Environmental and Antigen Receptor-Derived Signals Support Sustained Surveillance of the Lungs by Pathogen-Specific Cytotoxic T Lymphocytes (original) (raw)
Abstract
Viral infections often gain access to the body of their host by exploiting areas of natural vulnerability, such as the semipermeable surfaces of mucosal tissues which are adapted for adsorption of nutrients and other diffusible molecules. Once the microbes have crossed the epithelial barrier, they can disperse to other tissues where eradication may not be possible. The best opportunity for successful immune intervention is immediately after infection while the pathogen is confined to a localized area of the body. Cytotoxic T lymphocytes (CTL) which reside at the site where the infection begins can make an important contribution to immunity by reducing early dissemination of the infection. Because the lungs provide easy access points for many pathogens to enter the body, they require protection from many complementary mechanisms, including pathogen-specific cytotoxic T cells. In this study we show that an enduring response to pathogen-derived peptide antigens facilitates sustained surveillance of the lungs by pathogen-specific CTL during the recovery from influenza virus infection. Our studies show that these processed peptide antigens reinforce expression of two homing receptors (CD69 and CD103) which help recently activated virus-specific CTL colonize the lungs during a mild inflammatory response. We suggest that this requirement for prolonged antigen presentation to reinforce local CTL responses in the lungs explains why protective cellular immunity quickly declines following influenza virus infection and other viral infections that enter the body via mucosal tissues.
INTRODUCTION
Many different pathogens enter the lungs via the conducting airways which descend to progressively branching bronchi and terminate in thin-walled alveoli in the lung parenchyma. Epithelial cells which line the airways and alveoli play a critical role in susceptibility to infection with influenza virus because they express a unique enzyme that is required to cleave hemagglutinin and produce new infectious virus. Because the respiratory tract is vulnerable to so many different infections, the immune system has developed a complex array of defense mechanisms to protect the lungs, including mucus-secreting and ciliated epithelial cells that help expel inhaled antigens via the mucociliary escalator. Pathogens that are able to cross the outer mucus barrier trigger innate immune activation and induce an inflammatory response which is essential for rapid recruitment of other leukocytes to the site of infection. Once the infection has become established in the lungs, eradication requires antibodies and/or T cells (13, 36), which recognize antigens that are carried to the local lymphoid tissues by cells of the innate immune system. The newly activated T cells then quickly mobilize to the site of viral replication. The relative importance of the two branches of adaptive immunity to the recovery of the host is determined by the severity of the infection (13).
Cytotoxic T lymphocytes (CTL) can accelerate the rate of viral clearance from the lungs and provide some cross-reactive immunity between different strains of influenza virus (23). Since epithelial cells in the airways and alveoli produce the highest concentrations of infectious virus, they are the primary targets of the protective CTL. Comparisons between different routes of infection and other methods of vaccination indicate that preexisting populations of virus-specific CD8 T cells in the lungs can make a valuable contribution to immunity (1, 9); however, the mechanisms that support sustained surveillance of the mucosal surface by the responding CTL have not been clearly defined. In this paper we examine the contribution of antigen-induced activation antigens in CD8 T cell migration to the lungs after influenza virus infection. It was previously known that although very few circulating memory CD8 T cells enter the lung airways in the absence of an inflammatory response, the antigen-specific CTL that reside in the airways during the recovery from influenza virus infection are replenished by T cells from the circulation long after most symptoms of inflammation have resolved (8, 47). Our data show that the late recruitment of these additional pathogen-specific CD8 T cells into the lungs is greatly facilitated by a response to recent antigen stimulation, which reinforces expression of two adhesion molecules (CD69 and CD103) that together enhance T cell transit into lungs and retention at the mucosal surface. Although CTL lose much of their lytic activity during prolonged residence in the lung airways (16, 44), they can make a valuable contribution to immunity through the local release of inflammatory mediators which trigger rapid recruitment of lytic cells from other tissues including the lung parenchyma.
MATERIALS AND METHODS
Mice and reagents.
C57BL/6 (B6) and congenic CD45.1+ mice were purchased from Charles River through the National Cancer Institute (NCI) animal program. The CD69 knockout (CD69KO) (30) and CD103KO (37) mice and mice expressing a dominant negative form of the transforming growth factor β (TGF-β) receptor II (dnTGFβRII) (10) were generously provided by other investigators. The cross-bred F5 and OTI T cell receptor (TCR) transgenic mouse lines were generated at the University of Connecticut Health Center, and experiments were performed in accordance with regulations by the American Association for Accreditation of Laboratory Animal Care, as well as federal and state agencies. At 8 to 14 weeks of age, female mice were anesthetized by intraperitoneal (i.p.) injection with 2,2,2-tribromoethanol (Avertin) before intranasal (i.n.) infection with 600 50% egg infectious doses (EID50) of E61-13-H17 influenza virus, 106 EID50 of an X31 virus expressing a peptide from the chicken ovalbumin (OVA) gene (X31-OVA) (28), or 103 PFU of WSN-OVAI. Virus stocks were grown in chicken eggs, titers were determined, and stocks were stored as described previously (4). Major histocompatibility complex class I (MHC-I) tetramers that are specific for the OVA, NP31 (endogenous nucleoprotein), and acid polymerase (PA) epitopes have been described previously (2, 12, 17).
Transfer experiments and antibody treatment.
Spleens and peripheral lymph nodes were dissociated to form single-cell suspensions and depleted of red blood cells. For in vivo competition assays, recipient animals received a total of 106 mixed lymphocytes by tail vein injection. Alternatively, lymphocytes were labeled with 1 μM carboxyfluorescein succinimidyl ester (CFSE) dye at 37°C for 10 min, and 2 × 105 cells were transferred to the recipients (24). To block antigen presentation, mice were treated twice with 25D1.16 or control antibodies at 20 days postinfection ([dpi] 100 μg i.n. plus 400 μg intravenously [i.v.]) and 23 dpi (250 μg i.v.).
Sample preparation for flow cytometry.
