Impact of the Conjugation Method on the Immunogenicity of Streptococcus pneumoniae Serotype 19F Polysaccharide in Conjugate Vaccines (original) (raw)
Abstract
7vCRM (Pfizer, Inc.) and PHiD-CV (GlaxoSmithKline Biologicals) are two pneumococcal conjugate vaccines licensed for the prevention of invasive pneumococcal disease and acute otitis media caused by the vaccine serotypes of Streptococcus pneumoniae. Neither vaccine contains serotype 19A, but both contain the closely related serotype 19F. No decrease in the incidence of serotype 19A disease has been observed following the introduction of 7vCRM, suggesting that this serotype 19F-containing vaccine provides limited cross-protection against serotype 19A. To investigate the impact that conjugation methods may have on antipolysaccharide immune responses and to determine whether this limited cross-protection is characteristic of the serotype 19F polysaccharide or rather of the 19F-CRM (cross-reacting material) conjugate, we compared naturally induced antibodies against serotypes 19F and 19A with antibodies induced after vaccination with different pneumococcal conjugate vaccines. We found that conjugation of the serotype 19F polysaccharide using reductive amination (as in 7vCRM) resulted in the formation of at least one additional epitope that is not present in the native form of the 19F polysaccharide or following 19F conjugation using a bifunctional spacer (as in the prototype vaccine 7vOMPC) or cyanylation (as in PHiD-CV). We also found that pneumococcal vaccines conjugated using cyanylation induce more opsonophagocytic antibodies against serotype 19F and a considerably higher level of cross-opsonophagocytic antibodies against serotype 19A than vaccines conjugated using reductive amination. In conclusion, these results suggest that the conjugation method can influence the functionality of the antibodies induced against the homologous serotype 19F and the cross-reactive serotype 19A of S. pneumoniae.
7vCRM (Prevenar/Prevnar; Pfizer, Inc.) contains capsular polysaccharides of Streptococcus pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, each conjugated to the nontoxic cross-reacting mutant diphtheria toxin CRM197. Serotype 19A was not included in 7vCRM, because it was expected that the immunological similarities with vaccine serotype 19F would elicit sufficient cross-protection, as observed for serotype 6B and the related serotype 6A (26, 38, 48, 52). Serotypes 19F and 19A are indeed closely related biochemically (38, 52). Serotype 19A and 19F polysaccharides are composed of similar trisaccharide units polymerized through phosphate diester groups and differ only in the position of the linkage to the α-l-rhamnose residue: α(1→2) for 19F and α(1→3) for 19A (19, 29).
Among the seven serotypes included in 7vCRM, the lowest protection against disease is observed for serotype 19F. For invasive pneumococcal disease due to serotype 19F, the reported effectiveness of 7vCRM ranges from 67% to 87% (4, 22, 48), whereas for acute otitis media due to serotype 19F, the efficacy of 7vCRM is reported to be 25% (95% confidence interval [CI], −14% to 51%) (11). Despite this apparently weaker protection against serotype 19F than against the other serotypes, disease due to serotype 19F is well controlled by vaccination with 7vCRM. In contrast, a rise in the incidence of disease caused by the vaccine-related serotype 19A has been observed after the introduction of 7vCRM, especially in the United States, suggesting that 7vCRM provides no or limited cross-protection against serotype 19A (16, 32, 45).
Following the successful introduction of 7vCRM in 2000, two additional pneumococcal conjugate vaccines (PCVs) were licensed on the basis of immunological noninferiority to 7vCRM: the pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV [Synflorix]; GlaxoSmithKline [GSK] Biologicals) and 13vCRM (Prevnar 13; Pfizer, Inc.) (17, 49, 50). PHiD-CV targets pneumococcal serotypes 1, 5, and 7F in addition to those targeted by 7vCRM (37, 46). Eight of the 10 polysaccharides in PHiD-CV are conjugated to the nontypeable H. influenzae protein D, and the remaining 2 are conjugated to tetanus and diphtheria toxoids. As with 7vCRM, serotypes 6A and 19A were not included in PHiD-CV because the related serotypes 6B and 19F, which are included in PHiD-CV, were expected to provide cross-protection. 13vCRM contains serotypes 3, 6A, and 19A in addition to those targeted by PHiD-CV (5).
Licensure based on immunological noninferiority requires serological assays that reflect clinical protection. However, the classically used enzyme-linked immunosorbent assays (ELISAs) determine the antibody concentrations but do not necessarily reflect the functional potential of the antibodies. Because opsonophagocytosis is the primary mechanism of protection against S. pneumoniae infections, the in vitro opsonophagocytosis activity (OPA) assay is acknowledged as the best surrogate for evaluating the protection provided by pneumococcal vaccines (18, 39, 47).
Divergent estimates of vaccine efficacy could thus arise from these two assays. It was recently shown that although ELISA results indicate that 7vCRM induces antibodies against serotype 19F above threshold levels in a high proportion of children (99% [95% CI, 98% to 100%]), only 91% of children (95% CI, 88% to 94%) had functional antibodies (OPA titer, ≥8) against this serotype (37). The latter estimate seems to correspond better with the observed effectiveness of 7vCRM in the United States (87% [95% CI, 65% to 95%]) (48). Furthermore, serotype 19F required the highest antibody concentration to obtain 50% killing in the OPA assay (14). For the vaccine-related serotype 19A, three doses of 7vCRM yielded only 2% (95% CI, 1% to 4%) of sera with OPA titers of ≥8 (37), the threshold considered to correlate with clinical effectiveness (14, 37, 50). Similar results were found subsequently in other studies (20, 26).
Because polysaccharides must be chemically modified before covalent linking to a carrier protein, the conjugation chemistry could alter the polysaccharide structure and, consequently, the exposure of epitopes. In 2002, Lee suggested that the conjugation method using reductive amination, in which pneumococcal polysaccharides are first oxidized by periodate to create aldehyde groups, modified the antigenic properties of some serotypes, including serotype 19F, from those of the native polysaccharides (25).
Given the limited cross-protection against serotype 19A following the implementation of 7vCRM, we investigated the immune responses induced by PCVs containing serotype 19F conjugates but not serotype 19A in order to determine whether this low level of cross-protection is characteristic of the 19F polysaccharide (and therefore also applicable to PHiD-CV) or rather of the 19F-CRM conjugate used in 7vCRM (and therefore possibly different from the cross-protection provided by the 19F-diphtheria toxoid conjugate used in PHiD-CV). We thus compared the impact of different conjugation chemistries on the antipolysaccharide immune responses. To determine whether the conjugation method alters the polysaccharide structure and consequently the expression of epitopes, we used ELISAs and OPA assays to analyze the antipolysaccharide immune responses induced by different serotype 19F polysaccharide conjugates. We compared the functionalities of the antibodies against the homologous serotype 19F and the cross-reactive antibodies against the related serotype 19A induced after the vaccination of children with PCVs manufactured using reductive amination versus cyanylation conjugation chemistries.
