Chemoprevention in familial adenomatous polyposis (original) (raw)
. Author manuscript; available in PMC: 2013 Feb 12.
Published in final edited form as: Best Pract Res Clin Gastroenterol. 2011 Aug;25(0):607–622. doi: 10.1016/j.bpg.2011.08.002
Abstract
Familial adenomatous polyposis (FAP) predictably leads to adenomas and eventual adenocarcinomas in the lower gastrointestinal tract and less frequently, the upper gastrointestinal tract. Chemopreventive strategies have been studied in FAP patients to delay the development of adenomas in the upper and lower gastrointestinal tract, as well as to prevent recurrence of adenomas in the retained rectum of patients after prophylactic surgery with colectomy and ileorectal anastamosis (IRA). The nonsteroidal anti-inflammatory drug (NSAID) sulindac and selective cyclooxygenase-2 (COX-2) inhibitor celecoxib reduce polyposis of the retained rectum after colectomy with IRA. Reports of cardiovascular risks of some NSAIDs and selective COX-2 inhibitors have led to promising studies of lower doses in combination with ursodeoxycholic acid, statin, and difluoromethylornithine. Curcumin and eicosapentaenoic acid show efficacy in small clinical trials of FAP chemoprevention. This article will review the concept of chemoprevention and the current clinical literature in FAP chemoprevention.
Keywords: Familial adenomatous polyposis, Adenomas, Chemoprevention, Sulindac, Celecoxib, Curcumin, Eicosapentaenoic acid
Introduction
Familial adenomatous polyposis
Familial adenomatous polyposis (FAP) is an autosomal dominant disease caused by mutation in the Adenomatous Polyposis Coli (APC) gene, located on chromosome 5 [1]. This germline defect accelerates the initiation of the adenoma–carcinoma sequence, resulting in the development of numerous adenomatous colorectal polyps at young age. Polyposis inevitably progresses to colorectal cancer if left untreated. Given the predictable development of colorectal cancer in patients with FAP, the safest preventative strategy is surgical resection of the colon when polyposis develops. The two main prophylactic surgeries are colectomy with ileorectal anastamosis (IRA) and proctocolectomy with ileal pouch-anal anastamosis (IPAA) [2]. Colectomy with IRA is a straightforward operation with less functional side effects compared to proctocolectomy with IPAA [3]. However, patients who undergo colectomy with IRA are at a 25% risk of developing cancer in the retained rectum after 20 years [4]. These patients require regular endoscopic surveillance to identify and treat recurrent adenomas in the rectum.
Adenomatous polyps also occur in the upper gastrointestinal tract, especially in the duodenum, and may progress to malignancy, albeit at a lower rate [5]. Regular endoscopic surveillance of the upper gastrointestinal tract usually detects duodenal disease at a premalignant state [2].
Chemoprevention
Chemoprevention is the use of pharmaceutical or natural agents to prevent or delay the development of cancer in healthy patients. Chemoprevention should ideally be well tolerated, low in toxicity, cheap, and effective. Patients with FAP develop visible, countable, precursor lesions in the form of adenomatous polyps prior to transformation to cancer. Consequently, FAP patients are an ideal group to assess the efficacy of various chemopreventive agents. There are three main roles that chemoprevention can play in patients with FAP: (1) to delay prophylactic colectomy; (2) to prevent cancer development in the retained rectum in patients after colectomy with IRA; and (3) to prevent cancer development in the upper gastrointestinal tract, especially the duodenum. Nonsteroidal anti-inflammatory drugs (NSAIDs), ursodeoxycholic acid, statins, difluoromethylornithine (DFMO), and various dietary supplements have been studied as potential chemopreventive agents (Table 1).
Table 1.
Potential chemopreventive agents in FAP
| NSAIDs |
|---|
| Sulindac |
| Celecoxib |
| Aspirin |
| Combination therapy with NSAIDs |
| Difluoromethylornithine |
| Ursodiol |
| Statin |
| Eicosapentaenoic acid |
| Curcumin |
| Vitamin C |
| Fiber |
| Calcium |
Lower gastrointestinal tract chemoprevention
Nonsteroidal anti-inflammatory drugs
NSAIDs have been studied extensively as a chemopreventive agent in patients with FAP. The efficacy of NSAIDs has been demonstrated in clinical trials and animal studies. NSAIDs inhibit cyclooxygenase (COX), a key enzyme in the conversion of arachidonic acid to prostaglandins and other eicosanoids. Prostaglandins appear to play a key role in the adenoma–carcinoma sequence by altering cell adhesion, inhibiting apoptosis, and promoting angiogenesis [6,7]. Elevated prostaglandin levels are found in many premalignant and malignant lesions including colorectal adenomas and adenocarcinomas [8].
There are two cyclooxygenase enzymes present in humans, COX-1 and COX-2. COX-1 is constitutively expressed, but COX-2 is absent under physiologic conditions. COX-2 is increasingly induced in the adenoma–carcinoma sequence [6,7]. The importance of COX-2 in the development of adenomas is demonstrated in the Apc Δ716 mouse, a murine model for human FAP. COX-2 deficiency in these mice partly suppresses the adenomatous phenotype [9]. NSAIDs appear to exert most of their anti-neoplastic effect via inhibition of COX-2. However, other evidence suggests NSAIDs may have an anti-neoplastic effect independent of COX-2 suppression (Fig. 1). Other proposed targets include βcatenin, PPARδ, TGFβ, Ras, and NF-κB [10–14].
Fig. 1.
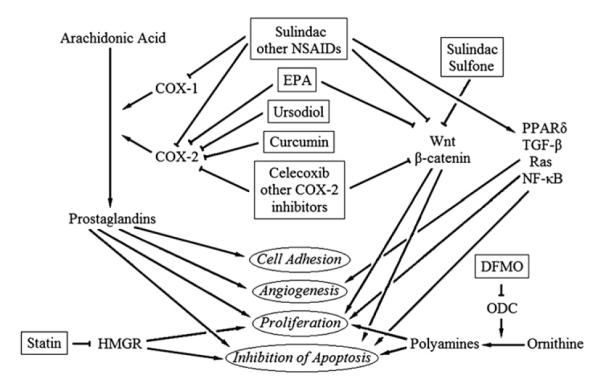
Mechanisms of chemoprevention.
Sulindac
The efficacy of NSAIDs as chemopreventive agents in patients with FAP was first suggested in a non-randomized study of four members of a family with FAP complicated by desmoid tumours. In attempting to treat desmoid tumours with sulindac, an NSAID, the number of adenomas in the retained rectum in three of the patients drastically decreased with oral sulindac. One patient had an intact colon which also responded favourably to the sulindac regimen [15].
This finding was confirmed in a single institution, randomized, double-blinded, placebo-controlled trial conducted by Giardiello et al.. The study involved 22 FAP patients, of which only four had undergone previous colectomy with IRA. In a treatment span of 9 months, 150 mg of sulindac twice a day showed statistically significant reduction in polyp count and diameter compared to placebo. When only the 18 patients with intact colons were analyzed, the results still remained statistically significant at 3 months, 6 months, and 9 months. However, after the discontinuation of the drug at 9 months, partial recrudescence of polyps was noted. No significant adverse effects were experienced by the study subjects [16].
In a trial involving FAP patients who have undergone colectomy with IRA, sulindac for an average of 63 months significantly reduced rectal polyp number in all 12 patients. Higher-grade adenoma (tubulovillous, villous adenomas) recurrence was also significantly reduced. The most common side effect was rectal mucosal erosions [17]. Several clinical trials have verified these findings (Tables 2 and 3) [16–31].
Table 2.
