LPS from Porphyromonas gingivalis Sensitizes Capsaicin-Sensitive Nociceptors (original) (raw)
. Author manuscript; available in PMC: 2012 Jan 1.
Abstract
Although odontogenic infections are often accompanied by pain, little is known about the potential mechanisms mediating this effect. In this study, we tested the hypothesis that trigeminal nociceptive neurons are directly sensitized by lipopolysaccharide (LPS) isolated from an endodontic pathogen, Porphyromonas gingivalis (P. gingivalis). In vitro studies conducted with cultures of rat trigeminal neurons demonstrated that pretreatment with LPS produced a significant increase in the capsaicin-evoked release of calcitonin gene-related peptide (CGRP) when compared to vehicle pretreatment, thus showing sensitization of the capsaicin receptor, TRPV1, by LPS. Furthermore, confocal microscopic examination of human tooth pulp samples showed the colocalization of the LPS receptor (toll-like receptor 4; TLR4) with CGRP containing nerve fibers. Collectively, these results suggest the direct sensitization of nociceptors by LPS at concentrations found in infected canal systems as one mechanism responsible for the pain associated with bacterial infections.
Introduction
Odontogenic infections, such as acute periradicular abscess, are generally polymicrobial and dominated by anaerobic bacteria. Although several different microorganisms can colonize the root canal system, some specific species have been related to features of periradicular diseases. For example, the presence of Porphyromonas gingivalis is often accompanied by painful periradicular lesions, including abscesses (1, 2).
Porphyromonas gingivalis (P. gingivalis) is a nonfermentative black-pigmented Gram-negative, obligate anaerobic rod (3), which has its primary ecological niche in the human oral cavity and is well known as an important periodontal pathogen (4). However, this microorganism is frequently present in root canal infections and other odontogenic abscesses of patients not suffering from periodontal disease, which confirms its pathogenic role (1). P. gingivalis is found in 30% of teeth with primary root canal infections associated with painful symptoms (2). Furthermore, there is a positive association between Porphyromonas spp. and pain, mechanical allodynia, swelling and purulent exudates in root canals (5,6).
P. gingivalis possesses a large number of putative virulence determinants including fimbriae, haemagglutinin, capsule, outer membrane vesicles, powerful hydrolytic enzymes and lipopolysaccharide complex (LPS). These virulence factors can initiate host defense mechanisms, leading to tissue destruction (7). Among these factors, LPS is a remarkably potent stimulant of the host’s innate immune response against gram-negative bacterial infections. LPS mediates its biological functions via activation of the toll-like receptor 4 (TLR4) and cluster of differentiation receptor-14 (CD-14) (8,9). Thus, cells that express TLR4 and/or CD14 have the potential for detecting bacterially derived LPS.
The mechanisms for pain due to odontogenic infections remain unclear. In general, it is possible that bacterial byproducts indirectly activate nociceptors via the release of host factors (eg., prostaglandins, leukotrienes, cytokines, etc) triggered by activation of pathways such as the receptors of the innate immune system expressed by nearby cells (eg., macrophages, neutrophils, etc). Alternatively, it is possible that bacterial byproducts, or even bacteria themselves, directly activate nociceptors. Recent evidence in favor of this latter hypothesis has been generated by the observation that TLR4 and CD14 are expressed by trigeminal ganglion nociceptors that express the capsaicin receptor, transient receptor potential V1 (TRPV1) (10).
Although this prior anatomical study demonstrated that nociceptor cell bodies in the trigeminal ganglion express TLR4 and CD14, it is not known whether pulpal terminals of capsaicin-sensitive nociceptors express the TLR4 receptor, nor has it been demonstrated that LPS activates nociceptors or sensitizes their responses to noxious stimuli such as capsaicin. To address this gap in knowledge, we conducted the following study in two parts. First, we evaluated whether human dental pulp neurons co-express both TLR4 and TRPV1. Second, we evaluated whether pretreatment with LPS purified from Porphyromonas gingivalis altered either basal or capsaicin-evoked release of calcitonin gene-related peptide (CGRP) from primary cultures of trigeminal neurons. CGRP was selected as a marker for neuronal activation since prior studies have demonstrated that it mediates the initiation of neurogenic inflammation and is released from capsaicin-sensitive pulpal neurons (11–13).
