Mitochondrial Ca2+ and apoptosis (original) (raw)
Abstract
Mitochondria are key decoding stations of the apoptotic process. In support of this view, a large body of experimental evidence has unambiguously revealed that, in addition to the well-established function of producing most of the cellular ATP, mitochondria play a fundamental role in triggering apoptotic cell death.
Various apoptotic stimuli cause the release of specific mitochondrial pro-apoptotic factors into the cytosol. The molecular mechanism of this release is still controversial, but there is no doubt that mitochondrial calcium (Ca2+) overload is one of the pro-apoptotic ways to induce the swelling of mitochondria, with perturbation or rupture of the outer membrane, and in turn the release of mitochondrial apoptotic factors into the cytosol.
Here, we review as different proteins that participate in mitochondrial Ca2+ homeostasis and in turn modulate the effectiveness of Ca2+-dependent apoptotic stimuli. Strikingly, the final outcome at the cellular level is similar, albeit through completely different molecular mechanisms: a reduced mitochondrial Ca2+ overload upon pro-apoptotic stimuli that dramatically blunts the apoptotic response.
Keywords: Calcium, Mitochondria, Apoptosis, Cell death, Endoplasmic reticulum, MAM
1. Introduction
The term apoptosis, from a Greek word meaning ‘falling leaves’, was initially proposed in 1972 [1] to describe a form of natural cell death occurring in multicellular organisms. It had become necessary to distinguish between the different types of cell death and this kind exhibited a characteristic morphology and what seemed to be an endogenously regulated process. Over the past decades since the introduction of the term, scientific knowledge of this biological process has been greatly expanded.
Apoptosis, that involves altruistic suicide of individual cells in favour of the organism as a whole, was thus defined as programmed cell death of type I characterized by blebbing, cell shrinkage and extensive chromatin condensation and DNA degradation [2–5].
Apoptotic activity is essential for organ homeostasis by keeping under control cell number and tissue tropism both in physiological and pathological circumstances [4,6]. Inappropriate activation of apoptosis has been recognized to be at the basis of many common pathologies [7,8].
Apoptosis in mammals can have one of two initiation phases: the death receptor pathway (extrinsic apoptotic pathways) and the mitochondrial pathway (intrinsic apoptotic pathways), which pathway is selected depends on the nature of the death signal to be integrated [9,10].
There is no doubt that cell death belongs to the numerous cell functions on which Ca2+ exerts a complex regulatory role [11–13]. It has long been known that in neurons and other cell types an unchecked increase in cytosolic Ca2+ concentration ([Ca2+]c) can trigger apoptosis [14] and likewise, agents able to release Ca2+ from intracellular stores have been shown to be pro-apoptotic [15].
Importantly, Ca2+ is a critical sensitizing signal in the pro-apoptotic transition of mitochondria that plays a key role in the regulation of cell death [16]. Mitochondrial Ca2+ overload is one of the pro-apoptotic ways to induce the swelling of mitochondria, with perturbation or rupture of the outer membrane, and in turn the release of mitochondrial apoptotic factors into the cytosol [17].
The mitochondrial intermembrane space (IMS) contains many pro-apoptotic factors such as cytochrome c, apoptosis inducing factor (AIF), procaspase-9, Smac/DIABLO as well as endonuclease G [18–22]. These are released from mitochondria to the cytosol in response to apoptotic signals, like DNA damage, oxidative stress, energetic catastrophe, viral infection, endoplasmic reticulum (ER) stress or xenobiotic intoxication. Their release is preceded by the outer mitochondrial membrane (OMM) permeabilization, a crucial step in apoptosis, but how this is exactly performed is not yet clear [23]. Several B-cell CLL/lymphoma-2 (Bcl-2)-protein family members can affect the permeability of the OMM, for example, by binding to the mitochondrial voltage-dependent anion channels (VDACs) and regulating its properties or by forming multimeric channel complexes [24]. OMM rupture, and thus release of apoptogenic factors, can also occur subsequently to a long-lasting increase in inner mitochondrial membrane (IMM) permeability, also called mitochondrial permeability transition (MPT). MPT is supposedly due to the opening of a permeability transition pore (PTP), the molecular nature of which is however still unresolved [25]. A role for VDAC in the PTP has been proposed, but this is still highly controversial. An important point hereby was the demonstration that all VDAC isoforms are dispensable for the induction of MPT [26]. Independently of the mechanism by which the increase in permeability of the OMM is achieved, it allows the release of the apoptogenic factors present in the IMS to the cytoplasm and the progression of apoptosis [25,27–29].
Released pro-apoptotic proteins can initiate three signalling cascades leading to apoptosis: (i) released cytochrome c, together with apoptosis protease activating factor 1 (APAF-1), preexisting in the cytosol, forms “apoptosome” which results in the activation of procaspase-9, and in turn activation of effector caspases (caspases-3, -6, and -7); (ii) released from the IMS, both Smac/DIABLO and Omi/HtrA2 favour caspase activation by antagonizing the endogenous inhibitors of caspases, the cytosolic inhibitor of apoptosis proteins (IAPs); and (iii) released AIF and endonuclease G favour DNA fragmentation and chromatin condensation [28]. At the early phase of apoptosis, simultaneously with cytochrome c release (before caspases activation), fragmentation of the mitochondrial network has been observed [30]. Moreover, execution of mitochondria-related apoptosis can be connected with mitochondrial dysfunction including loss of mitochondrial membrane potential (MMP), increased reactive oxygen species (ROS) production, decreased ATP production and alteration of mitochondrial Ca2+ homeostasis.
2. Oncogene and oncosuppressor proteins regulation of mitochondrial Ca2+ homeostasis in control of apoptosis
A critical link between Ca2+ and apoptosis was established while studying the oncoprotein Bcl-2 and its mechanism of action. Bcl-2 is a central regulator of apoptosis, able to block or delay apoptosis in different cell types, from haematopoietic to neural [31]. The immediate interest generated around this protein's functions led to the discovery of several other proteins displaying sequence homology. These are also active in the control of apoptosis and have given rise to a whole family of Bcl-2 proteins.
To date, this family comprises at least thirteen members that can be easily classified, regarding their control of apoptosis, as proapoptotic and antiapoptotic [32]. The antiapoptotic members conserve the higher sequence homology with Bcl-2 and especially within four highly conserved Bcl-2 Homology domains (BH1-4), like the Bcl-2-related gene A1, BCL-XL, BCL-w, and MCL-1. The proapoptotic family members instead can be subdivided into effector proteins, such as BAK and BAX, that were originally described to contain only BH1-3, and into the BH3-only proteins that share homology only in the third domain and comprise BAD, BID, BIM, BIK, PUMA, Noxa and others [32].
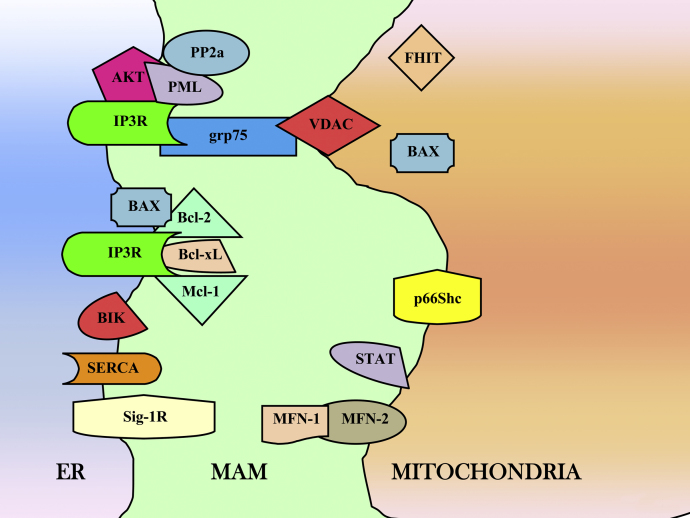
Through their localization to cytosol, ER and mitochondria, they allow regulation of apoptosis at different stages and during different pathways, ranging from the direct control of OMM permeability or unfolded protein response [32,33] to the control of intracellular Ca2+ homeostasis (Fig. 1).
