PHOSPHATIDYLINOSITOL-4,5-BISPHOSPHATE PROMOTES BUDDING YEAST SEPTIN FILAMENT ASSEMBLY AND ORGANIZATION (original) (raw)
. Author manuscript; available in PMC: 2011 Jun 10.
Published in final edited form as: J Mol Biol. 2010 Oct 15;404(4):711–731. doi: 10.1016/j.jmb.2010.10.002
Abstract
Septins are a conserved family of GTP-binding proteins that assemble into symmetric linear hetero-oligomeric complexes, which, in turn, are able to polymerize into apolar filaments and higher-order structures. In budding yeast (Saccharomyces cerevisiae) and other eukaryotes, proper septin organization is essential for processes that involve membrane remodeling, such as the execution of cytokinesis. In yeast, four septin subunits form a Cdc11-Cdc12-Cdc3-Cdc10-Cdc10-Cdc3-Cdc12-Cdc11 hetero-octameric rod that polymerizes into filaments that are thought to form a collar around the bud neck in close contact with the inner surface of the plasma membrane. To explore septin-membrane interaction, we examined the effect of lipid monolayers on septin organization at the ultrastructural level using electron microcopy. Using this methodology we have acquired new insights concerning the potential effect of septin-membrane interactions on filament assembly, and more specifically on the role of phosphoinositides. Our studies demonstrate that budding yeast septins interact specifically with phosphatidylinositol-4,5-_bis_phosphate (PIP2) and indicate that the N-terminus of Cdc10 makes a major contribution to the interaction of septin filaments with PIP2. Furthermore, we found that presence of PIP2 promotes filament polymerization and organization on monolayers, even under conditions or for mutants that prevent filament formation in solution. In the extreme case of septin complexes lacking the normally terminal subunit Cdc11, or the normally central Cdc10 doublet, the combination of the PIP2-containing monolayer and nucleotide permitted filament formation in vitro via atypical Cdc12-Cdc12 and Cdc3-Cdc3 interactions, respectively.
Keywords: septins, phosphoinositides, yeast, lipid monolayers, mutations
Introduction
Septins were discovered nearly 40 years ago via the large-scale screening for cell division cycle mutants in the budding yeast Saccharomyces cerevisiae 1 and are highly conserved from fungi to humans 2. In yeast, septins are essential for cytokinesis, in part, because they are involved in recruitment of the protein machinery required for septation and, in part, because they establish a diffusion barrier between the mother cell and the daughter bud that helps maintain cell polarity (reviewed in 3; 4). In higher eukaryotes, septins have similar roles in demarcating subcellular boundaries, for example, at the base of dendritic spines in neurons 5; 6; 7, in the annulus separating the tail from the midbody in spermatozoa 8; 9, and in formation of the compartment in which phagosomes engulf opsinized bacteria 10. Thus, in general, septin structures are found in intimate association with membranes and are implicated in processes where membrane remodeling occurs.
We have determined 11 that the four essential budding yeast septins are arranged in a linear, symmetric hetero-octamer: Cdc11-Cdc12-Cdc3-Cdc10-Cdc10-Cdc3-Cdc12-Cdc11 (Fig. 1A). At physiological ionic strength, these octameric rods (Fig. 1B, panel 1) further self-assemble end-to-end into non-polar paired filaments, resembling "railroad tracks" (Fig. 1B, panel 2). C. elegans septins 12 and human septins 13 also assemble into symmetric complexes and apolar filaments in vitro, strongly supporting the notion that a non-polar axial arrangement is a conserved feature of the architecture of septin complexes and filaments. Each septin has a common organization of structural domains 14: a central GTP-binding domain immediately preceded by an alpha-helix (α0) containing a stretch of basic residues and further upstream an N-terminal extension of variable length, and followed by a C-terminal extension (CTE) of variable length that usually contains a segment predicted to have the capacity to form a coiled-coil. As originally deduced from the crystal structure of a human hetero-hexameric septin complex (Sept7-Sept6-Sept2-Sept2-Sept6-Sept7) 13, and as we showed for the yeast septin rod 11, the contacts responsible for subunit-subunit interactions in septin complexes alternate between a "G interface" (involving residues in and around the GTP-binding domains of the adjacent monomers) and an "NC interface" (involving residues in and around the N- and C-terminal segments of the adjacent monomers) (Fig. 1A). Thus, because of the additional terminal subunit, polymerization of S. cerevisiae septin complexes involves formation of an NC interface between the Cdc11 subunits at the end of neighboring rods, whereas polymerization of the human hetero-hexameric complex is mediated by a G interface between the Sept7 subunits at the end of neighboring rods.
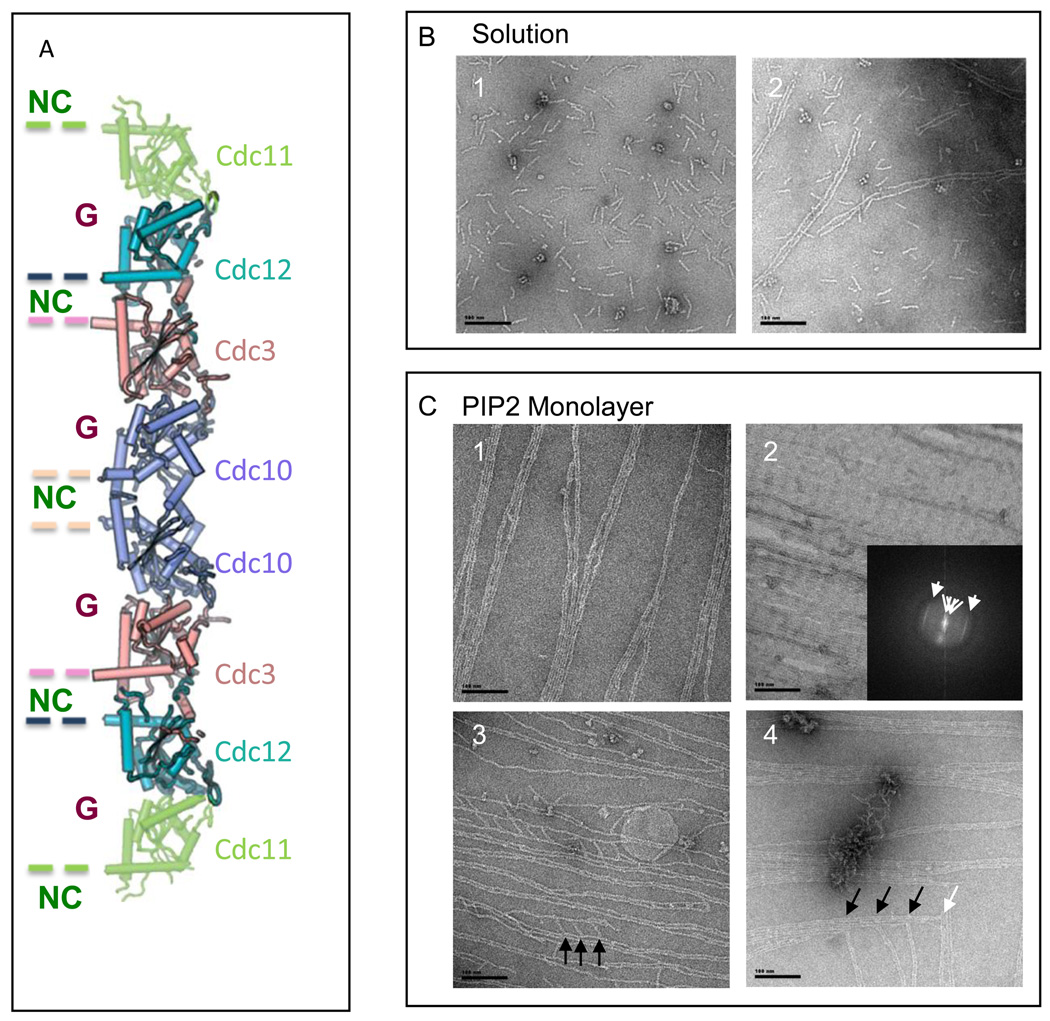
Figure 1. Effect of PI(4,5)P2-DOPC lipid monolayers on the assembly and organization of septin filaments. Scales bars: 100 nm.
A. Schematic representation of the arrangement of the septin monomers into the octameric complex. The alternation of G (GTP or GDP binding) and NC interfaces are visualized.
B. Wild type septins in vitro. Panel 1.Octameric budding yeast septin complexes in high salt (Tris-HCl pH 8, 50 mM, NaCl 300 mM) and negatively stained. Panel 2. Paired filaments, “Railroad tracks”, of septins in low salt conditions (Tris-HCl pH 8, 50 mM).
C. Wild type septins on lipid monolayers. Panel 1 Filaments of septins at an initial concentration in solution of 0.01 mg.mL−1, with a weight PI(4,5)P2 concentration of 20 %. Septin filaments are tightly paired and start forming sheets of filaments. Panel 2. Filaments of septins organized into a flat sheet of filaments, at an initial concentration in solution of 0.03 mg.mL−1, with a weight PI(4,5)P2 concentration of 20 %. The Fourier transform of the image shows two repeats: 376 and 44.3 Å, corresponding to a septin octameric and monomeric repeat, respectively. Panel 3 Filaments of septin observed after an overnight incubation, an initial septin concentration of 0.005 mg.mL−1 and a PI(4,5)P2 concentration of 20 %. Tight paired filaments are connected by short rods, which length is 36.7 ± 7 nm and which are 36.5 ± 5 nm apart. Short octameric septin rods connect sets of parallel paired filaments orthogonally. Panel 4. Filaments of septin observed after an overnight incubation, an initial septin concentration of 0.02 mg.mL−1 and a PI(4,5)P2 concentration of 20 %. Arrows point connections between the ends of paired filaments to another perpendicular paired filament and are about 70 nm apart, which is the size of two septin octamers.
Localization of yeast septins by indirect immunofluorescence or using septins fused to a fluorescence reporter protein have shown that, under normal growth conditions, these proteins are always closely associated with the inner surface of the plasma membrane, even though their structural organization changes during passage through the cell cycle. During normal mitosis, septins first localize to a patch at the incipient budding site that rapidly resolves into a ring and then expands into an hourglass-shaped collar within the bud neck, which eventually splits in half concomitant with cytokinesis, separating a mother from its new daughter cell. The collar in budding cells has been visualized in situ by electron microscopy (EM) in S. cerevisiae 15 and in Candida albicans 16. In both organisms, the septins appear to be assembled into filaments approximately 10 nm in diameter and arranged in concentric rings that are closely apposed to the inner surface of the cell membrane. Only under starvation conditions, such as those caused by nutrient limitation or those necessary to trigger meiosis, are septins found in the cytoplasm, where they co-localize with microtubules 17.
Having previously analyzed and defined the subunit arrangement and assembly properties of yeast septin complexes in vitro in solution 11, we felt it was now important to investigate septin organization under conditions that more closely resemble those present in situ. Given the intimate association of septins with the cell membrane observed in vivo, and as a first step to understanding the interplay between the plasma membrane and septin structure, we used a lipid monolayer assay to characterize septin-lipid interaction and its effect on septin filament assembly and organization. In this regard, we were particularly interested in determining whether phosphoinositides play a significant role in septin-membrane interaction. This class of lipids has functions in membrane trafficking (both exocytosis and endocytosis) 18 and in a variety of signaling pathways 19. In particular, PIP2 controls various aspects of actin cytoskeleton dynamics at the cell membrane 20; 21; 22. This phosphoinositide is almost exclusively restricted to the plasma membrane in budding yeast, and during mitosis is particularly enriched at the bud neck 23. Moreover, when PIP2 is converted to phosphatidylinositol-3,4,5-_tris_phosphate by ectopic expression of mammalian PI 3-kinase in yeast, septin filaments no longer localize to the bud neck, but instead form small rings in the cytoplasm 24, suggesting that PIP2 per se is essential for association of septins with the cytoplasmic membrane. Consistent with this conclusion, it has been shown that certain budding yeast septins and a human septin (Sept4) interact with phosphoinositides in vitro 25; 26, and it was postulated 25; 26 that this binding involves the basic residues found in what we now know constitutes the α0 segment in the N-terminus of a septin 13. Also in agreement with this view, it has been reported that a subset of human septins (Sept2/Sept6/Sept7) found in brain extracts is able to modify the morphology of phosphoinositide-containing giant liposomes by inducing the formation of protruding tubules, and EM revealed that an organized array of septin filaments formed on these protrusions 27.
