IGF-I 3′ Untranslated Region: Strain-Specific Polymorphisms and Motifs Regulating IGF-I in Osteoblasts (original) (raw)
Abstract
Reduced IGF-I is associated with low bone mass in humans and mice. C3H/He/J (C3H) mice have higher skeletal IGF-I and greater bone mass than C57BL/6J (B6). We hypothesized that strain-related genotypic differences in Igf1 affected skeletal function. The Igf1 coding region is nonpolymorphic, but its 3′ untranslated region (UTR) is polymorphic between C3H and B6. Luciferase-Igf1 3′ UTR reporter constructs showed that these polymorphic regions did not affect UTR function. IGF-I splice variants give rise to a common mature IGF-I peptide, but different E peptides. We identified two splice products, exon 4+6 (Ea) and exon 4+5+6 (Eb, mechano-growth factor) and found that their abundance was unchanged during osteoblastic differentiation. The Igf1 3′ UTR encoded by exon 6 contains alternative polyadenylation sites. Proximal site use produces a short 3′ UTR of approximately 195 bases, whereas distal site usage results in an approximately 6300-base UTR. Although Igf1 mRNA levels did not change during osteoblastic differentiation, distal polyadenylation site usage was increased in B6 cells but not in C3H. The resulting long Igf1 RNA isoform is less stable and has decreased translation efficiency, which may be one mechanism contributing to decreased IGF-I in B6 vs. C3H mice. Although the long UTR contains a conserved [GU]18 repeat, which is a positive regulator of UTR activity, it is also targeted by negative regulators, miR-29 and miR-365. These microRNAs are increased in B6 and C3H cells during osteoblastic differentiation. Differential expression of the long Igf1 3′ UTR isoform may be a possible mechanism for enhanced IGF-I regulation in B6 vs. C3H mice.
IGF-I plays a critical role in skeletal growth, serving as an autocrine, paracrine, and endocrine regulator of bone mass. Animals and humans with deficits in IGF-I display decreased peak bone mass and impaired linear growth. Indeed, serum IGF-I and volumetric bone mineral density are complex traits that cosegregate in both mice and humans. The majority of serum IGF-I is synthesized by the liver, and it functions as an endocrine regulator of cell growth, differentiation, and survival in multiple target tissues. Although serum IGF-I levels have a strong impact on the skeleton, the local production of IGF-I by skeletal cells is vital for bone mass acquisition (reviewed by Ref. 1).
In mice, the Igf1 gene gives rise to multiple mRNA isoforms, ranging in size from approximately 1 to 7.5 kb. These isoforms arise from usage of alternative promoters (in exon 1 or exon 2), alternative splicing of exon 5, and differential usage of polyadenylation signals within exon 6 (http://genome.ucsc.edu/) (2). Similar Igf1 transcript variants are observed in humans (3). The mature IGF-I protein is encoded by sequences within exons 3 and 4, with alternative E peptides (Ea or Eb) encoded by inclusion or exclusion of exon 5 (4). The function of the endogenous E peptides is somewhat controversial, although it is known that the Eb splice variant is induced in injured skeletal muscle, followed by Ea variant up-regulation (reviewed by Ref. 3). Aside from skeletal muscle studies, there is little information about the distribution and regulation of IGF-I splice variants in other tissues.
The major size difference in Igf1 mRNAs is due to the length of its 3′ untranslated region (UTR). Use of a proximal polyadenylation site results in a short 3′ UTR of approximately 200 bases, whereas use of a distal polyadenylation site results in a 3′ UTR of approximately 6300 bases. The smaller Igf1 mRNA isoforms are more stable than the large, and the smaller isoforms may be preferentially translated (5). The relative ratio of large to small Igf1 mRNAs can differ with tissue type. For example, the smaller Igf1 isoforms are predominant in liver, whereas in long bone, the large Igf1 isoforms are more abundant (6).
Inbred strains of mice expressing higher levels of skeletal IGF-I tend to have higher bone mass. This concept is well illustrated when comparing the C3H/He/J (C3H) and C57BL/6J (B6) mouse strains. C3H mice have higher skeletal IGF-I levels, greater bone formation, and more bone mass than B6 mice (7). Further, a B6.C3H congenic carrying a C3H locus containing the IGF-I gene also has higher skeletal IGF-I levels and bone mass compared with B6 mice (8). We hypothesized that polymorphisms in the Igf1 gene could play a role in the differential expression of this protein in B6 vs. C3H mice. Although the Igf1 coding region is not polymorphic, its 3′ UTR has three regions that are polymorphic between C3H and B6 (summarized in Fig. 1). In addition, it was recently demonstrated that the circadian deadenylase, Nocturnin, interacts with Igf1 mRNA. In osteoblasts overexpressing Nocturnin, the activity of the IGF-I 3′ UTR from B6 mice is significantly decreased, whereas activity of the corresponding 3′ UTR fragment from C3H mice was unaffected by Nocturnin expression levels (9). Here, we report further characterization of the 3′ UTR from the Igf1 gene in B6 and C3H mice and identified microRNAs (miRNAs) that regulate IGF-I expression.
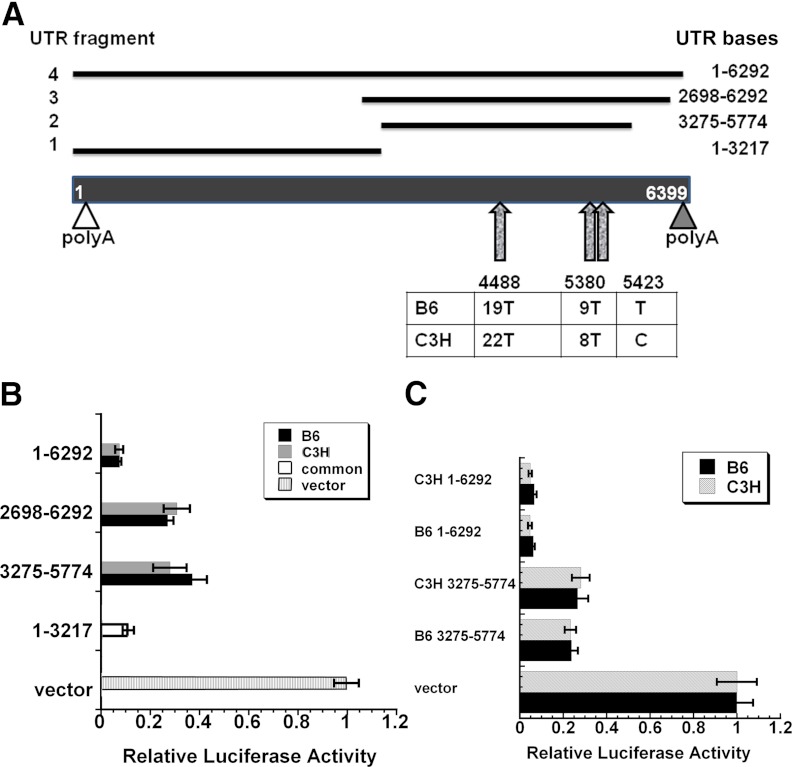
Fig. 1.
