Preferential reductions in intermuscular and visceral adipose tissue with exercise-induced weight loss compared with calorie restriction (original) (raw)
Abstract
Intermuscular adipose tissue (IMAT) and visceral adipose tissue (VAT) are associated with insulin resistance. We sought to determine whether exercise-induced weight loss (EX) results in greater reductions in IMAT and VAT compared with similar weight loss induced by calorie restriction (CR) and whether these changes are associated with improvements in glucoregulation. Sedentary men and women (50–60 yr; body mass index of 23.5–29.9 kg/m2) were randomized to 1 yr of CR (n = 17), EX (n = 16), or a control group (CON; n = 6). Bilateral thigh IMAT and VAT volumes were quantified using multi-slice magnetic resonance imaging. Insulin sensitivity index (ISI) was determined from oral glucose tolerance test glucose and insulin levels. Weight loss was comparable (P = 0.25) in the CR (−10.8 ± 1.4%) and EX groups (−8.3 ± 1.5%) and greater than in the control group (−2.0 ± 2.4%; P < 0.05). IMAT and VAT reductions were larger in the CR and EX groups than in the CON group (P ≤ 0.05). After controlling for differences in total fat mass change between the CR and EX groups, IMAT and VAT reductions were nearly twofold greater (P ≤ 0.05) in the EX group than in the CR group (IMAT: −45 ±5 vs. −25 ± 5 ml; VAT: −490 ± 64 vs. −267 ± 61 ml). In the EX group, the reductions in IMAT were correlated with increases in ISI (r = −0.71; P = 0.003), whereas in the CR group, VAT reductions were correlated with increases in ISI (r = −0.64; P = 0.006). In conclusion, calorie restriction and exercise-induced weight loss both decrease IMAT and VAT volumes. However, exercise appears to result in preferential reductions in these fat depots.
Keywords: calorie restriction, exercise, weight loss, insulin resistance, muscle fat infiltration
intermuscular adipose tissue (IMAT) is adipose tissue (i.e., adipocytes) that is interspersed between muscle cells and bundles and within the boundary of the whole muscle, as defined by the epimysium fascia. Cross-sectional studies have shown that IMAT is a strong determinant of insulin resistance, possibly as good a marker as visceral adipose tissue and better than whole-body fat mass (4). Intervention studies have shown that decreases in whole-body and visceral adipose tissue are associated with decreases in insulin resistance (13); however, it is not clear whether reductions in IMAT are associated with decreases in insulin resistance.
Calorie restriction (CR) and exercise (EX), when sufficient in volume (i.e., 60 min of daily vigorous cardiovascular exercise) both result in weight loss and improve glucoregulatory function (24, 25, 30). However, it is not known whether one of these approaches to weight loss leads to greater reductions in IMAT. Single-leg immobilization in humans has been shown to increase IMAT volume in as little as 4 wk (18). Furthermore, physical activity has been shown to protect against age-dependent accumulation of IMAT (15). Therefore, it is conceivable that, with similar reductions in whole-body fat mass, EX could result in greater reductions in IMAT compared with CR.
The primary purpose of the present study was to test the hypothesis that both CR- and EX-induced weight loss decrease IMAT volumes but that EX results in greater reductions when changes in whole-body fat mass are similar. Additionally, we had previously reported that EX does not result in a preferential loss of VAT compared with CR (23). However, in that report, we did not account for small but important differences between groups in total fat mass; therefore, a secondary purpose of the present study was to determine whether EX results in preferential VAT reductions after statistically adjusting for small differences in total body fat mass. The data reported in this article were obtained as part of an investigation [Phase I Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)] of the feasibility of long-term CR on potential markers of aging and risk factors for age-related disease. This paper reports site-specific findings from the Washington University site of the CALERIE studies. Other results from this study have been published previously (23, 27, 29–31).
METHODS
Participants.
Men and postmenopausal women aged 50–60 yr with a body mass index of 23.5–29.9 kg/m2 were recruited from the St. Louis, MO, metropolitan area. Potential subjects were excluded if they had a history of diabetes or fasting blood glucose value of ≥126 mg/dl or a resting blood pressure of >170 mmHg systolic or >100 mmHg diastolic. Other exclusion criteria included a history or clinical evidence of coronary artery disease, stroke, or lung disease as well as a recent history or evidence of malignancy. Subjects had to be nonsmokers and sedentary (defined as exercising <20 min/day, twice per week during the 6 mo before baseline testing). Participants were randomly assigned with stratification for sex to 12 mo of CR, EX, or to “healthy lifestyle” control group (CON). All participants gave their informed, written consent to participate in the study, which was approved by the Human Research Protection Committee at Washington University School of Medicine.