Here we utilized lipid monolayers and recombinant septin complexes to mimic the conditions under which budding yeast septins would interact with the cell membrane. In our studies, we felt it was important to examine full, reconstituted septin complexes because prior analysis of septin-lipid interaction had examined only individual septins and their ability to bind to single lipids immobilized on filter strips 25. Moreover, we used our lipid monolayer approach to analyze the potential role and specificity of PIP2 in promoting the interaction between septins and the surface of the cell membrane. All samples were visualized by EM to monitor the effect of the lipids on septin assembly and organization at the ultrastructural level. As we describe, we found that PIP2, and under certain conditions the addition of guanine nucleotide, has a remarkable capacity to promote the polymerization of septin complexes into filaments and to influence their arrangement in ways that we did not previously observe in solution.
RESULTS
Refined molecular model of the yeast septin complex
In solution, purified recombinant Cdc11-Cdc12-Cdc3-Cdc10-Cdc10-Cdc3-Cdc12-Cdc11 complexes are short rods at high salt (above 200 mM NaCl) (Fig. 1B, panel 1); when the salt concentration is lowered below 200 mM, the rods associate end-on-end to form long paired filaments (separated by ~10 nm) (Fig. 1B, panel 2) 11. Using single-particle methodology, we examined many thousands of negatively-stained rods. Using the resulting images, we generated a 3-dimensional reconstruction of the yeast hetero-octamer at 27 Å resolution (the same structure was reproducibly obtained using projection matching to distinct initial models) (Fig. S1). The yeast hetero-octamer displays a narrow face (Fig. S1A) and a wider face (Fig. S1B), as was also observed in the crystal structure of the human hetero-hexamer 13. This sidedness, together with our previous characterization of the NC and G interfaces between the subunits in the yeast rod 11, enabled us to dock the crystal structure of human Sept2 within the envelope for each monomer (Figs. S1A and S1B) to produce a molecular model of the yeast hetero-octamer (Fig. 1A), which has helped to guide the design of structure-based mutants and interpretation of our data.
Lipid monolayer assay to analyze septin-membrane interaction
Lipid monolayers were prepared as described by Taylor et al. 28. A drop of lipids dissolved in chloroform was deposited on the meniscus of a solution of purified septin complex in the well of a Teflon block. The lipid mixtures used contained 1,2-dioleoyl-_sn_-glycero-3-phosphocholine (DOPC) alone or doped with different amounts of various phosphoinositides. Because of its two unsaturated fatty acyl chains, DOPC generates a more fluid monolayer than phosphatidylcholine derivatives containing saturated fatty acyl chains (like dimyristoyl-PC or dipalmitoyl-PC); this greater fluidity facilitates incorporation of other phospholipids, including phosphoinositides 29. After a given incubation time, the resulting lipid monolayer was picked up by touching the carbon-coated face of an grid to the meniscus and the material on the grid was then either negatively stained or for examination by EM. The best hydrophobic support for the lipid monolayers was provided by perforated (holey) carbon-coated grids because continuity of the monolayer was maintained across the air-water interface above the holes. In this study, the proportion of phosphoinositide in the lipid monolayer is given in weight percent (and molar ratio versus DOPC can be obtained, for example for PIP2, by dividing the weight percent by 1.56). Each condition tested was repeated and examined independently at least three times.
PIP2 is specifically required for septin association with the lipid monolayer
When lipid monolayers contained DOPC alone, no septins were associated with the grid (Fig. S2A, panel 1), even after overnight incubation. Likewise, when the DOPC was doped with phosphatidylinositol 4-phosphate (PIP), even at concentrations as high as 20%, no septins were associated with the grids (Fig. S5A, panel 1, and Fig. S5B, panel 1). By contrast, when the DOPC was doped with 20% PIP2, robust septin deposition on the monolayer was observed (see further below). To rule out that the recruitment of septins to the monolayer was merely an electrostatic effect due to the fact that PIP2 has a higher net negative charge than PIP, we tested phosphatidylinositol-3,4,5-_tris_phosphate (PIP3). At 20% PIP3 (or even higher concentrations (30–50%), very few septin structures were found associated with the grids, even at relatively high septin concentration and even after overnight incubation (Fig. S5A, panel 2–4 , and Fig. S5B, panel 2–4). Thus, PIP2 was unique in its ability to promote highly efficient septin binding to the DOPC monolayer.
Given the striking and specific effect of PIP2, we explored in a systematic manner a range of conditions and concentrations to optimize the DOPC lipid monolayer assay. First, the effect of various PIP2 concentrations from 2.5 to 75% were tested at a constant septin concentration (0.02 mg-mL−1) with overnight incubation (Fig. S2A). We also tested the effect of septin concentration (from 0.005 mg-mL−1 to 0.1 mg-mL−1) at a constant PIP2 concentration (20%) with overnight incubation (Fig. S2B). To assess the kinetics of septin deposition and organization, we used lipid monolayers containing 20% PIP2 and a septin concentration of 0.03 mg-mL−1 (Fig. S3), and varied the time after the lipid was applied (from 10 sec to overnight) before the monolayer was transferred from the meniscus to the grid.
Effect of PIP2-containing monolayers on the organization of septin filaments
Even at the lowest amount of PIP2 tested (2.5%), clumps of aggregated septins were associated with the surface of the grid (Fig. S2A, panel 2). As the amount of PIP2 was increased (5%–50%), both the amount and level of organization of the septins increased dramatically (Fig. S2A, panels 3 and 4). We found that 5% PIP2 was sufficient to induce assembly of the yeast septin complexes into paired filaments, and at 10% PIP2 and above further changes in septin organization were induced. In general, we found 20% PIP2 to be optimal in terms of the clarity of visualization and degree of organization exhibited.
Most strikingly, on a lipid monolayer containing 20% PIP2, the filaments form tight pairs instead of the more loosely paired "railroad track"-like filaments observed for filaments captured from solution (Fig. 1C, panel 1). These double filaments were observed at protein concentrations starting as low as 0.005 mg-mL−1 (Fig. S2B, panel 1), equivalent to the concentration that gives rise to paired filaments in solution. Although shorter and sparser, tightly paired filaments were observed at incubation times as short as 10 sec; by 1 min, tightly paired filaments increased dramatically in length and number, and became much more concentrated by 30 min (Fig. S3). The filaments in these tight pairs run side-by-side on the plane of the lipid without any visible separation between them, and such double filaments tend to associate laterally with neighboring double filaments. Moreover, at increasing septin concentration (>0.02 mg-mL−1) (Fig. 1C, panel 2; see also Fig. S2, panels 3 and 4) and longer incubation time (>1 hr) (Fig. SD), the capacity for lateral association permits the tightly-paired filaments to organize into extended sheets. In these sheets, lateral association of the filaments seems to involve homotypic interactions between septins, based on the apparent register of an extra feature that recurs at a octamer-length position along the long axis of the filament (Fig. 1C, panel 4, and Fig. 2A). Indeed, Fourier transforms of large sheets of filaments formed after overnight incubation show diffraction peaks at 44 and 370 Å (Fig. 1C, panel 2), which correspond to the repeat expected for a septin monomer and the entire septin hetero-octamer, respectively, further supporting the notion that in the sheets the septin octamers in juxtaposed filaments are arranged in register. By contrast, the few filaments seen on monolayers with 20% PIP3 were neither paired rail-road tracks nor tight pairs, but rather associated into small, very compact bundles and no organized sheets were ever observed (Fig. S5A, panel 2–4 , and Fig. S5B, panel 2–4).
Figure 2. PI(4,5)P2 lipid monolayers facilitate filament formation.
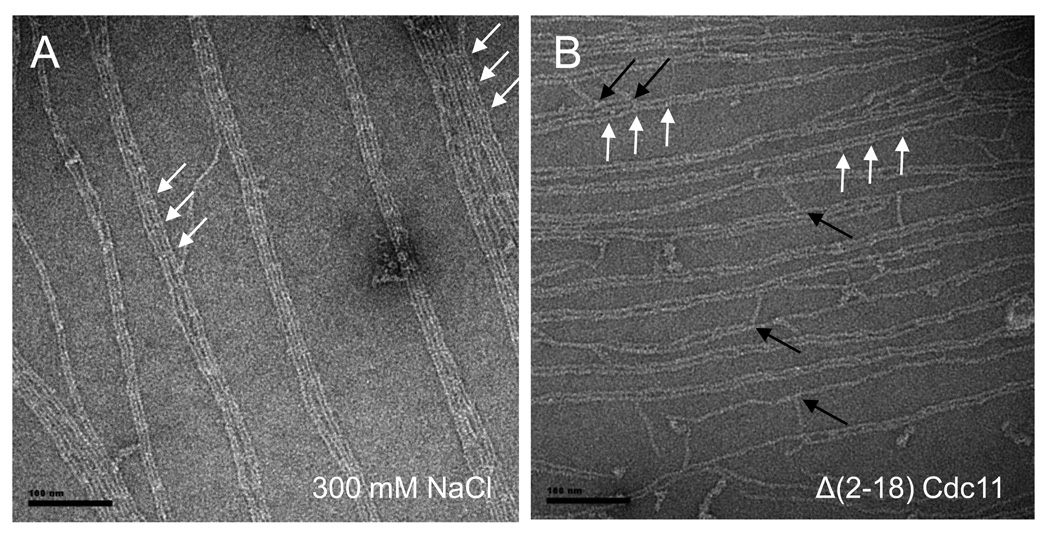
Scale bars: 100 nm long. 2.A. Filaments and sheets of filaments of septins in high salt conditions (Tris-HCl, pH8, 50 mM, NaCl 300 mM) with a starting concentration of 0.02 mg.mL−1 on DOPC lipid monolayers doped with 20 % PI(4,5)P2. 2.B. Δα0Cdc11 septin complexes in low salt (Tris-HCl 50 mM, with a starting concentration of 0.005 mg.mL−1 can form filaments on PIP2 (20% in DOPC) lipid monolayers. A periodic and octameric decoration can be visualized (white arrows). Short octameric rods are able to connect tight paired filaments (black arrows).
Under the same conditions (20% PIP2), but at lower protein concentration (<0.02 mg-mL−1) (Fig. S2B, panel 2) or at short incubation times (<30 min) (Fig. S3), the tightly paired filaments coexist with somewhat thicker filaments that appear to correspond to a tight pair in which the filaments appear twisted around each other in rope-like fashion. None of the distinct, lipid-induced structures discussed above were present in mock incubations lacking the purified septins (Fig. S2B, panel 1).
Lipid monolayer does not affect the overall rate of filament formation
Given the striking effects of the PIP2-containing lipid monolayer on the arrangement of septin filaments, we also wanted to assess whether the rate of filament formation was also affected. Hence, we compared the kinetic behavior we observed for filament polymerization on the lipid monolayers (Fig. S3) to septin filament formation in solution. To measure the rate of septin filament formation in solution, we using a stopped-flow apparatus and monitored light scattering of the solution because filament formation and elongation cause a readily detectable increase in scattering intensity (Fig. S4A). To initiate polymerization, septins at the same concentration used in our lipid monolayer assays (0.02 mg-mL−1) were diluted from high salt to low salt at various temperatures (5–20 °C). We found that nucleation/initiation of polymerization in solution took less than 1 min (Sup. Fig. 4A) and was temperature-dependent, as expected for a diffusion-dependent reaction (Fig. S4A). To corroborate that the change observed by light scattering corresponded to authentic filament formation, samples of the 5 and 20 °C solutions were withdrawn at different time points (5 and 30 sec) and examined by EM (Fig. S4B–F). Indeed, by 30 sec, long paired filaments were observed at both temperatures. At 5 sec and at 4 °C, apparent polymerization intermediates were found, which we quantified (Fig. S4C). In brief, in solution and at room temperature, long paired filaments were formed readily within 5 sec (Fig. S4E), and on the lipid monolayer somewhat shorter paired filaments were found bound within the same time frame. We conclude that association with the lipid monolayer does not have a major effect (either stimulatory or inhibitory) on the rate at which septin hetero-octamers polymerize into filaments.
Lipid monolayer reveals septin filaments can adopt a mesh-like arrangement
A mode of septin filament organization not previously observed in solution was frequently seen on the lipid monolayers containing 20% PIP2 after overnight incubation, especially at lower protein concentrations (e.g., 0.005 mg-mL−1). In this unique arrangement, shorter septin filament doublets originate within and run orthogonal to longer paired filaments or filament bundles (Fig. 1C, panel 3). These perpendicular septin filaments can also span from one set of filaments to another creating an interlocked mesh-like structure. The length of the shortest of these cross-bridge filaments is 36.7 ± 7 nm, consistent with the length of a single septin hetero-octamer, suggesting that these rods are interacting via their terminal Cdc11 subunit, with Cdc11 subunits present at periodic sites in the paired filaments. Consistent with this view, the spacing between cross-bridges is 36.5 ± 5 nm (or multiples thereof), again corresponding to the length of a hetero-octamer. Also in agreement with the conclusion that the contacts responsible for the cross-bridges are mediated via Cdc11-Cdc11 interaction is the fact that we were occasionally able to visualize paired filament ends making direct connection with the end of a perpendicular paired filament (Fig. 1C, panel 4, arrows). Finally, such meshes were never observed for septin complexes containing a Cdc11 mutant lacking its CTE (see below), suggesting that the Cdc11-Cdc11 interaction required for mesh formation is mediated via its C-terminal coiled-coil element.
