Effects of age and beta-amyloid on cognitive changes in normal elderly people (original) (raw)
. Author manuscript; available in PMC: 2013 Dec 1.
Abstract
Age-related decline is common in multiple cognitive domains. β-amyloid (Aβ) deposition, a pathological hallmark of Alzheimer’s disease, is also associated with cognitive changes in many older people. In this study, we examined a wide range of cognitive function in order to differentiate the effect of age and Aβ on cognition during aging. Using PET imaging with the radiotracer Pittsburgh compound B (PIB), we classified normal older subjects as High PIB-Old and Low PIB-Old and applied sequential multivariate analyses (i.e., principal components analysis [PCA] and discriminant analysis) to obtain summary measures of cognitive tests encompassing multiple cognitive domains. Among 5 cognitive components, a significant age effect was observed in component scores of visual memory and executive functions, regardless of the level of Aβ. Discriminant scores (weighted scores of the 5 cognitive components) revealed a significant effect of both age and Aβ and were further associated with quantitative PIB counts. The results of the current study highlight both effects of age and Aβ on cognitive changes in normal elderly.
Keywords: Age, beta-amyloid, PIB-PET, cognition, principal component analysis, discriminant analysis
1. Introduction
Advanced age is commonly associated with lower performance on diverse cognitive tasks and structural and functional brain changes (Buckner, 2004; Grady & Craik, 2000). One of the cognitive processes that is detrimentally affected by advanced age is episodic memory, which refers to the conscious mental process of forming, retaining, and recalling information along with the spatio-temporal context in which the information was acquired (Grady & Craik, 2000; Tulving, 1983). Previous findings show that a network of brain regions including the medial temporal lobe (MTL), prefrontal cortex (PFC), parietal cortex, and sensory association cortices supports episodic memory with a particularly critical role of the hippocampus (Eichenbaum, 2000; Sperling et al., 2003; Wagner, Shannon, Kahn, & Buckner, 2005). Consistent with these results, studies on anatomical changes during normal aging indicate that advanced age is associated with widespread cortical thinning as well as volumetric reduction, but more prominent atrophy in PFC and MTL including the hippocampus (Rajah, Kromas, Han, & Pruessner, 2010; Raz et al., 1997).
Although a substantial amount of research has detected behavioral, structural, and functional changes associated with advanced age, the striking heterogeneity of the aging population poses a fundamental limit to understanding normal aging processes. One of the pathological changes that is found in cognitively intact older individuals is accumulation of β-amyloid (Aβ), a pathological hallmark of Alzheimer’s disease (AD), which is behaviorally characterized by severe memory decline (Gandy, 2005). Accumulation of amyloid can now be measured in vivo using positron emission tomography (PET) with radiotracers such as [11C]-Pittsburg Compound B (PIB) that bind to amyloid (Klunk et al., 2004). Post-mortem studies have indicated that about 30% of cognitively intact older adults harbor a high level of amyloid deposition that is roughly equivalent to that of AD patients (Bennett et al., 2006; J. L. Price & Morris, 1999)). Consistent with autopsy findings, in vivo PIB-PET studies have shown that accumulation of Aβ is present in a similar proportion of cognitively intact older people (Aizenstein et al., 2008). Such individuals show reduced cortical thickness and altered functional activity compared to those with low PIB binding (Dickerson et al., 2009; Fotenos, Mintun, Snyder, Morris, & Buckner, 2008; Sperling et al., 2009). Considering that a relatively large proportion of older adults with AD pathology may be found in a population traditionally viewed as “normal aging”, it remains unresolved to what extent age and Aβ pathology contributed to previous findings of age-related changes in cognition as well as brain structure and function.
With respect to the effect of Aβ deposition on cognition in older adults without dementia or mild cognitive impairment, the results are mixed. Aizenstein et al. (2008) examined the relationship of amyloid deposition and cognitive function in clinically unimpaired older adults and found no significant relationship between the two factors. Similar results from cross-sectional and longitudinal data showing no difference in cognition and rates of cognitive decline in relation to amyloid deposition also come from other studies (Bourgeat et al., 2010; Driscoll et al., 2006; Rowe et al., 2010). On the other hand, some report a significant difference in cognition or rates of cognitive decline as a function of Aβ deposition (Pike et al., 2007; Resnick et al., 2010; Villemagne et al., 2008) or Aβ – related brain atrophy (Mormino et al., 2009; Oh et al., 2011). A partial explanation of these varied results may be that neuropsychological testing measures might have not been sensitive enough to detect the difference in cognition due to Aβ deposition.
In order to examine effects of advanced age and Aβ on cognition, we developed a global measure that combined individual neuropsychological test scores while capturing differences driven by group membership by employing sequential multivariate analyses: principal components analysis (PCA) and discriminant analysis (DA). PCA allowed us to remap the individual’s scores to fewer scores in a more parsimonious way while ensuring greater reliability in measuring cognitive performance. Next, we applied DA that combined the component scores and improved the discriminatory power of the neuropsychological tests by group membership. The first aim of the present study was to examine the age-related difference in cognition by looking at group differences between healthy young and older adults who show little evidence of Aβ deposition. The second aim of the study was to examine whether, and by what cognitive aspects, older people with and without evidence of enhanced amyloid deposition may differ. Our primary goal was not, in fact, to define a set of neuropsychological tests that could be used to detect β-amyloid, but rather to compare the effects of age and Aβ on cognition in normal elderly.
