BCG Lymphadenitis and Recurrent Oral Candidiasis in an Infant with a New Mutation Leading to Interleukin-12 Receptor Beta-1 Deficiency (original) (raw)
. Author manuscript; available in PMC: 2012 May 29.
Published in final edited form as: J Investig Allergol Clin Immunol. 2011;21(5):401–404.
Abstract
Mendelian susceptibility to mycobacterial diseases (MSMD) is a rare syndrome characterized by the predisposition to infections caused by weakly virulent mycobacteria, such as bacille Calmette-Guérin (BCG) vaccines and environmental mycobacteria. Salmonellosis has been reported in almost half of affected patients. Patients are also vulnerable to Mycobacterium tuberculosis. Various other infectious diseases occurred, albeit each rarely, but mucocutaneous candidiasis have been reported in more patients. Interleukin-12 receptor β1 (IL-12Rβ1) deficiency is the most frequent genetic cause of MSMD. Here, we describe an infant with a single episode of BCG lymphadenitis (BCG-itis) who also suffered from recurrent oral candidiasis. Genetic analysis revealed a new homozygous mutation (64+1G>T) in the IL12RB1 gene that caused complete IL-12Rβ1 deficiency. IL12Rβ1 deficiency should be considered in patients with BCG infection, even a single episode of BCG-itis or recurrent mucocutaneous candidiasis. Nevertheless, awareness should be increased in countries where BCG vaccination is performed.
Keywords: BCG lymphadenitis, IL-12Rβ1 deficiency, Mendelian susceptibility to mycobacterial diseases, Mucocutaneous candidiasis
Introduction
Mendelian susceptibility to mycobacterial disease (MSMD) (OMIM 209,950) is a rare congenital syndrome conferring a predisposition to infections caused by weakly virulent mycobacteria, such as bacille Calmette-Guérin (BCG) vaccines and nontuberculous environmental mycobacteria, in otherwise healthy individuals [1]. Patients are also susceptible to Mycobacterium tuberculosis [2]. Severe disease caused by non-typhoidal Salmonella serovars is also observed in nearly half of the cases [3]. The study of patients with MSMD has demonstrated the key role of the IL-12/IFN-γ axis in immunity to mycobacteria in humans [4]. There are five disease causing autosomal genes, including IFNGR1, IFNGR2, STAT1, IL12B, and IL12RB1, and one X-linked gene, NEMO, and allelic heterogeneity accounts for the existence of 13 defined disorders, all of which result in impaired IFN-γ mediated immunity [2, 3]. IL-12Rβ1 deficiency is the most frequent known genetic etiology of MSMD, found in about 40% of the patients with a known genetic defect [3]. Here we present an infant with BCG lymphadenitis (BCG-itis) and recurrent oral candidiasis due to complete IL-12Rβ1 deficiency caused by a new homozygous mutation (64+1G>T) in the IL12RB1 gene.
Case Description
A 7-month-old boy presented with left axillary lymphadenopathy persisting for two to three weeks. His past medical history was unremarkable with the exception of recurrent oral candidiasis. His parents were first-degree relatives. Family history was negative for genetic disorders or tuberculosis. He was vaccinated with BCG at the age of three months. On admission, routine physical examination was unremarkable with the exception of lymphadenopathy with a diameter of 5×5 cm in the left axillary region. There was a BCG vaccine scar on his left shoulder.
