Titration to target dose of bisoprolol vs. carvedilol in elderly patients with heart failure: the CIBIS-ELD trial (original) (raw)
Abstract
Aims
Various beta-blockers with distinct pharmacological profiles are approved in heart failure, yet they remain underused and underdosed. Although potentially of major public health importance, whether one agent is superior in terms of tolerability and optimal dosing has not been investigated. The aim of this study was therefore to compare the tolerability and clinical effects of two proven beta-blockers in elderly patients with heart failure.
Methods and results
We performed a double-blind superiority trial of bisoprolol vs. carvedilol in 883 elderly heart failure patients with reduced or preserved left ventricular ejection fraction in 41 European centres. The primary endpoint was tolerability, defined as reaching and maintaining guideline-recommended target doses after 12 weeks treatment. Adverse events and clinical parameters of patient status were secondary endpoints. None of the beta-blockers was superior with regards to tolerability: 24% [95% confidence interval (CI) 20–28] of patients in the bisoprolol arm and 25% (95% CI 21–29) of patients in the carvedilol arm achieved the primary endpoint (_P_= 0.64). The use of bisoprolol resulted in greater reduction of heart rate (adjusted mean difference 2.1 b.p.m., 95% CI 0.5–3.6, _P_= 0.008) and more, dose-limiting, bradycardic adverse events (16 vs. 11%; _P_= 0.02). The use of carvedilol led to a reduction of forced expiratory volume (adjusted mean difference 50 mL, 95% CI 4–95, _P_= 0.03) and more, non-dose-limiting, pulmonary adverse events (10 vs. 4%; P < 0.001).
Conclusion
Overall tolerability to target doses was comparable. The pattern of intolerance, however, was different: bradycardia occurred more often in the bisoprolol group, whereas pulmonary adverse events occurred more often in the carvedilol group.
This study is registered with controlled-trials.com, number ISRCTN34827306.
Keywords: Heart failure, Beta-blocker, Elderly, Tolerability, Target dose, Lung function
Introduction
Chronic heart failure is a growing epidemic associated with high mortality, morbidity, and quality of life (QoL) impairment and is a substantial burden on health systems.1 Three key trials have randomized nearly 9000 patients with systolic heart failure to beta-blocker (bisoprolol, carvedilol, or metoprolol succinate controlled release) or placebo and demonstrated a consistent 30% reduction in mortality and a 40% reduction in hospitalizations.2–4 Nevertheless, recent large international surveys have shown that only 20–40% of heart failure patients are taking beta-blockers and the mean dose is half the recommended target.5,6
The underuse and underdosing of beta-blockers may reflect a reluctance to change practice stemming from their long-standing contraindication in heart failure. Conversely, it may reflect a true lack of tolerability of beta-blockers in patients who are typically relatively old, have co-morbidities, and are taking a range of other drugs. It is noteworthy that many previous beta-blocker trials included heart failure patients who were younger (mean age 61–64) than those encountered in routine practice (mean age 71–75).2–4,7,8
Class effects may not be uniform and tolerability may differ between the commonly used beta-blockers, reflecting their distinct pharmacological profiles such as selectivity for the β1-adrenoceptor subtype (bisoprolol) or vasodilatory activity (carvedilol). However, differences in tolerability have not been systematically studied. If one proven beta-blocker were better tolerated than another it could be of considerable public health importance. Results of the Carvedilol Or Metoprolol European Trial (COMET), suggested that overall tolerability of carvedilol vs. metoprolol tartrate does not differ,8 but it has been suggested that this interpretation is problematic because doses were not equivalent.9 The second Carvedilol Open-Label Assessment found good tolerability for carvedilol in older heart failure patients,10 but no previous double-blind randomized trial had tolerability as the primary endpoint.
Beta-blocker therapy in patients with preserved left ventricular ejection fraction (LVEF) is associated with an improvement in echocardiographic parameters11 and international guidelines provide an expert-based recommendation of heart rate lowering using beta-blockers in these patients12 despite a lack of proven reduction in mortality.
We therefore designed the Cardiac Insufficiency Bisoprolol Study in Elderly (CIBIS-ELD) to investigate the tolerability of two of the most widely used beta-blockers in elderly heart failure patients with impaired and preserved LVEF. This is the first randomized, double-blind trial to have as its primary endpoint the tolerability of bisoprolol vs. carvedilol when used at their guideline-recommended target doses.
Methods
Trial design and patients
We undertook this investigator-initiated, randomized, double-blind, parallel-group trial in 21 centres in Germany, 1 in Montenegro, 15 in Serbia, and 4 in Slovenia. The CIBIS-ELD protocol was approved by all relevant federal institutes for drugs and medical devices as well as by national and local ethics committees. Patients provided written informed consent and the trial conforms to the principles outlined in the Declaration of Helsinki. Details of the CIBIS-ELD trial design have been published elsewhere.13 This study is registered with controlled-trials.com, number ISRCTN34827306.
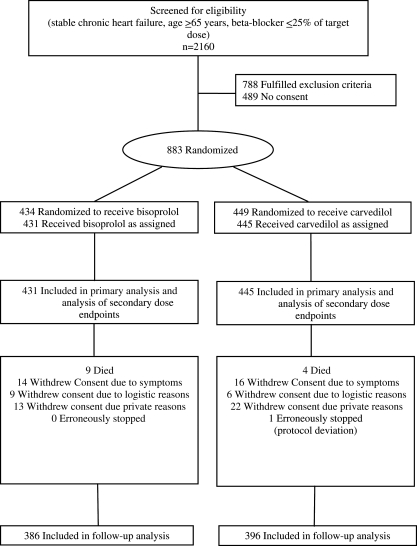
Patients were recruited between April 2005 and April 2008 (Figure 1). Eligible patients were 65 years or older with symptomatic chronic heart failure consistent with New York Heart Association (NYHA) functional class ≥II at time of enrolment or with an LVEF ≤ 45%. At baseline, participants had to be beta-blocker naïve or on ≤25% of the guideline-recommended target or equivalent dose.14 Patients who had been on suboptimal doses previously were included to investigate titration success following pretreatment. Patients had to be clinically stable and on stable medication for 2 weeks prior to randomization.