Material and Methods
Human dental pulp
This study was approved by the University of Texas Health Science Center at San Antonio IRB, and patients provided written informed consent. Patients who satisfied the inclusion criteria of a normal tooth scheduled for extraction, and reported a short-lasting response to a thermal test, no spontaneous pain, no caries or restoration, and no periradicular radiolucency were selected; all teeth were erupted and had fully developed roots. Following extraction, the tooth was split in half, and the dental pulp was carefully removed. The pulpal tissue was fixed in 4% paraformadehyde in 0.1 M phosphate buffer (PB) for 30 minutes, rinsed three times (10 minutes each) in 0.1M PB, and left overnight in 30% sucrose in 0.1 M PB at 4°C. The next day the tissue was embedded in Neg-50 (Richard-Allen Scientific; Kalamazoo, MI, USA) and 30µm thick sections were cut in the longitudinal plane with a cryostat. The pulpal sections were placed on Superfrost Plus slides (Fisher Scientific; Pittsburgh, PA, USA) and stored at −20 °C.
Immunohistochemistry
All steps described below were performed at room temperature. The slides with pulpal tissue were rinsed three times in 0.1M phosphate buffered saline (PBS) for 10 minutes each. Non-specific binding was decreased by the incubation of the tissue in 0.05M PBS with 0.3 % Triton X-100 (Fisher Scientific), 2% bovine gamma-globulin (BGG; Sigma; St. Louis, MO, USA), and 4% normal goat serum (NGS- Sigma) for one hour as a blocking solution. The tissue was incubated in primary antibodies (anti-human TLR4 raised in the rabbit, Santa Cruz Biotech, Santa Cruz, CA, USA at 1:100; anti-human CGRP raised in the guinea-pig, Neuromics, Northfield, MN, USA at 1:100; and with N52/GAP43 raised in the mouse, Neuromics, Northfield, MN, USA at 1:2,000/1:500) diluted in blocking solution overnight. The N52 and GAP43 (14) antibodies were used to identify a wide spectrum of nerve fibers in the dental pulp. The following day the tissue was washed three times in 0.1M PBS, at ten minutes each rinse. Secondary antibodies [anti-rabbit-conjugated AlexaFluor-568 (red), anti-guinea-pig-conjugated AlexaFluor-633 (blue) and anti-mouse-conjugated AlexaFluor-488 (green); Molecular Probes, Eugene, OR, USA] were diluted 1:100 in blocking solution and applied to the tissue for one hour while protected from the light. The tissue was rinsed three times in 0.1M PBS at ten minutes each in the dark, rinsed briefly in H2O, air-dried, coverslipped with Vectashield, (Vector Laboratories, Burlingame, CA, USA) and stored in a refrigerator at 4 °C. Confocal microscopy images were acquired using a Nikon Eclipse 90 microscope with a Nikon C1si confocal scanner (Melville, NY, USA).
Animals
Adult male Sprague Dawley (Charles River Laboratories, Wilmington, MA) rats weighing 250–300 g were used in this study. All animal study protocols were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and conformed to the International Association for the Study of Pain and federal guidelines. Animals were housed for 1 week before sacrifice with food and water available ad libitum.
Rat trigeminal ganglia primary culture
As described (15), rat trigeminal ganglia (TG) were removed quickly after decapitation, placed in ice-cold calcium- and magnesium-free HBSS (Invitrogen, Paisley, UK), and washed twice with HBBS. Ganglia were then treated with 5 mg/ml collagenase (Sigma) for 30 min and 0.1% trypsin (Sigma) for 15 min before homogenization. The TG were then centrifuged at 671 g for 2 min and resuspended in DMEM (Invitrogen), which also contained 1x penicillin–streptomycin (Invitrogen), 1x glutamine (Invitrogen), 10% fetal calf serum (Invitrogen), and 100 ng/ml nerve growth factor (NGF; Harlan, Indianapolis, IN, USA). The tissue was triturated gently, and cells from six ganglia were plated into a 48-well poly-D-lysine-coated plate (BD Biosciences, Bedford, MA), yielding ~4000 cells per well. The media were replaced at the end of 24 h and then 48 h later.