CR intervention.
The objective of the CR intervention was for participants to decrease calorie intake by 16% during the first 3 mo and by 20% during the remaining 9 mo. Prescriptions were based on total daily energy intake, which was assumed to equal total daily energy expenditure as measured by using doubly labeled water over two consecutive 2-wk assessment periods (methodology described below). Participants met with study dietitians once a week for body weight checks and for education on reducing portion sizes and replacing high-energy density foods with lower energy density foods. The participants kept diaries of their food intake on a regular basis during the study; information from the diaries was used by the dietitians as the basis for dietary counseling and for subjective monitoring of the subjects' progress.
EX intervention.
The EX intervention was designed to induce the same energy deficit as was induced by the CR intervention. Thus participants increased their energy expenditure from baseline by 16% during the first 3 mo and by 20% during the remaining 9 mo. Prescriptions were based on total daily energy expenditure assessments performed by using doubly labeled water as described below. Participants met with an exercise trainer on a weekly basis, and exercise energy expenditure goals were established at this time. Subjects were encouraged to use cardiovascular exercise as the most time-efficient approach to meeting the energy expenditure prescriptions. Participants were able to exercise at the site's facility or on their own with no specifications for frequency, duration, or intensity. Participants used heart rate monitors to measure and record daily and weekly gross exercise energy expenditure, exercise duration, and exercise heart rate. Heart rate monitor data were downloaded and reviewed each week by the exercise trainers to determine compliance. Exercise compliance data are presented in results below.
CON intervention.
Participants in the CON group had minimal contact with the research team and were not provided with a diet or exercise prescription. General information on a healthy diet was provided if requested by the participants, and free access to yoga classes was provided in the form of class passes/vouchers. Although requests for dietary advice and yoga class passes were not documented and/or quantified, requests for these benefits were minimal.
Doubly labeled water assessments of total energy expenditure.
Doubly labeled water (DLW) assessments were used to assess total energy expenditure (TEE) at baseline and served as the basis for the calculated goal for increases in TEE (i.e., 16–20% increase) in the EX group. Because the subjects were weight stable during the DLW assessment period, baseline energy intake was assumed to equal TEE, and this was used as the basis for calculating the goal for reductions in energy intake (i.e., 16–20% decrease) in the CR group. DLW assessments were made after two baseline urine samples were obtained and then an oral dose of DLW was administered (0.20 g of H218O, 0.12 g of 2H2O per kilogram of total body water); total body water was determined by using bioelectrical impedance. Urine samples were collected 4.5 h, 6 h, 7 days, and 14 days after dosage and were measured in duplicate for isotope abundance by using isotope ratio mass spectrometry.
Anthropometry and body composition.
Body weight was measured in duplicate in the morning after an overnight fast with the participant only wearing underwear and a hospital gown. Body weight for each assessment period (i.e., baseline and 12 mo) was calculated as the average of measures made on 3–5 different days. Height was measured to the nearest 0.1 cm at baseline. Fat mass, fat-free mass, and percentage body fat were measured with dual-energy X-ray absorptiometry (DXA) (Delphi W, Hologic, Waltham, MA; software version 11.2).
Abdominal visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) were measured with magnetic resonance imaging (MRI; Siemens, Iselin, NJ) using 10 serial 10-mm axial images beginning at L1 and moving downward. Images were analyzed with HIPPO software (version 1.3; Pisa, Italy) (21), and slice fat volumes were summed to give total visceral and subcutaneous adipose tissue volumes. Additional methodological details for MRI image acquisition and analysis for our laboratory have been previously reported (1, 23).