Fig. 1.
Schematic intracellular distribution of the reported proteins involved in ER–mitochondria Ca2+ cross-talk. Abbreviations: AKT, v-akt murine thymoma viral oncogene homologue; BAX, Bcl-2-associated X; Bcl-xL, B-cell lymphomas extra long; Bcl-2, B-cell lymphomas 2; BIK, Bcl-2 interacting killer; ER, endoplasmic reticulum; FHIT, fragile histidine triad; grp75, glucose-regulated protein 75; IP3R, inositol 1,4,5-trisphosphate receptor; MAMs, mitochondria-associated membranes; mcl-1, myeloid cell leukaemia-1; MFN-1/-2, mitofusin-1, -2; p66Shc, 66-kDa isoform of the growth factor adapter shc; PML, promyelocytic leukaemia protein; PP2a, protein phosphatase 2a; SERCA, sarco-endoplasmic reticulum Ca2+ ATPase; Sig-1R, Sigma-1 receptor; STAT, Signal Transducer and Activator of Transcription; VDAC, voltage-dependent anion channel.
Our group demonstrated how Bcl-2 over-expression was able to reduce the steady state of Ca2+ levels within the ER, with resulting reduced Ca2+ transfer to mitochondria during apoptotic stimulation and reduced triggering of mitochondrial fragmentation and apoptosis initiation [34,35]. Other groups have reported similar data, confirming how Bcl-2 could mediate an augmented leak from the compartment without affecting activity of ER Ca2+ ATPase [36,37]. Our group also addressed the hypothesis that its putative pore forming activity could mediate regulation of ER Ca2+ release [38]. Some controversy still remains, however, regarding the mechanism through which Bcl-2 reduces the Ca2+ transfer from ER to mitochondria. Bassik et al. showed how Bcl-2 alters the phosphorylation of IP3R, promoting its basal leaking [39]. A few years later, the group of Distelhorst proposed the direct interaction between the BH4 domain of Bcl-2 and the IP3R, reducing its opening probability and conferring resistance to apoptosis during prolonged exposure to the T cell receptor [40]. These data were recently confirmed in glioma cell lines. In this elegant work, it was shown how the interaction between Bcl-2 and IP3R is able not only to inhibit apoptosis, but also to inhibit its propagation through several cells in a Ca2+- and IP3-dependent manner [41].
Not only Bcl-2, but also Bcl-XL is able to bind IP3R, apparently determining its sensitivity for IP3 in a phosphorylation-dependent manner [42]. Recently, similar observations were extended to Mcl-1 [43].
Nonetheless recently this concept has been discussed. Monaco and coworkers show how the interaction between IP3Rs and Bcl-2 depend on its BH4 domain and a single critical residue of difference in the sequence compared to the once of Bcl-XL will not allow to the latter to maintain this interaction [44]. It should be noticed that Li and coworkers clearly show how Bcl-XL is involved in regulation of IP3Rs activity, by alternatively coexpressing Bcl-XL and the single IP3R isoform in an IP3R ko background (DT40 cells). A possible explanation of this point is that Bcl-XL could regulate IP3R by an indirect interaction. Moreover Monaco at al. performs experiments in cell types with mixed population of IP3R isoform (MEFs, C6 glioma cells), while Bcl-XL apparently regulates the channel activity of isoform 3 (see [42]).
Interestingly, pro-apoptotic members of the Bcl-2 family display an opposite behaviour. BAX and BAK where shown to control apoptosis from mitochondria and ER during Ca2+-dependent stimuli. Initially, it was shown that BAX was capable of inducing Ca2+ transfer from ER to mitochondria by promoting the release of a mitochondrial proapoptotic factor [45]. Later, Korsmeyer and colleagues generated a double knock out for both BAX and BAK displaying a strong reduction in the ER levels of Ca2+, as well as reduced cytoplasmic Ca2+ waves and mitochondrial uptake. At the same time, these cells were strongly resistant to different apoptotic stimuli. In the same cell lines, reintroduction of mitochondrial BAX restored mitochondrial apoptosis induction following exposure to tBID, but resistance to Ca2+-dependent stimuli was still observed. Recovery of ER Ca2+ levels by expressing SERCA in double knock out cells for both BAX and BAK also restored sensitivity to Ca2+-triggered apoptosis. This strongly suggested that, BAX and BAK where able to control Ca2+ homeostasis in the ER and mitochondria [46]. Recently, the relation between pro- and antiapoptotic members in the regulation of Ca2+ was explained through the activity of BI-1 (Bax inhibitor 1). This is a potent inhibitor of cell death during Bax activation, and also a regulator of ER [Ca2+]. Recently, it was shown how the C-terminus of BI-1 is able to generate a Ca2+ channel on the ER membrane responsible for the Ca2+ leak and that this activity is conserved in animals but not in plant and yeast [47].
Furthermore, it was demonstrated that Ca2+-dependent apoptotic stimuli can engage different BH3-only members of the family: ceramide induces dephosphorylation of the BH3-only protein BAD. Dephosphorylated BAD sensitizes the PTP to Ca2+ through a Bcl-XL-sensitive and VDAC-mediated process that does not require BAX or BAK [48]. Another BH3-only regulator has been shown to regulate Ca2+ homeostasis, BIK. This protein has been shown to promote BAX localization to ER and to increase the Ca2+ released in the cytoplasm during thapsigargin stimulation [49].
In a similar way to Bcl-2, another anti-apoptotic factor, the serine/threonine kinase Akt, is able to regulate mitochondrial Ca2+ levels through a direct activity on the ER side [50] (Fig. 1). Like Bcl-2, Akt protects from apoptosis by diminishing the Ca2+ flux from ER, without affecting the total Ca2+ content of the store [51]. This is possible by a strong inhibition of IP3R releasing properties, probably due to a phosphorylation event [52]. In fact, all three subtypes of IP3R present, in their C-terminal tail, a robust phosphorylation motif R_X_R_XX_(S/T), highly conserved in different species [53], which is a substrate for Akt kinase activity [52,53]. Thus, Akt function on IP3R and the consequent minor Ca2+ transfer from ER to mitochondria is the way through which Akt works to protect from Ca2+-mediated apoptosis. Interestingly, Akt can localize also into mitochondria [54] and intramitochondrial active Akt results in efficient protection against apoptotic signalling [55]. However, the exact role of this kinase inside the organelle has not been fully understood.
Recent evidence indicated that the crosstalk between ER and mitochondria is regulated also by another important oncosupressor: the promyelocytic leukaemia (PML) protein, encoded by a tumour suppressor gene implicated in the pathogenesis of leukaemia and cancer [56]. PML has been previously recognized as a critical and essential regulator of multiple apoptotic responses [57,58]. Nevertheless, how PML would exert such a broad and fundamental role in apoptosis remained a mystery for long time. The reported role of PML in the modulation of p53 transcription [59] failed to reconcile this issue, as well as the role played by PML in the transcription-independent early apoptotic response.
Within the cell, PML isoforms display both nuclear and cytosolic distribution. In the nucleus, PML is present in a multiprotein nuclear structure, the PML-nuclear bodies [60]. Recent data defined a novel and unexpected extra-nuclear PML-dependent pathway for the control of Ca2+ homeostasis and in turn Ca2+-dependent apoptosis [61].
Interestingly, in the cytosol, PML was found to localize to the ER and the mitochondria associated membranes, MAMs [62] (Fig. 1). MAMs are specialized domains selectively enriched in mitochondrial Ca2+ signalling elements, where Ca2+ transfer between ER and mitochondria takes place [63].
In particular, we obtained direct evidence that PML modulates intracellular Ca2+ homeostasis via PP2A phosphatase recruitment at the ER and at the MAMs to inactivate Akt kinase-dependent phosphorylation of IP3R3s. In so doing, PML is able to regulate Ca2+ mobilization into mitochondria induced by Ca2+-dependent stimuli (e.g., oxidative stress), which then triggers the cell death programme. Conversely, in the absence of PML, PP2A does not accumulate in the complexes with IP3R3 and Akt, and this results in an accumulation of activated Akt (phospho-Akt). Once activated, Akt can hyper-phosphorylate IP3R3 thus inhibiting ER Ca2+ release towards mitochondria and thus inhibiting the mitochondrial Ca2+ overload necessary to execute the cell death programme [62].
