THE EMERGING ROLE OF THE THIOREDOXIN SYSTEM IN ANGIOGENESIS (original) (raw)
. Author manuscript; available in PMC: 2011 Nov 1.
Published in final edited form as: Arterioscler Thromb Vasc Biol. 2010 Aug 26;30(11):2089–2098. doi: 10.1161/ATVBAHA.110.209643
Abstract
Notwithstanding a multitude of studies, the mechanisms of angiogenesis remain incompletely understood. Increasing evidence suggests that cellular redox homeostasis is an important regulator of angiogenesis. The thioredoxin (TRX) system functions as an endogenous antioxidant that can exert influence over endothelial cell (EC) function via modulation of cellular redox status. It has become apparent that the cytosolic thioredoxin-1 (TRX1) isoform participates in both canonical and novel angiogenic signaling pathways, and may represent an avenue for therapeutic exploitation. Recent studies have further identified a role for the mitochondrial isoform thioredoxin-2 (TRX2) in ischemia-induced angiogenesis. Thioredoxin interacting protein (TXNIP) is the endogenous inhibitor of TRX redox activity that has been implicated in growth factor-mediated angiogenesis. As TXNIP is strongly induced by glucose, this molecule could be of consequence to disordered angiogenesis manifest in diabetes mellitus. This review will focus on data implicating the TRX system in EC homeostasis and angiogenesis.
Keywords: Thioredoxin, Thioredoxin Interacting Protein, Angiogenesis, Ischemia, Endothelial cell
Introduction
Angiogenesis is a tightly controlled process originally observed during embryogenesis as new blood vessels arise from the primitive vascular plexus. Postnatal angiogenesis is now a recognized adaptive response to ischemia and hypoxia, in which the thioredoxin (TRX) system is increasingly implicated.1, 2 Angiogenesis may also be dysregulated - a key feature of tumor growth and other diseases, including atherosclerosis and the vascular complications arising from diabetes mellitus. Despite great promise, the ability to regulate angiogenesis therapeutically remains a largely unrealized goal.
The endothelial cell (EC) is central to angiogenesis. Notably, the endothelial cell senses low oxygen tensions, in part due to the inhibition of prolyl hydroxylase. Under normoxic conditions this enzyme hydroxylates proline groups in the oxygen degradation domain of the transcription factor hypoxia inducible factor-1α (HIF1α), leading to its degradation. As hypoxia inhibits prolyl hydroxylase, HIF1α is stabilized, and can translocate to the nucleus to induce the transcription of angiogenic genes, such as vascular endothelial growth factor (VEGF).3, 4
Cellular redox homeostasis is also an important regulator of angiogenesis. In ECs, the NADPH oxidases generate reactive oxygen species (ROS), which at low levels are important signaling moieties but at high levels have deleterious consequences.5–7 More recent work directly implicates the thioredoxin (TRX) system and its endogenous inhibitor, thioredoxin interacting protein (TXNIP) in EC homeostasis and key angiogenic processes including EC migration, proliferation and survival.8–11 Herein, we review the TRX system with a particular focus on the emerging evidence of its critical role in the modulation of angiogenesis.
The Thioredoxin System
In conjunction with glutathione and glutaredoxin, the TRX system maintains the reducing environment of the cell and detoxifies ROS. The antioxidant activity of the TRX system is primarily exerted by peroxiredoxins, which are reduced by TRX to enable scavenging of ROS.12 The TRX system is reproduced in distinct cellular compartments in the cytosol and nucleus is found the traditionally described thioredoxin-1 (TRX1) isoform, whereas in the mitochondria is found the more recently identified thioredoxin-2 (TRX2) isoform. Homozygous knockout of either isoform in mouse is embryonically lethal13, 14 and TRX molecules are retained throughout evolution, indicating that the TRX system is essential for life.15–18 Both isoforms are ubiquitously expressed and contain the highly conserved (Trp-Cys-Gly-Pro-Cys) catalytic motif that reduces oxidized proteins and ROS. In concert with these oxidoreductases are the selenium-dependent enzymes, thioredoxin reductase-1 (TRXR1) and thioredoxin reductase-2 (TRXR2), which utilize the electron donor NADPH to regenerate the TRX isoforms (Figure 1A). The ability of both TRX1 and TRX2 to undergo reversible oxidation may be modulated by TXNIP, which interacts with the catalytic motif to form a stable mixed disulfide that inhibits TRX activity. This property has led to the notion that TXNIP is the endogenous inhibitor of the TRX system, a finding that has been confirmed in multiple cell types in vitro19–21 albeit not consistently recapitulated in vivo.22, 23
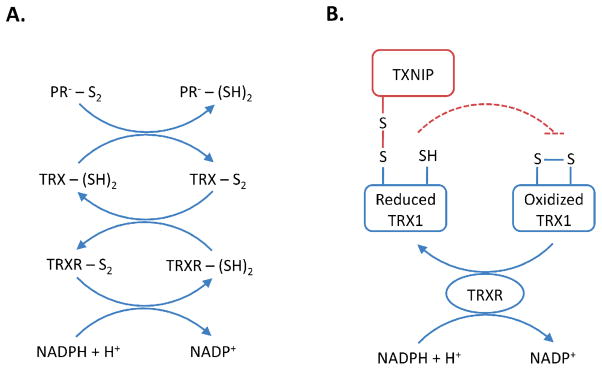
Figure 1. The Thioredoxin System.
(A) Thioredoxin (TRX) in the oxidized (TRX-S2) or reduced (TRX-(SH)2) state. In the reduced state, TRX directly reduces disulfides in oxidized substrate proteins (PR--S2). The resultant oxidation of TRX in this process is reversible and maintained by thioredoxin reductase (TRXR) and the electron donor NADPH. (B) Thioredoxin interacting protein (TXNIP) can form a mixed disulfide with reduced thioredoxin-1 (TRX1), inhibiting the ability of TRX1 to reduce disulfides of other protein substrates and/or undergo reversible oxidation.
The ability of TRX1 and TRX2 to interact with cysteine residues across a host of proteins entrenches the TRX system in a diverse range of cellular processes supra to its canonical role as a cytoprotective measure against oxidative stress. These include protein structure/folding, reductive and metabolic enzymes, energy utilization, transcription factors and immune modulation.24 The report that TRX1 was associated with increased tumor angiogenesis in a murine xenograft model11 has since led a number of investigators to explore the role of the TRX system in angiogenesis. Recent studies involving transgenic animal models and whole genome microarray analyses have enhanced our understanding of the role for the TRX system in angiogenesis, although significant gaps in our knowledge also remain.
Thioredoxin-1 and Angiogenesis
TRX1is a 12 kDa ubiquitous oxidoreductase originally isolated in Escherichia coli as a hydrogen donor for ribonucleotide reductase16 that is the rate-limiting enzyme in DNA synthesis (Figure 2). Homozygous knockout of Trx1 results in absorption of conceptuses after implantation, with cells derived from the inner cell mass unable to proliferate ex vivo.13 Conversely, mice overexpressing Trx1 develop normally, although they exhibit greater resistance to ischemic neuronal injury25 and adriamycin-induced cardiotoxicity.26 These data suggest that TRX1 expression conveys a cytoprotective effect to a variety of stressors, enhancing cell survival and function. TRX1may also regulate key EC activities relevant to physiological and tumor angiogenesis, including EC migration, proliferation and vascular network formation, and apoptosis and cell survival.