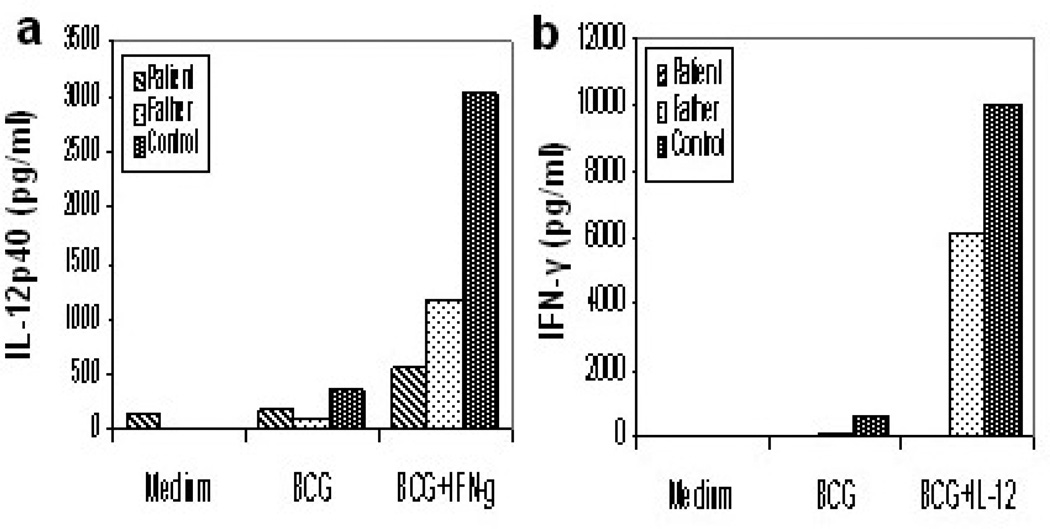
The tuberculin skin test was positive (15×15 mm). Thoracic computed tomography and abdominal ultrasonography were normal. The lymph node biopsy was performed and pathohistological examination showed a widespread infiltration of lymphohistiocytes. A few small granulomas without caseous necrosis were present. Ziehl Neelsen stain for acid-fast bacilli showed the presence of multibacillar histiocytes. M. tuberculosis complex PCR and culture were negative. Routine immunological evaluation including serum IgG, IgA, IgM, IgE, peripheral blood lymphocyte subsets and phagocyte respiratory burst assay were normal. Whole blood samples were in vitro activated with BCG, BCG plus IL-12 and BCG plus IFN-γ as described previously [4]. IL12p40 production was increased after stimulation with BCG plus IFN-γ (Figure, a). In contrast, IFN-γ production to BCG plus IL-12 was blunted (Figure, b). IL-12Rβ1 molecule expression on activated T cells was found to be negative by flow cytometric analysis (data not shown). DNA sequencing of the IL12RB1 gene revealed a new homozygous mutation (64+1G>T). The patient’s parents and brother were heterozgyous for this mutation and healthy. So, following the genetic analysis, the healthy brother was vaccinated with BCG at the age of three months.
Figure. Impaired IFN-γ production.
a, Whole blood samples were stimulated with BCG and BCG plus IFN-γ. Secreted IL12p40 was measured by ELISA.b, Whole blood samples were stimulated with BCG and BCG plus IL-12. Secreted IFN-γ was measured by ELISA.
The patient was treated with isoniazide (INH), rifampicin (RIF) and ethambutol for the first two months and then treatment continued with INH and RIF for another six months. Drainage of purulent material from the operation wound discontinued at the fourth month of the treatment. Eight months passed since the end of treatment and he is doing well with the exception of oral candidiasis.
Discussion
We reported an infant with BCG-itis and recurrent oral candidiasis due to complete IL-12Rβ1 deficiency caused by a new homozygous mutation (64+1G>T) in the IL12RB1 gene.
IL-12 depended IFN-γ secretion is essential to the control of intracellular pathogens, especially mycobacteria. IL-12 and IL-23 produced by macrophages and dendritic cells, bind to their receptors consisting of IL-12Rβ1 and IL-12Rβ2 that are expressed on natural killer cells and T lymphocytes. This stimulation causes IFN-γ production. IFN-γ binds to IFN-γ receptor and leads to phosphorylation of a signal transducer and activator of transcription type 1 (STAT-1). Defects in any pathway of the IL-12/IFN-γ axis cause susceptibility to mycobacteria or other infectious pathogens in humans [5, 6, 7]. IL-12Rβ1 deficiency is the most common form of MSMD. It has been demonstrated in a total of 141 affected patients from 30 country and 54 mutant alleles have been identified [7]. IL12Rβ1 deficiency which is a relatively common disease in Turkey is not due to a single mutation [6, 8–11].