Figure 1.
Participant flow through the study. Target dose based on the 2005 European Society of Cardiology guidelines.
Major exclusion criteria were: known contraindications to beta-blocker treatment, such as hypotension with a resting systolic blood pressure <90 mmHg, severe pulmonary disease or severe asthma, heart rate <55 b.p.m. prior to commencement of therapy, second or third degree sinoatrial block (without pacemaker), and known sick sinus syndrome.
Procedures
Patients were recruited through primary care physicians, and secondary and tertiary care hospitals. Upon enrolment, they were randomly assigned to either bisoprolol or carvedilol. For each centre, a random sequence of permuted blocks of variable length was generated by the Clinical Trial Centre Leipzig. Patients, investigators, and study personnel were blinded to treatment assignment for the duration of the trial.
During the initial titration phase of the study, patients were seen at fortnightly intervals. According to the titration scheme [based on the 2005 European Society of Cardiology (ESC) guidelines],14 the dose was scheduled to double at every visit to reach the target dose of 10 mg bisoprolol once daily or 25 mg carvedilol twice daily within 6 weeks (50 mg twice daily within 8 weeks for patients >85 kg). Investigators were free to delay titration or reduce the dose if clinically indicated. The titration phase was followed by a maintenance period lasting 4 weeks and the final visit was at 10 weeks (12 weeks for patients >85 kg).
Outcome measures
The primary endpoint of tolerability was defined as reaching the target dose through the process of fortnightly doubling with no more than one delayed increase and with the target dose maintained for at least 10 days. Titration failure was defined as failure to up-titrate more than once or as down-titration after receiving the target dose level. Predefined secondary endpoints were the percentage of target dose achieved at the end of the study, and the dose achieved prior to first titration failure. Occurrences of adverse events were recorded and their association with dose adjustment was assessed by odds ratios. For titration failures with no simultaneous adverse event, we implemented a blinded endpoint committee consisting of experts with sound clinical experience in heart failure therapy and research who evaluated the circumstances of titration failure based on data from all visits and additional investigator comments. Multiple reasons could be specified by the endpoint committee (Appendix).
Predefined clinical secondary endpoints were: NYHA functional class, heart rate, blood pressure (measured prior to dose titration at each visit) and LVEF, assessment of diastolic function, 6 min walk distance, 1 s forced expiratory volume (FEV1) and the physical and psychosocial component scores on the short-form QoL health survey (SF36) at the end of the study, adjusted for baseline.
Statistical analysis
All analyses were carried out by intention to treat, including all patients who received the first dose of allocated study medication. Patients who died or prematurely stopped treatment were judged not to have fulfilled the conditions for the primary endpoint. A sensitivity analysis was performed counting deaths as drop-outs. The primary null hypothesis that equal percentages of patients would tolerate the target doses of the two agents was tested against the two-sided alternative by Fisher's exact test. This was designed to discover the superiority of one beta-blocker assuming 60 vs. 50% tolerability to target doses. Doses achieved at follow-up were compared by the Mann–Whitney U test. Percentages of patients achieving the target dose free of titration failure are presented as Kaplan–Meier analyses. Prespecified baseline variables were examined for being predictors for achievement of target dose by multiple logistic regression. Only 26 patients (bisoprolol n = 11, carvedilol n = 15) reached the higher dose level applicable to patients >85 kg; therefore data for this group were not analysed separately.
Changes in clinical endpoints are presented as mean differences and their significance assessed within each treatment group by paired _t_-test. Comparison across groups was carried out by analysis of covariance (ANCOVA) with the follow-up measurement as dependent variable, the randomized agent as factor, and the baseline measurement as covariate (or as categorical co-factor in case of NYHA class). Patients with a pacemaker were excluded from the analysis of change in heart rate. Percentages of patients who had an adverse event were compared using Fisher's exact test. Analyses were performed using SPSS Version 15 (SPSS Inc., Chicago, IL, USA).
Sample size
The study was designed to detect a 10% difference between arms with a power of 80–90% on the assumption that at least 50% of all patients would meet the criterion for tolerability. We therefore needed to recruit 760–1040 patients at a significance level of 5%. In April 2008, we had enrolled 883 patients, leading to a power of 85%. For the detection of 25 vs. 35% tolerability, power was 90%. Since the primary endpoint was defined for all patients, no adjustment for drop-outs was necessary.
Results
A total of 883 patients were randomized (Germany n = 300, Montenegro n = 18, Serbia n = 535, and Slovenia n = 30), 876 of whom received the first dose of the study medication. One patient was erroneously excluded from further trial participation by an investigator; no patient was lost to follow-up (Figure 1). Baseline characteristics are shown in Table 1 and there were no imbalances between treatment groups.
Table 1.