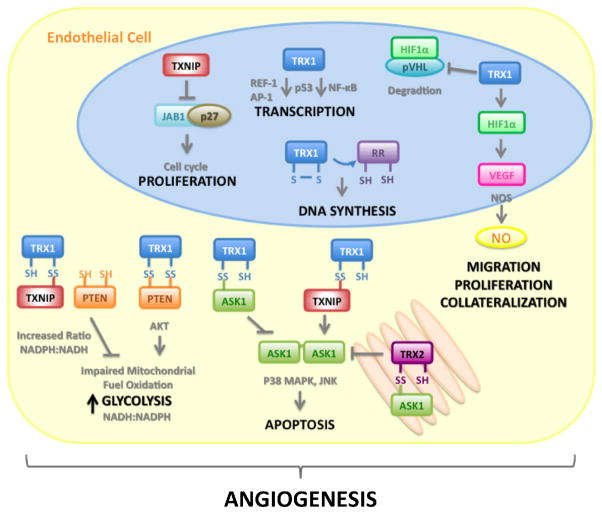
Figure 2. The Thioredoxin System and Angiogenesis.
Thioredoxin-1 (TRX1) and its endogenous inhibitor Thioredoxin Interacting Protein (TXNIP) are involved in multiple signaling pathways and cellular processes that confluence in mediation of angiogenesis. The TRX1/TXNIP system can modulate cell growth and proliferation by transcriptional mechanisms such as redox factor-1 (REF1) and NF-kB, JAB1/p27kip1 translocation, DNA synthesis via ribonucleotide reductase and energy metabolism/glycolysis through reductive inhibition of PTEN. Akt signaling facilitates cell survival. Apoptosis is modulated through the interaction of either cytosolic TRX1 or mitochondrial thioredoxin-2 (TRX2) with apoptosis signaling kinase-1 (ASK1) and competitive inhibition by TXNIP. In endothelial cells TRX1 prevents von Hippel-Lindau (pVHL) mediated degradation of the transcription factor hypoxia inducible factor-1α (HIF1α) leading to induction of vascular endothelial growth factor (VEGF) expression. The VEGF signaling cascade results in the activation of endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) release that facilitates key angiogenic events.
TRX1affects a number of transcriptional pathways involved in EC angiogenic function (Figure 2). Activation of the transcription factor NF-κB modulates a variety of angiogenesis-related proteins and genes, including Akt activation of VEGF that is critical for EC migration, proliferation and survival.27, 28 In the cytoplasm TRX1 blocks NF-κB activation by preventing its nuclear translocation, whereas in the nucleus TRX1 increases NF-κB transactivation through reduction of the Cys62 residue of the p50 DNA binding motif.29, 30 Thus TRX1 can modulate NF-κB activity to up-regulate or downregulate angiogenic gene transcription. Redox factor-1 (REF1) is a transcriptional co-activator in the HIF1α complex that binds to the VEGF promoter31, 32 and TRX1 has been demonstrated to enhance the binding activity of REF1 to the transcription factors fos, jun and AP1.33–35 The action of HIF1α is dependent on TRX1 redox activity with overexpression of TRX1 leading to increased HIF1α, VEGF, and nitric oxide synthase-2 expression in cancer cell lines.11 Nitric oxide (NO) production from VEGF signaling and endothelial nitric oxide synthase activation directly facilitates pro-angiogenic events including migration and proliferation.36, 37 The production of NO further enhances TRX activity by S-nitrosylation at Cys69, thereby increasing post-transcriptional pro-angiogenic signaling.38 The ability of TRX1 to interact with these transcription factors and undergo favorable post-translational modification is consistent with a central role for the TRX system in angiogenesis signaling.
Apoptosis and cell survival are important components of angiogenesis and vascular remodeling that can be modulated by the TRX system and ROS. TRX1interacts with apoptosis signaling kinase-1 (ASK1), preventing its homodimerization and suppressing its pro-apoptotic function by flagging ASK1 for ubiquitin-mediated degradation (Figure 2).39, 40 The interaction between TRX1 and ASK1 is dependent on TRX1 existing in the reduced state, with oxidation of TRX1 causing dissociation from ASK1.39, 40 Activation of ASK1 is reinforced by sustained elevated concentrations of ROS that induce cathepsin-D degradation of TRX1, while short-term exposure to low concentrations of ROS induces TRX1 expression and is anti-apoptotic.41 In response to ROS and oxidative damage, ASK1 activates JNK and p38 mitogen activated protein kinases leading to EC apoptosis and death. Indeed, when ASK1was overexpressed in human ECs, VEGF-induced cell migration and vascular network formation were inhibited.42 However, disparate phenotypes are reported when ASK1 is deleted in mouse. In one study, Ask1 null mice exhibited increased limb perfusion and VEGF receptor-2 signaling in a hindlimb ischemia model.43 By contrast another study demonstrated Ask1 deletion to reduce ischemia-induced neovascularization and VEGF expression in the hindlimb ischemia model.44 To date, the reasons underlying these conflicting observations are unclear.
TRX1and VEGF are overexpressed in a variety of cancers including mesothelioma,45 lung,46 colorectal,47, 48 liver,49 prostate,50 breast11 and leukemia.51 Elevated levels of TRX1 correlate with poor prognosis.46, 48, 52 When MCF7 breast carcinoma cells overexpressing TRX1 cells were xenotransplanted in mouse there was increased formation of solid tumors, while cells overexpressing a redox-impaired C32S/C35S TRX1 mutant had reduced tumorigenicity.53 Murine lymphoma xenografts overexpressing TRX1 also formed solid tumors with enhanced capillary networks and increased VEGF levels.11 The influence of TRX1 on hypoxia-mediated EC angiogenic signaling in the cancer milieu is illustrated by pharmacologic modulation of TRX activity. The TRX1 inhibitor AW464 was found to impair TRX1 and HIF1α activity, and specifically reduce EC proliferation and vascular network formation in vitro.54 Treatment of breast and colon cancer cells with the TRX1 signaling inhibitors, pleurotin and PX-12, decreased TRX1 levels and activity, inhibited increases in HIF1α protein and reduced levels of VEGF - ultimately resulting in decreased tumor capillary density.55 Blockade of TRX1 using the putative anticancer drug PX-12 has shown promising results in phase I clinical trials with decreased plasma concentrations of the angiogenic growth factor VEGF noted in PX-12 treated patients.56
Targeted pharmacological induction of TRX1 activity may prove useful in therapeutic modulation of angiogenesis in cardiovascular disease. For example, in a rat myocardial infarct model the red-wine compound resveratrol was demonstrated to enhance ischemia-mediated angiogenesis via TRX1 induction.57 Subsequent increases in expression of VEGF and the stress response enzyme heme oxygenase-1 were associated with enhanced angiogenesis and improved cardiac function.57 Resveratrol or exogenous TRX1 administration alone also increased EC vascular network formation in a Matrigel assay in vitro. In a similar myocardial infarction model in diabetic rats, enforced expression of TRX1 in the myocardium by adenovirus led to a reduction in oxidative stress and apoptosis, with improvements in cardiac function.58 Interestingly, these findings were associated with increased angiogenesis, arteriogenesis, and heme oxygenase-1 and VEGF expression. These initial studies indicate that modulation of the TRX system in ischemic myocardium represents an avenue of future investigation.