Randomized, placebo-controlled clinical trials of lower gastrointestinal tract chemoprevention in FAP patients
| Ref. # | First author | Patients | Patient characteristics | Treatment regimen | Duration | Outcome | Side effects |
|---|---|---|---|---|---|---|---|
| [16] | Giardiello | 22 | All patients had historyof polyposis. 18 patientshad intact colons. Fourpatients had undergonesubtotal colectomy withileorectal anastamosis. | Sulindac 150 mgtwice a day | 9 months | At nine months, sulindac group haddecrease in number of polyps by56% (p = 0.014) and size of polyps by65% (P < 0.001). 3 months after thediscontinuation of sulindac, therewas increase in number and size of polpys. | None observed |
| [18] | Labayle | 10 | All patients had historyof colectomy andileorectal anastamosis. | Sulindac 100 mgthree times a day | 4 months | Statistically significant decrease inrectal polyps in sulindac vs. placebo(P < 0.01). Recurrence of polyps afterdiscontinuation of sulindac. | None observed |
| [19] | Nugent | 24 | All patients had advancedduodenal polyposis andprevious prophylacticcolectomy. | Sulindac 200 mgtwice a day | 6 months | Rectal polyps improved in fiveof seven patients (p = 0.01) takingsulindac. Duodenal polyps improvedin five of 12 patients (p = 0.12)taking sulindac. | One patient stoppedsulindac for indigestionwithin 6 weeks. |
| [20] | Giardiello | 41 | Genotypically affected FAPpatients without polyposisat time of randomization. | Weight 20–44 kg –Sulindac 75 mgtwice a day. Weight>44 kg – Sulindac150 mg twice a day | 48 months | No significant differences in themean number or size of polypsbetween sulindac and placebo.Five of 21 withdrawn fromsulindac group and six of 20withdrawn from placebo group. | Few adverse effects.One withdrawn fromstudy for possibledrug-induced persistentneutropenia. |
| [46] | Steinbach | 77 | All patients had five or morepolyps at randomization.Twenty-five had intact colons. | Celecoxib 100 mgtwice daily or 400mg twice daily | 6 months | Mean number of colorectal polypsreduced by 28% (p = 0.003), rectalpolyps reduced by 22.5% (p = 0.01),and polyp burden reduced by 30.7%(p = 0.001) in the high-dosecelecoxib group compared to placebo. | No significant differencein adverse events amonglow-dose celecoxib,high-dose celecoxib, andplacebo. One patientwithdrew from study dueto dyspepsia. Anotherpatient withdrew due toacute allergic reaction.Another patient with priorpsychiatric historycommitted suicide. |
| [47] | Lynch | 18 | All patients, aged 10 to14 years-old, had APC mutationsand/or adenomas with familyhistory of FAP. | Celecoxib 16 mg/kgper day | 3 months | Reduction in colorectal polypsby 44.2% (p = 0.01) | No significant differencein adverse events betweencelecoxib and placebo. |
| [48] | Higuchi | 21 | Thirteen patients had previouscolectomy with IRA. | Rofecoxib 25 mg daily | 9 months | At 9 months, 9.9% decrease inpolyp number compared to placebo(p = 0.004) and reduction in polypsize compared to placebo (−16.2%versus 1.5%, p < 0.001). | No significant differencein adverse events. |
| [49] | Iwama | 61 | All patients had diagnosis ofFAP as defined by 100 or moreadenomas in the colonand rectum.Twenty-four patients hadintact colons. | Tiracoxib 150 mgdaily or 200 mg daily | 26 weeks | No significant difference in polypnumber or size noted betweentiracoxib and placebo group | No difference in adverseevents noted betweentiracoxib and placebo group |
| [61] | Burn | 206 | All patients were youngpatients aged 10 to 21years-old, who had confirmedFAP-associated APC mutationor high probability of carryingthe mutation based on linkedDNA markers or presence ofmlutiple colonic polyps. Allpatients had intact colons. | Aspirin 300 mg twice aday and/or resistantstarch 15 g twice a day | Median17 months | No significant reduction in polypcount in the rectosigmoid colonwith aspirin or resistant starch.Trend towards smaller size oflargest polyp in aspirin comparedto nonaspirin group (3.8 mm versus5.5 mm, p = 0.09). Significant decreasein largest polyp size in aspirin groupcompared to nonaspirin if treated >1 year(3.0 mm versus 6.0 mm, p = 0.02). | No serious adverse effectsrecorded. One patientwithdrew from aspirin/resistant starch groupdue to persistent epistaxis. |
| [86] | West | 55 | All patients had previouscolectomy with ileorectalanastamosis. | Eicosapentaenoicacid 1 g twice daily | 6 months | Number of rectal polyps reduced by22.4% (p = 0.012) and sum of polypdiameter reduced by 29.8% (p = 0.027). | No significant differencein adverse events betweeneicosapentaenoic acidand placebo. |
| [95] | Bussey | 36 | All patients had previouscolectomy with ileorectalanastamosis. | Vitamin C 3 g daily | 15–24months | A non-significant trend towards reductionin rectal polyp number noted in vitaminC compared to placebo group at 9,12, and 15 months. | None reported |
| [96] | DeCosse | 58 | All patients had previouscolectomy with ileorectalanastamosis. | Vitamin C 4 g andVitamin E 400 mgdaily or grainfiber 22.5 g daily | 4 years | No difference in rectal polyp numberbetween Vitamin C/E group and placebo.Trend towards decrease in rectal polypnumber in fiber group compared toplacebo at 9 and 33 months. | Diarrhoea more frequentin fiber group (p < 0.05). |
| [98] | Thomas | 25 | All patients had previouscolectomy with ileorectalanastamosis. | Calcium carbonate1500 mg daily | 6 months | No significant difference in rectal polypnumber, progression, or distributionbetween calcium and placebo group. | None reported |
Table 3.
Non-randomized clinical trials of lower gastrointestinal tract chemoprevention in FAP patients
| Ref.# | First author | Patients | Patient characteristics | Treatment regimen | Duration | Outcome | Side effects |
|---|---|---|---|---|---|---|---|
| [17] | Cruz-Correa | 12 | All patients had previouscolectomy with IRA. | Sulindac mean dosageof 158 mg daily | 14–98months | At 12 months, 74% reductionof polyp number (p = 0.02).Significant reduction ofhigh-grade adenomas(p = 0.004). One patientdeveloped stage III rectalcancer after 35 months | Rectal mucosal erosionsin six patients. |
| [21] | Waddell | 10 | Four patients hadintact colons. | Sulindac 150 mg or200 mg twice a day | 12-85months | Decrease or disappearance ofpolyps in all patients. | None reported |
| [22] | Rigau | 7 | Three patients had FAP. Onepatient had Gardnersyndrome. One patient hadnon-familial adenomatouspolyposis. Two patientshad multiple hyperplasticpolyposis. | Sulindac 200 mgtwice per day | 12–36months | Reduction in number and sizeof polyps after 6 months inall patients.No development of carcinomaduring follow-up. | None observed |
| [23] | Spagnesi | 20 | Fourteen patients hadprevious colectomy withIRA. Six had intact colons. | Sulindac 100 mgtwice per day | 60 days | Significant decrease in sizeand number of polyps (p < 0.01) | None reported |
| [24] | Ishikawa | 6 | Five patients had FAP. Onepatient had morethan 30 colorectaladenomatous polyps. | Sulindac 300 mg daily | 6 months | Three of six patients hadreduction in polyp number. | One patient developed multipleileorectal anastamotic ulcers despitereduction in dose. One patient hadperforation of a gastric ulcer. Onepatient had oligospermia improvedafter stopping sulindac. One patienthad subcutaneous abscess. |
| [25] | Winde | 28 | All patients had previouscolectomy with IRA. | Sulindac 150 mgsuppository twice a daywith dose reduction | 3–48months | All patients had reduction inpolyposis at 24 weeks. | No withdrawals due toadverse effects. Twopatients had mild gastritis. |
| [26] | Tonelli | 15 | All patients had previouscolectomy with IRA. | Sulindac 100 mg twice per day | 12–124 months | Significant regression of polypsafter 6 months in all patients(p < 0.02). Polyposis increased.After a mean of 48.6 months,number and size of polypsincreased again with statisticaldifference with baseline. Onepatient developed rectal cancerafter 106 months. | One patient withdrewdue to gastric bleedingafter 49 months. |
| [27] | Fernandez-Lopez | 29 | All patient had previouscolectomy with IRA. | Sulindac 150 mgtwice a day | 6 months | All patients had regressionof polyps after 6 months exceptfor one patient who eventuallydeveloped rectal cancer. | None reported |