2. Methods
2.1. Subjects
We performed PCA on a group of 297 participants. This included 189 cognitively intact older adults (mean age: 73.2 ± 7.3 years, range: 60–91 years, 126 females, mean MMSE = 28.9 ± 1.2) and 108 young adults (mean age: 24.7 ± 3.7 years, range: 20–34 years, 46 females, mean MMSE = 29.4 ± 0.9). All subjects were recruited from the community via advertisements and a subgroup completed PIB – PET, functional magnetic resonance imaging (MRI), structural MRI scans and genotyping for Apolipoprotein E (APOE) ε4 carrier status. All subjects underwent a medical interview and a detailed battery of neuropsychological tests. In order to be classified as cognitively intact older participants for the study, subjects were required to be 60 years or older, live independently in the community without neurological or psychiatric illness, and have no major medical illness or medication that could influence cognition. Fifty-two older subjects and 11 young subjects in the present study also participated in our previous study examining the relationship between amyloid deposition, gray matter volume, and cognition in cognitively intact older adults (Oh et al., 2011). All subjects provided informed consent in accordance with the Institutional Review Boards of the University of California, Berkeley and the Lawrence Berkeley National Laboratory prior to their participation.
Forty-four young and 52 older subjects who were included in the preceding PCA were subsequently included in the DA analysis (Table 1). The older subjects were selected if they had completed PIB-PET and structural MRI scans and the young adults were included if they had completed structural MRI scans. Eleven of 44 young adults also completed PIB-PET scans. Older adults were further grouped into High PIB - Old or Low PIB - Old as specified in the section 2.5.2, resulting in 18 older adults being classified as High PIB-Old and 34 as Low PIB-Old.
Table 1.
Characteristics of participants
| Young (n = 44) | Low PIB-Old (n = 34) | High PIB-Old (n = 18) | ||||
|---|---|---|---|---|---|---|
| M (Range) | SD | M (Range) | SD | M (Range) | SD | |
| Age (yrs) | 25.4(20.0 – 33.0) | 3.1 | 73.8 (61.4 – 88.2) | 5.8 | 74.8 (61.7– 87.3) | 6.6 |
| Education (yrs) | 16.6(14.0 – 20.0) | 1.9 | 17.5 (12.0 – 20.0) | 1.9 | 16.7 (13.0 – 20.0) | 2.0 |
| Gender (n, F/M)a | 23/21 | 20/14 | 14/4 | |||
| PIB index | 1.01(0.97 – 1.07)b | 0.03 | 1.02 (0.90 – 1.07) | 0.03 | 1.30(1.08 – 1.77) | 0.25 |
| APOE-ε4 (n[%])c | 11 (27.5%) | 9 (26.5%) | 7 (41.2%) | |||
| TIV | 1535.0(1184.2 – 1933.4) | 169.1 | 1611.0(1226.6–2019.9) | 172.3 | 1540.4(1284.9 – 1798.1) | 137.1 |
| MMSE | 29.3 (26.0 – 30.0) | 1.0 | 29.0 (26.0–30.0) | 1.2 | 29.2 (27.0–30.0) | 0.9 |
2.2. Neuropsychological testing
All subjects included in PCA completed 17 cognitive performance test measures. The tests included Free Recall Trials 1–5 (FR 1–5), Short-Delay Free Recall (SDFR), Short-Delay Cued Recall (SDCR), Long-Delay Free Recall (LDFR), and Long-Delay Cued Recall (LDCR) of California Verbal Learning Test (CVLT) II (Delis, Kramer, Kaplan, & Ober, 2000), Stroop Test (Golden, 1978), “Trail B minus A” scores from Trail Making Test (TMT) A and B (Reitan, 1958), Symbol Digit Modalities Test (Smith, 1982), category “Vegetables” and “Animals” from Category Fluency Test (Benton, Hamsher, & Sivan, 1983), Digit span forward and backward, Immediate Recall (VRI Recall Total), Delayed Recall (VRII Recall Total), Retention (VR % Retention) and Recognition (VR Recognition Total) from the Visual Reproduction Test in the Wechsler Memory Scale-Third Edition (WMS-III) (Wechsler, 1997), and Recall of Story A and Story B from Logical Memory (WMS-III; Wechsler, 1997).
