Inhalation Exposure to Smoke from Synthetic “Marijuana” Produces Potent Cannabimimetic Effects in Mice (original) (raw)
. Author manuscript; available in PMC: 2013 Dec 1.
Abstract
BACKGROUND
Use of synthetic “marijuana” has increased in recent years, produced adverse effects and prompted the temporary DEA ban of five specific cannabinoid analogs, including JWH-018. The objectives of the current study include determining the chemical content of the herbal product, Buzz, assessing its behavioral effects upon inhalation exposure to mice, determining whether CB1 receptors mediate its pharmacological activity, and ascertaining its biodisposition in blood and various organs.
METHODS
Using a nose-only exposure system, mice were exposed to smoke produced from combustion of an herbal incense product, Buzz, which contained 5.4% JWH-018. Cannabimimetic effects following smoke exposure were evaluated using the tetrad procedure, consisting of the following indices: hypomotility, antinociception, catalepsy, and hypothermia. Additionally, blood and tissues were collected for JWH-018 quantification.
RESULTS
Inhalation exposure to Buzz produced dose-related tetrad effects similar to marijuana as well as dose-related increased levels of JWH-018 in the blood, brain, heart, kidney, liver, lung, and spleen. The behavioral effects were blocked by rimonabant, a CB1 receptor antagonist. Effects produced by Buzz were similar in magnitude and time-course to those produced by marijuana, though equipotent doses of Buzz and marijuana yielded considerably lower brain levels of JWH-018 than THC for the respective materials.
CONCLUSIONS
Inhalation exposure to a product containing JWH-018 penetrates into the brain and other organs and produces CB1 receptor-mediated behavioral pharmacological effects in mice. The increased potency of JWH-018 compared to THC, the variable amount of drug added to various herbal products, and unknown toxicity, undoubtedly contribute to public health risks of synthetic cannabinoids.
Keywords: JWH-018, marijuana, inhalation, cannabinoid, herbal incense products, tetrad
1. Introduction
Marijuana is the most commonly used illicit substance in the world, with incidence of its use continually on the rise as teenage “regular use” has increased by over 10 percent in recent years (Patrick et al., 2011). The primary active constituent of this plant material Δ9-tetrahydrocannabinol (THC) binds to and activates two cannabinoid receptors, termed CB1 and CB2. More than 70 structurally-related chemicals have been identified in marijuana (Elsohly and Slade, 2005), though not all of these compounds bind to cannabinoid receptors. Additionally, endogenous cannabinoids, including N-arachidonyl ethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) are enzymatically synthesized on demand and degraded throughout the CNS and periphery. Because of efforts to create research tools to investigate the endogenous cannabinoid system as well as to develop potential cannabinoid medications, medicinal chemists have made hundreds, if not thousands, of synthetic cannabinoids. The synthesis of many of these compounds appears in the scientific literature. Over the last few years, several synthetic cannabinoids have been produced by clandestine laboratories throughout the world, infused into herbal blends, and sold through internet sources as well as by certain convenience stores in many countries throughout the world. The active ingredients found in many of these recreational products were first synthesized by Huffman and colleagues and named using a numbering system with the prefix of JWH (Huffman et al., 1994). Individuals using these products, marketed as “K2”, “Spice” and “Buzz”, report feeling marijuana-like effects, though standard drug tests do not screen for these designer cannabinoids. More troubling is the high incidence of adverse effects caused by these products, including delusions, paranoia, anxiety, tachycardia, hypertension, and in some cases, violent outbursts and agitation (Simmons et al., 2011; Vearrier and Osterhoudt, 2010). It has also been reported that consumption of herbal incense products over time produces development of tolerance and dependence, accompanied with physical withdrawal symptoms after abrupt discontinuation of use in humans (Zimmermann et al., 2009). On March 1, 2011, the FDA placed an emergency ban on five specific chemicals commonly found in these incense products: JWH-018, JWH-073, JWH-200, CP-47, 497, and cannabicyclohexanol (Leonhart, 2011). Herbal incense products containing cannabinoids are still being produced and sold, although the chemical components have been substituted with other cannabimimetic compounds that are not banned. The use of these products has increased dramatically in recent years due to the ease of legally obtaining the product and their inability to be detected using conventional drug screening procedures.
Behavioral data related to JWH-018 is limited, but there are some preclinical evaluations as well as published human case reports of adverse events. Wiley and colleagues noted that injected JWH-018 produces behavioral pharmacological effects similar to THC in mice (Wiley et al., 1998). Injections of JWH-018 and JWH-073 also substitute for THC in monkeys trained to discriminate THC from vehicle (Ginsburg et al., 2011), suggesting that they produce THC-like subjective effects. However, humans typically smoke the synthetic cannabinoid incense products and no studies to date have evaluated the effects of inhalation of smoke from these incinerated herbal incense products. Consequently, evaluating the effects of herbal incense products via inhalation in mice will increase the understanding of their in vivo properties and contribute to translational research in this field. Thus, using a nose-only exposure system, mice were exposed to pyrolyzed products to compare the pharmacological effects of a product containing synthetic cannabinoids with marijuana.
In the present study, we obtained via internet sources an herbal incense product called Buzz that had been presumably adulterated with synthetic cannabinoids. We first ascertained that the product contained JWH-018 as well as trace amounts of JWH-073 and JWH-398 (Poklis et al., 2012a). The second objective of this study was to determine whether inhalation exposure to smoke from Buzz produces cannabimimetic behavioral activity in the tetrad test, a widely used assay to investigate cannabimimetic properties of THC and other cannabinoids, including 1) antinociception, 2) catalepsy, 3) hypothermia, and 4) hypomobility (Martin et al., 1991). Other measures of cannabinoid activity, including hyperreflexia (Long et al., 2009; Patel and Hillard, 2001), straub tail (Cutler and Mackintosh, 1975; Jacob et al., 1981), ptosis (i.e., drooping eyelids; Aceto et al., 1998), and loss of righting reflex were also assessed. The third goal of this project was to examine whether CB1 receptors mediate the pharmacological effects elicited by Buzz, using the CB1 receptor antagonist/inverse agonist rimonabant. The final objective of this study was to ascertain the biodisposition of JWH-018 in blood as well as various organs, including brain, lung, kidney, liver, and spleen.