At much higher protein concentration (e.g., 0.5 mg-mL−1), we observed large arrays that appear to be two complete sets of paired filaments running orthogonally to each other (Fig. S2B, panel 4); however, in this case, the two sets of filaments simply criss-cross each other and do so with a repeat that does not correspond to any septin dimension, suggesting that this pattern may represent an overlay of one sheet of filaments upon another, a perhaps non-specific interaction somehow facilitated by the monolayer.
PIP2-containing lipid monolayers promote septin filament assembly under conditions that preclude filament assembly in solution
Another striking effect of the PIP2-containing lipid monolayer was that it permitted polymerization of yeast septin complexes into filaments under conditions in which they do not polymerize in solution. At high ionic strength (typically, 300 mM monovalent salt), yeast septin complexes exist in solution as hetero-octameric rods as defined previously 11 (Fig. 1A) and only polymerize to form paired filaments when the salt concentration is lowered by dialysis or dilution, suggesting that high salt prevents polymerization by masking electrostatic contacts necessary for filament formation. Unexpectedly, after deposition of septin complexes in high salt solution onto the 20% PIP2 monolayer, EM analysis revealed that robust filament formation occurred (Fig. 2A). This result is remarkable because, unlike the situation where the monolayer is layered onto a septin solution in low salt (where paired filaments are already preformed), in the high salt solution the septin complexes are presumably present as free octamers and therefore must be recruited to and self-assemble into filaments on the surface of monolayer. In fact, comparing otherwise identical conditions (20% PIP2, 0.02 mg-mL−1 protein), except for whether the septin complexes used were present in low or high salt, the filaments displayed on the carbon-coated grids appeared equally or even more uniform when they were recovered from high-salt solution than from low-salt solution (compare Figure 2A to Fig. 1C, panel 1). At significantly higher protein concentration (e.g., 0.1 mg-mL−1,), there was little or no difference between the septin structures recovered from low and high salt solution, although both were not quite as well-organized as those produced at lower protein concentration. The robust assembly of septin complexes recovered from high salt solution into numerous well-ordered paired filaments on PIP2-containing monolayers was specific to this phosphoinositide because it was not observed with monolayers containing equivalent concentrations of either PIP or PIP3 (Fig. S5B).
As a means to demonstrate that hetero-octamer polymerization requires contacts at the NC interface between the terminal Cdc11 subunits in adjacent rods, we have shown previously that septin complexes containing a mutant, Cdc11(Δ2–18), that lacks its α0 helix abolished self-assembly in solution without having any significant effect on the overall structure or stability of the hetero-octamer itself 11. In marked contrast, septin complexes containing the same mutant clearly assembled into filaments in the presence of the 20% PIP2-containing monolayer support (Fig. 2B). The resulting filaments seem quite similar in organization to those formed from wild-type septin complexes, even including the orthogonal filament cross-bridges (Fig. 2B, black arrows) and the prominent feature that occurs with octamer periodicity (Fig. 2B, white arrows). In addition, assembly of these mutant complexes into filaments exhibited equivalent kinetics, and displayed the same dependency on protein concentration and PIP2 concentration, as wild-type complexes. Because the residues (2–18) deleted in this Cdc11 mutant contain an especially prominent tract of basic residues (R12KRKHLKR19) (Fig. S7), these experiments rule out that this segment is required for the effects of PIP2 on septin organization.
Lipid monolayers reveal novel septin-septin contacts mediated by their globular domains
To explain the railroad track-like nature of the paired filaments observed in solution at low salt, we proposed that the observed gap between the filaments and their side-by-side association was mediated by formation of anti-parallel coiled-coils between the CTEs of septins on one filament and the CTEs of partner septins on the other filament 11. However, in the filament doublets we observed on the surface of the PIP2-containing monolayers, the lateral interaction between the filaments seems to involve direct contact between the globular domains of septins on one filament and the globular domains of the corresponding septins on the other filament because the filaments are tightly paired and in register. Alternatively, on the lipid monolayer, the coiled-coil contacts could collapse, adopting a conformation that nonetheless provides the majority of the binding energy necessary to stabilize the interfilament contacts. To distinguish between these possibilities, we examined the ability of septin mutants that lack their CTE (including their coiled-coil region) to form the hetero-octameric complex and to undergo filament pairing and elongation both in solution and on 20% PIP-containing lipid monolayers (Fig. 3).
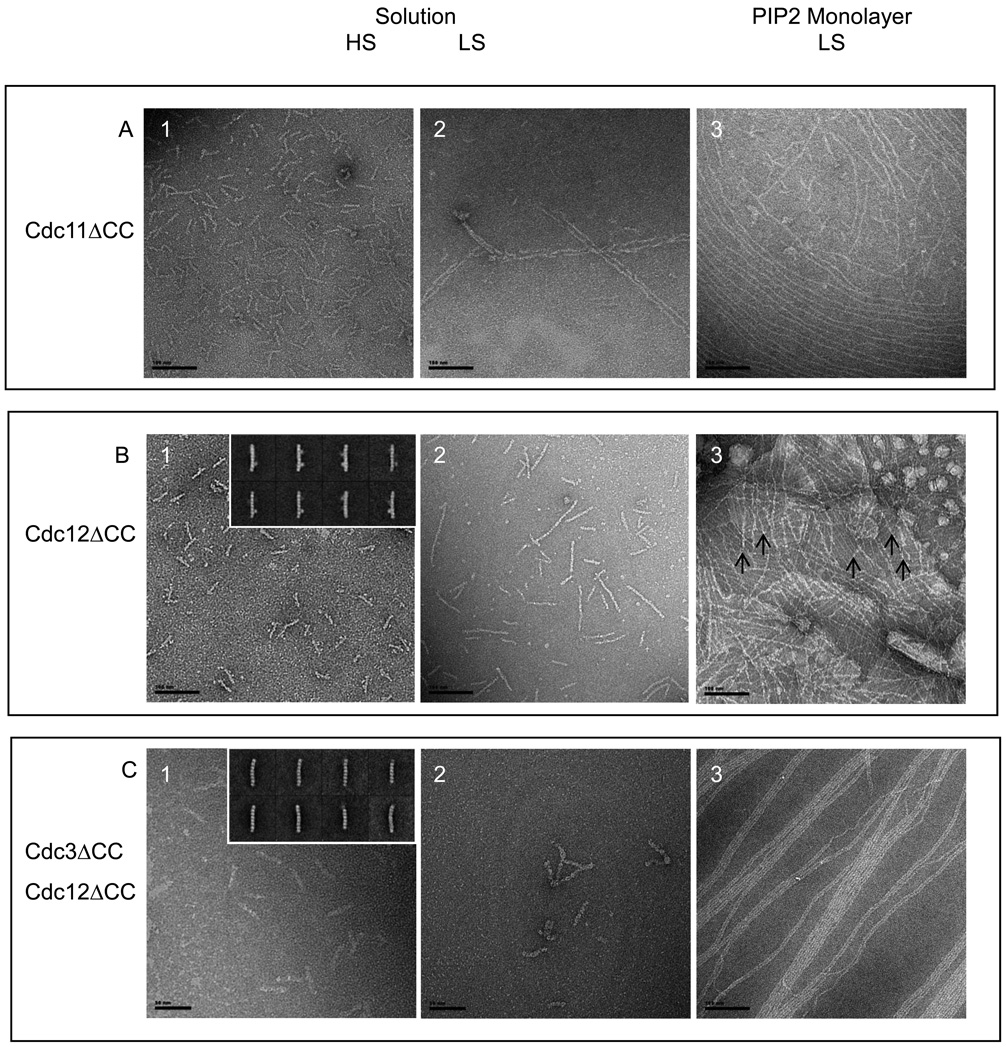
Figure 3. Assembly of ΔCTE septins.
Scale bars: 100 nm. In solution (A, panels 1–2, B, panels 1–2, C, panels 1–2) and on lipid monolayers (A, panel 3;B, panel 3;C, panel 3) for Cdc11 (Δ 306–415)-Cdc3-Cdc12-Cdc10 (A), MBP-Cdc12 (Δ 318–407) -Cdc3 - Cdc10- Cdc11 (B) and (Cdc12 (Δ 318–407) -Cdc3 (Δ 419–520)- Cdc10- Cdc11 (C). For all lipid monolayers assay, DOPC lipid monolayers contained 20% PIP2 and proteins were incubated overnight with lipids.
A, panel 1. Cdc11 (Δ 306–415)-Cdc3-Cdc12-Cdc10 septins form octameric rods in high salt conditions and paired filaments in low salt (A, panel 2). A, panel 3. On lipid monolayers, septin complexes with a missing coiled coil at the C-terminus of Cdc11 form filaments. The octameric decoration of the filaments is not visible and sheets of tight filaments do not form.
B, panel 1. MBP-Cdc12 (Δ 318–407) -Cdc3- Cdc10- Cdc11 septin form stable octameric rods,in high salt conditions. Some of the class averages are shown on the inset, where Cdc12 displays an extra density that corresponds to the MBP tag. B, panel2. Septins without the Cdc12 C-terminal extension, in low salt solution do not assemble into long paired filaments. Instead they form short single filaments (of the size of 3–4 multiple of octamers), which can occasionally pair. B, panel3. Septins without the Cdc12 C-terminal extension, in low salt solution on lipid monolayers. Septins can form filaments and lattices of interconnected and perpendicular ones. C, panel 1. Septins without both of Cdc12 and Cdc3 C-terminal extensions, in high salt solution still form octameric rods. From 2D classification and averaging from a dataset of 1,400 particles, 50 % of octamers, 31.5 % of heptamers and 18.5% of shorter hexamers are obtained. Class averages are shown on the inset. C, panel 2. Septins without both of Cdc12 and Cdc3 C-terminal extensions, in low salt solution do not assemble into paired filaments. C, panel 3. Septins without both of Cdc12 and Cdc3 C-terminal extensions, in low salt solution on a PIP2-DOPC lipid monolayers assemble into tight paired filaments and extended sheets.
In agreement with our previous findings 11; 30, septin complexes containing a Cdc11 mutant lacking its entire CTE (residues 306–415) (Cdc11ΔCC) formed hetero-octamers and polymerized into paired filaments both in solution and on the PIP2-containing monolayer (Fig. 3A). However, compared to wild type, these paired filaments show a reduced propensity to associate laterally and never displayed the cross-bridging filaments seen in the mesh-like structures exhibited by wild-type septins. Moreover, the characteristic feature that occurs with octamer periodicity that we found in wild-type filaments (and in all other mutants capable of filament formation on the lipid monolayer) was absent in the filaments containing Cdc11ΔCC. These observations show that that the 110-residue CTE of Cdc11 constitutes the element responsible for the octameric repeat seen along the filament axis in our images and that homotypic Cdc11-Cd11 interaction mediated by its CTE is necessary for formation of the cross-braced mesh-like organization produced by polymerized wild-type septin filaments.
Prior studies suggested that the linchpin of the septin hetero-octamer is a Cdc3-Cdc12 heterodimer and that coiled-coil formation between the CTEs of these two subunits is necessary for their stable association 30. Moreover, available evidence suggested that these coiled-coils formed the anti-parallel cross-filament connections in paired filaments formed in solution because the Cdc11 CTE was not necessary for formation of paired filaments and Cdc10 lacks a CTE 11. However, septin complexes containing a Cdc12 mutant lacking its entire CTE (residues 318 to 407) including its coiled-coil element (residues 377 to 407) (Cdc12ΔCC) (Fig. 3B, panel 1), or septin complexes containing both Cdc12ΔCC and a Cdc3 mutant lacking its entire CTE (residues 419–520) (Cdc3ΔCC) (Fig. 3C, panel 1), formed hetero-octamers, indicating that, when Cdc10 and Cdc11 are present, hetero-octamer formation does not require interaction of the Cdc3 and Cdc12 CTEs. The Cdc12ΔCC mutants were tagged with a MBP at their N terminus. The tag is clearly visible in the inset of fig.3B, panel 1. Nonetheless, the resulting hetero-octamers were detectably less stable than wild-type complexes; after two dimensional averaging and classification of images of the complexes containing both Cdc3ΔCC and Cdc12ΔCC, the distribution among 1,400 particles was 50% octamers, 32% heptamers, and 18% hexamers (Fig. 3C, panel 1, inset), whereas wild-type septin complexes are almost exclusively octamers 11. Although the CTEs of Cdc3 and Cdc12 seem to project far from the G interface between Cdc12 and Cdc11 (Fig. 1A), the large proportion of heptamers indicates that the lack of the Cdc3 CTE-Cdc12 CTE interaction weakens the Cdc12-Cdc11 interface, presumably due to conformational destabilization of Cdc12. The observed hexameric complexes could arise from conformational destabilization of the Cdc3-Cdc12 interface, leading to loss of both Cdc11 and Cdc12 from one end of a rod. In any event, these results demonstrate that the Cdc3 CTE-Cdc12 CTE interaction, while not essential for hetero-octamer assembly, provides additional contacts that help stabilize the hetero-octamer. Moreover, in solution, the Cdc12ΔCC complex formed only very short and unpaired filaments (2–3 hetero-octamers long) and the complex containing both Cdc3ΔCC and Cdc12ΔCC was unable to form any filaments.