CGRP release assay
All culture experiments were performed on days 5–7, at 37°C, using modified Hanks (Gibco, Carlsbad, CA, USA) buffer (10.9 mM Hepes, 4.2 mM sodium bicarbonate, 10 mM dextrose and 0.1% bovine serum albumin were added to 1× Hanks). After two initial washes, a 15 min baseline sample was collected. The cells then were exposed to either vehicle or Porphyromonas gingivalis LPS (#05H23-SV; Invivogen, San Diego, CA) (2mg/ml) for 15 min (pre-treatment) and then stimulated with capsaicin (30 nM) for 15 min (co-treatment). All the supernatants were collected for analysis of immunoreactive calcitonin gene-related peptide (iCGRP) content by radioimmunoassay (RIA).
iCGRP RIA
As previously described (16), primary antibody against CGRP (final dilution of 1:1,000,000; kindly donated by Dr. M. J. Iadarola, National Institutes of Health, Bethesda, MD) was added in the tubes containing media from cultured rat TG and incubated at 4°C for 24 h. Then 100 µl of [125I-]-Tyr0-CGRP28–37 (~20,000 cpm) and 50 µl of goat anti-rabbit antisera coupled to ferric beads (PerSeptive Diagnostics, Cambridge, MA) were added to these tubes. The tubes were incubated for another 24 h at 4°C. The assay was stopped using immunomagnetic separation of bound from free tracer. All compounds used in experiments were tested for interference with the RIA. The minimum detectable levels for CGRP for this assay are 3 fmol and with 50% displacement at 28 fmol.
Results
Immunohistochemistry
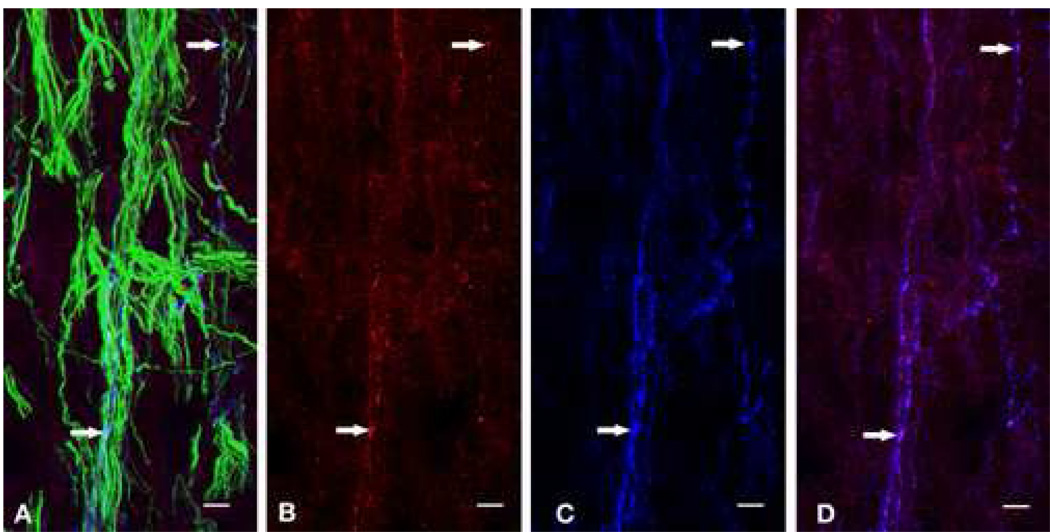
Confocal microscopic evaluation of the human dental pulp samples that had been stained with the TLR4, CGRP and N52/GAP43 antibodies demonstrated the colocalization of TLR4-immunoreactivity (IR) with CGRP-IR in a subset of the N52/GAP43 identified nerve fibers (Figure 1). This colocalization was particularly apparent within the small axons located within the subodontoblastic layer located next to the pulp horns (Figure 1). The CGRP staining was punctuate in character, and the TLR4 staining was especially striking within these same puncta.
Figure 1.
Confocal micrograph of the subodontoblastic plexus near a human pulp horn stained for N52/GAP43 (green), TLR4 (red), CGRP (blue) immunoreactivities (A). TLR4 and CGRP immunoreactivities are separately shown in B and C, respectively. A subset of the N52/GAP43 identified nerve fibers show the colocalization of CGRP and TRL4 (D). The white horizontal arrows show representative areas of colocalization. Scale bar is 7.5 µm.