2.3. Multivariate assessment of neuropsychological tests
To develop a more concise and reliable summary measure from multiple neuropsychological test measures, we conducted PCA followed by DA. PCA is a multivariate method that extracts underlying, unobserved components among observed variables that are correlated with each other to varying degrees, resulting in a reduction of a large number of variables into a smaller number of variables (Tabachnick & Fidell, 2001). First, cognitive test scores were entered into a PCA with varimax rotation. In order to ensure that cognitive test scores included in PCA are correlated with each other enough for components to emerge, the correlation matrix of cognitive test scores was examined to verify that a majority of correlations exceed R = .30 (Tabachnick & Fidell, 2001). Components were extracted with a criterion of an eigenvalue greater than 1 (Kaiser, 1960). Based on the individual tests that expressed the highest component loading scores for the component, each component was named as follows: executive function (EXE), verbal episodic memory (EM), semantic memory (SM), working memory (WM), and visual episodic memory (VM) (Table 2). Each subject’s score for each component was determined by regression of a subject’s raw test scores into the component loading scores for the component. The component scores are independent of each other so that they may be used as predictors in other analyses such as DA without the issue of multicollinearity (Tabachnick & Fidell, 2001). In order to evaluate the group differences in cognitive component scores without an inflated Type I error, we conducted multivariate analysis of variance (MANOVA) where group membership (Young, Low PIB - Old, and High PIB - Old) was an independent measure and 5 component scores were dependent measures. Once MANOVA verified that there was a significant effect of group membership on the dependent measures, univariate ANOVAs were conducted for each component, and the ANOVA results were evaluated at p < .05 with Bonferroni correction. Multiple regressions with PIB index being a predictor of interest and age and education as nuisance covariates were followed to assess the relationship between PIB index and component scores. Standardized beta values of PIB index were evaluated at p < .05.
Table 2.
Component loadings of individual cognitive measures contributing to each component
| Neuropsychological tests | Component loadings | ||||
|---|---|---|---|---|---|
| EM | VM | EXE | SM | WM | |
| CVLT LDCR | .916 | .227 | .119 | .167 | .014 |
| CVLT SDCR | .911 | .201 | .079 | .172 | .034 |
| CVLT LDFR | .898 | .258 | .154 | .149 | .017 |
| CVLT SDFR | .876 | .248 | .150 | .180 | .046 |
| CVLT 1–5 FR | .794 | .330 | .081 | .209 | .141 |
| LM A plus B1 | .340 | .414 | −.260 | .305 | .299 |
| VRII Recall Total | .278 | .902 | .150 | .102 | .080 |
| VR % Retention | .259 | .825 | .024 | .061 | −.036 |
| VRI Recall Total | .240 | .733 | .294 | .152 | .182 |
| VR Recognition Total | .218 | .732 | .165 | .110 | .038 |
| Symbol Digit | .233 | .566 | .514 | .211 | .153 |
| TrailBminusA | −.186 | −.197 | −.785 | −.123 | −.205 |
| Stroop Correct in 60 s | .167 | .486 | .541 | .271 | .203 |
| Vegetables Total | .319 | .054 | .113 | .819 | .095 |
| Animals Total | .231 | .310 | .212 | .769 | .076 |
| DS Forward | .033 | .083 | .109 | .033 | .844 |
| DS Backward | .042 | .061 | .163 | .106 | .834 |
DA was conducted with the 5 resulting component scores as independent measures and group membership (i.e., Young, Low PIB - Old, and High PIB - Old) as the dependent measure by the linear discriminant procedure. DA is a multivariate method that produces a weighted summary measure of a set of predictors in a way that maximizes discrimination of the dependent variables (Tabachnick & Fidell, 2001). We evaluated whether the resulting discriminant functions significantly discriminate the dependent measures by Wilk’s Lambda (Λ). Once the significance of discriminant functions was verified, we conducted a one-way ANOVA on the resulting discriminant scores between groups followed by multiple regression with PIB index being a predictor of interest and age and education as nuisance covariates. DA further allows us to test whether discriminant functions generated in one sample can be generalized to the population through a cross-validation procedure. In order to do this, we adopted a leave-one-out approach, rather than using an independent validation sample, due to the small sample size in our current data. Additionally, the linear discriminant procedure was followed by the stepwise discriminant procedure to confirm what predictor in the discriminant functions significantly contributed to the resulting discriminant scores. Assumptions of linearity were verified.
In addition to the cross-validation procedure using the leave-one-out method, we conducted receiver operating characteristics (ROC) analysis to evaluate the accuracy of classification using the discriminant scores. ROC methods allow us to test what proportion of cases are classified as true positives (i.e., sensitivity) vs. true negatives (i.e., specificity), and ROC curves are a graphical form of representing both sensitivity and specificity. The Area Under the Curve (AUC), the probability that a classifier will rank a randomly chosen positive instance higher than a randomly chosen negative one in the ROC graph was evaluated as a measure of the proportion of correct classifications (Metz, 1978). The cognitive data analyses were performed with SPSS v18.0 (SPSS Inc., Chicago, IL).
2.4. Imaging data acquisition
2.4.1. PIB-PET
All PET scans were performed at Lawrence Berkeley National Laboratory (LBNL) using a Siemens ECAT EXACT HR PET scanner in three-dimensional acquisition mode. Dynamic acquisition frames (total 34 frames) were obtained over 90 min as follows: 4x15, 8x30, 9x60, 2x180, 8x300 and 3x600 s. A detailed procedure for PIB-PET imaging data acquisition is described in a previously published study (Oh, et al., 2011).