Baseline characteristics
| All patients | Bisoprolol | Carvedilol | |
|---|---|---|---|
| (n = 876) | (n = 431) | (n = 445) | |
| Women, no. (%) | 329 (38) | 167 (39) | 162 (36) |
| Age, mean (SD), years | 72.8 (5.5) | 72.9 (5.6) | 72.7 (5.5) |
| NYHA class | |||
| I | 34 (4) | 15 (4) | 19 (4) |
| II | 575 (66) | 272 (63) | 303 (68) |
| III | 258 (30) | 139 (32) | 119 (27) |
| IV | 9 (1) | 5 (1) | 4 (1) |
| Hospitalization for heart failure during the past 12 months, no. (%) | 314 (36) | 143 (33) | 171 (38) |
| Heart rate on ECG, mean (SD), b.p.m. | 73 (14) | 74 (15) | 73 (14) |
| Blood pressure, mean (SD), mmHg | |||
| Systolic | 137 (21) | 137 (21) | 137 (22) |
| Diastolic | 80 (12) | 80 (12) | 80 (12) |
| LVEF, mean (SD), % | 42 (14) | 42 (14) | 42 (13) |
| LVEF > 45%, no. (%) | 250 (29) | 123 (29) | 127 (29) |
| 6 min walk distance, mean (SD), m | 322 (110) | 319 (103) | 325 (116) |
| NT-pro-BNP, median (IQR), pg/mL | 609 (255–1614) | 596 (236–1699) | 630 (284–1587) |
| Haemoglobin, mean (SD), g/dL | 13.7 (1.6) | 13.7 (1.6) | 13.7 (1.6) |
| FEV1, mean (SD), mL | 2192 (675) | 2185 (712) | 2197 (638) |
| FEV1, predicted for age and sex (%), mL | 90.8 (23.9) | 90.4 (24.8) | 91.2 (23.1) |
| Peripheral oedema, no. (%) | 183 (21) | 88 (20) | 95 (21) |
| Body mass index, mean (SD), kg/m² | 27.7 (4.9) | 28.0 (5.0) | 27.6 (4.7) |
| Medical history, no. (%) | |||
| Current smoker | 76 (9) | 41 (10) | 35 (8) |
| Myocardial infarction | 347 (40) | 163 (38) | 184 (41) |
| PCI and/or CABG | 196 (22) | 90 (21) | 106 (24) |
| Pacemaker and/or ICD | 56 (6) | 23 (5) | 33 (7) |
| Co-morbiditiesa | |||
| Hypertension | 724 (83) | 353 (82) | 371 (84) |
| Diabetes mellitus | 223 (26) | 107 (25) | 116 (26) |
| Hyperlipidaemia | 548 (63) | 261 (61) | 287 (65) |
| Peripheral vascular disease or stroke | 121 (14) | 59 (14) | 62 (14) |
| Atrial fibrillation | 164 (19) | 83 (19) | 81 (18) |
| COPD | 65 (7) | 28 (7) | 37 (8) |
| Renal dysfunction [GFR < 60] | 338 (39) | 165 (38) | 173 (39) |
| Anaemia [male: Hb < 13 g/dL; female: Hb < 12 g/dL] | 181 (21) | 86 (20) | 95 (21) |
| Depression | 73 (8) | 34 (8) | 39 (9) |
| Cardiovascular medication, no. (%) | |||
| Beta-blocker | |||
| None | 349 (40) | 175 (41) | 174 (39) |
| 12.5% of target dose equivalent | 149 (17) | 75 (17) | 74 (17) |
| 25% of target dose equivalent | 378 (43) | 181 (42) | 197 (44) |
| ACE inhibitor and/or ARB | 741 (85) | 374 (87) | 367 (83) |
| Aldosterone receptor antagonist | 275 (31) | 145 (34) | 130 (29) |
| Diuretic | 649 (74) | 323 (75) | 326 (73) |
| Cardiac glycoside | 129 (15) | 64 (15) | 65 (15) |
| Calcium channel blocker | 143 (16) | 80 (19) | 63 (14) |
| Nitrate | 277 (32) | 131 (30) | 146 (33) |
| Antiarrhythmic | 95 (11) | 48 (11) | 47 (11) |
| Statin | 342 (39) | 159 (37) | 183 (41) |
| Antiplatelet | 582 (66) | 287 (66) | 295 (66) |
| Anticoagulant | 220 (25) | 102 (24) | 118 (26) |
| QoL, mean (SD) | |||
| SF-36 physical component score | 38.2 (9.5) | 37.9 (9.3) | 38.5 (9.7) |
| SF-36 psychosocial component score | 45.4 (12.1) | 44.5 (11.8) | 46.2 (12.4) |
Primary endpoint
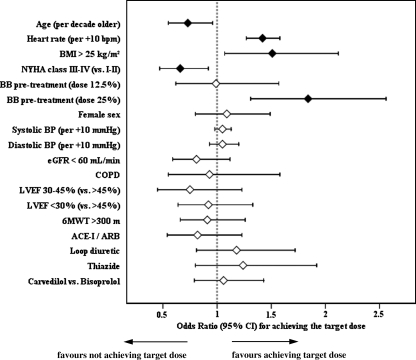
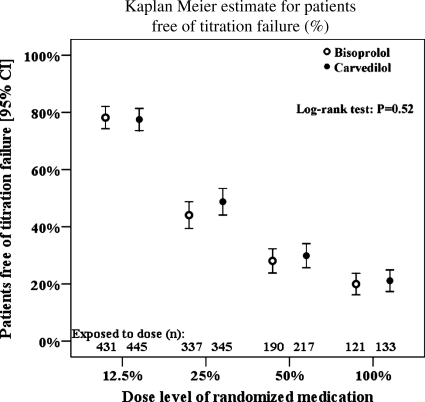
None of the beta-blockers was superior with regards to tolerability according to the primary endpoint of reaching the respective target doses when following the recommended titration scheme (Table 2). This result remained the same when adjusting for treatment effect covariates (Figure 2). Kaplan–Meier estimates show that the percentage of patients reaching the ascending dose levels in line with the titration scheme did not differ between groups (Figure 3). Overall, 31% of patients reached the full, and 55% tolerated at least half of the target doses (Table 2). The mean daily dose reached at follow-up was 5.0 mg for bisoprolol and 23.9 mg for carvedilol in patients ≤85 kg (47.7 mg in patients >85 kg). Factors associated with reaching the primary endpoint are shown in Figure 2.