Polymorphisms in the Igf1 3′ UTR from B6 and C3H mice do not affect UTR function. A, Schematic representation of the Igf1 3′ UTR from exon 6 and the 3′ UTR fragments cloned into pMIR-REPORT Luciferase for functional analysis. Fragment 1 is nonpolymorphic. Fragments 2–4 are polymorphic, and strain-specific constructs were created. The proximal (open triangle) and distal (filled triangle) polyadenylation sites are indicated. The three polymorphic regions are denoted by arrows. B, Activity of luciferase-3′ UTR constructs in transiently transfected MC3T3 cells. All Igf1 3′ UTR constructs tested had significantly lower luciferase activity compared with the vector alone (P < 0.01). C, Activity of strain-specific luciferase-3′ UTR constructs in transiently transfected B6 or C3H stromal cells. Luciferase activity is expressed as a percentage of β-gal activity (mean ± sem).
Materials and Methods
Cell culture
The murine osteoblastic cell line MC3T3-E1 (MC3T3) was obtained from American Type Culture Collection (Manassas, VA). MC3T3 cells were cultured in α-MEM (Invitrogen, Fredrick, MD) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA).
For culture of marrow stromal cells (MSCs), bone marrow was flushed from the long bones of 8-wk-old B6 and C3H mice by centrifugation (10). Cells were cultured in α-MEM + 10% fetal bovine serum for 6 d. At confluence (wk 0), culture medium was supplemented with 5 mm β-glycerophosphate and 50 μg/ml ascorbic acid, to promote osteoblastic differentiation. Cells were trypsinized and subcultured once for transfections. Transcription arrest experiments were performed in the presence of 75 μm 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (Sigma, St. Louis, MO) (11). These studies were approved by the Institutional Animal Care and Use Committee at the University of Connecticut Health Center.
Constructs
PCR and appropriate primer sets were used to amplify Igf1 3′ UTR fragments of interest from the genomic DNA of B6 or C3H mice. UTR fragments were subcloned into the cytomegalovirus promoter-luciferase reporter pMIR-REPORT (Ambion, Austin, TX) using _Mlu_I and _Sac_I restriction enzyme sites. Deletion mutants were made by overlap extension. Constructs were verified by sequencing.
Transfections
MC3T3 cells were plated at 18,000 cells/cm2, and FuGENE 6 (FuGENE:DNA ratio, 3:2; Roche, Indianapolis, IN) was used to cotransfect luciferase-Igf1 3′ UTR constructs and a constitutively expressed β-galactosidase (β-gal) construct, as a control for transfection efficiency (Promega, Madison, WI). Primary stromal cells were plated at a similar density and were also transfected with FuGENE 6 (FuGENE:DNA ratio, 3:1).
Cotransfection of MC3T3 cells with luciferase-UTR constructs, β-gal expression plasmid, and miRNA hairpin inhibitors (Dharmacon/Thermo Fisher Scientific, Auburn, AL) was accomplished using X-tremeGENE reagent (X-tremeGENE:nucleic acid ratio, 5:1; Roche). A negative control inhibitor, the sequence of which was determined not to interact with any know miRNAs, was also obtained from Dharmacon (12).
Twenty-four hours after transfection, cells were serum deprived overnight, and cell lysates were prepared using Reporter Lysis buffer (Promega), according to the manufacturer's instructions. Equal aliquots of cell lysate were used to determine luciferase activity (Luciferase assay system; Promega) and β-gal activity (Galacton chemiluminescent assay system; Tropix, Bedford, MA). Luciferase activity was expressed as a percentage of the β-gal activity. Transient transfections were performed using six replicates per experiment, and each experiment was performed at least three times. More than one DNA preparation for each construct was tested.
Transfection of miRNA inhibitors (50 nm) alone was accomplished using Oligofectamine reagent (Oligofectamine:nucleic acid ratio, 3:1; Invitrogen). Forty-eight hours after transfection, cells were switched to serum-free medium. The serum-free conditioned medium was harvested 18 h later. IGF-I levels were quantified by RIA (ALPCO, Windham, NH) (13).
RNA analysis
RNA was isolated from cultured cells using TRIzol (Invitrogen) or Qiazol (QIAGEN, Valencia, CA) and quantified spectroscopically. For quantitative RT-PCR (qRT-PCR), aliquots of RNA were treated with deoxyribonuclease I before analysis, to exclude any potential signal from DNA contamination. To quantify mRNA levels in total RNA, deoxyribonucleased RNA was reverse transcribed with Moloney Murine Leukemia Virus-reverse transcriptase, and using random hexamer primers (Invitrogen), followed by qPCR with iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) in a MiQ qPCR cycler (Bio-Rad). The primer sets used are shown in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. RNA levels were determined using standard curves and were normalized to 18S rRNA.
To quantify miR-29a levels in total RNA, the _mir_Vana qRT-PCR miRNA Detection kit (Ambion) was used for B6 mice. Briefly, duplicate aliquots of RNA were reverse transcribed and subjected to PCR amplification using the MiQ qPCR cycler. RNA levels were calculated using standard curves, and miR-29 levels were expressed relative to those of 5S. To quantify miR-29a in C3H mice and miR-365 levels in both mouse strains, the TaqMan Small RNA Assay (Invitrogen) was used. Briefly, duplicate aliquots of RNA were reverse transcribed and subjected to PCR amplification using the MiQ qPCR cycler. RNA levels were calculated using standard curves, and miR-365 levels were expressed relative to those of Sno202 small nucleolar RNA.
For Northern blot analysis, 7.5 μg of total RNA were denatured and subjected to electrophoresis through a 1% formaldehyde-agarose gel, and blotted onto a Gene Screen Plus membrane, as directed by the manufacturer (DuPont, Wilmington, DE). Triplicate cultures were analyzed. A cDNA fragment corresponding to exons 3 and 4 of mouse Igf1 and murine 18S rRNA (American Type Culture Collection) were labeled with [α-32P]dCTP (3000 Ci/mmol; NEN Life Science Products, PerkinElmer, Boston, MA) (Ready-to-Go labeling kit, Amersham, Piscataway, NJ) and used to probe the blots (11). Specific hybridization was detected by autoradiography, and relative band densities were determined using ImageJ software (http://rsb.info.nih.gov/ij/download.html).
For rapid amplification of 3′ cDNA ends (3′ RACE), RNA was reverse transcribed using Moloney Murine Leukemia Virus- reverse transcriptase and a 3′ RACE adapter primer (Ambion) (14). cDNA products were amplified by PCR, using a forward primer located in Igf1 exon 4 and 3′ RACE outer primer, as detailed in Supplemental Table 1. PCR products were cloned via T/A cloning into pCR4-TOPO, according to the manufacturers' instructions (Invitrogen). Eight clones containing insert were subjected to sequence analysis.
Data analysis
Data are presented as mean ± sem. Data were analyzed by Student's t test or one-way ANOVA with Tukey post hoc test as appropriate (KaleidaGraph, Synergy Software, Reading, PA).
Results
Igf1 3′ UTR polymorphisms do not cause differences in UTR activity
The predominant 3′ UTR for Igf1 mRNA is encoded by exon 6 of the mouse gene and contains three regions that are polymorphic between B6 and C3H. One region, starting at 3′ UTR base 4488 in B6, has 19 T, but C3H has 22 T. The second region, starting at base 5380 in B6, has 9 T, whereas the corresponding region in C3H has 8 T. The last polymorphic region, at base 5423 in B6, has a T, whereas a C is found at the corresponding region in C3H (Fig. 1A). We created a series of luciferase-Igf1 3′ UTR reporter constructs for the purpose of testing UTR function. Fragment 1, which was not polymorphic, contained 3′ UTR bases 1–3217. Fragment 2 spanned bases 3275–5774 and contained all polymorphic regions. Fragment 3 contained the 3′ end of the UTR, from bases 2698 to 6292. Lastly, fragment 4 contained the full 3′ UTR, bases 1–6292.