2.4.2. Structural MRI
High-resolution structural MRI scans were collected at LBNL on a 1.5 T Magnetom Avanto system (Siemens Inc., Iselin, NJ) with a 12 channel head coil run in triple mode. Three high-resolution T1-weighted magnetization-prepared rapid gradient echo (MPPAGE) scans were collected axially for each subject (TR = 2110 ms, TE = 3.58 ms, flip angle: 15°, field of view = 256 X 256 mm, matrix size: 256 X 256 mm, slices: 160, voxel size = 1 X 1 X 1 mm3).
2.5. Imaging data analysis
2.5.1. PIB-PET
PIB-PET imaging data analysis methods are described in a previously published study (Oh, et al., 2011). Briefly, all PET images were preprocessed using Statistical Parametric Mapping 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm/). All 34 frames collected over 90 min were realigned to the middle frame (17th frame) and coregistered to the subject’s structural MRI image. A PIB distribution volume ratio (DVR) was calculated using Logan graphical analysis and a gray matter-masked cerebellum as reference region (Logan et al., 1996; J. C. Price et al., 2005).
2.5.2. Structural MRI
Region of Interest (ROI) labeling of structural MRI images was implemented using the FreeSurfer version 4.4 software package (http://surfer.nmr.mgh.harvard.edu) in order to create a gray-matter cerebellar reference region and to calculate a global PIB index encompassing cortical ROIs including frontal, temporal, and parietal cortices and anterior and posterior cingulate cortices. Older subjects were classified as High PIB - Old or Low PIB - Old based on the global PIB index based on a cutoff score obtained from 11 young control subjects. That is, older subjects were classified as High PIB – Old if their global PIB index fell 2 SD above the mean PIB index in 11 young control subjects (Mormino et al., 2012). The resulting cutoff score for classifying older subjects as High PIB – Old was 1.08. Although the cutoff score value is relatively low, the proportion of older subjects classified as High PIB – Old was approximately 35%, which is equivalent to those reported in other studies (Bennett, et al., 2006; Sperling, et al., 2009; Villemagne et al., 2011)). This very conservative threshold ensured that the Low PIB – Old group was unlikely to contain individuals with significant amyloid accumulation.
3. Results
3.1. Characteristics of participants
Demographics, global PIB index, and Mini Mental State Examination (MMSE) scores for all older and young subjects are summarized in Table 1. High PIB – Old vs. Low PIB - Old groups did not differ on any measures such as age, gender, education, APOE genotype, total intracranial volume (TIV), and MMSE. There was no significant difference in PIB index between the Low PIB – Old group and the young subjects for whom PIB data were available (Low PIB – Old: 1.02 ± 0.03; Young: 1.01 ± 0.03, p > .2).
3.2. Group means of neuropsychological tests
For 12 out of the 17 measures, a one-way ANOVA showed a significant group effect at p < .05 (df 2, 90), while 5 measures (CVLT short delay cued recall, CVLT long delay cued recall, Vegetables total, Digit span forward, and Logical memory A plus B1) did not show any significant differences across groups. Post-hoc t-tests with Bonferroni correction generally showed that group differences were the result of an effect of age rather than amyloid deposition (Supplementary Table 1). In a subset of measures, however, a differential group effect was shown. For CVLT long delay free recall and digit span backward scores, a significant difference was found between Young and High PIB - Old groups while no difference was found between Young and Low PIB - Old groups. No significant difference was found between High PIB – Old and Low PIB – Old groups on any raw test scores (Supplementary Table 1).
3.3. Neuropsychological components measured by PCA
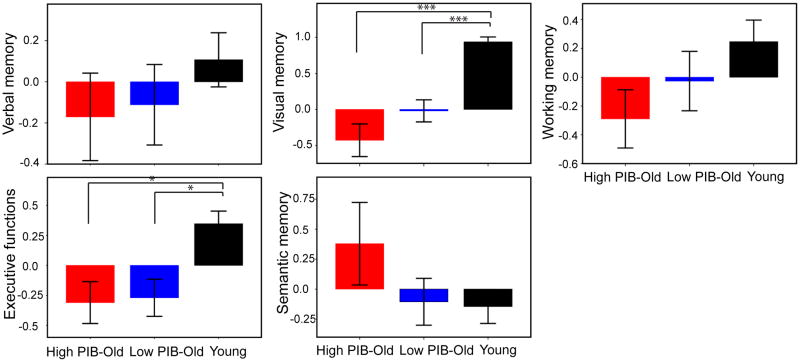
PCA revealed 5 components, which are verbal episodic memory (EM), visual episodic memory (VM), semantic memory (SM), working memory (WM), and executive functions (EXE). These 5 components accounted for 77.8 % of the total variance of the data. The component loadings are shown in Table 2 and the mean component scores for each group are depicted in Figure 1. Using group as independent measures and 5 component scores as dependent measures, MANOVA revealed that 3 groups were significantly different in a combination of 5 component scores, Wilk’s Lambda (Λ) = .41, F(10, 172) = 9.52, p < .001. Among 5 components, univariate ANOVAs produced a significant group effect for visual memory and executive function component scores (visual memory: F[2, 90] = 29.04, p < .001; executive function: F[2, 90] = 7.83, p < .01). As shown in Figure 1, post-hoc t tests further indicated that the differences between groups could be attributed to age effects rather than amyloid deposition in both components. Even though these cognitive domains did not show a group effect of amyloid deposition, we conducted multiple regressions in order to assess whether these cognitive domains affected by age are associated with amyloid deposition as measured by PIB index. By multiple regression controlling for age and years of education, PIB index significantly predicted visual episodic memory scores, β = −.28, p < .05, but not executive function scores (p > .10).