Table 2.
Tolerability and dose endpoints
| Patients in treatment groups | _P_-value | ||
|---|---|---|---|
| Bisoprolol | Carvedilol | ||
| (n = 431) | (n = 445) | ||
| Primary endpoint achieveda, no. (%) | 102 (24) | 112 (25) | 0.64 |
| 95% CI for rate | 20–28 | 21–29 | |
| Dose level at follow-up, no. (%) | 0.58 | ||
| 0 (study medication stopped before follow-up) | 46 (11) | 51 (11) | |
| 12.5% (1.25 mg bisoprolol or 3.125 mg carvedilol) | 47 (11) | 45 (10) | |
| 25% (2.5 mg bisoprolol or 6.25 mg carvedilol) | 108 (25) | 97 (22) | |
| 50% (5 mg bisoprolol or 12.5 mg carvedilol) | 98 (23) | 110 (25) | |
| 100% (10 mg bisoprolol or 1–2×25 mg carvedilol) | 132 (31) | 142 (32) |
Figure 2.
Predictors of tolerability. Filled diamonds indicate factors significantly related to outcome.
Figure 3.
Kaplan–Meier estimate showing that the percentage of patients reaching the ascending dose levels in line with the titration scheme did not differ between groups. White circles indicate bisoprolol and black circles indicate carvedilol.
Safety and reasons for titration failure
In total, 668 patients (75.7%) did not reach the primary endpoint and experienced at least one titration failure. While there was no overall difference between the two groups, bradycardia (defined as heart rate <55 b.p.m. or a heart rate below 60 b.p.m. plus a decrease of more than 15%) was the most common reason for titration failure and occurred more often in the bisoprolol group (Table 3). These episodes were associated with more dose reductions (P = 0.003) as well as a lower likelihood of achieving the target dose (P < 0.001).
Table 3.
Adverse events and relationship to target dose
| Number of adverse events | ||||
|---|---|---|---|---|
| Bisoprolol | Carvedilol | _P_-value | ||
| Any adverse event, no. (%) | 281 (65) | 284 (64) | 0.67 | |
| Death | 9 (2) | 4 (1) | 0.17 | |
| Hospitalization | 13 (3) | 14 (3) | 1.00 | |
| Worsening heart failure | 95 (22) | 94 (21) | 0.74 | |
| Bradycardia | 70 (16) | 47 (11) | 0.02 | |
| New AV block | 46 (11) | 39 (9) | 0.36 | |
| Hypotension | 37 (9) | 44 (10) | 0.56 | |
| Fatigue/ drowsiness | 46 (11) | 23 (5) | 0.003 | |
| Vertigo | 32 (7) | 32 (7) | 1.00 | |
| Pulmonary | 16 (4) | 44 (10) | 0.01 | |
| Renal dysfunction | 36 (8) | 29 (7) | 0.31 | |
| Anaemia | 29 (7) | 52 (12) | 0.01 | |
| Hyperuricaemia | 20 (5) | 20 (5) | 1.00 | |
| Hyperlipidaemia | 22 (5) | 23 (5) | 1.00 | |
| Odds ratio for relationship of BB titration with AE | ||||
| Bisoprolol | Carvedilol | |||
| Any titration failure | Dose reduction | Any titration failure | Dose reduction | |
| Any adverse event | 2.10** | 2.88*** | 2.08** | 1.04 |
| Death | † | |||
| Hospitalization | 0.79 | 6.62*** | 3.58 | 3.75* |
| Worsening heart failure | 1.19 | 1.36 | 1.53 | 1.52 |
| Bradycardia | 1.60 | 3.04*** | 4.35** | 0.91 |
| New AV block | 1.75 | 1.09 | 2.49 | 0.92 |
| Hypotension | 1.66 | 0.87 | 2.23 | 1.28 |
| Fatigue/ drowsiness | 12.75*** | 3.90*** | 2.93 | 4.58*** |
| Vertigo | 1.38 | 2.30* | 2.03 | 3.15** |
| Pulmonary | 1.08 | 1.26 | 1.23 | 0.66 |
| Renal dysfunction | 1.04 | 0.64 | 0.83 | 0.29* |
| Anaemia | 0.77 | 0.42 | 1.32 | 0.09*** |
| Hyperuricaemia | 2.31 | 0.91 | 0.80 | 0.46 |
| Hyperlipidaemia | 1.13 | 0.26 | 0.75 | 0.39 |
Pulmonary adverse events, which included a change in FEV1 of ≥20%, or clinical symptoms such as breathing difficulty, obstructive ventilatory disorders, and bronchospasm occurred more often in the carvedilol group than among patients taking bisoprolol. However, pulmonary adverse events were not dose-limiting nor led to withdrawal of carvedilol. Anaemia occurred more often in patients taking carvedilol (Table 3). A decrease in mean haemoglobin was seen in the carvedilol group (Table 4), and this effect was more pronounced in patients who were beta-blocker naïve at baseline (interaction term: P < 0.01). Other adverse events with no difference between groups were worsening heart failure, hypotension, hospital admission, and mortality (Table 3).