| [28] | Guldenschuh | 17 | Seven patients had previouscolectomy with IRA. | Sulindac 300 mg daily | 4 months | Statistically significant decreasein number of adenomas(120 ± 112 to 28 ± 64, p = 0.007).Six months after cessation oftherapy, number of adenomasincreased to 48 ± 44.5. | None observed |
| [29] | Matsumoto | 7 | No patients had priorproctocolectomy. | Sulindac 100 mg threetimes per day | 12 months | Protrusion index measuredby double-contrast barium enemaexamination improved from3.0 ± 1.1 to 1.1 ± 0.8/cm2 indistal colon (p < 0.02) and from3.4 ± 2.4 to 0.9 ± 1.3/cm2 inproximal colon (p < 0.02). | None observed |
| [30] | Hirota | 8 | All patients had previouscolectomy with IRA. | Indomethacin suppository50 mg once or twiceper day | 4–8 weeks | In six of eight patients, numberof polyps decreased. Number ofpolyps increased afterindomethacin discontinuation. | Tenesmus occurred inone patient aftersuppository insertion. |
| [31] | Akasu | 7 | All patients had priorcolectomy with IRA | Indomethacin sustained-release 75 mg–100 mgdaily | 81–345 days | Reduction of rectal polyp numberseen in all patients (p = 0.023).Increase in size and number ofrectal polyps after cessation oftreatment in six patients aftera median of 373 days. | Two patients hadanaemia due to lowerintestinal ulcers andtreatment discontinued. |
| [50] | Dolara | 7 | All patients had previouscolectomy with IRA. | Nimesulide 2 mg/kgper day | 2.5 months | No change in mucosalproliferation by biopsy | One patient hadgeneralized oedemaand muscle pain. |
| [51] | Hallak | 8 | Five patients had previouscolectomy with IRA. | Rofecoxib 25 mg daily | 18–30months | Reduction in polyp formationfrom 15.1 ± 11.7 to 6.0 ± 5.8after 12 months (p = 0.002) andfurther decrease to 1.6 ± 1.6 atend of follow-up (p = 0.001). | No major side effects |
| [40] | van Stolk | 18 | All patients had previouscolectomy with IRA. | Sulindac sulfone 200 mg,300 mg, or 400 mg daily | 6 months | No significant decrease in polypnumber or cellular proliferation. | Reversible hepatotoxicitynoted in eight of 18 patients. |
| [94] | Cruz-Correa | 5 | Four patient had previouscolectomy with IRA andone patient had previousproctocolectomy with IPAA | Curcumin 480 mg andquercetin 20 mg threetimes per day | 3–9months | Decrease in rectal and ileal polypnumber by 60.4% (p = 0.043) andsize by 50.9% (p = 0.039)from baseline. | One patient had self-limiteddiarrhoea and, anotherpatient reported mild nauseaand sour taste with treatment. |
Given its efficacy in regression of adenomas, sulindac’s potential as a primary chemopreventive agent in patients with FAP was studied. Forty-one patients with APC mutations (genotypically affected), who were not yet phenotypically affected, were randomized to placebo or sulindac as a primary prophylaxis against the development of colorectal adenomas. After four years of treatment, no significant difference between the two groups was seen. Adenomas emerged in nine of 21 patients receiving sulindac (43%) and 11 of 20 patients receiving placebo (55%, p = 0.54). No significant difference in mean number of polyps or size was noted between the two groups [20]. Of note, patients who were polyp-free had significantly lower prostaglandin levels measured in rectal mucosa after treatment than those who developed polyps [32]. The measurement of mucosal prostaglandin level may be helpful to assess compliance as well as effectiveness of sulindac.
The ultimate goal of chemoprevention in FAP is to prevent the inevitable development of colorectal cancer among these patients. Despite evidence that sulindac may regress adenomas in the rectum after colectomy with IRA, no evidence exists that the drug delays or prevents the development of malignancy in these rectal segments. In fact, several patients maintained on sulindac with polyp regression have developed colorectal adenocarcinoma [33–35].
Sulindac can be given to delay the progression of polyposis in the retained rectum among patients after colectomy with IRA but should be used in conjunction with a strict endoscopic surveillance regimen. Currently, sulindac is not recommended as a primary chemopreventive agent.
Sulindac sulfone
Sulindac is a prodrug that is metabolized in vivo to sulindac sulfide and sulindac sulfone. Sulindac sulfide has anti-neoplastic effects via inhibition of COX and prostaglandin synthesis which can also results in side effects such as gastrointestinal ulceration and bleeding. Sulindac sulfone (exisulind) does not suppress COX activity yet is able to induce apoptosis in colon adenocarcinoma in vitro [36]. In an azoxymethane-induced colon cancer model, sulindac sulfone reduced the tumour incidence, multiplicity, and tumour burden without significant inhibition of cyclooxygenase, lipoxygenase, or phospholipase A2 [37]. Sulindac sulfone may induce apoptosis via suppression of cyclic guanosine monophosphate (cGMP) phosphodiesterase and subsequent increase in cGMP-dependent protein kinase G with resultant programmed cell death [38].
In a randomized, placebo-controlled study conducted by Arber et al., 281 patients with sporadic adenomatous polyps were given placebo or sulindac sulfone 100 or 200 mg twice per day for 12 months. The higher dose of sulindac sulfone showed significant reduction in median polyp size compared to placebo (50% versus 25%, p = 0.03). There was also a lower rate of disease progression in the sulindac sulfone group compared to placebo (6.1% versus 27.9%, p = 0.02). However, significant increase in liver enzymes (8.4%) and abdominal pain (14.7%) were reported in the sulindac sulfone group [39]. Eighteen FAP patients with prior colectomy with IRA were given sulindac sulfone at a dose of 200, 300, and 400 mg orally twice per day. No significant decrease in polyp number or significant effect on cellular proliferation was noted after 6 months of treatment. Reversible hepatic toxicity marked by elevated transaminase and bilirubin was noted in eight of 18 patients, requiring dose reduction or drug cessation. Generally mild gastrointestinal side effects including nausea, vomiting, abdominal pain, indigestion, decreased appetite, and change in bowel habits were reported in 14 of 18 patients [40]. Sulindac sulfone appears to have minimal effect in FAP patients, and is associated with hepatotoxicity.
Cyclooxygenase-2 inhibitors
Interest in COX-2 inhibitors as chemopreventive agents in FAP was prompted by the gastrointestinal toxicity noted with a long-term use of non-selective NSAIDs [41,42]. Several randomized, placebo-controlled trials with COX-2 inhibitors showed reduction of sporadic colorectal adenoma recurrence[43–45].
In a double-blind, placebo-controlled study, 77 FAP patients (25 with intact colons) were randomized to celecoxib at 100 mg or 400 mg twice daily or placebo for 6 months. Endoscopic evaluation performed at the beginning and end of the 6 months demonstrated a 28% reduction in mean number of polyps (p = 0.003) and 30.7% reduction in polyp burden (p = 0.001) in the high-dose celecoxib group compared to the placebo group [46].
The efficacy of celecoxib as primary chemopreventive agent was suggested in a study by Lynch et al. involving a cohort of 18 young patients with APC gene mutations and/or adenomas with a family history of FAP. Celecoxib at a dose of 16 mg/kg/day, corresponding to an adult dose of 400 mg twice per day, was well tolerated and significantly reduced the number of colorectal polyps by 44.2% at 3 months (p = 0.01) [47]. Although promising, further studies are needed to determine the safety of a long-term COX-2 inhibitor use in children with FAP, and whether this drug can safely delay prophylactic colectomies in these patients.
Despite apparent effectiveness, reports of potential cardiovascular toxicity with COX-2 inhibitors limit their use in FAP. Dose-dependent increase in incidence of deaths from cardiovascular causes, nonfatal myocardial infarction, stroke, and heart failure have been noted in patients receiving COX-2 inhibitors [52–54]. Based on this data, rofecoxib (Vioxx) was voluntarily removed by Merck from the United States market in 2004. Celecoxib remains available but with a United States Food and Drug Administration (FDA)-mandated black box warning. Cardiovascular complications do not appear to be limited to COX-2 inhibitor use. Non-selective NSAIDs, including sulindac and naproxen, have been suggested to increase cardiovascular thrombotic events [55,56].