Mitochondrial Thioredoxin-2 and Ischemia-Induced Neovascularization
Mitochondrial dysfunction and excessive ROS generation are apparent in cardiovascular disease, leading to perturbations in endothelial function and apoptotic signaling, in part by inhibiting NO bioavailability. As deletion of TRX2 is embryonically lethal coinciding with mitochondrial maturation stage14 TRX2 may play a regulatory role in the spectrum of endothelial function, including angiogenesis. Indeed, in EC-specific Trx2 transgenic mice, both NO bioavailability and mitochondrial ROS scavenging were increased and there was an overall reduction in oxidative stress.59 When crossed with Apolipoprotein E null mice atherosclerotic lesions were reduced. Subsequent investigations demonstrated that EC-specific Trx2 transgenesis enhanced both angiogenesis and arteriogenesis in a murine model of hindlimb ischemia.8 Induction of ASK1-dependent apoptosis by ROS was reduced in ECs isolated from Trx2 transgenics. When Trx2 transgenics were crossed with eNOS knockout mice (that have a severely impaired ischemia-induced angiogenic response) Trx2 overexpression dramatically abrogated the deleterious eNOS null phenotype. These data demonstrate a role for TRX2 in facilitating ischemia-induced angiogenesis. The recent observation that TRX2 reduces hypertension and ROS-generating NADPH oxidases emphasizes the importance of the TRX system in the broader context of endothelial biology.60
Thioredoxin Reductases
Thioredoxin reductase-1 is a selenium dependent enzyme localized to the cytosol. In mouse, the TrxR1 null phenotype is embryonically lethal61 and siRNA-mediated knockdown of TRXR1 renders ECs susceptible to oxidative stress and apoptosis.62 The selenoprotein is also implicated in angiogenesis. Low levels of selenium have been shown to increase VEGF expression and tumor angiogenesis in vivo,63 while inhibition of TRXR1 activity in selenium replete ECs increases VEGF production leading to enhanced migration, proliferation and tubulogenesis in vitro. (this seems inconsistent with the angiogenic effects of TRXR1) 64 Congruent with these observations, the TRXR1 inhibitor PX-916 displays marked anti-cancer activity with reductions in TRX1 activity, HIF1α and VEGF levels in tumors.65 The TRXR1 inhibitor auranofin has been implicated in reduced (?)EC damage and anti-angiogenic activity in rheumatoid arthritis,66, 67 while other gold compounds inhibited angiogenesis in zebrafish (mention mechanism).68 No studies have explicitly examined the role of mitochondrial TRXR2 in angiogenesis, although it is speculated to have roles in the endothelium.69
Thioredoxin Interacting Protein as a Modulator of Angiogenesis Signaling
Thioredoxin interacting protein (TXNIP) was isolated as a 50 kDa Vitamin-D3 inducible protein (Vitamin-D3 upregulated protein-1) by yeast two-hybrid screen for TRX1 interacting proteins in cervical cancer cells.70 This interaction requires that TRX1 is in the reduced state71, 72 and is significant in that TXNIP is the only currently known endogenous cofactor that inhibits TRX1 and TRX2 redox activity.72–74 Patwari et al, elucidated that an intra-molecular disulfide bond between Cys63 and Cys247 in TXNIP is susceptible to disulfide exchange with reduced TRX1 at Cys247, leading to a stable mixed disulfide (Figure 1B).71, 75 While yet to be confirmed by crystallography this mixed disulfide at the catalytic motif would significantly impair the ability of TRX1 to interact with a number of pro-angiogenic molecules, leading to modulation of angiogenic signaling.
In cancer biology, TXNIP has been described as a metastasis suppressor76 that is strongly downregulated in a variety of tumor tissues and cell lines.77–80 Mice deficient in TXNIP also have increased incidence of hepatocellular carcinoma.81 Conversely, when TXNIP was over-expressed in the HEp-2 epithelial cell line, xenotransplants displayed reduced tumorigenesis in mouse.82 TXNIP is known to interact with a variety of molecules to modulate cell cycle progression and growth (Figure 2). Overexpression of TXNIP leads to an enhanced interaction with α-importin-1 (RCH1) and nuclear translocation of the complex suppressing growth activity in MCF7 breast cancer cells.83 Thioredoxin interacting protein can also competitively inhibit Jab1-mediated nuclear export of p27kip1 to the cytoplasm leading to cell cycle arrest84 (Figure 2), which is critical to halting tumor progression.85, 86 In the cytoplasm TXNIP interacts with TRX1 and inhibits its nuclear translocation, blocking TRX1-dependent gene transcription87 and further influencing the expression of cell death and survival genes.88 The cell cycle inhibitor and tumor suppressor p21(WAF1) also suppresses TXNIP gene transcription leading to increases in TRX activity, EC migration, vascular network formation and invasion.89
Recent work from our laboratory revealed a central role for TXNIP in angiogenic growth factor-mediated EC migration.10 To gain insights into the transcriptional events necessary for angiogenesis, we used whole genome cDNA microarray analysis to identify and characterize concordant gene expression programs triggered by administration of three angiogens: VEGF, basic fibroblast growth factor and nicotine. In human microvascular ECs, TXNIP was consistently repressed by all three angiogenic factors. Co-repression of TXNIP was associated with stimulation of TRX activity. Interestingly, hexamethomium, a nicotinic acetylcholine receptor antagonist, abrogated growth factor-related effects on TXNIP and TRX1, suggesting a role for the nicotinic acetylcholine receptor in growth factor signaling via modulation of TXNIP/TRX1. Gene knockdown of TRX1 by siRNA abrogated the stimulatory effects of all three angiogenic factors on EC migration. Gene silencing of TXNIP alone, without angiogenic factors, induced TRX activity and profoundly stimulated EC migration a pro-angiogenic effect. More recently, we explored the effect of TRX1/TXNIP modulation on EC migration and vascular network formation using a Matrigel assay. Downregulation of TRX1 by siRNA in human coronary artery ECs inhibited migration and vascular network formation in a Matrigel assay, while siRNA inhibition of TXNIP increased migration and vascular network formation.90
Fluid shear stress is a principal trigger for arteriogenesis. Normal laminar flow has been demonstrated to inhibit TXNIP expression and increase TRX activity in rabbit aorta and ECs.91 Shear stress also enhances S-nitrosylation (and thereby the activity) of TRX1 and increases the production of NO in ECs92 leading to suppression of TXNIP expression in smooth muscle cells.20 These observations implicate the TRX system, particularly TXNIP, as a regulator of biomechanical signaling in the vasculature. Interestingly, ischemia was found to induce TXNIP expression in a rat myocardial infarction model, while DNAzyme to TXNIP led to reduced apoptosis and improved cardiac function.93 The mechanisms underlying the relationship between TXNIP and endothelial function require further investigation.
Despite the demonstration that TXNIP inhibits TRX activity in multiple cell types in vitro including ECs,10 vascular smooth muscle cells,20 breast cancer cells,21 lens epithelial cells94 and mesangial cells,95 and in aortic tissue ex vivo91 this observation has not been consistently demonstrated in vivo. This is particularly true of the total and tissue-specific TXNIP knockout mice,22, 23, 96, 97 and TXNIP-deficient mouse models available,98 which demonstrate no significant changes in TRX activity. The inability of current techniques to specifically and sensitively measure TRX redox moieties in vivo could explain this remaining controversy. Indeed, the glutathione system represents the primary redox buffer, and competes with the TRX system for reducing equivalents (NADPH).99 While some investigations in the TXNIP deficient and knockout mice have noted minor changes in the reduced to oxidized glutathione ratio and NADP/NADPH ratio,96, 100 others have not22, 23 and these discrepancies highlight a major outstanding issue in the field that needs to be addressed.