Thigh adipose tissue volumes were quantified by using MRI. Ten transverse images, 10 mm thick, were obtained just superior to the patella with no intersection gap between slices with a 1.5-T superconducting MRI instrument (Siemens, Iselin, NJ) and a T1-weighted pulse sequence. Subcutaneous adipose tissue and IMAT volumes of the thighs were quantified using Analyze Direct software (version 10.0; Mayo Clinic, Rochester, MN), which distinguishes muscle from adipose tissue based on pixel brightness. The technician manually designated the boundary between muscle and subcutaneous compartments by tracing the muscle fascia (epimysium) on the MR image; by using a similar method, the bone area was excluded from further analysis. Then, a bright white pixel known to be fat was designated as such by planting a “seed” in that region. The software then identified all other pixels of equal or greater brightness as fat and summed the pixel area as an estimate of two-dimensional fat area. As needed based on visual inspection of the selected fat regions, the technician adjusted the brightness threshold, thereby allowing the software to better capture and quantify fat regions and/or exclude nonfat regions. Cross-sectional areas for each slice were multiplied by slice thickness to obtain volume data. Volume data from the most proximal eight slices were summed to give thigh adipose tissue volumes in the 8-cm region of interest. All thigh adipose tissue volumes are reported as the sum of the right and left thighs.
Oral-glucose-tolerance test and fasting blood analyses.
Two-hour, 75-g oral glucose tolerance tests (OGTTs) were performed in the morning after a 12-h fast. Subjects were advised to refrain from exercise for ≥48 h before the OGTT. Additionally, subjects were given specific instructions by the study dietitian to consume at least 11 servings per day of carbohydrate-rich foods (i.e., fruits, breads, and/or dairy equivalent to ≥150 g/day carbohydrate) for 3 days before the OGTT but were otherwise advised to maintain their usual diet; small or moderated amounts of alcohol and caffeine were permitted if it was part of the subject's habitual diet. At 1600–1800 in the evening before the OGTT, the participants were admitted to the General Clinical Research Center and ate a standardized evening meal, which did not contain alcohol or caffeine. Thereafter, nothing was consumed except water until the OGTT commenced the next morning. Blood samples were acquired before and every 30 min after ingestion of the glucose beverage. Plasma glucose was measured with the glucose oxidase method (YSI Stat Plus; YSI, Yellow Springs, OH). Plasma insulin was measured with a double-antibody radioimmunoassay. Total areas under the curve (AUCs) were calculated for the plasma glucose and insulin responses using the trapezoidal rule. Insulin sensitivity index was calculated according to Matsuda and Defronzo (19) and was based on all measures of glucose and insulin made during the OGTT.
Statistical analyses.
Analyses were performed with data from all subjects who underwent both baseline and 12-mo thigh MRI scans. Baseline characteristics were compared by using ANOVA for quantitative measures and by using Fisher's exact tests for categorical variables. Study outcomes were analyzed by using analysis of covariance (ANCOVA) in which change scores (i.e., final minus initial values) were used as the dependent variable, study group was used as the independent variable, and baseline values were included as a covariate. Because there were small (but nonsignificant) differences in fat mass changes between the CR and EX groups (indicating differences between groups in terms of the year-long energy deficit), additional ANCOVA analyses were performed, which included only the CR and EX groups; in addition to the baseline values, fat mass change was included as a covariate in these analyses so that the effects of the CR and EX interventions on outcomes could be evaluated as if fat mass changes were identical. Furthermore, although our sample size was not optimal for including additional covariates, we also performed exploratory analyses that included age and sex as covariates. Post hoc paired comparisons were performed by using least significant difference (protected F test). Parametric correlation analyses were performed by using Pearson's correlations to determine relationships among outcomes that changed in response to the interventions; non-parametric Spearman rank correlation analyses were also performed, since it was questionable whether some of the data distributions were suitable for parametric analyses. Analyses were performed with SAS for Windows version 9.2. All statistical tests were two-tailed, and significance was accepted at P ≤ 0.05. Data are reported as means ± SE unless otherwise noted.
RESULTS
Participants.
Forty-eight participants started the study and were randomized to CR (n = 19), EX (n = 19), or CON (n = 10). Two subjects (CR, n = 1; EX, n = 1) dropped out before undergoing follow-up testing. Furthermore, seven subjects (CR, n = 1; EX, n = 2; CON, n = 4) were not included in the present report because of missing MRI data due to technical problems, participant refusal to undergo the MRI scan, or inability to perform MRI scans on participants with metallic implants or prostheses. Therefore, data from 39 subjects were included in the present analysis.
Sex and racial distributions were similar in all groups as presented in Table 1. On average, subjects in the exercise group were slightly older than in the other groups (Table 1). Average body mass index values were in the overweight range for all groups. Baseline values for whole-body and regional body composition did not differ between groups (Table 1).
Table 1.
