Smoking and Incidence of Atrial Fibrillation: Results from the Atherosclerosis Risk in Communities (ARIC) Study (original) (raw)
. Author manuscript; available in PMC: 2012 Aug 1.
Published in final edited form as: Heart Rhythm. 2011 Mar 15;8(8):1160–1166. doi: 10.1016/j.hrthm.2011.03.038
Abstract
Background
Cigarette smoking increases the risk of coronary heart disease, but whether smoking increases atrial fibrillation (AF) is uncertain.
Objectives
To determine the association of cigarette smoking with incident AF in a population-based cohort of blacks and whites.
Methods
We determined the risk of incident AF through December 2002 in relation to baseline (1987–1989) smoking status and cigarette-years of smoking in over 15,000 participants of the prospective Atherosclerosis Risk in Communities study.
Results
Over a mean follow-up of 13.1 years, 876 incident AF events were identified. Compared to never smokers, the multivariable-adjusted hazard ratios (HR) for AF were 1.32 (95% CI, 1.10–1.57) in former smokers, 2.05 (95% CI, 1.71–2.47) in current smokers, and 1.58 (95% CI, 1.35–1.85) in ever smokers. In the highest tertile of accumulated smoking amount (>675 cigarette-years), the incidence of AF was 2.10-times greater (95% CI, 1.74–2.53) than those who never smoked. Associations were similar by gender, race, and type of event (AF and atrial flutter), and also when only AF events identified by study exam ECGs were included. Finally, individuals who quit smoking exhibited a trend indicating a slightly lower risk of developing AF (HR, 0.88; 95% CI, 0.65–1.17) compared to those who continued to smoke.
Conclusions
Smoking was associated with the incidence of AF, with more than a 2-fold increased risk of AF attributed to current smoking. In addition, a trend toward a lower incidence of AF appeared among smokers who quit compared to continued smokers.
Keywords: smoking, cigarette, arrhythmia, atrial fibrillation
INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia in clinical practice and currently affects more than 2.2 million Americans.1 Risk factors for AF include increasing age, male gender, white race, obesity, hypertension, and diabetes;2 more recently, the metabolic syndrome has also been implicated in the development AF.3 However, other important cardiovascular risk factors, such as elevated cholesterol and cigarette smoking are less clearly related to AF. Specifically, cigarette smoking has been shown to predict one’s individual risk for AF in a risk score developed using the Atherosclerosis Risk in Communities (ARIC) study;4 however, results from other prospective studies investigating the association between smoking and AF have provided inconsistent results.
In the Framingham Heart Study, cigarette smoking conferred a 40% increased odds of developing AF among women, but there was no association among men.5 Furthermore, current smoking was not a significant predictor for AF in the Framingham risk score for AF.6 Compared with never smokers, the Rotterdam study reported a 51% and 49% increased risk of incident AF among current and former smokers, respectively, which did not differ by gender.7 A 37% increased risk of AF among ever smokers was reported in the Manitoba Follow-Up Study.8 Yet, no association was found between smoking and AF in the Danish Diet, Cancer, and Health Study9 or the Multifactor Primary Prevention Study.10
Though cigarette smoking increases oxidative stress,11 inflammation,11 and atrial fibrosis,12 all mechanisms potentially involved in the etiology of AF,13 these limited and inconsistent data suggest further investigation of the association between smoking and AF is warranted. Thus, we assessed in detail the risk of incident AF in relation to smoking status and amount in the ARIC study.
METHODS
Study Population
The ARIC study is a prospective investigation aimed to identify risk factors for atherosclerosis and cardiovascular disease. ARIC recruited adults aged 45–64 years from four US communities: Forsyth County, NC; Jackson, MS; Minneapolis suburbs, MN; and Washington County, MD.14 Blacks and whites were recruited from Forsyth County, only blacks from Jackson, and predominantly whites from the other two communities. Between 1987 and 1989, 15,792 participants (8710 women) were enrolled, completing a home interview and clinic visit. Three triennial follow-up clinic visits were conducted, and annual telephone interviews and active surveillance of the ARIC community hospitals are conducted to follow-up participants. The ARIC study was approved by institutional review boards at each participating center, and informed consent was obtained from all participants.