The Thioredoxin System and Diabetes-Related Impairment of Angiogenesis
In diabetes mellitus, hyperglycemia induces ROS that contribute to the pathogenesis of diabetic vascular complications. Reactive oxygen species have profound effects on the vasculature, leading to endothelial dysfunction, accelerated atherosclerosis, microvascular and peripheral arterial disease.101 A striking characteristic of the diabetic state is the heterogeneity of angiogenic dysregulation. For example, VEGF is upregulated in the diabetic eye whereas VEGF signaling is impaired in the peripheral arterial and microvascular environments.102 To date, the mechanisms underlying dysregulated angiogenesis and impaired VEGF signaling in diabetes mellitus remain unclear.
Microarray studies identified TXNIP as the gene most strongly induced by glucose in pancreatic beta cells.103 A carbohydrate response element within the TXNIP promoter underlies this striking feature.104 Thioredoxin interacting protein is induced by sugars in a variety of other cell types including vascular smooth muscle cells,105 ECs,90, 106 hepatoma,107 and breast cancer.21 It is associated with increased apoptosis in pancreatic beta islets108, 109 and cardiomyocytes by competitive inhibition of the TRX1/ASK-1 complex.93, 110, 111 Hyperglycemic induction of TXNIP in vascular smooth muscle cells and ECs in vitro represses TRX activity, induces ROS accumulation,105 and inhibits vascular network formation.90 Knockdown of TXNIP by siRNA or adenoviral overexpression of TRX1 abrogates glucose-induced ROS accumulation in smooth muscle cells.105 Furthermore, in streptozotocin-induced diabetic rats, TXNIP expression was increased in the vasculature and TRX activity reduced.105 As impaired angiogenesis and endothelial dysfunction are the hallmark of diseases such as diabetes mellitus, hyperglycemia-mediated induction of TXNIP could have important consequences for ROS-induced endothelial dysfunction, impaired angiogenesis and dysregulated biomechanical signaling.
We recently identified a critical role for hyperglycemia-mediated induction of TXNIP in diabetes-related impairment of ischemia-mediated angiogenesis using a murine model of hindlimb ischemia.(Dunn et al, TBA). Moreover, TXNIP knockdown to non-diabetic levels rescued diabetes-related impairment of ischemia-mediated angiogenesis and limb functional recovery. Diabetes-related inhibition of VEGF was also attenuated by TXNIP knockdown. The demonstration that adenoviral overexpression of TRX1 in the infarcted myocardium of diabetic rats enhances angiogenesis and improves cardiac function58 is consistent with our proposal that dysregulation of the TRX system is a critical mechanism underlying disordered angiogenesis in diabetes mellitus. Interestingly, there appears to be a link between diabetes mellitus and TRX2. In rats with streptozotocin-induced diabetes, aortic expression of TRX2 is reduced and in vitro siRNA knockdown of TRX2 in ECs results in increased glucose toxicity with up-regulation of the endothelial glucose transporter, GLUT1.112 As previously mentioned, the interaction between TXNIP and TRX2 within the mitochondria is particularly intriguing78 given the metabolic aberrations of diabetes mellitus.
The Thioredoxin System: Redox Modulation of Angiogenesis?
Despite structural and functional similarities re-iterated between the TRX systems such as redox metabolism and apoptosis, ablation of either the respective TRX1/2 or TRXR1/2 isoforms results in cessation of embryogenesis,13, 14, 61, 113 surprisingly demonstrating little functional redundancy between the two systems. Thioredoxin-1 has been shown to stabilize HIF1α protein by causing the dissociation of von Hippel-Lindau protein thereby preventing HIF1α ubiquitination and degradation (Figure 2).9 Yet opposing roles for TRX1 and TRX2 have been described in HEK-293 cells submitted to hypoxic treatment.114, 115 Overexpression of TRX1 resulted in increased HIF1α accumulation and activity under hypoxia, normoxia and NO treatments. Conversely, when TRX2 was overexpressed, hypoxia-induced HIF1α accumulation and activity were reduced with increases in mitochondrial ROS.114 Overexpression of TRXR2 also reduced NO-induced HIF1α accumulation and activity.115 As mitochondria facilitate ATP production the investigators went on to demonstrate that TRX1 expression enhances ATP levels thereby increasing HIF1α protein translation, while TRX2 or TRXR2 overexpression attenuated ATP levels and protein translation.115 These findings suggest an association between TRX2 and the mitochondrial respiratory chain, with ROS entering the cytosol and counterbalancing TRX1 enhanced translation of HIF1α protein.115 Recent studies in pancreatic beta cells identify TXNIP as a mechanism underlying the TRX1/TRX2 balance of power.74 Under normal conditions Saxena et al, found TXNIP to be strongly localized to the nucleus. However, under conditions of oxidative stress TXNIP is shuttled to the mitochondria where it out-competes ASK1 binding to TRX2 leading to induction of apoptosis via the mitochondrial pathway.
The divergent effects of the reductases TRXR1 and TRXR2 strengthen the concept that cellular homeostasis requires a delicate balance between TRX1 and TRX2. In cardiac development a similar balancing act is evident for TRXR1 and TRXR2. Embryos null for TrxR1 are not viable in vivo and ECs isolated from embryos do not proliferate in vitro.61 However, isolated cardiomyocytes are not affected and cardiac specific deletion does not affect embryo development.61 By contrast, cardiac-specific TrxR2 deletion leads to cardiomyopathy and death in the embryo.116 The independent features of the two TRX systems are intriguing and suggests that redox control of hypoxia- and metabolically-mediated cellular function extends to development as well as cellular physiology and disease.
Conclusion
The paradigm of TRX1 as a simple redox regulator has shifted in recent times with TRX activity proven to extend to many aspects of vascular function, including angiogenesis. In the EC, TRX1 exerts pro-angiogenic effects throughout a broad range of cellular activities relevant to angiogenic function, including transcription, post-translational modification, migration, proliferation, vascular network formation, apoptosis and intracellular signaling. Mitochondrial TRX2 also plays a critical role in ischemia-induced angiogenesis and arteriogenesis. These potent effects may be modulated by TXNIP in vitro although the effect of TXNIP on TRX activity in vivo requires further clarification. Intriguingly, normalization of hyperglycemia-induced TXNIP expression to non-diabetic levels rescues diabetes-related impairment of ischemia-mediated angiogenesis. The ability of TXNIP to further regulate cell cycle progression and metastasis clearly identifies TXNIP as an important target for therapeutic angiogenesis. The onus of future investigations rests on precisely delineating the inter-relationship of these TRX system members so that effective modulation of this system as an angiogenic therapy can be realized.
Acknowledgments
The authors would like to acknowledge funding support from the National Health and Medical Research Council of Australia (GRANT No. 512299). Dr Louise Dunn is the recipient of a National Health and Medical Research Council of Australia Postdoctoral Training Fellowship (GRANT No. 537537). Dr. Cooke is supported by grants from the National Institutes of Health (RC2HL103400 and 1U01HL100397), and the Tobacco Related Disease Research Program of the University of California (18XT-0098).