The benefit of regular use of COX-2 inhibitors and non-selective NSAIDs in FAP patients with cardiovascular risk factors needs to be weighed against the potential cardiovascular adverse events of these medications.
Aspirin
Given the potential cardiovascular side effects of NSAIDs, focus has turned to aspirin as a candidate for chemoprevention in FAP. Unlike sulindac and COX-2 inhibitors, aspirin not only has a favourable cardiovascular profile but is used as primary pharmacotherapy in patients with cardiovascular risk factors. Several, randomized, placebo-controlled trials have shown reduction in sporadic adenomas in patients using aspirin with prior history of adenomas [57–59].
In the Colorectal Adenoma/Carcinoma Prevention Programme 1 (CAPP1) study, aspirinwas studied in combinationwith resistant starch (RS), a dietary fiber, in patients with FAP. Starch consumption had been described to inversely correlate with colon cancer incidence [60]. In the CAPP1 study, 206 FAP patients with intact colons were randomized to one of four arms: aspirin 600 mg orally daily, RS 30 g orally daily, aspirin plus RS, or placebo plus placebo. After median treatment duration of 17 months, no significant reduction in polyp count or size was noted with either intervention. However, a trend towards a decrease in polyp count and size was noted in the aspirin groups but not the resistant starch groups [61].
With favourable cardiovascular profile, aspirin holds appeal as a primary chemopreventive agent for colorectal cancer, but effectiveness in FAP remains unclear.
Combination therapy
The concern for potential toxicity of NSAIDs has spurred interest in combination therapy that may allow for lower doses of NSAIDs in patients with FAP. Several animal studies have demonstrated potential roles of these combination therapies.
Difluoromethylornithine and nonsteroidal anti-inflammatory drugs
Polyamines (putresceine, spermidine, and spermine) are low-molecular-weight, organic cations that are ubiquitous in all higher eukaryotes. They are important for normal cellular growth and differentiation [62]. Polyamine levels are elevated in neoplastic tissues compared to normal tissues and in presymptomatic patients with FAP. Activity of ornithine decarboxylase (ODC), the first enzyme in the polyamine synthesis, is also significantly elevated in presymptomatic patients with germline APC mutations [63]. Difluoromethylornithine (DFMO) is an enzyme-activated irreversible inhibitor of ODC with antiproliferative qualities. The clinical use of DFMO has been limited by side effects found at high doses, including hearing loss, diarrhoea, abdominal pain, emesis, anaemia, leukopenia, and thrombocytopenia [64]. Low-dose DFMO can be combined with NSAIDS to limit toxicity, and the combination inhibits intestinal carcinogenesis in mice and rats [65–68]. In a human study, 375 patients with history of previously removed sporadic adenomas were randomized to receive placebo or combination of DFMO 500 mg and sulindac 150 mg daily for 36 months. Follow-up colonoscopy in 3 years showed significant decrease in recurrence of one or more adenomas in the DFMO/sulindac group (P < 0.001). There was also significant reduction in advanced adenomas (8.5% versus 0.7%, P < 0.001) and multiple adenomas (13.2% versus 0.7%, P < 0.001) in patients receiving the combination therapy versus placebo. No significant difference in adverse events was noted in the trial [69]. Trials comparing celecoxib versus celecoxib and DFMO in patients with FAP are currently under investigation.
Ursodeoxycholic acid and nonsteroidal anti-inflammatory drugs
Ursodeoxycholic acid or ursodiol is a tertiary bile acid, minimally present in human bile. When given orally, this bile acid decreases rates of colonic neoplasia in rats and patients with ulcerative colitis and primary sclerosing cholangitis [70–72]. Ursodiol has an inhibitory effect on COX-2 [73]. In a study involving ApcMin mice, ursodiol, in combination with low-dose sulindac, caused a dose-dependent decrease in the number of small bowel and colonic polyp tumours. The combined regimen of sulindac and ursodiol was more effective than either agent alone [74].
Statin and cyclooxygenase-2 inhibitor
Two large randomized, controlled trials studying the effect of hydroxyl-3-methylglutaryl CoA reductase (HMGR) inhibitors or statins on cardiovascular outcomes suggested a possible role of statins as a colorectal chemopreventive agent. Patients taking pravastatin and simvastatin had 43% and 19% reduction in sporadic colorectal cancer incidence, respectively [75,76]. In a case–control study of 1953 patients with sporadic colorectal cancer and 2015 controls, the use of statins was associated with a significant reduction in relative risk of colorectal cancer [77]. These observations let to animal studies in ApcMin mice, a murine model for human FAP. In ApcMin mice fed a combination of atorvastatin and celecoxib for 80 days, complete suppression of colonic polyp formation and 86% reduction in small intestinal polyps were noted. A synergistic effect occurred with combination therapy significantly greater than that of each agent alone [78].
Eicosapentaenoic acid
Omega (ω)-3 polyunsaturated fatty acids (ω-3 PUFAs) are naturally occurring fatty acids found predominantly in cold-water fish and include the essential fatty acid α-linolenic acid (ALA) and metabolites eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Epidemiologic studies suggest a role for ω-3 PUFAs as chemopreventive agents. Populations with higher consumption of fish and fish oil as compared to animal fat have lower incidence of colorectal cancer [79,80]. In ApcMin mice, EPA significantly suppressed polyp number and load in both the small and large intestine [81]. Both COX-2-dependent and -independent mechanisms appear to play a part in PUFA’s role as a chemopreventive agent [82–85].
West et al. randomized FAP patients with prior colectomy with IRA to either 1 g of EPA twice per day orally or placebo for six months. The group performed endoscopic evaluation of the retained rectum at time 0 and 6 months. At 6 months, there was 22.4% (p = 0.012) reduction in polyp number and 29.8% (p = 0.027) decrease in polyp size in the EPA group compared to the placebo group. No significant difference in adverse events was reported [86].
Curcumin
Curcumin (diferuloylmethane) is the major yellow pigment extracted from turmeric, the powdered root of Curcuma longa. Turmeric is commonly used as a spice in Asia, especially the Indian subcontinent where a low incidence of colorectal cancer exists [87]. Rodent studies show that curcumin may interfere with colon carcinogenesis [88,89], and ApcMin mice treated with curcumin have reduction in intestinal tumour formation [90]. The mechanism of chemoprevention appears to involve the upregulation of carcinogen-detoxifying enzymes such as glutathione S-transferase [91] and suppression of COX-2 expression [92]. In 15 patients with advanced colorectal cancer refractory to standard chemotherapies, radiologic stability of disease was noted in five patients treated with 2–4 months of curcumin [93].
The combination of curcumin and quercetin, a flavonoid recognized for antioxidant properties, was studied in five patients ranging in age from 21 to 51, who have undergone colectomy with IRA (four patients) or proctocolectomy with IPAA (one patient). In this uncontrolled trial, patients were given curcumin 480 mg and quercetin 20 mg orally three times per day for 3–9 months. This combination was associated with a mean decrease in rectal and ileal polyp number from baseline of 60.4% (p = 0.043) and polyp size from baseline of 50.9% (p = 0.039). The combination was tolerated well. One patient reported self-limited diarrhoea and another reported mild nausea and sour taste after ingestion of the pill which subsided after 3 days without recurrence [94]. Further randomized, double-blind, controlled studies in humans are needed to confirm these findings.
Vitamins and minerals
Nearly 30 years ago, Bussey et al. studied Vitamin C’s effect upon polyposis. In a study involving 36 FAP patients with colectomy and IRA, patients were randomized to vitamin C 3 g orally daily or placebo for 15–24 months. A non-significant trend towards reduction in rectal polyp number was noted in patients treated with vitamin C [95]. In a 4-year period, combination of daily vitamin C (4 g) and vitamin E (400 mg), with or without grain fiber (22.5 g) supplementation was studied in 58 FAP patients with prior colectomy and IRA. No difference in rectal polyp number was seen in patients taking the vitamins. However, a non-significant trend towards reduction in rectal polyp number was seen in the high-fiber group with increase in reduction in patients who were compliant with the treatment regimen [96].
