CB1 antagonism impairs the induction of epileptiform activity by group I metabotropic glutamate receptor activation (original) (raw)
. Author manuscript; available in PMC: 2010 Jul 23.
Summary
Exposure to the group I metabotropic glutamate receptor agonist dihydroxyphenylglycine (DHPG) induces epileptiform activity in the CA3 region of the hippocampus that persists following wash-out of DHPG. DHPG also can cause long term depression of synaptic transmission and at some synapses this may be mediated by endocannabinoids. We evaluated whether the selective cannabinoid receptor (CB1) antagonists AM 251 or SR 141716 could modify induction of epileptiform activity produced by DHPG exposure. The induction of epileptiform activity by DHPG exposure was significantly reduced by either CB1 antagonists, SR 141716 or AM 251. Minimal effects on epileptiform activity were noted once the activity had been induced. In control slices, exposure to DHPG for 30 minutes produced long term depression (LTD) of synaptic transmission, on average about a 70% reduction in slope of the field EPSP. When slices were exposed to both DHPG and SR 141716 (3 μM) LTD did not occur and the population EPSP remained at control values or greater. These results suggest that CB1 receptors mediate some of DHPG effects that result in persistent epileptiform activity, and antagonism of CB1 receptors has anti-epileptogenic properties. Paradoxically DHPG also caused long term depression of excitatory synaptic transmission in the CA3 region and CB1 antagonism prevents the depression. We hypothesize that the ictal activity induced by DHPG requires depression of synaptic strength and CB1 antagonism prevents this depression and the induction of ictal activity.
Keywords: long term depression (LTD), dihydroxyphenylglycine (DHPG), endocannabinoid, CA3, SR 141716, AM 251
A number of pathologies that produce epilepsy are associated with elevated glutamate levels and glutamate levels increase in humans before the onset of temporal lobe seizures (During and Spencer 1993). When glutamate release is great, juxtasynaptic group I metabotropic glutamate receptors are activated. Activation of these synapses turns on a number of second messenger systems (Hermans and Challiss 2001) that result in long-lasting changes in excitability that are protein synthesis dependent (Merlin, et al. 1998) and likely contribute to the development of epilepsy, epileptogenesis (Wong, et al. 2002).
Transient exposure of hippocampal slices to the group I mGluR agonist dihydroxyphenylglycine (DHPG) results in a persistent increase of spontaneous CA3 network activity that includes brief interictal epileptiform discharges and more prolonged periods of neuronal synchrony that resembles that seen during seizures (ictal activity) (Wong, Chuang and Bianchi 2002, Sayin and Rutecki 2003, Karr and Rutecki 2008). This provides an in vitro model of group I mGluR mediated epileptogenesis that can be used to assess for interventions that may reduce the development of epilepsy.
We hypothesized that the endocannabinoid receptors, particularly CB1 receptors, may be involved in the induction of epileptiform activity by group I mGluRs because at some central nervous system synapses group I mGluRs cause presynaptic inhibition mediated by retrograde endocannabinoid transmission (Heifets and Castillo 2009) and recent findings that CB1 antagonists may depress epileptogenesis following febrile seizures or head trauma (Chen, et al. 2007, Echegoyen, et al. 2009). To test this hypothesis we evaluated the effect of CB1 antagonists on the induction and maintenance of epileptiform activity produced by DHPG. Furthermore we tested whether the SR 141716, a CB1 antagonist altered the depression of evoked synaptic activity produced by DHPG.
Induction of epileptiform activity
Hippocampal slices (500 μ thick) were prepared from Sprague Dawley rates (30–40 day old) and then they were then either transferred to an interface recording chamber that was perfused with warmed (32–34°) artificial cerebrospinal fluid (aCSF) that had an extracellular potassium concentration of 5 mM or incubated in small bottles containing 10 ml of aCSF.
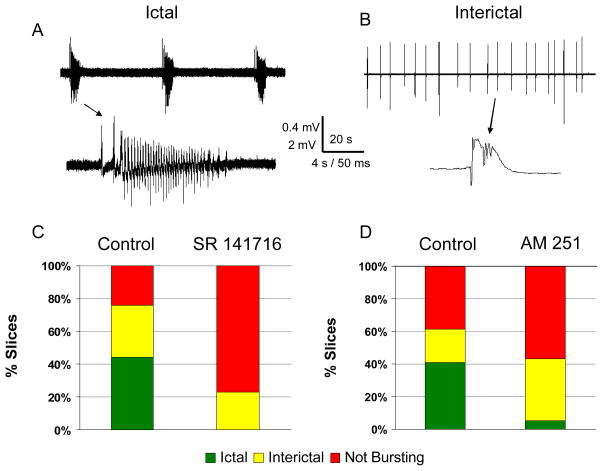
Slices transferred to bottles were then incubated at room temperature in the presence of either 50 μM S-DHPG alone or in combination with a CB1 antagonist that was added before the DHPG. Slices were transferred to an interface chamber and incubated for 60 minutes in warmed aCSF. Following this incubation, extracellular recordings were made from the CA3 region for 5 minutes to characterize spontaneously occurring activity. The activity recorded was considered as not bursting, interictal bursting, or displaying ictal activity defined as episodes of greater than 2 seconds duration of synchronous rhythmic extracellular field activity (Figure 1A&B).
