Double-core-hole spectroscopy for chemical analysis with an intense X-ray femtosecond laser (original) (raw)
Abstract
Theory predicts that double-core-hole (DCH) spectroscopy can provide a new powerful means of differentiating between similar chemical systems with a sensitivity not hitherto possible. Although DCH ionization on a single site in molecules was recently measured with double- and single-photon absorption, double-core holes with single vacancies on two different sites, allowing unambiguous chemical analysis, have remained elusive. Here we report that direct observation of double-core holes with single vacancies on two different sites produced via sequential two-photon absorption, using short, intense X-ray pulses from the Linac Coherent Light Source free-electron laser and compare it with theoretical modeling. The observation of DCH states, which exhibit a unique signature, and agreement with theory proves the feasibility of the method. Our findings exploit the ultrashort pulse duration of the free-electron laser to eject two core electrons on a time scale comparable to that of Auger decay and demonstrate possible future X-ray control of physical inner-shell processes.
Keywords: multi-photon ionization, ultrafast, two-photon spectroscopy
Advances in the understanding of the structure and dynamics of matter using lasers (1) and synchrotron radiation (2) were made possible by X-ray photoelectron spectroscopy based on single-core-hole (SCH) ionization. However, this widely used technique has limitations when it comes to investigating molecules with nearly equivalent atoms such as carbon, oxygen, or nitrogen atoms in hydrocarbons (3), aromatic molecules (4), or nucleobases such as uracil (5).
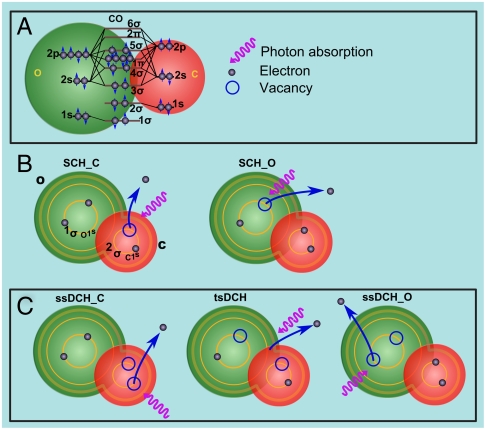
Double-core-hole (DCH) states are not easy to produce because of their short lifetime but they can remove such ambiguity. As schematically shown for CO in Fig. 1, in a molecular system, two types of DCHs states are expected (3); double-core-hole ionization on a single site (ssDCH) and double-core-hole ionization with single vacancies on two different sites (tsDCH). About two decades ago, Cederbaum et al. (3) demonstrated by calculating SCH and DCH binding energies that tsDCH spectra should show an enhanced chemical shift, allowing site-specific identification of nearly equivalent atoms with different chemical environments. Cederbaum et al. (3) predicted (1) that the ability to distinguish binding energies associated with tsDCH will provide a sensitive probe of the local chemical environment of the atoms in molecules. This is a substantial development of the general principle of electron spectroscopy for chemical analysis (ESCA), which until now has been mostly limited to SCH ionization.
Fig. 1.
Schematic illustration of (A) the electronic structure of the CO molecule, (B) the SCH ionization at the C K-edge (SCH_C) and the SCH ionization at the O K-edge (SCH_O), and (C) the ssDCH ionization at the C K-edge (ssDCH_C), the ssDCH ionization of the O K-edge (ssDCH_O) and the tsDCH ionization.
Santra et al. (4) extended the original idea of Cederbaum et al. (3) by calculating the inner-shell double ionization spectrum of paraaminophenol that was found to be more sensitive to chemical effects than the inner-shell single ionization spectrum. They suggested (4) that intense, ultrashort pulses could allow such investigations via a nonlinear two-photon absorption process. Such experimental observations were not possible until the recent (6, 7) advent of ultrafast X-ray free-electron lasers (FELs), which are revolutionizing spectroscopy (8–12) and imaging (13).