Conceptually, similar data were also obtained with other unrelated anti-apoptotic proteins. The most striking example was provided by an oncogene expressed in a human hepatocarcinoma. This oncogene is generated by the integration of the Hepatitis B virus genome in the gene encoding the protein SERCA1 (Sarco/ER Ca2+ ATPase type 1). Viral activation was shown to _cis_-activate SERCA1 chimeric transcripts with splicing of exon 4 and/or exon 11. Splicing of exon 11 creates a frameshift and a premature stop codon in exon 12. The encoded protein lacks most of the cytosolic N and P domains and critical Ca2+-binding regions of the transmembrane region. This protein is incapable of active Ca2+ pumping [64], and is causally involved in the neoplastic phenotype. Although the molecular mechanism of this effect has not been elucidated yet, it may be speculated that the mutated SERCA could either interfere with the activity of endogenous pumps and/or could act as a Ca2+ leak pathway from the ER. These data are consistent with the observations that overexpression of SERCA in HeLa cells increases the susceptibility of cells to apoptotic agents [65,66]. This notion was further reinforced by the study of the enterovirus 2B protein, a small membrane-integral replication protein with an amphipathic α-helix that can build membrane-integral pore forming multimeric transmembrane bundles [67]. This Coxsackie viral protein 2B was shown to be anti-apoptotic and to reduce ER Ca2+ levels [68] and, consequently, the amount of Ca2+ transfer from the ER to mitochondria [68]. The data obtained suggest that the reduction of the Ca2+ content in ER and the resulting down regulation of Ca2+ fluxes between ER and mitochondria is the major component of the viral antiapoptotic programme.
3. MAMs as a hot spot for transducing Ca2+ cell death signals from the ER to mitochondria
Other proteins localized at the MAMs (Fig. 1) are emerging as key players in the control of Ca2+ homeostasis and apoptosis [69–73]. VDAC mediates trafficking of small molecules and ions, including Ca2+, across the OMM. Multicellular organisms and mammals have three VDAC isoforms (VDAC1, VDAC2, and VDAC3), owing isoform-specific functions [74].
VDACs are localized in a crucial position and form the main interface between cytosol and mitochondria, playing an important role in many cellular processes, ranging from metabolism regulation to cell death [75]. Moreover, VDACs are present in contact sites between mitochondria and ER [76,77], forming an important interface for Ca2+ signalling delivery between the two organelles. VDAC1 allows rapid Ca2+ diffusion across OMM, leading to increased Ca2+ concentration in the mitochondria and higher susceptibility to apoptosis [78]. It was also demonstrated that the mitochondrial chaperone grp75 (glucose-regulated protein 75) induces a physical coupling between the IP3R and VDAC1 [77]: in this way, it forms an ER–mitochondria Ca2+ tunnel that allows for a better transfer of the Ca2+ ions from the ER across the OMM.
Recently, De Stefani et al. revealed an interesting scenario in which each VDAC isoform has similar Ca2+ channelling properties, despite their different contribution to cell death: pro-apoptotic for VDAC1 [78,79], anti-apoptotic for VDAC2 [80] and no significant influence on apoptosis for VDAC3 [81]. To conciliate this observation with the classic paradigm linking mitochondrial Ca2+ to apoptosis [82], it was demonstrated that VDAC1, by selectively interacting with the IP3Rs, is preferentially involved in the transmission of the low-amplitude Ca2+-dependent apoptotic signals to mitochondria [81].
The disruption of the physical platform for the interplay between the ER and mitochondria sites has profound consequences for cellular function, such as imbalances of intracellular Ca2+ signalling and disrupted apoptosis progression.
The interactions between the two organelles are modulated by different proteins, such as the mitochondria-shaping proteins MFN-1/-2 (mitofusin-1/-2). In particular, the absence of MFN-2 not only changes the morphology of the ER but also decreases by 40% the interactions between ER and mitochondria, thus affecting the transfer of Ca2+ signals to mitochondria.
In fact, up-regulation of MFN-2 is necessary and sufficient for the induction of apoptosis in vascular smooth muscle cell [83]; in addition, it was recently observed that the MFN-2 interacting protein Trichoplein/mitostatin is downregulated in several human tumours [84] and deeply regulates mitochondrial morphology and tethering with ER, with consequent control in apoptosis levels [85]. Finally, it has also been demonstrated that the presence of MFN-2 is important for the execution of the mechanism whereby mitochondria depolarization leads to the inhibition of the STIM1 puncta-structure formation (that correspond to ER–plasma membrane junctions), with subsequent blocking in Ca2+ release-activated Ca2+ (CRAC) channels activity, a crucial step for the regulation of gene expression and controlling of cell survival [86].
Interestingly, also ER chaperones were found to be compartmentalized at the MAMs. The Sigma-1 receptor (Sig-1R) has been identified as selectively residing at the MAMs, forms a Ca2+-sensitive chaperone complex with BiP/GRP78 and associates with isoform 3 of IP3R [87]. The first effect of this complex is a prolonged Ca2+ signalling from ER to mitochondria, with consequent modulation of the apoptotic programme. As demonstration of this, small molecules able to block the Sig-1R lead to the activation of caspase-dependent apoptosis [88]. Previously, another multifunctional protein, PACS-2 (phosphofurin acidic cluster sorting protein 2) was identified as necessary for the intimate association of mitochondria with the ER. The absence of PACS-2 causes mitochondria fragmentation, disruption of the ER–mitochondria axis and affects Ca2+ homeostasis and apoptosis [89]. In particular, the depletion of PACS-2 determines the mislocalization of IP3Rs and consequent alteration of the correct Ca2+ transfer from ER to mitochondria [90]. Moreover, PACS-2 also affects ER Ca2+ homeostasis, regulating the distribution of the Ca2+-binding chaperones calnexin, through a mechanism that contemplates the direct interaction between PACS-2 and calnexin [91]. In addition, when the apoptotic programme is initiated, PACS-2 binds BID, leading to its translocation into mitochondria, where it is cleaved to tBID. Subsequently, cytochrome c is release and caspase-3 activated [89].
4. Deadly liaisons: ROS and mitochondrial Ca2+ homeostasis in the control of cell death
As much as Ca2+ appears to be involved, there is no doubt other “pro-apoptotic” conditions must be met for apoptosis to occur. Indeed, mitochondria can handle large Ca2+ loads in normal physiological conditions (e.g., in cardiac myocytes, significant amounts of Ca2+ are accumulated at every heartbeat), with no deleterious effects [92].
As to these additional “apoptotic signals”, the most important is considered to be oxidative stress [93–95].
A two hits model can thus be proposed, similar to that suggested by Hajnoczky and coworkers where the physiological mitochondrial uptake of Ca2+ caused by IP3-producing agonists, is turned into an apoptotic signal in the presence of ceramide, possibly via opening of the PTP [96].
The apoptotic stimulus can directly or indirectly damage the mitochondria, but this effect is marginal or totally ineffective, if the mitochondrial are not contemporarily exposed to an elevated [Ca2+]. In other words, mitochondria appear to act as “coincidence detectors”, where only the contemporary application of both signals can be transduced into an effective triggering signal of apoptosis.
In this section, we will summarize recent discoveries about the effects of four proteins, not directly related to each other, with a common outline in terms of ROS perturbation, mitochondrial Ca2+ deregulation and sensitivity to apoptosis.