Baseline characteristics of the Study Participants
| Calorie Restriction (n = 17) | Exercise (n = 16) | Control (n = 6) | P Value | |
|---|---|---|---|---|
| Sex | ||||
| Men | 6 (35%) | 6 (38%) | 2 (33%) | 1.00 |
| Women | 11 (65%) | 10 (62%) | 4 (67%) | |
| Race | ||||
| White | 16 (94%) | 15 (94%) | 5 (83%) | 0.54 |
| Other | 1 (6%) | 1 (6%) | 1 (17%) | |
| Age, yr | 55.0 ± 0.7 | 59.0 ± 0.7* | 55.7 ± 1.2 | 0.0009 |
| Weight, kg | 78.1 ± 2.5 | 76.8 ± 2.6 | 81.2 ± 4.2 | 0.68 |
| BMI, kg/m2 | 26.7 ± 0.5 | 26.8 ± 0.5 | 27.2 ± 0.8 | 0.88 |
| Total fat mass, kg | 26.8 ± 1.3 | 25.3 ± 1.4 | 25.9 ± 2.3 | 0.73 |
| Percent body fat, % | 35.1 ± 1.9 | 33.8 ± 2.0 | 33.1 ± 3.3 | 0.84 |
| Abdominal VAT, ml | 828 ± 136 | 1126 ± 140 | 1158 ± 228 | 0.25 |
| Abdominal SAT, ml | 2455 ± 134 | 1920 ± 138 | 1833 ± 225 | 0.34 |
| Thigh IMAT, ml | 108 ± 9 | 96 ± 9 | 77 ± 15 | 0.25 |
| Thigh subcutaneous fat, ml | 971 ± 90 | 845 ± 93 | 882 ± 152 | 0.62 |
| Thigh muscle volume, ml | 1233 ± 210 | 1582 ± 216 | 1301 ± 354 | 0.50 |
Compliance.
Compliance data have been previously reported in greater detail (23, 30). The average prescribed increase in physical activity energy expenditure in the EX group was 563 kcal/day. Based on measured 7-day physical activity recall questionnaires, physical activity energy expenditure increased by 408 kcal/day (P ≤ 0.05) during the EX intervention and did not change significantly in the CR (−93 kcal/day) or CON (+128 kcal/day) groups. Based on data retrieved from portable heart rate monitors, the EX group participants exercised 6 days/wk for 62 min/session at 71% of maximal heart rate; this corresponded with a net exercise energy expenditure of 228 kcal/day, which is likely an underestimate because ∼14% of the exercise session were not recorded on the heart rate monitors. The most frequently used modes of exercise were walking, elliptical machine exercise, cycling, and running. On average, the prescribed reduction in energy intake for participants in the CR group was 524 kcal/day. According to 7-day food diaries, energy intake in the CR group decreased by ∼318 kcal/day (P < 0.05) during the intervention and did not change in the EX (+48 kcal/day) or CON (−44 kcal/day) groups.
Body weight and composition.
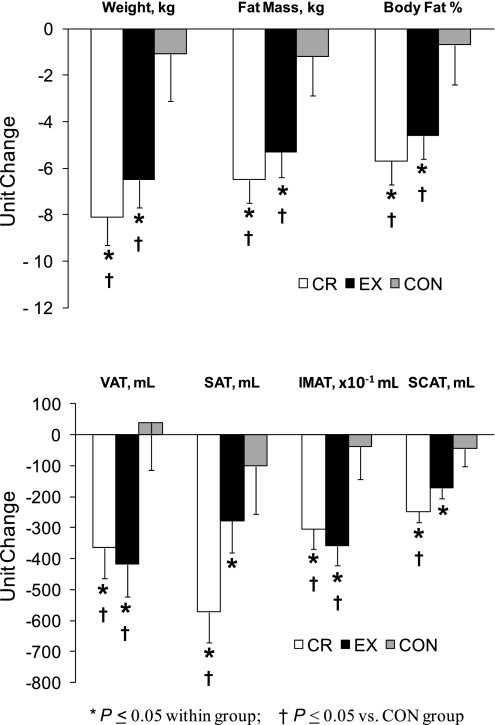
Body weight, whole-body adiposity, and abdominal adiposity data have been reported previously (23, 28) but are presented here (Table 1; Fig. 1) for the smaller subset of subjects used in the present report to assist in interpreting the IMAT data. Body weight, whole body adiposity, and VAT decreased significantly in the CR and EX groups but did not change in the CON group. SAT decreased in the CR and EX groups; however, only the CR group changes were significantly different from those in the CON group (Fig. 1). Thigh IMAT volume decreased significantly in the EX and CR groups and remained unchanged in the control group (Fig. 1, bottom). Thigh subcutaneous fat volume decreased significantly in the EX and CR groups; however, only the change in the CR group differed significantly from that for the CON group (Fig. 1, bottom). None of these findings was affected by the inclusion of age and sex as covariates in the analyses except for the VAT comparisons in which the ANCOVA P value became 0.06, likely due to the loss of statistical power resulting from the added covariates.