Here, we report measurements of tsDCH using CO as the showcase, and support them by our calculations (14). Although recently ssDCH in molecules were measured with the Linac Coherent Light Source (LCLS) (8, 9) FEL, and synchrotrons (15, 16), tsDCH spectra have remained elusive. The work described here demonstrates an important enhancement of ESCA using FELs such as the LCLS (6) and illustrates unambiguously the ability for X-ray control of inner-shell processes. The breakthrough in our investigation is the unique capability to manipulate inner-shell ionization processes, in particular to control femtosecond Auger decay processes. This population control is achieved by tuning the X-ray pulse intensity and duration. We chose intense X-ray pulses with duration comparable to the intrinsic Auger decay time of the atoms constituting the molecular species. The intensity of the pulse ensures that the fluence is high enough to allow two core electrons to each absorb a photon to create two core holes. By choosing the ultrashort X-ray pulse duration to be shorter than the typical SCH state lifetime, the Auger cascade decay is suppressed and the formation of DCH versus SCH states is, thus, actively controlled. We characterize the production and decay of these states by photoelectron and Auger-electron spectroscopy, and demonstrate the practicability of the method for future chemical applications.
The double ionization potential of tsDCH states includes structural information (i.e., Coulomb repulsion by core holes at two sites of known separation) and chemical information (i.e., the interatomic relaxation energy specific to the chemical environment within the molecule). The latter effect is of greatest interest in DCH spectroscopy (14).
Results and Discussion
Observation of tsDCH and ssDCH States in CO Using Photoelectron Spectroscopy.
The interaction of intense ultrashort X-ray pulses with matter is dominated by core-shell excitation and by the competition between Auger relaxation and multiple inner-shell ionization. To unravel the SCH and DCH ionization processes in molecular CO and its components C and O we use photoelectron spectroscopy (see Materials and Methods). The X-ray laser pulse duration is comparable to the core lifetimes of C and O (4–7 fs) and this allows a second photon to be absorbed by the singly charged ion, prepared by the first photon, before Auger decay sets in. The latter process would favor multiple valence ionization leading to high charge states and spectral congestion. To have sufficient X-ray laser pulse intensity to enable DCH ionization, we carried out the present measurements with approximately 10 fs pulse duration. Although very short, this time is still sufficient to allow other processes to occur, and the main goal of this work was to identify the tsDCH states among the background of other competing processes.
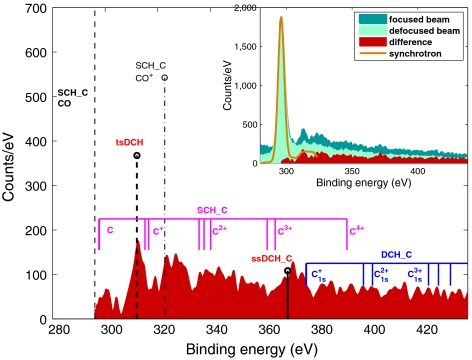
Fig. 2 reveals various ionization mechanisms. At binding energy higher than the SCH state, there is a continuum of emission, with several peaks, indicating that many processes are occurring. Fig. 2, Inset shows the photoelectron spectra recorded at 700 eV photon energy obtained with an electron time-of-flight (e-TOF) spectrometer aligned at 54.7° with respect to the polarization axis of the LCLS X-ray beam. The yellow (solid line) spectrum was taken with synchrotron X-rays* and is shown for comparison. The dark green filled spectrum corresponds to the measurement carried out with the highest X-ray laser pulse intensity, 1017 W/cm2 (focused; see Materials and Methods) whereas the light green filled spectrum corresponds to the weakest pulse intensity, 4.5 × 1014 W/cm2 (defocused). The red filled spectrum corresponds to the difference between the dark and light green spectra and this spectrum is shown separately in the main plot. We display the measurements in this fashion because the focused spectra are very rich in SCH and DCH structures. Subtracting the defocused from the focused spectrum emphasizes the DCH ionization processes, shown clearly in the red filled spectrum. Predicted electron energies for several SCH and DCH photo-processes are indicated in the figure by vertical lines.
