The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury (original) (raw)
Abstract
The human miR-29 family of microRNAs has three mature members, miR-29a, miR-29b, and miR-29c. miR-29s are encoded by two gene clusters. Binding sites for several transcriptional factors have been identified in the promoter regions of miR-29 genes. The miR-29 family members share a common seed region sequence and are predicted to target largely overlapping sets of genes. However, the miR-29 family members exhibit differential regulation in several cases and different subcellular distribution, suggesting their functional relevance may not be identical. miR-29s directly target at least 16 extracellular matrix genes, providing a dramatic example of a single microRNA targeting a large group of functionally related genes. Strong antifibrotic effects of miR-29s have been demonstrated in heart, kidney, and other organs. miR-29s have also been shown to be proapoptotic and involved in the regulation of cell differentiation. It remains to be explored how various cellular effects of miR-29s determine functional relevance of miR-29s to specific diseases and how the miR-29 family members may function cooperatively or separately.
Keywords: microRNA, kidney, heart
microRNAs are endogenous, small, noncoding RNA molecules that regulate the expression of protein-coding genes. Readers are referred to several previous articles for detailed reviews of the biogenesis and action of microRNAs in general (3, 10, 38, 43). As of September 26, 2011, the miRBase database (36) lists 1,424 identified microRNAs in human, 720 in mouse, and 408 in rat. MicroRNAs have been reported to impact on numerous biological processes and diseases including renal and cardiovascular physiology and diseases (4, 8, 34, 49, 55, 79, 81, 103). While many microRNAs remain poorly understood or controversial, recent studies have provided significant insights into the biology of the miR-29 family of microRNAs and the relevance of miR-29s to renal and cardiovascular injury.
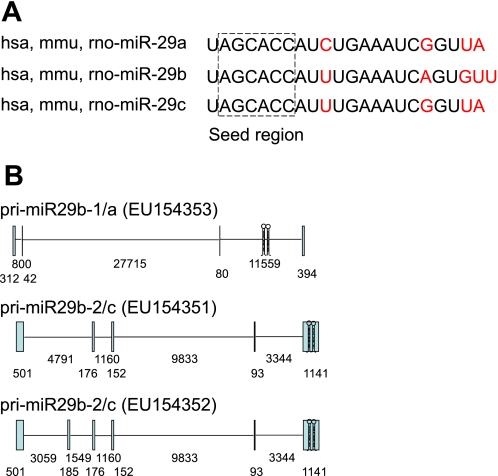
The miR-29 family in human includes hsa-miR-29a, hsa-miR-29b-1, hsa-miR-29b-2, and hsa-miR-29c. miR-29b-1 and miR-29b-2 have identical mature sequences, which are together called miR-29b. Mature miR-29s are highly conserved in human, mouse, and rat. Mature miR-29s share identical sequences at nucleotide positions 2–7, the seed region that plays a key role in determining which protein-coding genes a microRNA would target (Fig. 1_A_). Therefore, predicted target genes for the miR-29 family members largely overlap.
Fig. 1.
Mature sequences and primary transcripts of the miR-29 family. A: mature sequences of the miR-29 family members are conserved in human (hsa-), mouse (mmu-), and rat (rno-), and share identical seed regions. Nucleotides that differ among the miR-29 family members are shown in red. B: primary transcripts of the miR-29 gene clusters in human. Exons and introns are shown as vertical boxes and horizontal lines, respectively. The hairpins indicate the locations of the sequences encoding precursors of miR-29s. Numbers indicate the nucleotide length of each exon or intron.
GENOMIC ORGANIZATION
The gene encoding the precursors of miR-29b-1 and miR-29a is located on chr. 7q32.3 in human, while the gene encoding miR-29b-2 and miR-29c is on chr. 1q32.2. The sequences encoding the two microRNAs in each cluster are separated by <1 kb (11, 21, 61). RACE and RT-PCR analyses have confirmed that miR-29b-1 and miR-29a are transcribed together as a polycistronic primary transcript (11, 61). Likewise, miR-29b-2 and miR-29c are transcribed together (Fig. 1_B_). Both primary transcripts are transcribed from the [−] strand, with miR-29b-1 and miR-29b-2 being upstream of miR-29a and miR-29c, respectively.
Human miR-29b-2 and miR-29c are encoded by the last exon of the miR-29b-2/c primary transcript (GenBank accession numbers: EU154351 and EU154352) (11). In contrast, the precursors of miR-29b-1 and miR-29a are processed from the last intron of the primary transcript EU154353 (11) (Fig. 1_B_). A study informed by an expressed sequence tag (BI768447) identified a new splice variant indicating that the precursors of miR-29b-1 and miR-29a can be generated from the last exon of a different primary transcript (GU321462) (61). Cell type and other factors may determine which splicing pattern dominates. While both intronic and exonic coding will eventually yield mature miR-29b and miR-29a, the alternative splicing may influence the regulation of the expression of these microRNAs.
The genomic organization of miR-29 genes in rodents is less well characterized but appears to share many characteristics of the human genes, including the gene clusters. For example, rat miR-29b-1 and miR-29a are clustered on chr. 4 and miR-29b-2 and miR-29c on chr. 13.