References
- 1.Banai S, Shweiki D, Pinson A, Chandra M, Lazarovici G, Keshet E. Upregulation of vascular endothelial growth factor expression induced by myocardial ischaemia: implications for coronary angiogenesis. Cardiovasc Res. 1994;28:1176–1179. doi: 10.1093/cvr/28.8.1176. [DOI] [PubMed] [Google Scholar]
- 2.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 3.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW, Ratcliffe PJ. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1997;94:8104–8109. doi: 10.1073/pnas.94.15.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang SS, Zheng RL. Biphasic regulation of angiogenesis by reactive oxygen species. Pharmazie. 2006;61:223–229. [PubMed] [Google Scholar]
- 6.Remacle J, Raes M, Toussaint O, Renard P, Rao G. Low levels of reactive oxygen species as modulators of cell function. Mutat Res. 1995;316:103–122. doi: 10.1016/0921-8734(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 7.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 8.Dai S, He Y, Zhang H, Yu L, Wan T, Xu Z, Jones D, Chen H, Min W. Endothelial-specific expression of mitochondrial thioredoxin promotes ischemia-mediated arteriogenesis and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:495–502. doi: 10.1161/ATVBAHA.108.180349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim WJ, Cho H, Lee SW, Kim YJ, Kim KW. Antisense-thioredoxin inhibits angiogenesis via pVHL-mediated hypoxia-inducible factor-1alpha degradation. Int J Oncol. 2005;26:1049–1052. [PubMed] [Google Scholar]
- 10.Ng MK, Wu J, Chang E, Wang BY, Katzenberg-Clark R, Ishii-Watabe A, Cooke JP. A central role for nicotinic cholinergic regulation of growth factor-induced endothelial cell migration. Arterioscler Thromb Vasc Biol. 2007;27:106–112. doi: 10.1161/01.ATV.0000251517.98396.4a. [DOI] [PubMed] [Google Scholar]
- 11.Welsh SJ, Bellamy WT, Briehl MM, Powis G. The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res. 2002;62:5089–5095. [PubMed] [Google Scholar]
- 12.Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J Biol Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- 13.Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol. 1996;178:179–185. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 14.Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke FM, Orozco C, Perkins AV, Cock I, Tonissen KF, Robins AJ, Wells JR. Identification of molecules involved in the ‘early pregnancy factor’ phenomenon. J Reprod Fertil. 1991;93:525–539. doi: 10.1530/jrf.0.0930525. [DOI] [PubMed] [Google Scholar]
- 16.Laurent TC, Moore EC, Reichard P. Enzymatic Synthesis of Deoxyribonucleotides. Iv. Isolation and Characterization of Thioredoxin, the Hydrogen Donor from Escherichia Coli B. J Biol Chem. 1964;239:3436–3444. [PubMed] [Google Scholar]
- 17.Moore EC. A thioredoxin--thioredoxin reductase system from rat tumor. Biochem Biophys Res Commun. 1967;29:264–268. doi: 10.1016/0006-291x(67)90446-9. [DOI] [PubMed] [Google Scholar]
- 18.Wakasugi H, Rimsky L, Mahe Y, Kamel AM, Fradelizi D, Tursz T, Bertoglio J. Epstein-Barr virus-containing B-cell line produces an interleukin 1 that it uses as a growth factor. Proc Natl Acad Sci U S A. 1987;84:804–808. doi: 10.1073/pnas.84.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxena G, Chen J, Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem. 285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulze PC, Liu H, Choe E, Yoshioka J, Shalev A, Bloch KD, Lee RT. Nitric oxide-dependent suppression of thioredoxin-interacting protein expression enhances thioredoxin activity. Arterioscler Thromb Vasc Biol. 2006;26:2666–2672. doi: 10.1161/01.ATV.0000248914.21018.f1. [DOI] [PubMed] [Google Scholar]
- 21.Turturro F, Friday E, Welbourne T. Hyperglycemia regulates thioredoxin-ROS activity through induction of thioredoxin-interacting protein (TXNIP) in metastatic breast cancer-derived cells MDA-MB-231. BMC Cancer. 2007;7:96. doi: 10.1186/1471-2407-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chutkow WA, Patwari P, Yoshioka J, Lee RT. Thioredoxin-interacting protein (Txnip) is a critical regulator of hepatic glucose production. J Biol Chem. 2008;283:2397–2406. doi: 10.1074/jbc.M708169200. [DOI] [PubMed] [Google Scholar]
- 23.Yoshioka J, Imahashi K, Gabel SA, Chutkow WA, Burds AA, Gannon J, Schulze PC, MacGillivray C, London RE, Murphy E, Lee RT. Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ Res. 2007;101:1328–1338. doi: 10.1161/CIRCRESAHA.106.160515. [DOI] [PubMed] [Google Scholar]
- 24.Lillig CH, Holmgren A. Thioredoxin and related molecules--from biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 25.Takagi Y, Mitsui A, Nishiyama A, Nozaki K, Sono H, Gon Y, Hashimoto N, Yodoi J. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc Natl Acad Sci U S A. 1999;96:4131–4136. doi: 10.1073/pnas.96.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shioji K, Kishimoto C, Nakamura H, Masutani H, Yuan Z, Oka S, Yodoi J. Overexpression of thioredoxin-1 in transgenic mice attenuates adriamycin-induced cardiotoxicity. Circulation. 2002;106:1403–1409. doi: 10.1161/01.cir.0000027817.55925.b4. [DOI] [PubMed] [Google Scholar]
- 27.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 28.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 29.Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 30.Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrero P, Okamoto K, Coumailleau P, O’Brien S, Tanaka H, Poellinger L. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1alpha. Mol Cell Biol. 2000;20:402–415. doi: 10.1128/mcb.20.1.402-415.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, Gallick GE. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24:3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- 33.Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc Natl Acad Sci U S A. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. Embo J. 1992;11:653–665. doi: 10.1002/j.1460-2075.1992.tb05097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xanthoudakis S, Miao G, Wang F, Pan YC, Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. Embo J. 1992;11:3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ, Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooke JP. NO and angiogenesis. Atheroscler Suppl. 2003;4:53–60. doi: 10.1016/s1567-5688(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 38.Haendeler J, Hoffmann J, Tischler V, Berk BC, Zeiher AM, Dimmeler S. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat Cell Biol. 2002;4:743–749. doi: 10.1038/ncb851. [DOI] [PubMed] [Google Scholar]