Nonadherent cells were collected from the lungs by lavage five times in Hank's balanced salt solution (HBSS). Mice were exsanguinated or perfused with phosphate-buffered saline (PBS) with 75 U/ml heparin until the lungs were white. Ground-glass microscope slides were used to make cell suspensions from spleens and peripheral lymph nodes. Red blood cells were lysed with ammonium chloride, and lymphocytes were filtered through cell strainers. Lymphocytes were released from the chopped lung tissues by digestion with 150 U/ml collagenase (Life Technologies, Rockville, MD) in RPMI medium supplemented with 1 mM MgCl2, 1 mM CaCl2, and 5% fetal calf serum (FCS) at 37°C for 90 min. Cells were pushed through strainers and spun on 44/67% Percoll gradients at 400 × g for 20 min. Washed lymphocytes were stained with tetramers that are specific for the influenza virus epitopes NP366-374 and PA324-333 presented in the context of H-2Db (i.e., NP366-374/Db and PA324-333/Db) as described previously (2, 11). OVA tetramers were made at the University of Connecticut. All other tetramers were supplied by the NIH Tetramer Facility (Emory University Vaccine Center at Yerkes, Atlanta, GA). Lymphocytes were stained with allophycocyanin (APC)-conjugated tetramers and anti-CD8 (clone 53.6.72) for 1 h at room temperature. All other markers were stained at 4°C using monoclonal antibodies (MAb) specific for CD45.1, CD45.2, CD69, CD44, CD127, PD-1, and CD62L (EBioscience or BD Pharmingen, San Diego, CA). Fixed samples were analyzed on a Becton-Dickinson FACSCalibur or LSR-II flow cytometer and analyzed using Flowjo software (Tree Star Inc.).
Histology.
Lungs were inflated with 1 ml of 4% formaldehyde in phosphate-buffered saline and fixed at room temperature for 1 h. Lung tissue was embedded in paraffin, and 4-μm sections were stained with hematoxylin and eosin (H&E) using a standard procedure.
Statistics.
Unless otherwise stated, all experiments were repeated at least twice using a minimum of three animals per group. Statistical significance was determined using an unpaired two-tailed Student's t test. The comparisons that were used to generate P values are indicated by horizontal lines in the figures.
RESULTS
Antigen persistence supports CD8 T cell activation in the lungs.
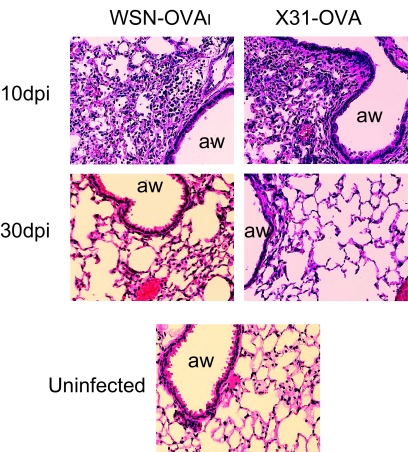
Influenza virus infections induce robust inflammatory responses in the lungs which are characterized by massive accumulations of leukocytes in and around the airways and alveolar spaces (Fig. 1). The symptoms of inflammation in the lungs decline as infectious influenza virus is cleared at ∼10 dpi, when most activated granulocytes are eliminated from the airways (Y.-T. Lee, unpublished data). Residual populations of activated virus-specific CTL persist for several more weeks (16, 25) and are replenished by additional CD8 T cells from the circulation (8, 47). The late timing of their arrival in the airways indicates that the new pathogen-specific T cells are endowed with enhanced migratory properties, which are not characteristic of other circulating memory CD8 T cells that are specific for unrelated pathogens. We have previously shown that the gradual disappearance of activated virus-specific CD8 T cells from the lung airways occurs soon after processed influenza virus peptides are cleared from the local lymph nodes (47). Although bystander CD8 T cells can be recruited into the lungs during heterologous respiratory virus infections (7), they do not persist or maintain an activated phenotype without ongoing inflammation (47). We have further examined the CD8 T cell response to influenza virus infection to determine whether recent antigen stimulation directly aids transit and/or retention of antigen-specific CD8 T cells in the lungs during times of reduced inflammation.
Fig. 1.
Inflammation in lungs declines after viral clearance. Mice were infected with WSN-OVAI or X31-OVA. The lungs were harvested from groups of three animals at either 10 dpi, which is the peak of CD8 effector response, or 30 dpi, when very few activated granulocytes remained. The lungs were inflated with 4% formaldehyde and fixed for 1 h. The lung tissues were embedded in paraffin, and 4-μm sections were stained with hematoxylin and eosin. Photographs were taken at a magnification of ×10 (aw, airway). Tissues from representative animals are shown.
CD69 is a C-type lectin of unknown function which is briefly expressed on T cells soon after antigen stimulation (40). Recently, CD69 has been implicated in having a role in the pathogenesis of allergic airway inflammation during the response to asthma by enhancing the size of the CD4 T cell response in the lungs (27). Only two mechanisms are known to induce transient CD69 expression on murine CD8 T cells: recent TCR signaling and exposure to type I interferon (5, 40). In addition, antigen-specific CD8 T cells which localize to the epithelial surface of the gastrointestinal tract universally express CD69 without any apparent requirement for antigen stimulation (45), indicating that an additional mechanism supports prolonged CD69 expression on CD8+ T cells in some mucosal tissues. Sustained CD69 expression is also a characteristic of many virus-specific CD8 T cells that colonize the lungs after respiratory virus infections (16, 25, 31) but is not maintained on bystander memory CD8 T cells which are recruited into the airways during heterologous infections (7, 47). These data indicate that the lung environment does not provide nonspecific stimuli which can induce CD69 expression on T cells without antigen or a strong inflammatory response.
Our previous studies showed that processed peptide antigens were present in the draining lymph nodes (DLN) until at least 2 months after primary influenza virus infection, when changes in local CD8 T cell migration were detected during parabiosis experiments (47). Antigen persistence has been detected by several independent groups using different strains of influenza virus, indicating that it is a common characteristic of this pathogen (20, 39, 47); however, some isolates of recombinant influenza virus do not support prolonged antigen presentation leading to complete clearance of antigenic peptides within ∼15 dpi (28). How the prolonged presence (or absence) of peptide antigens changes the phenotype of virus-specific CD8 T cells in the lungs has not been carefully examined. Here, MHC-I tetramer analysis is used to determine which activation antigens and adhesion molecules are expressed by virus-specific CD8 T cells in the lungs while influenza virus-derived peptide antigens are present in the mediastinal lymph nodes (MLN).