Figure 1.
Bar graphs represent mean factor scores by Young, Low PIB-Old, and High PIB-Old groups. Significant differences were detected on visual memory and executive function factor scores between Young and Low PIB-Old groups and between Young and High PIB-Old groups. * p < .05, *** p < .001.
Other component scores were not significantly different between groups. The component scores for the 3 groups were retained for discriminant analysis.
3.4. Discriminant function scores
In order to further reduce the cognitive data to obtain one global composite score encompassing 5 components, we conducted discriminant function analysis where two discriminant functions were obtained with the 5 components, chosen by the linear discriminant procedure for 3 levels of the dependent measure (i.e., group membership: Young, Low PIB – Old and High PIB – Old groups). The first discriminant function was statistically significant, Λ = .41, χ2 = 77.55, p < .001, and accounted for 98.0 % of the total between-group variability, showing that the created discriminant function correctly classified a total of 71 of 96 subjects (74%). The second discriminant function accounted for the remaining 2.0 % of variability, but was not significant, Λ = .97, χ2 = 2.44, p = .66. As shown in Table 3, higher weights of the first discriminant function were placed on visual memory and executive function scores. This result was further confirmed by the stepwise discriminant procedure showing that the addition of visual memory followed by executive functions and working memory to the function at each step resulted in significant Wilk’s Λs (Visual memory: Λ = .61, p < .001; Visual memory + Executive functions: Λ = .51, p < .001; Visual memory + Executive functions + Working memory: Λ = .46, p < .001). Because the second discriminant function was not significant in discriminating groups and the variance explained by it was minimal, we used scores from the first discriminant function only to represent cognitive function scores (i.e., discriminant scores in the following text) for subsequent analyses.
Table 3.
Linear discriminant function coefficients for discriminant analysis
| Component | Function coefficient | |
|---|---|---|
| Function 1 | Function 2 | |
| Verbal memory | .391 | −.084 |
| Visual memory | .933 | −.087 |
| Executive functions | .662 | .549 |
| Semantic memory | .025 | .905 |
| Working memory | .479 | −.155 |
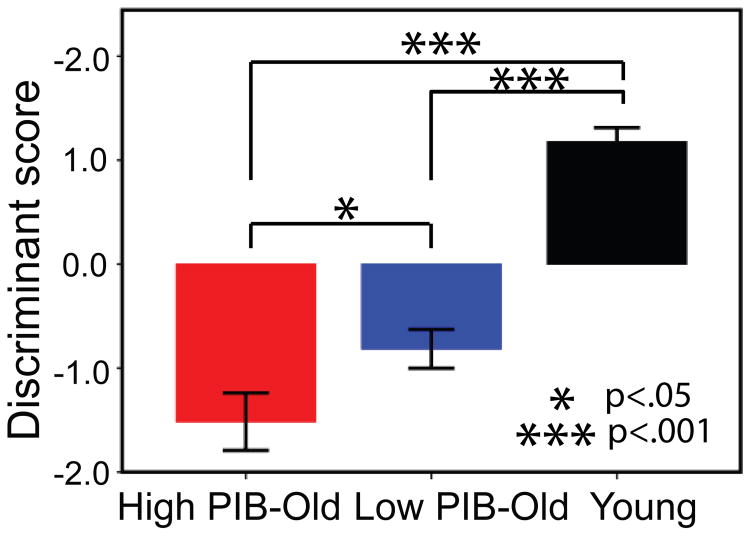
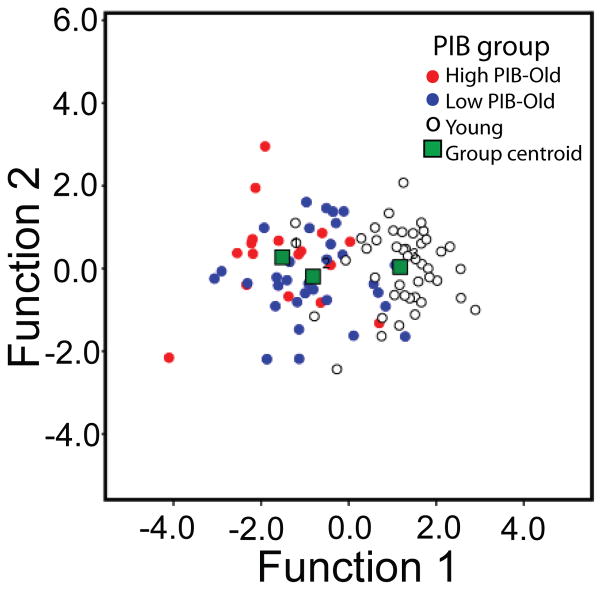
The significance of differences in resulting discriminant scores across groups was evaluated by a one-way ANOVA that showed that groups differed significantly on discriminant scores, F[2, 90] = 60.66, p < .001. Post-hoc t-tests with Bonferroni correction further confirmed significant differences between Young and Low PIB – Old (Young: M = 1.18, SD = 0.90; Low PIB – Old: M = − 0.81, SD = 1.06, p < .001) and between Young and High PIB – Old groups (Young: M = 1.18, SD = 0.90; High PIB – Old: M = − 1.51, SD = 1.14, p < .001). The difference between High PIB – Old and Low PIB – Old adults was also significant, (p = .032, once age was controlled for). Figure 2 depicts group mean differences in discriminant scores. Figure 3 shows a detailed distribution of subjects’ two discriminant function scores and their group memberships. Figure 3 clearly shows that only the first discriminant function separates the 3 groups, as mentioned previously, whereas no separation is made by the second discriminant function.