In marked contrast, when analyzed using the 20% PIP2-containing monolayer, both complexes formed long, tightly paired filaments, indicating that the lateral contacts observed in the filament doublets formed on the lipid monolayer are not mediated by and do not require the CTEs of either Cdc3 or Cdc12, consistent with a role for direct interactions between the globular domains in the observed lateral filament-filament association. On the lipid monolayer, the filaments formed by the Cdc12ΔCC complex typically were arranged as perpendicular arrays; however, unlike such arrays of wild-type filaments, the mutant filaments contained both "thin" and "thick" filaments, presumably corresponding to single (unpaired) and paired filaments, and the thin filaments seem to cross the thick ones at a tetramer-length rather than at an octamer-length distance (Fig. 3B, panel 3, crossing shown by arrows), suggesting the interfilament cross-connections may be mediated via the free Cdc3 CTEs. In agreement with this viewpoint, the filaments formed by the complexes containing both Cdc3ΔCC and Cdc12ΔCC were much longer, straighter, and did not exhibit the tendency to form the orthogonal arrays.
PIP2-containing lipid monolayer promotes nucleotide-dependent polymerization of Cdc11-less and Cdc10-less septin complexes
Cdc3 and Cdc12 are essential for survival of S. cerevisiae cells, whereas it is possible to isolate yeast variants that can survive in the absence of either Cdc10 or Cdc11, although the latter septin-deficient mutants display temperature-sensitive growth, certain morphological abnormalities, and cytokinesis defects 31; 32. In high salt, septin complexes lacking Cdc11 (Cdc11-less complexes) form stable hetero-hexamers that are missing the terminal subunit at each end, but are unable to polymerize into filaments when the salt concentration is lowered 11; 30. However, at high protein concentration (0.15 mg-mL−1) and in the presence of exogenously added GTP (0.5 mM), there is some tendency for the rods to associate end-to-end to form rudimentary filaments 33. Strikingly, this tendency was dramatically promoted on the surface of the 20% PIP2-containing monolayer, where long tightly paired filaments were formed, and further enhanced by guanine nucleotide (Fig. 4A). Compared to the absence of nucleotide, both GDP and GTP, and even GTPγS, had similar effects. Presumably, this rod-rod association is mediated via interaction of the G interface of the Cdc12 subunits exposed when Cdc11 is absent. If so, this Cdc12-Cdc12 G interface is insensitive to the phosphorylation state of the bound nucleotide, despite the fact that Cdc12 is competent to bind and hydrolyze exogenously added GTP 33.
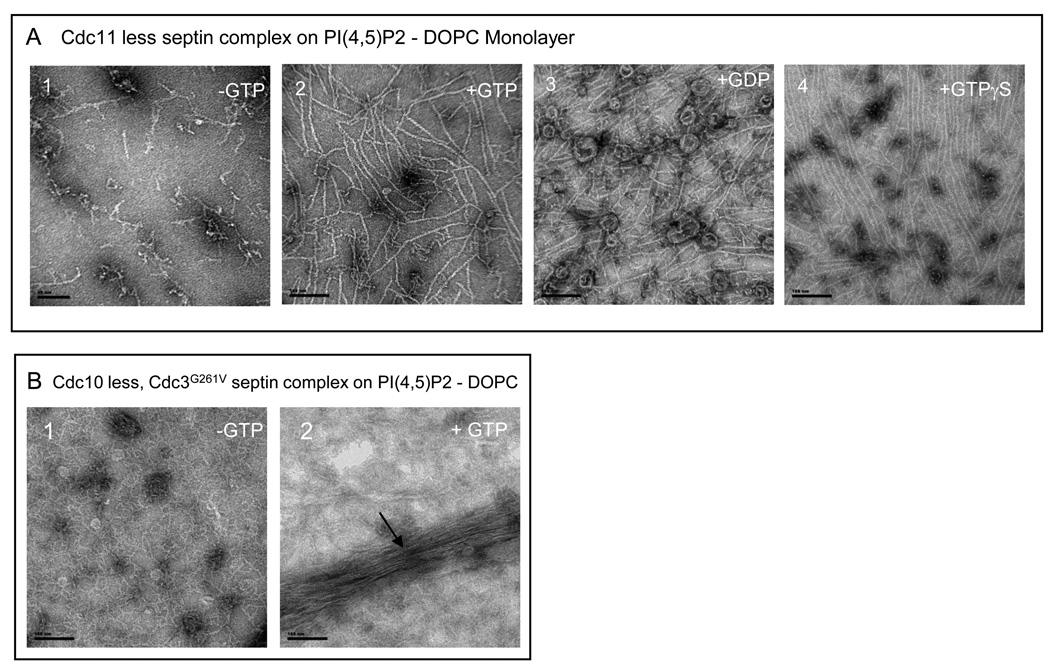
Figure 4. Effect of nucleotides and PI(4,5)P2 on the assembly of the Cdc11-less and Cdc10-less septin.
Scale bars:100 nm.
A. Cdc11-less septins organized on lipid monolayers with a PIP2 concentration of 20 % and a septin concentration 0f 0.03 mg.mL−1, in low salt conditions. A, panel 1. No nucleotide is added. A, panel 2. GTP 0.5mM is present. In A, panel 3 and B, panel 4, GDP 0.5 mM and GTP gamma-S are respectively added at a concentration of 0.5 mM. B. Cdc10-less, Cdc11-Cdc12-Cdc3(G261V) septin complexes organized on lipid monolayers with a PI(4,5)P2 concentration of 20% and a septin concentration of 0.02 mg.mL−1, in low salt conditions with (B, panel 2) or without (B, panel 1) additional GTP 0.5 mM.
In high salt, septin complexes lacking Cdc10 (Cdc10-less complexes) form stable hetero-trimers that are missing the central pair of subunits 11, and are unable to form filaments when the salt concentration is lowered 11, although others have observed bundles of filamentous material 30; 32. In the course of our studies of septin-septin interactions, and as described in detail elsewhere (McMurray, Bertin et al., unpublished results), we isolated a mutation in Cdc3, Cdc3(G261V), that significantly improves the growth of Cdc10-less cells at elevated temperature. Interestingly, single-particle analysis of Cdc10-less complexes also containing Cdc3(G261V) showed that, in addition to the expected hetero-trimers, a readily detectable number of hetero-tetramers were observed [as well as some pentamers and hexamers] (McMurray, Bertin et al., unpublished results). Assuming no reshuffling of the standard subunit order (Fig. 1A), these observations suggested that the G261V mutation increases the stability of Cdc3-Cdc3 interaction mediated by their G interface. If stabilized, Cdc11-Cdc12-Cdc3(G261V)-Cdc3(G261V)-Cdc12-Cdc11 hexamers should be capable of Cdc11-Cdc11-mediated polymerization. Indeed, at high protein concentration (0.15 mg-mL−1) and on the surface of the 20% PIP2-containing monolayer, Cdc10-less complexes exhibited robust formation of bundles of disorganized filaments in a guanine nucleotide-dependent manner (Fig. 4B).
Septin-monolayer interaction
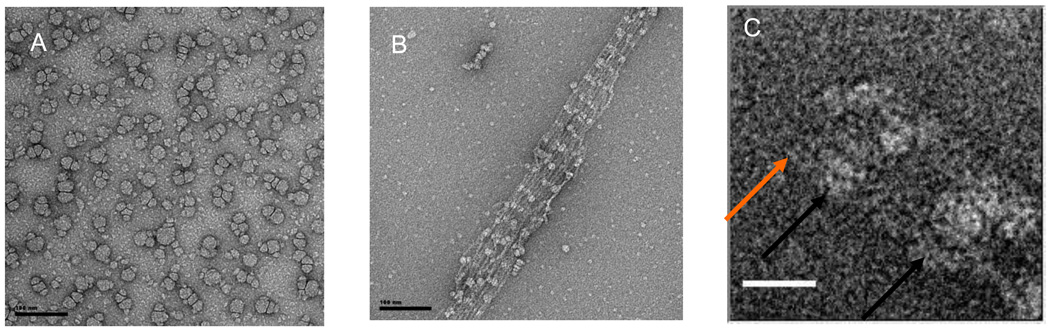
In the absence of DOPC, PIP2 applied to the septin-containing solution and then transferred to the carbon-coated grid did not exhibit a monolayer-like distribution, but appeared in the EM as small micelles (or, occasionally, tubes) (Fig. 5A). Septin complexes polymerized under low-salt conditions in the presence of such PIP2 micelles yielded filament bundles decorated periodically with these lipid structures (Fig. 5B). The distance between these marks was 320±20 nm, which is the length of the hetero-octamer, suggesting that under these conditions PIP2 binds most avidly to either Cdc11 or Cdc10. We have shown previously 11 that the paired filaments in such bundles are in register and that ends of the filaments in the sheaf all terminate with Cdc1l, as expected for end-on-end assembly of Cdc11-Cdc12-Cdc3-Cdc10-Cdc10-Cdc3-Cdc12-Cdc11 rods. Analysis of the images of the PIP2-decorated bundles at higher magnification (Fig. 5C) indicated that the micelles are not bound at the end of the bundle (Fig. 5C, orange arrow), but rather about half an octamer length from the end (Fig. 5C, black arrow), indicating that the micelle is likely interacting primarily with Cdc10. Consistent with this conclusion, we observed over the course of many experiments that the amount of septin material recoverable on the 20% PIP2-containing DOPC monolayer was distinctly less for the Cdc10-less complexes than for all of the other variations we examined.
Figure 5. Periodic decoration of septin bundles by PI(4,5)P2 micelles.
Scale bars in A and B: 100 nm, scale bar in C: 20 nm
A. Micelles of PIP2 in low salt conditions. B. Decoration of septin bundles by PIP2 micelles with an octameric repeat. C. The orange arrows point at the bundle tips while the black arrows point at the lipid micelles.