Fig. 2.
Photoelectron spectra of carbon recorded at 700 eV photon energy, approximately 10 fs pulse duration. Calculated state energies and intensities are marked by vertical thick lines with head markers: tsDCH (dashed line), ssDCH (solid line), and SCH of CO+ with a valence hole (dash-dotted). The calculated energies for atomic ions are marked by a group of solid vertical lines without head markers. The spectra are calibrated to the known experimental binding energy value of the CO SCH that is marked by the thin dashed line (see text and Materials and Methods for details).
Although the X-ray beam is very intense, the absolute number of counts is not high, because the beam is focused to a very small spot. Thus the interaction volume does not contain a very large number of molecules, and in addition, the angular acceptance of the e-TOF is limited to a cone of ± 3 degrees.
The electron spectrum in Fig. 2 (red filled) reflects a sequence of ionization events produced by Auger decay and fragmentation of CO and its ions as well as multiple ionization of C and O fragments, in a single pulse. We observe:
- SCH ionization of neutral molecules and molecular ions with a valence hole. Whereas the SCH CO peak results from single-photon processes, the SCH CO+ peak results from sequential absorption of two photons, where the first photon ionizes a valence electron and the second photon ionizes a core electron. The calculated positions of these peaks are shown in the main panel of Fig. 2 as a thin black dotted-dashed line (CO+) and a thin black dashed line (CO).
- SCH ionization from atomic C_q_+ fragments (initial state with q valence holes). These peaks correspond to multiple photon processes. The initial atomic ions are prepared by fragmentation following either Auger decay or direct valence ionization or by further ionization/Auger decay of atomic ions. The calculated energies are indicated by the group of solid purple lines.
- DCH ionization from atomic C_q_+ (1 s) (with a single-core hole and the remaining holes in the valence shells). This process involves sequential ionization faster than Auger decay. The calculated energies of these multiply ionized states are shown by the group of solid blue lines. However, there are no peaks in the data corresponding to these or the above atomic ion lines: The intensity is observed in a continuum.
- DCH ionization of CO molecules. Photoelectrons from core-shell ionization of CO+(1 s)-1 with a single hole in the 1 s orbital of the C atom, ssDCH_C, and the second electron ejected from the same atom (as depicted in Fig. 1). These energies are indicated by the solid thick black line.
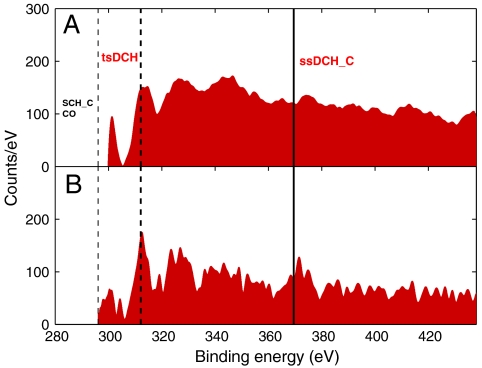
The most intense peak in Fig. 2 corresponds to the tsDCH with its energy indicated by the dashed thick black line. This was the goal of our investigation and the experimental finding agrees very well with our calculations as shown in Table 1. To check our tsDCH results, we used an alternative approach. Instead of subtracting defocused spectra from focused spectra, we carried out two measurements with focused beams and with photon energies above (700 eV) and below (500 eV) the O K-edge. The latter measurements allow all DCHs at 700 eV to be detected but only the ssDCH on C at 500 eV to be observed. The subtraction of the two spectra enables us to remove single-photon ionization events. This is shown in Fig. 3A, revealing consistent observation of the tsDCH when compared to Fig. 3B (which is the main panel of Fig. 2).
Table 1.