Table 4.
Clinical endpoints
| Bisoprolol (B) | Carvedilol (C) | Difference B–C from ANCOVA | |
|---|---|---|---|
| NYHA functional class | (n = 386) | (n = 396) | |
| Mean change (95% CI) | −0.29 (−0.35 to −0.24) | −0.25 (−0.30 to −0.20) | −0.01 (−0.08 to +0.05) |
| _P_-value | <0.001 | <0.001 | 0.71 |
| Heart rate on ECGa, b.p.m. | (n = 367) | (n = 369) | |
| Mean change (95% CI) | −8.4 (−9.8 to −7.0) | −6.0 (−7.2 to −4.7) | −2.1 (−3.6 to −0.5) |
| _P_-value | <0.001 | <0.001 | 0.008 |
| Systolic blood pressure, mmHg | (n = 386) | (n = 396) | |
| Mean change (95% CI) | −9.3 (−11.4 to −7.3) | −9.5 (−11.7 to −7.3) | +0.6 (−1.7 to +2.9) |
| _P_-value | <0.001 | <0.001 | 0.60 |
| Diastolic blood pressure, mmHg | (n = 386) | (n = 396) | |
| Mean change (95% CI) | −4.7 (−5.9 to −3.5) | −4.2 (−5.4 to −3.0) | −0.3 (−1.6 to +1.1) |
| _P_-value | <0.001 | <0.001 | 0.69 |
| LVEF, % | (n = 383) | (n = 394) | |
| Mean change (95% CI) | +3.0 (+2.3 to +3.7) | +2.7 (+2.0 to +3.4) | +0.4 (−0.5 to +1.4) |
| _P_-value | <0.001 | <0.001 | 0.36 |
| 6-min-walk distance, m | (n = 357) | (n = 358) | |
| Mean change (95% CI) | +19 (+11 to +26) | +13 (+6 to +19) | +5 (−4 to +14) |
| _P_-value | <0.001 | <0.001 | 0.25 |
| Haemoglobin, g/dL | (n = 358) | (n = 373) | |
| Mean change (95% CI) | –0.07 (−0.20 to +0.06) | −0.24 (−0.37 to −0.11) | +0.15 (−0.02 to +0.32) |
| _P_-value | 0.28 | <0.001 | 0.07 |
| FEV1, mL | (n = 349) | (n = 365) | |
| Mean change (95% CI) | +3 (−32 to +39) | −42 (−73 to −11) | +50 (+4 to +95) |
| _P_-value | 0.86 | 0.007 | 0.03 |
| SF-36 physical component score | (n = 289) | (n = 295) | |
| Mean change (95% CI) | +2.4 (+1.6 to +3.3) | +2.0 (+1.1 to +2.9) | +0.4 (−0.7 to +1.5) |
| _P_-value | <0.001 | <0.001 | 0.49 |
| SF-36 psychosocial component score | (n = 289) | (n = 295) | |
| Mean change (95% CI) | +3.5 (+2.4 to +4.7) | +2.6 (+1.5 to +3.7) | +0.4 (−1.0 to +1.7) |
| _P_-value | <0.001 | <0.001 | 0.61 |
Potential reasons for down-titration, slowed titration, or discontinuation defined by the blinded endpoint committee included undesirable reduction in heart rate ≤60 b.p.m. (n = 70, 8.0%); undesirable reduction in blood pressure ≤100 mmHg systolic/≤60 mmHg diastolic (n = 21, 2.4%), logistical reasons (n = 14, 1.6%), and patient refusing the medication for unknown reasons (n = 31, 3.5%). Patients in the bisoprolol group were more likely to be affected by an undesirable reduction in heart rate [n = 45 (12%) vs. carvedilol n = 25 (6%); P = 0.01]. There were no differences between bisoprolol and carvedilol with regards to the other reasons for down-titration, slowed titration, or discontinuation.
Change in New York Heart Association class, left ventricular ejection fraction, 6 min walk distance, quality of life, heart rate, and 1 s forced expiratory volume
New York Heart Association functional class, LVEF, 6 min walk distance and QoL improved to the same extent over the period of the study in each treatment group; blood pressure was lowered equally. Heart rate decreased in both groups from baseline to follow-up, but the reduction was greater in the bisoprolol group. Mean FEV1 decreased in the carvedilol group whereas it remained stable in the bisoprolol group (Table 4).
Discussion
In this first head-to-head comparison trial of two approved beta-blockers in elderly heart failure patients, we found no superiority of bisoprolol vs. carvedilol or vice versa with regards to tolerability to target doses, but the reasons for not reaching the primary endpoint and the clinical reaction to the beta-blockers differed.
Pharmacological differences and heart rate
Recent publications have confirmed heart rate reduction as an important target in the treatment of heart failure.15,16 The selective β1-adrenoceptor-blocker bisoprolol was associated with a larger heart rate reduction and more bradycardic adverse events than the non-selective α1-, β1-, and β2-adrenoceptor-blocker carvedilol. Sole alpha-blockade is known to increase heart rate and the combination of alpha- and beta-blockade in one molecule appears to weaken its heart rate lowering effect.17 Although this difference in selectivity may explain our results, CIBIS-ELD is the first comparison trial to provide evidence of its clinical relevance in heart failure patients. In multivariate analysis, higher baseline heart rate predicted better tolerability of target doses, regardless of treatment group. Of note, the baseline mean heart rate was relatively low in this study (73 b.p.m.) when compared with other heart failure trials such as CIBIS II (80 b.p.m.),2 CIBIS III (79 b.p.m.),18 and COMET (81 b.p.m.).8 The lower baseline heart rate of patients in this trial may explain at least in part why the mean daily dose reached (bisoprolol: 5.0 mg; carvedilol: 23.9 mg for patients ≤85 kg; and 47.7 mg for patients >85 kg) was lower than that observed, for example, in CIBIS III (mean bisoprolol dose 8.3 mg),18 and in COMET (mean carvedilol dose 41.8 mg, compared with a target of 50 mg).8
Pharmacological differences and pulmonary function
Beta-blockers are frequently not up-titrated or even withheld for fear of bronchoconstriction.