X31-OVA and WSN-OVAI are recombinant viruses which have been engineered to express the SIINFEKL peptide from the chicken ovalbumin gene in the stalk region of their neuraminidase coat proteins (18, 41). CD8 T cells from RAG−/− OTI TCR transgenic mice (17) were labeled with 5-and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE) and used to compare the kinetics of antigen clearance after sublethal infection with each virus (28, 47). The naïve CD45.1+ OTI cells (OTI-WT, where WT is wild type) were transferred to recipient mice 20 or 30 days after influenza virus infection, and the MLN were analyzed for dividing OTI cells 1 week later, using the CD45.1 congenic marker and CFSE dye (see Fig. S1 in the supplemental material). Most of the OTI cells that were recovered from the MLN of the WSN-OVAI-infected mice completed several rounds of cell division at each time point analyzed, confirming that processed peptide antigens were present at >30 dpi (47). Additional dividing OTI cells were also found in the cervical lymph nodes (CLN) (data not shown). Many OTI cells also initiated cell division in the mice that had been infected with X31-OVA when the transfers were performed at 10 dpi (Y.-T. Lee, unpublished); however, very few dividing cells were detected when the transfers were performed at later time points (20 and 30 dpi), indicating that most processed OVA peptides were cleared from these animals between 10 and 20 dpi, as reported previously (28). We have previously shown that naïve OTI cells do not initiate cell division after transfer to mice that have been infected with WSN-OVA0 at 30 dpi since the virus carries a point mutation in the OVA peptide that prevents CD8 T cell activation (19). Titration experiments also showed that 105 was the optimal number of naïve CD8 T cells to follow residual antigen presentation in the MLN at 30 dpi. The use of larger numbers of cells did not increase the size of the response, suggesting that low concentrations of antigen were the rate-limiting step.
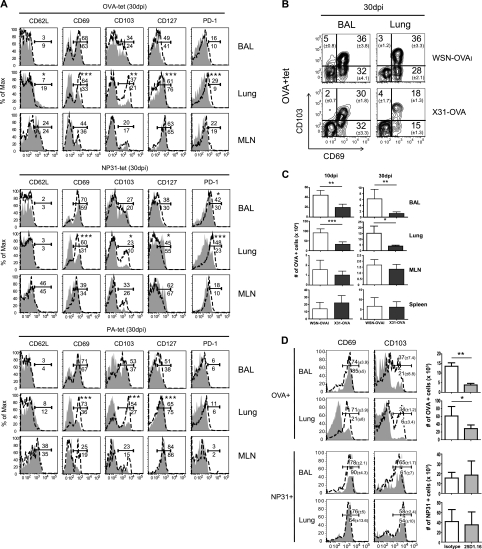
Once we had confirmed that the two viruses produced substantially different kinetics of antigen clearance (see Fig. S1 in the supplemental material), we used MHC-I tetramers to analyze the phenotype of the endogenous virus-specific CD8 T cells in the lungs (Fig. 2A; see also Fig. S2). Both viruses induced large effector CD8 T cell responses by 10 dpi, which included endogenous nucleoprotein (NP31), acid polymerase (PA), and OVA-specific CD8 T cells in the lungs. There were no significant differences in the level of CD8 T cell activation between the two groups of animals at this time (see Fig. S2). As the response progressed, the phenotypes of the virus-specific CD8 T cells in the lungs began to change, and the most dramatic differences were found for CD69 and CD103 expression on tetramer-positive CD8 T cells in the lung parenchyma, where large percentages of the tetramer-positive CD8 T cells maintained CD69 and CD103 expression >30 dpi with WSN-OVAI (Fig. 2A). In contrast, relatively small percentages of the tetramer-positive CD8 T cells that were in the lung parenchyma of the X31-OVA-infected animals expressed CD69+ and/or CD103+ (Fig. 2A and B). Although there was variation between the NP31-, PA-, and OVA-specific CD8 T cells, all three tetramers revealed consistently higher percentages of CD69+ and CD103+ cells in the lungs of the WSN-OVAI-infected mice than in the other animals. We also examined CD44, CD11a, and KLRG1 expression, but no significant differences between the two groups of animals were found (data not shown). The transfer experiments with naïve OTI cells indicated that most OVA peptides were cleared from the MLN of the X31-OVA-infected mice within 20 dpi (see Fig. S1 in the supplemental material). However, since small numbers of activated endogenous virus-specific CD8 T cells were detected in the lungs at 30 dpi (Fig. 2B), the possibility that trace quantities of antigen remained in other tissues below the level of detection by this assay cannot be excluded. Since previous studies have shown that virus-specific CD8 T cells in the lung airways are replenished during late antigen presentation (8, 47), we also compared the numbers of OVA-specific CD8 T cells in the tissues of the X31-OVA- and WSN-OVAI-infected mice at different times after infection (Fig. 2C). The WSN-OVAI and X31-OVA viruses both induced robust responses by virus-specific CD8 T cells by 10 dpi, as shown by the cumulative numbers of OVA-specific CD8 T cells in the different tissues. However, we found some difference in the homing properties of the responding OVA-specific CD8 T cells since higher numbers of tetramer-positive cells were detected in the spleens of some X31-OVA-infected animals, while the numbers in the lungs were substantially lower than in the WSN-OVAI-infected mice. This difference was more pronounced by 30 dpi. These data suggested that there may be a defect in CD8 T cell migration to this site after peptide antigens were cleared from the MLN.
Fig. 2.