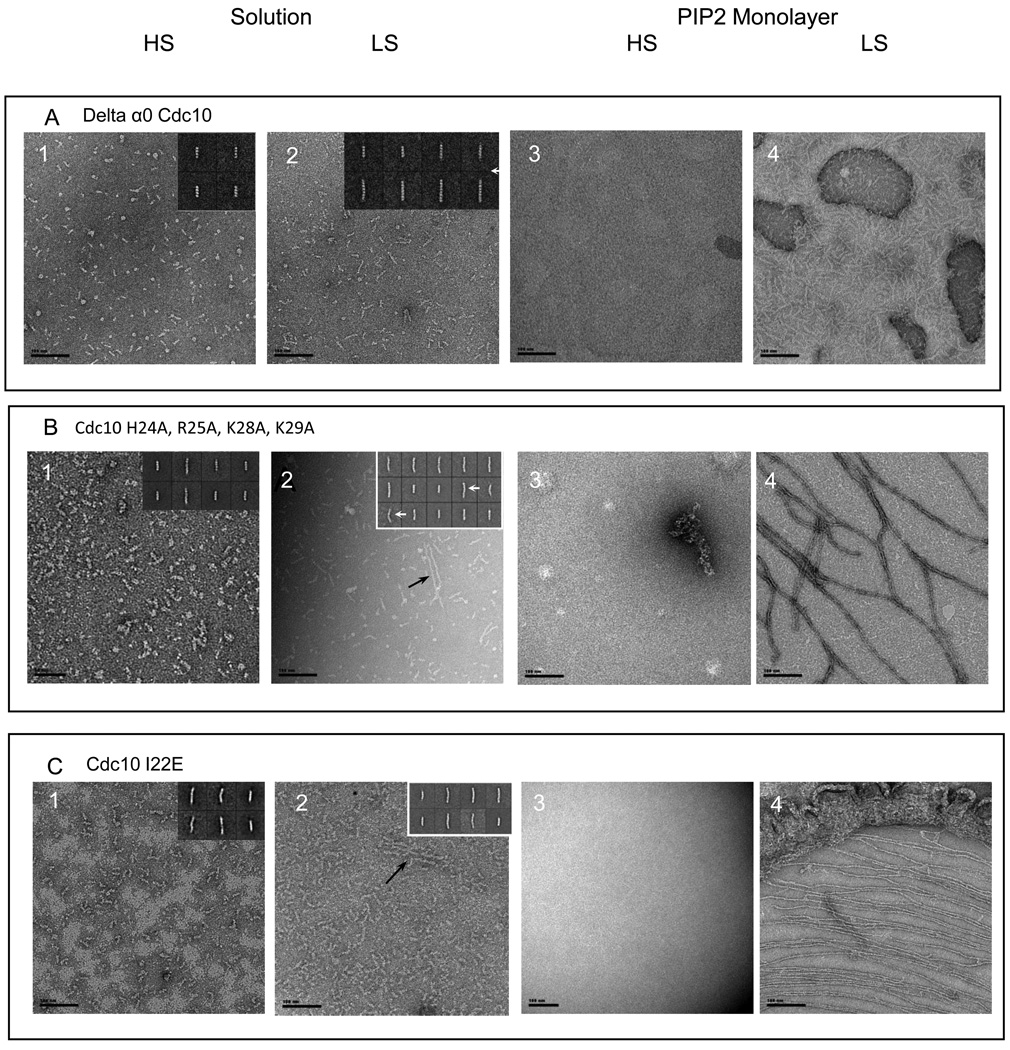
To further analyze the role of Cdc10 in the association of septin filaments with the PIP2-containing lipid monolayer, three kinds of Cdc10 mutants were generated: a deletion removing nearly all of its α0 helix, Cdc10(Δ12–28) (Fig. S7); point mutations that eliminated all four of the basic residues in α0, Cdc10(H24A R25A K28A K29A); and, replacement of a hydrophobic residue in α0 that is situated within the NC interface of the Cdc10-Cdc10 dimer with a charged reside, Cdc10(I22E). As expected, based on the importance of side chains in α0 for providing the contacts that make up a substantial portion of the buried surface area of the NC interface 13 that holds the central Cdc10-Cdc10 dimer in the hetero-octamer together (Fig. 1A), septin complexes containing Cdc10(Δ12–28) formed exclusively Cdc11-Cdc12-Cdc3-Cdc10(Δ12–28) hetero-tetramers in high salt (Fig. 6A, panel 1, inset, and Table 1) and were only able to form "inside-out" Cdc10(Δ12–28)-Cdc3-Cdc12-Cdc11-Cdc11-Cdc12-Cdc3-Cdc10(Δ12–28) rods when the salt concentration was lowered (Fig. 6A, panel 2, and Table 1). In the presence of the PIP2-containing monolayer, this same pattern was observed (Fig, 6A, panels 3 and 4). Interestingly, compared to the Cdc10-less complex, it appeared that much more of the Cdc10(Δ12–28) complex was recovered on the PIP2-containing monolayer. Interestingly, a single basic residue of those in α0, K29, remains in the Cdc10(Δ12–28) mutant, which may explain its greater tendency to associate with the PIP2-containing surface, in comparison to complexes lacking Cdc10 altogether. Alternatively, even the incorporation of such a mutant Cdc10 into hetero-octamers may favorably influence how basic residues in other septins in the rod are presented to the PIP2-containing surface.
Figure 6. mutations in the alpha 0 helix of Cdc10.
Scale bars: 100 nm.
Cdc10 in complex with Cdc3, Cdc11 and Cdc12 was mutated in its alpha0 helix. The alpha0 helix was either deleted from amino acid 12 to 28 (A) or point mutations were designed. Basic residues were mutated to alanine (H24A, R25A, K28A, K29A Cdc10) (B). Isoleucine 28 was mutated to glutamic acid (C)). Those complexes were analyzed either in solution (A, panels 1–2; B, panels 1–2; C, panels 1–2) or on lipid monolayers of 20 % PIP2 in DOPC (A, panels 3–4; B, panels 3–4, C, panels 3–4). Representative 2-D class averages are shown on the right hand inset of each image when septin complexes are observed in solution. In solution and high salt, tetrameric complexes are mainly observed. The point mutants display a small percentage of longer rods as compared with the deletion of the whole alpha0 helix (A, panel 1; B, panel 1; C, panel 1). In solution and low salt conditions, short paired filaments can rarely be seen for the point mutants (B, panel 2 and C, panel 2, black arrows). Class averages show a kink in the middle of some of the longer rods (white arrows). On 20% PIP2 lipid monolayers with a starting septin concentration of 0.02 mg.mL−1, filaments can be recovered for the point mutants, in low salt (B, panel 4; C, panel 4). However those filaments were more rarely observed for the I22E mutation (C, panel 4). The Cdc10(Δα0)-Cdc11-Cdc12-Cdc3 does not polymerize on lipid monnolayers (A, panel 4).
Table 1.
| Septin | mutation | Solution(high salt) | Solution(Low Salt) | Lipidmonolayer(High Salt) | Lipidmonolayer(low salt) |
|---|---|---|---|---|---|
| Cdc10 | Δ alpha 0 (Δ12–28) | Trimers 11.7%Tetramers 88.3% | Tetramers 41.3%Pentamers 8.03%Hexamers 10.8%Heptamers 18.2%Octamers 21.8% | Noorganization | Noorganization |
| H24A, R25A,K28A, K29A | Tetramers 63.4%Pentamers 19.2%Hexamers 3.1%Heptamers 14.2% | Tetramers 28.6%Pentamers 7.2 %Hexamers 23.1%Heptamers 10.6%Octamers 30.5%Occasional Shortpaired filaments | Noorganization | organized | |
| I22E | Tetramers 31.9%Pentamers 31.8%Hexamers 21.6%Heptamers 24.9 %Octamers 11.1 % | Tetramers 26%Pentamers 6.2 %Hexamers 6.2%Heptamers 8.8%Octamers 52.8%Short pairedfilaments | Noorganization | Rareorganization | |
| Cdc11 | Δ alpha 0 (Δ2–18) | Heptamers 5.4%Octamers 94.6% | Octamers mainly | organized | organized |
| R12A, K13A, R14A,K15A, K16A | Hexamers 5 %Heptamers 28 %Octamers 67 % | Hexamers 11 %Heptamers 46%Octamers 43 % | organized | organized | |
| Δ C-ter (Δ306–415) | Octamers mainly | Paired filaments | organized | organized | |
| Cdc3 | R111A, R112A,K115A | Octamers mainly | Paired filaments | Noorganization | organized |
| K90A, R93A, R94A | Octamers mainly | Paired filaments | Noorganization | organized |
Unlike complexes containing Cdc10(Δ12–28), complexes containing Cdc10(H24A R25A K28A K29A) formed a much greater proportion of rods of greater than tetramer length (Table 1), a considerable proportion (~25%) of which displayed a visible kink (Fig. 6B, panel 2, inset), indicating that these point mutations weakened, but did not destroy, the ability of Cdc10 to dimerize via its NC interface. In solution, these Cdc10(H24A R25A K28A K29A)-containing septin complexes displayed only a weak ability for end-to-end polymerization when the salt concentration was lowered (Fig. 6B, compare panel 2 to panel 1). However, at low salt, but on the surface of the PIP2-containing lipid monolayer, these complexes assembled into long tightly paired filaments. However, we noted that these filaments were always organized into rather sparse and loose networks and never formed compact sheets, in agreement with our other evidence that the highly basic character of the Cdc10 α0 is important for efficient association of septin complexes with the PIP2-containing lipid monolayer and/or for lateral packing of filament doublets.
Like septin complexes containing Cdc10(H24A R25A K28A K29A), septin complexes containing Cdc10(I22E) formed a significant fraction of rods of greater than tetramer length (Table 1), which displayed only a weak ability for end-to-end polymerization when the salt concentration was lowered (Fig. 6C, compare panel 2 to panel 1). However unlike septin complexes containing Cdc10(H24A R25A K28A K29A), assembly of Cdc10(I22E)-containing complexes into paired filaments on the surface of the lipid monolayer at low salt was very inefficient. Images, like that shown in Fig. 6C, panel 4, were observed only very rarely. Thus, in addition to weakening the NC interface of the Cdc10-Cdc10 pair at the center of the hetero-octameric rod, it is possible the I22E increases charge repulsion between Cdc10 and the acidic head group in PIP2.
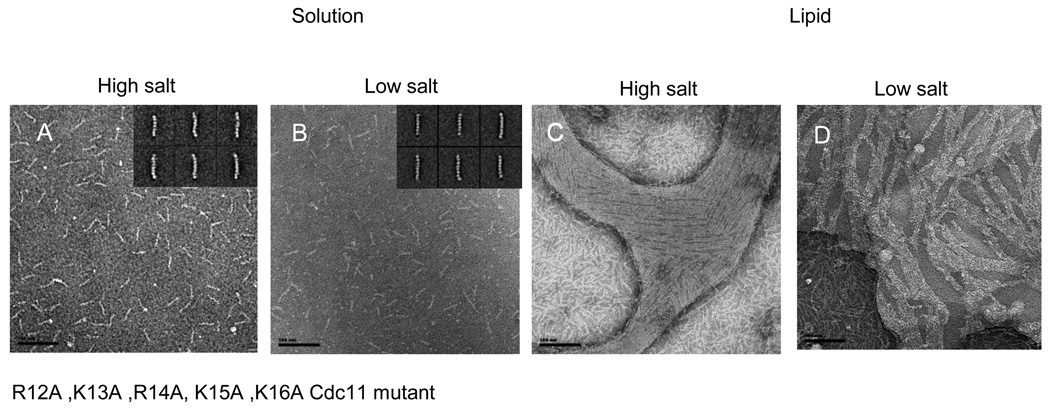
Although Cdc10 appears to bind PIP2 with the highest affinity and specificity in our lipid monolayer assay, other septins (in particular, the basic residues in their α0) may also contribute additional contacts with the PIP2-containing surface (Fig. S7). To explore this possibility, we constructed additional mutations in other septins. For septin complexes containing Cdc11(R12A K13A R14A K15A K16A), in which five of the seven basic residues in the Cdc11 α0 have been mutated, hetero-octamer formation was only moderately reduced (Table 1), but, as observed before for Cdc11(Δα0) 11, rod polymerization into filaments in low-salt solution was eliminated (Figs. 7A and 7B), as expected for an alteration that weakens the Cdc11-Cdc11 NC interface. Once again, however, with the additional stabilization provided by association with the PIP2-containing lipid monolayer, the Cdc11(R12A K13A R14A K15A K16A)-containing complexes were able to efficiently form arrays of closely packed, tightly paired filaments (Figs. 7C and 7D).
Figure 7. Mutation in the alpha0 helix of Cdc11.
Scale bars: 100 nm.
The Cdc11 (R12A K13A R14A K15A K16A) Cdc11 mutant was analyzed in solution (A–B) and on lipid monolayers (C–D) in high salt (A and C) and low salt conditions (B and D). In solution, octameric rods are mainly observed in both high salt and low salt. Typical class averages are shown on the insets. On lipid monolayers tight paired filaments can be seen.