In a meta-analysis of three randomized trials involving individuals with prior sporadic adenomas, supplemental calcium was effective for the prevention of recurrent adenomas [97]. However, in a trial involving 25 patients with FAP with prior colectomy and IRA, daily calcium carbonate at 1500 mg orally showed no effect on the number, size, or distribution of rectal polyps [98].
Upper gastrointestinal tract chemoprevention
Since colorectal cancer can largely be prevented by prophylactic colectomy and regular polyp surveillance in the retained rectum, duodenal cancer has become the leading cause of death in patients with FAP who have already undergone prophylactic colectomy [99]. Nearly 90% of patients with FAP will develop duodenal polyps, the precursor lesions of duodenal adenocarcinoma [100] and 4.5% will develop duodenal adenocarcinoma in their lifetime [5]. In contrast to the colon, prophylactic surgical resection of the ampulla and/or duodenum is accompanied by significant morbidity. Duodenal surgery is currently indicated for patients with severe duodenal polyposis or duodenal carcinoma. Chemoprevention would be ideal to induce regression or stabilization of these premalignant lesions.
Twenty-four FAP patients with prophylactic colectomy and advanced duodenal polyposis were randomized to sulindac at 200 mg orally, twice daily or placebo. After 6 months of treatment, no significant qualitative improvement in duodenal polyposis was seen compared to placebo [18]. However, there were significantly fewer small polyps, 2 mm or less, noted in the sulindac treatment compared to placebo group [101]. In a study comparing the efficacy of sulindac to calcium and calciferol in 18 patients with upper gastrointestinal polyps with prior colectomy, no significant change in polyp number was seen in either group after 6 months of treatment [102]. A prospective study composed of eight patients with prior large duodenal polyposis, were given sulindac 150 mg orally twice per day for a mean of 8.75 months. No significant benefit was seen in these patients, and one developed an invasive periampullary carcinoma while on sulindac [103].
Phillips et al. randomized 83 FAP patients to receive 100 mg celecoxib twice daily, 400 mg celecoxib twice daily, or placebo. After 6 months of treatment, there was no significant difference among the groups in number of polyps. However, there was significant qualitative improvement in polyposis for those taking the high-dose celecoxib when the patient’s endoscopies were reviewed independently by five physicians [104]. Despite this positive study, chemoprevention studies of duodenal polyposis with NSAIDs have been disappointing (Table 4). Resistance of FAP duodenal polyposis to NSAIDs in contrast to FAP colorectal polyposis may, in part, be explained by differential COX-2 expression. COX-2 is expressed at higher levels in the duodenum than colon in FAP patients [106]. Thus, higher dosages of NSAIDs may be needed to suppress polyposis in the duodenum. However, future studies with higher dosages may be limited by the potential cardiovascular toxicity of non-selective NSAIDs and COX-2 inhibitors [52–56].
Table 4.
Clinical trials of duodenal polyposis chemoprevention in FAP patients
| Ref. # | First author | Type of study | Patients | Study drug | Duration | Outcome | Side effects |
|---|---|---|---|---|---|---|---|
| [19] | Nugent | Randomized, double-blind,placebo-controlled | 24 | Sulindac 200 mg twice a day | 6 months | Qualitative polyp status improvedin five patients, worsened in oneand was unchanged in five(p = 0.12 compared to placebo) | One patient stopped sulindacwithin 6 weeks due toindigestion without endoscopicevidence of duodenal ulceror erosion. |
| [102] | Seow-Cheon | Randomized crossover,double-blind | 18 | Calcium carbonate380 mg/calciferol 500 mgdaily or sulindac 300 mg daily | 6 months | No significant difference in gastricor duodenal polyposis noted ineither treatment group. | Not reported |
| [103] | Richard | Prospective | 8 | Sulindac 150 mg twice a day | Mean 8.75months | No significant benefit seen. Onepatient progressed to invasiveduodenal adenocarcinoma.Another patient showedrecurrence of polyp with severedysplasia requiring apancreaticoduodenectomy. | Two patients discontinuedsulindac due to abdominalcramps. Another stopped dueto documented gastritis,duodenal ulcer, andprogression of polyps tovillous changes. |
| [104] | Phillips | Randomized, double-blind,placebo-controlled | 83 | Celecoxib 100 mg or400 mg twice a day | 6 months | When reviewed by fiveendoscopists, celecoxib 400 mgtwice daily showed qualitativeimprovement in duodenalpolyposis (p = 0.033). Noquantitative improvement seen. | One patient had an allergicreaction to celecoxib.Second patient withdrewfrom study with symptomsof dyspepsia without evidenceof peptic ulcer. Another withpsychiatric historycommitted suicide. |
| [105] | Wallace | Randomized,placebo-controlled | 26 | Ranitidine 300 mg daily | 6 months | No difference seen in duodenalpolyp number compared toplacebo (p = 0.9). | None reported |
Along with positive effect on colonic polyposis, EPA and combination NSAIDs with DFMO, ursodiol, or statin reduce small intestinal polyposis in ApcMin mice and show promise as potential chemopreventive candidate regimens in humans for duodenal polyposis [67,74,78,81].
Summary
Many drugs and dietary supplements have been studied as potential agents for chemoprevention in familial adenomatous polyposis. The NSAID sulindac and the COX-2 inhibitor celecoxib have been studied most extensively and show efficacy in reducing polyp burden in patients after colectomy with IRA. Celecoxib also shows benefit in patients prior to colectomy and possibly in patients with duodenal polyposis.
Chemoprevention with sulindac 150 mg twice daily or celecoxib 400 mg twice daily can be considered in FAP patients following initial prophylactic surgery and retained rectal segments as an adjunct to endoscopic surveillance. It is unclear whether Celecoxib 400 mg twice daily is beneficial in patients with duodenal polyposis. The benefits of these agents in long-term use need to be closely weighed against the risk of potential gastrointestinal and cardiovascular side effects. Any use of chemopreventive agents requires vigilant endoscopic surveillance as breakthrough malignancies are documented in patients despite being maintained on a chemopreventive regimen.
Combination therapy with NSAIDs along with dietary supplements such as curcumin and PUFAs shows promise as chemopreventive agents but needs further randomized, controlled trials to verify efficacy and safety. As knowledge in the pathophysiology of FAP advances, more effective drugs and drug combinations will no doubt present themselves as potential chemopreventive agents.
Key points.
- The NSAID sulindac and COX-inhibitor celecoxib can reduce adenoma burden in the retained rectum of patients after colectomy with ileorectal anastamosis.
- The COX-2 inhibitor celecoxib may reduce diminutive duodenal adenomas.
- Although chemopreventive agents cause polyp regression, whether alteration of the progression to adenocarcinoma occurs is unclear, as case reports exist of patients developing malignancy despite chemopreventive regimens.
- If utilized, chemopreventive regimens should be accompanied by vigilant endoscopic surveillance and supplemented by ablation therapies.
- Combination therapy and dietary supplementation show promise in reducing polyposis in animal studies and small human trials.
Footnotes
Conflict of interest statement None.