LPS Modulation of Capsaicin-evoked CGRP release
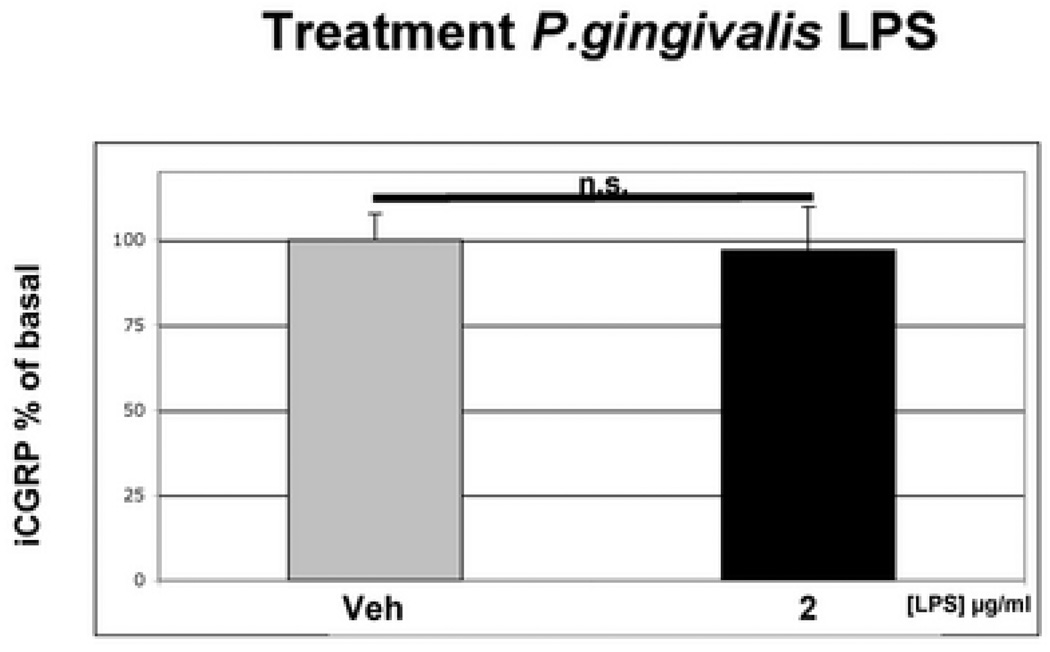
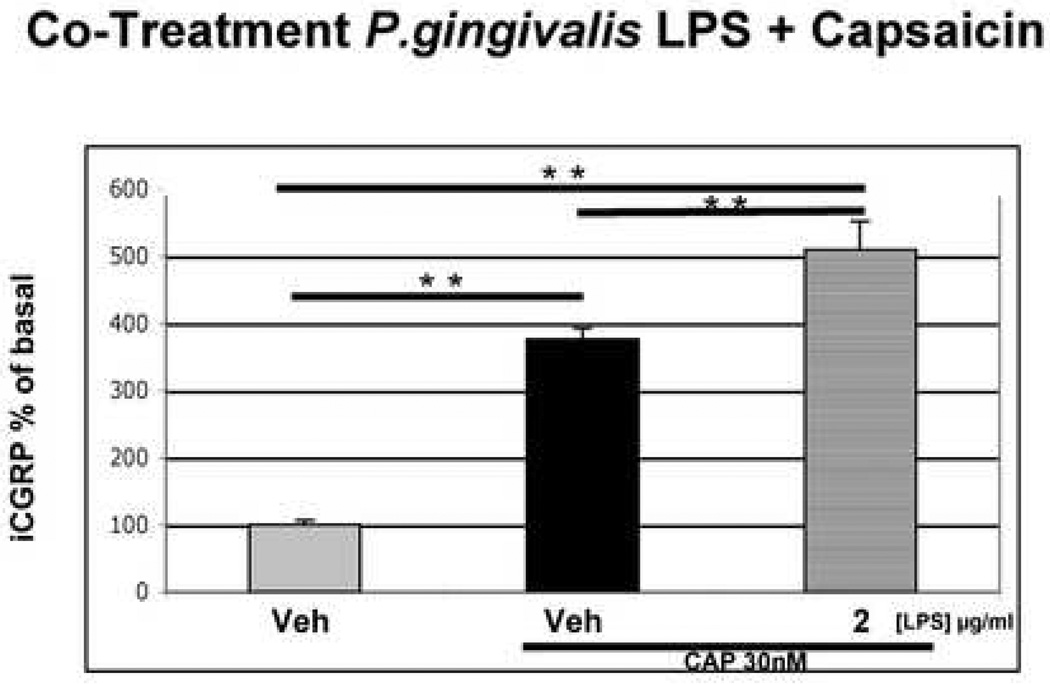
We next evaluated whether LPS alters the functional activity of TG neurons. Pretreatment of cultured TG neurons with vehicle or LPS from Porphyromonas gingivalis (2 µg/ml) for 15 minutes did not alter basal rates of CGRP release compared to vehicle pre-treatment (Fig 2). However, LPS did significantly enhance capsaicin activation of the same TG neuronal cultures (Fig 3), with LPS pre-treatment producing a significant increase in CGRP release as compared to the vehicle treated group (p <0.01, Fig. 3).