Atrial Fibrillation Ascertainment
Electrocardiograms (ECGs) during the baseline visit were used to exclude individuals with prevalent AF or atrial flutter. Incident AF diagnoses were identified from ECGs performed during study follow-up visits through 1998, and hospital discharge records and death certificates through 2005.
ARIC examination ECGs were recorded using MAC PC Personal Cardiographs (Marquette Electronics, Inc., Milwaukee, WI). At each clinic visit, a standard supine 12-lead resting ECG was recorded, at least one hour after smoking tobacco or ingestion of caffeine. ECGs were transmitted by modem to the ARIC ECG Reading Center for computer coding, and those computer-coded as AF were visually re-checked by a cardiologist to confirm the diagnosis.15
Annual follow-up telephone calls to cohort participants and survey of local hospitals identified hospitalizations or deaths. Hospital discharge records were gathered from all hospitalizations, and AF was identified by an ICD-9 discharge code of 427.31 or 427.32 among any of the discharge diagnoses. AF was also identified when any listed cause of death on a death certificate was coded as AF (ICD-9 code 427.3 or ICD-10 code I48). AF occurring simultaneously with heart revascularization surgery (ICD-9 code 36.X) or other cardiac surgery involving heart valves or septa (ICD-9 code 35.X) was not considered an incident event. Within the ARIC cohort, the sensitivity and specificity of hospital discharge diagnoses for AF was found to be 84% and 98%, respectively.16
Smoking Assessment
Cigarette smoking status and amount were self-reported. Participants were asked whether they ever smoked cigarettes, and if so, the age of smoking initiation, number of years of smoking, number of cigarettes smoked per day, and whether they currently smoked, and if not, the age of cessation. From responses to these questions, participants were categorized as current, former, or never smokers, and cigarette-years of smoking were calculated for ever smokers.
We categorized smoking status using three classifications: 1) current, former, never (reference), 2) ever, never (reference), and 3) current, non-current (reference). Cigarette-years of smoking were categorized into tertiles for ever smokers, and never smokers served as the reference group. In addition, current and former smokers were dichotomized at 800 cigarette-years of smoking (equivalent to 40 pack-years), with never smokers as the reference.
Additional Baseline Measurements
Race, education level, and alcohol drinking status were determined by self-report. The sports index for physical activity during leisure time ranged from 1 (low) to 5 (high), and was based on the questionnaire developed by Baecke et al.17 Body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared. A participant was categorized as diabetic if he/she had a fasting glucose ≥126 mg/dL (or non-fasting glucose of ≥200 mg/dL), reported a physician diagnosis of diabetes, or was currently taking medication for diabetes. The average of the last 2 of 3 blood pressure measurements of ≥140 mmHg systolic and/or ≥90 mmHg diastolic, or blood pressure medication use in the past 2 weeks defined hypertension. Prevalent coronary heart disease (CHD) included a history of myocardial infarction (MI), MI indicated on the baseline ECG, or history of coronary bypass or angioplasty. Prevalent heart failure (HF) was identified by the Gothenburg criteria18 or self-report of HF medication use in the past 2 weeks.
Statistical Analysis
Analyses were conducted using SAS version 8.2 (SAS Institute, Cary, NC). We excluded individuals who were not of black or white race (N=48), blacks from Minneapolis and Washington County (N=55), prevalent AF or missing AF status at baseline (N=261), and those with unreadable baseline ECGs (N=85). We additionally excluded those with missing or unknown smoking status (N=14) and/or missing cigarette-years of smoking (N=265) at baseline in models where appropriate.