4.1. FHIT
The FHIT gene spans the FRA3B fragile site at chromosome 3p14.2 and is inactivated (through gene deletions, abnormal transcripts and promoter hypermethylation) in >50% of human cancers [97]. Its product, Fhit, is a typical dinucleoside 5′,5‴-P1,P3-triphosphate (Ap3A) hydrolase that acts as a tumour suppressor in vivo and in vitro, enhancing susceptibility to apoptosis [98]. Fhit−/− mice are highly susceptible to carcinogens, and reintroduction of FHIT by gene transfer reduces the tumour burden by triggering apoptosis [99]. Studies have confirmed that FHIT over-expression leads to the activation of the extrinsic pathway [100], as well as the intrinsic pathway by alteration of MMP and enhanced efflux of cytochrome c from the organelle [101]. Fhit has been located in the mitochondria (Fig. 1), although it was first identified as a cytosolic protein. Trapasso et al. [102] have shown that Fhit can be directed to mitochondria upon interaction with Hsp60 and Hsp10. The mitochondrial localization determines the binding with and stabilization of ferredoxin reductase (Fdxr), a flavoprotein transactivated by p53. The FHIT/Fdxr complex generates ROS and increases Ca2+ uptake into mitochondria [103]. Fhit sensitizes the low-affinity Ca2+ transporters of mitochondria, potentiating the effect of apoptotic agents. This effect has been attributed to the fraction of Fhit sorted to mitochondria, as shown by a chimeric mitochondrial Fhit that retains the Ca2+ signalling properties of Fhit (and the pro-apoptotic activity of the native protein) while completely losing the effects on cell cycle [103].
4.2. p66Shc
Shc-like molecules function as ‘adaptor proteins’, which receive signals from growth factor receptors, which are phosphorylated on tyrosines. Three Shc isoforms have been identified in mammals, p46Shc, p52Shc and p66Shc [104]. Similarly to p46Shc and p52Shc, p66Shc binds mitogenic transducer protein but does not stimulate cell proliferation [105], while it participates in intracellular pathways that control oxidative stress and apoptosis.
Cells from which p66shc is depleted are resistant to apoptotic death induced by oxidative stress and p66Shc−/− mice are resistant to paraquat, living about 30% longer than controls [106]. Moreover, it has been observed that after challenge with hydrogen peroxide, p66Shc translocates from the cytosol to mitochondria. Serine 36 is a critical regulatory site for the apoptotic activity of p66Shc and PKC isoform β is required for phosphorylation [107]. In fact, it has been shown that PKCβ, activated by oxidative challenges, causes the phosphorylation and protein import of p66Shc into mitochondria, where it acts as oxidoreductase. This initiates a feedforward cycle of ROS production that leads to the opening of mPTP, release of pro-apoptotic cofactors and triggering of cell death [107]. Phosphorylated p66Shc is then recognized by the prolyl isomerase Pin1 that catalyzes its cis_–_trans isomerization, allowing the import into mitochondria (Fig. 1) after dephosphorylation by type 2 protein serine/threonine phosphatase (PP2A). Within mitochondria, p66Shc then binds cytochrome c, shuttling electrons from cytochrome c to molecular oxygen [108], generating H2O2. This event in turn perturbs mitochondrial structure and function (Ca2+ uptake and ATP production).
The key role of p66Shc as molecular sentinel that controls cellular stress and mitochondrial physiology has been confirmed recently in transgenic mice modelling amyotrophic lateral sclerosis (ALS), where the neurotoxicity mediated by mutSOD1 (G93A-SOD1) is exerted by mitochondrial redox activity of p66Shc [109]. In cells expressing mutSOD1, accumulation of phosphorylated p66Shc (on serine 36) has been demonstrated, associated with decreases in mitochondrial Ca2+ uptake capacity, ATP production, and mitochondrial integrity. This it is further supported by the observation that overexpression of dominant negative, functionally inactive p66Shc proteins protects cells against mutSOD1 toxicity. Most importantly, the deletion of p66Shc ameliorates mitochondrial function, delays onset, improves motor performance and prolongs survival in ALS transgenic mice model [109].
4.3. KRIT1
Cerebral Cavernous Malformations (CCM) is one of the major cerebrovascular diseases [110]. It is characterized by abnormally enlarged and often leaky capillary cavities in the brain, leading to an increased risk of hemorrhagic stroke, seizures, and focal neurological deficits [111,112]. KRIT1 is one of the three genes responsible for causing CCM [113], and it was recently shown that KRIT1 plays an important role in molecular mechanisms involved in the maintenance of ROS homeostasis to prevent oxidative cellular damage [114]. Ablation of KRIT1 leads to a significant increase of ROS levels due to the reduction of the expression of the antioxidant protein SOD2, as well as of the transcription factor FoxO1. The regulation of FoxO by KRIT1 has been also demonstrated in C. elegans, where kri-1, the worm orthologue of KRIT1/CCM1, is required for germline removal-dependent lifespan extension through regulation of DAF-16, the worm orthologue of mammalian FoxO genes [115]. Indeed, KRIT1 knock-out cells present enhanced levels of mitochondrial superoxide and a consequent decline of mitochondrial energy metabolism, characterized by a strong reduction in MMP, mitochondrial Ca2+ levels and ATP production. In this case, a downregulation of mitochondrial Ca2+ levels cannot be linked to apoptosis resistance, because when mitochondria are sensitized by oxidative stress, small arises in mitochondrial [Ca2+] could promote opening of the mPTP [116,117]. Indeed, KRIT1 loss is associated with an increased susceptibility to caspase 3 activation during oxidative challenge.
4.4. STAT3
Signal Transducer and Activator of Transcription 3 (STAT3) is a latent cytosolic transcription factor activated by cytokines, growth factors and oncogenes [118] and, recently, emerged as a regulator of cell cycle and apoptosis [119]. In addition to its canonical nuclear function, which requires tyrosine phosphorylation, DNA binding and transcriptional activity, STAT3 has been reported to exert non-nuclear functions. It was shown to localize to mitochondria (Fig. 1), where it regulates cellular respiration [120]. Gough and colleagues demonstrated that exclusive mitochondrial targeting of STAT3 appears to contribute to RAS-dependent cellular transformation, by augmenting electron transport chain activity, particularly at the level of complexes II and V [121].
On the contrary, in cells expressing physiological levels of the nuclear constitutively active STAT3C mutant, the mitochondrial Ca2+ uptake, mitochondrial ATP production and basal respiratory chain activity are reduced [122].
Furthermore, these cells show reduced MMP, diminished ROS production and high resistance to apoptosis. These data highlighted the role of STAT3C to enhance cell survival and protection from apoptosis via specific regulation of mitochondrial functions.
Although the roles played by nuclear or mitochondrial STAT3 may seem contradictory, it must be borne in mind that specific phosphorylation on tyrosine or serine occurs (mitochondrially localized STAT3 is not phosphorylated on tyrosine 705, the hallmark of transcriptional activation, but on Serine 727) upon distinct stimuli and under distinct physiological or pathological conditions [122], leading to two functionally distinct molecular effects.
