riggs BL 2002 Clinical review 144: Estrogen and the male skeleton. J Clin Endocrinol Metab 87:1443–1450 [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Nielson CM, Marshall LM, Lapidus JA, Barrett-Connor E, Ensrud KE, Hoffman AR, Laughlin G, Ohlsson C, Orwoll ES 2009 The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab 94:3337–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SM, Hryb DJ, Nakhla AM, Romas NA, Rosner W 2002 Sex hormone-binding globulin is synthesized in target cells. J Endocrinol 175:113–120 [DOI] [PubMed] [Google Scholar]
- Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA 1999 Androgen and estrogen signaling at the cell membrane via G-proteins and cyclic adenosine monophosphate. Steroids 64:100–106 [DOI] [PubMed] [Google Scholar]
- Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, Metzger J, Schweigert FJ, Luppa PB, Nykjaer A, Willnow TE 2005 Role of endocytosis in cellular uptake of sex steroids. Cell 122:751–762 [DOI] [PubMed] [Google Scholar]
- Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K 2005 Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study: a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26:569–585 [DOI] [PubMed] [Google Scholar]
- Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR 2005 Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 26:557–568 [DOI] [PubMed] [Google Scholar]
- Södergård R, Bäckström T, Shanbhag V, Carstensen H 1982 Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem 16:801–810 [DOI] [PubMed] [Google Scholar]
- Cawthon PM, Ewing SK, McCulloch CE, Ensrud KE, Cauley JA, Cummings SR, Orwoll ES; Osteoporotic Fractures in Men (MrOS) Research Group 2009 Loss of hip BMD in older men: the Osteoporotic Fractures in Men (MrOS) Study. J Bone Miner Res 24:1728–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn RA, Ficker JL 1999 Physical Activity Scale for the Elderly (PASE): the relationship with activity measured by a portable accelerometer. J Sports Med Phys Fitness 39:336–340 [PubMed] [Google Scholar]
- Block G, Subar AF 1992 Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc 92:969–977 [PubMed] [Google Scholar]
- Durrleman S, Simon R 1989 Flexible regression models with cubic splines. Stat Med 8:551–561 [DOI] [PubMed] [Google Scholar]
- Devlin TF, Weeks BJ, 1986 Spine functions for logistic regression modeling. Proc Eleventh Annual SAS Users Group International, SAS Institute, Cary, NC, 1986, pp 646–651 [Google Scholar]
- Turner RT, Wakley GK, Hannon KS 1990 Differential effects of androgens on cortical bone histomorphometry in gonadectomized male and female rats. J Orthop Res 8:612–617 [DOI] [PubMed] [Google Scholar]
- Lorentzon M, Swanson C, Andersson N, Mellström D, Ohlsson C 2005 Free testosterone is a positive, whereas free estradiol is a negative, predictor of cortical bone size in young Swedish men: the GOOD study. J Bone Miner Res 20:1334–1341 [DOI] [PubMed] [Google Scholar]
- Lapauw BM, Taes Y, Bogaert V, Vanbillemont G, Goemaere S, Zmierczak HG, De Bacquer D, Kaufman JM 2009 Serum estradiol is associated with volumetric BMD and modulates the impact of physical activity on bone size at the age of peak bone mass: a study in healthy male siblings. J Bone Miner Res 24:1075–1085 [DOI] [PubMed] [Google Scholar]
- Szulc P, Joly-Pharaboz MO, Marchand F, Delmas PD 2004 Insulin-like growth factor I is a determinant of hip bone mineral density in men less than 60 years of age: MINOS study. Calcif Tissue Int 74:322–329 [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton 3rd LJ, Robb RA, Camp JJ, Atkinson EJ, Oberg AL, Rouleau PA, Riggs BL 2005 Relationship of volumetric BMD and structural parameters at different skeletal sites to sex steroid levels in men. J Bone Miner Res 20:730–740 [DOI] [PubMed] [Google Scholar]
- Mellström D, Vandenput L, Mallmin H, Holmberg AH, Lorentzon M, Odén A, Johansson H, Orwoll ES, Labrie F, Karlsson MK, Ljunggren O, Ohlsson C 2008 Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res 23:1552–1560 [DOI] [PubMed] [Google Scholar]
- Amin S, Zhang Y, Felson DT, Sawin CT, Hannan MT, Wilson PW, Kiel DP 2006 Estradiol, testosterone, and the risk for hip fractures in elderly men from the Framingham Study. Am J Med 119:426–433 [DOI] [PubMed] [Google Scholar]
- Szulc P, Delmas PD 2007 Bone loss in elderly men: increased endosteal bone loss and stable periosteal apposition. The prospective MINOS study. Osteoporos Int 18:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink HA, Ewing SK, Ensrud KE, Barrett-Connor E, Taylor BC, Cauley JA, Orwoll ES 2006 Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab 91:3908–3915 [DOI] [PubMed] [Google Scholar]