Antigen is required for sustained CD69 and CD103 expression on CD8 T cells in the lungs. Recipient B6 mice were infected with WSN-OVAI or X31-OVA. (A) MHC-I tetramer analysis was used to phenotype the virus-specific CD8 T cells in the lungs at 30 dpi. Overlaid histograms (representative animals from groups of five) show gated populations of tetramer-positive CD8 T cells from X31-OVA-infected mice (gray shading) and WSN-OVAI-infected mice (dashed line). The percentages of cells that were found within the markers (averages from five animals) are shown for WSN-OVAI-infected (above the line) and X31-OVA-infected (below the line) animals. Statistical significance was determined using an unpaired Student's t test. Three independent experiments gave very similar results. (B) Large percentages of OVA-specific CD8 T cells in the lungs expressed both CD69 and CD103 30 days after WSN-OVAI infection. (C) Different numbers of OVA-specific CD8 T cells were recovered at 30 dpi from the lungs of the X31-OVA-infected mice and WSN-OVAI-infected animals. Means ± standard deviations from three individual animals are shown. (D) WSN-OVAI-infected mice were treated twice with 25D1.16 (or isotype control) antibodies at 20 and 23 dpi. MHC-I tetramer analysis was used to phenotype virus-specific CD8 T cells in the lungs 6 days later. Overlaid histograms (representative animals from groups of three) show tetramer-positive cells within the CD8 gate. The percentages of cells within the marked regions (averages from three animals) are shown for 25D1.16-treated mice (below the marker and gray shading) and control animals (above the marker and dashed lines). Two independent experiments gave very similar results. Bar charts are means ± standard deviations of OVA-specific CD8 T cells from three individual animals. Max, maximum; tet, tetracycline; BAL, bronchoalveolar lavage specimen. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To further verify whether the activated phenotype of the virus-specific CD8 T cells in the lungs was dependent on recent antigen stimulation, we used antibodies (Clone25D1.16) to prevent the TCR from binding to the SIINFEKL/Kb complex (32). The mice were first infected with WSN-OVAI and later treated with the 25D1.16 (or isotype control antibodies) on days 20 and 23 after infection. Six days after the first antibody treatment, MHC-I tetramer analysis was used to compare the phenotypes of the OVA- and NP-specific CD8 T cells in the lungs (Fig. 2D). The lung parenchyma of the 25D1.16-treated mice contained much smaller percentages of OVA-specific CD8 T cells that expressed CD69 and CD103 than the control animals. There was also a substantial reduction in the percentage of OVA-specific CD8 T cells that expressed CD103 in the lung airways. The phenotypes of the NP31-specific CD8 T cells were unchanged by the antibody treatments, showing that the blocking activity was specific. In addition, the 25D1.16 antibody treatments reduced the numbers of OVA-specific CD8 T cells that were recovered from the lungs (Fig. 2D), which further suggested that the pathogen-derived antigens enhanced CD8 T cell migration to the lungs during the late stage of the response.
Access to the lungs is supported by CD69 expression on virus-specific CD8 T cells.
The experiments shown in Fig. 2 indicated that late antigen stimulation facilitated recruitment of antigen-specific CD8 T cells to the lungs but did not reveal how the responding virus-specific CD8 T cells were able to reach the mucosal surface after most of the inflammation had resolved. Recently, activated effector T cells were reported to disseminate to peripheral tissues (including the mucosal surfaces of the gut) during the peak of the inflammatory response (26), but very little is known about their migratory properties after inflammation declines. Since CD69 expression is often upregulated on T cells in mucosal tissues and is very tightly regulated during antigen stimulation (3), we used an in vivo competition assay to determine whether CD69 supports sustained surveillance of the lungs by pathogen-specific CTL during the late stage of the response to influenza virus infection.
To generate supplies of virus-specific CD8 T cells that are deficient for CD69 expression, we crossed the OTI mice with CD69KO mice (30). Other CD69KO mice were crossed with F5 mice (29) which express a transgenic TCR that is specific for a peptide that is encoded in the nucleoprotein (NP) gene from the E61-13-H17 virus (42), which is another strain of influenza virus that supports prolonged antigen presentation (19, 47). Mixed populations of naïve F5-WT and F5-CD69KO cells were transferred to the naïve recipients 1 day before infection, and the congenic CD45.1/CD45.2 markers were used to compare the ratios of transferred F5 cells in different tissues as the infection progressed. It was essential to analyze both cell populations in the same recipients to ensure that they were exposed to identical concentrations of antigen and inflammatory molecules. This approach also eliminates the need to count cells in separate mice after purification and thus reduces experimental variability.
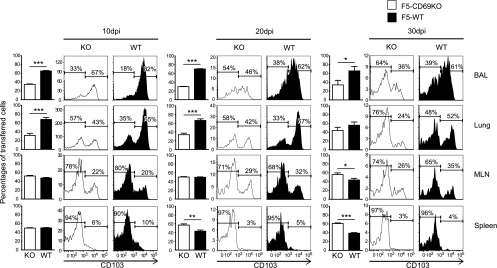
An earlier study suggested that CD69 expression may influence the kinetics of T cell activation in lymphoid tissues (38). To determine whether different rates of T cell activation were a variable in our experiments, we used CFSE analysis to follow the early response of the transferred F5 cells to influenza virus infection. The recipient mice received equal numbers of CFSE-labeled RAG−/− CD45.1+ CD45.2+ F5-WT and CD45.1+ F5-CD69KO cells 1 day before E61-13-H17 infection. On days 3, 5, 7, and 9 after infection, lymphocytes were recovered from different tissues, and the transferred F5 cells were analyzed for CFSE dilution (see Fig. S3 in the supplemental material). This study revealed no differences in the rates of proliferation by the two transferred T cell populations at any time point analyzed. Approximately equal ratios of F5-WT and F5-CD69KO cells were also recovered from most tissues at 10 dpi (including the inguinal and cervical lymph nodes, liver, and blood) (data not shown), which was the peak of the CD8 effector T cell response (Fig. 3). The only exception to this pattern was found in the lungs, where the F5-WT cells outnumbered the F5-CD69KO cells by a ratio of more than 2:1. The congenic markers were exchanged for some experiments to ensure that the transferred T cells were not being rejected. Similar discrepancies in the ratios of transferred F5 cells were also found in the lungs at 20 and 30 dpi, where lower percentages of F5-CD69KO cells than F5-WT cells expressed CD103. These data showed that recently activated F5 cells which lacked CD69 expression failed to reach the lung mucosa very efficiently. More F5-CD69KO than F5-WT cells were found in the spleens at 20 and 30 dpi (Fig. 3), which suggested that some activated CD8 T cells may have been redirected to this site. Similar results were obtained when OTI-WT and OTI-CD69KO cells were transferred to B6 mice before WSN-OVAI infection (41) (see Fig. S4 in the supplemental material).