(This study was presented in part at the 3rd International Symposium on Pneumococci and Pneumococcal Diseases, Anchorage, AK, May 2002; the 5th International Symposium on Pneumococci and Pneumococcal Diseases, Alice Springs, Australia, April 2006; and the 7th International Symposium on Pneumococci and Pneumococcal Diseases, Tel Aviv, Israel, March 2010.)
MATERIALS AND METHODS
Study design.
The design of the clinical studies used in this assessment is summarized in Table 1. The protocol of these studies was approved by the institutional review boards of the participating centers or the appropriate independent ethics committees. The studies were conducted according to good clinical practice and the Declaration of Helsinki. Written informed consent was obtained from the parents or legal guardians of all subjects before enrollment. All studies were randomized and controlled. Studies C, F, and G were double blind; studies A and D were single blind; and studies B and E were open label. In studies A to F, the immunogenicity and safety of PHiD-CV were compared with those of 7vCRM. In study G, the immunogenicity, safety, and efficacy of the 11-valent prototype vaccine 11Pn-PD were analyzed in comparison to a control group receiving a hepatitis A vaccine. Detailed results with regard to the immunogenicity and safety of 11Pn-PD (33, 35, 36, 43), PHiD-CV (2, 6, 46, 51), and the coadministered vaccines (24) have been reported previously. All studies were conducted with healthy children between the ages of 6 and 16 weeks at the time of the first vaccine dose and between the ages of 11 and 18 months at the time of the booster dose.
TABLE 1.
Overview of the clinical studies used in this analysis
| Type of study and countries | Vaccine groupsa | Primary vaccination | Booster vaccination | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | References | Schedule | No. of children vaccinated | Study | Reference(s) | Schedule (mo) | No. of children vaccinated | ||
| PHiD-CV vaccination studies | |||||||||
| Finland, France, Poland | PHiD-CV + DTPa-HBV-IPV/Hib; 7vCRM + DTPa-HBV-IPV/Hib | A (study 001) | 6, 24, 46 | 2, 3, 4 mo | 1,650 | D (study 007) | 6, 24, 46 | 12-18 | 1,112 |
| Germany, Poland, Spain | PHiD-CV + DTPa-HBV-IPV/Hib + MenC-CRM; PHiD-CV + DTPa-HBV-IPV/Hib + MenC-TT; PHiD-CV + DTPa-HBV-IPV + Hib-MenC-TT; 7vCRM + DTPa-HBV-IPV + Hib-MenC-TT | B (study 011) | 6, 24, 51 | 2, 4, 6 mo | 1,548 | E (study 017) | 6, 24, 51 | 11-18 | 1,437 |
| The Philippines | PHiD-CV + DTPw-HBV/Hib + OPV; 7vCRM + DTPw-HBV/Hib + OPV | C (study 012) | 2, 6, 24 | 6, 10, 14 wk | 400 | F (study 018) | 1 | 12-18 | 373 |
| Poland | PHiD-CV + DTPw-HBV/Hib + IPV; 7vCRM + DTPw-HBV/Hib + IPV | 2, 4, 6 mo | 406 | 12-18 | 383 | ||||
| Experimental vaccine study (Czech Republic, Slovakia) | 11Pn-PD + DTPa-HBV-IPV/Hib; HAV + DTPa-HBV-IPV/Hib | G (POET) | 33, 35, 36, 42, 43 | 3, 4, 5, 12-15 mo | 4,968 |
Vaccines.
The following vaccines were used: 7vCRM (Prevenar/Prevnar; Pfizer Inc., New York, NY); PHiD-CV (Synflorix; GSK Biologicals, Rixensart, Belgium); two PHiD-CV predecessor vaccine formulations, 4Pn-PD and 11Pn-PD (GSK Biologicals); a prototype vaccine conjugated to the outer membrane protein complex of Neisseria meningitidis serogroup B (7vOMPC; Merck & Co., Inc., Whitehouse Station, NJ); and the 23-valent nonconjugated polysaccharide vaccine (23vPS) (Pneumovax 23; Merck & Co., Inc.). 7vCRM contains capsular polysaccharides from seven pneumococcal serotypes (2 μg each of the capsular polysaccharides for serotypes 4, 9V, 14, 18C, 19F, and 23F; 4 μg of the capsular polysaccharide for serotype 6B) conjugated to the nontoxic diphtheria CRM197 toxin. The polysaccharides in 7vCRM were activated by oxidation with sodium periodate, followed by linkage to the carrier protein by reductive amination (41). PHiD-CV contains 1 μg each of the capsular polysaccharides of S. pneumoniae serotypes 1, 5, 6B, 7F, 9V, 14, and 23F and 3 μg of the serotype 4 capsular polysaccharide, each conjugated to a recombinant nonlipidated form of nontypeable H. influenzae protein D, along with 3 μg of serotype 18C capsular polysaccharide conjugated to tetanus toxoid and 3 μg of serotype 19F capsular polysaccharide conjugated to diphtheria toxoid. 4Pn-PD contains 1 μg each of serotype 6B, 14, 19F, and 23F capsular polysaccharides (30), and 11Pn-PD contains 1 μg each of serotype 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F capsular polysaccharides, all conjugated individually to protein D. Conjugation for the PHiD-CV, 4Pn-PD, and 11Pn-PD vaccines was performed by cyanylation using 1-cyano-4-dimethylamino-pyridinium tetrafluoroborate (CDAP) (27). 7vOMPC contains the following amounts of capsular polysaccharides: 1 μg each for pneumococcal serotypes 4 and 14, 1.5 μg for serotype 9V, 2 μg each for serotypes 18C and 19F, 3 μg for serotype 23F, and 5 μg for serotype 6B. Each polysaccharide was individually conjugated to the outer membrane protein complex (OMPC) using a bigeneric spacer covalently linked by a multistep conjugation method (28).
Sera.
Serum samples from children vaccinated in studies A to G were collected and stored at −20°C until analysis at GSK Biologicals (Rixensart, Belgium). Selected sera from two other clinical studies were analyzed at the National Institute for Health and Welfare (THL, Helsinki, Finland): the Undeca-Pn-008 study, a phase II study that evaluated the immunogenicity and safety of the 11Pn-PD prototype vaccine in infants and toddlers (31), and the Finnish Otitis Media vaccine efficacy trial, which compared in parallel a 7vCRM and a 7vOMPC vaccine group to a control hepatitis B vaccine group (8, 11, 21).