Figure 1.
Induction of epileptiform activity by DHPG was suppressed by CB1 antagonists. Slices exposed to DHPG resulted in ictal patterns of activity (A), interictal activity (B) or no spontaneously occurring network activity (not bursting). Ictal activity consisted of recurrent population discharges that occurred for longer than 2 s at greater than 2 Hz (A, scale bar with 0.4 mV and time scale of 20 s for top trace and 4 s for bottom trace). Interictal activity consisted of recurrent brief (less than 500 ms) discharges (B, scale bar of 2 mV and time scale of 20 s and 50 ms). C: Following exposure to DHPG and after a 1 hour wash period, control slices showed ictal activity in 44% of slices (green) with 32% demonstrating interictal activity (yellow), and 24 % not bursting (red) (n = 26). When SR 141716 (3 μM) was applied prior to and with the DHPG no slices showed ictal discharges and 23% had interictal discharges (p < 0.001). D: Similar findings occurred with AM 521 (5 μM) with only 5% of slices demonstrating ictal discharges (n = 37) compared to 41% of control slices (n = 39, p =0.001).
The CB1 antagonist SR1417116 (3μM) depressed DHPG’s induction of epileptiform activity significantly (p < 0.001 by Chi square) with no slices showing ictal patterns, 23% interictal patterns, and 77% of slices without spontaneously occurring bursting (n = 26 slices). In slices exposed to DHPG alone 44% demonstrated ictal patterns of activity with 32% having interictal patterns, and 24% not bursting (n = 25 slices, Figure 1C).
AM 251 (5 μM), another CB1 antagonist, also suppressed the induction of epileptiform activity by DHPG. In slices incubated in the presence of AM251 and DHPG, 5% of slices had ictal activity, 38% interictal discharges, and 57% without bursting (n = 37); compared to control DHPG-exposed slices that resulted in 41% having ictal activity, 21 % interictal activity, and 38% without bursting (n = 37, p =0.001 by Chi square, Figure 1D).
Maintenance of epileptiform activity
We evaluated the effects of SR141716 and AM251 at concentrations that inhibited the induction of epileptiform activity by DHPG on epileptiform activity that had already been induced. AM 521 had no significant effect on the patterns of activity once induced. Of 27 slices that had been exposed to DHPG, 5 had no bursting, 5 had interictal bursting, and 17 displayed ictal activity. When AM 251 was added to the bathing saline, the proportions were unchanged with 3 slices demonstrating no bursting, 8 interictal bursting, and 16 ictal patterns (p > 0.05 by Chi square). SR 141716 was applied to 18 slices that demonstrated ictal activity following DHPG exposure and 10 continued to displayed ictal patterns, 5 converted to interictal patterns and 3 stopped. Of the 10 slices that continued to demonstrate ictal activity the duration of discharges was not changed (6.5 ± 0.9 s vs. 6.7 ± 1.0 s, mean ± SEM, p > 0.05 by paired t-test) and the interval between ictal discharges was not significantly different (65.3 ± 25.8 s vs. 37.1 ± 12.2 s, p> 0.05).
Long Term Depression following DHPG exposure
A brief, 5 minute, application of DHPG causes a long term depression of Schaffer collateral input to CA1 neurons (Huber, et al. 2001). We evaluated a more prolonged application of DHPG, 30 minutes, which can induce long term changes in network excitability, on CA3 recurrent collateral synaptic function. The aCSF included 0.3–1 μM DCG-IV, a group II mGluR antagonist that blocks mossy fiber synaptic transmission (Yoshino, et al. 1996). This was done to isolate the recurrent excitatory synaptic from mossy fiber synaptic activity. A bipolar stimulating electrode was placed in the stratum radiatum of the CA3 region and a recording electrode was placed at the same distance from the pyramidal cell layer as the stimulating electrode. Test stimulation was given at 50% of intensity that produced a maximal response and administered every 30 s. To test if the reduction in amplitude was related to a decrease in synaptic release probability, we assessed if there was changed in synaptic facilitation that occurs at inter-stimulus intervals of 50 ms (Zalutsky and Nicoll, 1990).