Baseline participant characteristics by smoking status were compared using the chi-square statistic and analysis of variance (ANOVA). Kaplan-Meier methods were used to estimate the cumulative incidence of AF. Age- and sex-adjusted incidence rates for AF were calculated using Poisson regression. Hazard ratios for AF were estimated using Cox proportional hazards regression after adjusting for baseline age, sex, race, ARIC field center, education (less than high school, high school graduate to vocational school, any college), BMI, alcohol drinking status (current, former, never), sports index (≥3.5, <3.5), diabetes, and hypertension.
In a number of additional analyses, we tested the sensitivity of our results to the definition of AF event. First, we conducted separate analyses for AF events identified from hospital discharge codes and from ECGs in the study exams. Second, we explored whether association of smoking was different for events coded as atrial flutter (ICD9 427.32) or AF only (ICD9 427.31). Finally, we excluded AF events associated with non-cardiac chest or abdominal surgery.
We also created an additional five cohorts using exams 2–4 and follow-up telephone contacts 3 and 6 years after exam 4 as baseline. For each cohort, we excluded individuals with prevalent AF and began follow-up at the date of the exam/telephone contact. We adjusted for the same potential confounders as measured during the follow-up exams, using exam 4 confounders for the cohorts 3 and 6 years after exam 4. For a particular cohort, we categorized individuals as quitting smoking if they were current smokers at the previous exam and former at the current exam; current smokers at both exams served as the reference. These five cohorts were pooled into one Cox model using a robust variance estimator to take into account within-individual correlations since study participants could be included more than once.19
To test the proportional hazards assumption for the Cox regression models, interaction terms between smoking status/amount and log of follow-up time were tested and the log-log survival curves were plotted. The proportional hazards assumption was met for the first 16 years of follow-up, but not thereafter. Therefore, we did administrative censoring at December 31, 2002 for all analyses.
RESULTS
After exclusions, 15,329 ARIC participants were available for the smoking status analysis, and 15,078 were available for the cigarette-years and the combined smoking status and amount analyses. Current and former smokers were less well educated, more likely to be current drinkers, were less physically active, and had more prevalent CHD and HF than never smokers (Table 1).
Table 1.
Baseline Participant Characteristics by Smoking Status, ARIC 1987–89
| Current (N=4005) | Former (N=4950) | Never (N=6374) | |
|---|---|---|---|
| Age, years | 53.6 (5.7) | 54.8 (5.8) | 54.0 (5.8) |
| Male | 1900 (47.4) | 3054 (61.7) | 1915 (30.0) |
| Black | 1214 (30.3) | 962 (19.4) | 1880 (29.5) |
| Education | |||
| < High school | 1243 (31.1) | 1075 (21.7) | 1302 (20.4) |
| HS to vocational school | 1649 (41.2) | 1948 (39.4) | 2661 (41.8) |
| Any college | 1107 (27.7) | 1924 (38.9) | 2403 (37.8) |
| Drinking status | |||
| Current | 2535 (63.6) | 3141 (63.7) | 2883 (45.4) |
| Former | 879 (22.0) | 1170 (23.7) | 835 (13.2) |
| Never | 574 (14.4) | 623 (12.6) | 2629 (41.4) |
| BMI, kg/m2 | 26.4 (5.0) | 28.0 (5.1) | 28.3 (5.7) |
| Sports index | |||
| <3.5 | 3581 (89.7) | 4067 (82.4) | 5582 (87.9) |
| ≥3.5 | 410 (10.3) | 870 (17.6) | 768 (12.1) |
| Diabetes | 421 (10.6) | 588 (11.9) | 803 (12.7) |
| Hypertension | 1291 (32.2) | 1713 (34.8) | 2316 (36.5) |
| Coronary heart disease | 208 (5.3) | 367 (7.5) | 160 (2.6) |
| Heart failure | 217 (5.5) | 235 (4.8) | 257 (4.1) |
| Cigarette-years of smoking | 670.8 (430.9) | 463.6 (434.1) | 0 (0) |
Over a mean follow-up of 13.1 years, 876 incident AF events were identified. Of these, 749 were ascertained by hospital discharge diagnoses alone, 17 from a study ECG alone, 105 from both a study ECG and a hospital discharge diagnosis, 1 from a death certificate alone, 3 from hospital discharge diagnoses and a death certificate, and 1 from all 3 sources. AF was the primary diagnosis in 351 of 858 events identified from hospitalizations.