Figure 2.
Bar graph representing mean discriminant scores obtained from the first discriminant function by Young, Low PIB-Old, and High PIB-Old groups. * p < .05, *** p < .001.
Figure 3.
Scatterplot illustrating subjects’ group memberships as a function of two discriminant function scores.
In order to validate the generalizability of discriminant functions obtained from the present sample, we conducted cross-validation using a leave-one-out method that allows us to test the generalizability of the results without the requirement of an independent sample. The accuracy of classification in the original data and the cross-validation analysis is shown in Supplementary Table 2. In the cross-validated data, 66 of 96 subjects (68.8 %) were correctly classified as their original group membership. The classification results of cross-validation are much higher compared to the proportion of each group in the total sample, which is 18.8%, 35.4% and 45.8% for High PIB – Old, Low PIB – Old, and Young groups, respectively.
Because discriminant scores showed significant effects of both age and amyloid deposition, we further assessed whether discriminant scores are associated with quantitative PIB indices of all older subjects. Multiple regressions with older subjects’ PIB index being a predictor of interest and age being a nuisance variable of no interest revealed that PIB index significantly predicted discriminant scores, β = −.28, p < .05.
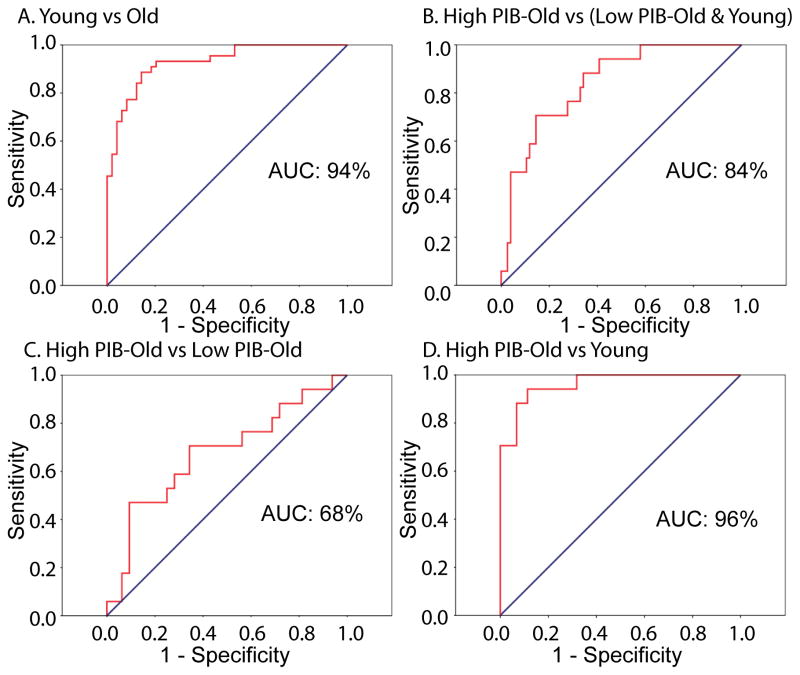
In order to further quantify the accuracy of classification by discriminant scores, we additionally conducted receiver operating characteristic (ROC) analysis. As shown in Figure 4, the area under the curve (AUC) ranged between 68% and 96% and was significantly greater than chance (p’s < .05). However, discriminant scores did better in separating Young from Older groups than High PIB from Low PIB groups: AUC classification of Young vs Old was 94% while AUC classification of High PIB vs Low PIB was 68%.
Figure 4.
Sensitivity and specificity plots of discriminant scores created by the first discriminant function. Red lines represent the ROC curve.
4. Discussion
In the present study, we found that, among 5 PCA-driven cognitive composite scores encompassing multiple cognitive domains, young adults performed significantly better on visual memory and executive function compared to both High PIB and Low PIB older adults. By combining the 5 component scores with the discriminant function analysis method, we further reduced the cognitive data into one composite score by which it was demonstrated that High PIB and Low PIB older adults significantly differ in cognitive performance. Older subjects’ PIB index was further significantly associated with component scores of visual memory and disciriminant scores when age and education were controlled for. Using cross-validation analysis, classification by the discriminant scores was shown to be above chance. Overall, we found separate effects of age and Aβ deposition on cognition in normal elderly.
