A Biobehavioral Home-Based Intervention and the Well-being of Patients With Dementia and Their Caregivers: The COPE Randomized Trial (original) (raw)
. Author manuscript; available in PMC: 2014 Jul 10.
Published in final edited form as: JAMA. 2010 Sep 1;304(9):983–991. doi: 10.1001/jama.2010.1253
Abstract
Context
Optimal treatment to postpone functional decline in patients with dementia is not established.
Objective
To test a nonpharmacologic intervention realigning environmental demands with patient capabilities.
Design, Setting, and Participants
Prospective 2-group randomized trial (Care of Persons with Dementia in their Environments [COPE]) involving patients with dementia and family caregivers (community-living dyads) recruited from March 2006 through June 2008 in Pennsylvania.
Interventions
Up to 12 home or telephone contacts over 4 months by health professionals who assessed patient capabilities and deficits; obtained blood and urine samples; and trained families in home safety, simplifying tasks, and stress reduction. Control group caregivers received 3 telephone calls and educational materials.
Main Outcome Measures
Functional dependence, quality of life, frequency of agitated behaviors, and engagement for patients and well-being, confidence using activities, and perceived benefits for caregivers at 4 months.
Results
Of 284 dyads screened, 270 (95%) were eligible and 237 (88%) randomized. Data were collected from 209 dyads (88%) at 4 months and 173 (73%) at 9 months. At 4 months, compared with controls, COPE patients had less functional dependence (adjusted mean difference, 0.24; 95% CI, 0.03–0.44; _P_=.02; Cohen _d_=0.21) and less dependence in instrumental activities of daily living (adjusted mean difference, 0.32; 95% CI, 0.09–0.55; _P_=.007; Cohen _d_=0.43), measured by a 15-item scale modeled after the Functional Independence Measure; COPE patients also had improved engagement (adjusted mean difference, 0.12; 95% CI, 0.07–0.22; _P_=.03; Cohen _d_=0.26), measured by a 5-item scale. COPE caregivers improved in their well-being (adjusted mean difference in Perceived Change Index, 0.22; 95% CI, 0.08–0.36; _P_=.002; Cohen _d_=0.30) and confidence using activities (adjusted mean difference, 0.81; 95% CI, 0.30–1.32; _P_=.002; Cohen _d_=0.54), measured by a 5-item scale. By 4 months, 64 COPE dyads (62.7%) vs 48 control group dyads (44.9%) eliminated 1 or more caregiver-identified problems (χ12=6.72, _P_=.01).
Conclusion
Among community-living dyads, a nonpharmacologic biobehavioral environmental intervention compared with control resulted in better outcomes for COPE dyads at 4 months. Although no group differences were observed at 9 months for patients, COPE caregivers perceived greater benefits.
Among the more than 5 Million dementia patients in the United States, most live at home, cared for by family members.1 Functional decline, a core disease feature, represents a risk factor for poor quality of life, high health care costs, institutionalization, and mortality.2–4 With disease progression, families increasingly provide hands-on physical assistance with activities of daily living (ADLs) and instrumental ADLs (IADLs). This often results in heightened caregiver distress, a risk factor for patient nursing home placement.5
Few large randomized trials evaluate treatments for supporting physical function of patients with dementia. Trials of antidementia medications show few if any benefits for physical function or caregiver burden and have substantial adverse effects.6–8 In 1 study, twice-yearly comprehensive care planning in memory clinics showed no additional positive effects on functional decline.9 Previous nonpharmacologic intervention trials (exercise, use of pleasant activities, home environmental modifications) had promising findings, yet studies reported small effect sizes and outcomes other than functional dependence or required replication.10,11 Recent psychosocial caregiver interventions showed caregiver improvements, but benefits either did not extend to patients with dementia or did not address functional dependence.12,13
Building on previous nonpharmacologic approaches and best clinical practices,14 we designed the Care of Persons with Dementia in their Environments (COPE) trial to test a nonpharmacologic, biobehavioral approach to support physical function and quality of life for patients with dementia and the well-being of their caregivers. The COPE program targeted modifiable environmental stressors to decrease sensorial, physical, and cognitive demands and align with patient capabilities and also ruled out underlying medical conditions that could lead to reduced patient functioning. The intervention sought to re-engage patients in daily activities and increase functionality, thereby alleviating caregiver burden.
We hypothesized that COPE patients, compared with those in a control group, would show reduced functional dependence, improved quality of life, and enhanced engagement in activities at 4 months (main study end point). We also hypothesized that COPE caregivers, compared with control caregivers, would report improved well-being and confidence using activities at 4 months. Also considered was whether COPE reduced occurrences of agitated behavior and eliminated problem areas identified by caregivers. Because the COPE study included a brief medical screen to rule out undiagnosed medical conditions, prevalence of these conditions are reported for intervention patients. Secondarily, we evaluated long-term effects (at 9 months).
METHODS
Study Population
Patients with dementia and family caregivers were recruited from March 2006 to June 2008 through media announcements and mailings by social agencies targeting caregivers. Study procedures were explained to interested caregivers contacting the research team (telephone, return postcard), and a brief telephone eligibility screen was administered. Eligible patients had a physician diagnosis of probable dementia (using criteria from NINCDS/ADRDA [National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association]) or a Mini-Mental State Examination (MMSE)15 score less than 24; they also were 21 years or older and English speaking, needed help with daily activities or had behavioral symptoms, and lived with or within 5 miles of family caregivers. Eligible caregivers provided oversight or care for 8 or more hours weekly, planned to live in the area for 9 months, were not seeking nursing home placement, and reported difficulty managing patient functional decline or behaviors.
Exclusion criteria for dyads were terminal illnesses with life expectancy of less than 9 months, active treatments for cancer, more than 3 acute hospitalizations in the past year, or involvement in another caregiver trial. Patients were excluded if they had schizophrenia or bipolar disorder, had dementia secondary to probable head trauma, or had an MMSE score of 0 and were bed-bound.
Written informed consent was obtained from caregivers prior to baseline interviews using forms approved by the institutional review board. Caregivers provided proxy patient consent and patient assent was obtained for each patient-related assessment using scripts approved by the institutional review board. Families were compensated $20 at each interview for their participation.