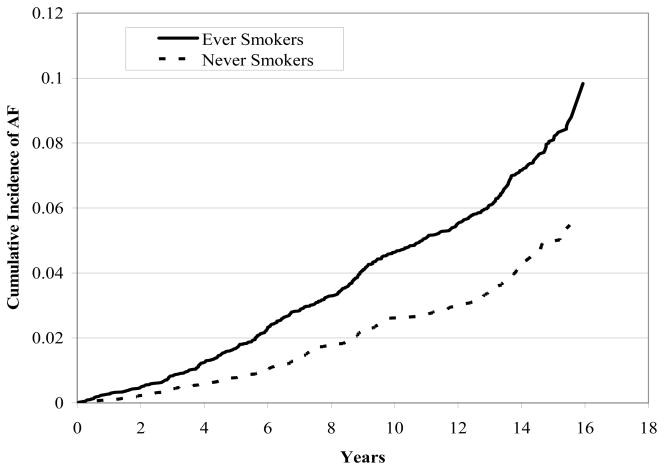
The cumulative incidence of AF was 5.7% in never smokers and 9.8% in ever smokers (Figure 1). The age- and sex-adjusted incidence rates for AF were 28 and 41 per 10,000 person-years in never and ever smokers, respectively (Table 2). The incidence of AF was 1.58-times (95% CI, 1.35–1.85) higher in ever compared to never smokers. In addition, the risk of AF was 1.32-times (95% CI, 1.10–1.57) greater among former smokers and was 2-fold higher in current smokers (HR, 2.05; 95% CI, 1.71–2.47) than never smokers. Hazard ratios were similar by race (HR, 1.57; 95% CI, 1.32–1.88 in whites and HR, 1.60; 95% CI, 1.12–2.29 in blacks for ever vs. never smoking), although the incidence rates were lower in blacks than whites (Table 3).
Figure 1.
Cumulative Incidence of Atrial Fibrillation among Ever vs. Never Smokers at Baseline, ARIC 1987–2002.
Table 2.
Incidence Rates and Hazard Ratios for Atrial Fibrillation by Smoking Status and Cigarette-Years of Smoking Categories, ARIC 1987–2002
| Number of Events | Person-years | Incidence Rate* | Hazard Ratio† | 95% CI | |
| Smoking Status | |||||
| Never (ref.) | 270 | 86,307 | 28 | 1.00 | ref. |
| Ever | 606 | 115,048 | 41 | 1.58 | (1.35–1.85) |
| Former | 333 | 64,887 | 36 | 1.32 | (1.10–1.57) |
| Current | 273 | 50,161 | 48 | 2.05 | (1.71–2.47) |
| Non-Current (ref.) | 603 | 151,194 | 31 | 1.00 | ref. |
| Current | 273 | 50,161 | 48 | 1.76 | (1.52–2.05) |
| Cigarette-Years | |||||
| Zero (ref.) | 270 | 86,307 | 28 | 1.00 | ref. |
| ≤300 | 120 | 39,660 | 28 | 1.04 | (0.83–1.30) |
| >300 to ≤675 | 180 | 37,422 | 41 | 1.60 | (1.30–1.95) |
| >675 | 285 | 34,759 | 55 | 2.10 | (1.74–2.53) |
Table 3.