We determined whether the exon 6 3′ UTR from B6 and C3H had inherent differences in activity by transfecting luciferase-3′ UTR reporter constructs of each genotype into the MC3T3 mouse osteoblastic cell line (Fig. 1B). We found that all 3′ UTR fragments tested caused a significant decrease in luciferase activity, in comparison with the vector alone (P < 0.01). The constructs with the full-length exon 6 3′ UTR had the lowest luciferase activity, likely reflecting the higher complement of negative regulatory elements in this 6-kb region. Notably, the activities of the UTRs carrying regions polymorphic between B6 and C3H were identical, suggesting that the polymorphic regions do not cause inherent differences in 3′ UTR activity.
It is possible that the polymorphic regions of the Igf1 exon 6 3′ UTR may modulate activity in a manner that is dependent on strain-specific differences in _trans_-acting factors. To test this, we transfected polymorphic luciferase-3′ UTR constructs (fragments 2 and 4) into primary cultures of B6 or C3H MSCs (Fig. 1C). The 3′ UTR constructs did not display differences in activity, regardless of whether they were transfected into B6 or C3H cells. 3′ UTR activity in the stromal cell cultures was similar to that observed in MC3T3. Overall, these data indicate that the polymorphic regions in the exon 6 3′ UTR do not affect function. Lastly, to test the relative stability of the endogenous Igf1 mRNA, we performed transcription arrest experiments in confluent cultures of MSCs isolated from B6 and C3H mice. Analysis of long and short Igf1 isoforms by Northern blotting and qRT-PCR did not demonstrate significant strain-specific differences in RNA stability. In the transcriptionally arrested cells, the half-life of the long Igf1 RNAs was approximately 7 h, and that of the shorter transcripts was approximately 13 h (data not shown).
Igf1 splice variants in osteoblastic cells
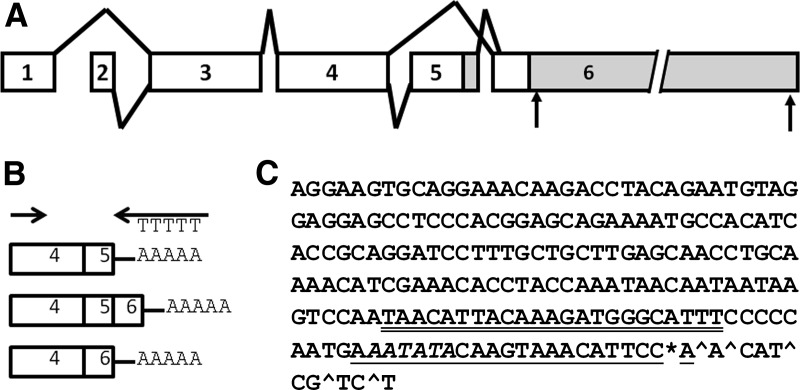
Igf1 has alternative splice variants, which can give rise to alternative E peptides, as well as alternative 3′ UTRs. The alternatively spliced RNAs could contain exons 4+6, 4+5+6, or exon 4+5 (without 6) (Fig. 2, A and B). Further, the short form of IGF-I could contain a 3′ UTR from exon 5 or a short 3′ UTR from exon 6. An analysis of the splice variants expressed in bone cells has not been previously reported and could impact IGF-I regulation and E peptide activity. We used 3′ RACE to identify splicing variants and to determine which of the shorter 3′ UTRs, exon 5 or exon 6, were found in Igf1 RNA isolated from MC3T3 cells (14). RNA was reverse transcribed using a 3′ RACE adapter primer, and cDNA products were amplified using a forward primer within exon 4 (Fig. 2B). This resulted in the amplification of two major bands of approximately 600 and 650 bp. These bands were subsequently cloned, and eight individual clones were sequenced. We found that the short Igf1 mRNA species in MC3T3 cells contained both exon 4+6 and exon 4+5+6 splice products but not exon 4+5 only. Further, the short 3′ UTRs from exon 6 displayed a cluster of termination sites within a span of nine bases, the most common of which was within the putative binding site for miR-1/206 (Fig. 2C).
Fig. 2.
Igf1 splice variants in osteoblasts and identification of 3′ ends for short Igf1 mRNAs. A, Diagram of alternative splicing patterns for mouse Igf1. Polyadenylation sites in exon 6 are indicated by arrows, and UTRs are indicated in gray. B, 3′ RACE design. C, Short Igf1 mRNA ends identified. The most common site of polyA addition is denoted by the asterisk, and less common sites are denoted by the caret. The miR-1/206 binding site is underlined, and a miR-365 site is double underlined. The polyA signal is in italic.