Clinical features of these patients are not restricted to a predisposition to mycobacterial disease. Salmonellosis has been reported in almost half of affected patients, and significant number of patients present with isolated salmonellosis [1, 7, 11]. De Beaucoudrey et al found that 84 out of the 108 patients with IL-12Rβ1 deficiency vaccinated with BCG developed BCG disease, which presented as a localized disease in 17 patients. [7]. Disseminated tuberculosis has been reported in several patients [7, 10]. Recurrent oral candidiasis was also observed in our patient. Mucocutaneous candidiasis has been reported that 32 (24%) of the 132 symptomatic patients by de Beaucoudrey et al [7]. The clinical features of candidiasis in IL-12Rβ1 deficient patients will be reported elsewhere (Rodriguez-Gallego et al, unpublished data) [12]. Despite the high frequency of mucocutaneous candidiasis, disseminated fungal infections such as histoplasmosis and paracoccidioidomycosis have been diagnosed in a single patient [7, 13]. Other infectious diseases (toxoplasmosis, nocardiosis, and leishmaniasis) occured mostly in one patient, but klebsiellosis was also reported in five patients. [7, 14]. In addition, IL-12Rβ1 deficiency, and other disorders of the IL-12/IFN-γ axis might even predispose to cancer [15, 16]. So MSMD, and particularly IL-12Rβ1 deficiency, may be more heterogeneous than previously thought.
In most cases, patients are treated conservatively with prolonged antibiotic treatment, and in those who do not respond well, additional recombinant IFN-γ treatment has been shown to be effective [1, 9]. Our patient responded well to antituberculosis treatment and did not require IFN-γ therapy. Abdominal surgery has often been required to remove splenic and mesenteric lesions, which seem to be poorly accessible to antibiotics and IFN-γ [3]. 15% of patients with mutations in IL12B and IL12RB1 were found to have some kind of a mycobacterial recurrence, probably due to the reactivation of the initial mycobacterial agent, in most cases, upon premature treatment cessation [17].
The mortality rate among symptomatic patients was 32%, which is somewhat higher than the value previously reported (15%) and with a less favourable outcome than previously thought [2, 7].
In conclusion, IL12Rβ1 deficiency should be considered in patients with BCG infection, even a single episode of BCG-itis. In addition, patients with recurrent mucocutaneous candidiasis should also be evaluated for IL12Rβ1 deficiency. Nevertheless, awareness should be increased in countries where BCG vaccination is performed.
Footnotes
The authors declare that they have no conflict of interest and financial relationship with any organization or drug industry.
References
- 1.Al-Muhsen S, Casanova JL. The genetic heterogeneity of mendelian susceptibility to mycobacterial diseases. J Allergy Clin Immunol. 2008;122:1043–1051. doi: 10.1016/j.jaci.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 2.Fieschi C, Dupuis S, Catherinot E, Feinberg J, Bustamante J, Breiman A, Altare F, Baretto R, Le Deist F, Kayal S, Koch H, Richter D, Brezina M, Aksu G, Wood P, Al-Jumaah S, Raspall M, Da Silva Duarte AJ, Tuerlinckx D, Virelizier JL, Fischer A, Enright A, Bernhöft J, Cleary AM, Vermylen C, Rodriguez-Gallego C, Davies G, Blütters-Sawatzki R, Siegrist CA, Ehlayel MS, Novelli V, Haas WH, Levy J, Freihorst J, Al-Hajjar S, Nadal D, De Moraes Vasconcelos D, Jeppsson O, Kutukculer N, Frecerova K, Caragol I, Lammas D, Kumararatne DS, Abel L, Casanova JL. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor beta1 deficiency: medical and immunological implications. J Exp Med. 2003;197:527–535. doi: 10.1084/jem.20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, Jouanguy E, Boisson-Dupuis S, Fieschi C, Picard C, Casanova JL. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18:347–361. doi: 10.1016/j.smim.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Feinberg J, Fieschi C, Doffinger R, Feinberg M, Leclerc T, Boisson-Dupuis S, Picard C, Bustamante J, Chapgier A, Filipe-Santos O, Ku CL, de Beaucoudrey L, Reichenbach J, Antoni G, Baldé R, Alcaïs A, Casanova JL. Bacillus Calmette Guerin triggers the IL-12/IFN-gamma axis by an IRAK-4- and NEMO-dependent, non-cognate interaction between monocytes, NK, and T lymphocytes. Eur J Immunol. 2004;34:3276–3284. doi: 10.1002/eji.200425221. [DOI] [PubMed] [Google Scholar]
- 5.Casanova JL, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol. 2002;20:581–620. doi: 10.1146/annurev.immunol.20.081501.125851. [DOI] [PubMed] [Google Scholar]
- 6.Tanir G, Dogu F, Tuygun N, Ikinciogullari A, Aytekin C, Aydemir C, Yuksek M, Boduroglu EC, de Beaucoudrey L, Fieschi C, Feinberg J, Casanova JL, Babacan E. Complete deficiency of the IL-12 receptor beta1 chain: three unrelated Turkish children with unusual clinical features. Eur J Pediatr. 2006;165:415–417. doi: 10.1007/s00431-005-0078-8. [DOI] [PubMed] [Google Scholar]
- 7.de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J, Al Muhsen S, Janniere L, Rose Y, Desurenaim M, Kong XF, Filipe-Santos O, Chapgier A, Picard C, Fischer A, Dogu F, Ikinciogullari A, Tanir G, Al Hajjar S, Al Jumaah S, Frayha HH, AlSum Z, Al Ajaji S, Alangari A, Al-Ghonaium A, Adimi P, Mansouri D, Ben Mustapha I, Yancoski J, Garty BZ, Rodriguez-Gallego C, Caragol I, Kutukculer N, Kumararatne DS, Patel S, Doffinger R, Exley A, Jeppsson O, Reichenbach J, Nadal D, Boyko Y, Pietrucha B, Anderson S, Levin M, Schandene L, Schepers K, Efira A, Mascart F, Matsuoka M, Sakai T, Siegrist CA, Frecerova K, Bluetters-Sawatzki R, Bernhoft J, Freihorst J, Baumann U, Richter D, Haerynck F, De Baets F, Novelli V, Lammas D, Vermylen C, Tuerlinckx D, Nieuwhof C, Pac M, Haas WH, Muller-Fleckenstein I, Fleckenstein B, Levy J, Raj R, Cleary Cohen A, Lewis DB, Holland S, Yang KD, Wang X, Wang W, Jiang L, Yang X, Zhu C, Xie Y, Pui Wah Lee P, Chan KW, Chen TX, Castro G, Ivelisse N, Codoceo A, King A, Bezrodnik L, Di Giovani D, Gaillard MI, de Moraes-Vasconcelos D, Grumach AS, da Silva Duarte AJ, Aldana R, Espinosa-Rosales FJ, Bejaoui M, Bousfiha AA, El Baghdadi J, Ozbek N, Aksu G, Keser M, Somer A, Hatipoglu N, Aydogmus C, Asilsoy S, Camcioglu Y, Gulle S, Ozgur TT, Ozen M, Oleastro M, Bernasconi A, Mamishi S, Parvaneh N, Rosenzweig S, Barbouche R, Pedraza S, Lau YL, Ehlayel MS, Fieschi C, Abel L, Sanal O, Casanova JL. Revisiting human IL-12Rβ1 deficiency: a survey of 141 patients from 30 countries. Medicine (Baltimore) 2010;89:381–402. doi: 10.1097/MD.0b013e3181fdd832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aksu