Fig. 1.
Changes in body weight and composition in response to 12 mo of weight loss induced by calorie restriction or exercise and in the control group. Values are means ± SE and have been adjusted for baseline values. CR, calorie restriction group; EX, exercise-induced weight loss group; CON, control group; VAT, visceral adipose tissue volume; SAT, subcutaneous adipose tissue volume; IMAT, thigh intermuscular adipose tissue volume; SCAT, thigh subcutaneous adipose tissue volume.
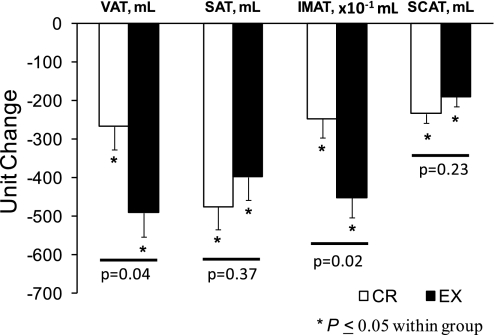
Although the reductions in whole body fat mass in the CR and EX groups were not significantly different, there were somewhat greater fat mass reductions in the CR group. Therefore, additional analyses of the changes in VAT and IMAT were performed in an analysis that only included the CR and EX groups and included changes in whole-body fat mass as a covariate. After adjusting for differences in whole-body fat mass reductions, the EX group had twofold greater reductions in VAT and IMAT compared with the CR group (Fig. 2). The addition of age and sex as covariates did not alter the significance of these findings. Furthermore, although our study was not optimally powered to evaluate differential changes in VAT and IMAT between men and women, we saw no evidence for differential effects in men and women (study group by sex interaction effects: VAT, P = 0.76; IMAT, P = 0.87).
Fig. 2.
Abdominal and thigh adipose tissue volumes in response to 12 mo of calorie restriction or exercise-induced weight loss. Values are means ± SE and have been adjusted for baseline values and for between-group differences in total fat mass reductions.
Insulin action and oral glucose tolerance.
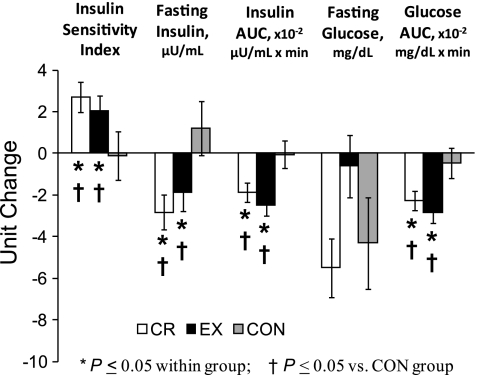
Changes in insulin action have been previously presented (30) but are presented briefly here for the slightly smaller sample used in the present study (Fig. 3). OGTT glucose AUC decreased in the CR and EX groups and remained unchanged in the CON group. These reductions in postprandial glucose concentrations occurred despite lower fasting and postprandial insulinemia in the CR and EX groups. Accordingly, insulin sensitivity index increased in the EX and CR groups but did not change in the CON group (Fig. 3). These findings were not altered by the inclusion of age and sex as covariates.
Fig. 3.
Changes in indexes of glucoregulatory function in response to calorie restriction and exercise-induced weight loss, and in the control group. Values are means ± SE and have been adjusted for baseline values. Insulin sensitivity index values were log transformed for statistical analysis; however, untransformed, baseline-adjusted means are presented for ease of interpretation. AUC, area under the curve during the 2-h oral glucose tolerance test.