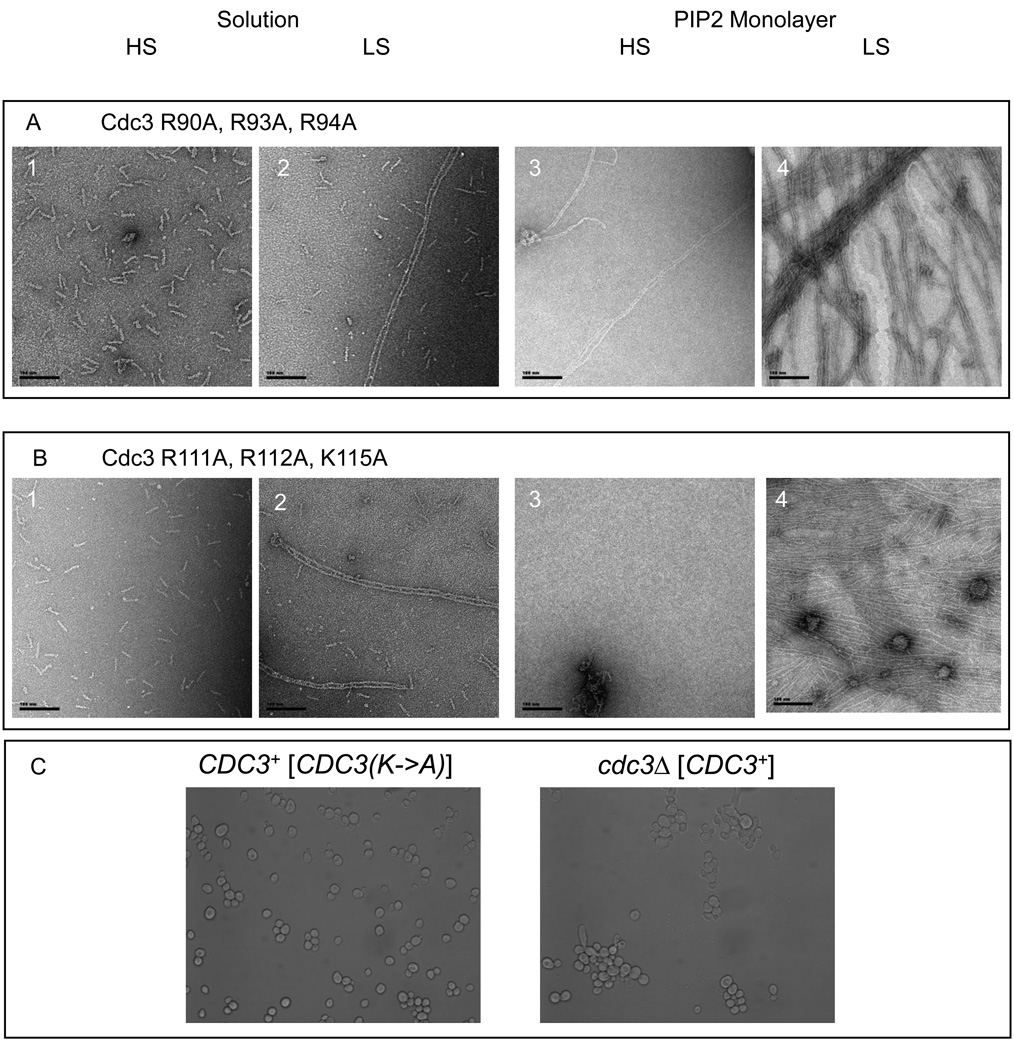
In addition to five basic residues in its α0, Cdc3, has a short track of three additional basic residues just 12 residues upstream. Hence, these regions were independently mutated to produce two different mutants: Cdc3(K90A R93A R94A) and Cdc3(R111A R112A K115A). In conjunction with the other septins, both of these mutants behaved nearly indistinguishably from wild-type septin complexes— they formed almost exclusively stable hetero-octamers in high-salt solution (Table1), polymerized into paired filaments in low-salt solution (Figs. 8A and 8B, compare panels 2 to panels 1), and, on the PIP2-containing lipid monolayers, polymerized into tightly paired filaments, although the bundles and sheets were less well organized and association with the monolayer was more efficient when the complexes were recruited from low-salt solution than from high-salt solution (Figs. 8A and 8B, panels 3 and panels 4). Therefore, these basic segments in Cdc3 make only a modest contribution to the ability of the septin complex to associate with and organize upon the PIP2-containing monolayer. Consistent with this conclusion, even when a sextuple mutant lacking both sets of basic residues, Cdc3(K90A R93A R94A R111A R112A K115A), was expressed as the sole source of this septin in vivo, the cells displayed a wild-type phenotype with regard to both growth and morphology (Fig. 8C).
Figure 8. Cdc3 mutants.
Scale bars: 100 nm.
Cdc3 (R90A, R93A, R94A)–Cdc10–Cdc11-Cdc12 (A) and Cdc3 (R111A, R112A, K115A)–Cdc10–Cdc11-Cdc12 (B) were analyzed both in solution (A, panels 1–2; B, panels 1–2) and on DOPC lipid monolayers doped with PIP2 20 % (A, panels 3–4; B, panels 3–4). In solution, both of the mutants assemble into octameric rods in high salt (A, panel 1 and B, panel 1) and into paired filaments (A, panel 2 and B, panel 2) in low salt conditions. Samples were incubated on lipid monolayers, overnight, with a starting septin concentration of 0.02 mg.mL−1 in either low salt (A, panel 3; B, panel 3) or high salt (A, panel 4; B, panel 4) conditions. The Cdc3 (R90A, R93A, R94A)–Cdc10–Cdc11-Cdc12 mutant can form filaments and even sheets of filaments on lipid monolayers (A, panels 3–4). Cdc3 (R111A, R112A, K115A)–Cdc10–Cdc11-Cdc12 assembles into filaments on lipid monolayers (B, panels 3–4) at 0.02 mg.mL−1 as well. C. Budding yeast cells grown at 37 °C, wild type (C, panel 2) or mutants Cdc3(R90A, R93A, R94A R111A, R112A, K115A) (C, panel 1). No difference is discernible.
We did not generate any mutations in this same region of Cdc12, because compared to the other yeast septins, its α0 is the least basic (Fig. 7S) and because in experiments testing the ability of individual purified septins to bind to single lipids immobilized on filter strips, Cdc12 exhibited the poorest affinity for phosphoinositides compared to the other septins tested (our unpublished results).
Discussion
PIP2-containing lipid monolayers facilitate septin polymerization
Our studies demonstrate that the supramolecular architecture of septin filaments is dramatically influenced by interaction with PIP2 on the surface of a lipid monolayer. First, certain aspects of filament organization are different. Paired septin filaments deposited from solution onto carbon-coated grids were joined laterally with a gap between them, which is spanned by thin periodic connections attributed to CTE-CTE interactions between Cdc3 and Cdc12 on one filament with those on the neighboring filament, yielding a railroad track appearance 11. By contrast, when the solution was exposed to the PIP2-containing DOPC solution and the lipid-associated material transferred to the carbon-coated grid, the resulting filaments were seen to be paired very tightly, with no gap, and mutagenesis studies showed that the CTEs of neither Cdc3 nor Cdc12 were necessary for this arrangement. Thus, on the PIP2-containing lipid surface, lateral association of the globular domains was markedly enhanced. Moreover, the tightly paired filaments are arranged in register, i.e. Cdc11 in one filament pairs with Cdc11 in the neighboring filament, Cdc12 pairs with Cdc12, and so forth. This configuration at the filament level of organization is a dramatic manifestation of the propensity to self-associate that is displayed by individual purified septins in the absence of preferred partners 13; 30; however, in filaments, the NC and G interfaces that mediate septin-septin association in the rod are all occupied, and thus the side-by-side homotypic interaction of septins in the tightly paired filaments must be occurring by a novel interface. Mutagenesis of residues outside of the NC and G contact surfaces may help define this new interface.
A second striking difference between filaments recovered from solution and those deposited on the PIP2-containing monolayer is that the tightly paired filaments had a strong tendency to further self-associate laterally into packed sheets. This behavior was enhanced at higher protein concentration and at longer incubation time. This level of organization seems to be dependent on the CTE of Cdc11 because the only septin complexes that fail to display formation of such sheets were complexes containing Cdc11ΔCC. Thus, Cdc11 appears to be able to engage in three types of self-association: the NC interaction necessary for rod assembly into filaments; lateral association of its globular domain to form tightly paired filaments; and, CTE-mediated "cross-linking" of filaments into meshes. Moreover, in the absence of the CTE-dependent interlinking of filaments, for example, when the protein concentration is low and the number of tightly paired filaments is sparse, the filaments in a pair have a tendency to entwine around each other to give a rope-like appearance.
A third remarkable difference between solution and the lipid monolayer is that we frequently observed that the tightly paired filaments organized into an array that was interconnected by orthogonal, periodically positioned septin rods, creating a mesh-like network. We have never observed such an arrangement in filaments recovered from solution. However, septin-containing structures comprising tightly paired, rope-like filaments in a gauze-like arrangement were reported in EM images of the yeast cell cortex obtained by rapid-freeze and deep-etch of yeast spheroplasts [28]. Thus, although our PIP2-containing DOPC monolayers obviously do not represent the full lipid (or protein) complexity of the native yeast plasma membrane, our assay system nonetheless appears to mimic aspects of how the cell membrane influences septin filament organization. Therefore, another conclusion to be drawn from our simplified system is that PIP2 in the plasma membrane appears to be a major factor for recruitment of septins to the plasma membrane and for organizing septin structures there. Consistent with this view, in S. cerevisiae, PIP2 is confined almost exclusively to the plasma membrane and is enriched at the bud neck 23. Hence, our monolayer procedure resembles more closely than solution methods the conditions that these proteins encounter in vivo.
Nevertheless, the level of PIP2 we routinely used in our assays (20% by weight; i.e., 13.2:86.8::PIP2:DOPC) may seem rather high, given that most estimates of the total phosphatdylinositol concentration in the yeast plasma membrane indicate no more than 10% 34; 35 and thus the PIP2 concentration at a significantly lower value 36. However, we did observe effects of PIP2 on septin recruitment to and organization on the lipid monolayers even at a much lower concentration (5%). Moreover, some estimates of the total phosphatidylinositol content of the yeast plasma membrane are much closer to the range we used 37, varying from 17.7 % 38 to 27.7 % 39. Furthermore, the major complex sphingolipids in yeast are decorated with inositol-phosphate 39 and, in addition, the PIP2 distribution can be non-uniform and locally concentrated by its incorporation into lipid rafts 40; 41. Therefore, the level of PIP2 at septin assembly sites in vivo could approach the concentration we routinely used and achieve similar effects to those we have observed in vitro.
Another prominent difference between septin filament assembly and organization in solution and that on the PIP2-containing monolayers was that, on this surface, filament formation was observed for certain mutant septin complexes that are unable to polymerize into filaments in solution. Presumably, deposition on the monolayer achieves three things. First, the local concentration of proteins is increased and the frequency of their encounter enhanced when they are confined to a two-dimensional surface. This may explain how the PIP2-containing monolayer promotes assembly of hetero-octamers captured directly from high-salt conditions. In fact, the high concentration of charges on the monolayer surface (+ on DOPC and − on PIP2) may efficiently displace the monovalent counter-ions. Second, contact with PIP2 may induce conformational changes in specific septins that allosterically alter the inter-septin contact surfaces, exposing residues that compensate for the alterations in the mutants. Third, even if no conformational change is induced, the sum of many additional, although individually weak, contacts of the septins with the lipid headgroups may stabilize native septin conformation and thereby overcome unfolding or other deleterious effects caused by the mutations. In these ways, alterations that otherwise cripple septin complex organization and/or filament assembly in solution can be "rescued." A corollary of these considerations is that those mutations for which filament formation could be restored on the PIP2-containing lipid monolayer cannot be total loss-of-function alleles. A particularly dramatic example that supports this conclusion is the fact that rods containing the Cdc11(Δ𢀓18) mutation, which totally abrogates filament formation in solution 11, do polymerize on the PIP2-containing lipid monolayer. Thus, the Cdc11(Δ2–18) deletion weakens, but does not totally destroy, the NC contact surface and presence of the lipid monolayer provides additional contacts that help stabilize this otherwise weakened interface. Conversely, and for the preceding reasons, assembly in solution could be considered a more stringent condition for assessing the effects of septin alterations.
Most striking in this regard is the fact that even septin complexes lacking certain core subunits altogether could form filaments on the PIP2-containing monolayer. In agreement with the conclusion that Cdc11 provides the links responsible for both the mesh-like organization and formation of the laterally packed sheets, Cdc11-less complexes formed tightly paired filaments that were disorganized and dispersed. Such Cdc11-less complexes are stable heterohexamers 11 with Cdc12 as the terminal subunit. Consistent with the filaments arising from polymerization of these hexameric rods via Cdc12-Cdc12 interaction mediated by their G interface, filament formation by Cdc11-less complexes was strongly guanine nucleotide dependent. Similarly, Cdc10-less complexes, in particular those also containing the Cdc3(G261V) mutation (McMurray, Bertin et al., unpublished results), were able to polymerize into paired filaments, albeit much less efficiently than the Cdc11-less complexes, and, also unlike the Cdc11-less complexes, what filaments that did form had a tendency to form disorganized bundles. However, like the Cdc11-less complexes, filament formation by Cdc10-less complexes was also guanine-nucleotide dependent. This nucleotide requirement and the necessity of the Cdc3(G261V) mutation to observe detectable filament formation by Cdc10-less complexes in our assay is consistent with filament formation arising from polymerization of the complexes via Cdc3-Cdc3 interaction mediated by their G interface. Taken together, these in vitro findings provide a mechanistic confirmation of our conclusion based on genetic analysis, described in detail elsewhere (McMurray, Bertin et al., unpublished results), that cells lacking Cdc10 or Cdc11 are able to survive because they are still able to form filaments in vivo via atypical Cdc3-Cdc3 and Cdc12-Cdc12 interactions.