In this trial carvedilol was associated with more pulmonary adverse events than bisoprolol and with a reduction of FEV1, which is in line with its pharmacodynamic properties. Incidence of pulmonary adverse events was nonetheless moderate in both groups. In contrast to pre-existing opinions, neither these adverse events nor the reduction in FEV1 with carvedilol were a significant limitation for up-titration. Furthermore, the presence of chronic obstructive pulmonary disease (COPD), which was the most powerful independent predictor of beta-blocker underutilization in the EuroHeart Failure Survey,6 was not predictive of less ability to titrate dose upwards in our trial. In a study that looked specifically at heart failure patients with COPD receiving bisoprolol or placebo, bisoprolol was associated with a 5% reduction in FEV1.19 However, this did not cause pulmonary symptoms or impair QoL.
Tolerability of beta-blocker therapy in CIBIS-ELD
Titration scheme
The observation that only 31% of patients reached their target dose contrasts with findings from previous trials, in which 42–87% of patients reached the recommended target doses.2–4,8,18,20 However, these previous trials enrolled younger patients (60–63 years), allowed a longer duration of titration (10–16 weeks and longer if clinically indicated), and also allowed more than one delay in titration, and/or intermediate dose steps (for bisoprolol 3.75 and 7.5 mg) instead of a doubling of the dose every fortnight as recommended by the 2005 ESC guidelines. The new 2008 ESC guidelines adopt the titration schemes of these larger beta-blocker trials without citing new evidence, but our findings appear to support this change.21
Target doses
Although a dose-related reduction in mortality and hospitalization rates was shown in younger patients receiving carvedilol,22 a recent meta-analysis of 23 beta-blocker trials failed to show an association between beta-blocker dose and survival benefit in heart failure.16 Our study was not designed to address the relationship of dose benefit; however, it does clearly raise the question of the achievability of currently recommended targets.
Predictors of tolerability
In agreement with other investigators, our findings show that younger age and NYHA functional class II predicted patients’ ability to tolerate higher beta-blocker doses.10,23 Beta-blocker pretreatment was a further predictor of tolerability in CIBIS-ELD, which may be in favour of a slower approach to titration and is in line with clinical observations that a quarter of the recommended beta-blocker dose is a hurdle to be overcome. A body mass index >25 kg/m2 being predictive of achieving higher doses may be due to adverse effects probably being linked to the volume of distribution, but is an observation that to our knowledge has not been reported before.
Adverse effects
We expected that the vasodilatory effects of carvedilol might lead to lower tolerability as a result of hypotension. In a review of selective vs. non-selective beta-blockers, the most frequent adverse effects were reported to be worsening heart failure with bisoprolol, and hypotension and dizziness with carvedilol.24 Our results do not confirm these findings. Anaemia as an adverse event was observed more frequently in patients receiving carvedilol. These results are in line with findings from the COMET trial.25
Limitations
A correlation between tolerability to the target doses or titration success and mortality cannot be established on the basis of our data due to the short follow-up. Another limitation might be that there is no recommended beta-blocker target dose for patients with preserved LVEF. However, despite a lack of proven reduction in mortality, there is an expert-based recommendation of heart rate lowering using beta-blockers in diastolic heart failure and for reasons of comparison, we used the same dose.26 In addition, recent data from SENIORS suggest that beta-blockers may possibly be effective in patients with LVEF >35%.27 It may be considered a limitation that only 25% of patients reached the primary endpoint though a target of 50% was planned. However, this should first be seen as an unexpected outcome which deserves consideration when speaking about the meaning of target dose, and second, it is not a true limitation as the power of the study was not reduced. Further, it was not mandatory for CIBIS-ELD investigators to document reasons for titration failure. Therefore, a blinded endpoint committee assessed the patients’ clinical data at the time of titration failure (for failures unrelated to adverse events) and potential reasons for titration failure were recorded where possible.
Conclusion and clinical implications
In CIBIS-ELD, we found no difference in achieved doses and tolerability to target doses between bisoprolol and carvedilol in elderly patients with heart failure, although the patterns of adverse effects differed. With both agents, it appears that clinicians should follow an individualized, slower, titration scheme. For patients with low resting heart rates, physicians might prefer prescription of carvedilol, and for patients with lung disease, the favourable beta-blocker might be bisoprolol.
Funding
This work was supported by the German Federal Ministry of Education and Research (grant number 01GI0205). Sponsor according to ICH-GCP was the Charité-Universitätsmedizin in Berlin, Germany. Merck KGaA gave an unrestricted grant without any rights to influence trial design, data collection, data analysis, and interpretation or publication.
Conflict of interest: H.-D.D. reported receiving research grant support and travel support from Merck KGaA, and equipment provision support from Merck KgaA, Roche, and Biosite. S.I. reported receiving travel support from Merck KGaA. E.T. reported receiving support from Merck KGaA, Getemed AG and ResMed. R.D. reported receiving research grant support from Merck KGaA, and equipment provision support from Merck KgaA, Roche, and Biosite. For all other authors, there is nothing to declare.