References
- [1].Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–5. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- [2].Vasen HFA, Moslein G, Alonso A, Aretz S, Bernstein I, Bertario L, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP) Gut. 2008;57:704–13. doi: 10.1136/gut.2007.136127. [DOI] [PubMed] [Google Scholar]
- [3].Aziz O, Athanasiou T, Fazio VW, Nicholls RJ, Darzi AW, Church J, et al. Meta-analysis of observational studies of ileorectal versus ileal pouch-anal anastomosis for familial adenomatous polyposis. Br J Surg. 2006;93:407–17. doi: 10.1002/bjs.5276. [DOI] [PubMed] [Google Scholar]
- [4].Heiskanen I, Jarvinen HJ. Fate of the rectal stump after colectomy and ileorectal anastomosis for familial adenomatous polyposis. Int J Colorectal Dis. 1997;12:9–13. doi: 10.1007/s003840050070. [DOI] [PubMed] [Google Scholar]
- [5].Bulow S, Bjork J, Christensen IJ, Fausa O, Jarvinen H, Moesgaard F, et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut. 2004;53:381–6. doi: 10.1136/gut.2003.027771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- [7].Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, Dubois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–16. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- [8].Giardiello FM, Offerhaus GJA, DuBois RN. The role of nonsteroidal anti-inflammatory drugs in colorectal cancer prevention. Eur J Cancer. 1995;31A:1071–6. doi: 10.1016/0959-8049(95)00137-8. [DOI] [PubMed] [Google Scholar]
- [9].Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–9. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- [10].McEntee MF, Chiu CH, Whelan J. Relationship of β-catenin and Bcl-2 expression to sulindac-induced regression of intestinal tumors in Min mice. Carcinogenesis. 1999;20:635–40. doi: 10.1093/carcin/20.4.635. [DOI] [PubMed] [Google Scholar]
- [11].He T, Chan TA, Vogelstein B, Kinzler KW. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–45. doi: 10.1016/s0092-8674(00)81664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-β superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol. 2001;59:901–8. [PubMed] [Google Scholar]
- [13].Herrmann C, Block C, Geisen C, Haas K, Weber C, Winde G, et al. Sulindac sulfide inhibits Ras signaling. Oncogene. 1998;17:1769–76. doi: 10.1038/sj.onc.1202085. [DOI] [PubMed] [Google Scholar]
- [14].Stark LA, Reid K, Sansom OJ, Din FV, Guichard S, Mayer I, et al. Aspirin activates the NF-κB signaling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer. Carcinogenesis. 2007;28:968–76. doi: 10.1093/carcin/bgl220. [DOI] [PubMed] [Google Scholar]
- [15].Waddell W, Loughry RW. Sulindac for polyposis of the colon. J Surg Oncol. 1983;24:83–7. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- [16].Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–6. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- [17].Cruz-Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002;122:641–5. doi: 10.1053/gast.2002.31890. [DOI] [PubMed] [Google Scholar]
- [18].Labayle D, Fischer D, Vielh P, Drouhin F, Pariente A, Bories C, et al. Sulindac causes regression of rectal polyps in familial adenomatous polypois. Gastroenterology. 1991;101:635–9. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- [19].Nugent KP, Farmer KCR, Spigelman AD, Williams CB, Phillips RKS. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993;80:1618–9. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- [20].Giardiello FM, Yang VW, Hylind LM, Krush AJ, Petersen GM, Trimbath JD, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med. 2002;346:1054–9. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Waddell WR, Ganser GF, Cersie EJ, Loughry RW. Sulindac for polyposis of the colon. Am J Surg. 1989;157:175–9. doi: 10.1016/0002-9610(89)90442-x. [DOI] [PubMed] [Google Scholar]
- [22].Rigau J, Pique JM, Rubio E, Planas R, Tarrech JM, Bordas JM. Effects of long-term sulindac therapy on colonic polyposis. Ann Intern Med. 1991;115:952–4. doi: 10.7326/0003-4819-115-12-952. [DOI] [PubMed] [Google Scholar]
- [23].Spagnesi MT, Tonelli F, Dolara P, Caderni G, Valanzano R, Anastasi A, et al. Rectal proliferation and polyp occurrence in patients with familial adenomatous polyposis after sulindac treatment. Gastroenterology. 1994;106:362–6. doi: 10.1016/0016-5085(94)90593-2. [DOI] [PubMed] [Google Scholar]
- [24].Ishikawa H, Akedo I, Suzuki T, Narahara H, Otani T. Adverse effects of sulindac used for prevention of colorectal cancer. J Natl Cancer Inst. 1997;89:1381. doi: 10.1093/jnci/89.18.1381. [DOI] [PubMed] [Google Scholar]
- [25].Winde G, Schmid KW, Brandt B, Muller O, Osswald H. Clinical and genomic influence of sulindac on rectal mucosa in familial adenomatous polyposis. Dis Colon Rectum. 1997;40:1156–69. doi: 10.1007/BF02055161. [DOI] [PubMed] [Google Scholar]
- [26].Tonelli F, Valanzano R, Messerini L, Ficari F. Long-term treatment with sulindac in familial adenomatous polyposis: is there an actual efficacy in prevention of rectal cancer? J Surg Oncol. 2000;74:15–20. doi: 10.1002/1096-9098(200005)74:1<15::aid-jso4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- [27].Fernandez-Lopez F, Conde-Freire R, Cadarso-Suarez C, Garcia-Iglesias J, Puente-Dominguez JL, Potel-Lesquereux J. Sulindac in familial adenomatous polyposis: evaluation by nuclear morphometry. Eur J Surg. 2001;167:375–81. doi: 10.1080/110241501750215285. [DOI] [PubMed] [Google Scholar]
- [28].Guldenschuh I, Hurlimann R, Muller A, Ammann R, Mullhaupt B, Dobbie Z. Relationship between APC genotype, polyp distribution, and oral sulindac treatment in the colon and rectum of patients with familial adenomatous polyposis. Dis Colon Rectum. 2001;44:1090–9. doi: 10.1007/BF02234627. [DOI] [PubMed] [Google Scholar]
- [29].Matsumoto T, Nakarmura S, Esaki M, Yao T, Iida M. Effect of the non-steroidal anti-inflammatory drug sulindac on colorectal adenomas of uncolectomized familial adenomatous polyposis. J Gastroenterol Hepatol. 2006;21:251–7. doi: 10.1111/j.1440-1746.2006.04181.x. [DOI] [PubMed] [Google Scholar]
- [30].Hirota C, Iida M, Aoyagi K, Matsumoto T, Tada S, Yao T. Effects of indomethacin suppositories on rectal polyposis in patients with familial adenomatous polyposis. Cancer. 1996;78:1660–5. [PubMed] [Google Scholar]
- [31].Akasu T, Yokoyama T, Sugihara K, Fujita S, Moriya Y, Kakizoe T. Peroral sustained-release indomethacin treatment for rectal adenomas in familial adenomatous polyposis: a pilot study. Hepatogastroenterology. 2002;49:1259–61. [PubMed] [Google Scholar]
- [32].Giardiello FM, Casero RA, Hamilton SR, Hylind LM, Trimbath JD, Geiman DE, et al. Prostanoids, ornithine decarboxylase, and polyamines in primary chemoprevention of familial adenomatous polyposis. Gastroenterology. 2004;126:425–31. doi: 10.1053/j.gastro.2003.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lynch HT, Thorson AG, Smyrk T. Rectal cancer after prolonged sulindac chemoprevention. Cancer. 1995;75:936–8. doi: 10.1002/1097-0142(19950215)75:4<936::aid-cncr2820750407>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- [34].Niv Y, Fraser GM. Adenocarcinoma in the rectal segment in familial polyposis coli is not prevented by sulindac therapy. Gastroenterology. 1994;107:854–7. doi: 10.1016/0016-5085(94)90136-8. [DOI] [PubMed] [Google Scholar]
- [35].Giardiello FM, Sannhake EW, Duobois RN, Hylind LM, Robinson CR, Hubbard WC, et al. Prostaglandin levels in human colorectal mucosa. Dig Dis Sci. 1998;43:311–6. doi: 10.1023/a:1018898120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Piazza GA, Rahm AK, Finn TS, Fryer BH, Li H, Stoumen AL, et al. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997;57:2452–9. [PubMed] [Google Scholar]