Serum samples from adults were stored at −20°C and were analyzed at the Center for Biologics Evaluation and Research (CBER; U.S. Food and Drug Administration, Bethesda, MD). Sera from healthy, unimmunized adults were obtained from the National Institutes of Health blood bank (Bethesda, MD). Paired pre- and postvaccination sera from adults receiving 23vPS were kindly provided by David Goldblatt (Institute of Child Health, London, United Kingdom); paired sera from adults receiving 7vCRM were kindly provided by D. V. Madore (Wyeth Vaccines/Pfizer, Inc., Pearl River, NY) and M. H. Nahm (University of Alabama at Birmingham); and sera from adults receiving 7vOMPC were kindly provided by H. Ukwu (Merck & Co., Inc., Whitehouse Station, NJ). Finally, sera from adults receiving one dose of the 4Pn-PD or 11Pn-PD vaccine were also included in the analysis.
Antipneumococcal IgG ELISAs.
For pediatric sera, antipneumococcal IgG concentrations were measured by ELISA with 22F preadsorption as previously described (15, 44). The cutoff value for the 22F-ELISA used at GSK (see Fig. 3) was 0.05 μg/ml IgG for all serotypes, which is an arbitrary value greater than the limit of quantitation of the assay for each of the serotypes. In this assay, an antibody concentration of 0.2 μg/ml was shown to be equivalent to 0.35 μg/ml by use of the reference ELISA (without 22F preadsorption) (15, 34), which is the concentration recommended by the World Health Organization for noninferiority comparison of infant immune responses after primary immunization with a PCV (50). For the ELISA used at the THL laboratory (44) (see Table 3), the limits for quantitation were 0.1 and 0.16 μg/ml for serotypes 19A and 19F, respectively.
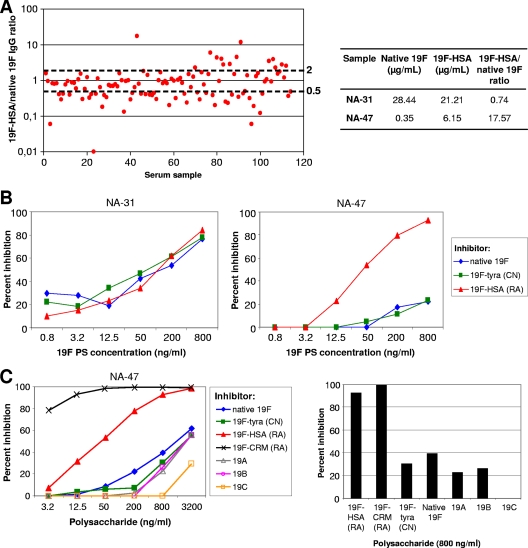
For adult sera, antipneumococcal IgG concentrations were measured at the CBER using an ELISA with 22F preadsorption as described previously (7) (see Fig. 1A and 2). Competitive inhibition ELISAs (see Fig. 1B and C) were performed at the CBER using 22F adsorption as described previously (7). The antigens used for coating microtiter plates in these experiments are described in Table 2. For the native serotype 19F polysaccharide, plates were coated with a combination of 5 μg/ml 19F polysaccharide and 5 μg/ml methylated human serum albumin (HSA) to improve attachment. Only sera containing ≥2 μg/ml of antibody to either the 19F polysaccharide or the 19F-HSA antigen were used for the inhibition experiments. The ratio of the concentration of IgG antibody against 19F-HSA to the concentration of IgG antibody against native 19F was calculated, and a ratio between 0.5 and 2 was considered as indicating no difference.
FIG. 1.
Antibody binding to 19F polysaccharide antigens. (A) Concentrations of IgG antibodies to native 19F polysaccharide and to the 19F polysaccharide conjugated to human serum albumin (19F-HSA) by reductive amination in sera from healthy unvaccinated blood donors (n = 113) were measured by 22F-ELISA as described previously (7). The 19F-HSA/native 19F IgG concentration ratio was calculated for each donor. (B) The inhibition of anti-19F polysaccharide (PS) binding was measured in two sera (one [NA-31] with similar concentrations of antibodies to native 19F and 19F-HSA and the other [NA-47] with a high concentration of the antibody to 19F-HSA) from healthy unimmunized adults by inhibition ELISA, as described previously (7). ELISA plates were coated with either native 19F polysaccharide, the 19F polysaccharide linked to HSA (19F-HSA) by reductive amination (RA), or the 19F polysaccharide linked to tyramine (19F-tyra) by cyanylation (CN). (C) The binding of different 19F conjugates and polysaccharides from the related serotypes 19A, 19B, and 19C was measured in serum NA-47 as for panel B.
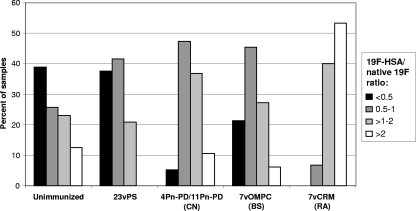
FIG. 2.
Comparative distribution of ratios of the concentration of IgG against 19F-HSA to the concentration of IgG against native 19F polysaccharide. The 19F-HSA/native 19F IgG antibody concentration ratio was calculated for sera from healthy unimmunized adults (n = 113) or from adults vaccinated with either 23vPS (n = 24), 7vCRM (a vaccine conjugated using reductive amination [RA]) (n = 15), 7vOMPC (a prototype vaccine conjugated using a bigeneric spacer [BS]) (n = 33), or one of the two experimental vaccines 11Pn-PD and 4Pn-PD (both vaccines conjugated using cyanylation [CN]) (n = 19). The 22F-ELISA was performed as described previously (7).
TABLE 2.
Pneumococcal polysaccharide antigens used in ELISA
| Coating antigen | Carrier | Conjugation methoda |
|---|---|---|
| Native 19F | None | None |
| 19F-HSA | Human serum albumin | RA |
| 19F-CRM | CRM197 | RA |
| 19F-tyra | Tyramine | CN |
OPA assays.
For pediatric sera, the OPA assay used by the GSK laboratory (see Fig. 3) was adapted from the method described by Romero-Steiner et al. (40) and was performed as described previously (14). Similarly, the THL laboratory used a modification of the Romero-Steiner OPA assay (40) (see Table 3). The OPA titer was defined as the reciprocal of the lowest serum dilution that induced ≥50% bacterial killing relative to the levels in the control wells. For both OPA assays, the lowest serum dilution tested (assay cutoff) for all serotypes was 1:8, which corresponds to the titer that has been shown to correlate with protection against invasive pneumococcal disease (50).