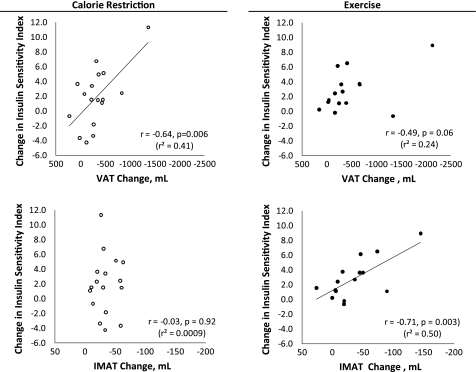
Correlations.
At baseline for all groups combined, higher levels of VAT were associated with lower ISI (r = −0.63; P = 0.0001). In contrast, baseline IMAT values (all groups combined) were not correlated with ISI (r = −0.21; P = 0.25). In response to the CR intervention, reductions in VAT but not IMAT changes were correlated with increases in ISI (Fig. 4). In response to the EX intervention, there was a marginal (but nonsignificant) association between VAT reductions and improvements in ISI (Fig. 4). However, decreases in IMAT in the EX group were strongly associated with increases in ISI (Fig. 4). Because of outlier values in the data used for correlation analyses (Fig. 4), non-parametric Spearman rank correlation analyses were also performed. The marginal correlation in the EX group between changes in VAT and improvements in ISI became more clearly nonsignificant (P = 0.12). The significance/nonsignificance of all other correlations was not altered by performing non-parametric analyses.
Fig. 4.
Associations between changes in visceral or intermuscular adipose tissue volume and changes in insulin sensitivity index in the calorie restriction and exercise groups. Correlation coefficients are from Pearson correlations. Because of several outlier values, non-parametric Spearman rank correlation analyses were also performed. The marginally significant correlation between changes in VAT and improvements in ISI in the EX group (top right) became more clearly nonsignificant (P = 0.12). The significance/nonsignificance of all other correlations was not altered by performing non-parametric analyses.
DISCUSSION
Findings from the present study indicate that both CR and EX-induced weight loss result in decreases in intermuscular adipose tissue volume in the thigh muscles. However, when matched for reductions in total body fat mass, exercise results in a substantially larger reduction in thigh IMAT volume. Furthermore, we had previously reported that visceral adipose tissue volume decreases similarly in response to CR and EX-induced weight loss (23). However, there were small differences between the CR and EX groups with respect to whole body fat mass reductions. In the present study, after statistically accounting for whole body fat mass changes, the reductions in VAT in response to exercise-induced weight loss were significantly greater than those seen in response to CR. Taken together, these results indicate that exercise results in preferential reductions in thigh IMAT and VAT compared with CR, provided that the exercise volume is sufficient to induce weight loss. These findings add to existing literature in showing that, at least for some outcomes, exercise may provide larger benefits than those which are attainable through CR alone (11, 29).
Other studies support the notion that exercise affects IMAT levels, independent of changes in whole body mass. For example, 4 wk of single leg immobilization in healthy humans, which was accomplished by having the subjects use crutches to make one leg non-weight-bearing, resulted in increases in IMAT in the immobilized limb despite a small but significant reduction in whole body mass (18). In another study, a year-long physical activity intervention that included cardiovascular, strength, and balance exercises protected 70- to 89-yr-old men and women from increases in IMAT, as were seen in the nonexercising control group, despite no differences between groups with respect to changes in whole-body mass (15). In contrast, another study reported that an intervention of CR and EX, compared with similar body mass and fat mass reductions induced by CR alone, did not result in significantly greater reductions in IMAT (although the absolute IMAT reduction was ∼70% greater in the combined intervention group) (5). However, the exercise volume (3 days/wk, 60–75 min/session) was approximately half of that used in the present study and may not have been sufficient for large effects on body weight and composition.
Acute vigorous exercise stimulates lipolysis in adipocytes through increases in sympathetic adrenergic activity (3, 17, 32). Although this effect is systemic, visceral adipose tissue is especially sensitive to lipolytic activation by the adrenal system (2). Therefore, it seems plausible that the exercise-induced adrenal activity is at least partly responsible for the preferential reductions in VAT seen in our study. Whether this contributes to preferential reductions in IMAT induced by exercise is not clear, since it is not known whether IMAT, like VAT, has high sensitivity to adrenergic lipolytic activation. However, from a biological perspective, it is logical that IMAT would serve as an energy source for contracting muscle because of its proximity to muscle cells. If this is the case, repeated bouts of exercise would be expected to decrease IMAT volume in active muscle, whereas IMAT in inactive muscle would remain unchanged. Future studies might be able to gain insights into this possibility by comparing changes in IMAT in active vs. inactive muscles during exercise-induced weight loss.