Figure 2.
Effect of LPS on Basal CGRP Release. Rat TG neurons were cultured for 7 days, washed and then exposed to either vehicle or LPS derived from P.gingivalis (2 µg/ml) for 15 min prior to collection of media and assay for CGRP by radioimmunoassay. Treatment Data are presented as mean ± SEM percentage of basal release (n = 6 per group; n.s _p_>0.05, one-way ANOVA with Bonferroni’s post hoc test).
Figure 3.
Effect of LPS on Capsaicin-Evoked CGRP Release. Cultured TG neurons were exposed to either vehicle or P.gingivalis LPS (2 µg/ml for 15 min) and then exposed to either vehicle/capsaicin (30nM) or LPS/capsaicin for an additional 15 min. Treatment Data are presented as mean ± SEM percentage of basal release (n = 6 per group; **p < 0.01; *** p < 0.001, one-way ANOVA with Bonferroni’s post hoc test).
Discussion
Recent studies have been focused on the relationship between the concentration of LPS present in necrotic root canals of infected teeth and associated signs and symptoms in endodontic patients. One recent study (19) verified that LPS was detectable in all the samples from root canals of 50 patients with a concentration range 0.48–4.42 µg/ml. The mean concentration of endotoxin in samples from patients with spontaneous pain was 3.7µg/ml, while in asymptomatic cases it was 2.4µg/ml, showing a positive correlation between the concentration of endotoxin in the root canal and the presence of endodontic signs and symptoms. Other investigators examined the endotoxin content of samples obtained from single root canals of 30 teeth with pulpal necrosis and apical periodontitis (20). Their results demonstrated that greater LPS levels were correlated with clinical symptoms and the presence of exudate from the canal systems. These correlational studies are consistent with the hypothesis that LPS in clinical infections is related to the production of spontaneous pain and mechanical allodynia. This hypothesis is strengthened by preclinical studies demonstrating that injection of LPS into rats produces nocifensive behavior and mechanical allodynia (21). Taken together, these data implicate LPS as a mediator of spontaneous pain and mechanical allodynia in patients with odontogenic infections.
In the present study, we used LPS from P. gingivalis, one of the most pathogenic species in the group of black-pigmented Gram-negative anaerobes (22). Other investigators have found P. endodontalis in 53% and P. gingivalis in 12% of periapical abscesses with endodontic origin (4). In addition, samples collected from 70 root canals of abscessed teeth were found to contain Porphyromonas spp., and especially P. gingivalis, which was significantly correlated with mechanical allodynia and purulent exudates (6).
It is well accepted that the innate immune response against bacteria leads to the release of certain inflammatory mediators, such as cytokines, that can sensitize nociceptors. Therefore, it is possible that pain due to odontogenic infections might be mediated indirectly by the sensitization of nociceptors. However, it has been demonstrated recently that trigeminal neurons express both LPS receptors (TLR4 and CD14), leading to the hypothesis that bacterial byproducts might directly activate or sensitize trigeminal nociceptors (10). The present study confirmed these results by demonstrating that TLR4 is co-localized with CGRP in fibers innervating human dental pulp.