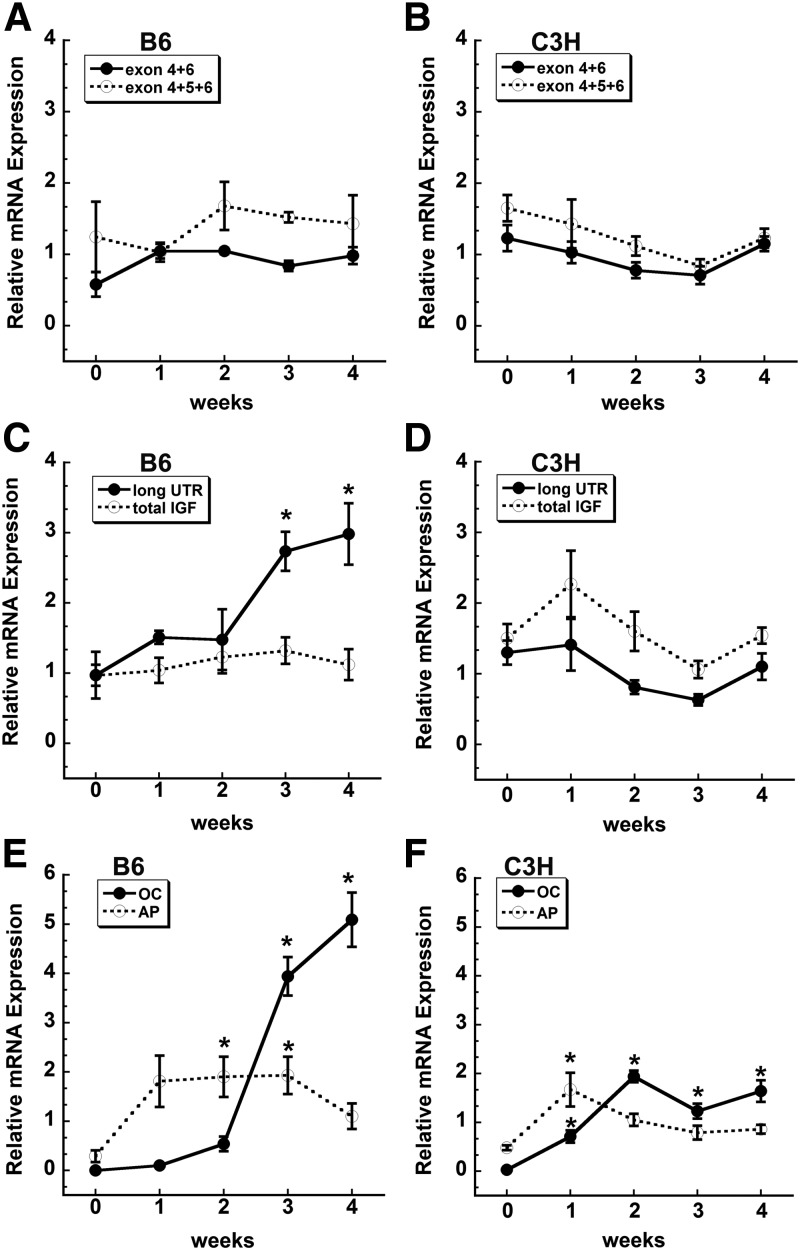
Igf1 alternative splicing is regulated during muscle cell differentiation (3). To determine whether splicing patterns might also be regulated during osteoblastic differentiation, we quantified exon 4+6 and 4+5+6 products in B6 vs. C3H bone MSCs during differentiation. We found that the relative expression of the two different splice variants did not significantly change during osteoblastic differentiation in either mouse strain (Fig. 3, A and B). Further, the total amount of Igf1 mRNA did not significantly change with osteoblastic differentiation in both mouse strains. However, the relative abundance of the long Igf1 3′ UTR-containing RNA was significantly increased during wk 3 and 4 of differentiation in B6 mice but not in C3H (Fig. 3, C and D). This increase in the long Igf1 3′ UTR-containing RNA in B6 mice mirrored that of osteocalcin mRNA, indicating increased usage of the distal polyadenylation signal with osteoblastic differentiation (Fig. 3, C and E). The fold increase in osteocalcin mRNA appeared to be higher in B6 mice, compared with C3H. However, direct comparison of osteocalcin mRNA levels in confluent stromal cell cultures showed that C3H had 11-fold higher osteocalcin mRNA compared with B6 (relative quantity 2.81 ± 0.9 vs. 0.25 ± 0.01, respectively; P ≤ 0.05; cycle threshold 22.8 vs. 19.4). Overall, these data suggest that Igf1 polyadenylation site usage changes with osteoblastic differentiation in B6 mice but not in C3H.
Fig. 3.
Evaluation of Igf1 splice variants and 3′ UTR length between C3H and B6 cells during osteoblast differentiation in vitro. B6 and C3H stromal cells were grown to confluence (0) and then cultured for up to 4 wk after confluence in osteoblast differentiation medium. RNA abundance was quantified by qRT-PCR and expressed relative to 18S rRNA. *, Significantly different from confluence, P < 0.05. A and B, Splice variants. C and D, Total Igf1 mRNA (amplified by an exon 4 primer set) compared with mRNA containing the long exon 6 3′ UTR. E and F, Alkaline phosphatase (AP) and osteocalcin (OC) mRNA levels, documenting the differentiation state of the cultures.
Conserved GU-rich motif in the Igf1 3′ UTR
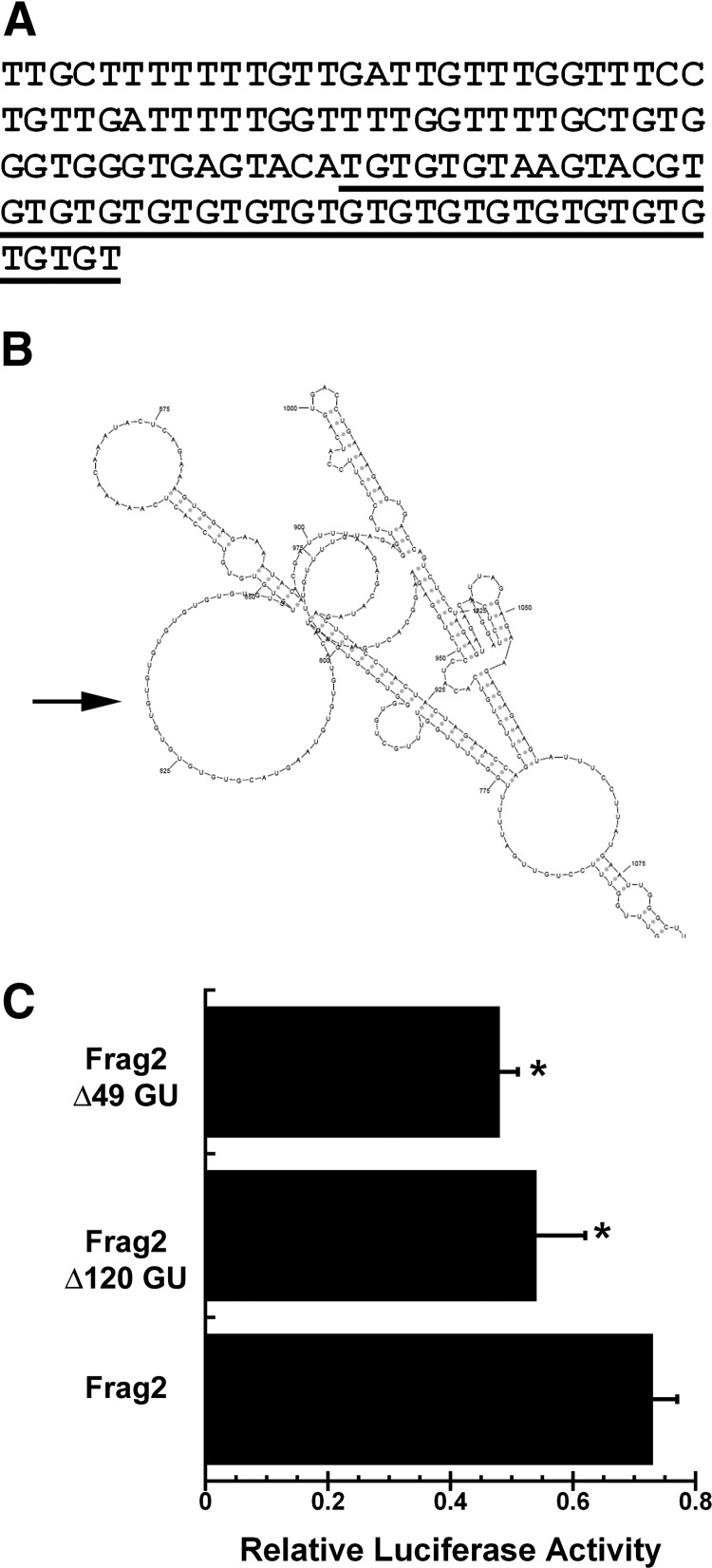
The long Igf1 3′ UTR contains several sequence motifs that are conserved between human and mouse. One motif consists of a 120-base region, starting at B6 base 4022, which is approximately 90% GU (Fig. 4A). This region includes a series 18 GU (GU18) repeats, and the human Igf1 3′ UTR also contains a GU19 repeat element (http://genome.ucsc.edu/). This was a region of interest, because there are several RNA binding proteins that recognize GU-rich elements, and which regulate RNA splicing, deadenylation, and mRNA decay (15–19). Further, many eukaryotic RNA binding proteins recognize loops in stem-loop structures or short unstructured sequences, and RNA secondary structure analysis suggests that the Igf1 3′ UTR GU-repeat may be located within a loop structure (sFOLD; http://sfold.wadsworth.org/cgi-bin/index.pl) (Fig. 4B) (20, 21). To test the function of GU-rich region, we created two deletion constructs, in the context of the C3H 3275–5774 base UTR (fragment 2). Transfection of constructs carrying deletion of the GU18 element or deletion of the entire 120-base GU-rich region resulted in a significant decrease in luciferase activity, compared with the parent construct. The smaller 49-base deletion containing the GU18 repeat had activity similar to that of the larger 120-base deletion, suggesting that the GU-repeat element may be the most active motif in this region (Fig. 4C).