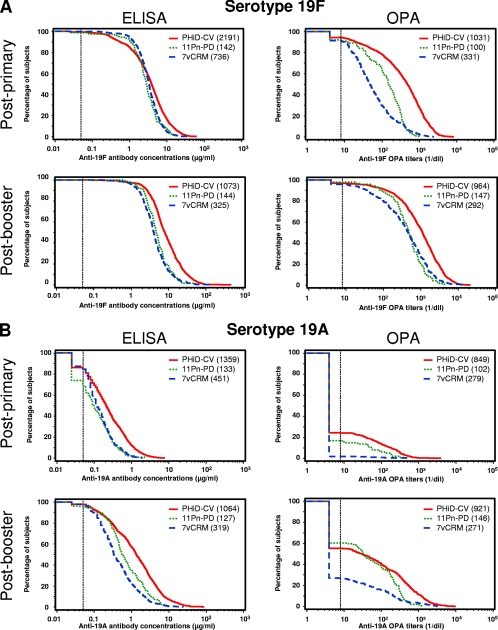
FIG. 3.
Reverse cumulative distribution curves of antipolysaccharide IgG concentrations and OPA titers measured in pediatric sera after vaccination with 7vCRM, PHiD-CV, or 11Pn-PD. (A) Serotype 19F; (B) serotype 19A. To prepare the reverse cumulative distribution curves for primary vaccination, the data from the PHiD-CV groups in studies A to C were pooled; the data from the 7vCRM groups in studies A to C were pooled; and the data from the POET study group (study G) were used for 11Pn-PD. For the booster vaccination, the data from the PHiD-CV groups in studies D to F were pooled; the data from the 7vCRM groups in studies D to F were pooled; and the data from the POET study group (study G) were used for 11Pn-PD. The dotted lines represent an assay cutoff of 0.05 μg/ml for the 22F-ELISA and a titer of 8 for the OPA assay. The numbers of sera tested are given in parentheses in the keys.
TABLE 3.
Antibody concentrations and OPA titers in selected sera from Finnish children vaccinated with 7vCRM, 7vOMPC, or 11Pn-PD at the ages of 2, 4, 6, and 12 to 15 monthsa
| Vaccine (n)b | 22F-ELISA | OPA assay | ||||||
|---|---|---|---|---|---|---|---|---|
| % of samples with antibody concns of ≥0.35 μg/ml (95% CI) | GMC (μg/ml) (95% CI) | % of samples with OPA titers of ≥8 (95% CI) | OPA GMT (95% CI) | |||||
| 19F | 19A | 19F | 19A | 19F | 19A | 19F | 19A | |
| 7vCRM (29-30) | 100 (88-100) | 72 (53-87) | 9.3 (7.2-11.9) | 0.7 (0.5-1.0) | 100 (88-100) | 10 (2-27) | 55.7 (39.9-77.8) | 4.9 (3.8-6.3) |
| 7vOMPC (29-30) | 100 (88-100) | 62 (42-79) | 9.3 (7.5-11.6) | 0.4 (0.3-0.6) | 87 (69-96) | 20 (8-39) | 35 (22-55) | 4.9 (4.2-5.8) |
| 11Pn-PD (25-26) | 100 (86-100) | 44 (24-65) | 11.9 (8.8-16.0) | 0.4 (0.3-0.7) | 100 (86-100) | 68 (47-85) | 99.6 (74.6-133.3) | 14.6 (9.0-24.1) |
Statistical analysis.
The percentage of serum samples with an IgG antibody concentration of ≥0.2 μg/ml according to GSK's 22F-ELISA and the percentage of serum samples with an OPA titer of ≥8 were computed with the 95% confidence interval (CI) on the according-to-protocol cohorts for immunogenicity.
The geometric mean antibody concentrations (GMCs) and the geometric mean OPA titers (GMTs) were calculated with the 95% CIs by taking the antilog of the mean of the log concentration/titer transformations. Reverse cumulative distribution curves of the antibody concentrations and OPA titers were plotted on the according-to-protocol cohorts for immunogenicity. Data were pooled across the three primary vaccination studies (studies A to C) and the three booster studies (studies D to F) for both 7vCRM and PHiD-CV vaccine recipients. The results for the 11Pn-PD group of study G were included in the graphs for comparison. Antibody concentrations and OPA titers below the assay cutoff were assigned an arbitrary value of half the assay cutoff (i.e., 0.025 μg/ml for GSK's 22F-ELISA and 4 for GSK's OPA assay) for calculation of GMCs and GMTs, as well as for the reverse cumulative distribution curves.
RESULTS
Conjugation by reductive amination induces additional epitopes on the 19F polysaccharide.
The ability of naturally occurring antibodies to bind the 19F polysaccharide was assessed at the CBER by measuring, in sera from unimmunized adults, the concentrations of antibodies to the native serotype 19F polysaccharide and to 19F-HSA, where the 19F polysaccharide is linked to human serum albumin by reductive amination. Approximately half (55/113 [48.7%]) of the sera had a 19F-HSA/native 19F IgG concentration ratio between 0.5 and 2, suggesting that they were equally reactive toward 19F-HSA and native 19F (Fig. 1 A). A large proportion of sera (44/113 [38.9%]) bound more to native 19F polysaccharide than to 19F-HSA (ratio, <0.5). Surprisingly, 14 of 113 (12.4%) sera from unimmunized adults had a 19F-HSA/native 19F antibody concentration ratio above 2, suggesting stronger binding to the 19F-HSA conjugate than to the native 19F polysaccharide. Thus, it appears that the reductive amination conjugation method utilizing periodate treatment modifies the antigenic properties of the 19F polysaccharide.
A competitive inhibition ELISA (7) was subsequently used to assess whether the binding to 19F-HSA could be inhibited by native 19F polysaccharide, 19F-HSA, or the 19F polysaccharide conjugated to tyramine by cyanylation (19F-tyra). Two different patterns of inhibition were found in these sera from unimmunized adults. The first pattern occurred in sera with similar levels of binding to 19F-HSA and native 19F (19F-HSA/native 19F antibody concentration ratios between 0.5 and 2), where preincubation with the native 19F, 19F-HSA, or 19F-tyra resulted in equivalent inhibition. An example of this pattern is shown in Fig. 1B (left): serum sample NA-31 has a 19F-HSA/native 19F antibody concentration ratio of 0.74. The second pattern occurred in sera with stronger binding to 19F-HSA (ratios, >2), where preincubation with 19F-HSA, but not with native 19F or 19F-tyra, strongly inhibited binding in a dose-dependent manner. An example of this pattern is shown in Fig. 1B (right): serum sample NA-47 has a 19F-HSA/native 19F antibody concentration ratio of 17.57. These results and the inhibition data for sera from additional healthy adults (data not shown) suggest that the 19F polysaccharide activated by periodate and linked to HSA by reductive amination expresses at least one additional epitope that is not present on either the native 19F polysaccharide or the 19F polysaccharide linked to tyramine by cyanylation. The 19F polysaccharide linked to CRM197 using reductive amination (19F-CRM, obtained from the licensed 7vCRM vaccine) also strongly inhibited binding to 19F-HSA, suggesting that this putative additional epitope on 19F-HSA is also present on the 19F-CRM conjugate (Fig. 1C).