Following baseline interviews, dyads were randomized to the COPE or control group and reassessed by telephone at 4 and 9 months by interviewers masked to participant group. Consistent with other trials,13,16 caregivers of patients placed in nursing homes prior to 4 months (n=7) were reassessed at 4 months (but not 9 months) in areas amenable to reporting. Caregivers of patients who died (n=21) were not reassessed at 4 months (n=9) or 9 months (n=12) nor included in analyses, as outcome measures were not relevant.
Randomization
Dyads were stratified by living arrangement (alone/together) and randomized within each stratum using random permuted blocks to control for possible changes in participant mix over time. The blocking number was developed by the project statistician and unknown to others. Randomization lists and 2 sets of randomization forms were prepared using opaque envelopes. The project director randomized each dyad within 48 hours of the baseline interview.
Treatment Conditions
The COPE program sought to support patient capabilities by reducing environmental stressors and enhancing caregiver skills. In this multicomponent intervention, all COPE dyads received exposure to each treatment element: assessments (patient deficits and capabilities, medical testing, home environment, caregiver communication, and caregiver-identified concerns); caregiver education (patient capabilities, potential effects of medications, pain, constipation, dehydration); and caregiver training to address caregiver-identified concerns and help them reduce stress. Training in problem-solving, communication, engaging patients in activities, and simplifying tasks was tailored to address caregiver-identified concerns and patient capabilities.
COPE dyads received up to 10 sessions over 4 months with occupational therapists and 1 face-to-face session and 1 telephone session with an advance practice nurse. Occupational therapists initially interviewed caregivers to identify patient routines, previous and current roles, habits and interests, and caregiver concerns. They also conducted cognitive and functional testing to identify patient strengths and deficits in attention, initiation and perseveration, construction, conceptualization, and memory.17,18 Occupational therapists then trained caregivers to modify home environments, daily activities, and communications to support patient capabilities; use problem-solving to identify solutions for caregiver-identified concerns; and reduce stress. For each targeted concern, a written action plan was provided11,13,16 describing treatment goals, patient strengths, and specific strategies. In a home visit, the nurse provided caregivers health-related information (pain detection, hydration), obtained patient blood and urine samples, and examined patients for signs of dehydration. Laboratory evaluations included complete blood cell count, blood chemistry, thyroid testing of serum samples, and culture and sensitivity testing of urine samples. Patient medications were reviewed for appropriateness, polypharmacy, and dosing using published guidelines.19 Caregivers were informed of results by telephone and mailed copies to share with the patients’ physicians.
Dyads assigned to the control group received up to three 20-minute telephone calls from trained research staff members (not occupational therapists or nurses). Using scripts, staff asked caregivers about care challenges, mailed relevant informational brochures, and reviewed the materials in subsequent calls. Materials included tips from the Alzheimer’s Association and government agencies on home safety and managing patient behaviors, functional decline, and caregiver stress. This controlled for professional attention and tailoring of information.
Treatment Implementation
Interventionists for both treatment groups were independently trained in protocols through readings, didactic sessions, and practices. For the COPE group, treatment fidelity was monitored through twice-monthly supervision and audiotapes submitted by interventionists, which were reviewed by investigators. For the control group, randomly selected telephone calls were monitored for protocol adherence in real time. In both groups, interventionists completed documentation of duration and delivery content for each contact, which was reviewed for adherence. The COPE interventionists did not have contact with the control group interventionists.
Measures
Characteristics of dyads that were assessed included living arrangement (alone/together), sex, education, race, age, financial difficulty (1, not very difficult, to 3, very difficult paying for basics like food), and use of 10 formal services (eg, home health aide). To describe the racial background of participants, caregivers identified themselves and the patient with dementia as Caucasian/white, black/African American, or other.
Patient Outcomes
For functional dependence, we used a 15-item measure modeled after the Functional Independence Measure,20 previously shown as psychometrically sound and corresponding to objective determinations of dependence and assistance required.21,22 Items included 8 IADLs (telephone, shopping, meal preparation, housework, laundry, travel, medicine, managing finances) and 7 self-care ADLs (bathing, dressing upper/lower body, toileting, grooming, eating, getting in/out of bed). For each item, caregivers chose a score to indicate the following: patients were completely independent (a score of 7); there was a safety concern, excessive time required, or assistive devices used (6); patients needed supervision, setup, or cueing but no physical help (5); or patients needed physical help (4 for a little help, 25% assistance; 3 for moderate, 50% assistance; 2 for a lot of help, 75% assistance; or 1 for complete help, >75% assistance). A total mean functional dependence score was derived by summing across items and dividing by number of items (actual range of means, 1.0–6.3). Lower scores represented greater dependence (α = .92). Subscale scores for IADL dependence (α =.81) and ADL dependence (α = .93) were similarly derived.
We used the 12-item validated Quality of Life–Alzheimer Disease scale to assess caregiver perceptions of patient quality of life (1, poor, to 4, excellent).23 Overall mean response was calculated by summing across items and dividing by number of items. Higher scores indicated better quality of life (α = .78).
Activity engagement was measured using a validated 5-item scale24 (eg, “showed signs of pleasure/enjoyment”), with items rated 1 for never to 3 for often. Scores were derived by summing across items and dividing by number of items, with 1 item reverse coded (actual range of means, 1.0–2.8). Higher scores indicated greater engagement (α = .62).
We used the 16-item Agitated Behavior in Dementia scale to assess agitated behaviors in the past month.25 At baseline, caregivers indicated whether agitated behaviors occurred (yes/no) and, if yes, the number of times. Total number of agitated behaviors was derived by summing yes items; a mean frequency score was derived by summing across items and dividing by number of items (actual range, 0.0–121.1). Higher scores indicated greater number of agitated behaviors and frequency.
Caregiver Outcomes
Caregiver well-being (improvement/worsening) was evaluated using the 13-item Perceived Change Index,26 fashioned after pharmacologic trial measures and shown to have strong psychometric properties. Caregivers rated change in ability to manage dementia, emotional status (anger, distress), and somatic symptoms (energy, sleep quality) in the past month using 5-point scales (1, got much worse, to 5, improved a lot). Total mean score was derived by summing across items and dividing by number of items. Higher scores indicated greater improvement (α = .86).