Memory research has indicated that episodic memory is among the cognitive processes that decline with advancing age (Grady & Craik, 2000). Because a relatively large proportion of cognitively older adults harbor a high level of Aβ deposition that is roughly equivalent to that of AD patients suffering from episodic memory deficits and substantial damage of the hippocampus (Eichenbaum, 2000), it was unclear whether age-related episodic memory impairment seen in previous aging studies is solely due to the effect of age. When we examined the relation between age and cognition while excluding older adults who harbor Aβ deposition, older adults without evidence of Aβ deposition performed significantly worse on visual episodic memory than young adults. The degree of amyloid deposition measured by PIB-PET was further negatively associated with visual episodic memory scores within all older subjects. It is rather surprising, however, that we did not find any effect of age on verbal episodic memory. When we looked for ceiling or floor effects in the neuropsychological test scores with higher weights on this component, we did not find any such effect. Therefore, it is possible that our highly educated older subjects simply show preserved verbal episodic memory capacity equivalent to that of young adults. In addition, it could be argued that visual memory deficits may be due to sensory, perceptual, or attention differences rather than memory. Because the executive function component that is statistically independent from the visual episodic memory component consist of neuropsychological tests such as the Stroop test, Trail B minus A, and Symbol Digit tests with higher weights, these differences might have been accounted for by the executive function component. Therefore, the present results may indicate that an amyloid-independent aging process relates to age-associated cognitive changes, especially in the cognitive domain of visual episodic memory.
Behavioral and neuroimaging studies have also found age effects on working memory and executive function in which one needs to maintain and manipulate information in memoranda to achieve task goals (see Reuter-Lorenz & Sylvester, 2005, for a review). We did find a significant effect of age on executive function, although the age effect on working memory did not reach significance perhaps because we included a digit span forward task that previous reports note is not greatly affected by age (Craik & Jennings, 1992). In sum, the present findings together suggest that normal aging involves reduced cognitive functions in both visual episodic memory and executive functions regardless of the status of amyloid deposition.
Previous findings on the effect of Aβ deposition on cognition in normal older adults are mixed. Some report no relationship between amyloid deposition and cognitive function in clinically normal older adults (Aizenstein, et al., 2008; Driscoll, et al., 2006; Rowe, et al., 2010). Other studies, on the other hand, report higher amyloid deposition measured by PIB-PET in association with poorer cognition, especially in episodic memory function (Pike, et al., 2007; Villemagne, et al., 2011; Villemagne, et al., 2008). Longitudinal studies have reported that Aβ deposition is associated with exacerbated cognitive decline over a period of time, although these studies did not find an association between amyloid deposition and concurrent cognitive performance (Driscoll, et al., 2006; Resnick, et al., 2010; Storandt, Mintun, Head, & Morris, 2009). These varied results may have several explanations. One might be that, across studies, samples might have been at different stages of Aβ deposition. Another might be that a mediating factor such as cognitive reserve might have obscured a possible relationship Aβ deposition and cognition. By examining the relationship between amyloid deposition, a multitude of neuropsychological tests, and cognitive reserve, Rentz et al. (2010) reported a significant interaction effect of amyloid deposition and cognitive reserve on neuropsychological test performance in normal older adults. That is, while there was no relation between amyloid deposition and cognitive performance in subjects with higher cognitive reserve, a significantly negative relation between the two factors was found for those with low cognitive reserve. The results suggest that a different level of cognitive reserve in older adults could obscure an effect of amyloid deposition on cognition in the preclinical older population. The third potential explanation may be that the neuropsychological test measures might have lacked sensitivity in detecting cognitive differences between normal older adults with and without amyloid deposition. The present findings provide evidence supporting this possibility.
Findings from the present study are particularly intriguing in light of multivariate methods that provided increased sensitivity in detecting cognitive differences between High PIB and Low PIB older adults. When we examined the individual neuropsychological test scores for group differences, we found a prominent age effect (i.e., Young subjects perform better than either High PIB or Low PIB older adults), but no effect of amyloid deposition. Using component scores, we still did not find any significant group-wise effect of amyloid deposition, although visual memory component scores were associated with quantitative PIB index. When we combined the 5 component scores into discriminant scores that weight component scores in order to maximize discriminatory power of group membership, a significant group difference in cognition between High PIB and Low PIB older adults emerged. These discriminant scores further showed a significantly negative association with quantitative PIB index. These results are consistent with findings showing increased sensitivity and specificity in diagnosing AD from controls by applying a series of multivariate methods on neuropsychological test measures (Chapman et al., 2010). Greater loadings placed on visual memory and executive functions in the discriminant function further suggest that the two cognitive functions are particularly sensitive to both age and Aβ pathology.
A differential effect of amyloid deposition on cognition in cognitively normal older adults, however, is not attributed to APOE genotypes. When we examined the effect of APOE e4 allele on cognition, there was no significant difference in cognition as measured in discriminant function scores. The number of ApoE-e4 homozygotes in our sample, however, was very small. Therefore, our conclusion concerning the effect of APOE genotypes on cognition may be limited. Future studies with a larger proportion of APOE4 homozygotes will be needed to fully address this issue.