REGULATION OF miR-29 EXPRESSION
Transcriptional Regulation
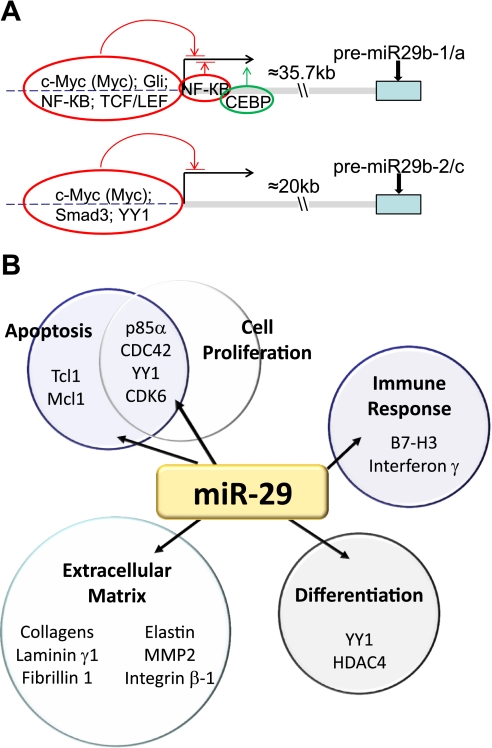
Like most microRNAs and mRNAs, miR-29s are transcribed by RNA polymerase II. Recent studies have identified several critical cis elements in the proximal region of miR-29 gene promoters (Fig. 2_A_). Chromatin immunoprecipitation analysis has identified strong myc binding in the vicinity of the transcription start site of both miR-29b-1/a and miR-29b-2/c clusters (11, 61). Other studies have confirmed additional transcriptional factor binding sites including a Gli binding site at −424 and three NF-κB binding sites at −561, −110, and +134 in the human miR-29b-1/a promoter (61); a Smad3 binding site in a highly conserved region ∼22 kb upstream of miR-29b-2 (72); at least one Yin-Yang-1 (YY1) binding site in the miR-29b-2/c promoter (92); a CEBP binding site located at +15 to +29 bp immediately downstream of the miR-29b-1/a transcription start site (21); and two TCF/LEF binding site within the proximal promoter of miR-29b-1/a (33).
Fig. 2.
Transcriptional regulators and target genes of miR-29s. A: transcriptional factors with experimentally supported binding sites in miR-29 promoter regions. B: several cellular processes and genes that have been reported to be targeted by miR-29s.
In addition to the presence of these binding sites, miR-29 expression has been shown to be regulated by various transcriptional regulators and signaling pathways. Kapinas et al. (33) reported that miR-29a transcription was regulated by Wnt signaling, which is important in human osteoblast differentiation. Aberrant expression of miR-29s, resulting from dysregulation of signaling pathways, contributes to the development of disease processes. For example, NF-κB plays a central role in the regulation of myoblasts proliferation and differentiation in part by regulating the YY1-miR-29 negative regulatory circuit. Constitutive activation of NF-κB-YY1 pathway in rhabdomyosarcoma suppresses miR-29b/c. As a result, the uncontrolled YY1 level promotes tumor development (92). In cholangiocarcinoma cells, activation of c-myc, hedgehog, and TLR/NF-κB signaling pathways suppresses the miR-29b-1/a promoter activity (61). Other factors, such as CCAAT/enhancer binding protein alpha (CEBPA), may activate miR-29 expression. Loss of the activation by CEBPA in acute myeloid leukemia leads to silencing of miR-29b (21). Developmental changes and cell type-specific distribution of miR-29 expression, such as that reported in the murine lung (18), further support the presence of dynamic regulation of miR-29 expression.
Posttranscriptional Regulation
It appears that the three mature miR-29s may be regulated by distinct mechanisms in some cases, even though they are cotranscribed in two primary transcripts. For example, miR-29a is constitutively expressed in HeLa cells, while miR-29b exhibits low-level expression, with rapid degradation, except for during mitosis (29). miR-29c is not expressed at any significant level. In the renal medulla of the Dahl salt-sensitive (SS) rat and a consomic rat strain derived from it, miR-29a appears to be more abundant than miR-29b and miR-29c and the three miR-29s respond differently to 3 days of a high-salt diet (53).
The molecular mechanism underlying differential regulation of miR-29 family members remains to be further explored. Posttranscriptional processing or stability of mature miR-29s may contribute importantly to the observed differential regulation. Utilization of alternative promoters could also play a role. Pulse-chase analysis in HeLa cells indicated that miR-29b and miR-29c mimics were degraded faster than miR-29a mimic. Mutational analysis suggested that the rapid decay of miR-29b might involve uracil at nucleotide positions 9–11 (102).
Subcellular Distribution: Nucleus vs. Cytoplasm
In an elegant study by Hwang et al. (29), it was discovered that in HeLa cells different miR-29s have distinctly different subcellular localization. miR-29a is primarily localized to the cytosoplasm with some nuclear presence. In contrast, miR-29b is significantly enriched in the nucleus (29). The six nucleotides on the 3′-end of miR-29b were found to be required for nuclear localization. Mutations within this region impaired nuclear localization of exogenously delivered miR-29b oligonucleotides, while other internal deletion mutations do not prevent nuclear localization. Furthermore, the importance of this six-nucleotide sequence was evident when addition to the 3′-end of an unrelated siRNA induced its nuclear enrichment (29).
The nuclear localization of miR-29b is intriguing, but not unique. Recently deep sequencing of small RNAs in nuclear and cytoplasmic fractions of human nasopharyngeal carcinoma cells found that most mature miRNAs are imported into the nucleus to some degree (51). However, it is also clear that several miRNAs, including miR-29b, are actually enriched in the nucleus. In this analysis miR-29b had a 4.54-fold higher abundance in the nucleus than in the cytoplasm. Only miR-32 and miR-148 were enriched to a greater extent. This analysis also detected miR-29c, finding that it was enriched 2.84-fold in the nucleus (51). Cytosolic and nuclear miRNAs may interact with different proteins and elicit different biological effects that remain to be elucidated.
CELLULAR EFFECTS
Regulation of Extracellular Matrix
An exciting possibility that we and others have postulated is that a microRNA may have significant impact on a functional phenotype by regulating multiple genes that fall into the same or related pathways (47). The regulation of extracellular matrix by the miR-29 family is a dramatic example of a single microRNA family regulating a large set of functionally related genes. Our laboratory and other investigators have collectively shown that miR-29 family members target at least 16 genes related to extracellular matrix. These genes code for several of the key proteins involved in the physiological or pathological formation of extracellular matrix, including a large number of collagen isoforms, laminin γ1, fibrilin 1, elastin, matrix metalloproteinase 2, and integrin β1 (46, 53, 78, 88) (Fig. 2_B_).