Calculated electron binding energies (14) and the corresponding experimental binding energies. BE indicates binding energy. The experimental values are calibrated by adjusting the BE_SCH value to a known value (14)
| Theory (3) | Experiment | |
|---|---|---|
| BE_SCH | 298.2 eV | 296.5 eV ± 0.5 eV |
| BE_DCHSS | 369.6 eV | 371.4 eV ± 3.5 eV |
| BE_DCHTS | 314.2 eV | 312.8 eV ± 0.7 eV |
| BE_diff_DCHSS_SCH | 71.4 eV | 74.9 eV ± 4.0 eV |
| BE_diff_DCHTS_SCH | 16.0 eV | 16.3 eV ± 1.2 eV |
Fig. 3.
Photoelectron spectra demonstrating consistent structures measured with two experimental methods. (A, Upper) Photoelectron spectrum resulting from the subtraction of spectra measured above and below the O-K-edge. (B, Lower) Represents Fig. 2 (red filled part) and is compared to the Upper panel to demonstrate the observation of tsDCH in the photoelectron spectrum.
We note the presence of a manifold of emission from atomic states in Fig. 2, an effect that was previously reported (8) for N2. This overlaps the continuous emission due to shake-off processes. Although our pulses are very short (about 10 fs), it has been established that nuclear motion can occur in molecules of similar molecular weight and Auger lifetimes, in particular O2 (17). This reflects the complexity of the dynamics on very short time scales, and shows that many more studies are needed to unravel the complexity of interaction of X-rays with matter on very short time scales.
Observation of tsDCH and ssDCH States of CO and Auger-Electron Spectroscopy.
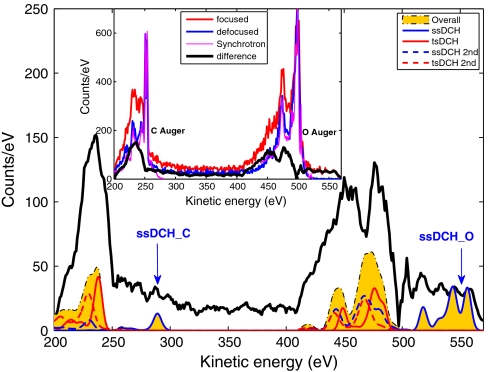
Fig. 4 shows Auger-electron spectra. The Inset displays the focused (red curve) and defocused (blue curve) spectra, along with synchrotron data (purple curve) for comparison*. The black curve shows their difference, also displayed in the main plot along with our calculation (14). The yellow shaded area represents the total calculation (all Auger and secondary Auger processes from both ssDCH and tsDCH states). Whereas the red solid and dashed curves represent tsDCH Auger and secondary Auger processes, respectively, the blue solid and dashed curves represent ssDCH Auger and secondary Auger processes. The Auger data are consistent with the presence of the tsDCH signal for C and O and also show clearly the ssDCH signal. The agreement between the measurements and the calculations is again very good, although our theory may underestimate the relative intensity of the tsDCH features. Auger decays of highly charged SCH states and molecular fragments may contribute to the discrepancy between the experimental and the theoretical results.
Fig. 4.
Auger spectra compared to theoretical DCH Auger spectra. Measurements taken at different X-ray intensities with a focused beam (red curve in Inset) and a defocused beam (blue curve in Inset). The purple curve was taken with synchrotron radiation and is shown for comparison. The difference of the two X-ray intensities is shown as the black curve. Theoretical Auger spectra are shown in the main panel: overall calculation (yellow shaded area); ssDCH (solid blue curve); tsDCH (solid red curve); ssDCH secondary processes (dashed blue curve); tsDCH secondary processes (dashed red curve).
Conclusion
The seminal prediction of Cederbaum et al. (3) has set in motion the idea to measure tsDCH and its realization, to achieve unambiguous chemical analysis. Our reported photo- and Auger-electron spectra demonstrate the feasibility of this unique method (4). The possibility to sensitively probe the chemical environment by measuring the binding energies associated with tsDCH, is now available, but our work also demonstrates that the processes occurring are very complex with many competing channels of ionization and dissociation.