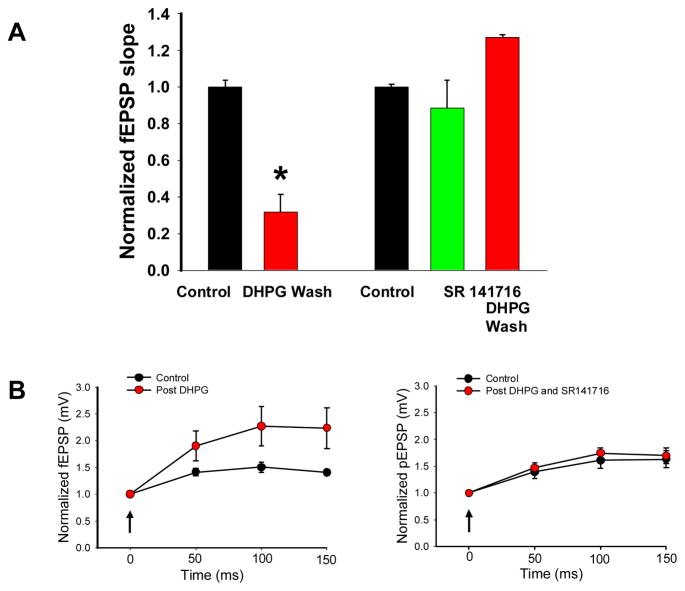
In control slices, bath application of DHPG (50 μM) for 30 minutes resulted in a long lasting depression of evoked synaptic field EPSPs (fEPSPs). One hour after the wash out of DHPG the slope of the fEPSP was depressed significantly to 32% of the original control value (Figure 2A).
Figure 2.
A: SR 141716 (3 μM) blocked long term depression that followed DHPG exposure. In control slices a 30 minute exposure to DHPG followed by a 60 minute wash to aCSF resulted in a significant reduction in the fEPSP slope (n = 9, p < 0.001 *) whereas SR141716 prevented the long term depression associated with DHPG exposure (n =7). SR 141716 depressed the fEPSP by about 11% (green bar, not significant). B: The induction of long term depression was associated with enhanced facilitation of the fEPSP compared to the control condition. Stimulation in stratum radiatum was given at 20 Hz (4 pulses) at the arrow and the population EPSP (pEPSP) amplitude was normalized to the first response. Enhanced facilitation was not noted when slices were exposed to DHPG in the presence of SR 141716.
The reduction in slope also was accompanied by a relative increase in facilitation of 4 responses delivered at 20 Hz (figure 2). This result suggested that the decrease in the evoked synaptic response was related to a decrease in the probability of synaptic release or a relative greater decrease in inhibitory mechanisms evoked by the stimulus. One hour after the 30 minute DHPG exposure 2 slices demonstrated ictal discharges, 5 interictal discharges, and 2 were not bursting.
When SR141716 (3 μM) was added to aACSF, a small decrease in the slope of the fEPSP was noted (figure 2A); however, following 30 minute exposure of DHPG and SR141716 and subsequent wash of DHPG for 1 hour in SR141716, depression of the fEPSP slope did not occur, nor was there a change in facilitation to repetitive stimulation (figure 2B). We hypothesize that the facilitation seen following DHPG alone is mediated by activation of CB1 receptors that depress presynaptic release of transmitter. None of the slices demonstrated ictal patterns, although 4 of the 7 had interictal discharges induced by DHPG exposure.
Discussion
Postsynaptic activation of group I mGluRs can lead to retrograde transmission by endocannabinoids and resultant presynaptic inhibition of both excitatory and inhibitory synapses (Heifets and Castillo, 2009). Our results show that CB1 antagonism suppressed the induction of ictal epileptiform activity that followed DHPG exposure and demonstrated anti-epileptogenesis in this in vitro model. Once induced, CB1 antagonism had less of an effect on epileptiform activity (anticonvulsant effects). These findings are complimentary to those described in a model of febrile convulsion and traumatic brain injury models of hyperexcitabity (Chen, et al, 2007, and Echegoyen, et al, 2009) and implicate a possible role of group I mGluRs in the increased excitability in other models.