The interaction between miR-29s and mRNAs encoding these extracellular matrix genes has been shown in many cases to be mediated by seed region binding in the 3′-untranslated region (UTR) (53). Bioinformatic analyses, primarily using TargetScan with support from PicTar and in some cases miRanda, indicate that the 3′-UTRs of 20 collagen genes contain predicted, conserved binding sites for miR-29s (53). The large number of collagen isoforms as predicted targets of miR-29s is not because of any extensive sequence homology among 3′-UTRs of collagen isoforms. Instead, the phenomenon is unique to miR-29s because no other miRNA was predicted to target more than 11 of the 20 collagen genes (53). Targeting by miR-29s often results in decreases of mRNA abundance of extracellular matrix genes, suggesting the effect of miR-29s on these target genes is at least in part mediated by decreasing mRNA stability. Analysis of target mRNA abundance is a viable approach for studying cellular effects of miR-29s in this case. Analysis of target proteins or proteomes (37, 86) would be physiologically more relevant but technically more challenging.
The regulation of extracellular matrix by miR-29s has been implicated in the development of fibrosis in many organs including heart (88), kidney (53, 72), lung (67), and liver (75, 77), as well as systemic sclerosis (59). Interestingly, it has been suggested that miR-29b can also prevent liver fibrosis by blocking activation of hepatic stellate cells through cell arresting mechanisms (77). Transforming growth factor (TGF)-β, an important transcriptional stimulator of many extracellular matrix genes (40, 44, 63, 85), may be an important factor that downregulates miR-29s in fibrotic conditions including in renal epithelial cells (20, 72), human fetal lung fibroblasts (18), and human trabecular meshwork cells (57). The relevance of miR-29 regulation of extracellular matrix to tissue fibrosis will be discussed in detail later in this article.
Regulation of Cell Proliferation, Differentiation, and Apoptosis
The importance of miR-29s in the regulation of cell proliferation, differentiation, and apoptosis is best illustrated by the role of miR-29s in cancer. miR-29s apparently serve as tumor suppressors in several cases, although they could be oncogenic in some cases. Downregulation of miR-29 family members has been correlated with many types of cancer including leukemia (9, 25, 26, 71), melanoma (64), and liver (96), colon (17), cervical (45), and lung (99) cancer. In many studies downregulation of miR-29 correlated with more aggressive forms of cancer or relapse (9, 71, 104), suggesting therapeutic restoration of miR-29 may improve disease prognosis. A beneficial effect of exogenous miR-29a and miR-29b treatment has been demonstrated experimentally in acute myeloid leukemia. Induced expression of miR-29a and miR-29b slowed cell growth and induced apoptosis of leukemia cells in vitro and exogenous delivery of miR-29b to xenografted K562 cell tumors was effective in reducing their size (26).
One of the mechanisms by which miR-29s suppress tumor growth is by relieving the suppression of p53. The p53 transcription factor is important in controlling expression of genes that regulate cell growth, senescence, apoptosis, and genome integrity in response to stress (2, 39, 74, 90, 91). Suppression or inactivation of p53 is a common characteristic in many types of cancer (28, 66, 84, 93). All three miR-29 family members can target p85α and CDC42, genes that normally suppress p53 expression (68) (Fig. 2_B_).
Several miR-29 targets are oncogenes or anti-apoptotic genes (Fig. 2_B_). It has been suggested that loss of miR-29 regulation of Tcl-1 facilitate the upregulation of Tcl-1 observed in aggressive B-chronic lymphocytic leukemia (71). The Tcl-1 proto-oncogene is an important coactivator of Akt, mediating antiapoptotic signaling in B and T cells (41, 70). Another important miR-29 family target, whose regulation would impact malignant cell survival, is the Bcl-2 family member, Mcl-1 (60, 96). Mcl-1 is an antiapoptotic protein that is overexpressed in acute myeloid leukemia (35). Expression of the oncogene CDK6, targeted by all miR-29 family members, is required for cell cycle to progress into S-phase (104). Other mechanisms possibly underlying the tumor-suppressive effect of miR-29s include regulation of aberrant DNA methylation by targeting DNA methyltransferases 3A and 3B (22, 26), promotion of proper myoblast differentiation but not rhabdomyosarcomagenesis by targeting YY1(92), modulation of immunomodulatory molecule B7-H3 to suppress the immune escape by solid tumors (97), suppression of interferon γ (58), and targeting B-Myb that is involved with cell proliferation and apoptosis(1, 31). In HPV-mediated cervical cancer restoration of miR-29a and miR-29b expression might aid in preventing malignant transformation of cervical cells by blocking the cell cycle at G1 and facilitating apoptosis through regulation of YY1 and CDK6 (45). Interestingly, loss of the regulation of extracellular matrix by miR-29s, besides its major effect on fibrosis, may also contribute to cancer cell migration and metastasis. For example, the downregulation of miR-29c in nasopharyngeal carcinomas might contribute to the metastatic tumor invasion through reduced regulation of extracellular matrix targets or related proteins (78).
Upregulation of miR-29s has also been shown to occur in some types of cancer. In breast cancer the miR-29a upregulation may induce malignancy by suppression of tristetraprolin (27). Moreover, miR-29 transgenic mice develop an indolent B-cell chronic lymphocytic leukemia phenotype (69, 76).
miR-29s may also contribute to normal tissue differentiation (Fig. 2_B_). In a cell model of myogenic differentiation, upregulation of miR-29 can attenuate the inhibitory action of TGF-β on myogenesis through targeting HDAC4, a key inhibitor of muscle differentiation (95). Derepression of miR-29 was shown to accelerate skeletal myogenesis by targeting its repressor YY1 (92). miR-29s regulate osteoblast differentiation targeting antiosteogenic factors and extracellular matrix proteins (46) as well as Wnt signaling antagonists (33).