Acknowledgements
We would like to thank the study participants for their time and commitment; the members of the steering and endpoint committees for their continued guidance and encouragement; Charité pharmacy staff Cornelia Eberhardt and Christiane Schwintzer for dispensing the trial medication; and Rob Stepney, medical writer, for assisting in the editing of the manuscript.
Appendix
Steering Committee
Hans-Jürgen Becker, German Heart Foundation, Frankfurt, Germany; Hans-Dirk Düngen, Charité-Universitätsmedizin, Campus Virchow-Klinikum, Department of Internal Medicine—Cardiology, Berlin, Germany; Thomas Eschenhagen, University Medical Center Hamburg Eppendorf, Institute for Experimental and Clinical Pharmacology, Hamburg, Germany; Roland Hardt, St Hildegardis Hospital, Department of Geriatric Medicine, Mainz, Germany; Friedrich Luft, Charité-Universitätsmedizin, Campus Buch, Experimental and Clinical Research Center, Berlin, Germany; Bernhard Rauch, Clinical Center Ludwigshafen, Center for Rehabilitation, Ludwigshafen, Germany; Elisabeth Steinhagen-Thiessen, Center for Geriatric Medicine, Berlin, Germany; Ruth Strasser, Technische Universität Dresden, Department of Internal Medicine—Cardiology, Dresden, Germany; Finn Waagstein, Sahlgrenska University Hospital, Wallenberg Laboratory, Göteborg, Sweden.
Clinical Endpoints Committee
Thomas Rau, University Medical Center Hamburg Eppendorf, Institute for Experimental and Clinical Pharmacology, Hamburg, Germany; Wolfram Döhner, Charité-Universitätsmedizin, Campus Virchow-Klinikum, Center for Stroke Research, Berlin, Germany; Felix Mehrhof, Charité-Universitätsmedizin, Campus Virchow-Klinikum, Department of Internal Medicine—Cardiology, Berlin, Germany.
Data Safety Monitoring Board
Ulrike Bauer, Competence Network for Congenital Heart Defects, Berlin, Germany; Stephan Beckmann, Center for Rehabilitation, Berlin, Germany; Jürgen Waigand, Charité-Universitätsmedizin, Campus Virchow-Klinikum, Department of Internal Medicine—Cardiology, Berlin, Germany.
CIBIS-ELD trial investigators (numbers in parentheses after country indicate number of randomized patients)
Germany (300) S. Baumbach (Apolda), St Beckmann (Berlin), St Czischke (Rosenheim), W. Dausch (Fritzlar), H.-Ch. Deyda (Verden), A. Dietze-Richter (Radebeul), H.-D. Düngen (Berlin), R. Erbel (Essen), E. Fleck (Berlin), E. Frohburg (Freiberg), Ch. Gerischer (Berlin), O. Hagen (Bochum), F. Hartmannn (Lübeck), J. Heckmann (Bad Münster am Stein-Ebernburg), J. Honneth (Essen), H.-U. Kreider-Stempfle (Bad Tölz), I. Kruck (Ludwigsburg), H. Leinberger (Erbach), A. Mügge (Bochum), E. Müller (Freiberg), M. Oeff (Brandenburg), B. Pieske (Göttingen), N. Proskynitopoulos (Nienburg), H.-E. Sarnighausen (Lüneburg), Ch. Schmitt (Berlin), K.-H. Schöll (Trier), R.-J. Schulz (Berlin), H.-Y. Sohn (München), R. Strasser (Dresden), H. Streich (Neubrandenburg), J. Taggeselle (Markkleeberg), P. Weismüller (Hagen), S. Zimmermann (Dippoldiswalde), R. Zotz (Herford). Montenegro (18) A. Bošković (Podgorica), B. Knežević (Podgorica). Serbia (535) S. Apostolović (Nis), S. Ćatović (Novi Pazar), V. Čelić (Belgrade), S. Dimković (Belgrade), D. Košević (Belgrade), M. Krotin (Belgrade), M. Miloradović (Kragujevac), J. Milosavljević (Jagodina), Z. Naumović (Sabac), M. Pavlović (Niš), V. Petrović (Vršac), B. Putniković (Belgrade), D. Sakač (Sremska Kamenica), N. Trifunović (Užice), Z. Vasiljević (Belgrade), S. Živković (Ćuprija). Slovenia (30) M. Lainščak (Golnik), D. Kovač (Murska Sobota), A. Marolt (Slovenj Gradec), N. Škrabl-Moćnik (Celje).