CGRP is a neuropeptide synthesized and released by a capsaicin-sensitive subpopulation of trigeminal nociceptors when activated. In peripheral tissue, this neuropeptide is involved in the development of neurogenic inflammation leading to vasodilation and contributing to the formation of edema. Therefore, levels of CGRP released from TG neurons can be used as a dependent measure of neuronal activation. In addition, capsaicin (the pungent ingredient of hot chili peppers) selectively activates TRPV1 (23), permitting it to be used during in vitro experiments to evaluate mechanisms regulating the activity of this important class of nociceptors.
TRPV1 is a non-selective ionotropic channel that is exclusively expressed in sensory neurons by nociceptors and is physiologically activated by heat (>43°C), low pH (<5.5) and certain lipophilic ligands (20). Interestingly, heat and low pH (acidity) are characteristics of the inflammatory milieu. Also, TRPV1 is known to be sensitized by numerous inflammatory mediators (21). Indeed, the crucial role of TRPV1 in inflammatory pain has been underscored by studies using mice carrying a genetic deletion of the TRPV1 gene. In these knockout mice, thermal hyperalgesia/allodynia does not develop following inflammation (25, 26).
TLR4 function as pattern recognition receptors for innate immune detection of tissue levels of bacterial-derived factors such as LPS (8, 9). The finding that capsaicin-sensitive nociceptors (i.e., TRPV1-positive) express TLR4 is consistent with the hypothesis that LPS can sensitize nociceptors via direct activation of this receptor. Therefore, we evaluated whether LPS derived from P. gingivalis directly sensitized these neurons. In this study, we found that LPS did not activate these neurons, but significantly sensitized them to an enhanced response to a chemical stimulus. Thus, LPS is capable of directly interacting with capsaicin-sensitive neurons, leading to exaggerated responses to noxious stimuli.
The TRPV1 channel is known to be sensitized by intracellular signaling pathways such as protein kinase C, phosphatidyl Inositol 3 kinase and others (27, 28). Therefore, the LPS-TLR4 interaction may trigger intracellular signaling cascades in the nociceptors leading to the sensitization of TRPV1. These findings might have considerable significance in understanding mechanisms of severe or prolonged inflammatory pain associated with bacterial infections (29).
Collectively, these data demonstrate for the first time that LPS from P. gingivalis, at concentrations found in infected canal systems, significantly sensitizes TRPV1 responses in trigeminal neurons. These findings have clear scientific and clinical implications and may shed light on the mechanism by which bacteria can directly sensitize trigeminal nociceptors.
Acknowledgments
This study was supported by: UTHSCSA President’s Council Endowed Chair in Research; NIH Grant DE015576 and CNPq-Brazil (PDE 200880/2005-5)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sundqvist G, Johansson E, Sjogren U. Prevalence of black-pigmented bacteroides species in root canal infections. J Endod. 1989;15:13–19. doi: 10.1016/S0099-2399(89)80092-5. [DOI] [PubMed] [Google Scholar]
- 2.Rocas IN, Siqueira JF, Jr, Andrade AFB, Uzeda M. Identification of selected putative oral pathogens in primary root canal infections associated with symptoms. Anaerobe. 2002;8:200–208. [Google Scholar]
- 3.Shah HN, Collins MD. Proposal for reclassification of Bacteroides asaccharolyticus, Bacteroides gingivalis, and Bacteroides endodontalis in a new genus Porphyromonas. Int J Syst Bacteriol. 1988;3:128–131. [Google Scholar]
- 4.Van Winkelhoff AJ, Carlee AW, de Graaff J. Bacteroides endodontalis and other black-pigmented Bacteroides species in odontogenic abscesses. Infect Immun. 1985;49:494–497. doi: 10.1128/iai.49.3.494-497.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomes BP, Pinheiro ET, Gade-Neto CR, Sousa EL, Ferraz CC, Zaia AA, Teixeira FB, Souza-Filho FJ. Microbiological examination of infected dental root canals. Oral Microbiol Immunol. 2004;19:71–76. doi: 10.1046/j.0902-0055.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 6.Jacinto RC, Gomes BPFA, Shah HN, Ferraz CC, Zaia AA, Souza-Filho FJ. Incidence and antimicrobial susceptibility of Porphyromonas gingivalis isolated from mixed endodontic infections. Int Endod J. 2006;39:62–70. doi: 10.1111/j.1365-2591.2005.01053.x. [DOI] [PubMed] [Google Scholar]