RELEVANCE TO CARDIOVASCULAR AND RENAL PHYSIOLOGY AND DISEASE
Heart
In 2008, van Rooij et al. (88) reported a reduction in expression of all three miR-29 isoforms in the areas bordering the infarcted myocardium in mice and miR-29b in human samples. Upon further analysis it was determined that cardiac fibroblasts were the primary cell type responsible for miR-29 expression in the heart. Additionally, treatment of isolated mouse cardiac fibroblasts with TGF-β reduced expression of miR29a, miR-29b, and miR-29c (88). It has been known for some time that TGF-β is important for stimulating cardiac fibrosis (6). Knockdown of miR-29 family members relieves the suppression of many targeted extracellular matrix genes involved with fibrogenesis both in vivo and in vitro (88, 100). Cardiac pressure overloading and chronic calcineurin signaling have also been shown to reduce miR-29c expression in animal models (88, 89), while miR-29b is downregulated in end-stage dilated cardiomyopathy (62). In dilated cardiomyopathy the left ventricle is dilated and becomes more compliant while the activity of extracellular matrix degrading matrix metalloproteinase increases (7, 12, 82, 87). Matrix metalloproteinase 2 is a confirmed miR-29b target (53). In total, these studies indicate that expression of miR-29 family members may be important for regulating extracellular matrix expression during pathological remodeling in cardiac tissue.
Soci et al. (80) showed that an intensive exercise protocol upregulated miR-29c and reduced expression of several extracellular matrix genes. They found ventricular compliance was increased. This study suggests that expression of miRNAs can be altered beneficially under physiological conditions (80).
Other studies indicate that suppression of miR-29 genes may be ineffectual, or even cardioprotective, in some contexts. The miRNA expression patterns in response to treatment of adult cardiac fibroblasts with angiotensin II revealed an upregulation of miR-29b, which was mediated through the angiotensin II type 1 receptor (30). It would be interesting to see if the upregulation of miR-29b contributes to the injurious effects of angiotensin II or represents a compensatory response. Another study reported that in vivo suppression of miR-29a and miR-29c protected hearts from ischemia reperfusion injury (100, 101).
Kidney
An important role of miR-29b in renal injury was identified in our studies of the Dahl SS rat (53). The SS rat is a well-established model of human common, salt-sensitive forms of hypertension and renal injury (14, 15, 73). The consomic SS.13BN rat, in which chromosome 13 of the SS genome has been replaced by chromosome 13 from the Brown Norway (BN) rat, exhibits substantially attenuated hypertension and renal injury (16, 48, 50, 56). We found that miR-29b in the renal medulla was upregulated by 3 days of a high-salt diet much more in SS.13BN rats than in SS rats. In vivo knockdown experiments in SS.13BN rats using intravenous administration of locked nucleic acid-modified anti-miR and extensive in vitro experiments showed that miR-29b targeted several extracellular matrix genes (Col1a1, Col3a1, Col4a1, Col5a1, Col5a2, Col5a3, Col7a1, Col8a1, Mmp2, and Itgb1) and likely contributed to the protection against interstitial fibrosis in the SS.13BN rat (53).
Further evidence for renoprotective effects of miR-29s in renal fibrosis was reported in a study of a mouse model of obstructive nephropathy (72). Severe tubulointerstital fibrosis was associated with reduced expression of miR-29s. Overexpression of miR-29b attenuated renal fibrosis in this model. Study of cultured renal tubular cells showed that miR-29b was downregulated by TGF-β1 via Smad3 (42, 72). Studies in our laboratories showed that miR-29c was downregulated in a rat model of progressive renal failure and in patients with renal interstitial fibrosis and was restored by renoprotective treatments in the rat model (unpublished data).
Long et al. (54) found that miR-29c was upregulated in glomeruli from db/db mice and in kidney microvascular endothelial cells and podocytes cultured in high ambient glucose. Systemic treatment with 2′-_O_-methyl-modified antisense complementary to the mature miR-29c sequence reduced albuminuria and mesangial matrix accumulation in db/db mice. The injurious effect of miR-29c was associated with proapoptotic effects of miR-29c on cultured podocytes and targeting of Sprouty homolog 1. This is reminiscent of the well-established proapoptotic effect of miR-29s in cancer cells. In cultured HK-2 cells, a human kidney epithelial cell line, high glucose was reported to downregulate miR-29a, which might contribute to upregulation of collagen IV expression (20).
Vasculature and Circulation
Upregulation of miR-29 has been reported to play an important role in suppressing elastin and other extracellular matrix genes during aortic development in the mouse (65). The study further indicated that a change from TGF-β signaling to Wnt signaling during aortic development may be related to the changes in miRNA expression and mRNA expression that occur. miR-29 expression levels in the aorta continue to be important after development. An investigation of miRNA expression in human experiencing aortic dissection revealed a downregulation of miR-29a and miR-29c, which may play a role in pathological extracellular matrix expression and focal adhesion (52). Boon et al. (5) reported that the miR-29 family members were upregulated in the aorta in aged mice and mouse models of aortic aneurysms as well as in biopsies of human thoracic aneurysms. Knockdown of miR-29 attenuated angiotensin II-induced dilation of the aorta in mice. The anti-miR oligonucleotides used in this study matched nucleotide numbers 2–17 of miR-29b and miR-29c but had a mismatch with miR-29a at nucleotide number 10. However, all three miR-29s were shown to be efficiently knocked down by the anti-miR at the dosages used (5).
All three miR-29 family members have also been found to regulate the important clotting factor fibrinogen (24). Elevated fibrinogen levels have been associated with cardiovascular disease (19, 23, 32, 83, 94) and even reduced systolic function in otherwise healthy individuals (98). Fibrinogen is produced in the liver, where miR-29 family downregulation has been associated with fibrosis and disease (75). Perhaps the downregulation of miR-29 in the liver may impact the cardiovascular system indirectly by relieving the suppression of fibrinogen.