Caregiver confidence using activities over the past month was measured by 5 investigator-developed items (identify daily activities patient can do, involve patient in activities, use activities to distract patient, manage boredom, set up activities) with ratings from 0 for not confident to 10 for very confident.27 Mean scores were derived across items (actual range of means, 0.60–10.00), with higher scores indicating greater confidence (α = .87).
We used a targeted measurement approach employed in medical, pharmacologic, psychotherapeutic, and behavior management trials to capture the most challenging problems (eg, behaviors, dependence, respite) for caregivers. 28,29 For each identified problem at baseline, caregivers indicated at 4 months whether that problem had been eliminated.
At 9 months, we evaluated caregiver appraisal of study benefits using an 11-item survey.13,16,29 Items concerned satisfaction (yes/no) with participation (study clearly explained, treated respectfully, effort required, recommend to others); and used ratings of not at all, some, and a great deal for perceived benefits (overall benefit, dementia understanding, confidence managing care, enhanced skills, life easier) and perceived patient benefits (improved daily life, helped keep patient home).
Statistical Analysis
Based on previous research, we based sample size calculation on assumptions of 25.0% attrition by 4 months and study hypothesis tested at 90% power to detect moderate effect sizes (_d_=0.45). We used α = .05 level test. Given expected attrition, we planned to randomize 230 dyads.
χ2 and Wilcoxon rank-sum tests were used to compare intervention and control participants on baseline characteristics and to compare those who stayed in vs those who dropped out by 4 months (main end point). These procedures were also used to examine potential differences at screening between eligible dyads willing to participate and those not willing. Means, standard deviations, and ranges for outcome measures were computed. The normality assumption for each dependent measure was tested by examining the distribution of residuals.
For main treatment effects, the outcome measure was 4-month score with design variable, living arrangement (alone/together), and baseline value of the outcome measure entered as covariates. For the 4-month sample, we found statistically significant differences between treatment groups at baseline for caregiver education and number of agitated behaviors (Table 1). We ran additional analyses of covariance with these variables as covariates. As results did not differ from the primary analyses, they are not reported. Cohen d was calculated to measure effect size.
Table 1.
Characteristics of Patients With Dementia and Their Caregivers Who Completed 4-Month Assessment
| Characteristic | Control Group(n = 107) | Intervention Group(n = 102) | Total(N = 209) | χ2 | Z | _P_Value |
|---|---|---|---|---|---|---|
| Patients with dementia | ||||||
| Age, mean (SD), y | 81.8 (9.9) | 83.1 (7.8) | 82.4 (8.9) | −1.00 | .33 | |
| Sex, No. (%) | 3.42 | .06 | ||||
| Male | 40 (37.4) | 26 (25.5) | 66 (31.6) | |||
| Female | 67 (62.6) | 76 (74.5) | 143 (68.4) | |||
| Race, No. (%) | 2.18 | .34 | ||||
| White | 72 (67.3) | 75 (73.5) | 147 (70.3) | |||
| African American | 31 (29.0) | 26 (25.5) | 57 (27.3) | |||
| Other | 4 (3.7) | 1 (1.0) | 5 (2.4) | |||
| Living arrangement, No. (%) | 0.07 | .79 | ||||
| Alone | 5 (4.7) | 4 (3.9) | 9 (4.3) | |||
| With caregiver | 102 (95.3) | 98 (96.1) | 200 (95.7) | |||
| No. of agitated behaviors, mean (SD) | 6.0 (3.0) | 6.8 (3.0) | 6.4 (3.0) | −1.98 | .048 | |
| MMSE score, mean (SD) | 13.6 (7.9) | 13.1 (8.2) | 13.4 (8.1) | −0.51 | .61 | |
| Caregivers | ||||||
| Age, mean (SD), y | 62.4 (11.7) | 62.0 (12.4) | 62.2 (12.0) | −0.31 | .83 | |
| Sex, No. (%) | 0.62 | .43 | ||||
| Male | 10 (9.3) | 13 (12.7) | 23 (11.0) | |||
| Female | 97 (90.7) | 89 (87.3) | 186 (89.0) | |||
| Race, No. (%) | 5.27 | .07 | ||||
| White | 71 (66.4) | 75 (73.5) | 146 (69.9) | |||
| African American | 31 (29.0) | 27 (26.5) | 58 (27.8) | |||
| Other | 5 (4.7) | 0 | 5 (2.4) | |||
| Relationship to patient, No. (%) | 1.69 | .19 | ||||
| Spouse | 45 (42.1) | 34 (33.3) | 79 (37.8) | |||
| Nonspouse | 62 (57.9) | 68 (66.7) | 130 (62.2) | |||
| Education, No. (%) | 7.06 | .03 | ||||
| <High school | 26 (24.3) | 38 (37.3) | 64 (30.6) | |||
| Some college | 42 (39.3) | 24 (23.5) | 66 (31.6) | |||
| ≥College | 39 (36.4) | 40 (39.2) | 79 (37.8) | |||
| Time caregiving, mean (SD), y | 3.9 (2.8) | 4.0 (4.4) | 4.0 (3.7) | −0.57 | .84 | |
| Financial difficulty, mean (SD)a | 2.2 (1.0) | 2.2 (1.0) | 2.2 (1.0) | −0.65 | .52 | |
| No. of formal services used, mean (SD) | 2.39 (1.3) | 2.45 (1.27) | 2.42 (1.28) | −0.38 | .70 |
Consistent with other trials, to evaluate clinical significance for outcomes reaching statistical significance at 4 months, we used the criterion of a 0.50-SD improvement from baseline to follow-up.13 This also represents the upper end of the distribution of effect sizes reported in the literature. We calculated number of dyads improving by 0.50 SD or more from baseline to 4 months and compared proportions between treatment groups using Mantel-Haenszel χ2 analyses, controlling for living arrangement. We also compared proportion of COPE and control group caregivers eliminating 1 or more caregiver-identified problem by 4 months using χ2 analysis, controlling for living arrangement.