It seems likely that some of the improvements in insulin action seen in both the CR and EX groups were mediated by reductions in whole-body and/or regional adiposity. However, it is surprising that the larger reductions in VAT and IMAT seen in the exercise group were not associated with greater improvements in insulin action. This is even more perplexing when considering that exercise improves glucoregulation, even in the absence of weight loss (7, 8), although this weight loss-independent effect is likely acute and might have dissipated in the ≥48 h between the last exercise bout and the OGTT (16, 20). Several explanations are possible. First, it is possible that the glucoregulatory benefit of a greater reduction in VAT and IMAT in the exercise group was matched by a CR-specific effect. That is, although the overall improvements in glucoregulation in the CR and EX groups were similar, the improvements may have occurred through partly different mechanisms. Limited evidence for differential adaptive mechanisms between CR and EX comes from the correlation analyses in the present study, showing that the improvements in insulin action in the CR group were more tightly associated with VAT changes, whereas those in the EX group were more tightly associated with IMAT changes. To gain further insights into mechanistic differences between adaptations to CR and EX, we are currently performing a trial to determine whether CR, but not comparable weight loss through exercise, improves glucoregulation by altering postprandial incretin hormone responses [i.e., GIP and/or GLP-1, both of which are involved in glycemic control (9)].
Another explanation for the similar improvements in glucoregulation in the CR and EX groups, despite greater exercise-induced improvements in VAT and IMAT, is that there is no mechanistic link (i.e., cause-effect relationship) between glucoregulation and VAT and IMAT. Although this notion contradicts dogma, especially for VAT, research has shown that there is no difference in insulin sensitivity between subjects with low VAT volumes and those with a twofold greater VAT volume when the low and high VAT groups were matched for intrahepatic triglyceride content (10), implying no mechanistic connection between VAT and glucoregulation.
Although it is commonly thought that fat is a regulator of insulin action, inflammation, and oxidative stress, it has been proposed that the opposite is true for IMAT. That is, insulin resistance, inflammation, and oxidative stress regulate IMAT accumulation through effects on adipogenesis (26). The fact that we did not observe differences in insulin action and markers of systemic inflammation [i.e., IL-6, TNF-α, C-reactive protein, and free fatty acids, as published previously (12, 30)] whereas we did see differences in IMAT changes between groups argues against this possibility. Furthermore, it seems unlikely that alterations in adipogenesis could explain the differential effects of CR and EX on IMAT, since our subjects were in a net energy deficit, thereby making any effects on adipogenesis, which is an anabolic state, unlikely.
In conclusion, 1 year of exercise-induced weight loss results in greater reductions in IMAT and VAT than comparable weight loss induced by CR alone. Although this advantage of exercise-induced weight loss did not coincide with greater improvements in glucoregulation, these findings raise the possibility that CR and EX improve glucoregulation through partly distinct mechanisms, some of which do not involve VAT and IMAT. Furthermore, because VAT and IMAT have been linked to other outcomes such as oxidative stress (14, 22) and muscle contractile function (6, 18), respectively, it is possible that exercise-induced weight loss may have other advantages over CR alone.
GRANTS
This work was supported by National Institutes of Health Cooperative Agreement AG-20487, National Institutes of Health General Clinical Research Center Grant RR-00036, National Institute of Diabetes and Digestive and Kidney Diseases Research Training Center Grant DK-20579, and National Institute of Diabetes and Digestive and Kidney Diseases Clinical Nutrition Research Unit Grant DK-56341. E. P. Weiss was supported by National Institute on Aging Grant AG-00078 and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-080886.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.C.M., J.L.M., K.M., D.T.V., L.F., and E.P.W. conceptualized and designed the research; J.C.M. and E.P.W. performed experiments; J.C.M. and E.P.W. analyzed data; J.C.M., J.L.M., K.M., D.T.V., L.F., and E.P.W. interpreted results of experiments; J.C.M. and E.P.W. prepared figures; J.C.M. and E.P.W. drafted the manuscript; J.C.M., J.L.M., K.M., D.T.V., L.F., and E.P.W. edited and revised the manuscript; J.C.M., J.L.M., K.M., D.T.V., L.F., and E.P.W. approved the final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the study participants for cooperation and to the staff of the Applied Physiology Laboratory and the General Clinical Research Center at Washington University School of Medicine for skilled assistance.