Paradoxically, DHPG exposure leads to depression of evoked excitatory synaptic activity and abnormal spontaneous epileptiform activity. This long term depression was blocked by the CB1 antagonist SR 141716 at a concentration that blocked the induction of ictal discharges. One theory regarding the brief duration of interictal epileptiform activity in the CA3 region postulates that the interictal bursts results in a depletion of available synaptic vesicles for release (Staley, et al. 1998). DHPG-induced ictal epileptiform activity is converted to interictal activity by increasing synaptic transmission with 4-aminopyridine (Sayin and Rutecki 2003). Reducing synaptic release by CB1 mediated presynaptic mechanisms may contribute to the ability of the recurrent excitatory CA3 network to generate longer lasting ictal-like discharges. Also a reduction in synaptic strength was accompanied by more facilitation of subsequent synaptic input and would be expected to favor longer lasting discharges. It is possible that the reduction in the fEPSP slope represented an increase in feedforward inhibition; however, in most cases activation of group I mGluRs results in reduction of evoked inhibition (Heifets and Castillo, 2009). The facilitation of subsequent stimuli could be due to a decrease in recurrent synaptic drive by a decrease in the strength of excitatory synaptic drive onto interneurons or loss of the medium and slow afterhyperpolarization mediated by calcium activated potassium currents (Young et al, 2008). We have previously found that when DHPG reduces population spike amplitude, paired pulse inhibition of the second population spike is reduced at inter-stimulus intervals of 15 and 25 ms (Sayin and Rutecki, 2003) suggesting a reduction in network synaptic inhibition.
The prolonged discharges also appear to be promoted by long-lasting postsynaptic changes mediated by group I mGluR that include a cation current (Bianchi, et al. 2009) and loss of the medium and slow afterhyperpolarization mediated by calcium-activated potassium channels (Young, et al. 2008). A complex relationship exists between synaptic strength and neuronal synchronization. Changes in membrane excitability in conjunction with depressed synaptic transmission allows for longer network synchronization.
Our results showing CB1 antagonists blocking the induction of group I mGluR ictal epileptiform activity in the CA3 region of the hippocampus point to a “disease modifying” effect in conditions of excessive glutamate release and pathologic activation of group I mGluRs. This may translate to new therapies to prevent the development of epilepsy and modify the effects of seizures that create intractable epilepsy.
Acknowledgments
This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Service (grant to PAR). The contents do not represent the views of the Dept. of Veterans Affairs or the United States Government.
Footnotes
Conflict of Interest Statement: None of the authors have any conflicts of interest and the paper conforms to the ethical guidelines of Epilepsia for publication.
References
- Bianchi R, Chuang S-C, Zhao W, Young SR, Wong RKS. Cellular plasticity for group I mGluR-mediated epileptogenesis. J Neurosci. 2009;29:3497–3507. doi: 10.1523/JNEUROSCI.5447-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Neu A, Howard AL, Foldy C, Echegoyen J, Hilgenberg L, Smith M, Mackie K, Soltesz I. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J Neurosci. 2007;27:46–58. doi: 10.1523/JNEUROSCI.3966-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–1610. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- Echegoyen J, Armstrong C, Morgan RJ, Soltesz I. Single application of a CB1 receptor antagonist rapidly following head injury prevents long-term hyperexcitability in a rat model. Epilepsy Res. 2009;85:123–127. doi: 10.1016/j.eplepsyres.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Ann Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E, Challiss R. Structural, signaling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- Karr L, Rutecki PA. Activity-dependent induction and maintenance of epileptiform activity produced by group I metabotropic glutamate receptors in the rat hippocampal slice. Epilepsy Res. 2008;81:14–23. doi: 10.1016/j.eplepsyres.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Merlin LR, Bergold PJ, Wong RKS. Requirement of protein synthesis for group I mGluR-mediated induction of epileptiform discharges. J Neurophysiol. 1998;80:989–993. doi: 10.1152/jn.1998.80.2.989. [DOI] [PubMed] [Google Scholar]
- Sayin U, Rutecki PA. Group I metabotropic glutamate receptor activation produces prolonged epileptiform neuronal synchronization and alters evoked population responses in the hippocampus. Epilepsy Res. 2003;53:186–195. doi: 10.1016/s0920-1211(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Longacher M, Bains JS, Yee A. Presynaptic modulation of CA3 network activity. Nature Neurosci. 1998;1:201–209. doi: 10.1038/651. [DOI] [PubMed] [Google Scholar]
- Wong RK, Chuang SC, Bianchi R. Metabotropic Glutamate Receptors and Epileptogenesis. Epilepsy Curr. 2002;2:81–85. doi: 10.1046/j.1535-7597.2002.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino M, Sawada S, Yamamoto C, Kamiya H. A metabotropic glutamate receptor agonist DCG-IV suppresses synaptic transmission at mossy fiber pathway of the guinea pig hippocampus. Neurosci Lett. 1996;207:70–72. doi: 10.1016/0304-3940(96)12486-1. [DOI] [PubMed] [Google Scholar]
- Young SR, Bianchi R, Wong RK. Signaling mechanisms underlying group I mGluR-induced persistent AHP suppression in CA3 hippocampal neurons. J Neurophysiol. 2008;99:1105–1118. doi: 10.1152/jn.00435.2007. [DOI] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]