5. Conclusions
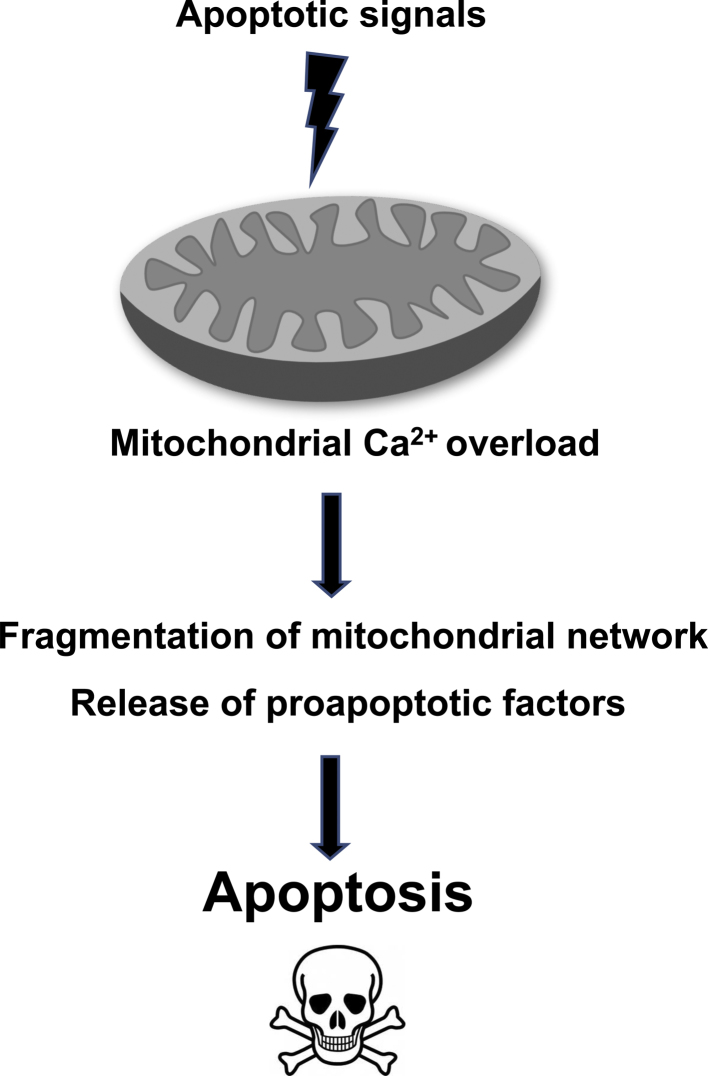
Regarding the site of action of many apoptotic Ca2+ signals, mitochondria emerge as a critical location (Fig. 2). Indeed, treatment with different Ca2+-dependent apoptotic stimuli, such as ceramide, arachidonic acid, oxidative stress, to name a few, causes the release of Ca2+ from the ER; this in turn induces dramatic changes in mitochondrial morphology, with a consequent release of mitochondria proteins involved in the apoptotic process, such as cytocrome c, AIF, Smac/Diablo (Fig. 2). If Ca2+ changes are prevented, mitochondrial morphology is preserved and the cells are protected from apoptosis. The full understanding of how mitochondria can sense, handle and decode various Ca2+ signals still represents an exciting challenge in biomedical sciences. Indeed, as summarize in this review, many proteins, some of theme completely unexpected, participate in mitochondrial Ca2+ homeostasis.
Fig. 2.
Schematic representation of mitochondrial Ca2+-dependent apoptosis.
However, it must be stressed that mitochondrial Ca2+ uptake is essential in rapidly adapting aerobic metabolism to the increased needs of a stimulated cell [123]. Moreover, in an elegant study, Foskett and coworkers, demonstrated as constitutive IP3R-dependent Ca2+ release from ER to mitochondria is an essential cellular process that is required for efficient mitochondrial respiration and maintenance of normal cell bioenergetics [124]. This means that while mitochondrial Ca2+ overload leads to apoptosis a controlled, and transient mitochondrial Ca2+ load is needed for survival.
All these observations lead to a really complex but fascinating picture, where mitochondrial Ca2+ signals represent crucial triggers of apoptosis.
Conflict of interest statement
This is to certify that there is no conflict of interest with this manuscript submission.
Acknowledgements
The authors apologize for any excessive bias and the inevitable omissions. The authors are also deeply indebted to past collaborators. This research was supported by the Ministry of Science and Higher Education, Poland, grant NN407 075 137 to M.R.W., the Italian Ministry of Health to A.R. and the Italian Association for Cancer Research (AIRC), Telethon (GGP09128 and GGP11139B), local funds from the University of Ferrara, the Italian Ministry of Education, University and Research (COFIN, FIRB and Futuro in Ricerca), the Italian Cystic Fibrosis Research Foundation and Italian Ministry of Health to P.P.
SMa was supported by a FIRC fellowship; AB was supported by a research fellowship FISM – Fondazione Italiana Sclerosi Multipla (Cod. 2010/B/1); SP was supported by a training fellowship FISM (Cod. 2010/B/13); JMS was also supported by a Ph.D. fellowship from The Foundation for Polish Science (FNP), UE, European Regional Development Fund and Operational Programme “Innovative economy”.
References
- 1.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alnemri E.S., Livingston D.J., Nicholson D.W., Salvesen G., Thornberry N.A., Wong W.W., Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson D.W., Thornberry N.A. Caspases: killer proteases. Trends Biochem. Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 4.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 5.Thornberry N.A., Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 6.Ellis R.E., Yuan J.Y., Horvitz H.R. Mechanisms and functions of cell death. Annu. Rev. Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 7.Thompson C.B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 8.Strasser A., O’Connor L., Dixit V.M. Apoptosis signaling. Annu. Rev. Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 9.Fulda S., Debatin K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 10.Green D.R. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 11.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 12.Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Rimessi A., Giorgi C., Pinton P., Rizzuto R. The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim. Biophys. Acta. 2008;1777:808–816. doi: 10.1016/j.bbabio.2008.05.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sattler R., Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J. Mol. Med. 2000;78:3–13. doi: 10.1007/s001090000077. [DOI] [PubMed] [Google Scholar]
- 15.Mattson M.P., Chan S.L. Calcium orchestrates apoptosis. Nat. Cell Biol. 2003;5:1041–1043. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- 16.Kroemer G., Reed J.C. Mitochondrial control of cell death. Nat. Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 17.Giorgi C., Romagnoli A., Pinton P., Rizzuto R. Ca2+ signaling, mitochondria and cell death. Curr. Mol. Med. 2008;8:119–130. doi: 10.2174/156652408783769571. [DOI] [PubMed] [Google Scholar]
- 18.Ferri K.F., Kroemer G. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 19.Martinou J.C., Desagher S., Antonsson B. Cytochrome c release from mitochondria: all or nothing. Nat. Cell Biol. 2000;2:E41–E43. doi: 10.1038/35004069. [DOI] [PubMed] [Google Scholar]
- 20.Joza N., Susin S.A., Daugas E., Stanford W.L., Cho S.K., Li C.Y., Sasaki T., Elia A.J., Cheng H.Y., Ravagnan L., Ferri K.F., Zamzami N., Wakeham A., Hakem R., Yoshida H., Kong Y.Y., Mak T.W., Zuniga-Pflucker J.C., Kroemer G., Penninger J.M. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- 21.Verhagen A.M., Ekert P.G., Pakusch M., Silke J., Connolly L.M., Reid G.E., Moritz R.L., Simpson R.J., Vaux D.L. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 22.Parrish J., Li L., Klotz K., Ledwich D., Wang X., Xue D. Mitochondrial endonuclease G is important for apoptosis in C. elegans. Nature. 2001;412:90–94. doi: 10.1038/35083608. [DOI] [PubMed] [Google Scholar]
- 23.Tait S.W., Green D.R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 24.Shoshan-Barmatz V., De Pinto V., Zweckstetter M., Raviv Z., Keinan N., Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Aspects Med. 2010;31:227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Rasola A., Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 26.Baines C.P., Kaiser R.A., Sheiko T., Craigen W.J., Molkentin J.D. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson M., Kornbluth S. Mitochondria in apoptosis and human disease. Curr. Mol. Med. 2001;1:91–122. doi: 10.2174/1566524013364239. [DOI] [PubMed] [Google Scholar]
- 28.Kroemer G., Galluzzi L., Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 29.Halestrap A.P. Mitochondrial permeability: dual role for the ADP/ATP translocator? Nature. 2004;430:1. doi: 10.1038/nature02816. [DOI] [PubMed] [Google Scholar]
- 30.Suen D.F., Norris K.L., Youle R.J. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsujimoto Y., Finger L.R., Yunis J., Nowell P.C., Croce C.M. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 32.Chipuk J.E., Moldoveanu T., Llambi F., Parsons M.J., Green D.R. The BCL-2 family reunion. Mol. Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez D., Rojas-Rivera D., Hetz C. Integrating stress signals at the endoplasmic reticulum: the BCL-2 protein family rheostat. Biochim. Biophys. Acta. 2011;1813:564–574. doi: 10.1016/j.bbamcr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Pinton P., Ferrari D., Magalhaes P., Schulze-Osthoff K., Di Virgilio F., Pozzan T., Rizzuto R. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J. Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinton P., Ferrari D., Rapizzi E., Di Virgilio F.D., Pozzan T., Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foyouzi-Youssefi R., Arnaudeau S., Borner C., Kelley W.L., Tschopp J., Lew D.P., Demaurex N., Krause K.H. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer A.E., Jin C., Reed J.C., Tsien R.Y. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chami M., Prandini A., Campanella M., Pinton P., Szabadkai G., Reed J.C., Rizzuto R. Bcl-2 and Bax exert opposing effects on Ca2+ signalling, which do not depend on their putative pore-forming region. J. Biol. Chem. 2004:54581–54589. doi: 10.1074/jbc.M409663200. [DOI] [PubMed] [Google Scholar]