Fig. 4.
GU-rich motif in long Igf1 3′ UTR. A, Sequence of GU-rich motif (B6 3′ UTR bases 4023–4143). Region deleted for the Δ49 GU construct is underlined. B, Potential secondary structure (sFOLD) of GU18 element, in the context of the 3275–5774 fragment 2 (Frag2). The potential secondary structure of this region was similar in the context of the 1–6292 fragment 4 and was not altered by strain-specific polymorphisms. C, Deletion of the entire GU-rich motif (Δ120 GU), or the region containing GU18 (Δ49 GU), decreases 3′ UTR-regulated luciferase activity, in the context of fragment 2 (3275–5779). *, Significantly different from intact fragment 2, P < 0.05.
miRNAs acting on Igf1
miRNAs are a class of small noncoding RNAs that can regulate mRNA stability and translation, through interaction with complementary regions within the target mRNA, frequently within the 3′ UTR (22, 23). The miRNA miR-1 was recently shown to down-regulate IGF-I expression in muscle (24). We used a bioinformatic approach (www.targetscan.org; http://cbio.mskcc.org/mirnaviewer/) to identify other potential miRNAs that may regulate the IGF-I 3′ UTR. We assembled a list of the most promising candidates based on evolutionary conservation and degree of complementarity between the 3′ end of the target and 5′ end of the miRNA (Supplemental Table 2).
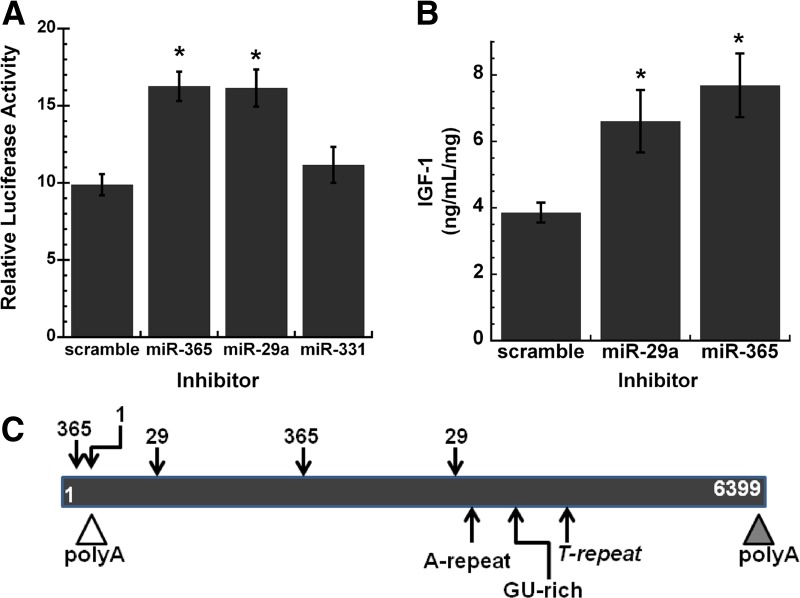
To determine whether specific miRNAs target the Igf1 3′ UTR, MC3T3 cells were cotransfected with luciferase-3′ UTR constructs and specific miRNA inhibitors. In cells transfected with the reporter carrying the 1–3217 3′ UTR (fragment 1) and inhibitors for miR-19b-1*, miR-15b, or miR-365 (also known as miR-365-3p), only miR-365 inhibitor caused a significant increase in reporter activity, indicating that this miRNA targets the Igf1 UTR (Supplemental Fig. 1A). In cells transfected with the reporter carrying the 3275–5674 3′ UTR (fragment 2, B6) and inhibitors for miR-29a, miR-26b, or miR-331, only miR-29a inhibitor caused a significant increase in reporter activity, indicating that this miRNA targets the Igf1 UTR (Supplemental Fig. 1B). In the process of examining other miRNAs that might be potential regulators of IGF-I, we also performed transfections with inhibitors for miR-1, miR-206, Let7a. However, these miRNA inhibitors did not consistently up-regulate the expression of the 3′ UTR reporter constructs tested, suggesting that they are not likely regulators of Igf1 mRNA in MC3T3 cells (data not shown).
The ability of miR-29a and miR-365 to negatively regulate the Igf1 3′ UTR was further confirmed using a luciferase construct containing the full-length UTR (fragment 4, B6) (Fig. 5A). Because miR-29a and miR-365 inhibitors caused a significant increase in Igf1 3′ UTR reporter activity, we determined whether these miRNAs down-regulated the expression of endogenous IGF-I in osteoblasts. MC3T3 cells were transiently transfected with the scrambled control or inhibitors for miR-29a or miR-365. IGF-I in osteoblast-conditioned medium was quantified by RIA. Transfection with miR-29a or miR-365 inhibitor caused a significant increase in IGF-I protein levels, confirming that these miRNAs are negative regulators of IGF-I (Fig. 5B). A schematic illustrating the relative locations of the predicted binding sites for miR-29, miR-365, and miR-1 within the long Igf1 3′ UTR is shown in Fig. 5C.
Fig. 5.
miR-29a and miR-365 target IGF-I. A, Activity of luciferase-1–6292 3′ UTR construct (fragment 4) in MC3T3 cells, transiently cotransfected with 50 nm miRNA inhibitor or scrambled control. *, Significantly different from scrambled control, P < 0.05. B, Quantity of IGF-I in conditioned medium from MC3T3 cells, transiently transfected with 50 nm miRNA inhibitor or scrambled control. *, Significantly different from scrambled control, P < 0.05. IGF-I was quantified by RIA and expressed as ng/ml·mg cell layer protein. C, Schematic representation of the Igf1 3′ UTR from exon 6, with the relative location of sequence motifs and binding sties for miR-365, miR-1, and miR-29.