To evaluate 9-month effects, intervention and control groups were compared on adjusted mean differences (baseline to 9 months) for each outcome using the same procedures as for 4-month effects. We also compared intervention and control group caregiver perceived benefit at 9 months using Mantel-Haenszel χ2 analyses, controlling for living arrangement.
Statistical analysis was performed with SPSS version 17.0 (SPSS Inc, Chicago, Illinois) with the significance level set at P < .05. All analyses were 2-sided. Analyses included all caregivers actively caregiving (not bereaved) and providing 4-month data. Following intention-to-treat principles, we included participants regardless of exposure level to treatment.
We adjusted for 6 outcome measures (functional dependence, activity engagement, quality of life, frequency of agitated behaviors, and caregiver well-being and confidence) using a method controlling for false discovery rate (ie, proportion of rejected hypotheses expected to be erroneous).30 Because .05 significance was used, we controlled the false discovery rate to be not more than 5%. Reported numerical P values were not corrected for multiple end points, but impact of adjustment is noted in Table 2.
Table 2.
Comparison of Intervention (n = 102) and Control (n = 107) Group Patients and Caregivers at 4 Monthsa
| Mean (SD) Score | AdjustedMean DifferenceBetween Groups(95% CI) | _P_Value | Cohen_d_ | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 4-Month Follow-up | ||||||
| Control Group | InterventionGroup | Control Group | InterventionGroup | ||||
| Patient outcomes | |||||||
| Overall functional dependenceb | 2.8 (1.3) | 3.0 (1.2) | 3.3 (1.3) | 3.7 (1.3) | 0.24 (0.03 to 0.44) | .02 | 0.21 |
| IADL dependence | 1.8 (1.0) | 1.8 (1.0) | 2.5 (1.1) | 2.8 (1.2) | 0.32 (0.09 to 0.55) | .007 | 0.43 |
| ADL dependence | 4.1 (1.8) | 4.3 (1.7) | 4.3 (1.7) | 4.6 (1.6) | 0.16 (−0.09 to 0.42) | .21 | |
| Activity engagement | 2.0 (0.4) | 1.9 (0.4) | 1.9 (0.5) | 2.0 (0.4) | 0.12 (0.07 to 0.22) | .03 | 0.26 |
| QOL-AD score | 2.1 (0.5) | 2.1 (0.4) | 2.1 (0.5) | 2.2 (0.5) | 0.10 (0.00 to 0.20) | .06 | 0.14 |
| ABID score | 9.8 (10.7) | 11.0 (14.6) | 5.5 (8.0) | 6.7 (10.6) | −0.65 (−3.05 to 1.74) | .59 | |
| Caregiver outcomes Perceived change in well-being | 2.8 (0.5) | 2.7 (0.5) | 2.9 (0.5) | 3.1 (0.6) | 0.22 (0.08 to 0.36) | .002 | 0.30 |
| Confidence using activitiesc | 7.0 (2.2) | 6.6 (2.1) | 6.9 (2.5) | 7.5 (1.9) | 0.81 (0.30 to 1.32) | .002 | 0.54 |
RESULTS
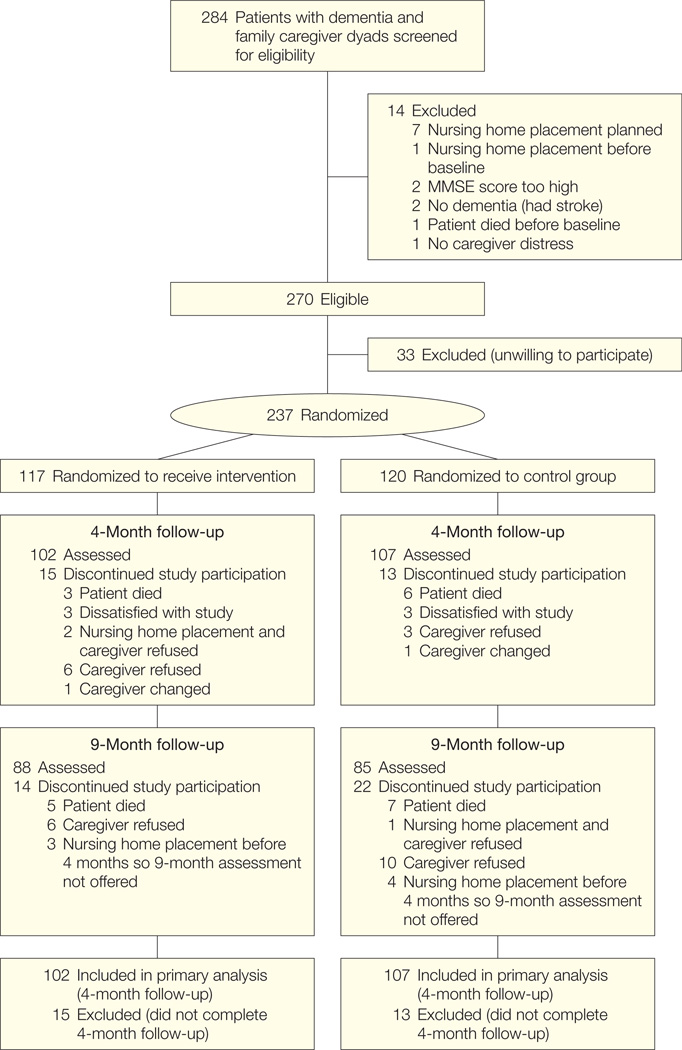
Of 284 screened, 270 dyads (95.1%) were eligible, of whom 237 (87.8%) were willing to participate. No statistically significant demographic differences were found between the enrolled dyads and the 33 dyads eligible but unwilling to participate. Study attrition was low, with 28 dyads (11.8%) lost by 4 months. A higher percentage of male caregivers (n = 12, 34.3%) dropped out compared with female caregivers (n = 16, 7.9%; χ12=19.9; P < .001) (Figure 1).
Figure 1.