The role of PIP2 in septin-membrane interaction
Several prior observations pointed to a role for phosphoinositides, and specifically PIP2, in septin filament organization and stability in vivo. First, as described previously 24 and confirmed here (Fig. S6A), conversion of plasma membrane PIP2 to PIP3 by induced expression of a plasma membrane-targeted derivative of the mammalian phosphatidylinositol 3-kinase p110, a class of enzyme that S. cerevisiae lacks, causes septin filaments (marked with Cdc10-GFP) to fragment, to be displaced from the bud neck, and to re-assemble in the cytosol as small rings and spirals. Second, shift of yeast temperature-sensitive mutants to restrictive temperature revealed (Fig. S6B) that continous synthesis of PIP2 by the sequential action of the plasma membrane-associated phosphatidylinositol 4-kinase Stt4 and the plasma membrane-associated phosphatidylinositol-4P 5-kinase Mss4, but not the Golgi body-associated phosphatidylinositol 4-kinase Pik1, is required for stability of the collar of septin filaments at the bud neck. However, in the cell, PIP2 could be playing roles at many levels. For example, Gic2, an effector of the small GTPase Cdc42 that is involved in initiating septin recruitment and assembly at the incipient bud site 42 is a PIP2-binding protein 43, and protein kinase Cla4, which is necessary for septin filament assembly 34, is stimulated by Cdc42, which is, in turn, activated by Cdc24, a guanine nucleotide exchange factor with a DH-PH domain that binds preferentially to PIP2 44. Regardless of what other effects PIP2 may have in vivo, what our results with the synthetic PIP2-containing monolayer and purified recombinant septin complexes demonstrate is that this lipid interacts directly with and has effects on the septins themselves. In this regard, conditions that generate elevated PIP2 production in vivo promote actin polymerization 45, in part by disrupting association between actin monomers and the actin-sequestering protein profilin 46. Since in yeast, as in animal cells, an actin-based contractile ring is assembled at the bud neck and participates in cytokinesis 47, and septins are found in close association even with purified actomyosin rings isolated from yeast cells 48, PIP2 may have a dual role in organizing both septin-and actin-based stuctures at the bud neck.
Characterization of the septin-PIP2 interaction
The most obvious and accessible chemical signature of PIP2 is the unique distribution of negative charges on its head group. The structural element in septins with the greatest concentration of positive charge is the tract of basic residues found in the α0 helix. Indeed, studies by others have implicated these residues in the ability of individual septins to bind to phosphoinositides 25; 26. However, except for Cdc11, the α0 helix in the other septins in the complex seem buried within the NC interfaces (Fig. 1A). To determine whether some of the side chains in the α0 might be able to contact the lipid surface, we constructed a homology-based model of yeast septin filament organization, based on the following considerations. First, it should be recalled that the yeast septin complex displays two distinct views in the EM, one wider than the other, which gives the rods and filaments a sidedness (Fig S1), a characteristic also observed at near-atomic resolution for the human septin heterohexameric complex (widest dimension, ~41 Å; narrower dimension, ~33 Å). Second, the peak derived from Fourier transform analysis of the packed sheets of tightly paired filaments on the PIP2-containing monolayer corresponds to a filament separation of ≥44 Å, suggesting that the filaments associate with the surface via their wide side. Using MODELLER 49 and the crystal structure of human Sept2 as the reference 50, the individual yeast septin subunits (Cdc3, Cdc10, Cdc11, Cdc12) in a filament were modeled and then superimposed onto the structure of the human filament (PDB ID = 2QAG) using RAPIDO 51. Our resulting model for the quaternary structure of a portion of a yeast septin filament is shown in Fig S7C (upper image). If the wide side of the filament model is laid on a membrane bilayer, the parallel CTEs at the NC interfaces project parallel to the surface and, most importantly, the Cα atoms of the basic residues in Cdc10 (highlighted as green balls) are positioned such that the corresponding side chains would appear to be within contact distance of the lipid headgroups. Moreover, a more recent crystal structure of human Sept2 shows that its α0 helix shifts its position when guanine nucleotide is bound at the G interface 50. Hence, one can imagine that nucleotide binding might facilitate interaction of the α0 helices with lipid; and, furthermore, through conformational coupling, this relationship is likely reciprocal, such that septin-lipid association may also promote guanine nucleotide binding and/or hydrolysis. In the same vein, since the Cdc10-Cdc10 NC dimer at the center of the hetero-octamer is flanked on each side by a Cdc3 subunit, the interaction of α0 in Cdc10 with lipid and the resulting effects on the conformation and/or nucleotide-bound state of Cdc10 could be communicated allosterically to Cdc3, and from cdc3 to the other subunits bound to it, further promoting adoption of orientations that optimize both overall septin-septin and filament membrane interactions.
In the above discussion, we have focused on the pivotal role of the α0 helix in Cdc10 in association of yeast septin filaments with membranes because we found that, of all the combinations and variations that we explored, complexes lacking Cdc10 altogether or complexes containing three different Cdc10 α0 mutations (Δ12–28; I22E; and, H24A R25A K28A K29A) interacted the most poorly and/or failed to assembly into filaments under conditions where wild-type complexes robustly formed filaments. By comparison, for example, an α0 deletion (Δ2–18) or mutation of five basic residues in α0 in Cdc11 caused virtually no discernible difference from wild-type filament formation and behavior on the PIP2-containing monolayer. For Cdc3, eliminating positive charge in α0 only reduced the ability of the septin complex to bind to lipids under high-salt conditions, but not under low-salt conditions. Therefore, the basic residues in α0 of Cdc3 apparently make some contribution, but only a modest one, to binding to the PIP2-containing monolayer. Nonetheless, given the extensive surface area of the wide side of a filament in contact with the lipid monolayer, many additional side chains from the globular domains of all of the septin subunits could be contributing many additional interactions with the lipid surface that, cumulatively, have an important influence. This possibility may explain why, aside from Cdc10, removal of basic residues from the α0 segments of other yeast septins had little or no (Cdc11) or only modest (Cdc3) effects. Likewise, this possibility may explain why a mutant human Sept7(K27A R28A)-Sept6-Sept2(K32A K33A) complex was still able to bind to PIP2-containing liposomes 27.
Our studies show that the CTEs of Cdc3 and Cdc12 contribute to the overall stability of the hetero-octamers, but are not required for their assembly or for their ability to assemble into tightly paired filaments or packed sheets on the surface of the PIP2-containing DOPC monolayer. Thus, under our assay conditions, the binding energy provided by the lipid support and the laterally interaction of their globular domains predominates and is sufficient to maintain rod structure and facilitate rod polymerization.
Conclusions
We have shown here that a PIP2-containing lipid surface specifically facilitates septin filament formation and dramatically influences both the structure of filaments and how the filaments are organized with respect to each other. Thus, this approach has provided a tool that has supplied considerable new information about what factors could influence septin organization in situ. Moreover, some of the different arrangements of septin filaments we observed may correspond to distinct organizational states in vivo as cells progress through the cell cycle. Although this membrane mimic has provided many new insights, the next challenge is to delineate at the ultrastructural level the actual organization of septins at the cortex of a yeast cell. The advent of EM tomography makes this goal potentially feasible 52.
Material and Methods
Septin expression and purification
Complexes containing wild-type Cdc10, Cdc11, and either (His)6-Cdc12 or (His)6-Cdc3, were expressed in Escherichia coli and then purified by immobilized metal affinity, size exclusion, and ion exchange chromatography, as described before 11. The following mutant complexes were prepared in the same way as the wild-type Cdc3–Cdc11–(His)6–Cdc12-Cdc10) complex by using appropriate vectors to express the indicated combinations of proteins: MBP–Cdc12(Δ318–407)–Cdc10–Cdc11–(His)6-Cdc3; Cdc12(Δ318–407)–Cdc10–Cdc11–(His)6-Cdc3(Δ419–520); Cdc12– (His)6-Cdc3–Cdc10–Cdc11(Δ2–18); Cdc12–(His)6-Cdc3–Cdc10–Cdc11(Δ306–415); Cdc3–Cdc11–(His)6–Cdc12-Cdc10Δ12–28; Cdc3–Cdc11(Δ13–29)–(His)6–Cdc12-Cdc10; Cdc3–Cdc11(R12A, K13A R14A K15A K16A)–(His)6–Cdc12-Cdc10; Cdc3(R111A R112A K115A)–Cdc11–(His)6–Cdc12-Cdc10; Cdc3(K90A R93A R94A)–Cdc11–(His)6–Cdc12-Cdc10; (His)6–Cdc12-Cdc10-Cdc3; Cdc3-(His)6–Cdc12-Cdc11; (His)6–Cdc12-Cdc11-Cdc3(G261V); Cdc3–Cdc11–(His)6–Cdc12-Cdc10(I22E); Cdc3–Cdc11–(His)6– Cdc12-Cdc10(H24A R25A K28A K29A). Purified complexes (yield ~1 mg per liter culture) were used immediately or were flash-frozen and stored at −80° C before examination in the EM.
The Cdc12ΔCC mutant examined in our experiments carried an E. coli MalE (maltose-binding protein; MBP) tag at its N-terminus, which does not interfere with hetero-octamer assembly (Fig. 3B, panel 1, inset) 11, but could potentially have influenced the behavior of the resulting mutant complexes. However, before testing its properties (Fig. 3B, panels 2 and 3), the MBP tag was cleaved off by exhaustive TEV protease digestion.
Electron microscopy and image processing of complexes in solution
The septin complexes were diluted to 0.01 mg/ml, adsorbed onto carbon-coated grids, and stained with 2% uranyl formate. Samples were examined by using a Tecnai T12 microscope (FEI) operated at 120 kV, and images were collected by using film (cat. no. S0163; Kodak) at a magnification of 30,000 and −1 µm underfocus or by using a Gatan 1024×1024 CCD camera. Micrographs were digitized on a Nikon Coolscan 8000 scanner with a pixel size of 4.23 Å at the sample level. Particles were classified and aligned to generate class averages by using SPIDER 53. Particles were windowed out into 135×135 pixels images by using the Boxer interface of EMAN and appended into a single SPIDER file, then normalized against the background. One round of reference-free alignment and classification was performed before references were selected from the first class averages. Several rounds of multireference alignment and classification were then performed, and new references were selected from the class averages until no further improvement was obtained. For the Cdc12(Δ318–407)–Cdc10–Cdc11–(His)6-Cdc3(Δ419–520) complex, 1,400 particles were collected and split into 25 classes after three rounds of successive alignment and classification For the MBP-Cdc12(Δ318–407)–Cdc10–Cdc11–(His)6-Cdc3 complex, 2,550 particles were collected and split into 65 classes after three rounds of successive alignment and classification. For the Cdc3–Cdc11–(His)6–Cdc12-Cdc10(Δ12–28) complex, in high salt, 1,240 particles were collected and split into 78 classes after three rounds of successive alignment and classification. For the Cdc3–Cdc11–(His)6–Cdc12-Cdc10(Δ12–28) complex, in low salt, 1,356 particles were collected and split into 61 classes after three rounds of successive alignment and classification. For the Cdc3–Cdc11–(His)6–Cdc12-Cdc10(H24A R25A K28A K29A) complex, in high salt, 2,546 particles were collected and split into 45 classes after three rounds of successive alignment and classification. For the Cdc3–Cdc11–(His)6–Cdc12-Cdc10(H24A, R25A, K28A, K29A), septin complex, in low salt, 3,169 particles were collected and split into 75 classes after three rounds of successive alignment and classification. For the Cdc3–Cdc11–(His)6–Cdc12-Cdc10(I22E) complex, in high salt, 2,096 particles were collected and split into 42 classes after three rounds of successive alignment and classification. For the Cdc3–Cdc11–(His)6–Cdc12-Cdc10(I22E) complex, in low salt, 2982 particles were collected and split into 56 classes after three rounds of successive alignment and classification. For the Cdc3–Cdc11(R12A K13A R14A K15A K16A)–(His)6–Cdc12-Cdc10 complex, in high salt, 3,201 particles were collected and split into 58 classes after three rounds of successive alignment and classification. For the Cdc3–Cdc11(R12A K13A R14A K15A K16A)–(His)6–Cdc12-Cdc10 complex, in low salt, 3,140 particles were collected and split into 62 classes after three rounds of successive alignment and classification.
Lipid monolayer assay
The wells of a Teflon block were filled to capacity (20 µL) with solutions of septin complexes in either high or low salt, and then a drop (~0.5 µL) of lipid solution in chloroform was applied to the surface of the solution. The Teflon block was placed in a humidified Petri dish and equilibrated, typically, overnight (but for at least a few hours) at 4° C. To capture the lipid monolayer and adsorbed proteins, a grid was placed gently on surface of the solution. Three types of grids were used: standard grids coated with a continuous hydrophobic carbon film; Quantifoil™ (Quantifoil, Inc.) holey grids; and, C-flat™ (Proto-chips, Inc.) holey grids. In all cases, the grids were placed so that the carbon side faced the solution, as described in Taylor et al. (2007) 28 and Kubalek et al. 54. The grids were then lifted from the air-water interface, and stained with a solution of 2% uranyl formate before being analyzed by electron microscopy. Homogeneity of the monolayer was best preserved over the holes in the holey grids.