2. Methods
2.1 Subjects
Male C57BL/6J mice (Jackson Laboratories, Bar Harbor, Maine) aged 6-8 weeks were utilized for these studies. Mice were maintained on a 12 h light /dark cycle at 22 +/− 2 C with food and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.2 Drugs
Marijuana (containing 7.4 % THC) and rimonabant were obtained from the National Institute on Drug Abuse (Rockville, MD). Placebo was composed of placebo marijuana plant material from the National Institute on Drug Abuse (Rockville, MD). Buzz (unknown material containing 5.4 % JWH-018) was obtained via the internet source www.imzonged.com accessed on 3/1/2009 (imzonged.com, 2009). Buzz, marijuana, or placebo was combusted and delivered to animals via smoke inhalation. Rimonabant was dissolved in a 1:1 mixture of ethanol and emulphor and diluted with saline for a ratio of 1:1:18 (ethanol: emulphor: saline) and injected via the subcutaneous (s.c.) route of administration.
2.3 Exposure system
The smoke exposure system was described in previous published studies (Lichtman et al., 2001). Plant material was burned in a corncob pipe and the smoke was drawn through tygon tubing to a manifold by a vacuum pump at a rate of 551 mL/min using a flow regulator. Mice were placed into holding tubes which exposed only their noses to smoke drawn through the manifold containing six mouse holding tube openings. Smoke was filtered post-inhalation (after manifold exposure) through 500 mg of glass-wool fiber. Mice were exposed to smoke until 2 min elapsed or when plant material was completely consumed.
2.4 Determination of JWH-018 concentrations in Buzz
Synthetic cannabinoids present in the herbal incense product, Buzz, were quantified by 72 h continuous methanol extraction of 50 mg Buzz. A 100 μL aliquot of the extract was mixed with 20 μL of d3-THC internal standard and then dried under dry nitrogen. The resultant residue was dissolved in 100 μL mobile phase and then analyzed using the instrument method described herein but applying non-extracted calibrators (Poklis et al., 2012a).
2.5 Behavioral measures
2.5.1 Locomotor activity
Locomotor activity was assessed using six photocell activity cages (6.5 x 11 inches) containing 8 photocell beams per chamber (Lichtman et al., 2001). Mice were placed into individual chambers 10 min after being removed from the smoke exposure system and activity was measured using a Digiscan Animal Activity Monitor (Med Associates, Inc., St. Albans, VT) which measured horizontal ambulatory behavior. All six mice were placed in the activity chambers (over an approximately 60 sec time period) and then the activity program was started. Activity was quantified by assessing the total number of beam interruptions in both the dose effect and CB1 receptor antagonism studies.
2.5.2 Antinociception
Nociception was assessed by measuring the tail-flick response latency to radiant heat in the dose effect experiment (D’Amour and Smith, 1941) using an apparatus manufactured by the VCU department of Pharmacology and Toxicology instrument maker containing a 150 watt projector bulb to produce heat, and a photocell to detect tail movement. The intensity of the radiant heat stimulus was held constant to yield baseline latencies of 2-4 s. In the experiment evaluating the time course of antinociception, we elected to use the warm water tail withdrawal test in order to minimize damage to the tail because of the repeated testing. The distal 2 cm of the tail was immersed into 52.0 °C water and latency to withdrawal the tail was recorded. In both the radiant heat tail-flick and tail withdrawal assays, a maximum cutoff of 10 s was used to minimize tail damage. Pre-exposure tail-flick or tail withdrawal latency was assessed for each mouse. All mice were tested serially (i.e., one mouse at a time) with either one tail flick apparatus or one warm water bath. Tail-flick or tail withdrawal testing took less than 30 sec per mouse. In all mice straub tail was not present when tail flick or tail withdrawal latencies were assessed after smoke exposure.
2.5.3 Body Temperature
Rectal temperature was measured to the nearest 0.1° C using a 2 cm long probe attached to a telethermometer (Yellow Spring Industries Inc., Yellow Springs, Ohio) in all experiments. Pre-exposure body temperature was assessed for each mouse. Body temperature was assessed serially (i.e., one mouse at a time). Testing of each group of six mice took approximately five min.
2.5.4 Catalepsy
Catalepsy was assessed using the ring test (Pertwee, 1972), in which subjects were placed on a 5.5 cm diameter steel ring elevated 16 cm over the table and observed for a 5-min period of time. Six mice were tested at the same time. The duration of time in which subjects remained motionless, except for respiratory movements, was summed. Catalepsy was measured in dose-effect and CB1 receptor antagonism studies.
2.5.5 Other Observable Characteristics
Mice were also observed for several other indices following inhalation exposure including: hyperreflexia, straub tail, ptosis, and loss of righting reflex.
2.6 Inhalation Exposure Procedure
In all studies, mice were allowed to acclimate to the experimental room for 60 min prior to testing. Pre-exposure body temperature and tail-flick/withdrawal latency were assessed for each mouse. All studies were conducted between 1100 and 1600 h each day, with the exception of the time course study (i.e., Study 3) which was conducted between 0900 h and 1900 h. After smoke exposure, subjects were removed from the apparatus and physical appearance was assessed. Presence or absence of hyperreflexia (i.e., reflexive jumping behavior; Long et al., 2009), straub tail, ptosis (drooping eyelids), and loss of righting reflex (i.e., mice remained on their sides and did not right themselves after being picked up by an observer blind to treatment conditions) was noted 0-5 min after placement in the home cage.
Study 1. An initial study assessed the dose response of Buzz (placebo, 10, 20 or 50 mg of Buzz, normalized to 50 mg plant material with placebo) in the tetrad test. Separate groups of naïve mice were used for each exposure condition. Six mice were tested in each exposure group and the exposures were completed in ascending order on two test days (i.e., On day 1 placebo and 50 mg of Buzz were tested and on day 2, 10 mg of Buzz and then 20 mg of Buzz were tested). Ten min after smoke exposure, locomotor activity was assessed for 10 min, tail flick (radiant heat) latency was assessed at 25 min, body temperature was measured at 30 min, and catalepsy was measured from 40-45 min post-exposure. Subjects were sacrificed 60 min after smoke exposure for blood and organ extraction.
Study 2. In order to test whether the tetrad effects observed following inhalation exposure to Buzz (20 mg) were mediated by a CB1 receptor mechanism of action, mice were administered rimonabant (3 mg/kg) or vehicle 10 min prior to smoke exposure. Separate groups of naïve mice were used for each exposure. Six mice were tested in each exposure group and the exposures were completed in ascending order (i.e., placebo and then 20 mg of Buzz) on two separate test days. In each exposure group three mice were pre-treated with vehicle and three mice were pre-treated with rimonabant. Locomotor activity, body temperature, and catalepsy were assessed at 10, 30, and 40 min, respectively. Antinociception was not assessed in this experiment because exposure to the smoke from Buzz (20 mg/kg) did not significantly affect radiant heat tail-flick latencies.