Several limitations in the current study need to be noted. First, the PCA that we used to develop a summary measure of neuropsychological tests does not allow us to test whether underlying constructs will generalize to other independent samples, because it only reduces a set of observed data into a smaller set of unobserved variables. In addition, due to the small sample size of older subjects who were scanned using PIB-PET, a validation test by splitting the sample was also not feasible, although we validated our discriminant functions using cross-validation (i.e., a leave-one-out method), an alternative method in the case of a small sample size. Thus, we cannot confidently state that the identical pattern of cognitive test performance will exist in independent samples of older High and Low PIB subjects. Studies with independent and larger samples or cross-validated factor analysis will be needed to generalize our cognitive components to different samples. Second, the use of extreme age groups such as young and old adults might have been less ideal than scanning individuals from the whole adult lifespan to fully investigate cognitive aging. For these reasons, as well as the relatively small sample, our results may be considered preliminary. Nevertheless, these results reveal age – and Aβ –related alterations in visual memory and executive function that could serve as a hypothesis- testing starting point in other studies that might use a multivariate approach to investigate this problem. In addition, while the specific pattern of cognitive performance may not necessarily be the best or most sensitive for detecting Aβ, the results do point out the relative size of cognitive decline that can be attributed to age as opposed to Aβ.
In this study, we examined whether and how age and amyloid deposition affect cognition in normal elderly. Our results suggest that effects of both age and β-amyloid are associated with cognitive decline, although cognitive performance of these older adults is within the normal range. It is likely that age-related cognitive decline in older adults with little Aβ deposition is due to the fact that Aβ is but once instance of neural burden that cannot account for all the variance in cognitive aging. The present results also may indicate that age-related cognitive decline could be accounted for by a host of other factors such as vascular disease, stress, and hormonal factors. Future studies will be needed to further understand the role of these factors in cognitive changes in normal elderly.
Supplementary Material
01
Acknowledgments
This study was supported by the Alzheimer’s Association and NIH grant (R01-AG034570) to W.J. Jagust.
Footnotes
Conflict of interest statement
Dr. Jagust has served as a consultant to GE Healthcare and Bayer Healthcare which manufacture amyloid imaging agents.
The other authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hwamee Oh, Email: hwameeoh@berkeley.edu.
Cindee Madison, Email: cindee@berkeley.edu.
Thaddeus J. Haight, Email: tad@berkeley.edu.
Candace Markley, Email: candace.markley@berkeley.edu.
William J. Jagust, Email: jagust@berkeley.edu.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual aphasia examination. Iowa City, IA: AJA Associates; 1983. [Google Scholar]
- Bourgeat P, Chetelat G, Villemagne VL, Fripp J, Raniga P, Pike K, et al. Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology. 2010;74(2):121–127. doi: 10.1212/WNL.0b013e3181c918b5. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Chapman RM, Mapstone M, Porsteinsson AP, Gardner MN, McCrary JW, DeGrush E, et al. Diagnosis of Alzheimer’s disease using neuropsychological testing improved by multivariate analyses. J Clin Exp Neuropsychol. 2010;32(8):793–808. doi: 10.1080/13803390903540315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FI, Jennings JM. Human memory. In: Craik FI, Salthouse TA, editors. The handbook of aging and cognition. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc; 1992. pp. 51–110. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Resnick SM, Troncoso JC, An Y, O’Brien R, Zonderman AB. Impact of Alzheimer’s pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006;60(6):688–695. doi: 10.1002/ana.21031. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1(1):41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol. 2008;65(1):113–120. doi: 10.1001/archneurol.2007.27. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test: A manual for clinical and experimental uses. Chicago: Stoelting; 1978. [Google Scholar]
- Grady CL, Craik FI. Changes in memory processing with age. Curr Opin Neurobiol. 2000;10(2):224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- Kaiser HF. The application of electronic computers to factor analysis. Educational and Psychological Measurement. 1960;20:141–151. [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16(5):834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8(4):283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Brandel MG, Madison CM, Rabinovici GD, Marks S, Baker SL, et al. Not quite PIB-positive, not quite PIB-negative: Slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage. 2012;59(2):1152–1160. doi: 10.1016/j.neuroimage.2011.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Mormino EC, Madison C, Hayenga A, Smiljic A, Jagust WJ. beta-Amyloid affects frontal and posterior brain networks in normal aging. Neuroimage. 2011;54(3):1887–1895. doi: 10.1016/j.neuroimage.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25(11):1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Rajah MN, Kromas M, Han JE, Pruessner JC. Group differences in anterior hippocampal volume and in the retrieval of spatial and temporal context memory in healthy young versus older adults. Neuropsychologia. 2010;48(14):4020–4030. doi: 10.1016/j.neuropsychologia.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74(10):807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digits Modalities Test. Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- Sperling RA, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, et al. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20(2):1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66(12):1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 4. Boston, MA: Allyn and Bacon; 2001. [Google Scholar]
- Tulving E. Elements of episodic memory. Oxford: Clarendon press; 1983. [Google Scholar]
- Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69(1):181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Darby D, Maruff P, Savage G, Ng S, et al. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer’s disease. Neuropsychologia. 2008;46(6):1688–1697. doi: 10.1016/j.neuropsychologia.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01