Race-Specific Incidence Rates and Hazard Ratios for Atrial Fibrillation by Smoking Status Categories, ARIC 1987–2002
| Number of Events | Person-years | Incidence Rate* | Hazard Ratio† | 95% CI | |
| Blacks | |||||
| Never (ref.) | 60 | 24,882 | 22 | 1.00 | ref. |
| Ever | 98 | 27,139 | 32 | 1.60 | (1.12–2.29) |
| Former | 44 | 12,357 | 29 | 1.32 | (0.86–2.02) |
| Current | 54 | 14,782 | 35 | 1.96 | (1.30–2.96) |
| Non-Current (ref.) | 104 | 37,239 | 24 | 1.00 | ref. |
| Current | 54 | 14,782 | 35 | 1.73 | (1.21–2.49) |
| Whites | |||||
| Never (ref.) | 210 | 61,425 | 29 | 1.00 | ref. |
| Ever | 508 | 87,909 | 44 | 1.57 | (1.32–1.88) |
| Former | 289 | 52,530 | 38 | 1.31 | (1.08–1.59) |
| Current | 219 | 35,379 | 53 | 2.09 | (1.70–2.56) |
| Non-Current (ref.) | 499 | 113,955 | 33 | 1.00 | ref. |
| Current | 219 | 35,379 | 53 | 1.79 | (1.51–2.12) |
In a sensitivity analysis including only AF events identified by ECG during ARIC exams, the increased risk of AF associated with smoking remained. The multivariable-adjusted hazard ratios for ECG-diagnosed AF were 1.78 (95% CI, 1.10–2.87) in former, 2.45 (95% CI, 1.44–4.16) in current, and 1.99 (95% CI, 1.27–3.12) in ever smokers compared to never smokers. Additional sensitivity analyses excluding AF events associated with non-cardiac chest or abdominal surgery, and subgroup analyses by gender and by type of event (AF, atrial flutter) yielded similar results as our primary analyses (data not shown).
Because CHD and HF may be mediators, yet may also confound the association between smoking and AF, we additionally ran models 1) censoring for CHD and HF during follow-up and 2) adjusting for prevalent CHD and HF at baseline. When excluding prevalent CHD/HF at baseline and censoring incident CHD or HF during follow-up, associations were attenuated, indicating that CHD and HF may account for some of the association of smoking with AF. Compared to never smokers, the hazard ratios (95% CI) were 1.23 (0.98–1.54) in former, 1.62 (1.27–2.06) in current, and 1.37 (1.12–1.67) in ever smokers. In models adjusting for CHD and HF at baseline, the hazard ratios (95% CI) of AF were 1.30 (1.09–1.55), 1.98 (1.64–2.38), and 1.54 (1.31–1.81) in former, current, and ever smokers compared to never smokers.
We also created tertiles of cigarette-years of smoking, and those in the lowest tertile had similar risk of developing AF as never smokers (Table 2). Those in the second tertile had an incidence rate of 41 per 10,000 person-years and a 60% increased risk of developing AF (95% CI, 30%–95%). The heaviest smokers (>675 cigarette-years) exhibited an incidence rate of 55 per 10,000 person-years and a hazard ratio of 2.10 (95% CI, 1.74–2.53) compared to never smokers. With further adjustment for prevalent CHD and HF at baseline, the hazard ratio for AF among the heaviest smokers was 1.94 (95% CI; 1.60–2.35).
We dichotomized current and former smokers into light/moderate versus heavy smokers (<800 vs. ≥800 cigarette-years) (Table 4). Former smokers had lower incidence rates and hazard ratios for AF compared to current smokers with similar cigarette-years of smoking. Among those with <800 cigarette-years, former and current smokers had hazard ratios of 1.16 (95% CI, 0.96–1.40) and 1.85 (95% CI, 1.49–2.30), respectively. In heavy smokers of ≥800 cigarette-years, former smokers had a 89% increased risk, whereas current smokers had a 131% increased risk of developing AF compared to never smokers.