Although there are only a few FEL sources operating in the world at the present time, we are on the threshold of a huge expansion of the number of such facilities. The Japan X-ray free-electron laser, as well as the European X-ray FEL in Hamburg will soon begin operation, and will be followed by more sources in Korea, China, Switzerland, Sweden, etc. It is therefore of utmost importance to establish which methods are viable at FEL sources. Here we have confirmed experimentally that tsDCH spectroscopy is feasible as theoretically predicted, and does indeed have the potential for wide application.
The reported measurements are accomplished with the unique ability to control the brightness and short pulse duration of the LCLS X-ray FEL. The physical phenomena are general and should be possible with any intense, X-ray femtosecond laser. Finally, these results will serve as a basis for producing selective configurations with single- or double-core holes on specific atoms in any molecule (for example, by varying pulse length), ultimately controlling how these states decay and how the molecule fragments.
Materials and Methods
Experiment.
The experiment was conducted using the atomic molecular and optical instrument (AMO) (11, 12, 18) and the LCLS (6) at the Stanford Linear Accelerator Center (SLAC) National Accelerator Laboratory. Five e-TOF spectrometers were used to record the spectra reported. The electron energy spectra were recorded by e-TOFs located at selected polar and azimuthal angles with respect to the polarization and propagation directions of the X-ray beam. For the present experiment, the LCLS produced pulsed X-ray laser radiation at 500 and 700(+/- ∼ 10) eV at a repetition rate of 60 Hz, with an estimated pulse energy of 0.007 mJ at 500 eV and 0.03 mJ at 700 eV, and with approximately 10 fs pulse duration. The molecular CO sample was introduced via a 100 μm pulsed nozzle into the interaction region where it crossed the LCLS photon beam. The photon energies were generated by 40 pC compressed electron bunches to acquire the approximately 7–10 fs pulse duration data. The pulse duration is difficult to determine for ultrashort pulses (< 10 fs) with compressed electron bunches (19). We used indirect estimates based on measurements of the peak electron current to derive X-ray pulse durations (6). Absolute pulse energy calibration is problematic, because the LCLS AMO chamber does not have a direct method to measure the energy. All pulse energy values given in this paper are the nominal accelerator values measured upstream from the beamline optics, and corrected by a factor of 0.15 to account for transmission losses by the beamline optics, as the transmission is estimated to be about 0.15 to 0.35 (11, 12). For the measurements in the “focused” conditions the LCLS photon beam was focused by Kirkpatrick–Baez (KB) mirrors to an elliptical profile with full-width half max (FWHM) lengths of the major and minor semi axes of 2.2 μm and 1.2 μm (20), respectively. The focus profile was determined during the commissioning phase of the LCLS by solid target depletion experiments (20). This focal size results in an X-ray beam intensity of about 1018 W/cm2 with 280 fs pulse duration and about 1017 W/cm2 with approximately 10 fs pulse duration. To produce the very short pulses (< 10 fs), only the very last 16–17 LCLS undulators, out of a total of 33 undulators were used. This configuration should not change the spot size based on accelerator data (6). For the measurements in the “defocused” condition, the curvature of the KB mirrors was adjusted to move the focus position 20 mm away from the optimal focal position, resulting in an intensity of approximately 4.5 × 1014 W/cm2 and an estimated elliptical profile with FWHM lengths of the major and minor semi axes of 37.5 µm and 20.6 µm respectively. The photoelectron spectra shown in Figs. 2 and 3 were measured at an e-TOF positioned in the plane perpendicular to the X-ray propagation, at the so called magic angle (where the signal intensity is independent of the angle of detection) to optimize the detection of photoelectrons. The Auger spectra shown in Fig. 4 were measured with e-TOF spectrometers positioned perpendicularly to the photon polarization direction to minimize the contribution of photoelectron peaks relative to Auger peaks. The synchrotron reference data shown in Figs. 2 (yellow solid line) and 4 (purple solid line) were taken at the ELETTRA synchrotron light source, Gas Phase beamline. The difference in resolution between the synchrotron-based spectrum and the FEL-based spectra is due to the LCLS photon energy jitter. We carried out the measurements in the focused and defocused conditions to directly take into account SCH ionization, including shake up and shake off (SUO) excitations. These effects proved to be problematic in a previous work (8) because using synchrotron-based ionization was not adequate in accounting for SUO or removing structures from highly charged states produced by FEL radiation. The subtraction of the focused-defocused spectra allowed us to generate cleaner spectra demonstrating unambiguously the production of the tsDCH. The experimental values listed in Table 1 were obtained by Gaussian fitting of the spectral peaks. The theoretical values are from ref. 14. We also carried out measurements with photon energies above and below the O K-edge to take into account the SCH/multiple ionization structures. The subtraction of the spectra led to the confirmation of the observation of the tsDCH because it appeared at the same position when we compared the below-above O K-edge (Fig. 3, Upper) with the focused-defocused method (Fig. 3, Lower or the main panel of Fig. 2). The spectra were normalized to the SCH peak.