The distribution of the ratios of concentrations of IgG against 19F-HSA to the concentrations of IgG against native 19F, as measured using the CBER's 22F-ELISA, was then compared for sets of sera from adults who were either left unvaccinated or vaccinated with different pneumococcal vaccines (Fig. 2). In adults vaccinated with 23vPS, the distribution of the ratios was relatively similar to that found for unvaccinated adults, with a large proportion of the ratios equal to or below 2. Most of the sera from adults vaccinated with 19F polysaccharides conjugated using cyanylation (4Pn-PD/11Pn-PD) or a bigeneric spacer (7vOMPC) bound equally to 19F-HSA and native 19F (ratios between 0.5 and 2), and only 6% to 10% of these sera had a 19F-HSA/native 19F antibody concentration ratio above 2. In contrast, the majority of samples (53%) from adults immunized with 7vCRM, produced using reductive amination, bound more to 19F-HSA (ratios, >2). These results suggest that antibodies against the additional epitope are induced mainly after vaccination with the 19F polysaccharide conjugated using reductive amination (as in 7vCRM), but not after vaccination with the native 19F polysaccharide (as in 23vPS) or the 19F polysaccharide conjugated using a bigeneric spacer (as in 7vOMPC) or cyanylation (as in PHiD-CV), where the levels of antibodies remain similar to those of naturally occurring antibodies to this additional epitope.
Finally, to determine whether other serotypes within pneumococcal serogroup 19 could be responsible for the antibodies recognizing the putative additional epitope, an inhibition ELISA was performed with polysaccharides from serotypes 19A, 19B, and 19C in sera from unimmunized adults (Fig. 1C). The level of inhibition of binding was similar to that obtained with 19F-tyra and native 19F polysaccharide. Thus, the heterologous serotypes 19A, 19B, and 19C were not the source of these naturally occurring antibodies.
Distribution of serotype 19F- and 19A-specific immune responses in vaccinated children.
Reverse cumulative distribution curves of the antibody concentrations and the OPA titers (as measured by GSK's 22F-ELISA and OPA assays) after vaccination with PHiD-CV, 11Pn-PD, or 7vCRM are shown in Fig. 3. Primary vaccination induced a strong antibody response against serotype 19F (Fig. 3A, left). The distributions of antibody concentrations were in the same range for the three PCVs. The response was further improved following booster vaccination. However, the functionalities of these antibodies differed when they were measured by the OPA assay (Fig. 3A, right). The OPA GMT against serotype 19F obtained after primary vaccination with 7vCRM (52.4 [95% CI, 44.9 to 61.1]; pooled results from studies A to C) was lower than the OPA GMT obtained after primary vaccination with the prototype 11Pn-PD vaccine, which, in turn, was lower than that obtained after primary vaccination with PHiD-CV (308.6 [95% CI, 277.3 to 343.4]; pooled results from studies A to C). These differences were attenuated after the booster dose, although the OPA GMT after vaccination with PHiD-CV remained higher than those obtained after vaccination with the other two vaccines (835.3 [95% CI, 754.9 to 924.3] for PHiD-CV versus 346.0 [95% CI, 285.9 to 418.7] for 7vCRM).
The concentrations of antibodies and OPA titers against the related serotype 19A were lower than those against serotype 19F (Fig. 3B). Following primary vaccination, PHiD-CV induced higher antibody concentrations against serotype 19A than 7vCRM or 11Pn-PD. Concentrations of antibodies against serotype 19A increased after the booster dose for all three vaccines. Although substantial amounts of anti-19A antibodies could be detected by ELISA after vaccination with all three PCVs, only a limited fraction of these antibodies were functional according to the OPA assay (Fig. 3B, right). Only 1.8% (95% CI, 0.6% to 4.1%) of the children had functional antibodies against 19A after primary vaccination with 7vCRM, whereas functional antibodies above the threshold were detected in 16.7% (95% CI, 10.0% to 25.3%) of the children after primary vaccination with 11Pn-PD and in 24.3% (95% CI, 21.4% to 27.3%) after primary vaccination with PHiD-CV. After the booster dose, the level of OPA increased for all three vaccines. Functional antibodies were detected in 26.9% (95% CI, 21.7% to 32.6%) of the children primed and boosted with 7vCRM, whereas 60.3% (95% CI, 51.9% to 68.3%) of the children vaccinated with 11Pn-PD and 55.2% (95% CI, 51.9% to 58.4%) of the children vaccinated with PHiD-CV had functional antibodies. The OPA GMT against serotype 19A after the booster vaccination was higher with PHiD-CV than with 7vCRM (41.2 [95% CI, 35.4 to 48.1] versus 10.3 [95% CI, 8.4 to 12.7]). These results showed that whereas serotype 19F conjugates induced high levels of functional antibodies against the homologous 19F serotype, only limited amounts of functional cross-reactive anti-serotype 19A antibodies could be detected.
Comparison of the antibodies to serotype 19F and 19A polysaccharides induced in children vaccinated with three PCVs.
To explore the impact of the conjugation method and the carrier protein on serotype 19F and 19A polysaccharide immunogenicity in children, we analyzed the quality of the antibodies against serotype 19F and the cross-reactive antibodies against serotype 19A induced after vaccination with three different PCVs (7vCRM, 7vOMPC, and 11Pn-PD). Serum samples from children who received three or four PCV doses were selected to represent similar anti-serotype 19F antibody concentrations in each vaccine group. The antibody concentrations ranged from 4.7 to 136.7 μg/ml for serotype 19F and from less than 0.1 to 9.0 μg/ml for serotype 19A. For serotype 19F, all of the samples tested from children vaccinated with 7vCRM or 11Pn-PD had OPA titers above the seropositivity cutoff (≥8), as did 87% of the samples from children vaccinated with 7vOMPC (Table 3). For serotype 19A, the rates of OPA seropositivity were 2 and 7 times higher in the 7vOMPC (20% [95% CI, 8% to 39%]) and 11Pn-PD (68% [95% CI, 47% to 85%]) groups, respectively, than in the 7vCRM group (10% [95% CI, 2% to 27%]). The 19A OPA GMTs were higher in the 11Pn-PD group (14.6 [95% CI, 9.0 to 24.1]) than in the two other groups (4.9 [95% CI, 3.8 to 6.3] for 7vCRM and 4.9 [95% CI, 4.2 to 5.8] for 7vOMPC). Thus, 7vCRM, 7vOMPC, and 11Pn-PD seem to have different abilities to induce functional cross-reactive antibodies against serotype 19A.