Study 3. In the time course experiment, mice were exposed to Buzz (20 or 50 mg), marijuana (200 mg), placebo smoke, or the nose-only exposure system in the absence of smoke. The air-exposure treatment group was assessed to ensure that exposure to the placebo had no effect on behavioral measures as we have shown previously (Varvel et al., 2005). Additionally, mice exposed to air only treatment served as a control for the “exposure” to the holding tubes (i.e., restraint control). Six naïve mice were tested in each exposure group. The exposures were completed in ascending order on two separate days. On day 1, a group of six mice underwent air exposure and then a separate group of mice was exposed to 200 mg of marijuana. On day 2, separate groups of mice were exposed to placebo smoke, 20 mg of Buzz, or 50 mg of Buzz (in this order). Tail withdrawal latencies (assessed in warm water) and rectal temperatures were measured before any smoke exposure and 0.5, 1, 2, 4, and 8 h after smoke exposure. Ring immobility and locomotor activity were not assessed due to their susceptibility to habituation effects following repeated testing.
2.7 Determination of blood and organ JWH-018 concentrations
Sixty min after Buzz exposure, mice from the dose-effect experiment were decapitated and blood, brain, heart, kidney, liver, lung, and spleen were collected for JWH-018 quantification. The extractions were performed using cold acetonitrile procedure as previously described for analysis of natural cannabinoids in blood (Poklis et al., 2012a; 2012b; 2010). Prior to extraction, 250 μL of mouse blood was diluted with 750 μL of drug-free human blood to create a sample size of 1 mL. The organs were homogenized in deionized water at a 1:4 dilution (w/w) with a handheld glass homogenizer. Half of the resulting homogenate by mass was extracted according to the procedure. A volume of 50 μL of internal standard containing 25 ng/mL of d3-THC was added to each sample, then mixed, and allowed to equilibrate overnight. The following day, 2 mL of ice cold acetonitrile was added dropwise to each sample while vortexing. The samples were centrifuged at 2054g for 10 min and then placed in −40°C freezer for at least 2 h. The top layer of each sample containing the acetonitrile was removed via a disposable glass pipette and placed in clean test tube. Extracts were then dried using a Savant AES1000. The resultant residues were reconstituted with 100 μL of mobile phase and placed in auto-sampler vials for high-performance liquid chromatography tandem mass spectrometry (HPLC/MS/MS) analysis. As previously described (Poklis et al., 2012a; 2012b), the HPLC/MS/MS system consisted of an Applied Bio systems 3200 Q trap with a turbo V source for TurbolonSpray that was attached to a Shimadzu SCL HPLC system controlled by Analyst 1.4.2 software. The chromatographic separation was conducted using a Zorbaz eclipse XDB-C 18 column, 4.6 x 75 mm, 3.5 μm (Agilent Technologies, USA).
2.8 Statistical Analysis
Body temperature was expressed as the difference between pre- and post-exposure values obtained from each mouse. Antinociception was calculated by transforming the tail-flick and tail withdrawal data to percent maximum possible effect [%MPE, where %MPE = 100 × (post-exposure latency – pre-exposure latency) ÷ (10 s (cut-off time) – pre-exposure latency)]. Percent catalepsy was calculated by dividing immobility time (s) by 300 s (i.e., total test time). Statistical analyses for antinociception, body temperature, and catalepsy consisted of one or two factor ANOVAs on %MPE, difference in body temperature from baseline (i.e., pre-exposure minus and post-exposure), and percent catalepsy, respectively. All other statistical analyses were also conducted using one or two factor ANOVA. Differences were considered significant at P<0.05. Post hoc analyses included Dunnett’s test and Bonferroni corrected t-tests and are indicated in the text.
3. Results
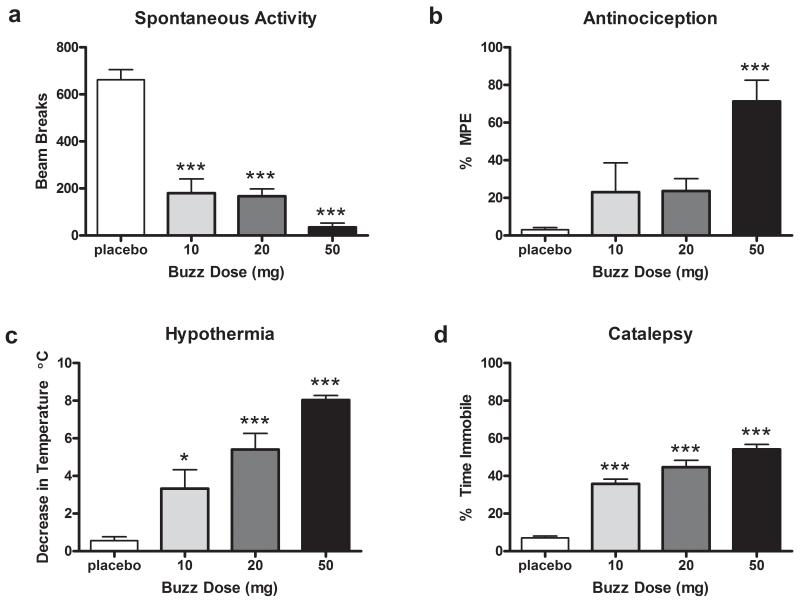
Study1. Buzz was found to contain 5.4% JWH-018. Two other synthetic cannabinoids (JWH-073 and JWH-398) were detected at less than 0.1% (Poklis et al., 2012a). Inhalation exposure to smoke from pyrolyzed Buzz (10, 20, or 50 mg) led to cannabimimetic effects in the behavioral tetrad test (Figure 1). Significant effects of hypomotility (F(3,20)=44.98, p<0.0001), hypothermia (_F_(3, 20)=21.9, _p_<0.0001), and catalepsy (_F_(3,20)=60.18, _p_<0.0001) were found, with Dunnett’s post hoc analysis revealing that 10 (_p_<0.001), 20 (_p_<0.001), and 50 (_p_<0.001) mg of Buzz differing from placebo. Buzz exposure also elicited a significant antinociceptive effect in the radiant heat tail-flick test (_F_(3,20)=8.18, _p_<0.001), although only 50 mg significantly differed from placebo (_p_<0.001). As shown in Table 1, Buzz also produced observable effects including ptosis, hyperreflexive responses, and straub tail in a dose-dependent manner. No significant differences between treatment conditions were found in baseline measures (see Table 2 for group means ± SEM) of body temperature or tail flick (p >.05).