Theory.
Single ionization potentials (IPs) and double-core-hole ionization potentials (DIPs) were calculated (14) using the complete active space self consistent field (CASSCF) method. The influence of the chemical environment on these DIPs was evaluated (14) for ssDCH and tsDCH states. Electron energies of the sequential Auger emissions following the double-core-hole creation were obtained from ab initio energies of triply charged states with one core hole and two valence holes and quadruply charged states with four valence holes. Auger intensities were estimated from the ab initio Auger rates and the initial-state populations obtained by solving rate equations. The IPs and DIPs of single- and double-core-hole states of CO were computed by means of the CASSCF method to evaluate the impact of the chemical environment on the respective ionization processes and provide guidance for the X-ray two-photon photoelectron (4) experiment reported here. Spectral distributions of the sequential Auger emission following the DCH creation were also computed as a guide in planning the present experiment and to interpret the present experimental spectra. For this purpose, we calculated the energies of triply charged states with one core hole and two valence holes (CVV) and quadruply charged states with four valence holes (VVVV), by the complete active space configuration interaction method with the orbitals obtained from the CASSCF calculations. The intensities of the Auger emissions from tsDCH and ssDCH states to the CVV states and subsequent Auger decay from CVV to VVVV states were evaluated from the atomic populations and the relative populations of the initial DCH states obtained by solving the rate equation where the FEL conditions were imposed.
Acknowledgments.
We thank all of the LCLS support staff, in particular J. C. Castagna, M. Swigger, and Dr. V. Feyer for assistance in acquiring reference data at Elettra. This work was funded by the US Department of Energy, Office of Science, Basic Energy Science, Chemical, Geosciences, and Biological Divisions. Funding from the Ministry for Infrastucture, Universities and Research, Italy (Grants FIRB-RBAP045JF2, FIRB-RBAP06AWK3), the Japan Ministry of Education, Culture, Sports, Science and Technology,and the Japan Science and Technology Agency, the Turku University Foundation, and the Swedish Research Council (VR) is gratefully acknowledged.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*This reference data was taken at the ELETTRA synchrotron light source, Gas Phase beamline using a hemispherical analyzer.