G, Tirpan C, Cavusoglu C, Soydan S, Altare F, Casanova JL, Kutukculer N. Mycobacterium fortuitum-chelonae complex infection in a child with complete interleukin-12 receptor beta 1 deficiency. Pediatr Infect Dis J. 2001;20:551–553. doi: 10.1097/00006454-200105000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Asilsoy S, Bilgili G, Turul T, Dizdarer C, Kalkan S, Yasli H, Can D, Genel F, Sanal O. Interleukin-12/-23 receptor beta 1 deficiency in an infant with draining BCG lymphadenitis. Pediatr Int. 2009;51:310–312. doi: 10.1111/j.1442-200X.2009.02818.x. [DOI] [PubMed] [Google Scholar]
- 10.Ozbek N, Fieschi C, Yilmaz BT, de Beaucoudrey L, Demirhan B, Feinberg J, Bikmaz YE, Casanova JL. Interleukin-12 receptor beta 1 chain deficiency in a child with disseminated tuberculosis. Clin Infect Dis. 2005;40:e55–e58. doi: 10.1086/427879. [DOI] [PubMed] [Google Scholar]
- 11.Sanal O, Turul T, De Boer T, Van de Vosse E, Yalcin I, Tezcan I, Sun C, Memis L, Ottenhoff TH, Ersoy F. Presentation of interleukin-12/-23 receptor beta1 deficiency with various clinical symptoms of Salmonella infections. J Clin Immunol. 2006;26:1–6. doi: 10.1007/s10875-006-7830-3. [DOI] [PubMed] [Google Scholar]
- 12.Pedraza-Sánchez S, Herrera-Barrios MT, Aldana-Vergara R, Neumann-Ordoñez M, González-Hernández Y, Sada-Díaz E, de Beaucoudrey L, Casanova JL, Torres-Rojas M. Bacille Calmette-Guérin infection and disease with fatal outcome associated with a point mutation in the interleukin-12/interleukin-23 receptor beta-1 chain in two Mexican families. Int J Infect Dis. 2010 doi: 10.1016/j.ijid.2009.11.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Moraes-Vasconcelos D, Grumach AS, Yamaguti A, Andrade ME, Fieschi C, de Beaucoudrey L, Casanova JL, Duarte AJ. Paracoccidioides brasiliensis disseminated disease in a patient with inherited deficiency in the beta1 subunit of the interleukin (IL)-12/IL-23 receptor. Clin Infect Dis. 2005;41:e31–e37. doi: 10.1086/432119. [DOI] [PubMed] [Google Scholar]
- 14.Sanal O, Turkkani G, Gumruk F, Yel L, Secmeer G, Tezcan I, Kara A, Ersoy F. A case of interleukin-12 receptor beta-1 deficiency with recurrent leishmaniasis. Pediatr Infect Dis J. 2007;26:366–368. doi: 10.1097/01.inf.0000258696.64507.0f. [DOI] [PubMed] [Google Scholar]
- 15.Cárdenes M, Angel-Moreno A, Fieschi C, Sologuren I, Colino E, Molinés A, García-Laorden MI, Campos-Herrero MI, Andújar-Sánchez M, Casanova JL, Rodríguez-Gallego C. Oesophageal squamous cell carcinoma in a young adult with IL-12R beta 1 deficiency. J Med Genet. 2010;47:635–637. doi: 10.1136/jmg.2009.071910. [DOI] [PubMed] [Google Scholar]
- 16.Toyoda H, Ido M, Nakanishi K, Nakano T, Kamiya H, Matsumine A, Uchida A, Mizutani H, de Beaucoudrey L, Vogt G, Boisson-Dupuis S, Bustamante J, Casanova JL, Komada Y. Multiple cutaneous squamous cell carcinomas in a patient with interferon gamma receptor 2 (IFN gamma R2) deficiency. J Med Genet. 2010;47:631–634. doi: 10.1136/jmg.2009.072108. [DOI] [PubMed] [Google Scholar]
- 17.Bousfiha A, Picard C, Boisson-Dupuis S, Zhang SY, Bustamante J, Puel A, Jouanguy E, Ailal F, El-Baghdadi J, Abel L, Casanova JL. Primary immunodeficiencies of protective immunity to primary infections. Clin Immunol. 2010;135:204–209. doi: 10.1016/j.clim.2010.02.001. [DOI] [PubMed] [Google Scholar]