Fig. 3.
CD69 is required for efficient CD8 T cell migration to the lungs. Equal numbers (106) of F5-WT and F5-CD69KO cells were transferred to B6 mice 1 day before E61-13-H17 infection. On the days shown the recipient mice were analyzed for ratios of transferred F5 cells using the congenic CD45.1 and CD45.2 markers. The bar graphs show percentages (with standard deviations) of mutant and WT F5 cells within the CD45.1 gate from three animals. The histograms show gated populations of transferred F5 cells from representative animals, with percentages of cells in marked regions (averaged from groups of three). Three independent experiments gave very similar results. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
CD103 aids CD8 T cell retention in the lungs.
CD103 has been implicated in having a role in CD8 T cell migration in mucosal tissues (37), but no specific analysis of pathogen-specific CTL in the lungs has been reported. Since antigen was required to maintain CD103 expression on the virus-specific CD8 T cells that colonized the lungs after influenza virus infection (Fig. 2), we investigated whether this molecule was required to help residual populations of pathogen-specific CTL remain in the lungs after viral clearance. First the F5 and OTI mice were crossed with CD103-deficient animals (30, 48), and virus-specific CD8 T cells from the progeny animals were used for in vivo competition studies with wild-type T cells, as described above (Fig. 4).
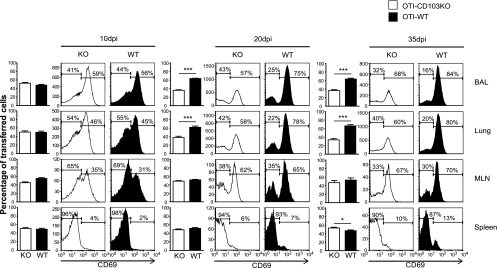
Fig. 4.
CD103 promotes CD8 T cell retention in the lungs. Equal numbers (106) of RAG−/− OTI-WT and OTI-CD103KO cells were transferred to B6 mice 24 h before they were infected with WSN-OVAI. On the days shown, the recipient mice were analyzed for ratios of transferred OTI cells using the congenic CD45.1 and CD45.2 markers. The bar graphs show percentages (with standard deviations) of mutant and WT OTI cells within the CD45.1 gate from three individual animals. The histograms show gated populations of transferred F5 cells from representative animals, with percentages of cells in marked regions (averaged from groups of three individual animals). Three independent experiments gave very similar results. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Approximately equal ratios of OTI-WT and OTI-CD103KO cells were recovered from all of the tissues at 10 dpi (including the inguinal and cervical lymph nodes) (data not shown), indicating that CD103 was not required for effector CD8 T cells to access the lungs. The ratios of transferred OTI cells remained constant in most tissues throughout the contraction of the effector response, showing that CD103 expression did not substantially influence CD8 T cell activation or survival. By 20 dpi there was a substantial change in the ratios of F5 cells in the lungs, where the wild-type OTI cells outnumbered the OTI-CD103KO cells by a ratio ∼2:1. Different percentages of CD69+ cells within the two transferred cell populations pointed to a selective loss of activated CD8 T cells from the lungs when CD103 was not expressed. All of the other tissues maintained relatively equal ratios of transferred cells at all time points analyzed. Similar results were obtained when F5-WT and F5-CD103KO cells were transferred to mice that had been previously infected with E61-13-H17 (see Fig. S5 in the supplemental material). These data indicated that CD103 expression helped the newly activated pathogen-specific CD8 T cells remain in the lungs after viral clearance.
Recently activated CD8 T cells show enhanced sensitivity to environmental factors which promote T cell retention in the lungs.
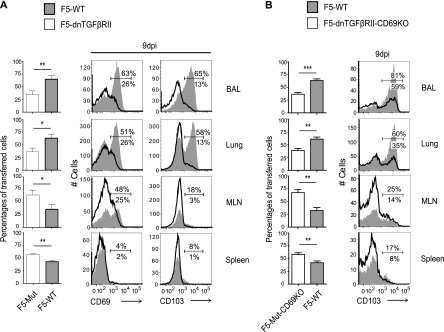
Our data show that CD69 and CD103 expression on influenza virus-specific CTL in the lungs was reinforced during continuing antigen stimulation (Fig. 2); however, previous studies have shown that CD103 expression by antigen-specific CD8 T cells in other tissues is dependent on local activation of transforming growth factor β (TGF-β) (6). To determine whether TGF-β-derived signals are required for influenza virus-specific CD8 T cells to maintain CD103 expression in the lungs, we crossed F5 and F5-CD69KO mice with other animals that express a dominant negative form of the TGF-β receptor II (dnTGFβRII) under the control of a T cell specific promoter (14). Equal numbers of CD45.1+ F5-WT and CD45.1/CD45.2+ F5-dnTGFβRII cells were transferred to naïve recipient mice 1 day before E61-13-H17 infection (Fig. 5A). A second group of animals received equal numbers of CD45.1/CD45.2+ F5-WT and CD45.1+ F5-dnTGFβRII-CD69KO cells (Fig. 5B). The tissues from the two groups of recipient mice were analyzed for ratios of transferred F5 cells at 9 dpi using the congenic CD45.1 and CD45.2 markers.
Fig. 5.