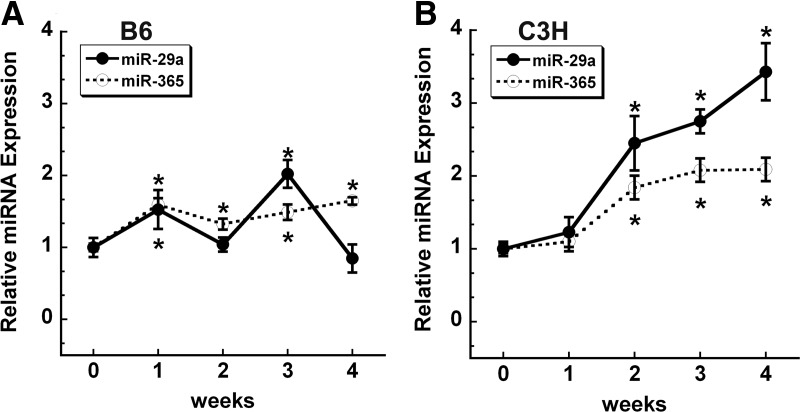
We previously reported that the expression of miR-29a/c family members was significantly increased in a mouse osteoblastic cell line undergoing differentiation (12). Here, we used primary cultures of B6 and C3H mouse bone MSCs to examine the expression of miR-29a and miR-365 during osteoblastic differentiation in vitro (Fig. 6, A and B). We found a modest, but significant, increase in miR-365 in differentiating cells from both mouse strains. In contrast, the expression pattern for miR-29a differed. In B6 cells, miR-29a expression was bimodal and was significantly increased after 1 and 3 wk of differentiation. This expression pattern for B6 miR-29a is similar to that reported for MC3T3, which are derived from mice with a B6 background (25). In C3H stromal cells, expression of miR-29a progressively increased, with an approximately 3-fold increase seen at 4 wk. Although miR-29 promotes osteoblastic differentiation, the role of miR-365 in this process is not yet determined (26).
Fig. 6.
miR-29a and miR-365 increase during osteoblastic differentiation in vitro. B6 (A) and C3H (B) stromal cells were grown to confluence (0) and then cultured for up to 4 wk after confluence in osteoblast differentiation medium. miRNA abundance was quantified by qRT-PCR and expressed relative to 5S rRNA (for miR-29a) or Sno202 (for miR-365). *, Significantly different from confluence, P < 0.05.
Discussion
This study provides additional data on potential mechanisms underlying the differences in IGF-I expression between B6 and C3H inbred strains. Although C3H mice express more IGF-I compared with B6, luciferase-UTR reporter experiments indicate that the three sets of polymorphisms identified in their Igf1 3′ UTRs did not impact expression. However, our work on the Igf1 3′ UTR polymorphisms allowed us to generate tools useful for studying basic posttranscriptional mechanisms regulating IGF-I in osteoblasts (Fig. 1). We defined the Igf1 splice variants expressed in osteoblasts, found differing expression of the long Igf1 3′ UTR in B6 vs. C3H stromal cultures, identified a GU-rich motif in the 3′ UTR as a positive-regulatory element, and identified two miRNAs that are negative regulators of IGF-I expression in these cells.
Alternative RNA isoforms
Alternative splicing is an important determinant for increasing diversity of function and regulation from a single gene. It is estimated that 85% of human genes produce at least two distinct populations of RNA isoforms (27, 28; reviewed by Ref. 29). However, only approximately 20% of alternative splicing events are conserved between mouse and human (30). The conservation between mouse and human splicing patterns for Igf1 suggests evolutionary pressure for preservation. In muscle, Igf1 alternative splicing is regulated by differentiation, injury, and mechanical loading (reviewed by Ref. 3). Although there is substantial literature on the Igf1 splice variants expressed in muscle, there is less known about the expression of these variants in osteoblasts or in other cell types. Moreover, the function of the alternative E peptides, arising from alternative splicing, remains a matter of controversy. In vitro studies in muscle cells suggest that the E peptides may increase IGF-I uptake from the medium (31). Different E peptides may also be associated during the proliferative state of the cell. For example, one study suggests an induction of the Eb peptide in prostate cancer cells (32). Some hypothesize that the E peptides may modify pro-IGF-I bioavailability, or ability to interact with IGF-binding proteins or IGF receptor; these hypotheses are yet to be tested (33).
Alternative polyadenylation is observed in RNAs from more than 50% of human genes, and 3′ end processing affects transcription termination, RNA stability, export to the cytoplasm, and translation efficiency (34). Use of one polyadenylation site over another can be influenced by the strength of the polyA signal, its context, and the complement of protein complexes responsible for 3′ end formation, including core polyadenylation factors (reviewed by Ref. 35). Generally, in proliferating cells, 3′ UTRs tend to be shorter, whereas increased 3′ UTR length is associated with differentiation (36, 37). We identified both positive and negative regulatory elements in the long Igf1 3′ UTR, and it is likely that this approximately 6300-base UTR contains other active motifs not yet characterized (Figs. 4 and 5). Because IGF-I is expressed in many tissues, it is possible that some _cis_-acting and _trans_-acting factors are active in a tissue-specific manner. Further, a longer UTR permits a greater number of regulatory motifs, and the combinatorial regulation of these motifs could provide fine-tuning of IGF-I protein levels in different cell types.
We found that, although the total amount of Igf1 mRNA did not significantly change during osteoblastic differentiation, the abundance of RNA containing the long Igf1 3′ UTR was significantly increased in B6, but not C3H, cells (Fig. 3, C and D). The long Igf1 mRNA isoform is less stable and has decreased translation efficiency, and this may be one mechanism contributing to lower levels of IGF-I in B6 vs. C3H mice (5).
Igf1 3′ UTR function
We identified a conserved [GU]18 repeat as a positive regulator of Igf1 3′ UTR activity in osteoblasts (Fig. 4). There are several candidate RNA binding proteins known to interact with GU-rich regions. One of these, CUG-repeat binding protein 1, is a regulator of splicing and can destabilize mRNAs by recruiting deadenylases (15, 17). Another possible candidate may be Far upstream element binding protein 3, which interacts with GU-repeats in the 3′ UTR of fibroblast growth factor 9 mRNA and may promote translation (18). A third candidate is muscle excess 3c, an RNA binding protein that binds GU-rich elements in Caenorhabditis elegans (19). Muscle excess 3c-null mice were recently shown to have growth retardation and IGF-I deficiency, mediated by decreased Igf1 mRNA translation (38). Identification of _trans_-acting factors interacting with the Igf1 GU-rich element is under investigation.
IGF-I and miRNAs
To our knowledge, only miR-1 and Let7f have been previously shown to target Igf1 RNA (24, 39, 40). miR-1 is frequently considered a muscle-specific miRNA, and we did not detect its expression in differentiating human osteoblasts (A. Delany, unpublished data); therefore, we did not test its activity in the present experiments. In our studies, using miRNA inhibitors to validate miRNA activity on the Igf1 3′ UTR, we did not detect de-repression of UTR activity with inhibitors for miR-206, which is predicted to interact with the same Igf1 UTR as miR-1 (data not shown). Because the expression of miR-206 is decreased during osteoblastogenesis, it is possible that miR-206 is not abundantly expressed in MC3T3 cells (41). Several Let7 family members are expressed in osteoblastic cells (25). Although we did not test inhibitor for Let7f in our system, we did test Let7a and found that it did not relieve repression of the Igf1 3′ UTR constructs (data not shown).
Here, we demonstrate that miR-29a and miR-365 are negative regulators of IGF-I and target its 3′ UTR (Fig. 5). Although our studies were limited to MC3T3 cells, due to the difficulty of transfecting primary cells, they document an important regulatory mechanism in cells that express these miRNAs and Igf1. Notably, the expression of miR-29a and miR-365 increased with osteoblastic differentiation, and in B6 cells, the abundance of RNAs containing the long Igf1 3′ UTR also increased. The combined increase in miRNAs that negatively regulate IGF-I expression and the increased proportion of the long form of Igf1 mRNA, which is targeted by these miRNAs, has the potential to decrease IGF-I protein expression during differentiation in B6. IGF-I is a mitogen for cells of the osteoblastic lineage, and it stimulates collagen synthesis (42). Cell replication and extracellular matrix deposition decrease with osteoblast maturation. It is possible that miRNA profile and polyadenylation site usage contribute to these effects.