Patients with cirrhotic livers have lower miR-29 expression in the tissue, as well as lower circulating miR-29a (75). Levels of miR-29b have been found to be higher in the plasma of smokers (13). While it has not been determined if these circulating miRNAs would be capable of effecting gene expression in end organs, circulating miRNAs may have diagnostic or prognostic values.
FUTURE DIRECTIONS
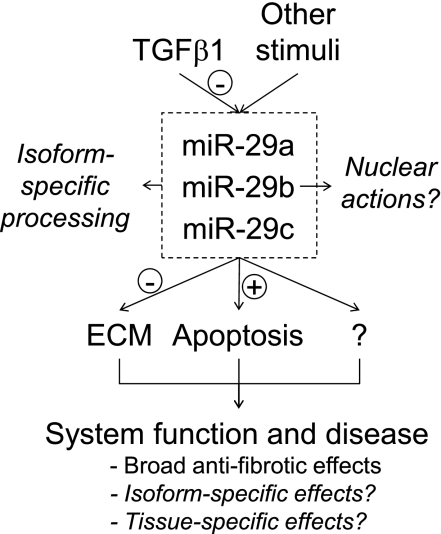
Recent progress in the understanding of the miR-29 family is beginning to paint a clear picture in which miR-29s play potent antifibrotic and proapoptotic roles in several disease processes (Fig. 3). The antifibrotic effect of miR-29s fits the observation that miR-29 expression is downregulated by TGF-β1, a key profibrotic factor. This, however, does not rule out the possibility that miR-29s could participate in other biological pathways. In fact, members of the miR-29 family exhibit highly diverse characteristics in the regulation of their expression and subcellular localization, suggesting complex functions of miR-29s that are likely to be isoform and tissue specific.
Fig. 3.
An overview of the biology and disease relevance of the miR-29 family.
Several important questions regarding the biology and disease relevance of miR-29s should be addressed in future studies (Fig. 3). It will be important to further explore the mechanisms underlying the differential regulation of expression and subcellular localization of miR-29 family members, as well as physiological and disease implications of such differential regulation. Strong evidence exists for antifibrotic and proapoptotic effects of miR-29s. It will be important to understand how these effects of miR-29s, and perhaps other cellular effects of miR-29s that are currently less appreciated, manifest in specific physiological or disease contexts. Finally, potential diagnostic and therapeutic values of miR-29s remain to be examined or established.
GRANTS
This work was supported by US National Institutes of Health Grants HL-085267, DK-084405, HL-082798, and HL-029587, a Clinical and Translational Science Institute grant (to M. Liang), and National Natural Science Foundation of China Grants 30871176 and 30971374 (to X. Ding).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Arturo S. B-MYB, a transcription factor implicated in regulating cell cycle, apoptosis and cancer. Eur J Cancer 41: 2479– 2484, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Aylon Y, Oren M. p53: Guardian of ploidy. Mol Oncol 5: 315– 323, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215– 233, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt K, Mi QS, Dong Z. microRNAs in kidneys: biogenesis, regulation, and pathophysiological roles. Am J Physiol Renal Physiol 300: F602– F610, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, Vinciguerra M, Rosenthal N, Sciacca S, Pilato M, van Heijningen P, Essers J, Brandes RP, Zeiher AM, Dimmeler S. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ Res 109: 1115– 1119, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med 331: 1286– 1292, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Brower GL, Janicki JS. Contribution of ventricular remodeling to pathogenesis of heart failure in rats. Am J Physiol Heart Circ Physiol 280: H674– H683, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol 23: 175– 205, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, Iuliano R, Palumbo T, Pichiorri F, Roldo C, Garzon R, Sevignani C, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Engl J Med 353: 1793– 1801, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell 136: 642– 655, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, Mendell JT. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 40: 43– 50, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coker ML, Thomas CV, Clair MJ, Hendrick JW, Krombach RS, Galis ZS, Spinale FG. Myocardial matrix metalloproteinase activity and abundance with congestive heart failure. Am J Physiol Heart Circ Physiol 274: H1516– H1523, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, Wagner DR, Staessen JA, Heymans S, Schroen B. Circulating microRNA-208b and microRNA-499 reflect myocardial damage in cardiovascular disease / clinical perspective. Circ Cardiovas Genet 3: 499– 506, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Cowley AW., Jr The genetic dissection of essential hypertension. Nat Rev Genet 7: 829– 840, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Cowley AW, Jr, Stoll M, Greene AS, Kaldunski ML, Roman RJ, Tonellato PJ, Schork NJ, Dumas P, Jacob HJ. Genetically defined risk of salt sensitivity in an intercross of Brown Norway and Dahl S rats. Physiol Genomics 2: 107– 115, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Cowley AW, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension 37: 456– 461, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Cummins JM, He Y, Leary RJ, Pagliarini R, Diaz LA, Jr, Sjoblom T, Barad O, Bentwich Z, Szafranska AE, Labourier E, Raymond CK, Roberts BS, Juhl H, Kinzler KW, Vogelstein B, Velculescu VE. The colorectal microRNAome. Proc Natl Acad Sci USA 103: 3687– 3692, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, Thannickal VJ, Cardoso WV, Lu J. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol 45: 287– 294, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, Goldbourt U, Willeit J, Kiechl S, Yarnell JW, Sweetnam PM, Elwood PC, Cushman M, Psaty BM, Tracy RP, Tybjaerg-Hansen A, Haverkate F, de Maat MP, Fowkes FG, Lee AJ, Smith FB, Salomaa V, Harald K, Rasi R, Vahtera E, Jousilahti P, Pekkanen J, D'Agostino R, Kannel WB, Wilson PW, Tofler G, Arocha-Pinango CL, Rodriguez-Larralde A, Nagy E, Mijares M, Espinosa R, Rodriquez-Roa E, Ryder E, Diez-Ewald MP, Campos G, Fernandez V, Torres E, Marchioli R, Valagussa F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Cremer P, Nagel D, Curb JD, Rodriguez B, Yano K, Salonen JT, Nyyssonen K, Tuomainen TP, Hedblad B, Lind P, Loewel H, Koenig W, Meade TW, Cooper JA, De Stavola B, Knottenbelt C, Miller GJ, Bauer KA, Rosenberg RD, Sato S, Kitamura A, Naito Y, Palosuo T, Ducimetiere P, Amouyel P, Arveiler D, Evans AE, Ferrieres J, Juhan-Vague I, Bingham A, Schulte H, Assmann G, Cantin B, Lamarche B, Despres JP, Dagenais GR, Tunstall-Pedoe H, Woodward M, Ben-Shlomo Y, Davey Smith G, Palmieri V, Yeh JL, Rudnicka A, Ridker P, Rodeghiero F, Tosetto A, Shepherd J, Ford I, Robertson M, Brunner E, Shipley M, Feskens EJ, Kromhout D, Dickinson A, Ireland B, Juzwishin K, Kaptoge S, Lewington S, Memon A, Sarwar N, Walker M, Wheeler J, White I, Wood A. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA 294: 1799– 1809, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Du B, Ma LM, Huang MB, Zhou H, Huang HL, Shao P, Chen YQ, Qu LH. High glucose down-regulates miR-29a to increase collagen IV production in HK-2 cells. FEBS Lett 584: 811– 816, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Eyholzer M, Schmid S, Wilkens L, Mueller BU, Pabst T. The tumour-suppressive miR-29a/b1 cluster is regulated by CEBPA and blocked in human AML. Br J Cancer 103: 275– 284, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C, Volinia S, Guler G, Morrison CD, Chan KK, Marcucci G, Calin GA, Huebner K, Croce CM. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA 104: 15805– 15810, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folsom AR, Wu KK, Rosamond WD, Sharrett AR, Chambless LE. Prospective study of hemostatic factors and incidence of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 96: 1102– 1108, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Fort A, Borel C, Migliavacca E, Antonarakis SE, Fish RJ, Neerman-Arbez M. Regulation of fibrinogen production by microRNAs. Blood 116: 2608– 2615, 2010. [DOI] [PubMed] [Google Scholar]
- 25.Garzon R, Garofalo M, Martelli MP, Briesewitz R, Wang L, Fernandez-Cymering C, Volinia S, Liu CG, Schnittger S, Haferlach T, Liso A, Diverio D, Mancini M, Meloni G, Foa R, Martelli MF, Mecucci C, Croce CM, Falini B. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci USA 105: 3945– 3950, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garzon R, Heaphy CE, Havelange V, Fabbri M, Volinia S, Tsao T, Zanesi N, Kornblau SM, Marcucci G, Calin GA, Andreeff M, Croce CM. MicroRNA 29b functions in acute myeloid leukemia. Blood 114: 5331– 5341, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep 10: 400– 405, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goh AM, Coffill CR, Lane DP. The role of mutant p53 in human cancer. J Pathol 223: 116– 126, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Hwang HW, Wentzel EA, Mendell JT. A hexanucleotide element directs microRNA nuclear import. Science 315: 97– 100, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Jeppesen PL, Christensen GL, Schneider M, Nossent AY, Jensen HB, Andersen DC, Eskildsen T, Gammeltoft S, Hansen JL, Sheikh SP. Angiotensin II type 1 receptor signaling regulates microRNA differentially in cardiac fibroblasts and myocytes. Br J Pharmacol 164: 394– 404, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joaquin M, Watson RJ. Cell cycle regulation by the B-Myb transcription factor. Cell Mol Life Sci 60: 2389– 2401, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kannel WB, Wolf PA, Castelli WP, D'Agostino RB. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA 258: 1183– 1186, 1987. [PubMed] [Google Scholar]
- 33.Kapinas K, Kessler C, Ricks T, Gronowicz G, Delany AM. miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J Biol Chem 285: 25221– 25231, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol 4: 1255– 1266, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling X, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10: 375– 388, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucl Acids Res 39: D152– D157, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kriegel AJ, Fang Y, Liu Y, Tian Z, Mladinov D, Matus IR, Ding X, Greene AS, Liang M. MicroRNA-target pairs in human renal epithelial cells treated with transforming growth factor beta 1: a novel role of miR-382. Nucleic Acids Res 38: 8338– 8347, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11: 597– 610, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Kruse JP, Gu W. Modes of p53 regulation. Cell 137: 609– 622, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuang PP, Zhang XH, Rich CB, Foster JA, Subramanian M, Goldstein RH. Activation of elastin transcription by transforming growth factor-β in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 292: L944– L952, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Laine J, Künstle G, Obata T, Sha M, Noguchi M. The protooncogene TCL1 is an Akt kinase coactivator. Mol Cell 6: 395– 407, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Lan HY, Chung AC. Transforming growth factor-beta and Smads. Contrib Nephrol 170: 75– 82, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843– 854, 1993. [DOI] [PubMed] [Google Scholar]
- 44.Lei J, Silbiger S, Ziyadeh FN, Neugarten J. Serum-stimulated α1 type IV collagen gene transcription is mediated by TGF-β and inhibited by estradiol. Am J Physiol Renal Physiol 274: F252– F258, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Wang F, Xu J, Ye F, Shen Y, Zhou J, Lu W, Wan X, Ma D, Xie X. Progressive miRNA expression profiles in cervical carcinogenesis and identification of HPV-related target genes for miR-29. J Pathol 224: 484– 495, 2011. [DOI] [PubMed] [Google Scholar]