Table 4.
Incidence Rates and Hazard Ratios for Atrial Fibrillation by Smoking Status and Cigarette-Years of Smoking Categories, ARIC 1987–2002
| Mean Cigarette-years | Number of Events | Person-years | Incidence Rate* | Hazard Ratio† | 95% CI | |
| Never (ref.) | 0 | 270 | 86,307 | 28 | 1.00 | ref. |
| Former, <800 | 302 | 221 | 52,458 | 33 | 1.16 | (0.96–1.40) |
| Former, ≥800 | 1197 | 99 | 10,526 | 55 | 1.89 | (1.47–2.42) |
| Current, <800 | 452 | 139 | 34,530 | 42 | 1.85 | (1.49–2.30) |
| Current, ≥800 | 1149 | 126 | 14,328 | 58 | 2.31 | (1.83–2.92) |
In an attempt to further explore the association of quitting smoking with AF risk, we created 5 cohorts, using exams 2, 3, and 4, and annual telephone contacts 3 and 6 years past exam 4 as baseline, and pooled them in a Cox model. This analysis included 5,607 individuals and 326 AF cases. Individuals who quit smoking had a slightly lower, although statistically nonsignificant, risk of developing AF (HR, 0.88; 95% CI, 0.65–1.17, p-value=0.38) compared to those who continued to smoke.
DISCUSSION
In this population-based prospective study with up to 16 years of follow-up, former and current smokers exhibited a 32% and 105% increased risk of developing AF compared to never smokers. The risk of incident AF increased with increasing cigarette-years of smoking, and appeared to be somewhat greater among current smokers than former smokers with similar cigarette-years of smoking.
Our results corroborate the findings reported in the Framingham Heart Study,5 the Rotterdam Study,7 and the Manitoba Follow-up Study,8 prospective studies with similar design and inclusion criteria to the ARIC cohort. However, our results indicate an even stronger association between current smoking and incidence of AF than those previously reported. In the ARIC study, we reported more than a 2-fold increased risk of AF among current vs. never smokers, an estimate 50% greater than that reported in the Rotterdam Study. In addition, we have found a much higher risk among current smokers compared to former smokers, whereas the Rotterdam Study reported almost identical associations for former and current smokers (HR of 1.51 (1.07–2.12) and 1.48 (1.12–1.96) for current and former smokers, respectively).
Although our results in the ARIC study support those found in 3 other cohort studies, 2 Scandinavian studies did not find an association between smoking and risk of incident AF. Several factors may have led to masking of associations between smoking and AF in the Scandinavian studies, however. First, the Multifactor Primary Prevention Study10 included an intervention for smoking cessation and only baseline data were used in analyses. Smokers may have been more likely to quit or decrease their amount of smoking over follow-up, potentially masking an association between smoking and AF. Second, the Danish Diet, Cancer, and Health Study9 had the shortest follow-up of all studies (mean of 5.7 years) and also the most stringent exclusion criteria. Participants who were hospitalized prior to baseline for endocrine diseases or cardiovascular diseases were excluded. These exclusions created a healthier cohort, and possibly eliminated those who smoked heavily or who may have been more susceptible to the effects of smoking.
Several acute effects of smoking may be involved in the initiation of AF. Nicotine in cigarettes increases heart rate and blood pressure,20 mainly as a consequence of increases in plasma catecholamine concentrations due to nicotine stimulating sympathetic neurotransmission.21 In addition, nicotine-induced alteration of atrial myocyte ion channel conduction, either by release of neurotransmitters or by direct interaction with ion channels, may increase vulnerability to fibrillation. Nicotine has been shown to block the transient outward K+ current (Ito), which governs the initial phase of cardiac repolarization and influences other currents and membrane transport processes.22 The blockage of Ito may be proarrhythmic due to a delay of ventricular repolarization or prolongation of the effective refractory period.