REFERENCES
- 1.Arif H, Racette SB, Villareal DT, Holloszy JO, Weiss EP. Comparison of methods for assessing abdominal adipose tissue from magnetic resonance images. Obesity 15: 2240–2244, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Ann Med 27: 435–438, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Berman DM, Nicklas BJ, Rogus EM, Dennis KE, Goldberg AP. Regional differences in adrenoceptor binding and fat cell lipolysis in obese, postmenopausal women. Metabolism 47: 467–473, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Boettcher M, Machann J, Stefan N, Thamer C, Haring HU, Claussen CD, Fritsche A, Schick F. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. J Magn Reson Imaging 29: 1340–1345, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Christiansen T, Paulsen SK, Bruun JM, Overgaard K, Ringgaard S, Pedersen SB, Positano V, Richelsen B. Comparable reduction of the visceral adipose tissue depot after a diet-induced weight loss with or without aerobic exercise in obese subjects: a 12-week randomized intervention study. Eur J Endocrinol 160: 759–767, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 90: 1579–1585, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dengel DR, Galecki AT, Hagberg JM, Pratley RE. The independent and combined effects of weight loss and aerobic exercise on blood pressure and oral glucose tolerance in older men. Am J Hypertens 11: 1405–1412, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol 81: 318–325, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Drucker DJ. The biology of incretin hormones. Cell Metab 3: 153–165, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA 106: 15430–15435, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age (Dordr) 32: 97–108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana L, Villareal DT, Weiss EP, Racette SB, Steger-May K, Klein S, Holloszy JO. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized, controlled trial. Am J Physiol Endocrinol Metab 293: E197–E202, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Gan SK, Kriketos AD, Ellis BA, Thompson CH, Kraegen EW, Chisholm DJ. Changes in aerobic capacity and visceral fat but not myocyte lipid levels predict increased insulin action after exercise in overweight and obese men. Diabetes Care 26: 1706–1713, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Gletsu-Miller N, Hansen JM, Jones DP, Go YM, Torres WE, Ziegler TR, Lin E. Loss of total and visceral adipose tissue mass predicts decreases in oxidative stress after weight-loss surgery. Obesity (Silver Spring) 17: 439–446, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, Kritchevsky SB, Pahor M, Newman AB. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol 105: 1498–1503, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King DS, Baldus PJ, Sharp RL, Kesl LD, Feltmeyer TL, Riddle MS. Time course for exercise-induced alterations in insulin action and glucose tolerance in middle-aged people. J Appl Physiol 78: 17–22, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Large V, Arner P. Regulation of lipolysis in humans. Pathophysiological modulation in obesity, diabetes, and hyperlipidaemia. Diabetes Metab 24: 409–418, 1998 [PubMed] [Google Scholar]
- 18.Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr 85: 377–384, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Oshida Y, Yamanouchi K, Hayamizu S, Nagasawa J, Ohsawa I, Sato Y. Effects of training and training cessation on insulin action. Int J Sports Med 12: 484–486, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Positano V, Gastaldelli A, Sironi AM, Santarelli MF, Lombardi M, Landini L. An accurate and robust method for unsupervised assessment of abdominal fat by MRI. J Magn Reson Imaging 20: 684–689, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O'Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 116: 1234–1241, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO, The Washington University School of Medicine CALERIE Group One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci 61: 943–950, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 133: 92–103, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, Nguyen-Duy TB, Lee S, Kilpatrick K, Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res 12: 789–798, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Vettor R, Milan G, Franzin C, Sanna M, DeCoppi P, Rizzuto R, Federspil G. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab 297: E987–E998, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Villareal DT, Fontana L, Weiss EP, Racette SB, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med 166: 2502–2510, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Weiss EP, Holloszy JO. Improvements in body composition, glucose tolerance, and insulin action induced by increasing energy expenditure or decreasing energy intake. J Nutr 137: 1087–1090, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Ehsani AA, Holloszy JO. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol 102: 634–640, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 84: 1033–1042, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss EP, Villareal DT, Racette SB, Steger-May K, Premachandra BN, Klein S, Fontana L. Caloric restriction but not exercise-induced reductions in fat mass decrease plasma triiodothyronine concentrations: a randomized controlled trial. Rejuvenation Res 11: 605–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A. Catecholamines and the effects of exercise, training and gender. Sports Med 38: 401–423, 2008 [DOI] [PubMed] [Google Scholar]