Figure 1.
Buzz dose-dependently produces cannabinoid behavioral tetrad responses including (a) decreases in spontaneous locomotor activity, (b) antinociception in the radiant heat tail-flick test, (c) hypothermia, and (d) catalepsy in the ring immobility test. Mice were exposed via a nose-only exposure system to smoke produced from the incineration of placebo (50 mg), 10, 20, or 50 mg Buzz plant material. Baseline body temperature and tail flick latencies are found in Table 2.*p<0.05; ***p<0.001 vs. mice treated with 50 mg placebo smoke; Dunnett’s post-hoc test. All data reflect mean ± SEM; n=6 mice/group.
Table 1.
Inhalation exposure to Buzz produces CB1 receptor mediated hyperreflexia, straub tail, and ptosis. Data are expressed as percentage of mice showing presence of observable effect (n = 6 mice per condition).
| Dose/Condition | Hyperreflexia | Straub Tail | Ptosis |
|---|---|---|---|
| 50 mg Placebo | 0% | 0% | 33.33% |
| 10 mg Buzz | 50% | 66.67% | 33.33% |
| 20 mg Buzz | 100% | 100% | 100% |
| 50 mg Buzz | 100% | 100% | 100% |
| Rimonabant/50 mg Placebo | 0% | 0% | 0% |
| Vehicle/50 mg Placebo | 0% | 0% | 0% |
| Rimonabant/20 mg Buzz | 0% | 0% | 0% |
| Vehicle/20 mg Buzz | 50%* | 66.67%* | 66.67%* |
Table 2.
Mean baseline (i.e., pre-exposure) measures for body temperatures and tail flick or tail withdrawal. Data are expressed as the mean of 6 mice/treatment condition ± SEM. No significant differences in baseline measures of body temperature or tail flick/ tail withdrawal were found between treatment groups in each study (One way ANOVAs; all studies p>0.05).
| Study 1: Dose effect | Body temperature (°C) | Tail Flick (sec) |
|---|---|---|
| Dose/Condition | Mean ± SEM | Mean ± SEM |
| 50 mg Placebo | 37.42 ± 0.20 | 2.38 ± 0.07 |
| 10 mg Buzz | 37.57 ± 0.07 | 2.17 ± 0.12 |
| 20 mg Buzz | 37.80 ± 0.12 | 2.12 ± 0.12 |
| 50 mg Buzz | 37.42 ± 0.15 | 2.25 ± 0.06 |
| Study 2: Mechanism of Action | Body temperature (°C) | |
| Dose/Condition | Mean ± SEM | |
| Vehicle/Placebo | 38.73 ± 0.12 | |
| Rimonabant/Placebo | 38.75 ± 0.10 | |
| Vehicle/20 mg Buzz | 38.92 ± 0.23 | |
| Rimonabant /20 mg Buzz | 38.60 ± 0.13 | |
| Study 3: Time Course | Body temperature (°C) | Tail Withdrawal (sec) |
| Dose/Condition | Mean ± SEM | Mean ± SEM |
| Air exposed | 38.20 ± 0.15 | 1.61 ± 0.20 |
| 50 mg Placebo | 38.62 ± 0.14 | 1.32 ± 0.20 |
| 20 mg Buzz | 38.55 ± 0.10 | 1.30 ± 0.20 |
| 50 mg Buzz | 38.60 ± 0.10 | 1.39 ± 0.20 |
| 200 mg Marijuana | 38.57 ± 0.15 | 1.60 ± 0.20 |
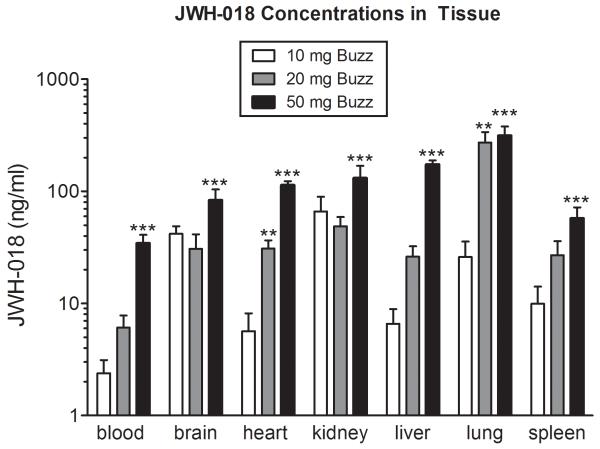
JWH-018 concentrations detected in the blood, brain, heart, kidney, liver, lung, and spleen are displayed in Figure 2. A dose-dependent relationship between JWH-018 tissue concentration and dose burned was revealed in the blood (F(3,19)=26.05, p<0.0001), brain (F(3,17)=7.78, p<0.01), heart (F(3,19)=85.39, p<0.0001), kidney (F(3,16)=6.89, p<0.01), liver(F(3,18)= 105.60, p<0.0001), lung (F(3,17)=13.44, p<0.0001), and spleen (F(3,19)=8.80, p<0.001). Results from Dunnett’s post hoc analyses revealed that mice exposed to 50 mg of buzz had significant increases in JWH-018 concentration in all tissues (p<0.001) as compared to mice exposed to placebo. Additionally, significant increases in JWH-018 concentration in mice exposed to 20 mg of buzz as compared to placebo-exposed mice were found in the heart (p<0.01) and lung (p<0.01).
Figure 2.
JWH-018 concentrations collected from blood, brain, heart, kidney, liver, lung, and spleen 1 h after mice were exposed to smoke produced from incineration of Buzz (10, 20, or 50 mg). JWH-018 was not detected in blood or tissue of mice exposed to placebo (not shown). **p<0.01; ***p<0.001 vs. mice treated with 50 mg placebo smoke; Dunnett’s post-hoc test. All data reflect mean ± SEM; n=4-6 mice/group of JWH-018 concentrations (ng/ml).