TGF-β supports a lung-homing phenotype for virus-specific CD8 T cells. Equal numbers of F5-WT and F5-dnTGFβRII cells (A) or F5-WT and F5-dnTGFβRII-CD69KO cells (B) were transferred to B6 mice 1 day before infection with E61-13-H17. Recipient mice were analyzed for ratios of transferred F5 cells at 9 dpi using the congenic CD45.1 and CD45.2 markers. Overlaid histograms show gated populations of transferred F5 cells. Means ± standard deviations from three individual animals are shown. Duplicate experiments gave similar results. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
A large percentage of F5-WT cells that reached the lungs of the recipient mice expressed CD103 as expected; however, much smaller percentages of F5-dnTGFβRII (Fig. 5A) and F5-dnTGFβRII-CD69KO cells (Fig. 5B) expressed CD103. Although the mutation was slightly leaky, these data confirmed that TGF-β-derived signals were required for CD103 expression on pathogen-specific CD8 T cells in the lungs. The cell ratios revealed higher numbers of F5-dnTGFβRII (or F5-dnTGFβRII-CD69KO) than F5-WT cells in most tissues at 9 dpi (Fig. 5B, bar graphs), which was consistent with resistance to apoptosis in the absence of TGF-β-induced signals (35). Although the mutant F5-dnTGFβRII and F5-dnTGFβRII-CD69KO cells were more numerous than the F5-WT cells in most tissues, they were relatively inefficient at reaching the lungs. Phenotypic data indicated a preferential loss of CD69+ F5-dnTGFβRII cells from the lungs when TGF-β signaling was blocked (Fig. 5A). These data indicated that the recently activated CD8 T cells were highly sensitive to TGF-β-derived signals and responded by upregulating CD103, which, in turn, prevented a selective loss of CD69+ CD8 T cells from the lungs. Additional time points could not be analyzed due to early rejection, as reported previously (34). Together, CD69 and CD103 provide a pathogen-specific mechanism of sustained CTL surveillance in the lungs which is not dependent on a vigorous inflammatory response.
DISCUSSION
We have previously shown that virally encoded peptide antigens persist in the MLN for at least 2 months after influenza virus infection even though most symptoms of inflammation in the lungs subside much earlier (Fig. 1) (47). These peptide antigens were presented to virus-specific CD8 T cells by CD11b+ dendritic cells (DC) (19, 20) and helped replenish residual populations of virus-specific CTL which reside near the site of infection (19, 47). More recently, other investigators found trace quantities of viral genome in the lungs at 30 dpi using PCR analysis (20), which suggests that a renewable source of antigen may have been present in the lungs during the late stage of the response. Although we have not been able to verify the presence of viral genome in the lungs at >20 dpi (47), our data show that many endogenous virus-specific CD8 T cells maintained an activated phenotype in the lung parenchyma throughout the time that the peptide antigens were present in the DLN (Fig. 2). A large percentage of the OVA-specific CD8 T cells that were recovered from the lungs during the late stage of the response to WSN-OVAI infection expressed CD69 and declined in numbers when antigen presentation was blocked with antibodies that were specific for the OVA-MHC complex (Fig. 2D). The mechanisms that support CD69 expression on T cells which reside at mucosal surfaces are controversial since CD8+ intraepithelial lymphocytes (IEL) in the gut universally express CD69 without any apparent requirement for antigen stimulation (46). CD69+ CD8 T cells also become greatly enriched in the lung airways after respiratory virus infections (16, 25, 31), but our parabiosis studies showed that the lung environment did not provide sufficient antigen-independent signals to induce de novo CD69 expression on memory CD8 T cells that entered the airways after most inflammation had declined (47) (see Fig. S6 in the supplemental material). Since CD69 expression was maintained on the residual populations of OVA-specific CD8 T cells that remained in the airways after treatment with 25D1.16 antibodies (Fig. 2D), it is possible that the mucosal tissues provided signals which prevented recently activated CD8 T cells from downregulating CD69 expression after they arrived in the airways (21). If such signals were present in the influenza virus-infected lungs, they were not sufficient to prevent the loss of large numbers of virus-specific CD8 T cells from the lungs by 30 dpi (47) (Fig. 2C). A large percentage of virus-specific CD8 T cells in the MLN also expressed CD69 during late antigen presentation, which likely replenished the activated virus-specific CD8 T cells in the lungs during the chronic stage of the response (8, 47) since transit into the lungs between 20 and 30 dpi was greatly facilitated by CD69 expression (Fig. 3). It is not known which route newly activated T cells use to reach the airways since the blood supply enters lungs via two separate circulatory systems. Newly activated T cells leave the MLN in the efferent lymph, which joins the venous blood supply at the thoracic duct. The venous blood then circulates through the parenchymal tissue of the lungs before returning to the heart, where oxygenated blood joins the systemic circulation and nourishes the bronchial walls.
E-cadherin is the only known ligand for CD103 and is expressed on epithelial cells which line the lung airways and alveoli. This restricted pattern of E-cadherin expression in the lungs is consistent with a role in the retention of pathogen-specific CTL in the mucosa rather than enhanced migration into the lungs. Our data suggest that rapid acquisition of CD103 expression helps newly arriving CD8 T cells avoid early elimination from the airways, which might otherwise be accelerated by the action of the mucociliary escalator. Our data also show that dual signals which are transmitted via the TCR and TGF-β receptor are required to maintain CD103 expression on the influenza virus-specific CTL in the lungs, as in other tissues (6). Although many virus-specific CD8 T cells expressed CD103 in the lungs of the influenza virus-infected mice, most bystander memory CD8 T cells maintained a resting phenotype when they circulated through the lungs during parabiosis (see Fig. S6 in the supplemental material). These data indicate that the resting memory T cells were relatively resistant to TGF-β stimulation and may explain why the virus-specific CTL were retained in the lungs more efficiently than bystander CD8 T cells in our earlier studies (47). It is unclear whether the CD69+ virus-specific CD8 T cells in the lungs of the donor animals failed to enter the circulation during parabiosis, downregulated CD69 during transit to the partner animals, or did not survive.