Some chronic effects of cigarette smoke may also play a role in the development of AF, as indicated by the increased risk of AF among former smokers in our study. For example, nicotine may contribute to the development of atrial fibrosis, which has been shown to favor the occurrence of atrial arrhythmias.12 Interstitial fibrosis causes a substantial slowing of electrical impulse propagation in cardiac tissue and affects chamber geometry.23 A recent canine model of atrial fibrillation showed that nicotine causes downregulation of atrial microRNA’s miR-133 and miR-590 in atrial fibroblasts, with an associated upregulation of transforming growth factors TGF-β1 and TGF-βRII and increased collagen production, inducing a proarrhythmic atrial fibrosis.24
Finally, smoking may predispose to AF indirectly through the development of other diseases. In some cases, the development of myocardial infarction due to a hypercoagulable state, enhanced thrombosis, or hemodynamic stress as a result of smoking25 may increase the risk of subsequently developing AF. In addition, reduced lung function and chronic obstructive pulmonary disease (COPD) have been reported to increase the risk of AF;26, 27 therefore, the association of smoking and AF may also be in part mediated by reduced lung function or COPD.
Our study has several strengths, including the large sample size, long follow-up, geographic diversity, and biracial composition of the ARIC cohort. The large number of AF events compares favorably with previous publications, and allowed to obtain more precise estimates of the association of smoking status and amount with AF risk. Also, ours is the first study to show an association between smoking and AF risk among blacks. However, we also acknowledge some limitations. Some incident AF events among individuals without symptoms, those who had paroxysmal AF, or those who were not hospitalized were probably missed. In our study, most AF events were ascertained by hospital discharge records, which could lead to AF underascertainment. However, in a sensitivity analysis including only AF events identified during exam ECGs, we found that the hazard ratios for AF by smoking status categories were slightly stronger than the associations found when considering all AF cases, suggesting that case ascertainment alone could not explain the observed associations. Two additional findings support the validity of AF ascertainment in our study. First, AF rates in ARIC are similar to those reported by other cohorts, including studies, such as the Framingham Heart Study, that relied more on study exam ECGs for ascertainment of AF events.5, 27–30 Second, the association of variants in the 4q25 chromosomal region with AF risk in the ARIC study is of similar magnitude to associations found in other cohorts that relied more on ECGs to identify AF events.31 Finally, although we attempted to investigate the impact of smoking cessation on AF risk, we lacked adequate information on reasons for quitting smoking and on confounders past ARIC visit 4. Thus, residual confounding might explain the weak association between smoking cessation and subsequent AF risk.
In conclusion, current smokers had twice the risk of developing AF over up to 16 years of follow-up compared to never smokers. In addition, the risk of developing AF increased with increasing cigarette-years of smoking, and current smokers appeared to have a greater risk of AF than former smokers with similar cigarette-years of smoking. We found that the associations between smoking and AF do not differ between blacks and whites, even though the incidence rates of AF are lower among blacks compared to whites. Furthermore, among smokers with similar cigarette-years of smoking, a nonsignificant trend toward a lower incidence of AF in smokers who quit compared to continued smokers appeared. Future studies should identify underlying biological mechanisms and determine the role of smoking cessation in the prevention of AF.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The authors thank the staff and participants of the ARIC study for their important contributions. AMC was supported by NHLBI grant T32-HL-007779. This study was further supported by American Heart Association grant 09SDG2280087 and NHLBI grant RC1HL099452.