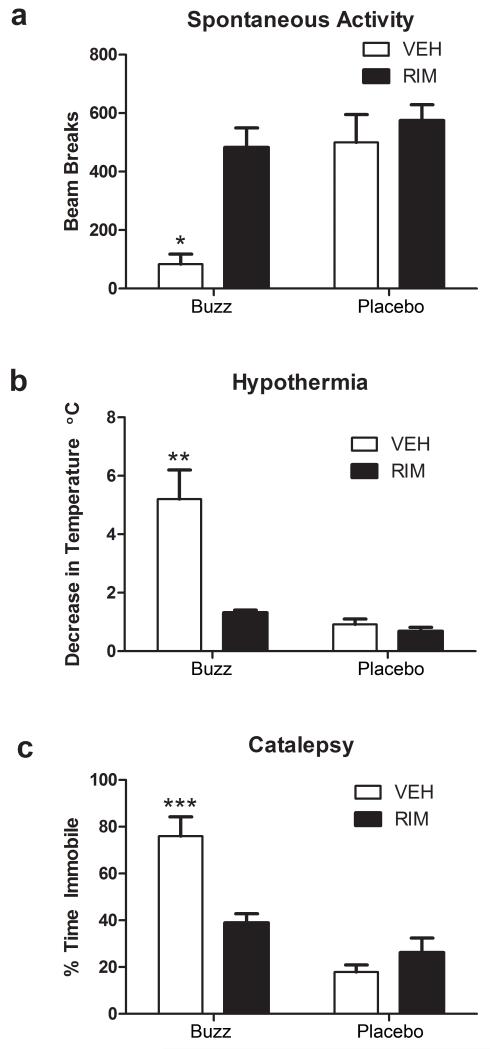
Study 2. As shown in Figure 3, the locomotor suppressant, hypothermic, and cataleptic effects of Buzz (20 mg) were blocked by rimonabant. Rimonabant also antagonized other behavioral effects of Buzz, as shown in Table 1. A significant interaction between rimonabant and inhalation treatment was found for spontaneous activity [Figure 3a; F(1,20)=5.53, p<0.05], hypothermia, [Figure 3b; F(1,20)=12.45, _p_<0.01], and catalepsy [Figure 3c; F(1,20)=15.70, _p_<0.001]. Bonferroni’s post hoc tests revealed that mice treated with vehicle+Buzz, but not mice treated with rimonabant+placebo or rimonabant+Buzz, had significant reductions in body temperature (_p_<0.001), compared to the vehicle+placebo group. Similarly, vehicle+Buzz-treated mice displayed decreased body temperature (_p_<0.01) and increased catalepsy scores (p<0.001) that significantly differed from all other treatment conditions. No significant difference between treatment conditions was found in baseline measures (see Table 2 for group means ± SEM) for body temperature (p >.05). Taken together, these results indicate that Buzz-induced hypothermia, hypomotility, and catalepsy are mediated through CB1 receptors.
Figure 3.
Pharmacological effects produced by inhalation of smoke from incinerated Buzz (20 mg) are CB1 receptor mediated. Rimonabant (3 mg/kg) significantly blocked Buzz-induced (a) decreases in spontaneous locomotor activity, (b) hypothermia, and (c) catalepsy in the ring immobility test. Baseline body temperatures are found in Table 2. *p<0.05; **p<0.01; ***p<0.001. Bonferroni’s multiple comparisons post-hoc test. All data reflect mean ± SEM; n=6 mice/group.
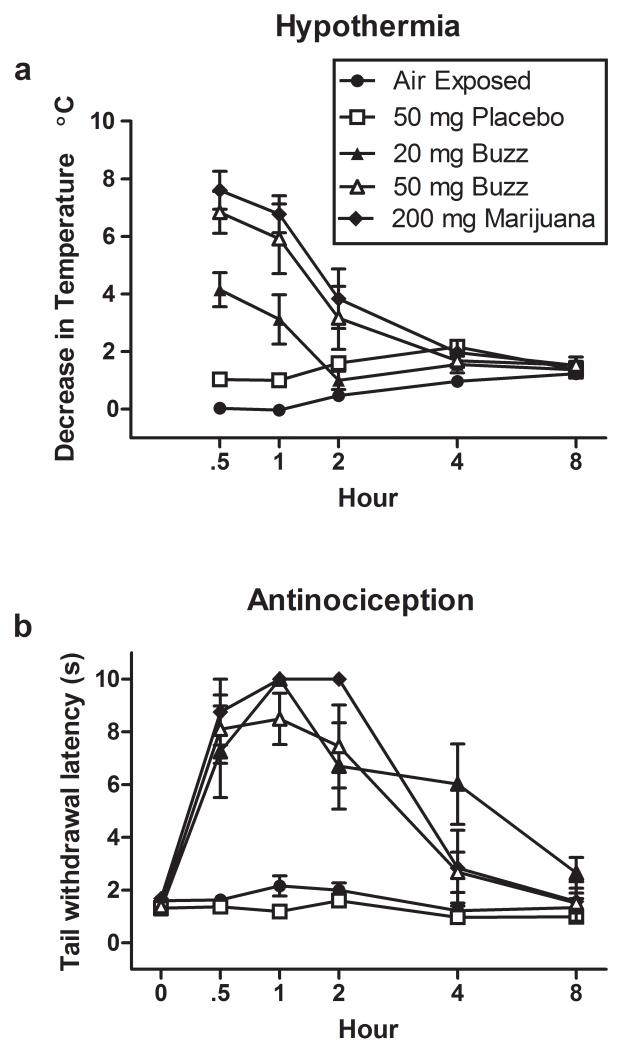
Study 3. The time-course of Buzz’s behavioral effects are shown in Fig. 4. Baseline measures for hypothermia and antinociception were first assessed. Then, hypothermia and antinociception were measured over an 8 h period (30, 60, 120, 240, and 480 min) following inhalation exposure to air, or smoke from pyrolyzed placebo, Buzz (20 or 50 mg), or marijuana (200 mg). A significant interaction was found between time and treatment condition for hypothermia, F(16, 100)=15.66, p<0.0001. Bonferroni’s test of multiple comparisons revealed significant decreases in temperature with doses of 20 and 50 mg Buzz and 200 mg marijuana 30 min after inhalation exposure (_p_<0.01, _p_<0.0001, and _p_<0.0001 respectively) as compared to placebo inhalation. Mice continued to exhibit hypothermia at 60 min after exposure to 50 mg Buzz and 200 mg marijuana (_p_<0.0001) as compared to placebo-treated mice. A significant interaction was also found between time and treatment condition for antinociception assessed using warm water tail withdrawal latency, _F_(20, 125)=6.854, _p_<0.0001. Bonferroni’s test of multiple comparisons revealed that tail withdrawal latencies were significantly increased with Buzz (20 or 50 mg) and marijuana (200 mg) as compared to placebo inhalation exposure at 30 and 60 min after inhalation exposure (_p_<0.0001). Tail withdrawal latencies at 120 min after inhalation exposure to Buzz (20 or 50 mg) and marijuana (200 mg) significantly differed from placebo (_p_<0.001; _p_<0.0001; _p_<0.0001 respectively), whereas at 240 min only 20 mg Buzz produced significant antinociception compared to placebo (p<0.001). No differences in body temperature or tail withdrawal latency were observed between placebo and air exposed mice at any time point. Additionally, no significant differences between treatment conditions were found in baseline measures (see Table 2 for group means ± SEM) of body temperature or tail withdrawal (p >.05).