References
- 1.Hannaford P, editor. Femtosecond laser spectroscopy, Kluwer ser. prog. lasers. Dynamics in real-time: Progress over a decade. Annu Rev Phys Chem. 1990;41:15–60. [Google Scholar]
- 2.Siegbahn K. New York: North Holland; 1969. ESCA Applied to Free Molecules. [Google Scholar]
- 3.Cederbaum LS, Tarantelli F, Sgamellotti A. On double vacancies in the core. J Chem Phys. 1986;85:6513–6523. [Google Scholar]
- 4.Santra R, Kryzhevoi N, Cederbaum LS. X-ray two-photon photoelectron spectroscopy: a theoretical study of inner-shell spectra of the organic para-aminophenol molecule. Phys Rev Lett. 2009;103:013002–013005. doi: 10.1103/PhysRevLett.103.013002. [DOI] [PubMed] [Google Scholar]
- 5.Feyer V, et al. Tautomerism in cytosine and uracil: an experimental and theoretical core level spectroscopic study. J Phys Chem A. 2009;113:5736–5742. doi: 10.1021/jp900998a. [DOI] [PubMed] [Google Scholar]
- 6.Emma P, et al. Femtosecond and subfemtosecond X-ray pulses from a self-amplified spontaneous-emission-based free-electron laser. Phys Rev Lett. 2004;92:074801. doi: 10.1103/PhysRevLett.92.074801. LCLS facility. [DOI] [PubMed] [Google Scholar]
- 7.Ackermann W, et al. Operation of a free-electron laser from the extreme ultraviolet to the water window. Nat Photonics. 2007;1:336–342. [Google Scholar]
- 8.Fang L, et al. Double core hole production in N2: Beating the Auger clock. Phys Rev Lett. 2010;105:083004. doi: 10.1103/PhysRevLett.105.083005. [DOI] [PubMed] [Google Scholar]
- 9.Cryan J, et al. Auger electron angular distribution of double core hole states in the molecular reference frame. Phys Rev Lett. 2010;105:083004. doi: 10.1103/PhysRevLett.105.083004. [DOI] [PubMed] [Google Scholar]
- 10.Berrah N, et al. Non-linear processes in the interaction of atoms and molecules with intense EUV and X-ray fields from SASE free electron lasers (FELs) J Mod Opt. 2010;57:1015–1040. Topical Review. [Google Scholar]
- 11.Hoener M, et al. Ultra-intense X-ray induced ionization, dissociation and frustrated absorption in molecular nitrogen. Phys Rev Lett. 2010;104:253002. doi: 10.1103/PhysRevLett.104.253002. [DOI] [PubMed] [Google Scholar]
- 12.Young L, et al. Femtosecond electronic response of atoms to ultraintense X-rays. Nature. 2010;466:56–61. doi: 10.1038/nature09177. [DOI] [PubMed] [Google Scholar]
- 13.Chapman H, et al. Femtosecond X-ray protein nanocrystallography. Nature. 2011;470:73–78. doi: 10.1038/nature09750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tashiro M, et al. Molecular double core-hole electron spectroscopy for chemical analysis. J Chem Phys. 2010;132:184302. [Google Scholar]
- 15.Eland JDH, et al. Double core hole creation and subsequent Auger decay in NH3 and CH4 molecules. Phys Rev Lett. 2010;105:3005. doi: 10.1103/PhysRevLett.105.213005. [DOI] [PubMed] [Google Scholar]
- 16.Lablanquie P, et al. Properties of hollow molecules probed by single-photon double ionization. Phys Rev Lett. 2011;106:063003. doi: 10.1103/PhysRevLett.106.063003. [DOI] [PubMed] [Google Scholar]
- 17.Björneholm O, et al. Direct and indirect methods for studying the energetics and dynamics of the Auger Doppler effect in femtosecond ultra-fast dissociation. J Chem Phys. 2001;115:4139–4149. [Google Scholar]
- 18.Bozek JD. AMO instrumentation for the LCLS X-ray FEL. Eur Phys J Spec Top. 2009;169:129–132. [Google Scholar]
- 19.Ding Y, et al. Measurements and simulations of ultralow emittance and ultrashort electron beams in the Linac Coherent Light Source. Phys Rev Lett. 2009;102:254801. doi: 10.1103/PhysRevLett.102.254801. [DOI] [PubMed] [Google Scholar]
- 20.Chalupski J, et al. Comparing different approaches to characterization of focused X-ray laser beams. Nucl Instrum Methods Phys Res A. 2011;631:130–133. [Google Scholar]