- [37].Piazza GA, Alberts DS, Hixon LJ, Paranka NS, Li H, Finn T, et al. Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res. 1997;57:2909–15. [PubMed] [Google Scholar]
- [38].Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, et al. Exisulind induction of apoptosis involves guanosine 3′,5′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated β-catenin. Cancer Res. 2000;60:3338–42. [PubMed] [Google Scholar]
- [39].Arber N, Kuwada S, Leshno M, Sjodahl R, Hultcrantz R, Rex D. Sporadic adenomatous polyp regression with exisulind is effective but toxic: a randomised, double blind, placebo controlled, dose-response study. Gut. 2006;55:367–73. doi: 10.1136/gut.2004.061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].van Stolk R, Stoner G, Haytone WL, Chan K, DeYoung B, Kresty L, et al. Phase I trial of exisulind (sulindac dulfone, FGN-1) as a chemopreventive agent in patients with familial adenomatous polyposis. Clin Cancer Res. 2000;6:78–89. [PubMed] [Google Scholar]
- [41].Wight NJ, Gottesdiener K, Garlick NM, Atherton CT, Novak S, Gertz BJ, et al. Rofecosib, a COX-2 inhibitor, does not inhibit human gastric mucosal prostaglandin production. Gastroenterology. 2001;120:867–73. doi: 10.1053/gast.2001.22432. [DOI] [PubMed] [Google Scholar]
- [42].Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet. 2004;364:665–74. doi: 10.1016/S0140-6736(04)16893-1. [DOI] [PubMed] [Google Scholar]
- [43].Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- [44].Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim KM, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- [45].Baron JA, Sandler RS, Bresalier RS, Quan H, Riddell R, Lanas A, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674–82. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- [46].Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- [47].Lynch PM, Ayers GD, Hawk E, Richmond E, Eagle C, Woloj M, et al. The safety and efficacy of celecoxib in children with familial adenomatous polyposis. Am J Gastroenterol. 2010;105:1437–43. doi: 10.1038/ajg.2009.758. [DOI] [PubMed] [Google Scholar]
- [48].Higuchi T, Iwama T, Yoshinaga K, Toyooka M, Taketo MM, Sugihara K. A randomized, double-blind, placebo-controlled trial of the effect of rofecoxib, a selective cyclooxygenase-2 inhibitor, on rectal polyps in familial adenomatous polyposis patients. Clin Cancer Res. 2003;9:4756–60. [PubMed] [Google Scholar]
- [49].Iwama T, Akasu T, Utsunomiya J, Muto T. Does a selective cyclooxygenase-2 inhibitor (tiracoxib) induce clinically sufficient suppression of adenomas in patients with familial adenomatous polyposis? A randomized double-blind placebo-controlled clinical trial. Int J Clin Oncol. 2006;11:133–9. doi: 10.1007/s10147-005-0548-z. [DOI] [PubMed] [Google Scholar]
- [50].Dolara P, Caderni G, Tonelli F. Nimesulide, a selective anti-inflammatory cyclooxygenase-2 inhibitor, does not affect polyp number and mucosal proliferation in familial adenomatous polyposis. Scand J Gastroenterol. 1999;34:1168. doi: 10.1080/003655299750025002. [DOI] [PubMed] [Google Scholar]
- [51].Hallak A, Alon-Baron L, Shamir R, Moshkowitz M, Bulvik B, Brazowski E. Rofecoxib reduces polyp recurrence in familial polyposis. Dig Dis Sci. 2003;48:1998–2002. doi: 10.1023/a:1026130623186. [DOI] [PubMed] [Google Scholar]
- [52].Solomon SD, McMurray JJV, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–80. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- [53].Baron JA, Sandler RS, Bresalier RS, Lanas A, Morton DG, Riddell R, et al. Cardiovascular events associated with rofecoxib: final analysis of the APPROVe trial. Lancet. 2008;372:1756–64. doi: 10.1016/S0140-6736(08)61490-7. [DOI] [PubMed] [Google Scholar]
- [54].Solomon SD, Wittes J, Finn PV, Fowler R, Viner J, Bertagnolli MM. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 2008;117:2104–13. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].ADAPT Research Group Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimter’s Disease Anti-Inflammatory Prevention Trial (ADAPT) PLoS Clin Trials. 2006;1:e33. doi: 10.1371/journal.pctr.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zell JA, Pelot D, Chen WP, McLaren CE, Gerner EW, Meyskens FL. Risk of cardiovascular events in a randomized placebo-controlled, double-blind trial of difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas. Cancer Prev Res. 2009;2:209–12. doi: 10.1158/1940-6207.CAPR-08-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- [58].Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, Piednoir B. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125:328–36. doi: 10.1016/s0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
- [59].Logan RFA, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134:29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- [60].Cassidy A, Bingham SA, Cummings JH. Starch intake and colorectal cancer risk: an international comparison. Br J Cancer. 1994;69:937–42. doi: 10.1038/bjc.1994.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Burn J, Bishop DT, Chapman PD, Elliott F, Bertario L, Dunlop MG, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res. 2011;4:655–65. doi: 10.1158/1940-6207.CAPR-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Pegg AE, McCann PP. Polyamine metabolism and function. Am J Physiol. 1982;43:CC2212–21. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- [63].Giardiello FM, Hamilton SR, Hylind LM, Yang VW, Tamez P, Casero RA. Ornithine decarboxylase and polyamines in familial adenomatous polyposis. Cancer Res. 1997;57:199–201. [PubMed] [Google Scholar]
- [64].Meyskens FL, Gerner EW. Development of difluoromethylornitine (DFMO) as a chemoprevention agent. Clin Cancer Res. 1999;5:945–51. [PubMed] [Google Scholar]
- [65].Nigro ND, Bull AW, Boyd M. Inhibition of intestinal carcinogenesis in rats: effect of difluoromethylornithine with piroxicam or fish oil. J Natl Cancer Inst. 1986;77:1309–13. [PubMed] [Google Scholar]
- [66].Lawson KR, Ignatenko NA, Piazza GA, Cui H, Gerner EW. Influence of K-ras activation on the survival response of Caco-2 cells to the chemopreventive agents sulindac and difluormethylornithine. Cancer Epidemiol Biomarkers Prev. 2000;9:1155–62. [PubMed] [Google Scholar]
- [67].Jacoby RF, Cole CE, Tutsch K, Newton MA, Kelloff G, Hwak ET, et al. Chemopreventive efficacy of combined piroxicam and difluoromethylornithine treatment of Apc mutant Min mouse adenomas, and selective toxicity against Apc mutant embryos. Cancer Res. 2000;60:1864–70. [PubMed] [Google Scholar]
- [68].Ignatenko NA, Besselsen DG, Stringer DE, Blohm-Mangone KA, Cui H, Gerner EW. Combination chemoprevention of intestinal carcinogenesis in a murine model of familial adenomatous polposis. Nutr Cancer. 2008;60:30–5. doi: 10.1080/01635580802401317. [DOI] [PubMed] [Google Scholar]
- [69].Meyskens FL, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res. 2008;1:32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Earnest DL, Holubec H, Wali RK, Jolley CS, Bissonette M, Bhattacharyya AK, et al. Chemoprevention of azoxymethane-induced colonic carcinogenesis by supplemental dietary ursodeoxycholic acid. Cancer Res. 1994;54:5071–4. [PubMed] [Google Scholar]
- [71].Tung BY, Emond MJ, Haggitt RC, Bronner MP, Kimmey MB, Kowdley KV. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med. 2001;134:89–95. doi: 10.7326/0003-4819-134-2-200101160-00008. [DOI] [PubMed] [Google Scholar]
- [72].Pardi DS, Loftus EV, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–93. doi: 10.1053/gast.2003.50156. [DOI] [PubMed] [Google Scholar]
- [73].Wali RK, Khare S, Tretiakova M, Cohen G, Nguyen L, Hart J, et al. Ursodeoxycholic acid and F6-D3 inhibit aberrant crypt proliferation in the rat azoxymethane model of colon cancer: roles of cyclin D1 and E-cacherin. Cancer Epidemiol Biomarkers Prev. 2002;11:1653–62. [PubMed] [Google Scholar]