Figure 4.
Incinerated Buzz and marijuana smoke dose-dependently produced cannabinoid behavioral responses including (a) hypothermia and (b) antinociception in the hot water bath tail withdrawal test. Mice were tested before exposure (i.e. 0 h or pre-exposure) and 30, 60, 120, 240 and 480 min after nose-only exposure to air, placebo, Buzz (20 or 50 mg) and marijuana (200 mg). Pre-exposure mean (± SEM) baseline temperatures were 38.20±0.15, 38.62±0.14, 38.55±0.10, 38.60±0.10, and 38.57± 0.15 °C for mice exposed to air, 50 mg placebo, 20 mg Buzz, 50 mg Buzz, and 200 mg marijuana, respectively. After 480 min (i.e. 8 h) mean (± SEM) body temperatures were 36.97±0.17, 37.25±0.28, 37.18 ±0.11, 37.07±0.15, and 37.05±0.18 °C for mice exposed to air, 50 mg placebo, 20 mg Buzz, 50 mg Buzz, and 200 mg marijuana, respectively. Baseline tail withdrawal latencies are found in Table 2. All data reflect mean ± SEM; n=6 mice/group.
4. Discussion
In the present study, we report that nose-only exposure to incinerated Buzz, containing 5.4 % of JWH-018, produces cannabimimetic effects in mice. The tetrad assay reveals four psychotropic properties of THC and other cannabinoids, including 1) antinociception 2) catalepsy 3) hypothermia, and 4) hypomobility (decreases in spontaneous locomotor activity; Martin et al., 1991). This spectrum of effects is typically indicative of cannabimimetic activity. The observations that rimonabant antagonized Buzz-induced hypothermia, reductions in spontaneous activity, and catalepsy indicate a CB1 receptor mechanism of action. Thus, we present the novel finding that exposure to smoke from the herbal incense product Buzz, containing 5.4% JWH-018, activates CB1 receptors producing behavioral effects that are typically observed following administration of THC and other cannabinoid receptor agonists in mice.
Other reports have demonstrated that injected JWH-018 produces antinociception, hypothermia, and hypomobility (Wiley et al., 2011) as well as produces CB1 receptor mediated decreases in body temperature and locomotor activity in mice (Brents et al., 2011). Additionally, JWH-018 and JWH-073 substitute for THC in monkeys trained to discriminate THC from vehicle (Ginsburg et al., 2011). Similarly, AM678, which is structurally identical to JWH-018, produces CB1 receptor mediated substitution for THC in rats (Jarbe et al., 2011a; 2011b; 2012) in the drug discrimination paradigm. Our results extend these findings in that we have shown exposure to the smoke from an herbal incense product containing JHW-018 produces CB1 receptor mediated cannabinoid effects.
We found that 50 mg Buzz (containing 2.7 mg JWH-018) and 200 mg marijuana (containing 14.8 mg THC) produce antinociception and hypothermia that are similar in magnitude and duration. Concentrations of JWH-018 (83 ± 42.8 ng/g) in the brain after inhalation of 50 mg of incinerated Buzz were lower than brain concentrations of THC (433 ± 66 ng/g) following 200 mg incinerated marijuana inhalation (Wilson et al., 2006) indicating that Buzz, and specifically JWH-018, is a potent intoxicant. These findings are consistent with reports showing that injected JWH-018 (Ki = 9 ± 5 nM) activates CB1 receptors with greater potency than THC (Ki = 41 nM; Atwood et al., 2010; Brents et al., 2011; Ginsburg et al., 2011; Wiley et al., 1998; 2011). One study also reported that JWH-018 produces greater maximal effects than THC in both in vitro and in vivo assays and found that its metabolites had affinity and activity at CB1 receptors as well (Brents et al., 2011).
The antinociceptive effects of Buzz and marijuana in the tail withdrawal test persisted for at least a 120 min duration. Interestingly, exposure to 20 mg Buzz (containing 1.08 mg JWH-018) produced a significant antinociceptive effect in the warm water tail withdrawal test, but did not alter radiant heat tail-flick latencies. The observation that a higher dose of Buzz was required to inhibit nociceptive behavior in the radiant heat tail-flick test than in the warm water tail withdrawal test can be accounted by the fact that radiant heat is a considerably more noxious stimulus than the warm water bath. Exposure to 20 mg and 50 mg Buzz produced respective body temperature decreases of 5° C and 8° C. Similarly, exposure to 200 mg marijuana resulted in an 8° C decrease in body temperature. The duration of hypothermic effects of Buzz (20 and 50 mg) and marijuana (200 mg) persisted for 60 min. In contrast, THC produced a longer duration of action than injected JWH-018 in monkeys trained in the THC drug discrimination assay (Ginsburg et al., 2011).
Tissue concentrations of JWH-018 in the blood, brain, heart, kidney, liver, lung, and spleen show a dose-dependent relationship between JWH-018 tissue concentration and quantity of Buzz burned. JWH-018 was widely distributed throughout all tissues assessed, although lower concentrations of JWH-018 were found in blood compared to all organs, which is likely due to the lipophilic nature of the compound. Interestingly, the highest concentrations of JWH-018 were found in the tissues associated with absorption (lung), metabolism (liver), and elimination (kidney).