The miR-29 family of miRNAs is highly conserved across species and has a common seed binding sequence, suggesting functional redundancy. miR-29 family members are transcribed from two distinct loci, the organization of which is conserved between mouse and human (43). This family of miRNAs negatively regulates the expression of extracellular matrix molecules, including several collagens and osteonectin (12, 22, 25, 44). In osteoblasts, miR-29 family members also target selected negative regulators of wnt signaling and additional negative regulators of osteoblastogenesis (25, 26). Moreover, transcription of the miR-29a locus is induced by canonical wnt signaling in osteoblasts, providing a positive feedback loop for amplification of wnt signaling (26).
The miR-29 family is expressed in many cell types, and its expression is often associated with differentiation and reduced cell proliferation (22, 45–47). Indeed, the expression of miR-29 family members is increased in aging liver and muscle, and in cultured fibroblasts undergoing senescence (48). Further, miR-29 and miR-1 are increased in the _Zmpste24_-null mouse, a model for Hutchinson-Gilford progeria syndrome (39, 48). These mutant mice have reduced circulating IGF-I, and it is likely that the increased levels of miR-29 and miR-1 contribute to this effect.
In contrast to miR-29, the function and regulation of miR-365 are less studied. However, like miR-29, expression of miR-365 is associated with reduced cell proliferation. For example, miR-365 targets cyclin D1 and B cell lymphoma 2 (49). The sequence of mature miR-365 is conserved between mouse and human, and two distinct loci give rise to identical mature miR-365. miR-365-1 (also known as miR-365-3p) is encoded by a gene on mouse chromosome 16 and is transcribed as a bicistronic pri-miRNA with miR-193b. This genomic organization is conserved in humans. miR-365-2 is encoded by a gene on mouse chromosome 11. One validated target for miR-365 is IL-6, which is a negative regulator of osteoblast differentiation and a stimulator of IGF-binding protein 5 expression (50, 51).
Further, miR-365 is up-regulated in response to cyclic loading in cultured chick chondrocytes. In the growth plate, it is expressed at higher levels in the prehypertropic and hypertropic zones, compared with the proliferating zone (52). In rat tibia, there was a report of a disconnect between the number of IGF-I positive hypertrophic zone chondrocytes observed by immunostaining compared with cells positive for Igf1 mRNA, visualized by in situ hybridization (53). It is possible that translational repression of Igf1 mRNA by miR-365 could play a role in this phenomenon; however, this must be validated experimentally. miR-365 targets histone deacetylase (HDAC) 4 and stimulates chondrocyte proliferation and differentiation (52). With regard to osteoblasts, because HDAC 4 is one of the corepressors required for the TGF-β-mediated negative regulation of Runx2 transcription, it is possible that miR-365 may promote osteoblast commitment and/or differentiation, in part, by targeting HDAC 4 (54). Lastly, miR-365 was recently reported to be part of an adipose-enriched miRNA cluster. Inhibition of miR-365 activity in precursor cells results in decreased adipogenesis, whereas ectopic expression of miR-365 inhibits myogenic differentiation (55). In the differentiation of multipotent precursor cells, miR-365 may be part of a molecular switch that represses myogenesis but allows progression of other lineages, such as osteoblastic, chondrogenic, or adipogenic.
In humans, allelic differences in IGF-I have been associated with obesity/body composition, osteoporosis, breast cancer, and colon cancer (56–59). Polymorphisms in the promoter region of the human Igf1 gene have been associated with hip bone geometry and risk of nonvertebral fracture in a cohort of women in Rotterdam (60). The human Igf1 3′ UTR is polymorphic, and polymorphisms occurring within binding sites for miRNAs or other _trans_-acting factors may provide potential mechanisms for various age-related phenotypes. In conclusion, we demonstrate that miR-29a and miR-365 are negative regulators of the Igf1 3′ UTR. Notably, the expression of these miRNAs in both B6 and C3H mice increases with osteoblastic differentiation, although there is differential expression of the long Igf1 3′ UTR isoform, suggesting a possible mechanism of enhanced IGF-I regulation in B6 vs. C3H mice. Future studies are required to understand the translational implications of these findings to chronic diseases, including osteoporosis.
Supplementary Material
Supplemental Data
Acknowledgments
We thank Julie Burgess for performing the IGF-I RIAs and Dr. Barbara Kream for thoughtful discussion of the data. We also thank the reviewers of our manuscript for their insightful comments.
This project was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health Grants AR45433 (to C.J.R.) and AR44877 (to A.M.D.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
Abbreviations:
B6
C57BL/6J
C3H
C3H/He/J
β-gal
β-galactosidase
GU18
18 GU
HDAC
histone deacetylase
MC3T3
MC3T3-E1
miRNA
microRNA
MSC
marrow stromal cell
qRT-PCR
quantitative RT-PCR
3′ RACE
rapid amplification of 3′ cDNA ends
UTR
untranslated region.