DISCUSSION
Our results showed that vaccination with 7vCRM or PHiD-CV induces high concentrations of antibodies against serotype 19F. However, higher levels of functional antibodies against 19F and 19A polysaccharides were induced by PHiD-CV, which uses cyanylation rather than reductive amination to conjugate polysaccharides to carrier proteins. One possible explanation is that conjugation of the 19F polysaccharide using reductive amination (as in 7vCRM) induced at least one additional epitope that is not present within the native form of the 19F polysaccharide or following 19F conjugation using cyanylation (as in PHiD-CV) or a bifunctional spacer (as in 7vOMPC).
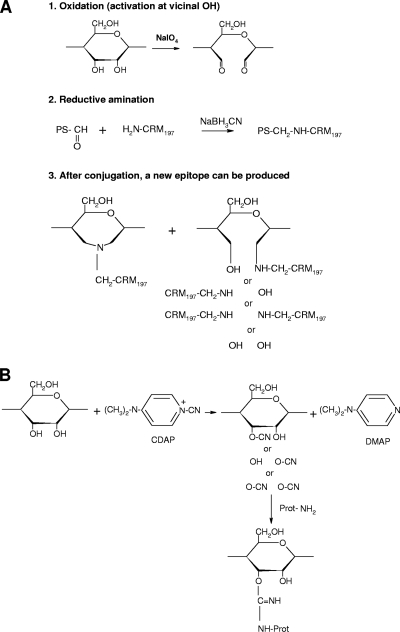
The pneumococcal polysaccharides must be chemically activated before conjugation with the carrier protein. The periodate oxidation step prior to reductive amination breaks and opens the hexasaccharide ring structure (Fig. 4 A), which may lead to ring-opened conjugates, depending on the serotype. This could result in increased flexibility of the polysaccharide and therefore a change in epitope exposure. In addition, the ring-opened polysaccharides may then reclose after the reductive-amination process, allowing the polysaccharides to form additional new conformations, some of which may result in new epitopes. In contrast, cyanylation, which activates the hydroxyl residues without altering the ring structure, allows better preservation of the native polysaccharide epitopes (Fig. 4B).
FIG. 4.
Preparation of polysaccharide-protein conjugates using reductive amination or cyanylation. (A) Conjugation involves oxidation of the polysaccharide with sodium periodate to introduce reactive aldehydes, followed by linkage to the carrier protein using reductive amination. This method breaks and opens the hexasaccharide ring structure. After conjugation, a new immunogenic epitope can be produced due to the binding of new groups to the hexasaccharide ring. (B) Cyanylation using CDAP introduces a cyanate group to hydroxyl groups, which forms a covalent bond to the amino or hydrazide group upon addition of the protein component. After cyanylation conjugation, the hexasaccharide ring remains intact and other chemical groups are not able to bind.
The periodate oxidation opens the hexasaccharide ring structures in most pneumococcal serotypes conjugated by this method, but the greatest effects are seen for serotypes 5, 18C, 19F, and 23F, because of the number of possible periodate activation sites on the polysaccharide (23). Thus, additional epitopes are probably generated by reductive amination in serotypes other than 19F.
We found substantial levels of antibodies specific for this additional 19F epitope in some sera from unimmunized adults, indicating that the induced epitope is probably also present on other natural immunogenic materials. The heterologous group 19 polysaccharides (serotypes 19A, 19B, and 19C) were not the source of these naturally occurring antibodies. The antibodies specific for the putative additional epitope could thus be specific for an epitope found on a common commensal organism. This is analogous to the periodate-activated group B streptococcal type III polysaccharide, which shares a common epitope (not present in the native type III polysaccharide) with the pneumococcal type 14 polysaccharide (3).
The putative additional epitope generated by reductive animation induced antibodies with lower (or no) functionality against serotype 19F, as revealed by the OPA titer, than those induced by a cyanylation-conjugated or native polysaccharide (Table 3). Differences in OPA titers may reflect variability not only in the quantity but also in the quality of the antibodies elicited by different conjugates. These results highlight the necessity of using OPA assays in addition to the reference ELISA recommended by WHO when one is evaluating immune responses induced by candidate PCVs (12, 50).
Previous studies have suggested that antibodies against serotype 19F have lower functionality than antibodies against the other serotypes included in 7vCRM (9, 11, 14, 37). Despite this apparently weaker protection against serotype 19F, invasive pneumococcal disease due to serotype 19F is well controlled by vaccination with 7vCRM. The functionality of the antibodies against serotype 19F induced after primary vaccination with the experimental 11Pn-PD vaccine was higher than that induced by 7vCRM. The efficacy of 11Pn-PD against acute otitis media caused by serotype 19F was demonstrated in the POET study (44.4% [95% CI, 8.3% to 66.3%]). To further improve the functionality of 19F-specific antibodies, alternative vaccine formulations were tested, and a diphtheria toxoid conjugate (19F-DT) was selected on the basis of improved OPA responses. PHiD-CV, which contains 19F-DT, demonstrated a better OPA response than 11Pn-PD and 7vCRM following primary vaccination, and also following the booster dose, when high OPA titers against 19F were obtained with all three PCVs (Fig. 3).
Our results showed that although vaccination with 7vCRM induces high concentrations of antibodies that cross-react with serotype 19A polysaccharide, these cross-reactive antibodies have a low opsonophagocytic capacity, in contrast to antibodies induced by vaccination with PCVs prepared using cyanylation. Similar results were obtained by Yu et al. (52). They showed that high antibody concentrations but low OPA titers were induced against serotype 19A after vaccination with two 5-valent prototype vaccines conjugated to CRM197 using reductive amination, whereas, in contrast, low antibody concentrations but high OPA titers were induced after vaccination with 7vOMPC, conjugated using a bigeneric spacer. These results agree with the poor concordance between the level of serotype 19A-specific antibodies estimated by ELISA and their opsonophagocytic capacity, and they support the idea that both types of assay should be used to evaluate immune responses induced by new PCVs.