ABBREVIATIONS
AF
atrial fibrillation
ARIC
Atherosclerosis Risk in Communities
BMI
body mass index
CHD
coronary heart disease
CI
confidence interval
ECG
electrocardiogram
HF
heart failure
HR
hazard ratio
ICD
International Classification of Diseases
MI
myocardial infarction
OR
odds ratio
Footnotes
CONFLICTS OF INTEREST
The authors have no relationships with industry and no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart Disease and Stroke Statistics--2008 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL American College of Cardiology/American Heart Association Task Force on Practice Guidelines. European Society of Cardiology Committee for Practice Guidelines. European Heart Rhythm Association . Heart Rhythm Society: ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain AM, Agarwal SK, Ambrose M, Folsom AR, Soliman EZ, Alonso A. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) Study. American Heart Journal. 2010;159:850–856. doi: 10.1016/j.ahj.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamberlain AM, Agarwal SK, Folsom AR, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] Study) American Journal of Cardiology. 2011;107:85–91. doi: 10.1016/j.amjcard.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 6.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heeringa J, Kors JA, Hofman A, van Rooij FJA, Witteman JCM. Cigarette smoking and risk of atrial fibrillation: the Rotterdam Study. American Heart Journal. 2008;156:1163–1169. doi: 10.1016/j.ahj.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. American Journal of Medicine. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 9.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. American Journal of Medicine. 2005;118:489–495. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. Journal of Internal Medicine. 2001;250:382–389. doi: 10.1046/j.1365-2796.2001.00902.x. [DOI] [PubMed] [Google Scholar]
- 11.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131:1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 12.Goette A, Lendeckel U, Kuchenbecker A, et al. Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart. 2007;93:1056–1063. doi: 10.1136/hrt.2005.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. Journal of the American College of Cardiology. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 14.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. American Journal of Epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) Study. American Heart Journal. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baecke J, Burema J, Frijters J. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. American Journal of Clinical Nutrition. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson H, Caidaul K, Larsson B, et al. Cardiac and pulmonary causes of dyspnoea--validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. European Heart Journal. 1987;8:1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 19.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. Journal of the American Statistical Association. 1989;84:1074–1078. [Google Scholar]
- 20.Benowitz NL, Jacob P3, Jones RT, Rosenberg J. Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. The Journal of Pharmacology and Experimental Therapeutics. 1982;221:368–372. [PubMed] [Google Scholar]
- 21.Haass M, Kubler W. Nicotine and sympathetic neurotransmission. Cardiovascular Drugs & Therapy. 1997;10:657–665. doi: 10.1007/BF00053022. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Shi H, Zhang L, et al. Nicotine is a potent blocker of the cardiac A-type K+ channels : effects on cloned Kv4.3 channels and native transient outward current. Circulation. 2000;102:1165–1171. doi: 10.1161/01.cir.102.10.1165. [DOI] [PubMed] [Google Scholar]
- 23.Goette A. Nicotine, atrial fibrosis, and atrial fibrillation: do microRNAs help to clear the smoke? [comment] Cardiovascular Research. 2009;83:421–422. doi: 10.1093/cvr/cvp188. [DOI] [PubMed] [Google Scholar]
- 24.Shan H, Zhang Y, Lu Y, et al. Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovascular Research. 2009;83:465–472. doi: 10.1093/cvr/cvp130. [DOI] [PubMed] [Google Scholar]
- 25.Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. Journal of the American College of Cardiology. 1997;29:1422–1431. doi: 10.1016/s0735-1097(97)00079-x. [DOI] [PubMed] [Google Scholar]
- 26.Buch P, Friberg J, Scharling H, Lange P, Prescott E. Reduced lung function and risk of atrial fibrillation in The Copenhagen City Heart Study. European Respiratory Journal. 2003;21:1012–1016. doi: 10.1183/09031936.03.00051502. [DOI] [PubMed] [Google Scholar]
- 27.Psaty BM, Manolio TAMHS, Kuller LHDH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 28.Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam Study. European Heart Journal. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 29.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 30.Stewart S, Hart CL, Hole DJ, McMurray JJ. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley Study. Heart. 2001;86:516–521. doi: 10.1136/heart.86.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamin EJ, Rice KM, Arking DE, et al. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nature Genetics. 2009;41:879–881. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]