- 39.Bassik M.C., Scorrano L., Oakes S.A., Pozzan T., Korsmeyer S.J. Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. EMBO J. 2004;23:1207–1216. doi: 10.1038/sj.emboj.7600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rong Y.P., Aromolaran A.S., Bultynck G., Zhong F., Li X., McColl K., Matsuyama S., Herlitze S., Roderick H.L., Bootman M.D., Mignery G.A., Parys J.B., De Smedt H., Distelhorst C.W. Targeting Bcl-2–IP3 receptor interaction to reverse Bcl-2's inhibition of apoptotic calcium signals. Mol. Cell. 2008;31:255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decrock E., Krysko D.V., Vinken M., Kaczmarek A., Crispino G., Bol M., Wang N., De Bock M., De Vuyst E., Naus C.C., Rogiers V., Vandenabeele P., Erneux C., Mammano F., Bultynck G., Leybaert L. Transfer of IP(3) through gap junctions is critical, but not sufficient, for the spread of apoptosis. Cell Death Differ. 2011 doi: 10.1038/cdd.2011.176. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li C., Wang X., Vais H., Thompson C.B., Foskett J.K., White C. Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eckenrode E.F., Yang J., Velmurugan G.V., Foskett J.K., White C. Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J. Biol. Chem. 2010;285:13678–13684. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monaco G., Decrock E., Akl H., Ponsaerts R., Vervliet T., Luyten T., De Maeyer M., Missiaen L., Distelhorst C.W., De Smedt H., Parys J.B., Leybaert L., Bultynck G. Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-Xl. Cell Death Differ. 2012;19:295–309. doi: 10.1038/cdd.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nutt L.K., Chandra J., Pataer A., Fang B., Roth J.A., Swisher S.G., O’Neil R.G., McConkey D.J. Bax-mediated Ca2+ mobilization promotes cytochrome c release during apoptosis. J. Biol. Chem. 2002;277:20301–20308. doi: 10.1074/jbc.M201604200. [DOI] [PubMed] [Google Scholar]
- 46.Scorrano L., Oakes S.A., Opferman J.T., Cheng E.H., Sorcinelli M.D., Pozzan T., Korsmeyer S.J. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 47.Bultynck G., Kiviluoto S., Henke N., Ivanova H., Schneider L., Rybalchenko V., Luyten T., Nuyts K., De Borggraeve W., Bezprozvanny I., Parys J.B., De Smedt H., Missiaen L., Methner A. The C terminus of Bax inhibitor-1 forms a Ca2+-permeable channel pore. J. Biol. Chem. 2012;287:2544–2557. doi: 10.1074/jbc.M111.275354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roy S.S., Madesh M., Davies E., Antonsson B., Danial N., Hajnoczky G. Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. Mol. Cell. 2009;33:377–388. doi: 10.1016/j.molcel.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathai J.P., Germain M., Shore G.C. BH3-only BIK regulates BAX,BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J. Biol. Chem. 2005;280:23829–23836. doi: 10.1074/jbc.M500800200. [DOI] [PubMed] [Google Scholar]
- 50.Vanderheyden V., Devogelaere B., Missiaen L., De Smedt H., Bultynck G., Parys J.B. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim. Biophys. Acta. 2009;1793:959–970. doi: 10.1016/j.bbamcr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marchi S., Rimessi A., Giorgi C., Baldini C., Ferroni L., Rizzuto R., Pinton P. Akt kinase reducing endoplasmic reticulum Ca2+ release protects cells from Ca2+-dependent apoptotic stimuli. Biochem. Biophys. Res. Commun. 2008;375:501–505. doi: 10.1016/j.bbrc.2008.07.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szado T., Vanderheyden V., Parys J.B., De Smedt H., Rietdorf K., Kotelevets L., Chastre E., Khan F., Landegren U., Soderberg O., Bootman M.D., Roderick H.L. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2427–2432. doi: 10.1073/pnas.0711324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan M.T., Wagner L., Yule D.I., Bhanumathy C., Joseph S.K. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J. Biol. Chem. 2006;281:3731–3737. doi: 10.1074/jbc.M509262200. [DOI] [PubMed] [Google Scholar]
- 54.Bijur G.N., Jope R.S. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J. Neurochem. 2003;87:1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mookherjee P., Quintanilla R., Roh M.S., Zmijewska A.A., Jope R.S., Johnson G.V. Mitochondrial-targeted active Akt protects SH-SY5Y neuroblastoma cells from staurosporine-induced apoptotic cell death. J. Cell. Biochem. 2007;102:196–210. doi: 10.1002/jcb.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rego E.M., Wang Z.G., Peruzzi D., He L.Z., Cordon-Cardo C., Pandolfi P.P. Role of promyelocytic leukemia (PML) protein in tumor suppression. J. Exp. Med. 2001;193:521–529. doi: 10.1084/jem.193.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi Y., Lallemand-Breitenbach V., Zhu J., de The H. PML nuclear bodies and apoptosis. Oncogene. 2004;23:2819–2824. doi: 10.1038/sj.onc.1207533. [DOI] [PubMed] [Google Scholar]
- 58.Bernardi R., Papa A., Pandolfi P.P. Regulation of apoptosis by PML and the PML-NBs. Oncogene. 2008;27:6299–6312. doi: 10.1038/onc.2008.305. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z.G., Ruggero D., Ronchetti S., Zhong S., Gaboli M., Rivi R., Pandolfi P.P. PML is essential for multiple apoptotic pathways. Nat. Genet. 1998;20:266–272. doi: 10.1038/3073. [DOI] [PubMed] [Google Scholar]
- 60.Bernardi R., Pandolfi P.P. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat. Rev. Mol. Cell Biol. 2007;8:1006–1016. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- 61.Pinton P., Giorgi C., Pandolfi P.P. The role of PML in the control of apoptotic cell fate: a new key player at ER–mitochondria sites. Cell Death Differ. 2011:1450–1456. doi: 10.1038/cdd.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giorgi C., Ito K., Lin H.K., Santangelo C., Wieckowski M.R., Lebiedzinska M., Bononi A., Bonora M., Duszynski J., Bernardi R., Rizzuto R., Tacchetti C., Pinton P., Pandolfi P.P. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science. 2010;330:1247–1251. doi: 10.1126/science.1189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giorgi C., De Stefani D., Bononi A., Rizzuto R., Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int. J. Biochem. Cell Biol. 2009:1817–1827. doi: 10.1016/j.biocel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chami M., Gozuacik D., Saigo K., Capiod T., Falson P., Lecoeur H., Urashima T., Beckmann J., Gougeon M.L., Claret M., le Maire M., Brechot C., Paterlini-Brechot P. Hepatitis B virus-related insertional mutagenesis implicates SERCA1 gene in the control of apoptosis. Oncogene. 2000;19:2877–2886. doi: 10.1038/sj.onc.1203605. [DOI] [PubMed] [Google Scholar]
- 65.Ma T.S., Mann D.L., Lee J.H., Gallinghouse G.J. SR compartment calcium and cell apoptosis in SERCA overexpression. Cell Calcium. 1999;26:25–36. doi: 10.1054/ceca.1999.0049. [DOI] [PubMed] [Google Scholar]
- 66.Pinton P., Ferrari D., Di Virgilio F., Pozzan T., Rizzuto R. Molecular machinery and signalling events in apoptosis. Drug Dev. Res. 2001;52:558–570. [Google Scholar]