- 46.Li Z, Hassan MQ, Jafferji M, Aqeilan RI, Garzon R, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem 284: 15676– 15684, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang M. MicroRNA: a new entrance to the broad paradigm of systems molecular medicine. Physiol Genomics 38: 113– 115, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang M, Lee NH, Wang H, Greene AS, Kwitek AE, Kaldunski ML, Luu TV, Frank BC, Bugenhagen S, Jacob HJ, Cowley AW. Molecular networks in Dahl salt-sensitive hypertension based on transcriptome analysis of a panel of consomic rats. Physiol Genomics 34: 54– 64, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Liang M, Liu Y, Mladinov D, Cowley AW, Jr, Trivedi H, Fang Y, Xu X, Ding X, Tian Z. MicroRNA: a new frontier in kidney and blood pressure research. Am J Physiol Renal Physiol 297: F553– F558, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang M, Yuan B, Rute E, Greene AS, Olivier M, Cowley AW., Jr Insights into Dahl salt-sensitive hypertension revealed by temporal patterns of renal medullary gene expression. Physiol Genomics 12: 229– 237, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Liao JY, Ma LM, Guo YH, Zhang YC, Zhou H, Shao P, Chen YQ, Qu LH. Deep sequencing of human nuclear and cytoplasmic small RNAs reveals an unexpectedly complex subcellular distribution of miRNAs and tRNA 3′ trailers. PLoS One 5: e10563, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao M, Zou S, Weng J, Hou L, Yang L, Zhao Z, Bao J, Jing Z. A microRNA profile comparison between thoracic aortic dissection and normal thoracic aorta indicates the potential role of microRNAs in contributing to thoracic aortic dissection pathogenesis. J Vasc Surg 53: 1341– 1349, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, Taylor NE, Lu L, Usa K, Cowley AW, Jr, Ferreri NR, Yeo NC, Liang M. Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension 55: 974– 982, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Long J, Wang Y, Wang W, Chang BHJ, Danesh FR. MicroRNA-29c is a signature microRNA under high glucose conditions that targets sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem 286: 11837– 11848, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lorenzen JM, Haller H, Thum T. MicroRNAs as mediators and therapeutic targets in chronic kidney disease. Nat Rev Nephrol 7: 286– 294, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Lu L, Li P, Yang C, Kurth T, Misale M, Skelton M, Moreno C, Roman RJ, Greene AS, Jacob HJ, Lazar J, Liang M, Cowley AW., Jr Dynamic convergence and divergence of renal genomic and biological pathways in protection from Dahl salt-sensitive hypertension. Physiol Genomics 41: 63– 70, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. Cross-talk between miR-29 and transforming growth factor-betas in trabecular meshwork cells. Invest Ophthalmol Visual Sci 52: 3567– 3572, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma F, Xu S, Liu X, Zhang QX, Xu X, Liu M, Hua M, Li N, Yao H, Cao X. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat Immunol 12: 861– 869, 2011. [DOI] [PubMed] [Google Scholar]
- 59.Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, Kowal-Bielecka O, Gay RE, Michel BA, Distler JH, Gay S, Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum 62: 1733– 1743, 2010. [DOI] [PubMed] [Google Scholar]
- 60.Mott JL, Kobayashi S, Bronk SF, Gores GJ. miR-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 26: 6133– 6140, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem 110: 1155– 1164, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naga Prasad SV, Duan ZH, Gupta MK, Surampudi VSK, Volinia S, Calin GA, Liu CG, Kotwal A, Moravec CS, Starling RC, Perez DM, Sen S, Wu Q, Plow EF, Croce CM, Karnik S. Unique microRNA profile in end-stage heart failure indicates alterations in specific cardiovascular signaling networks. J Biol Chem 284: 27487– 27499, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Neumann C, Yu A, Welge-Lüssen U, Lütjen-Drecoll E, Birke M. The effect of TGF-β2 on elastin, type VI collagen, and components of the proteolytic degradation system in human optic nerve astrocytes. Invest Ophthalmology Visual Sci 49: 1464– 1472, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen T, Kuo C, Nicholl MB, Sim MS, Turner RR, Morton DL, Hoon DS. Downregulation of microRNA-29c is associated with hypermethylation of tumor-related genes and disease outcome in cutaneous melanoma. Epigenetics 6: 388– 394, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ott CE, Grünhagen J, MJäger Horbelt D, Schwill S, Kallenbach K, Guo G, Manke T, Knaus P, Mundlos S, Robinson PN. MicroRNAs differentially expressed in postnatal aortic development downregulate elastin via 3′ UTR and coding-sequence binding sites. PLoS One 6: e16250, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ozaki T, Nakagawara A. p53: the attractive tumor suppressor in the cancer research field. J Biomed Biotechnol 2011: 603925, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pandit KV, Milosevic J, Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res 157: 191– 199, 2011. [DOI] [PubMed] [Google Scholar]
- 68.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85[alpha] and CDC42. Nat Struct Mol Biol 16: 23– 29, 2009. [DOI] [PubMed] [Google Scholar]
- 69.Pekarsky Y, Croce CM. Is miR-29 an oncogene or tumor suppressor in CLL? Oncotarget 1: 224– 227, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pekarsky Y, Koval A, Hallas C, Bichi R, Tresini M, Malstrom S, Russo G, Tsichlis P, Croce CM. Tcl1 enhances Akt kinase activity and mediates its nuclear translocation. Proc Natl Acad Sci USA 97: 3028– 3033, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pekarsky Y, Santanam U, Cimmino A, Palamarchuk A, Efanov A, Maximov V, Volinia S, Alder H, Liu CG, Rassenti L, Calin GA, Hagan JP, Kipps T, Croce CM. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res 66: 11590– 11593, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, Sung JJ, Lan HY. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol 22: 1462– 1474, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rapp JP. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension 4: 753– 763, 1982. [DOI] [PubMed] [Google Scholar]
- 74.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol 9: 402– 412, 2008. [DOI] [PubMed] [Google Scholar]