- 39.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. Embo J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 41.Haendeler J, Tischler V, Hoffmann J, Zeiher AM, Dimmeler S. Low doses of reactive oxygen species protect endothelial cells from apoptosis by increasing thioredoxin-1 expression. FEBS Lett. 2004;577:427–433. doi: 10.1016/j.febslet.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 42.Zhang R, He X, Liu W, Lu M, Hsieh JT, Min W. AIP1 mediates TNF-alpha-induced ASK1 activation by facilitating dissociation of ASK1 from its inhibitor 14-3-3. J Clin Invest. 2003;111:1933–1943. doi: 10.1172/JCI17790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, He Y, Dai S, Xu Z, Luo Y, Wan T, Luo D, Jones D, Tang S, Chen H, Sessa WC, Min W. AIP1 functions as an endogenous inhibitor of VEGFR2-mediated signaling and inflammatory angiogenesis in mice. J Clin Invest. 2008;118:3904–3916. doi: 10.1172/JCI36168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izumi Y, Kim-Mitsuyama S, Yoshiyama M, Omura T, Shiota M, Matsuzawa A, Yukimura T, Murohara T, Takeya M, Ichijo H, Yoshikawa J, Iwao H. Important role of apoptosis signal-regulating kinase 1 in ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2005;25:1877–1883. doi: 10.1161/01.ATV.0000174801.76234.bd. [DOI] [PubMed] [Google Scholar]
- 45.Kahlos K, Soini Y, Saily M, Koistinen P, Kakko S, Paakko P, Holmgren A, Kinnula VL. Up-regulation of thioredoxin and thioredoxin reductase in human malignant pleural mesothelioma. Int J Cancer. 2001;95:198–204. doi: 10.1002/1097-0215(20010520)95:3<198::aid-ijc1034>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 46.Kakolyris S, Giatromanolaki A, Koukourakis M, Powis G, Souglakos J, Sivridis E, Georgoulias V, Gatter KC, Harris AL. Thioredoxin expression is associated with lymph node status and prognosis in early operable non-small cell lung cancer. Clin Cancer Res. 2001;7:3087–3091. [PubMed] [Google Scholar]
- 47.Powis G, Kirkpatrick DL, Angulo M, Baker A. Thioredoxin redox control of cell growth and death and the effects of inhibitors. Chem Biol Interact. 1998;111–112:23–34. doi: 10.1016/s0009-2797(97)00148-8. [DOI] [PubMed] [Google Scholar]
- 48.Raffel J, Bhattacharyya AK, Gallegos A, Cui H, Einspahr JG, Alberts DS, Powis G. Increased expression of thioredoxin-1 in human colorectal cancer is associated with decreased patient survival. J Lab Clin Med. 2003;142:46–51. doi: 10.1016/S0022-2143(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 49.Noike T, Miwa S, Soeda J, Kobayashi A, Miyagawa S. Increased expression of thioredoxin-1, vascular endothelial growth factor, and redox factor-1 is associated with poor prognosis in patients with liver metastasis from colorectal cancer. Hum Pathol. 2008;39:201–208. doi: 10.1016/j.humpath.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 50.Xu W, Ngo L, Perez G, Dokmanovic M, Marks PA. Intrinsic apoptotic and thioredoxin pathways in human prostate cancer cell response to histone deacetylase inhibitor. Proc Natl Acad Sci U S A. 2006;103:15540–15545. doi: 10.1073/pnas.0607518103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shao L, Diccianni MB, Tanaka T, Gribi R, Yu AL, Pullen JD, Camitta BM, Yu J. Thioredoxin expression in primary T-cell acute lymphoblastic leukemia and its therapeutic implication. Cancer Res. 2001;61:7333–7338. [PubMed] [Google Scholar]
- 52.Jarvela S, Bragge H, Paunu N, Jarvela T, Paljarvi L, Kalimo H, Helen P, Kinnula V, Soini Y, Haapasalo H. Antioxidant enzymes in oligodendroglial brain tumors: association with proliferation, apoptotic activity and survival. J Neurooncol. 2006;77:131–140. doi: 10.1007/s11060-005-9030-z. [DOI] [PubMed] [Google Scholar]
- 53.Gallegos A, Gasdaska JR, Taylor CW, Paine-Murrieta GD, Goodman D, Gasdaska PY, Berggren M, Briehl MM, Powis G. Transfection with human thioredoxin increases cell proliferation and a dominant-negative mutant thioredoxin reverses the transformed phenotype of human breast cancer cells. Cancer Res. 1996;56:5765–5770. [PubMed] [Google Scholar]
- 54.Mukherjee A, Westwell AD, Bradshaw TD, Stevens MF, Carmichael J, Martin SG. Cytotoxic and antiangiogenic activity of AW464 (NSC 706704), a novel thioredoxin inhibitor: an in vitro study. Br J Cancer. 2005;92:350–358. doi: 10.1038/sj.bjc.6602338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welsh SJ, Williams RR, Birmingham A, Newman DJ, Kirkpatrick DL, Powis G. The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation. Mol Cancer Ther. 2003;2:235–243. [PubMed] [Google Scholar]
- 56.Baker AF, Dragovich T, Tate WR, Ramanathan RK, Roe D, Hsu CH, Kirkpatrick DL, Powis G. The antitumor thioredoxin-1 inhibitor PX-12 (1-methylpropyl 2-imidazolyl disulfide) decreases thioredoxin-1 and VEGF levels in cancer patient plasma. J Lab Clin Med. 2006;147:83–90. doi: 10.1016/j.lab.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39:813–822. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Samuel SM, Thirunavukkarasu M, Penumathsa SV, Koneru S, Zhan L, Maulik G, Sudhakaran PR, Maulik N. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in infarcted myocardium of diabetic rats. Circulation. 121:1244–1255. doi: 10.1161/CIRCULATIONAHA.109.872481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, Luo Y, Zhang W, He Y, Dai S, Zhang R, Huang Y, Bernatchez P, Giordano FJ, Shadel G, Sessa WC, Min W. Endothelial-specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. Am J Pathol. 2007;170:1108–1120. doi: 10.2353/ajpath.2007.060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Widder JD, Fraccarollo D, Galuppo P, Hansen JM, Jones DP, Ertl G, Bauersachs J. Attenuation of Angiotensin II-Induced Vascular Dysfunction and Hypertension by Overexpression of Thioredoxin 2. Hypertension. 2009 doi: 10.1161/HYPERTENSIONAHA.108.127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jakupoglu C, Przemeck GK, Schneider M, Moreno SG, Mayr N, Hatzopoulos AK, de Angelis MH, Wurst W, Bornkamm GW, Brielmeier M, Conrad M. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trigona WL, Mullarky IK, Cao Y, Sordillo LM. Thioredoxin reductase regulates the induction of haem oxygenase-1 expression in aortic endothelial cells. Biochem J. 2006;394:207–216. doi: 10.1042/BJ20050712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang C, Jiang W, Ip C, Ganther H, Lu J. Selenium-induced inhibition of angiogenesis in mammary cancer at chemopreventive levels of intake. Mol Carcinog. 1999;26:213–225. doi: 10.1002/(sici)1098-2744(199912)26:4<213::aid-mc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 64.Streicher KL, Sylte MJ, Johnson SE, Sordillo LM. Thioredoxin reductase regulates angiogenesis by increasing endothelial cell-derived vascular endothelial growth factor. Nutr Cancer. 2004;50:221–231. doi: 10.1207/s15327914nc5002_13. [DOI] [PubMed] [Google Scholar]