Flowchart of Study Design
Of the 102 dyads in the intervention group, 3 patients were placed in nursing homes and caregivers received a modified 4-month assessment. Of the 107 dyads in the control group, 4 patients were placed in nursing homes and caregivers received a modified 4-month assessment. MMSE indicates Mini-Mental State Examination.
By 9 months, an additional 36 dyads (17.2% from 4 months) were lost to follow-up. Total study attrition by 9 months was 64 dyads (27.0%). This included 20 deaths (13 control group patients [65%], 7 intervention patients [35%]) and 10 nursing home placements (5 control patients [50%], 5 intervention patients [50%]); group differences were not statistically significant.
For the 4-month sample, patients had a mean (SD) age of 82.4 (8.9) years and a mean (SD) MMSE score of 13.4 (8.1). Most were female (n=143, 68.4%) and lived with caregivers (n=200, 95.7%). Caregivers reported managing many agitated behaviors (mean [SD], 6.4 [3.0]) and high functional dependence (mean [SD], 2.9 [1.3]). Most patients were taking medications: 95 were taking antidepressants (40.1%); 77, medications to manage behavioral symptoms (32.5%); 108, pain medications (45.6%); and 173, memory enhancers (73.3%).
Caregivers were a mean (SD) age of 62.2 (12.0) years. Most were female (n = 186, 89.0%), white (n = 146, 69.9%), and nonspouses (n = 130, 62.2%; primarily adult sons and daughters [n=115, 88.5%]) (Table 1).
Treatment Implementation
Of 102 COPE dyads, 80 (78.4%) completed 8 to 12 sessions; 3 dyads (2.9%) had fewer than 3 sessions. Overall, dyads received a mean (SD) of 9.31 (1.54) face-to-face sessions (mean [SD] length, 68.24 [38.34] minutes) and 3.25 (0.79) telephone sessions (mean [SD] lengths, 20.15 [13.12] minutes for occupational therapists; 6.27 [16.50] minutes for nurses). Intervention cost was estimated as 537.05perdyadbasedonnationalhourlysalaryorfringeratesforoccupationaltherapists(537.05 per dyad based on national hourly salary or fringe rates for occupational therapists (537.05perdyadbasedonnationalhourlysalaryorfringeratesforoccupationaltherapists(42.83) and nurses ($74.41), patient laboratory costs ($120), and the mean number and length of contacts.31 Control group dyads received a mean (SD) of 2.83 (0.42) telephone contacts lasting 15 (8.39) minutes as per protocol.
Undiagnosed Medical Conditions
Among 117 COPE patients, nurse assessments were obtained for 107 patients (91.4%) and blood or urine samples for 92 patients (85.9%; 3 refused and samples were unattainable from 12). Undiagnosed illnesses occurred in 40 patients (37.3%); 3 patients (2.8%) had 2 or more coexisting undiagnosed medical illnesses. Conditions included bacteriuria (n=6; 15%), anemia (n=4; 9%), and hyperglycemia (n=2; 5%). For the 40 patients with undiagnosed medical illnesses, 39 caregivers (97.5%) followed up with physicians; 1 refused. Among the 39 caregivers following up with physicians, 1 patient was admitted to a hospital and 29 patients were outpatients.
4-Month Outcomes
Statistically significant improvements were observed in functional dependence for COPE patients (baseline to 4 months) compared with control group patients (adjusted mean difference, 0.24; 95% confidence interval [CI], 0.03–0.44; P = .02; Cohen d = 0.21), representing a small effect. Improvement occurred mostly for IADLs (adjusted mean difference, 0.32; 95% CI, 0.09–0.55; P = .007; Cohen d = 0.43), a moderate effect. COPE patients improved slightly more in ADL functioning than controls, but this was not statistically significant (Table 2). Similarly, we observed small but statistically significant improvements in engagement for COPE compared with control patients (adjusted mean difference, 0.12; 95% CI, 0.07–0.22; P = .03; Cohen d = 0.26). We did not find statistically significant benefits for frequency of agitated behaviors or quality of life.
Compared with control group caregivers, COPE caregivers reported improvement in well-being (adjusted mean difference, 0.22; 95% CI, 0.08–0.36; P = .002; Cohen d = 0.30) and enhanced confidence using activities (adjusted mean difference, 0.81; 95% CI, 0.30–1.32; P = .002; Cohen d = 0.54), small to moderate effects (Table 2).
Table 3 shows proportions of participants with clinically meaningful changes (≥0.50 SD) for statistically significant 4-month outcomes. Net improvement across measures favored COPE participants over controls, with differences reaching statistical significance for all except activity engagement. Differences in net improvements ranged from 14.6% to 26.5%. Of 112 caregivers (53.8%) reporting 1 or more caregiver-identified problems eliminated by 4 months, 64 (62.7%) were COPE and 48 (44.9%) were control group caregivers (χ12=6.72, P = .01).
Table 3.