The possibility that related mechanisms regulate CD103 and CD69 expression on CD8 T cells has not been directly explored, but earlier studies indicate that CD69 plays a nonredundant role in immune downregulation by a mechanism that requires TGF-β activation (33). This conclusion was supported by the observation that CD69 cross-linking leads to extracellular signal-regulated kinase (ERK) activation (49), which is required for TGF-β synthesis (15, 40). To become biologically active, a precursor form of TGF-β must be cleaved to release the positively charged low-molecular-weight peptide that is required for signal transduction. The physical properties of the charged peptide substantially limit its biological half-life in vivo and contain the sphere of influence of the cytokine to the immediate anatomical environment. We have shown that reduced numbers of F5 cells express CD103 in the lung airways when CD69 is not expressed. Others have shown that cross-linking CD69 with antibodies led to TGF-β activation in human T cells (22). Although these studies have not been reproduced in mice, it is possible that an autocrine pathway of TGF-β activation supports CD103 expression on CD8 T cells in the lungs, which relies on CD69-derived signals. Alternatively, TGF-β-sensitive CD8 T cells maybe excluded from the lungs in the absence of CD69 expression. Our data indicate that high concentrations of TGF-β, which are produced in the lungs after influenza virus infection (43), not only help contain the inflammatory response after viral clearance but also support immunity by helping populate the lungs with pathogen-specific CTL when other inflammatory cells are no longer present.
At this time we do not know why individual stocks of influenza virus vary in their abilities to support prolonged antigen presentation, but preliminary studies indicate that the X31-OVA virus induces a more vigorous cytokine response than WSN-OVAI within 2 dpi and may induce innate immune activation with faster kinetics. It is therefore possible that rapid innate immune activation interferes with antigen persistence; however, considerably more work will be required to completely resolve this issue.
In summary, our data show that CD69 and CD103 both promote surveillance of the lungs by pathogen-specific CTL during the recovery from respiratory virus infection. The role of CD69 is most evident during T cell transit to the lungs while the role of CD103 is consistent with subsequent retention in the airways, although there appears to be some redundancy in the action of the two molecules. Our data indicate that recent antigen stimulation directly promotes CD69 expression on the virus-specific CD8 T cells and increases their sensitivity to TGF-β, which is required for CD103 expression. Antigen-dependent mechanisms of T cell surveillance may be used to protect other mucosal tissues after vaccination or local infections.
Supplementary Material
[Supplemental material]
ACKNOWLEDGMENTS
Genetically modified mice and viruses were generously supplied by the following investigators: WSN-OVAI virus, David Topham (University of Rochester); E61-13-H17, Jonathon Yewdell (NIH); X31-OVA, Paul Thomas (University of Memphis); CD69KO mice, Steven Zeigler (Benaroya Research Institute Seattle); and dnTGFβRII mice, Richard Flavell (Yale University). The OVA and influenza virus tetramers were supplied by Leo Lefrançois and the NIAID tetramer facility. Histology was done by the University of Connecticut Histology Core facility. G.A.H. supplied CD103KO mice.
This work was supported by National Institutes of Health grants AI056172 and AI071213 (L.S.C.) and RO1 AI-36532 (G.A.H.).
Footnotes
▿
Published ahead of print on 23 February 2011.
REFERENCES
- 1.Belyakov I. M., Ahlers J. D. 2009. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J. Immunol. 183:6883–6892 [DOI] [PubMed] [Google Scholar]
- 2.Belz G. T., Xie W., Altman J. D., Doherty P. C. 2000. A previously unrecognized H-2Db-restricted peptide prominent in the primary influenza A virus-specific CD8+ T-cell response is much less apparent following secondary challenge. J. Virol. 74:3486–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craston R., et al. 1997. Temporal dynamics of CD69 expression on lymphoid cells. J. Immunol. Methods 209:37–45 [DOI] [PubMed] [Google Scholar]
- 4.Daly K., Nguyen P., Woodland D. L., Blackman M. A. 1995. Immunodominance of major histocompatibility complex class I-restricted influenza virus epitopes can be influenced by the T-cell receptor repertoire. J. Virol. 69:7416–7422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deblandre G. A., Leo O., Huez G. A., Wathelet M. G. 1992. CD69 is expressed on Daudi cells in response to interferon-alpha. Cytokine 4:36–43 [DOI] [PubMed] [Google Scholar]
- 6.El-Asady R., et al. 2005. TGF-β-dependent CD103 expression by CD8+ T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J. Exp. Med. 201:1647–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ely K. H., et al. 2003. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J. Immunol. 170:1423–1429 [DOI] [PubMed] [Google Scholar]
- 8.Ely K. H., Cookenham T., Roberts A. D., Woodland D. L. 2006. Memory T cell populations in the lung airways are maintained by continual recruitment. J. Immunol. 176:537–543 [DOI] [PubMed] [Google Scholar]
- 9.Estcourt M. J., Letourneau S., McMichael A. J., Hanke T. 2005. Vaccine route, dose and type of delivery vector determine patterns of primary CD8+ T cell responses. Eur. J. Immunol. 35:2532–2540 [DOI] [PubMed] [Google Scholar]
- 10.Filippi C. M., et al. 2008. Transforming growth factor-beta suppresses the activation of CD8+ T-cells when naive but promotes their survival and function once antigen experienced: a two-faced impact on autoimmunity. Diabetes 57:2684–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn K. J., et al. 1998. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 8:683–691 [DOI] [PubMed] [Google Scholar]
- 12.Flynn K. J., Riberdy J. M., Christensen J. P., Altman J. D., Doherty P. C. 1999. In vivo proliferation of naive and memory influenza-specific CD8+ T cells. Proc. Natl. Acad. Sci. U. S. A. 96:8597–8602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerhard W., Mozdzanowska K., Furchner M., Washko G., Maiese K. 1997. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol. Rev. 159:95–103 [DOI] [PubMed] [Google Scholar]
- 14.Gorelik L., Flavell R. A. 2000. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12:171–181 [DOI] [PubMed] [Google Scholar]