Supplementary Material
01
ACKNOWLEDGEMENTS
We thank Ho-Leung Ng and Tom Alber for the gift of plamids expressing MBP–Cdc12(Δ318–407)–Cdc10–Cdc11–(His)6-Cdc3, Cdc12(Δ318–407)–Cdc10–Cdc11–(His)6-Cdc3(Δ419–520) septin complexes. This work was supported by Jane Coffin Childs Postdoctoral Research Fellowship 61-1357 (to AB), NIH K99 grant GM86603 (to MAM), an NSF Predoctoral Fellowship (to GG), Howard Hughes Medical Institute (to EN), and NIH R01 grant GM21841 (to JT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hartwell LH. Genetic control of the cell division cycle in yeast. II. Genes controlling DNA replication and its initiation. J Mol Biol. 1971;59:183–194. doi: 10.1016/0022-2836(71)90420-7. [DOI] [PubMed] [Google Scholar]
- 2.Pan F, Malmberg RL, Momany M. Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol Biol. 2007;7:103. doi: 10.1186/1471-2148-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMurray MA, Thorner J. Reuse, replace, recycle. Specificity in subunit inheritance and assembly of higher-order septin structures during mitotic and meiotic division in budding yeast. Cell Cycle. 2009;8:195–203. doi: 10.4161/cc.8.2.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMurray MA, Thorner J. Septins: molecular partitioning and the generation of cellular asymmetry. Cell Div. 2009;4:18.1–18.40. doi: 10.1186/1747-1028-4-18. 26;4:18., C. D. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang CW, Fedchyshyn M, Harrison J, Xie H, Xue J, Robinson PJ, Wang LY, Trimble WS. Superfluous role of mammalian septins 3 and 5 in neuronal development and synaptic transmission. Mol Cell Biol. 2008;28:7012–7029. doi: 10.1128/MCB.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P, Kiebler MA. The GTP-binding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr Biol. 2007;17:1746–1751. doi: 10.1016/j.cub.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 7.Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr Biol. 2007;17:1752–1758. doi: 10.1016/j.cub.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steels JD, Estey MP, Froese CD, Reynaud D, Pace-Asciak C, Trimble WS. Sept12 is a component of the mammalian sperm tail annulus. Cell Motil Cytoskeleton. 2007;64:794–807. doi: 10.1002/cm.20224. [DOI] [PubMed] [Google Scholar]
- 9.Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, Goto M, Okubo K, Nishiyama H, Ogawa O, Takahashi C, Itohara S, Nishimune Y, Noda M, Kinoshita M. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8:343–352. doi: 10.1016/j.devcel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Huang YW, Yan M, Collins RF, Diciccio JE, Grinstein S, Trimble WS. Mammalian septins are required for phagosome formation. Mol Biol Cell. 2008;19:1717–1726. doi: 10.1091/mbc.E07-07-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertin A, McMurray MA, Grob P, Park SS, Garcia G, 3rd, Patanwala I, Ng HL, Alber T, Thorner J, Nogales E. Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc Natl Acad Sci U S A. 2008;105:8274–8279. doi: 10.1073/pnas.0803330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John CM, Hite RK, Weirich CS, Fitzgerald DJ, Jawhari H, Faty M, Schlapfer D, Kroschewski R, Winkler FK, Walz T, Barral Y, Steinmetz MO. The Caenorhabditis elegans septin complex is nonpolar. EMBO J. 2007;26:3296–3307. doi: 10.1038/sj.emboj.7601775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sirajuddin M, Farkasovsky M, Hauer F, Kuhlmann D, Macara IG, Weyand M, Stark H, Wittinghofer A. Structural insight into filament formation by mammalian septins. Nature. 2007;449:311–315. doi: 10.1038/nature06052. [DOI] [PubMed] [Google Scholar]
- 14.Versele M, Thorner J. Some assembly required: yeast septins provide the instruction manual. Trends Cell Biol. 2005;15:414–424. doi: 10.1016/j.tcb.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byers B, Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976;69:717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soll DR, Mitchell LH. Filament ring formation in the dimorphic yeast Candida albicans. J Cell Biol. 1983;96:486–493. doi: 10.1083/jcb.96.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pablo-Hernando ME, Arnaiz-Pita Y, Tachikawa H, del Rey F, Neiman AM, Vazquez de Aldana CR. Septins localize to microtubules during nutritional limitation in Saccharomyces cerevisiae. BMC Cell Biol. 2008;9:55. doi: 10.1186/1471-2121-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cockcroft S, De Matteis MA. Inositol lipids as spatial regulators of membrane traffic. J Membr Biol. 2001;180:187–194. doi: 10.1007/s002320010069. [DOI] [PubMed] [Google Scholar]
- 19.Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100:603–606. doi: 10.1016/s0092-8674(00)80696-0. [DOI] [PubMed] [Google Scholar]
- 20.Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, Sheetz MP, Meyer T. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100:221–228. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- 21.Sechi AS, Wehland J. The actin cytoskeleton and plasma membrane connection: PtdIns(4,5)P(2) influences cytoskeletal protein activity at the plasma membrane. J Cell Sci. 2000;113(Pt 21):3685–3695. doi: 10.1242/jcs.113.21.3685. [DOI] [PubMed] [Google Scholar]
- 22.Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 23.Garrenton LS, Stefan CJ, McMurray MA, Emr SD, Thorner J. Pheromone-induced anisotropy in yeast plasma membrane phosphatidylinositol-4,5-bisphosphate distribution is required for MAPK signaling. Proc. Natl. Acad. Sci. USA. 2010;107:11805–11810. doi: 10.1073/pnas.1005817107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Escudero I, Roelants FM, Thorner J, Nombela C, Molina M, Cid VJ. Reconstitution of the mammalian PI3K/PTEN/Akt pathway in yeast. Biochem J. 2005;390:613–623. doi: 10.1042/BJ20050574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casamayor A, Snyder M. Molecular dissection of a yeast septin: distinct domains are required for septin interaction, localization, and function. Mol Cell Biol. 2003;23:2762–2777. doi: 10.1128/MCB.23.8.2762-2777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Kong C, Xie H, McPherson PS, Grinstein S, Trimble WS. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr Biol. 1999;9:1458–1467. doi: 10.1016/s0960-9822(00)80115-3. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka-Takiguchi Y, Kinoshita M, Takiguchi K. Septin-mediated uniform bracing of phospholipid membranes. Curr Biol. 2009;19:140–145. doi: 10.1016/j.cub.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Taylor DW, Kelly DF, Cheng A, Taylor KA. On the freezing and identification of lipid monolayer 2-D arrays for cryoelectron microscopy. J Struct Biol. 2007;160:305–312. doi: 10.1016/j.jsb.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lentz BR, Barenholz Y, Thompson TE. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 2 Two-component phosphatidylcholine liposomes. Biochemistry. 1976;15:4529–4537. doi: 10.1021/bi00665a030. [DOI] [PubMed] [Google Scholar]
- 30.Versele M, Gullbrand B, Shulewitz MJ, Cid VJ, Bahmanyar S, Chen RE, Barth P, Alber T, Thorner J. Protein-protein interactions governing septin heteropentamer assembly and septin filament organization in Saccharomyces cerevisiae. Mol. Biol. Cell. 2004;15:4568–4583. doi: 10.1091/mbc.E04-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frazier JA, Wong ML, Longtine MS, Pringle JR, Mann M, Mitchison TJ, Field C. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J Cell Biol. 1998;143:737–749. doi: 10.1083/jcb.143.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farkasovsky M, Herter P, Voss B, Wittinghofer A. Nucleotide binding and filament assembly of recombinant yeast septin complexes. Biol Chem. 2005;386:643–656. doi: 10.1515/BC.2005.075. [DOI] [PubMed] [Google Scholar]
- 33.Versele M, Thorner J. Septin collar formation in budding yeast requires GTP binding and direct phosphorylation by the PAK, Cla4. J Cell Biol. 2004;164:701–715. doi: 10.1083/jcb.200312070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carman GM, Henry SA. Phospholipid biosynthesis in the yeast Saccharomyces cerevisiae and interrelationship with other metabolic processes. Prog. Lipid Res. 1999;38:361–399. doi: 10.1016/s0163-7827(99)00010-7. [DOI] [PubMed] [Google Scholar]
- 35.Daum G, Lees ND, Bard M, Dickson R. Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast. 1998;14:1471–1510. doi: 10.1002/(SICI)1097-0061(199812)14:16<1471::AID-YEA353>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 36.Odorizzi G, Babst M, S.D. E. Phosphoinositide signaling and the regulation of membrane trafficking in yeast. Trends Biochem. Sci. 2000;25:229–235. doi: 10.1016/s0968-0004(00)01543-7. [DOI] [PubMed] [Google Scholar]
- 37.van der Rest ME, Kamminga AH, Nakano A, Anraku Y, Poolman B, Konings WN. The plasma membrane of Saccharomyces cerevisiae: structure, function, and biogenesis. Microbiol Rev. 1995;59:304–322. doi: 10.1128/mr.59.2.304-322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, Daum G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol. 1991;173:2026–2034. doi: 10.1128/jb.173.6.2026-2034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patton JL, Lester RL. The phosphoinositol sphingolipids of Saccharomyces cerevisiae are highly localized in the plasma membrane. J Bacteriol. 1991;173:3101–3108. doi: 10.1128/jb.173.10.3101-3108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levitan I, Gooch KJ. Lipid rafts in membrane-cytoskeleton interactions and control of cellular biomechanics: actions of oxLDL. Antioxid Redox Signal. 2007;9:1519–1534. doi: 10.1089/ars.2007.1686. [DOI] [PubMed] [Google Scholar]
- 41.Martin TF. PI(4,5)P(2) regulation of surface membrane traffic. Curr Opin Cell Biol. 2001;13:493–499. doi: 10.1016/s0955-0674(00)00241-6. [DOI] [PubMed] [Google Scholar]
- 42.Iwase M, Luo J, Nagaraj S, Longtine M, Kim HB, Haarer BK, Caruso C, Tong Z, Pringle JR, Bi E. Role of a Cdc42p effector pathway in recruitment of the yeast septins to the presumptive bud site. Mol Biol Cell. 2006;17:1110–1125. doi: 10.1091/mbc.E05-08-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orlando K, Zhang J, Zhang X, Yue P, Chiang T, Bi E, Guo W. Regulation of Gic2 localization and function by phosphatidylinositol 4,5-bisphosphate during the establishment of cell polarity in budding yeast. J. Biol. Chem. 2008;283:14205–14212. doi: 10.1074/jbc.M708178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu JW, Mendrola JM, Audhya A, Singh S, Keleti D, DeWald DB, Murray D, Emr SD, Lemmon MA. Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol. Cell. 2004;13:677–688. doi: 10.1016/s1097-2765(04)00083-8. [DOI] [PubMed] [Google Scholar]
- 45.Shibasaki Y, Ishihara H, Kizuki N, Asano T, Oka Y, Yazaki Y. Massive actin polymerization induced by phosphatidylinositol-4-phosphate 5-kinase in vivo. J Biol Chem. 1997;272:7578–7581. doi: 10.1074/jbc.272.12.7578. [DOI] [PubMed] [Google Scholar]
- 46.Lassing I, Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985;314:472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- 47.Hales KG, Bi E, Wu JQ, Adam JC, Yu IC, Pringle JR. Cytokinesis: an emerging unified theory for eukaryotes? Curr. Opin. Cell Biol. 1999:717–725. doi: 10.1016/s0955-0674(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 48.Young BA, Buser C, Drubin DG. Isolation and partial purification of the Saccharomyces cerevisiae cytokinetic apparatus. Cytoskeleton. 2010;67:13–22. doi: 10.1002/cm.20412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eswar N, Eramian D, Webb B, Shen MY, Sali A. Protein structure modeling with MODELLER. Methods Mol Biol. 2008:145–159. doi: 10.1007/978-1-60327-058-8_8. [DOI] [PubMed] [Google Scholar]
- 50.Sirajuddin M, Farkasovsky M, Zent E, Wittinghofer A. GTP-induced conformational changes in septins and implications for function. Proc. Natl. Acad. Sci. USA. 2009;106:16592–16597. doi: 10.1073/pnas.0902858106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mosca R, Schneider TR. RAPIDO: a web server for the alignment of protein structures in the presence of conformational changes. Nucleic Acids Res. 2008;36:W42–W46. doi: 10.1093/nar/gkn197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leis ABR, Andrees L, Baumeisterc W. Visualizing cells at the nanoscale. Trends Biochem. Sci. 2009;34:60–70. doi: 10.1016/j.tibs.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 54.Kubalek EW, Kornberg RD, Darst SA. Improved transfer oft wo-dimensional crystals from the air/water interface to specimen support grids for high-resolution analysis by electron microscopy. Ultramicroscopy. 1991;35:295–304. doi: 10.1016/0304-3991(91)90082-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01