Clinical Significance of Main Outcomes at 4 Months
| Control Group, No. (%)a(n = 107) | Intervention Group, No. (%)a(n = 102) | Difference inNetImprovement(95% CI) | _P_Value | |||||
|---|---|---|---|---|---|---|---|---|
| Improved | Worsened | NetImprovement | Improved | Worsened | NetImprovement | |||
| Overall functional dependenceb | 41 (39.8) | 11 (10.7) | 30 (29.3) | 51 (51.5) | 3 (3.0) | 48 (48.5) | 19.2 (2.7 to 36.0) | .02 |
| IADL dependenceb | 52 (50.5) | 7 (6.8) | 45 (43.7) | 64 (64.6) | 3 (3.0) | 61 (61.6) | 17.9 (1.9 to 34.0) | .03 |
| Activity engagement | 40 (37.4) | 42 (39.3) | −2 (−1.9) | 44 (43.1) | 31 (30.4) | 13 (12.7) | 14.6 (−8.8 to 38.0) | .22 |
| Perceived change in well-being | 42 (39.3) | 21 (19.6) | 21 (19.6) | 58 (56.9) | 11 (10.8) | 47 (46.1) | 26.5 (7.2 to 45.8) | .007 |
| Confidence using activitiesc | 29 (27.4) | 24 (22.6) | 5 (4.7) | 41 (41.0) | 10 (10.0) | 31 (31.0) | 26.3 (7.9 to 44.7) | .005 |
9-Month Outcomes
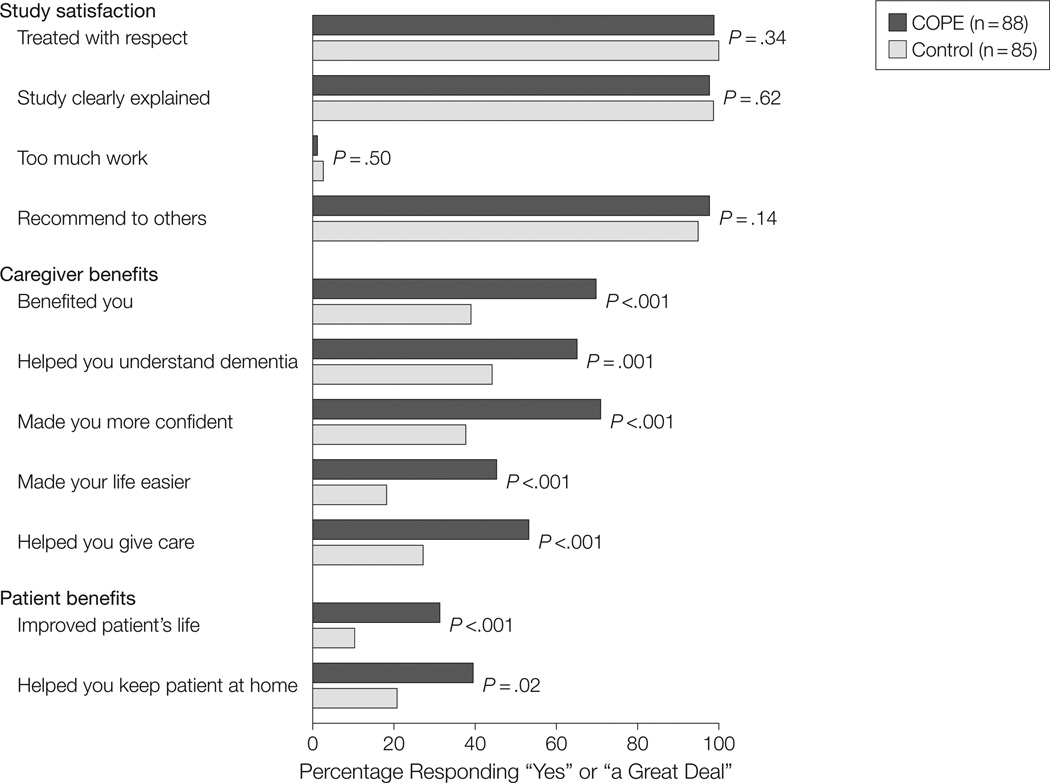
We did not find statistically significant differences between COPE and control group participants from baseline to 9 months for any outcome measure. Both intervention and control group caregivers considered study participation worthwhile and not time consuming, felt they were treated respectfully, and would recommend the study to others (all P ≥ .14). However, COPE compared with control caregivers reported a “great deal” of improvement in their lives overall (70.9% vs 38.5%, χ22=20.5, P < .001), disease understanding (66.3% vs 43.6%, χ22=15.0, P = .001), confidence managing behaviors (72.1% vs 37.2%, χ22=25.4, P < .001), made life easier (45.3% vs 17.9%, χ22=34.2, P < .001), ability to care for patients (54.7% vs 26.9%, χ22=25.7, P < .001), patients’ quality of life (32.6% vs 10.3%, χ22=17.0, P < .001), and ability to keep patients home (39.5 vs 20.8%, χ22=7.5, P = .02) (Figure 2).
Figure 2.
Perceived Benefits of Intervention and Control Group Caregivers at 9 Months
Percentages indicate those responding “yes” for Study Satisfaction items or “a great deal” for Caregiver or Patient Benefits items.
COMMENT
These findings add to an increasing evidentiary base for nonpharmacologic management of patients with dementia. We tested a multicomponent intervention that helped caregivers attend to patients’ medical well-being and simplify everyday tasks to align with patient capabilities. COPE addresses core elements of dementia care: optimizing physical health and function, engaging in daily activities, maintaining quality of life, and supporting caregivers.32 At 4 months, COPE improved patient functioning, especially IADLs; patient engagement; and caregiver well-being and confidence using activities. COPE did not improve caregiver ratings of patient quality of life or frequency of agitated behaviors, although change was in the right direction.
Improvement in patient function, albeit small, compares favorably with pharmacologic trials, yet with no adverse events or known risks. Although different functional measures were used, trials of dimebon33 and tarenflurbil6 showed no functional improvement, and benefits reported for donezepil were small (Cohen d < 0.10)34 compared with COPE (Cohen d = 0.21 for overall function, Cohen d = 0.43 for IADL). Other studies of cholinesterase inhibitors show statistically significant but small benefits for IADLs and a trend in ADL improvement, as in COPE.7 A multisite study found no differences in functioning from clinic-based treatments.9 In contrast, COPE decreased severity of overall dependence by 0.7 points and IADL dependence by 1 point. Control group caregivers also reported small functional gains of 0.5 points overall and 0.7 points for IADLs, although differences were statistically significant favoring intervention. As points on the scale reflect increments of 25% in physical assistance required by caregivers, a 1-point reduction may be clinically meaningful. Poor patient functioning is a predictor of disease progression, heightening risk of caregiver burden and nursing home placement.12 Also, dependencies are associated with increased health care costs.3 Thus, even small reductions in physical dependence may ease caregiver burden.
As to caregiver effects, pharmacologic interventions have shown only small benefits in caregiver burden (Cohen d = 0.18),8 whereas in this study COPE participants showed higher effects compared with controls, from Cohen d = 0.29 for well-being and d = 0.54 for confidence using activities to engage patients. These improvements appear to be clinically meaningful. More intervention dyads improved 0.50 SD or more than controls on outcome measures. Also, more COPE caregivers than controls reported eliminating at least 1 problem initially identified as challenging.