- 15.Grewal J. S., Mukhin Y. V., Garnovskaya M. N., Raymond J. R., Greene E. L. 1999. Serotonin 5-HT2A receptor induces TGF-β1 expression in mesangial cells via ERK: proliferative and fibrotic signals. Am. J. Physiol. 276:F922–F930 [DOI] [PubMed] [Google Scholar]
- 16.Hogan R. J., et al. 2001. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J. Immunol. 166:1813–1822 [DOI] [PubMed] [Google Scholar]
- 17.Hogquist K. A., et al. 1994. T cell receptor antagonist peptides induce positive selection. Cell 76:17–27 [DOI] [PubMed] [Google Scholar]
- 18.Jenkins M. R., Webby R., Doherty P. C., Turner S. J. 2006. Addition of a prominent epitope affects influenza A virus-specific CD8+ T cell immunodominance hierarchies when antigen is limiting. J. Immunol. 177:2917–2925 [DOI] [PubMed] [Google Scholar]
- 19.Khanna K. M., et al. 2008. In situ imaging reveals different responses by naive and memory CD8 T cells to late antigen presentation by lymph node DC after influenza virus infection. Eur. J. Immunol. 38:3304–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim T. S., Hufford M. M., Sun J., Fu Y. X., Braciale T. J. 2010. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J. Exp. Med. 207:1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohlmeier J. E., Miller S. C., Woodland D. L. 2007. Cutting edge: antigen is not required for the activation and maintenance of virus-specific memory CD8+ T cells in the lung airways. J. Immunol. 178:4721–4725 [DOI] [PubMed] [Google Scholar]
- 22.Lauzurica P., et al. 2000. Phenotypic and functional characteristics of hematopoietic cell lineages in CD69-deficient mice. Blood 95:2312–2320 [PubMed] [Google Scholar]
- 23.Liang S., Mozdzanowska K., Palladino G., Gerhard W. 1994. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J. Immunol. 152:1653–1661 [PubMed] [Google Scholar]
- 24.Lyons A. B., Parish C. R. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171:131–137 [DOI] [PubMed] [Google Scholar]
- 25.Marshall D. R., et al. 2001. Measuring the diaspora for virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. U. S. A. 98:6313–6318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masopust D., et al. 2004. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol. 172:4875–4882 [DOI] [PubMed] [Google Scholar]
- 27.Miki-Hosokawa T., et al. 2009. CD69 controls the pathogenesis of allergic airway inflammation. J. Immunol. 183:8203–8215 [DOI] [PubMed] [Google Scholar]
- 28.Mintern J. D., et al. 2009. Transience of MHC class I-restricted antigen presentation after influenza A virus infection. Proc. Natl. Acad. Sci. U. S. A. 106:6724–6729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moskophidis D., Kioussis D. 1998. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J. Exp. Med. 188:223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murata K., et al. 2003. CD69-null mice protected from arthritis induced with anti-type II collagen antibodies. Int. Immunol. 15:987–992 [DOI] [PubMed] [Google Scholar]
- 31.Ostler T., Hussell T., Surh C. D., Openshaw P., Ehl S. 2001. Long-term persistence and reactivation of T cell memory in the lung of mice infected with respiratory syncytial virus. Eur. J. Immunol. 31:2574–2582 [DOI] [PubMed] [Google Scholar]
- 32.Porgador A., Yewdell J. W., Deng Y., Bennink J. R., Germain R. N. 1997. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity 6:715–726 [DOI] [PubMed] [Google Scholar]
- 33.Sancho D., Gomez M., Sanchez-Madrid F. 2005. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 26:136–140 [DOI] [PubMed] [Google Scholar]
- 34.Sanjabi S., Flavell R. A. 2010. Overcoming the hurdles in using mouse genetic models that block TGF-beta signaling. J. Immunol. Methods 353:111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanjabi S., Mosaheb M. M., Flavell R. A. 2009. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity 31:131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scherle P. A., Palladino G., Gerhard W. 1992. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J. Immunol. 148:212–217 [PubMed] [Google Scholar]
- 37.Schon M. P., et al. 1999. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J. Immunol. 162:6641–6649 [PubMed] [Google Scholar]
- 38.Shiow L. R., et al. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440:540–544 [DOI] [PubMed] [Google Scholar]
- 39.Takamura S., et al. 2010. The route of priming influences the ability of respiratory virus-specific memory CD8+ T cells to be activated by residual antigen. J. Exp. Med. 207:1153–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Testi R., D'Ambrosio D., De Maria R., Santoni A. 1994. The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol. Today 15:479–483 [DOI] [PubMed] [Google Scholar]
- 41.Topham D. J., Castrucci M. R., Wingo F. S., Belz G. T., Doherty P. C. 2001. The role of antigen in the localization of naive, acutely activated, and memory CD8+ T cells to the lung during influenza pneumonia. J. Immunol. 167:6983–6990 [DOI] [PubMed] [Google Scholar]
- 42.Townsend A. R., Skehel J. J. 1984. The influenza A virus nucleoprotein gene controls the induction of both subtype specific and cross-reactive cytotoxic T cells. J. Exp. Med. 160:552–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uhl E. W., et al. 1996. Parainfluenza virus-induced persistence of airway inflammation, fibrosis, and dysfunction associated with TGF-beta 1 expression in brown Norway rats. Am. J. Respir. Crit. Care Med. 154:1834–1842 [DOI] [PubMed] [Google Scholar]
- 44.Vallbracht S., Unsold H., Ehl S. 2006. Functional impairment of cytotoxic T cells in the lung airways following respiratory virus infections. Eur. J. Immunol. 36:1434–1442 [DOI] [PubMed] [Google Scholar]
- 45.Wang H. C., Klein J. R. 2001. Multiple levels of activation of murine CD8+ intraepithelial lymphocytes defined by OX40 (CD134) expression: effects on cell-mediated cytotoxicity, IFN-γ, and IL-10 regulation. J. Immunol. 167:6717–6723 [DOI] [PubMed] [Google Scholar]
- 46.Wang H. C., Zhou Q., Dragoo J., Klein J. R. 2002. Most murine CD8+ intestinal intraepithelial lymphocytes are partially but not fully activated T cells. J. Immunol. 169:4717–4722 [DOI] [PubMed] [Google Scholar]
- 47.Zammit D. J., Turner D. L., Klonowski K. D., Lefrancois L., Cauley L. S. 2006. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity 24:439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziegler S. F., et al. 1994. The mouse CD69 gene. Structure, expression, and mapping to the NK gene complex. J. Immunol. 152:1228–1236 [PubMed] [Google Scholar]
- 49.Zingoni A., et al. 2000. CD69-triggered ERK activation and functions are negatively regulated by CD94/NKG2-A inhibitory receptor. Eur. J. Immunol. 30:644–651 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]