In the different studies that were carried out to assess the immunogenicity and safety of PHiD-CV (2, 46, 51), 20% to 30% of children had OPA titers of ≥8 against serotype 19A following a 3-dose primary vaccination with PHiD-CV, compared to less than 2% of children primed with 7vCRM. In addition, a booster dose in the second year of life resulted in serotype 19A OPA titers of ≥8 in 55% of PHiD-CV-immunized children and 27% of 7vCRM-immunized children (combined data set from this publication and references 46 and 51). It should also be noted that some serum samples were tested at both the GSK and THL laboratories. When the serotype 19A OPA results from the two laboratories were compared, GSK's OPA assay had a false-negative rate of 23.5% compared to THL's multiplexed OPA assay (10), whereas for serotype 19F, there was 100% agreement between the two assays (data not shown). Thus, GSK's OPA assay may underestimate the functional responses against serotype 19A compared to THL's multiplexed OPA assay.
Although an increase in the incidence of disease caused by serotype 19A has been observed since the introduction of 7vCRM, a recent analysis of the literature suggested that this vaccine could possibly provide some direct protection against serotype 19A disease in fully immunized children but that this modest effect might be masked by countervailing selection pressures, such as high antibiotic use (13). The magnitude of clinical cross-protection against serotype 19A disease that can be provided with the cross-reactive immune responses induced by PHiD-CV remains unknown at present.
In conclusion, our results suggest that the conjugation method can influence the immunogenicity of the serotype 19F polysaccharide and the functionality of the antibodies induced against serotype 19F and the cross-reactive serotype 19A. Furthermore, our results suggest that both an IgG ELISA and an OPA assay should be used to evaluate and compare the immune responses induced by PCVs.
Acknowledgments
We thank Patricia Lommel (GlaxoSmithKline Biologicals) for statistical analyses and critical reading of the manuscript and Frederik Fierens (GlaxoSmithKline Biologicals) for critical reading of the manuscript and helpful suggestions. Writing assistance was provided by Julie Harriague (4Clinics). Editorial assistance and manuscript coordination were provided by Valentine Wascotte and Véronique Mouton (GlaxoSmithKline Biologicals).
GSK Biologicals paid all costs associated with the development and publication of this report. All the authors had full access to the data and final responsibility for the submission of the publication.
C. Frasch and H. Käyhty received consulting fees and honoraria from GlaxoSmithKline Biologicals in the past 3 years. J. Poolman, R. Biemans, and L. Schuerman are employed by GlaxoSmithKline Biologicals and own stock. A. Nurkka has no conflict of interest to declare.
Synflorix is a trademark of the GlaxoSmithKline group of companies. Prevenar/Prevnar is a trademark of Pfizer, Inc. Pneumovax is a trademark of Merck & Co., Inc.
Footnotes
▿
Published ahead of print on 1 December 2010.
REFERENCES
- 1.Bermal, N., et al. 2011. Safety and immunogenicity of a booster dose of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) coadministered with DTPw-HBV/Hib and poliovirus vaccines. Pediatr. Infect. Dis. J. 30**:**69-72. [DOI] [PubMed] [Google Scholar]
- 2.Bermal, N., et al. 2009. The 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) co-administered with DTPw-HBV/Hib and poliovirus vaccines: assessment of immunogenicity. Pediatr. Infect. Dis. J. 28**:**S89-S96. [DOI] [PubMed] [Google Scholar]
- 3.Bhushan, R., B. F. Anthony, and C. E. Frasch. 1998. Estimation of group B streptococcus type III polysaccharide-specific antibody concentrations in human sera is antigen dependent. Infect. Immun. 66**:**5848-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, S., et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19**:**187-195. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2010. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb. Mortal. Wkly. Rep. 59**:**258-261. [PubMed] [Google Scholar]
- 6.Chevallier, B., et al. 2009. Safety and reactogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with routine childhood vaccines. Pediatr. Infect. Dis. J. 28**:**S109-S118. [DOI] [PubMed] [Google Scholar]
- 7.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8**:**266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekström, N., et al. 2005. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 73**:**369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekström, N., M. Vakevainen, J. Verho, T. Kilpi, and H. Kayhty. 2007. Functional antibodies elicited by two heptavalent pneumococcal conjugate vaccines in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 75**:**1794-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekström, N., et al. 2008. Establishment of the multiplexed opsonization assay (MOPA4) at KTL, abstr. P3, p. 133. 6th International Symposium on Pneumococci and Pneumococcal Diseases, Reykjavik, Iceland.
- 11.Eskola, J., et al. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344**:**403-409. [DOI] [PubMed] [Google Scholar]
- 12.Feavers, I., I. Knezevic, M. Powell, and E. Griffiths on behalf of the WHO Consultation on Serological Criteria for Evaluation and Licensing of New Pneumococcal Vaccines. 2009. Meeting report. Challenges in the evaluation and licensing of new pneumococcal vaccines, 7-8 July 2008, Ottawa, Canada. Vaccine 27**:**3681-3688. [DOI] [PubMed] [Google Scholar]
- 13.Hausdorff, W. P., B. Hoet, and L. Schuerman. 2010. Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A? BMC Pediatr. 10**:**4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henckaerts, I., N. Durant, D. De Grave, L. Schuerman, and J. Poolman. 2007. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine 25**:**2518-2527. [DOI] [PubMed] [Google Scholar]
- 15.Henckaerts, I., D. Goldblatt, L. Ashton, and J. Poolman. 2006. Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera. Clin. Vaccine Immunol. 13**:**356-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hicks, L. A., et al. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J. Infect. Dis. 196**:**1346-1354. [DOI] [PubMed] [Google Scholar]
- 17.Jódar, L., et al. 2003. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 21**:**3265-3272. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, S. E., et al. 1999. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in an infant mouse model of bacteremia for Streptococcus pneumoniae. J. Infect. Dis. 180**:**133-140. [DOI] [PubMed] [Google Scholar]
- 19.Katzenellenbogen, E., and H. J. Jennings. 1983. Structural determination of the capsular polysaccharide of Streptococcus pneumoniae type 19A (57). Carbohydr Res. 124**:**235-245. [DOI] [PubMed] [Google Scholar]
- 20.Kieninger, D. M., et al. 2008. Safety and immunologic non-inferiority of 13-valent pneumococcal conjugate vaccine compared to 7-valent pneumococcal conjugate vaccine given with routine vaccines in healthy infants, abstr. G-2117, p. 367. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 21.Kilpi, T., et al. 2003. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin. Infect. Dis. 37**:**1155-1164. [DOI] [PubMed] [Google Scholar]
- 22.Klugman, K. P., et al. 2008. Meta-analysis of the efficacy of conjugate vaccines against invasive pneumococcal disease, p. 317-326. In G. R. Siber, K. P. Klugman, and P. H. Mäkelä (ed.), Pneumococcal vaccines: the impact of conjugate vaccine. ASM Press, Washington, DC.