Consistent with recent studies,16,35 a high prevalence (close to 40%) was found of undiagnosed, treatable medical conditions for intervention patients with all but 1 dyad (97.5%) following up with physicians for treatment. However, effects of their treatment are unclear. A comparison of COPE patients with identified and treated medical problems (n=39) with COPE patients without identified medical problems or treatment (n = 63) showed similar 4-month gains. Nevertheless, managing physical health is an important aspect of dementia care. High rates of untreated conditions suggest the need for more frequent routine medical examinations because symptoms may present atypically and patients may not be able to report adequately.
At 9 months, there were no statistically significant differences in outcome measures. Nevertheless, perceived benefits favored intervention. Compared with controls, COPE caregivers reported a “great deal” of improvement in many areas, including managing care better and keeping patients home. Lack of findings for standardized measures contrasts with perceived benefits, highlighting the complexity of measuring improvements in quality of life.36
Of importance is that neither group reported finding the study burdensome, and both groups’ participants were equally willing to recommend it to others. Training and telephone education were equally well received.
Study limitations include an inability to determine active treatment components. The trial was not designed to answer this question and COPE reflects the integration of multiple components. COPE may primarily affect caregiver appraisals. As outcome measures relied on proxy report, it is difficult to rule out this pathway.
Another limitation is study generalizability. Because caregivers volunteered for participation, they may have been more aware of their role and more motivated to learn skills than nonvolunteers. 37 Only 15% of study caregivers were male and a higher proportion of male caregivers than female caregivers dropped out, so it is unclear how best to address their needs.1
A concern may be the placebo condition. Controls received information tailored to their needs,12 but the amount of time staff spent providing information was not equivalent to that in COPE. Nevertheless, our approach is an advance over previous studies employing no-treatment comparison groups.
Because most patients live at home with functional decline, a nonpharmacologic, biopsychosocial-environmental intervention may positively contribute to disease management. Future research needs to examine effects of underlying medical conditions, ways to boost treatment effects, cost-effectiveness, COPE in combination with pharmacologic treatments, and translational potential.
Acknowledgements
Funding/Support: Research reported was supported in part by funds from the National Institute on Aging and the National Institute on Nursing Research (RO1 AG22254) and the Pennsylvania Department of Health, Tobacco Settlement (SAP100027298).
Role of the Sponsor: Funding agencies had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Gitlin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Gitlin, Hauck.
Acquisition of data: Gitlin, Winter.
Analysis and interpretation of data: Gitlin, Winter, Dennis, Hodgson, Hauck.
Drafting of the manuscript: Gitlin, Winter.
Critical revision of the manuscript for important intellectual content: Gitlin, Dennis, Hodgson, Hauck.
Statistical analysis: Gitlin, Winter, Dennis, Hodgson, Hauck.
Obtained funding: Gitlin.
Administrative, technical, or material support: Gitlin, Winter.
Study supervision: Gitlin.
Financial Disclosures: None reported.
Additional Contributions: Barry Rovner, MD, Jefferson Hospital for Neuroscience, provided patient consultation, for which he did not receive additional compensation besides his salary. The interventionists who made important contributions were Michele Rifkin, MA, OTR/L, Health Through Action; Nicole Davis, MS, OTR/L; Lauren Lapin, OTR/L; Catherine Piersol, MA, OTR/L; Geri Shaw, OTR/L; and Tracey Vause-Earland, MA, OTR/L, and the nurse interventionist, Kathy Czekanski, RN, PhD. These individuals were employees or contractors for Thomas Jefferson University and were supported in part by funds from the listed granting agencies. We also acknowledge the contributions of our interviewing staff and thank the families for their study participation.
REFERENCES
- 1.2010 Alzheimer’s disease facts and figures. Alzheimer’s Association. doi: 10.1016/j.jalz.2010.01.009. http://www.alz.org/documents_custom/report_alzfactsfigures2010.pdf. Accessed August 4, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Atchison TB, Massman PJ, Doody RS. Baseline cognitive function predicts rate of decline in basic-care abilities of individuals with dementia of the Alzheimer’s type. Arch Clin Neuropsychol. 2007;22(1):99–107. doi: 10.1016/j.acn.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Hill J, Fillit H, Thomas SK, Chang S. Functional impairment, healthcare costs and the prevalence of institutionalisation in patients with Alzheimer’s disease and other dementias. Pharmacoeconomics. 2006;24(3):265–280. doi: 10.2165/00019053-200624030-00006. [DOI] [PubMed] [Google Scholar]
- 4.Soto ME, Andrieu S, Gillette-Guyonnet S, Cantet C, Nourhashemi F, Vellas B. Risk factors for functional decline and institutionalisation among community- dwelling older adults with mild to severe Alzheimer’s disease: one year of follow-up. Age Ageing. 2006;35(3):308–310. doi: 10.1093/ageing/afj059. [DOI] [PubMed] [Google Scholar]
- 5.Spillman BC, Long SK. Does high caregiver stress predict nursing home entry? Inquiry. 2009;46(2):140–161. doi: 10.5034/inquiryjrnl_46.02.140. [DOI] [PubMed] [Google Scholar]
- 6.Green RC, Schneider LS, Amato DA, et al. Tarenflurbil Phase 3 Study Group. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. JAMA. 2009;302(23):2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA. 2003;289(2):210–216. doi: 10.1001/jama.289.2.210. [DOI] [PubMed] [Google Scholar]
- 8.Lingler JH, Martire LM, Schulz R. Caregiverspecific outcomes in antidementia clinical drug trials: a systematic review and meta-analysis. J Am Geriatr Soc. 2005;53(6):983–990. doi: 10.1111/j.1532-5415.2005.53313.x. [DOI] [PubMed] [Google Scholar]