- 65.Powis G, Wipf P, Lynch SM, Birmingham A, Kirkpatrick DL. Molecular pharmacology and antitumor activity of palmarumycin-based inhibitors of thioredoxin reductase. Mol Cancer Ther. 2006;5:630–636. doi: 10.1158/1535-7163.MCT-05-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marzano C, Gandin V, Folda A, Scutari G, Bindoli A, Rigobello MP. Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Radic Biol Med. 2007;42:872–881. doi: 10.1016/j.freeradbiomed.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 67.Matsubara T, Ziff M. Inhibition of human endothelial cell proliferation by gold compounds. J Clin Invest. 1987;79:1440–1446. doi: 10.1172/JCI112972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ott I, Qian X, Xu Y, Vlecken DH, Marques IJ, Kubutat D, Will J, Sheldrick WS, Jesse P, Prokop A, Bagowski CP. A gold(I) phosphine complex containing a naphthalimide ligand functions as a TrxR inhibiting antiproliferative agent and angiogenesis inhibitor. J Med Chem. 2009;52:763–770. doi: 10.1021/jm8012135. [DOI] [PubMed] [Google Scholar]
- 69.Crane MS, Howie AF, Arthur JR, Nicol F, Crosley LK, Beckett GJ. Modulation of thioredoxin reductase-2 expression in EAhy926 cells: Implications for endothelial selenoprotein hierarchy. Biochim Biophys Acta. 2009;1790:1191–1197. doi: 10.1016/j.bbagen.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 70.Yamanaka H, Maehira F, Oshiro M, Asato T, Yanagawa Y, Takei H, Nakashima Y. A possible interaction of thioredoxin with VDUP1 in HeLa cells detected in a yeast two-hybrid system. Biochem Biophys Res Commun. 2000;271:796–800. doi: 10.1006/bbrc.2000.2699. [DOI] [PubMed] [Google Scholar]
- 71.Patwari P, Higgins LJ, Chutkow WA, Yoshioka J, Lee RT. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem. 2006;281:21884–21891. doi: 10.1074/jbc.M600427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishiyama A, Matsui M, Iwata S, Hirota K, Masutani H, Nakamura H, Takagi Y, Sono H, Gon Y, Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 73.Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000;164:6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- 74.Saxena G, Chen J, Shalev A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J Biol Chem. 2009 doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patwari P, Chutkow WA, Cummings K, Verstraeten VL, Lammerding J, Schreiter ER, Lee RT. Thioredoxin-independent regulation of metabolism by the alpha-arrestin proteins. J Biol Chem. 2009 doi: 10.1074/jbc.M109.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldberg SF, Miele ME, Hatta N, Takata M, Paquette-Straub C, Freedman LP, Welch DR. Melanoma metastasis suppression by chromosome 6: evidence for a pathway regulated by CRSP3 and TXNIP. Cancer Res. 2003;63:432–440. [PubMed] [Google Scholar]
- 77.Butler LM, Zhou X, Xu WS, Scher HI, Rifkind RA, Marks PA, Richon VM. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci U S A. 2002;99:11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dutta KK, Nishinaka Y, Masutani H, Akatsuka S, Aung TT, Shirase T, Lee WH, Yamada Y, Hiai H, Yodoi J, Toyokuni S. Two distinct mechanisms for loss of thioredoxin-binding protein-2 in oxidative stress-induced renal carcinogenesis. Lab Invest. 2005;85:798–807. doi: 10.1038/labinvest.3700280. [DOI] [PubMed] [Google Scholar]
- 79.Ikarashi M, Takahashi Y, Ishii Y, Nagata T, Asai S, Ishikawa K. Vitamin D3 up-regulated protein 1 (VDUP1) expression in gastrointestinal cancer and its relation to stage of disease. Anticancer Res. 2002;22:4045–4048. [PubMed] [Google Scholar]
- 80.Ohta S, Lai EW, Pang AL, Brouwers FM, Chan WY, Eisenhofer G, de Krijger R, Ksinantova L, Breza J, Blazicek P, Kvetnansky R, Wesley RA, Pacak K. Downregulation of metastasis suppressor genes in malignant pheochromocytoma. Int J Cancer. 2005;114:139–143. doi: 10.1002/ijc.20670. [DOI] [PubMed] [Google Scholar]
- 81.Sheth SS, Bodnar JS, Ghazalpour A, Thipphavong CK, Tsutsumi S, Tward AD, Demant P, Kodama T, Aburatani H, Lusis AJ. Hepatocellular carcinoma in Txnip-deficient mice. Oncogene. 2006;25:3528–3536. doi: 10.1038/sj.onc.1209394. [DOI] [PubMed] [Google Scholar]
- 82.Shin KH, Kim RH, Kang MK, Park NH. hnRNP G elicits tumor-suppressive activity in part by upregulating the expression of Txnip. Biochem Biophys Res Commun. 2008;372:880–885. doi: 10.1016/j.bbrc.2008.05.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nishinaka Y, Masutani H, Oka S, Matsuo Y, Yamaguchi Y, Nishio K, Ishii Y, Yodoi J. Importin alpha1 (Rch1) mediates nuclear translocation of thioredoxin-binding protein-2/vitamin D(3)-up-regulated protein 1. J Biol Chem. 2004;279:37559–37565. doi: 10.1074/jbc.M405473200. [DOI] [PubMed] [Google Scholar]
- 84.Jeon JH, Lee KN, Hwang CY, Kwon KS, You KH, Choi I. Tumor suppressor VDUP1 increases p27(kip1) stability by inhibiting JAB1. Cancer Res. 2005;65:4485–4489. doi: 10.1158/0008-5472.CAN-04-2271. [DOI] [PubMed] [Google Scholar]
- 85.Goukassian D, Diez-Juan A, Asahara T, Schratzberger P, Silver M, Murayama T, Isner JM, Andres V. Overexpression of p27(Kip1) by doxycycline-regulated adenoviral vectors inhibits endothelial cell proliferation and migration and impairs angiogenesis. FASEB J. 2001;15:1877–1885. doi: 10.1096/fj.01-0065com. [DOI] [PubMed] [Google Scholar]
- 86.Hwang CY, Ryu YS, Chung MS, Kim KD, Park SS, Chae SK, Chae HZ, Kwon KS. Thioredoxin modulates activator protein 1 (AP-1) activity and p27Kip1 degradation through direct interaction with Jab1. Oncogene. 2004;23:8868–8875. doi: 10.1038/sj.onc.1208116. [DOI] [PubMed] [Google Scholar]
- 87.Schulze PC, De Keulenaer GW, Yoshioka J, Kassik KA, Lee RT. Vitamin D3-upregulated protein-1 (VDUP-1) regulates redox-dependent vascular smooth muscle cell proliferation through interaction with thioredoxin. Circ Res. 2002;91:689–695. doi: 10.1161/01.res.0000037982.55074.f6. [DOI] [PubMed] [Google Scholar]
- 88.Minn AH, Pise-Masison CA, Radonovich M, Brady JN, Wang P, Kendziorski C, Shalev A. Gene expression profiling in INS-1 cells overexpressing thioredoxin-interacting protein. Biochem Biophys Res Commun. 2005;336:770–778. doi: 10.1016/j.bbrc.2005.08.161. [DOI] [PubMed] [Google Scholar]
- 89.Kuljaca S, Liu T, Dwarte T, Kavallaris M, Haber M, Norris MD, Martin-Caballero J, Marshall GM. The cyclin-dependent kinase inhibitor, p21(WAF1), promotes angiogenesis by repressing gene transcription of thioredoxin-binding protein 2 in cancer cells. Carcinogenesis. 2009;30:1865–1871. doi: 10.1093/carcin/bgp225. [DOI] [PubMed] [Google Scholar]