- 23.Kniskern, P. J., and S. Marburg. 1994. Conjugation: design, chemistry, and analysis, p. 37-69. In R. W. Ellis and D. M. Granoff (ed.), Development and clinical uses of Haemophilus b conjugate vaccines. Marcel Dekker, Inc., New York, NY.
- 24.Knuf, M., et al. 2009. Immunogenicity of routinely used childhood vaccines when coadministered with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV). Pediatr. Infect. Dis. J. 28**:**S97-S108. [DOI] [PubMed] [Google Scholar]
- 25.Lee, C. J. 2002. Quality control of polyvalent pneumococcal polysaccharide-protein conjugate vaccine by nephelometry. Biologicals 30**:**97-103. [DOI] [PubMed] [Google Scholar]
- 26.Lee, H., M. H. Nahm, R. Burton, and K. H. Kim. 2009. Immune response in infants to the heptavalent pneumococcal conjugate vaccine against vaccine-related serotypes 6A and 19A. Clin. Vaccine Immunol. 16**:**376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lees, A., B. L. Nelson, and J. J. Mond. 1996. Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate for use in protein-polysaccharide conjugate vaccines and immunological reagents. Vaccine 14**:**190-198. [DOI] [PubMed] [Google Scholar]
- 28.Marburg, S., et al. 1986. Biomolecular chemistry of macromolecules: synthesis of bacterial polysaccharide conjugates with Neisseria meningitidis membrane protein. J. Am. Chem. Soc. 108**:**5282-5287. [Google Scholar]
- 29.Miyazaki, T., and T. Yadomae. 1971. Polysaccharides of type XIX Pneumococcus. Part II. The type specific polysaccharide and its chemical behaviour. Carbohydr. Res. 16**:**153-159. [Google Scholar]
- 30.Novotny, L. A., et al. 2006. Passive immunization with human anti-protein D antibodies induced by polysaccharide protein D conjugates protects chinchillas against otitis media after intranasal challenge with Haemophilus influenzae. Vaccine 24**:**4804-4811. [DOI] [PubMed] [Google Scholar]
- 31.Nurkka, A., et al. 2004. Immunogenicity and safety of the eleven valent pneumococcal polysaccharide-protein D conjugate vaccine in infants. Pediatr. Infect. Dis. J. 23**:**1008-1014. [DOI] [PubMed] [Google Scholar]
- 32.Pelton, S. I., et al. 2007. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 26**:**468-472. [DOI] [PubMed] [Google Scholar]
- 33.Poolman, J., et al. 2009. Pneumococcal serotype 3 otitis media, limited effect of polysaccharide conjugate immunisation and strain characteristics. Vaccine 27**:**3213-3222. [DOI] [PubMed] [Google Scholar]
- 34.Poolman, J. T., et al. 2010. Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines. Clin. Vaccine Immunol. 17**:**134-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prymula, R., et al. 2008. Safety of the 11-valent pneumococcal vaccine conjugated to non-typeable _Haemophilus influenzae_-derived protein D in the first 2 years of life and immunogenicity of the co-administered hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated polio virus, Haemophilus influenzae type b and control hepatitis A vaccines. Vaccine 26**:**4563-4570. [DOI] [PubMed] [Google Scholar]
- 36.Prymula, R., et al. 2006. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 367**:**740-748. [DOI] [PubMed] [Google Scholar]
- 37.Prymula, R., and L. Schuerman. 2009. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev. Vaccines 8**:**1479-1500. [DOI] [PubMed] [Google Scholar]
- 38.Robbins, J. B., et al. 1983. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J. Infect. Dis. 148**:**1136-1159. [DOI] [PubMed] [Google Scholar]
- 39.Romero-Steiner, S., et al. 2006. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin. Vaccine Immunol. 13**:**165-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero-Steiner, S., et al. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4**:**415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanderson, C. J., and D. V. Wilson. 1971. A simple method for coupling proteins to insoluble polysaccharides. Immunology 20**:**1061-1065. [PMC free article] [PubMed] [Google Scholar]
- 42.Schuerman, L., R. Prymula, V. Chrobok, I. Dieussaert, and J. Poolman. 2007. Kinetics of the immune response following pneumococcal PD conjugate vaccination. Vaccine 25**:**1953-1961. [DOI] [PubMed] [Google Scholar]
- 43.Schuerman, L., R. Prymula, I. Henckaerts, and J. Poolman. 2007. ELISA IgG concentrations and opsonophagocytic activity following pneumococcal protein D conjugate vaccination and relationship to efficacy against acute otitis media. Vaccine 25**:**1962-1968. [DOI] [PubMed] [Google Scholar]
- 44.Simell, B., M. Lahdenkari, A. Reunanen, H. Kayhty, and M. Vakevainen. 2008. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin. Vaccine Immunol. 15**:**1391-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singleton, R. J., et al. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska Native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 297**:**1784-1792. [DOI] [PubMed] [Google Scholar]
- 46.Vesikari, T., et al. 2009. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr. Infect. Dis. J. 28**:**S66-S76. [DOI] [PubMed] [Google Scholar]
- 47.Vitharsson, G., I. Jonsdottir, S. Jonsson, and H. Valdimarsson. 1994. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J. Infect. Dis. 170**:**592-599. [DOI] [PubMed] [Google Scholar]
- 48.Whitney, C. G., et al. 2006. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 368**:**1495-1502. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. 2008. Meeting report. WHO/Health Canada Consultation on serological criteria for evaluation and licensing of new pneumococcal vaccines, 7 to 8 July 2008, Ottawa, Canada. World Health Organization, Geneva, Switzerland. http://www.who.int/biologicals/publications/meetings/areas/vaccines/pneumococcal/en/index.html.
- 50.World Health Organization. 2005. WHO Expert Committee on Biological Standardization. Recommendations for the production and control of pneumococcal conjugate vaccines, p. 64-98. WHO Technical Report Series, no. 927, annex 2. World Health Organization, Geneva, Switzerland. http://whqlibdoc.who.int/trs/WHO_TRS_927_eng.pdf. [PubMed]
- 51.Wysocki, J., et al. 2009. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when co-administered with different Neisseria meningitidis serogroup C conjugate vaccines. Pediatr. Infect. Dis. J. 28**:**S77-S88. [DOI] [PubMed] [Google Scholar]
- 52.Yu, X., et al. 1999. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants. J. Infect. Dis. 180**:**1569-1576. [DOI] [PubMed] [Google Scholar]