- 67.de Jong A.S., Wessels E., Dijkman H.B., Galama J.M., Melchers W.J., Willems P.H., van Kuppeveld F.J. Determinants for membrane association and permeabilization of the coxsackievirus 2B protein and the identification of the Golgi complex as the target organelle. J. Biol. Chem. 2003;278:1012–1021. doi: 10.1074/jbc.M207745200. [DOI] [PubMed] [Google Scholar]
- 68.Campanella M., de Jong A.S., Lanke K.W., Melchers W.J., Willems P.H., Pinton P., Rizzuto R., van Kuppeveld F.J. The coxsackievirus 2B protein suppresses apoptotic host cell responses by manipulating intracellular Ca2+ homeostasis. J. Biol. Chem. 2004;279:18440–18450. doi: 10.1074/jbc.M309494200. [DOI] [PubMed] [Google Scholar]
- 69.Patergnani S., Suski J.M., Agnoletto C., Bononi A., Bonora M., De Marchi E., Giorgi C., Marchi S., Missiroli S., Poletti F., Rimessi A., Duszynski J., Wieckowski M.R., Pinton P. Calcium signaling around Mitochondria Associated Membranes (MAMs) Cell Commun. Signal. 2011;9:19. doi: 10.1186/1478-811X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lynes E.M., Simmen T. Urban planning of the endoplasmic reticulum (ER): how diverse mechanisms segregate the many functions of the ER. Biochim. Biophys. Acta. 2011;1813:1893–1905. doi: 10.1016/j.bbamcr.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fujimoto M., Hayashi T. New insights into the role of mitochondria-associated endoplasmic reticulum membrane. Int. Rev. Cell Mol. Biol. 2011;292:73–117. doi: 10.1016/B978-0-12-386033-0.00002-5. [DOI] [PubMed] [Google Scholar]
- 72.Grimm S. The ER–mitochondria interface: the social network of cell death. Biochim. Biophys. Acta. 2012;1823:327–334. doi: 10.1016/j.bbamcr.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 73.Wieckowski M.R., Giorgi C., Lebiedzinska M., Duszynski J., Pinton P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat. Protoc. 2009;4:1582–1590. doi: 10.1038/nprot.2009.151. [DOI] [PubMed] [Google Scholar]
- 74.Messina A., Reina S., Guarino F., De Pinto V. VDAC isoforms in mammals. Biochim. Biophys. Acta. 2011 doi: 10.1016/j.bbamem.2011.10.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 75.Shoshan-Barmatz V., Israelson A., Brdiczka D., Sheu S.S. The voltage-dependent anion channel (VDAC): function in intracellular signalling, cell life and cell death. Curr. Pharm. Des. 2006;12:2249–2270. doi: 10.2174/138161206777585111. [DOI] [PubMed] [Google Scholar]
- 76.Shoshan-Barmatz V., Zalk R., Gincel D., Vardi N. Subcellular localization of VDAC in mitochondria and ER in the cerebellum. Biochim. Biophys. Acta. 2004;1657:105–114. doi: 10.1016/j.bbabio.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 77.Szabadkai G., Bianchi K., Varnai P., De Stefani D., Wieckowski M.R., Cavagna D., Nagy A.I., Balla T., Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rapizzi E., Pinton P., Szabadkai G., Wieckowski M.R., Vandecasteele G., Baird G., Tuft R.A., Fogarty K.E., Rizzuto R. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J. Cell Biol. 2002;159:613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abu-Hamad S., Sivan S., Shoshan-Barmatz V. The expression level of the voltage-dependent anion channel controls life and death of the cell. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5787–5792. doi: 10.1073/pnas.0600103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng E.H., Sheiko T.V., Fisher J.K., Craigen W.J., Korsmeyer S.J. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 81.De Stefani D., Bononi A., Romagnoli A., Messina A., De Pinto V., Pinton P., Rizzuto R. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ. 2012;19:267–273. doi: 10.1038/cdd.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pinton P., Giorgi C., Siviero R., Zecchini E., Rizzuto R. Calcium and apoptosis: ER–mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo X., Chen K.H., Guo Y., Liao H., Tang J., Xiao R.P. Mitofusin 2 triggers vascular smooth muscle cell apoptosis via mitochondrial death pathway. Circ. Res. 2007;101:1113–1122. doi: 10.1161/CIRCRESAHA.107.157644. [DOI] [PubMed] [Google Scholar]
- 84.Vecchione A., Fassan M., Anesti V., Morrione A., Goldoni S., Baldassarre G., Byrne D., D’Arca D., Palazzo J.P., Lloyd J., Scorrano L., Gomella L.G., Iozzo R.V., Baffa R. MITOSTATIN, a putative tumor suppressor on chromosome 12q24.1, is downregulated in human bladder and breast cancer. Oncogene. 2009;28:257–269. doi: 10.1038/onc.2008.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cerqua C., Anesti V., Pyakurel A., Liu D., Naon D., Wiche G., Baffa R., Dimmer K.S., Scorrano L. Trichoplein/mitostatin regulates endoplasmic reticulum–mitochondria juxtaposition. EMBO Rep. 2010;11:854–860. doi: 10.1038/embor.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singaravelu K., Nelson C., Bakowski D., de Brito O.M., Ng S.W., Di Capite J., Powell T., Scorrano L., Parekh A.B. Mitofusin 2 regulates STIM1 migration from the Ca2+ store to the plasma membrane in cells with depolarized mitochondria. J. Biol. Chem. 2011;286:12189–12201. doi: 10.1074/jbc.M110.174029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hayashi T., Su T.P. Sigma-1 receptor chaperones at the ER–mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 88.Spruce B.A., Campbell L.A., McTavish N., Cooper M.A., Appleyard M.V., O’Neill M., Howie J., Samson J., Watt S., Murray K., McLean D., Leslie N.R., Safrany S.T., Ferguson M.J., Peters J.A., Prescott A.R., Box G., Hayes A., Nutley B., Raynaud F., Downes C.P., Lambert J.J., Thompson A.M., Eccles S. Small molecule antagonists of the sigma-1 receptor cause selective release of the death program in tumor and self-reliant cells and inhibit tumor growth in vitro and in vivo. Cancer Res. 2004;64:4875–4886. doi: 10.1158/0008-5472.CAN-03-3180. [DOI] [PubMed] [Google Scholar]
- 89.Simmen T., Aslan J.E., Blagoveshchenskaya A.D., Thomas L., Wan L., Xiang Y., Feliciangeli S.F., Hung C.H., Crump C.M., Thomas G. PACS-2 controls endoplasmic reticulum–mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005;24:717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kottgen M., Benzing T., Simmen T., Tauber R., Buchholz B., Feliciangeli S., Huber T.B., Schermer B., Kramer-Zucker A., Hopker K., Simmen K.C., Tschucke C.C., Sandford R., Kim E., Thomas G., Walz G. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J. 2005;24:705–716. doi: 10.1038/sj.emboj.7600566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Myhill N., Lynes E.M., Nanji J.A., Blagoveshchenskaya A.D., Fei H., Carmine Simmen K., Cooper T.J., Thomas G., Simmen T. The subcellular distribution of calnexin is mediated by PACS-2. Mol. Biol. Cell. 2008;19:2777–2788. doi: 10.1091/mbc.E07-10-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rizzuto R., Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 93.Marchi S., Giorgi C., Suski J.M., Agnoletto C., Bononi A., Bonora M., De Marchi E., Missiroli S., Patergnani S., Poletti F., Rimessi A., Duszynski J., Wieckowski M.R., Pinton P. Mitochondria-ros crosstalk in the control of cell death and aging. J. Signal Transduct. 2012;2012:329635. doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Giorgi C., Agnoletto C., Bononi A., Bonora M., De Marchi E., Marchi S., Missiroli S., Patergnani S., Poletti F., Rimessi A., Suski J.M., Wieckowski M.R., Pinton P. Mitochondrial calcium homeostasis as potential target for mitochondrial medicine. Mitochondrion. 2012;12:77–85. doi: 10.1016/j.mito.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Di Lisa F., Kaludercic N., Carpi A., Menabo R., Giorgio M. Mitochondria and vascular pathology. Pharmacol. Rep. 2009;61:123–130. doi: 10.1016/s1734-1140(09)70014-3. [DOI] [PubMed] [Google Scholar]