Human users of synthetic cannabinoids report that these products share some but not all of the “subjective” effects marijuana (Schneir et al., 2011; Vardakou et al., 2010). Although these products are labeled “not for human consumption,” anecdotal and case reports indicate that they are indeed smoked to achieve marijuana-like effects. Moreover, anecdotal and case reports indicate that the use of “herbal spice” products is associated with a greater prevalence of adverse effects than marijuana use (Every-Palmer, 2010, 2011; Lapoint et al., 2011; Simmons et al., 2011; Vearrier and Osterhoudt, 2010; Young et al., 2011; Zimmermann et al., 2009). Although there are many human reports describing the use of herbal incense products containing synthetic cannabinoids, there have been no systematic laboratory studies using humans, which is due to potential toxicity concerns associated with these chemicals. For this reason, we chose to conduct this study using mice. Reports of adverse effects of products containing JWH-018 and/or other synthetic cannabinoids in humans include hypertension, anxiety, panic attacks, agitation, seizures, hallucinations, and psychoses (Auwarter et al., 2009; Every-Palmer, 2010; Muller et al., 2010a; 2010b; Vardakou et al., 2010). The high potency and efficacy by which JWH-018 activates CB1 receptors as compared to THC may account for intense adverse effects associated with synthetic cannabinoid herbal products. Additionally, JWH-018 metabolites activate CB1 receptors and have been shown to produce in vivo behavioral cannabinoid effects (Brents et al., 2011). Accordingly, both pharmacodynamic and pharmacokinetic properties of JWH-018 are likely to contribute to the increased adverse effects associated “synthetic cannabinoids” as compared to marijuana. Finally, given that JWH-018 is sprayed onto plant material, the amount of this compound on the product is likely to be uneven making it difficult for users to titrate the dose.
Given the adverse effects reported after using synthetic cannabinoid herbal incense products as well as unknown toxicology of these chemicals, their continued use represents a substantial public health concern. Lack of awareness of their untoward side effects coupled with the perception of their safety contributes to their prevalent use. Another driving factor in the use of these products may be the inability to detect their presence in urine using conventional drug screening methods. Individuals requiring urine drug screens may be particularly susceptible to abusing these products over marijuana. However, new techniques to detect JWH-018 and other synthetic cannabinoids in urine, blood, oral fluid, and plant material have recently been developed (Coulter et al., 2011; Dresen et al., 2011; ElSohly et al., 2011; Penn et al., 2011; Poklis et al., 2012a; 2012b). Further development and implementation of these and other testing procedures may help curb the use of herbal incense products. Public awareness of the dangers of these products will also play an important role in the reduction of their use.
Though the DEA placed an emergency ban on JWH-018 and four other synthetic cannabinoids in March of 2011, there are many other synthetic cannabinoids that could readily be manufactured and substituted for the banned compounds. Development of testing procedures for JWH-018 will drive the production of different herbal incense products in the future. Despite this temporary ban, Buzz and other popular herbal incense products are still available for purchase on the internet and various other sites. In addition, it is likely that other synthetic cannabinoids are replacing cannabinoids on the banned list (Phillips, 2011). Consequently, a challenge is to prevent the use of these other synthetic cannabinoids as well as the compounds that have been temporarily banned (Leonhart, 2011).
In conclusion, nose-only exposure to smoke from incinerated Buzz, containing 5.4% JWH-018, produces cannabimimetic effects in the tetrad that are mediated by CB1 receptors. Buzz (50 mg herbal material containing 2.7 mg JWH-018) and marijuana (200 mg containing 14.8 mg THC) produced antinociceptive and hypothermic effects that were similar in magnitude and duration. However, brain concentrations of JWH-018 (83 ± 42.8 ng/g) after inhalation of 50 mg incinerated Buzz were much lower than brain concentrations of THC (433 ± 66 ng/g) following inhalation of 200 mg marijuana (Wilson et al., 2006). These findings are consistent with the notion that the synthetic cannabinoid JWH-018 is a more potent intoxicant in eliciting CB1 receptor mediated effects than THC (Atwood et al., 2010; Brents et al., 2011; Ginsburg et al., 2011; Wiley et al., 1998; 2011).
Acknowledgements
We wish to acknowledge Carlotta B. Jackson and Mary E. Tokarz for excellent technical assistance.
Role of Funding Source
Funding for this study was provided by NIDA Grants 5P01DA009789 (A.H.L), R01DA03672 (A.H.L), P50DA005274 (A.H.L), and 1F31DA030869 (J.M.W.). The NIDA had no further role in designing the studies, conducting the studies, or submitting the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Contributors
Authors J.M. Wiebelhaus and L.E. Wise designed and conducted the studies, analyzed the data, and wrote the manuscript. Author A.H. Lichtman designed the studies and wrote the manuscript. Authors R.E. Vann, A. Poklis, and J.L. Poklis designed and conducted the studies. All authors contributed to and have approved the final manuscript.
References
- Aceto MD, Scates SM, Razdan RK, Martin BR. Anandamide, an endogenous cannabinoid, has a very low physical dependence potential. J. Pharmacol. Exp. Ther. 1998;287:598–605. [PubMed] [Google Scholar]
- Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br. J. Pharmacol. 2010;160:585–593. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auwarter V, Dresen S, Weinmann W, Muller M, Putz M, Ferreiros N. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? JMS. 2009;44:832–837. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PloS One. 2011;6:e21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter C, Garnier M, Moore C. Synthetic cannabinoids in oral fluid. J. Anal. Toxicol. 2011;35:424–430. doi: 10.1093/anatox/35.7.424. [DOI] [PubMed] [Google Scholar]
- Cutler MG, Mackintosh JH. Effects of delta-9-tetrahydrocannabinol on social behaviour in the laboratory mouse and rat. Psychopharmacologia. 1975;44:287–289. doi: 10.1007/BF00428908. [DOI] [PubMed] [Google Scholar]
- D’Amour FE, Smith DL. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941;72:74–79. [Google Scholar]
- Dresen S, Kneisel S, Weinmann W, Zimmermann R, Auwarter V. Development and validation of a liquid chromatography-tandem mass spectrometry method for the quantitation of synthetic cannabinoids of the aminoalkylindole type and methanandamide in serum and its application to forensic samples. JMS. 2011;46:163–171. doi: 10.1002/jms.1877. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Gul W, Elsohly KM, Murphy TP, Madgula VL, Khan SI. Liquid chromatography-tandem mass spectrometry analysis of urine specimens for K2 (JWH-018) metabolites. J. Anal. Toxicol. 2011;35:487–495. doi: 10.1093/anatox/35.7.487. [DOI] [PubMed] [Google Scholar]
- Elsohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Every-Palmer S. Warning: legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addiction. 2010;105:1859–1860. doi: 10.1111/j.1360-0443.2010.03119.x. [DOI] [PubMed] [Google Scholar]
- Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend. 2011;117:152–157. doi: 10.1016/j.drugalcdep.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Schulze DR, Hruba L, McMahon LR. JWH-018 and JWH-073: delta-9-THC like discriminative stimulus effects in monkeys. J. Pharmacol. Exp. Ther. 2011;340:37–45. doi: 10.1124/jpet.111.187757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JW, Dai D, Martin BR, Compton DR. Design, synthesis and pharmacology of cannabimimetic indoles. Bioorg. Med. Chem. Lett. 1994;4:563–566. [Google Scholar]
- [Accessed 3/1/2009];2009 imzonged.com.