- [74].Jacoby RF, Cole CE, Hwak ET, Lubet RA. Ursodeoxycholate/sulindac combination treatment effectively prevents intestinal adenomas in a mouse model of polyposis. Gastroenterology. 2004;127:838–44. doi: 10.1053/j.gastro.2004.06.003. [DOI] [PubMed] [Google Scholar]
- [75].Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- [76].Pedersen TR, Berg K, Cook TJ, Faergeman O, Haghfelt T, Kjekshus J, et al. Safety and tolerability of cholesterol lowering with simvastatin during 5 years in the Scandinavian Simvasatin Survival Study. Arch Intern Med. 1996;156:2085–92. [PubMed] [Google Scholar]
- [77].Poynter JN, Gruber SB, Higgins PDR, Almog R, Bonner JD, Rennert HS, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–92. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- [78].Swamy MV, Patlolla JMR, Steele VE, Kopelovich L, Reddy BS, Rao CV. Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCMin mice. Cancer Res. 2006;66:7370–7. doi: 10.1158/0008-5472.CAN-05-4619. [DOI] [PubMed] [Google Scholar]
- [79].Caygill CPJ, Hill MJ. Fish, n-3 fatty acids and human colorectal and breast cancer mortality. Eur J Cancer Prev. 1995;4:329–32. doi: 10.1097/00008469-199508000-00008. [DOI] [PubMed] [Google Scholar]
- [80].Norat T, Bingham S, Ferrari P, Slimani N, Jenab M, Mazuir M, et al. Meat, fish, and colorectal cancer risk: the European prospective investigation into cancer and nutrition. J Natl Cancer Inst. 2005;97:906–16. doi: 10.1093/jnci/dji164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Fini L, Piazzi G, Ceccarelli C, Daoud Y, Belluzzi A, Munarini A. Highly purified eicosapentaenoic acid as a free fatty acids strongly suppresses polyps in ApcMin/+ Mice. Clin Cancer Res. 2010;1:5703–11. doi: 10.1158/1078-0432.CCR-10-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Calviello G, Serini S, Piccioni E. n-3 polyunsaturated fatty acids and the prevention of colorectal cancer: molecular mechanisms involved. Curr Med Chem. 2007;14:3059–69. doi: 10.2174/092986707782793934. [DOI] [PubMed] [Google Scholar]
- [83].Hansen Petrik MB, McEntee MF, Chiu CH, Whelan J. Antagonism of arachidonic acid is linked to the antitumorigenic effect of dietary eicosapentaenoic acid in ApcMin/+ Mice. J Nutr. 2000;130:1153–8. doi: 10.1093/jn/130.5.1153. [DOI] [PubMed] [Google Scholar]
- [84].Dommels YEM, Haring MMG, Keestra NGM, Alink GM, van Bladeren PJ, van Ommen B. The role of cyclooxygenase in n-6 and n-3 polyunsaturated fatty acid mediated effects on cell proliferation, PGE2 synthesis and cytotoxicity in human colorectal carcinoma cell lines. Carcinogenesis. 2003;24:385–92. doi: 10.1093/carcin/24.3.385. [DOI] [PubMed] [Google Scholar]
- [85].Boudreau MD, Sohn KH, Rhee SH, Lee SW, Hunt JD, Hwang DH. Suppression of tumor cell growth both in nude mice and in culture by n-3 polyunsaturated fatty acids: mediation through cyclooxygenase-independent pathways. Cancer Res. 2001;61:1386–91. [PubMed] [Google Scholar]
- [86].West NJ, Clark SK, Phillips RKS, Hutchinson JM, Leiscester RJ, Belluzzi A, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918–25. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- [87].Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics. CA Cancer J Clin. 2000;2000(50):7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- [88].Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–66. [PubMed] [Google Scholar]
- [89].Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, et al. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597–601. [PubMed] [Google Scholar]
- [90].Mahmoud NN, Carothers AM, Grunberger D, Bilinski RT, Churchill MR, Martucci C, et al. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis. 2000;21:921–7. doi: 10.1093/carcin/21.5.921. [DOI] [PubMed] [Google Scholar]
- [91].Piper JT, Singhal SS, Salameh MS, Torman RT, Awasthi UC, Awasthi S. Mechanisms of anticarcinogenic properties of curcumin: the effect of curcumin on glutathione linked detoxification enzymes in rat liver. Int J Biochem Cell Biol. 1998;38:445–56. doi: 10.1016/s1357-2725(98)00015-6. [DOI] [PubMed] [Google Scholar]
- [92].Plummer SM, Holloway KA, Manson MM, Munks RJL, Kaptein A, Farrow S, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-κB activation via the NIK/IKK signaling complex. Oncogene. 1999;18:6013–20. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- [93].Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, et al. Pharmacodynamic and pharmacokinetic study of oral cucuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–900. [PubMed] [Google Scholar]
- [94].Cruz-Correa M, Shoskes DA, Sanchez P, Zhao Rhongua, Hylind LM, Wexner SD. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–8. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- [95].Bussey HJR, DeCosse JJ, Deschner EE, Eyers AA, Lesser ML, Morson BC, et al. A randomized trial of ascorbic acid in polyposis coli. Cancer. 1982;50:1434–9. doi: 10.1002/1097-0142(19821001)50:7<1434::aid-cncr2820500733>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [96].DeCosse JJ, Miller HH, Lesser ML. Effect of wheat fiber and vitamins C and E on rectal polyps in patients with familial adenomatous polyposis. J Natl Cancer Inst. 1989;81:1290–7. doi: 10.1093/jnci/81.17.1290. [DOI] [PubMed] [Google Scholar]
- [97].Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther. 2010;32:789–803. doi: 10.1016/j.clinthera.2010.04.024. [DOI] [PubMed] [Google Scholar]
- [98].Thomas MG, Thomson JPS, Williamson RCN. Oral calcium inhibits rectal epithelial proliferation in familial adenomatous polyposis. Br J Surg. 1993;80:499–501. doi: 10.1002/bjs.1800800432. [DOI] [PubMed] [Google Scholar]
- [99].Nugent KP, Spigelman AD, Phillips RK. Life expectancy after colectomy and ileorectal anastamosis for familial adenomatous polyposis. Dis Colon Rectum. 1993;36:1059–62. doi: 10.1007/BF02047300. [DOI] [PubMed] [Google Scholar]
- [100].Wallace MH, Phillips RKS. Upper gastrointestinal disease in patients with familial adenomatous polyposis. Br J Surg. 1998;85:742–50. doi: 10.1046/j.1365-2168.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- [101].Debinski HS, Trojan J, Nugent KP, Spigelman AD, Phillips RKS. Effect of sulindac on small polyps in familial adenomatous polyposis. Lancet. 1995;345:855–6. [PubMed] [Google Scholar]
- [102].Seow-Choen F, Vijayan V, Keng V. Prospective randomized study of sulindac versus calcium and claciferol for upper gastrointestinal polyps in familial adenomatous polyposis. Br J Surg. 1996;83:1763–6. doi: 10.1002/bjs.1800831232. [DOI] [PubMed] [Google Scholar]
- [103].Richard CS, Berk T, Bapat BV, Haber G, Cohen Z, Gallinger S. Sulindac for periampullary polyps in FAP patients. Int J Colorectal Dis. 1997;12:14–8. doi: 10.1007/s003840050071. [DOI] [PubMed] [Google Scholar]
- [104].Phillips RKS, Wallace MH, Lynch PM, Hawk E, Gordon GB, Saunders BP, et al. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut. 2002;50:857–60. doi: 10.1136/gut.50.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Wallace MH, Forbes A, Beveridge IG, Spigelman AD, Hewer A, Venitt S, et al. Randomized, placebo-controlled trial of gastric acid-lowering therapy on duodenal polyposis and relative adduct labeling in familial adenomatous polyposis. Dis Colon Rectum. 2001;44:1585–9. doi: 10.1007/BF02234376. [DOI] [PubMed] [Google Scholar]
- [106].Brosens LAA, Iacobuzio-Donahue CA, Keller JJ, Hustinx SR, Carvalho R, Morsink FH, et al. Increased cyclooxygenase-2 expression in duodenal compared with colonic tissues in familial adenomatous polyposis and relationship to the −765G → C COX-2 polymorphism. Clin Cancer res. 2005;11:4090–6. doi: 10.1158/1078-0432.CCR-04-2379. [DOI] [PubMed] [Google Scholar]