- 75.Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M, Tacke F, Trautwein C, Luedde T. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology 53: 209– 218, 2011. [DOI] [PubMed] [Google Scholar]
- 76.Santanam U, Zanesi N, Efanov A, Costinean S, Palamarchuk A, Hagan JP, Volinia S, Alder H, Rassenti L, Kipps T, Croce CM, Pekarsky Y. Chronic lymphocytic leukemia modeled in mouse by targeted miR-29 expression. Proc Natl Acad Sci USA 107: 12210– 12215, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sekiya Y, Ogawa T, Yoshizato K, Ikeda K, Kawada N. Suppression of hepatic stellate cell activation by microRNA-29b. Biochem Biophys Res Commun 412: 74– 79, 2011. [DOI] [PubMed] [Google Scholar]
- 78.Sengupta S, den Boon JA, Chen IH, Newton MA, Stanhope SA, Cheng YJ, Chen CJ, Hildesheim A, Sugden B, Ahlquist P. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci USA 105: 5874– 5878, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature 469: 336– 342, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soci UPR, Fernandes T, Hashimoto NY, Mota GF, Amadeu MA, Rosa KT, Irigoyen MC, Phillips MI, Oliveira EM. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol Genomics 43: 665– 673, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soifer HS, Rossi JJ, Saetrom P. MicroRNAs in disease and potential therapeutic applications. Mol Ther 15: 2070– 2079, 2007. [DOI] [PubMed] [Google Scholar]
- 82.Spinale FG, Coker ML, Thomas CV, Walker JD, Mukherjee R, Hebbar L. Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res 82: 482– 495, 1998. [DOI] [PubMed] [Google Scholar]
- 83.Stec JJ, Silbershatz H, Tofler GH, Matheney TH, Sutherland P, Lipinska I, Massaro JM, Wilson PFW, Muller JE, D'Agostino RB. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the Framingham offspring population. Circulation 102: 1634– 1638, 2000. [DOI] [PubMed] [Google Scholar]
- 84.Suzuki K, Matsubara H. Recent advances in p53 research and cancer treatment. J Biomed Biotechnol 2011: 978312, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sysa P, Potter JJ, Liu X, Mezey E. Transforming growth factor-beta1 up-regulation of human alpha(1)(I) collagen is mediated by Sp1 and Smad2 transacting factors. DNA Cell Biol 28: 425– 434, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tian Z, Greene AS, Pietrusz JL, Matus IR, Liang M. MicroRNA-target pairs in the rat kidney identified by microRNA microarray, proteomic, and bioinformatic analysis. Genome Res 18: 404– 411, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tyagi SC, Kumar SG, Haas SJ, Reddy HK, Voelker DJ, Hayden MR, Demmy TL, Schmaltz RA, Curtis JJ. Posttranscriptional regulation of extracellular matrix metalloproteinase in human heart end-stage failure secondary to ischemic cardiomyopathy. J Mol Cell Cardiol 28: 1415– 1428, 1996. [DOI] [PubMed] [Google Scholar]
- 88.Van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA 105: 13027– 13032, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci USA 103: 18255– 18260, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 408: 307– 310, 2000. [DOI] [PubMed] [Google Scholar]
- 91.Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell 137: 413– 431, 2009. [DOI] [PubMed] [Google Scholar]
- 92.Wang H, Garzon R, Sun H, Ladner KJ, Singh R, Dahlman J, Cheng A, Hall BM, Qualman SJ, Chandler DS, Croce CM, Guttridge DC. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell 14: 369– 381, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Whibley C, Pharoah PD, Hollstein M. p53 polymorphisms: cancer implications. Nat Rev Cancer 9: 95– 107, 2009. [DOI] [PubMed] [Google Scholar]
- 94.Wilhelmsen L, Svärdsudd K, Korsan-Bengtsen K, Larsson B, Welin L, Tibblin G. Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med 311: 501– 505, 1984. [DOI] [PubMed] [Google Scholar]
- 95.Winbanks CE, Wang B, Beyer C, Koh P, White L, Kantharidis P, Gregorevic P. TGF-beta regulates miR-206 and miR-29 to control myogenic differentiation through regulation of HDAC4. J Biol Chem 286: 13805– 13814, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y, Jia WH, Zhuang SM. Effects of MicroRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 51: 836– 845, 2010. [DOI] [PubMed] [Google Scholar]
- 97.Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7–H3: potential implications for immune based therapy of human solid tumors. Cancer Res 69: 6275– 6281, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan RT, Fernandes V, Yan AT, Cushman M, Redheuil A, Tracy R, Vogel-Claussen J, Bahrami H, Nasir K, Bluemke DA, Lima JAC. Fibrinogen and left ventricular myocardial systolic function: The Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 160: 479– 486, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, Calin GA, Liu CG, Croce CM, Harris CC. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 9: 189– 198, 2006. [DOI] [PubMed] [Google Scholar]
- 100.Ye Y, Hu Z, Lin Y, Zhang C, Perez-Polo JR. Downregulation of microRNA-29 by antisense inhibitors and a PPAR-γ agonist protects against myocardial ischaemia-reperfusion injury. Cardiovasc Res 87: 535– 544, 2010. [DOI] [PubMed] [Google Scholar]
- 101.Ye Y, Perez-Polo JR, Qian J, Birnbaum Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol Genomics 43: 534– 542, 2011. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Z, Zou J, Wang GK, Zhang JT, Huang S, Qin YW, Jing Q. Uracils at nucleotide position 9–11 are required for the rapid turnover of miR-29 family. Nucleic Acids Res 39: 4387– 4395, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhao Y, Srivastava D. A developmental view of microRNA function. Trends Biochem Sci 32: 189– 197, 2007. [DOI] [PubMed] [Google Scholar]
- 104.Zhao JJ, Lin J, Lwin T, Yang H, Guo J, Kong W, Dessureault S, Moscinski LC, Rezania D, Dalton WS, Sotomayor E, Tao J, Cheng JQ. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood 115: 2630– 2639, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]