- 90.Buckle A, Dunn LL, Ng MKC. Hyperglycaemia Inhibits Thioredoxin-Mediated Angiogenesis: Implications for Impairment of Neovascularisation in Diabetes Mellitus. Heart, Lung and Circulation. 2007;16:S214–S215. [Google Scholar]
- 91.Yamawaki H, Pan S, Lee RT, Berk BC. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J Clin Invest. 2005;115:733–738. doi: 10.1172/JCI200523001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoffmann J, Dimmeler S, Haendeler J. Shear stress increases the amount of S-nitrosylated molecules in endothelial cells: important role for signal transduction. FEBS Lett. 2003;551:153–158. doi: 10.1016/s0014-5793(03)00917-7. [DOI] [PubMed] [Google Scholar]
- 93.Xiang G, Seki T, Schuster MD, Witkowski P, Boyle AJ, See F, Martens TP, Kocher A, Sondermeijer H, Krum H, Itescu S. Catalytic degradation of vitamin D up-regulated protein 1 mRNA enhances cardiomyocyte survival and prevents left ventricular remodeling after myocardial ischemia. J Biol Chem. 2005;280:39394–39402. doi: 10.1074/jbc.M502966200. [DOI] [PubMed] [Google Scholar]
- 94.Liyanage NP, Fernando MR, Lou MF. Regulation of the bioavailability of thioredoxin in the lens by a specific thioredoxin-binding protein (TBP-2) Exp Eye Res. 2007;85:270–279. doi: 10.1016/j.exer.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng DW, Jiang Y, Shalev A, Kowluru R, Crook ED, Singh LP. An analysis of high glucose and glucosamine-induced gene expression and oxidative stress in renal mesangial cells. Arch Physiol Biochem. 2006;112:189–218. doi: 10.1080/13813450601093518. [DOI] [PubMed] [Google Scholar]
- 96.Hui ST, Andres AM, Miller AK, Spann NJ, Potter DW, Post NM, Chen AZ, Sachithanantham S, Jung DY, Kim JK, Davis RA. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc Natl Acad Sci U S A. 2008;105:3921–3926. doi: 10.1073/pnas.0800293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oka S, Liu W, Masutani H, Hirata H, Shinkai Y, Yamada S, Yoshida T, Nakamura H, Yodoi J. Impaired fatty acid utilization in thioredoxin binding protein-2 (TBP-2)-deficient mice: a unique animal model of Reye syndrome. FASEB J. 2006;20:121–123. doi: 10.1096/fj.05-4439fje. [DOI] [PubMed] [Google Scholar]
- 98.Donnelly KL, Margosian MR, Sheth SS, Lusis AJ, Parks EJ. Increased lipogenesis and fatty acid reesterification contribute to hepatic triacylglycerol stores in hyperlipidemic Txnip−/− mice. J Nutr. 2004;134:1475–1480. doi: 10.1093/jn/134.6.1475. [DOI] [PubMed] [Google Scholar]
- 99.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 100.Hui TY, Sheth SS, Diffley JM, Potter DW, Lusis AJ, Attie AD, Davis RA. Mice lacking thioredoxin-interacting protein provide evidence linking cellular redox state to appropriate response to nutritional signals. J Biol Chem. 2004;279:24387–24393. doi: 10.1074/jbc.M401280200. [DOI] [PubMed] [Google Scholar]
- 101.Schiekofer S, Balletshofer B, Andrassy M, Bierhaus A, Nawroth PP. Endothelial dysfunction in diabetes mellitus. Semin Thromb Hemost. 2000;26:503–511. doi: 10.1055/s-2000-13206. [DOI] [PubMed] [Google Scholar]
- 102.Duh E, Aiello LP. Vascular endothelial growth factor and diabetes: the agonist versus antagonist paradox. Diabetes. 1999;48:1899–1906. doi: 10.2337/diabetes.48.10.1899. [DOI] [PubMed] [Google Scholar]
- 103.Shalev A, Pise-Masison CA, Radonovich M, Hoffmann SC, Hirshberg B, Brady JN, Harlan DM. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology. 2002;143:3695–3698. doi: 10.1210/en.2002-220564. [DOI] [PubMed] [Google Scholar]
- 104.Minn AH, Hafele C, Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology. 2005;146:2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- 105.Schulze PC, Yoshioka J, Takahashi T, He Z, King GL, Lee RT. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem. 2004;279:30369–30374. doi: 10.1074/jbc.M400549200. [DOI] [PubMed] [Google Scholar]
- 106.Li X, Rong Y, Zhang M, Wang XL, LeMaire SA, Coselli JS, Zhang Y, Shen YH. Up-regulation of thioredoxin interacting protein (Txnip) by p38 MAPK and FOXO1 contributes to the impaired thioredoxin activity and increased ROS in glucose-treated endothelial cells. Biochem Biophys Res Commun. 2009;381:660–665. doi: 10.1016/j.bbrc.2009.02.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yamaguchi F, Takata M, Kamitori K, Nonaka M, Dong Y, Sui L, Tokuda M. Rare sugar D-allose induces specific up-regulation of TXNIP and subsequent G1 cell cycle arrest in hepatocellular carcinoma cells by stabilization of p27kip1. Int J Oncol. 2008;32:377–385. [PubMed] [Google Scholar]
- 108.Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A. Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes. 2008;57:938–944. doi: 10.2337/db07-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shalev A. Lack of TXNIP protects beta-cells against glucotoxicity. Biochem Soc Trans. 2008;36:963–965. doi: 10.1042/BST0360963. [DOI] [PubMed] [Google Scholar]
- 110.Chen J, Cha-Molstad H, Szabo A, Shalev A. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am J Physiol Endocrinol Metab. 2009;296:E1133–1139. doi: 10.1152/ajpendo.90944.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y, De Keulenaer GW, Lee RT. Vitamin D(3)-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem. 2002;277:26496–26500. doi: 10.1074/jbc.M202133200. [DOI] [PubMed] [Google Scholar]
- 112.Liang M, Pietrusz JL. Thiol-related genes in diabetic complications: a novel protective role for endogenous thioredoxin 2. Arterioscler Thromb Vasc Biol. 2007;27:77–83. doi: 10.1161/01.ATV.0000251006.54632.bb. [DOI] [PubMed] [Google Scholar]
- 113.Patenaude A, Ven Murthy MR, Mirault ME. Mitochondrial thioredoxin system: effects of TrxR2 overexpression on redox balance, cell growth, and apoptosis. J Biol Chem. 2004;279:27302–27314. doi: 10.1074/jbc.M402496200. [DOI] [PubMed] [Google Scholar]
- 114.Zhou J, Damdimopoulos AE, Spyrou G, Brune B. Thioredoxin 1 and thioredoxin 2 have opposed regulatory functions on hypoxia-inducible factor-1alpha. J Biol Chem. 2007;282:7482–7490. doi: 10.1074/jbc.M608289200. [DOI] [PubMed] [Google Scholar]
- 115.Zhou J, Eleni C, Spyrou G, Brune B. The mitochondrial thioredoxin system regulates nitric oxide-induced HIF-1alpha protein. Free Radic Biol Med. 2008;44:91–98. doi: 10.1016/j.freeradbiomed.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 116.Conrad M, Jakupoglu C, Moreno SG, Lippl S, Banjac A, Schneider M, Beck H, Hatzopoulos AK, Just U, Sinowatz F, Schmahl W, Chien KR, Wurst W, Bornkamm GW, Brielmeier M. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]