- Jacob JJ, Ramabadran K, Campos-Medeiros M. A pharmacological analysis of levonantradol antinociception in mice. J. Clin. Pharmacol. 1981;21:327S–333S. doi: 10.1002/j.1552-4604.1981.tb02611.x. [DOI] [PubMed] [Google Scholar]
- Jarbe TU, Deng H, Vadivel SK, Makriyannis A. Cannabinergic aminoalkylindoles, including AM678=JWH018 found in ‘Spice’, examined using drug (Delta(9)-tetrahydrocannabinol) discrimination for rats. Behav. Pharmacol. 2011a;22:498–507. doi: 10.1097/FBP.0b013e328349fbd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, LeMay BJ, Vemuri VK, Vadivel SK, Zvonok A, Makriyannis A. Central mediation and differential blockade by cannabinergics of the discriminative stimulus effects of the cannabinoid CB1 receptor antagonist rimonabant in rats. Psychopharmacology (Berl.) 2011b;216:355–365. doi: 10.1007/s00213-011-2226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbe TU, Tai S, Lemay BJ, Nikas SP, Shukla VG, Zvonok A, Makriyannis A. AM2389, a high-affinity, in vivo potent CB(1)-receptor-selective cannabinergic ligand as evidenced by drug discrimination in rats and hypothermia testing in mice. Psychopharmacology (Berl.) 2012;220:417–426. doi: 10.1007/s00213-011-2491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH. Severe toxicity following synthetic cannabinoid ingestion. Clin. Toxicol. 2011;49:760–764. doi: 10.3109/15563650.2011.609822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhart MM. Federal Register. Washington, DC: 2011. Schedules of controlled substances: temporary placement of five synthetic cannabinoids into schedule I of the Controlled Substances Act, 12201; pp. 11075–11078. [Google Scholar]
- Lichtman AH, Poklis JL, Poklis A, Wilson DM, Martin BR. The pharmacological activity of inhalation exposure to marijuana smoke in mice. Drug Alcohol Depend. 2001;63:107–116. doi: 10.1016/s0376-8716(00)00205-2. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nature Chem. Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol. Biochem. Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- Muller H, Huttner HB, Kohrmann M, Wielopolski JE, Kornhuber J, Sperling W. Panic attack after spice abuse in a patient with ADHD. Pharmacopsychiatry. 2010a;43:152–153. doi: 10.1055/s-0029-1243252. [DOI] [PubMed] [Google Scholar]
- Muller H, Sperling W, Kohrmann M, Huttner HB, Kornhuber J, Maler JM. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr. Res. 2010b;118:309–310. doi: 10.1016/j.schres.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Cannabinoid CB(1) receptor agonists produce cerebellar dysfunction in mice. J. Pharmacol. Exp. Ther. 2001;297:629–637. [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE, O’Malley PM, Johnston LD, Bachman JG. Adolescents’ reported reasons for alcohol and marijuana use as predictors of substance use and problems in adulthood. J. Stud. Alcohol Drugs. 2011;72:106–116. doi: 10.15288/jsad.2011.72.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn HJ, Langman LJ, Unold D, Shields J, Nichols JH. Detection of synthetic cannabinoids in herbal incense products. Clin. Biochem. 2011;44:1163–1165. doi: 10.1016/j.clinbiochem.2011.06.078. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The ring test: a quantitative method for assessing the ‘cataleptic’ effect of cannabis in mice. Br. J. Pharmacol. 1972;46:753–763. doi: 10.1111/j.1476-5381.1972.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. Changing flavor of ‘spice.’ Washington Post. Katharine Weymouth; Washington, D.C: 2011. [Google Scholar]
- Poklis JL, Amira D, Wise LE, Wiebelhaus JM, Haggerty BJ, Lichtman AH, Poklis A. Determination of naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in mouse blood and tissue after inhalation exposure to ‘buzz’ smoke by HPLC/MS/MS. Biomed. Chromatogr. 2012a doi: 10.1002/bmc.2710. Epub ahead of print. doi: 10.1002/bmc.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poklis JL, Amira D, Wise LE, Wiebelhaus JM, Haggerty BJ, Poklis A. Detection and disposition of JWH-018 and JWH-073 in mice after exposure to “Magic Gold” smoke. Forensic Sci. Int. 2012b doi: 10.1016/j.forsciint.2012.02.003. Epub ahead of print. doi: 10.1016/j.forsciint.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poklis JL, Thompson CC, Long KA, Lichtman AH, Poklis A. Disposition of cannabichromene, cannabidiol, and Delta-tetrahydrocannabinol and its metabolites in mouse brain following marijuana inhalation determined by high-performance liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2010;34:516–520. doi: 10.1093/jat/34.8.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneir AB, Cullen J, Ly BT. “Spice” girls: synthetic cannabinoid intoxication. J. Emerg. Med. 2011;40:296–299. doi: 10.1016/j.jemermed.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Simmons J, Cookman L, Kang C, Skinner C. Three cases of “spice” exposure. Clin. Toxicol. 2011;49:431–433. doi: 10.3109/15563650.2011.584316. [DOI] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou C. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol. Lett. 2010;197:157–162. doi: 10.1016/j.toxlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, Lichtman AH. Delta9-tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. J. Pharmacol. Exp. Ther. 2005;314:329–337. doi: 10.1124/jpet.104.080739. [DOI] [PubMed] [Google Scholar]
- Vearrier D, Osterhoudt KC. A teenager with agitation: higher than she should have climbed. Ped. Emerg. Care. 2010;26:462–465. doi: 10.1097/PEC.0b013e3181e4f416. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, Martin BR. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J. Pharmacol. Exp. Ther. 1998;285:995–1004. [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Martin BR, Huffman JW. 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depend. 2011;123:148–153. doi: 10.1016/j.drugalcdep.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM, Varvel SA, Harloe JP, Martin BR, Lichtman AH. SR 141716 (Rimonabant) precipitates withdrawal in marijuana-dependent mice. Pharmacol. Biochem. Behav. 2006;85:105–113. doi: 10.1016/j.pbb.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Young AC, Schwarz E, Medina G, Obafemi A, Feng SY, Kane C, Kleinschmidt K. Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am. J. Emerg. Med. 2011 doi: 10.1016/j.ajem.2011.05.013. doi: 10.1016/j.ajem.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold. Deutsches Arzteblatt Int. 2009;106:464–467. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]