- 96.Szalai G., Krishnamurthy R., Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huebner K., Croce C.M. Cancer and the FRA3B/FHIT fragile locus: it's a HIT. Br. J. Cancer. 2003;88:1501–1506. doi: 10.1038/sj.bjc.6600937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siprashvili Z., Sozzi G., Barnes L.D., McCue P., Robinson A.K., Eryomin V., Sard L., Tagliabue E., Greco A., Fusetti L., Schwartz G., Pierotti M.A., Croce C.M., Huebner K. Replacement of Fhit in cancer cells suppresses tumorigenicity. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13771–13776. doi: 10.1073/pnas.94.25.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zanesi N., Pekarsky Y., Croce C.M. A mouse model of the fragile gene FHIT: from carcinogenesis to gene therapy and cancer prevention. Mutat. Res. 2005;591:103–109. doi: 10.1016/j.mrfmmm.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 100.Deng W.G., Nishizaki M., Fang B., Roth J.A., Ji L. Induction of apoptosis by tumor suppressor FHIT via death receptor signaling pathway in human lung cancer cells. Biochem. Biophys. Res. Commun. 2007;355:993–999. doi: 10.1016/j.bbrc.2007.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Askari M.D., Vo-Dinh T. Implication of mitochondrial involvement in apoptotic activity of fragile histidine triad gene: application of synchronous luminescence spectroscopy. Biopolymers. 2004;73:510–523. doi: 10.1002/bip.10544. [DOI] [PubMed] [Google Scholar]
- 102.Trapasso F., Pichiorri F., Gaspari M., Palumbo T., Aqeilan R.I., Gaudio E., Okumura H., Iuliano R., Di Leva G., Fabbri M., Birk D.E., Raso C., Green-Church K., Spagnoli L.G., Venuta S., Huebner K., Croce C.M. Fhit interaction with ferredoxin reductase triggers generation of reactive oxygen species and apoptosis of cancer cells. J. Biol. Chem. 2008;283:13736–13744. doi: 10.1074/jbc.M709062200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Rimessi A., Marchi S., Fotino C., Romagnoli A., Huebner K., Croce C.M., Pinton P., Rizzuto R. Intramitochondrial calcium regulation by the FHIT gene product sensitizes to apoptosis. Proc. Natl. Acad. Sci. U.S.A. 2009;106:12753–12758. doi: 10.1073/pnas.0906484106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pelicci G., Dente L., De Giuseppe A., Verducci-Galletti B., Giuli S., Mele S., Vetriani C., Giorgio M., Pandolfi P.P., Cesareni G., Pelicci P.G. A family of Shc related proteins with conserved PTB, CH1 and SH2 regions. Oncogene. 1996;13:633–641. [PubMed] [Google Scholar]
- 105.Migliaccio E., Mele S., Salcini A.E., Pelicci G., Lai K.M., Superti-Furga G., Pawson T., Di Fiore P.P., Lanfrancone L., Pelicci P.G. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor–MAP kinase–fos signalling pathway. EMBO J. 1997;16:706–716. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Migliaccio E., Giorgio M., Mele S., Pelicci G., Reboldi P., Pandolfi P.P., Lanfrancone L., Pelicci P.G. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 107.Pinton P., Rimessi A., Marchi S., Orsini F., Migliaccio E., Giorgio M., Contursi C., Minucci S., Mantovani F., Wieckowski M.R., Del Sal G., Pelicci P.G., Rizzuto R. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- 108.Giorgio M., Migliaccio E., Orsini F., Paolucci D., Moroni M., Contursi C., Pelliccia G., Luzi L., Minucci S., Marcaccio M., Pinton P., Rizzuto R., Bernardi P., Paolucci F., Pelicci P.G. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 109.Pesaresi M.G., Amori I., Giorgi C., Ferri A., Fiorenzo P., Gabanella F., Salvatore A.M., Giorgio M., Pelicci P.G., Pinton P., Carri M.T., Cozzolino M. Mitochondrial redox signalling by p66Shc mediates ALS-like disease through Rac1 inactivation. Hum. Mol. Genet. 2011;20:4196–4208. doi: 10.1093/hmg/ddr347. [DOI] [PubMed] [Google Scholar]
- 110.Marchuk D.A., Srinivasan S., Squire T.L., Zawistowski J.S. Vascular morphogenesis: tales of two syndromes. Hum. Mol. Genet. 2003;12(Spec No 1):R97–R112. doi: 10.1093/hmg/ddg103. [DOI] [PubMed] [Google Scholar]
- 111.Gault J., Sarin H., Awadallah N.A., Shenkar R., Awad I.A. Pathobiology of human cerebrovascular malformations: basic mechanisms and clinical relevance. Neurosurgery. 2004;55:1–16. (discussion 16–17) [PubMed] [Google Scholar]
- 112.Clatterbuck R.E., Eberhart C.G., Crain B.J., Rigamonti D. Ultrastructural and immunocytochemical evidence that an incompetent blood–brain barrier is related to the pathophysiology of cavernous malformations. J. Neurol. Neurosurg. Psychiatry. 2001;71:188–192. doi: 10.1136/jnnp.71.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Laberge-le Couteulx S., Jung H.H., Labauge P., Houtteville J.P., Lescoat C., Cecillon M., Marechal E., Joutel A., Bach J.F., Tournier-Lasserve E. Truncating mutations in CCM1, encoding KRIT1, cause hereditary cavernous angiomas. Nat. Genet. 1999;23:189–193. doi: 10.1038/13815. [DOI] [PubMed] [Google Scholar]
- 114.Goitre L., Balzac F., Degani S., Degan P., Marchi S., Pinton P., Retta S.F. KRIT1 regulates the homeostasis of intracellular reactive oxygen species. PLoS One. 2010;5:e11786. doi: 10.1371/journal.pone.0011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berman J.R., Kenyon C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell. 2006;124:1055–1068. doi: 10.1016/j.cell.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 116.Jacobson J., Duchen M.R. Mitochondrial oxidative stress and cell death in astrocytes—requirement for stored Ca2+ and sustained opening of the permeability transition pore. J. Cell Sci. 2002;115:1175–1188. doi: 10.1242/jcs.115.6.1175. [DOI] [PubMed] [Google Scholar]
- 117.Huser J., Rechenmacher C.E., Blatter L.A. Imaging the permeability pore transition in single mitochondria. Biophys. J. 1998;74:2129–2137. doi: 10.1016/S0006-3495(98)77920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Aggarwal B.B., Kunnumakkara A.B., Harikumar K.B., Gupta S.R., Tharakan S.T., Koca C., Dey S., Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann. N.Y. Acad. Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bowman T., Garcia R., Turkson J., Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 120.Wegrzyn J., Potla R., Chwae Y.J., Sepuri N.B., Zhang Q., Koeck T., Derecka M., Szczepanek K., Szelag M., Gornicka A., Moh A., Moghaddas S., Chen Q., Bobbili S., Cichy J., Dulak J., Baker D.P., Wolfman A., Stuehr D., Hassan M.O., Fu X.Y., Avadhani N., Drake J.I., Fawcett P., Lesnefsky E.J., Larner A.C. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gough D.J., Corlett A., Schlessinger K., Wegrzyn J., Larner A.C., Levy D.E. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Demaria M., Giorgi C., Lebiedzinska M., Esposito G., D’Angeli L., Bartoli A., Gough D.J., Turkson J., Levy D.E., Watson C.J., Wieckowski M.R., Provero P., Pinton P., Poli V. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging (Albany, NY) 2010;2:823–842. doi: 10.18632/aging.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jouaville L.S., Pinton P., Bastianutto C., Rutter G.A., Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cardenas C., Miller R.A., Smith I., Bui T., Molgo J., Muller M., Vais H., Cheung K.H., Yang J., Parker I., Thompson C.B., Birnbaum M.J., Hallows K.R., Foskett J.K. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]