- 9.Nourhashemi F, Andrieu S, Gillette-Guyonnet S, et al. PLASA Group. Effectiveness of a specific care plan in patients with Alzheimer’s disease: cluster randomised trial (PLASA study) BMJ. 2010;340:c2466. doi: 10.1136/bmj.c2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teri L, Gibbons LE, McCurry SM, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003;290(15):2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- 11.Gitlin LN, Winter L, Corcoran M, Dennis MP, Schinfeld S, Hauck WW. Effects of the home environmental skill-building program on the caregivercare recipient dyad: 6-month outcomes from the Philadelphia REACH Initiative. Gerontologist. 2003;43(4):532–546. doi: 10.1093/geront/43.4.532. [DOI] [PubMed] [Google Scholar]
- 12.Sörensen S, Duberstein P, Gill D, Pinquart M. Dementia care: mental health effects, intervention strategies, and clinical implications. Lancet Neurol. 2006;5(11):961–973. doi: 10.1016/S1474-4422(06)70599-3. [DOI] [PubMed] [Google Scholar]
- 13.Belle SH, Burgio L, Burns R, et al. Resources for Enhancing Alzheimer’s Caregiver Health (REACH) II Investigators. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized, controlled trial. Ann Intern Med. 2006;145(10):727–738. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballard CG, Gauthier S, Cummings JL, et al. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol. 2009;5(5):245–255. doi: 10.1038/nrneurol.2009.39. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Minimental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Gitlin LN, Winter L, Dennis MP, Hodgson N, Hauck WW. Targeting and managing behavioral symptoms in individuals with dementia: a randomized trial of a nonpharmacological intervention [published online July 19, 2010] J AmGeriatr Soc. doi: 10.1111/j.1532-5415.2010.02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earhart CA. Allen Diagnostic Modules: Manual. 2nd ed. Colchester, CT: S&S Worldwide; 2006. [Google Scholar]
- 18.Miller JM, Pliskin NH. The clinical utility of the Mattis Dementia Rating Scale in assessing cognitive decline in Alzheimer’s disease. Int J Neurosci. 2006;116(5):613–627. doi: 10.1080/00207450600592164. [DOI] [PubMed] [Google Scholar]
- 19.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 20.Guide for the Uniform Data Set for Medical Rehabilitation (including the FIM instrument), version 5.1. Buffalo, NY: State University of New York at Buffalo; 1997. [Google Scholar]
- 21.Gitlin LN, Roth DL, Burgio LD, et al. Caregiver appraisals of functional dependence in individuals with dementia and associated caregiver upset: psychometric properties of a new scale and response patterns by caregiver and care recipient characteristics. J Aging Health. 2005;17(2):148–171. doi: 10.1177/0898264304274184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotter EM, Burgio LD, Roth DL, Gerstle J, Richardson P. Comparison of caregiver and occupational therapist ratings of dementia patients’ performance of activities of daily living. J Appl Gerontol. 2008;27(2):215–225. [Google Scholar]
- 23.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64(3):510–519. doi: 10.1097/00006842-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Albert SM, Del Castillo-Castaneda C, Sano M, et al. Quality of life in patients with Alzheimer’s disease as reported by patient proxies. J Am Geriatr Soc. 1996;44(11):1342–1347. doi: 10.1111/j.1532-5415.1996.tb01405.x. [DOI] [PubMed] [Google Scholar]
- 25.Logsdon RG, Teri L, Weiner MF, et al. Assessment of agitation in Alzheimer’s disease: the Agitated Behavior in Dementia scale: Alzheimer’s Disease Cooperative Study. J Am Geriatr Soc. 1999;47(11):1354–1358. doi: 10.1111/j.1532-5415.1999.tb07439.x. [DOI] [PubMed] [Google Scholar]
- 26.Gitlin LN, Winter L, Dennis MP, Hauck WW. Assessing perceived change in the well-being of family caregivers: psychometric properties of the Perceived Change Index and response patterns. Am J Alzheimers Dis Other Demen. 2006;21(5):304–311. doi: 10.1177/1533317506292283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP, Hauck WW. Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. Am J Geriatr Psychiatry. 2008;16(3):229–239. doi: 10.1097/JGP.0b013e318160da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Battle CC, Imber SD, Hoehn-Saric R, Nash ER, Frank JD. Target complaints as criteria of improvement. Am J Psychother. 1966;20(1):184–192. doi: 10.1176/appi.psychotherapy.1966.20.1.184. [DOI] [PubMed] [Google Scholar]
- 29.Schulz R, O’Brien A, Czaja S, et al. Dementia caregiver intervention research: in search of clinical significance. Gerontologist. 2002;42(5):589–602. doi: 10.1093/geront/42.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a new and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:1289–1300. [Google Scholar]
- 31.US Dept of Labor; 2006. May, Chartbook: occupational employment and wages. (2008, bulletin 2703). http://www.bls.gov/oes/. Accessed June 1, 2010. [Google Scholar]
- 32.Prince MJ, Acosta D, Castro-Costa E, Jackson J, Shaji KS. Packages of care for dementia in low- and middle-income countries. PLoS Med. 2009;6(11):e1000176. doi: 10.1371/journal.pmed.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfizer and Medivation announce results from two phase 3 studies in dimebon (latrepirdine*) Alzheimer’s disease clinical development program. Pfizer mediaroom. 2010 Mar 3; [Google Scholar]
- 34.Román GC, Wilkinson DG, Doody RS, Black SE, Salloway SP, Schindler RJ. Donepezil in vascular dementia: combined analysis of two large-scale clinical trials. Dement Geriatr Cogn Disord. 2005;20(6):338–344. doi: 10.1159/000088494. [DOI] [PubMed] [Google Scholar]
- 35.Löppönen MK, Isoaho RE, Räihä IJ, et al. Undiagnosed diseases in patients with dementia: a potential target group for intervention. Dement Geriatr Cogn Disord. 2004;18(3-4):321–329. doi: 10.1159/000080126. [DOI] [PubMed] [Google Scholar]
- 36.Rabins PV, Black BS. Measuring quality of life in dementia: purposes, goals, challenges and progress. Int Psychogeriatr. 2007;19(3):401–407. doi: 10.1017/S1041610207004863. [DOI] [PubMed] [Google Scholar]
- 37.Pruchno RA, Brill JE, Shands Y, et al. Convenience samples and caregiving research: how generalizable are the findings? Gerontologist. 2008;48(6):820–827. doi: 10.1093/geront/48.6.820. [DOI] [PubMed] [Google Scholar]