References
- 1.McMurray JJ, Stewart S. Heart failure: epidemiology, aetiology, and prognosis of heart failure. Heart. 2000;83:596–602. doi: 10.1136/heart.83.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 3.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in-congestive heart failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 4.Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann-Zalan I, DeMets DL. Effect of Carvedilol on the Morbidity of Patients with Severe Chronic Heart Failure: Results of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) study. Circulation. 2002;106:2194–2199. doi: 10.1161/01.cir.0000035653.72855.bf. [DOI] [PubMed] [Google Scholar]
- 5.Calvert MJ, Shankar A, McManus RJ, Ryan R, Freemantle N. Evaluation of the management of heart failure in primary care. Fam Pract. 2009;26:145–153. doi: 10.1093/fampra/cmn105. [DOI] [PubMed] [Google Scholar]
- 6.Komajda M, Follath F, Swedberg K, Cleland J, Aguilar JC, Cohen-Solal A, Dietz R, Gavazzi A, Van Gilst WH, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, Widimsky J, Freemantle N, Eastaugh J, Mason J Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology. The Euroheart Failure Survey programme—a survey on the quality of care among patients with heart failure in Europe: Part 2: treatment. Eur Heart J. 2003;24:464–474. doi: 10.1016/s0195-668x(02)00700-5. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, van Gilst WH, Widimsky J, Freemantle N, Eastaugh J, Mason J Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology. The EuroHeart Failure Survey programme—a survey on the quality of care among patients with heart failure in Europe: Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–463. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 8.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A Carvedilol Or Metoprolol European Trial Investigators . Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol or metoprolol European trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 9.Hjalmarson Å, Waagstein F. COMET: a proposed mechanism of action to explain the results and concerns about dose. Lancet. 2003;362:1077. doi: 10.1016/S0140-6736(03)14422-4. [DOI] [PubMed] [Google Scholar]
- 10.Krum H, Hill J, Fruhwald F, Sharpe C, Abraham G, Zhu JR, Poy C, Kragten JA. Tolerability of beta-blockers in elderly patients with chronic heart failure: the COLA II study. Eur J Heart Fail. 2006;8:302–307. doi: 10.1016/j.ejheart.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Bergström A, Andersson B, Edner M, Nylander E, Persson H, Dahlström U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-echocardiographic study (SWEDIC) Eur J Heart Fail. 2004;6:453–461. doi: 10.1016/j.ejheart.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in Collaboration With the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 13.Düngen HD, Apostolović S, Inkrot S, Tahirović E, Krackhardt F, Pavlović M, Putniković B, Lainscak M, Gelbrich G, Edelmann F, Wachter R, Eschenhagen T, Waagstein F, Follath F, Rauchhaus M, Haverkamp W, Osterziel KJ, Dietz R CIBIS-ELD Investigators . Bisoprolol vs. carvedilol in elderly patients with heart failure: rationale and design of the CIBIS-ELD trial. Clin Res Cardiol. 2008;97:578–586. doi: 10.1007/s00392-008-0681-6. [DOI] [PubMed] [Google Scholar]
- 14.Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, Hoes A, Jaarsma T, Korewicki J, Lévy S, Linde C, Lopez-Sendon JL, Nieminen MS, Piérard L, Remme WJ Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–1140. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 15.Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–894. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 16.McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–794. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 17.Stoschitzky K, Donnerer J, Klein W, Koshucharova G, Kraxner W, Lercher P, Maier R, Watzinger N, Zweiker R. Different effects of propranolol, bisoprolol, carvedilol and doxazosin on heart rate, blood pressure, and plasma concentrations of epinephrine and norepinephrine. J Clin Basic Cardiol. 2003;6:69–71. [Google Scholar]
- 18.Willenheimer R, van Veldhuisen DJ, Silke B, Erdmann E, Follath F, Krum H, Ponikowski P, Skene A, van de Ven L, Verkenne P, Lechat P CIBIS III Investigators. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by Enalapril, as compared with the opposite sequence: results of the randomized cardiac insufficiency bisoprolol study (CIBIS) III. Circulation. 2005;112:2426–2435. doi: 10.1161/CIRCULATIONAHA.105.582320. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins NM, MacDonald MR, Petrie MC, Chalmers GW, Carter R, Dunn FG, McMurray JJ. Bisoprolol in patients with heart failure and moderate to severe chronic obstructive pulmonary disease: a randomized controlled trial. Eur J Heart Fail. 2009;11:684–690. doi: 10.1093/eurjhf/hfp066. [DOI] [PubMed] [Google Scholar]
- 20.Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler-Soler J, Tavazzi L, Spinarova L, Toman J, Böhm M, Anker SD, Thompson SG, Poole-Wilson PA SENIORS Investigators. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 21.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL ESC Committee for Practice Guidelines. Task Force for Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of European Society of Cardiology. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European society of intensive care medicine (ESICM) Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 22.Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, Hershberger RE, Kubo SH, Narahara KA, Ingersoll H, Krueger S, Young S, Shusterman N. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation. 1996;94:2807–2816. doi: 10.1161/01.cir.94.11.2807. [DOI] [PubMed] [Google Scholar]
- 23.Dobre D, van Veldhuisen DJ, Mordenti G, Vintila M, Haaijer-Ruskamp FM, Coats AJ, Poole-Wilson PA, Flather MD SENIORS Investigators. Tolerability and dose-related effects of nebivolol in elderly patients with heart failure: data from the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure (SENIORS) trial. Am Heart J. 2007;154:109–115. doi: 10.1016/j.ahj.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Metra M, Nodari S, Dei Cas L. Beta-blockade in heart failure: selective versus nonselective agents. Am J Cardiovasc Drugs. 2001;1:3–14. doi: 10.2165/00129784-200101010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Komajda M, Anker SD, Charlesworth A, Okonko D, Metra M, Di Lenarda A, Remme W, Moullet C, Swedberg K, Cleland JG, Poole-Wilson PA. The impact of new onset anaemia on morbidity and mortality in chronic heart failure: results from COMET. Eur Heart J. 2006;27:1440–1446. doi: 10.1093/eurheartj/ehl012. [DOI] [PubMed] [Google Scholar]
- 26.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 27.van Veldhuisen DJ, Cohen-Solal A, Böhm M, Anker SD, Babalis D, Roughton M, Coats AJ, Poole-Wilson PA, Flather MD SENIORS Investigators. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data From SENIORS (Study of effects of Nebivolol Intervention on outcomes and rehospitalization in seniors with heart failure) J Am Coll Cardiol. 2009;53(23):2159–2161. doi: 10.1016/j.jacc.2009.02.046. [DOI] [PubMed] [Google Scholar]