- 7.Mayrand D, Holt SC. Biology of asaccharolytic black pigmented species. Annu Rev Microbiol. 1988;52:134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 2004;6:1361–1367. doi: 10.1016/j.micinf.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- 10.Wadachi R, Hargreaves KM. Trigeminal Nociceptors Express TLR-4 and CD14: a Mechanism for Pain due to Infection. J Dent Res. 2006;85:49–53. doi: 10.1177/154405910608500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hargreaves KM, Jackson DL, Bowles WR. Adrenergic regulation of capsaicin-sensitive neurons in dental pulp. J Endod. 2003;29:397–399. doi: 10.1097/00004770-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Hargreaves KM, Bowles WR, Garry G. An in vitro method to evaluate regulation of neuropeptide release from dental pulp. J Endod. 1992;18:597–600. doi: 10.1016/S0099-2399(06)81329-4. [DOI] [PubMed] [Google Scholar]
- 13.Kilo S, Harding-Rose C, Hargreaves KM, Flores CM. Peripheral CGRP release as a marker for neurogenic inflammation: a model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- 14.Pare M, Albrecht PJ, Noto CJ, Bodkin NL, Pittenger GL, Schreyer DJ, Tigno XT, Hansen BC, Rice FL. Differential hypertrophy and atrophy among all types of cutaneous innervation in the glabrous skin of the monkey hand during aging and naturally occurring type 2 diabetes. J Comp Neurol. 2007;501:543–567. doi: 10.1002/cne.21262. [DOI] [PubMed] [Google Scholar]
- 15.Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, Hargreaves KM. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci. 2006;26:8126–8136. doi: 10.1523/JNEUROSCI.0793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garry MG, Richardson JD, Hargreaves KM. Sodium nitroprusside evokes the release of immunoreactive calcitonin gene-related peptide and substance P from dorsal horn slices via nitric oxide-dependent and nitric oxide-independent mechanisms. J Neurosci. 1994;14:4329–4337. doi: 10.1523/JNEUROSCI.14-07-04329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang HW, Zhang W, Ren BP, Zeng JF, Ling JQ. Expression of Toll Like Receptor 4 in Normal Human Odontoblasts and Dental Pulp Tissue. J Endod. 2006;32:747–751. doi: 10.1016/j.joen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Botero TM, Shelburne CE, Holland GR, Hanks CT, Nör JE. TLR4 Mediates LPS-Induced VEGF Expression in Odontoblasts. J Endod. 2006;32:951–955. doi: 10.1016/j.joen.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Jacinto RC, Gomes BP, Shah HN, Ferraz CC, Zaia AA, Souza-Filho FJ. Quantification of endotoxins in necrotic root canals from symptomatic and asymptomatic teeth. J Med Microbiol. 2005;54:777–783. doi: 10.1099/jmm.0.45976-0. [DOI] [PubMed] [Google Scholar]
- 20.Horiba N, Maekawa Y, Abe Y, Ito M, Matsumoto T, Nakamura H. Correlations between endotoxin and clinical symptoms or radiolucent areas in infected root canals. Oral Surg Oral Med Oral Pathol. 1991;71:492–495. doi: 10.1016/0030-4220(91)90438-i. [DOI] [PubMed] [Google Scholar]
- 21.Cahill CM, Dray A, Coderre TJ. Priming enhances endotoxin-induced thermal hyperalgesia and mechanical allodynia in rats. Brain Res. 1998;12:13–22. doi: 10.1016/s0006-8993(98)00786-0. [DOI] [PubMed] [Google Scholar]
- 22.Sundqvist G. Pathogenicity and virulence of black-pigmented gram-negative anaerobes. FEMS Immunol Med Microbiol. 1993;6:125–137. doi: 10.1111/j.1574-695X.1993.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 23.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 24.Szolcsanyi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides. 2004;38:377–384. doi: 10.1016/j.npep.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 26.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 27.Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci U S A. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnington J, McNaughton P. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]