References
- 1.Yakar S, Courtland HW, Clemmons D. 2010. IGF-1 and Bone: new discoveries from mouse models. J Bone Miner Res 25:2543–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dreszer TR, Karolchik D, Zweig AS, Hinrichs AS, Raney BJ, Kuhn RM, Meyer LR, Wong M, Sloan CA, Rosenbloom KR, Roe G, Rhead B, Pohl A, Malladi VS, Li CH, Learned K, Kirkup V, Hsu F, Harte RA, Guruvadoo L, Goldman M, Giardine BM, Fujita PA, Diekhans M, Cline MS, Clawson H, Barber GP, Haussler D, James Kent W. 2012. The UCSC genome browser database: extensions and updates 2011. Nucleic Acids Res 40:D918–D923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matheny RW, Jr, Nindl BC, Adamo ML. 2010. Minireview: mechano-growth factor: a putative product of IGF-1 gene expression involved in tissue repair and regeneration. Endocrinology 151:865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton ER. 2006. The ABCs of IGF-1 isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab 31:791–797 [DOI] [PubMed] [Google Scholar]
- 5.Foyt HL, LeRoith D, Roberts CT., Jr 1991. Differential association of insulin-like growth factor I mRNA variants with polysomes in vivo. J Biol Chem 266:7300–7305 [PubMed] [Google Scholar]
- 6.He J, Rosen CJ, Adams DJ, Kream BE. 2006. Postnatal growth and bone mass in mice with IGF-1 haploinsufficiency. Bone 38:826–835 [DOI] [PubMed] [Google Scholar]
- 7.Rosen CJ, Dimai HP, Vereault D, Donahue LR, Beamer WG, Farley J, Linkhart S, Linkhart T, Mohan S, Baylink DJ. 1997. Circulating and skeletal insulin-like growth factor-I (IGF-1) concentrations in two inbred strains of mice with different bone mineral densities. Bone 21:217–223 [DOI] [PubMed] [Google Scholar]
- 8.Delahunty KM, Shultz KL, Gronowicz GA, Koczon-Jaremko B, Adamo ML, Horton LG, Lorenzo J, Donahue LR, Ackert-Bicknell C, Kream BE, Beamer WG, Rosen CJ. 2006. Congenic mice provide in vivo evidence for a genetic locus that modulates serum insulin-like growth factor-I and bone acquisition. Endocrinology 147:3915–3923 [DOI] [PubMed] [Google Scholar]
- 9.Kawai M, Delany AM, Green CB, Adamo ML, Rosen CJ. 2010. Nocturnin suppresses Igf1 expression in bone by targeting the 3′ untranslated region of Igf1 mRNA. Endocrinology 151:4861–4870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobson KR, Reading L, Haberey M, Marine X, Scutt A. 1999. Centrifugal isolation of bone marrow from bone: an improved method for recovery and quantitation of bone marrow osteoprogenitor cells from rat tibiae and femurae. Calcif Tissue Int 65:411–413 [DOI] [PubMed] [Google Scholar]
- 11.Delany AM, Canalis E. 1995. Transcriptional repression of insulin-like growth factor I by glucocorticoids in rat bone cells. Endocrinology 136:4776–4781 [DOI] [PubMed] [Google Scholar]
- 12.Kapinas K, Kessler CB, Delany AM. 2009. miR-29 suppression of osteonectin in osteoblasts: regulation during differentiation and by canonical Wnt signaling. J Cell Biochem 108:216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecka-Czernik B, Ackert-Bicknell C, Adamo ML, Marmolejos V, Churchill GA, Shockley KR, Reid IR, Grey A, Rosen CJ. 2007. Activation of peroxisome proliferator-activated receptor γ (PPAR γ) by rosiglitazone suppresses components of the insulin-like growth factor regulatory system in vitro and in vivo. Endocrinology 148:903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borson ND, Salo WL, Drewes LR. 1992. A lock-docking oligo(dT) primer for 5′ and 3′ race PCR. PCR Methods Appl 2:144–148 [DOI] [PubMed] [Google Scholar]
- 15.Vlasova IA, Tahoe NM, Fan D, Larsson O, Rattenbacher B, Sternjohn JR, Vasdewani J, Karypis G, Reilly CS, Bitterman PB, Bohjanen PR. 2008. Conserved GU-rich elements mediate mRNA decay by binding to CUG-binding protein 1. Mol Cell 29:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray D, Kazan H, Chan ET, Castilllo LP, Chaudhry S, Talukder S, Blencowe BJ, Morris Q, Hughes TR. 2009. Rapid and systematic analysis of the RNA recognition specificities of RNA-binding proteins. Nature Biotech 27:667–670 [DOI] [PubMed] [Google Scholar]
- 17.Lee JE, Lee JY, Wilusz J, Tian B, Wilusz CJ. 2010. Systematic analysis of cis-elements in unstable mRNAs demonstrates that CUGBP1 is a key regulator of mRNA decay in muscle cells. PLoS One 5:e11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gau B-H, Chen T-M, Shih Y-H J, Sun HS. 2011. FUBP3 interacts with FGF9 3′ microsatellite and positively regulates FGF9 translation Nucleic Acids Res 39:3582–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagano JM, Farley BM, Essien KI, Ryder SP. 2009. RNA recognition by the embryonic cell fate determinant and germline totipotency factor MEX-3. Proc Natl Acad Sci USA 106:20252–20257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding Y, Chan CY, Lawrence CE. 2004. _S_fold web server for statistical folding and rational design of nucleic acids. Nucleic Acids Res 32(Web Server issue):W135–W141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auweter SD, Oberstrass FC, Allain FH. 2006. Sequence-specific binding of single stranded RNA: is there a code for recognition? Nucleic Acids Res 34:4943–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kapinas K, Delany AM. 2011. MicroRNA biogenesis and regulation of bone remodeling. Arthritis Res Ther 13:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasinski AL, Slack FJ. 2011. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer 11:849–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elia L, Contu R, Quintavalle M, Varrone F, Chimenti C, Russo MA, Cimino V, De Marinis L, Frustaci A, Catalucci D, Condorelli G. 2009. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation 120:2377–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Hassan MQ, Jafferji M, Aqeilan RI, Garzon R, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB. 2009. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem 284:15676–15684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapinas K, Kessler C, Ricks T, Gronowicz G, Delany AM. 2010. miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J Biol Chem 285:25221–25231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. 2008. Alternative isoform regulation in human tissue transcriptomes. Nature 456:470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. 2008. Deep survey of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nature Genetics 40:1413–1415 [DOI] [PubMed] [Google Scholar]
- 29.Kalsotra A, Cooper TA. 2011. Functional consequences of developmentally regulated alternative splicing. Nature Reviews Genetics 12:715–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeo GW, Van Nostrand E, Holste D, Poggio T, Burge CB. 2005. Identification and analysis of alternative splicing events conserved in human and mouse. Proc Natl Acad Sci USA 102:2850–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeffer LA, Brisson BK, Lei H, Barton ER. 2009. The insulin-like growth factor (IGF)-I E-peptides modulate cell entry of the mature IGF-1 protein. Mol Biol Cell 20:3810–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armakolas A, Philippou A, Panteleakou Z, Nezos A, Sourla A, Petraki C, Koutsilieris M. 2010. Preferential expression of IGF-IEc (MGF) transcript in cancerous tissues of human prostate: evidence from a novel and autonomous growth factor activity of MGF E peptide in human prostate cancer cells. Prostate 70:1233–1242 [DOI] [PubMed] [Google Scholar]
- 33.Matheny RW, Jr, Nindl BC. 2011. Loss of IGF-1Ea or IGF-1Eb impairs myogenic differentiation. Endocrinology 152:1932–1934 [DOI] [PubMed] [Google Scholar]
- 34.Tian B, Hu J, Zhang H, Lutz CS. 2005. A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res 33:201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Giammartino DC, Nishida K, Manley JL. 2011. Mechanisms and consequences of alternative polyadenylation. Mol Cell 43:853–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. 2008. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science 320:1643–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shepard PJ, Choi EA, Lu J, Flanagan LA, Hertel KJ, Shi Y. 2011. Complex and dynamic landscape of RNA polyadenylation revealed by PAS-RAN seq. RNA 17:761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiao Y, Bishop CE, Lu B. 2012. Mex3c regulates insulin-like growth factor 1 (IGF1) expression and promotes post-natal growth. Mol Biol Cell 23:1404–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mariño G, Ugalde AP, Fernández AF, Osorio FG, Fueyo A, Freije JM, López-Otín C. 2010. Insulin-like growth factor 1 treatment extends longevity in a mouse model of human premature aging by restoring somatotroph axis function. Proc Natl Acad Sci 107:16268–16273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Selvamani A, Sathyan P, Miranda RC, Sohrabji F. 2012. An antagomir to microRNA let7f promotes neuroprotection in an ischemic stroke model. PLoS One 7:e32662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inose H, Ochi H, Kimura A, Fujita K, Xu R, Sato S, Iwasaki M, Sunamura S, Takeuchi Y, Fukumoto S, Saito K, Nakamura T, Siomi H, Ito H, Arai Y, Shinomiya K, Takeda S. 2009. A microRNA regulatory mechanism of osteoblast differentiation. Proc Natl Acad Sci USA 106:20794–20799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canalis E. 1980. Effect of insulin like growth factor I on DNA and protein synthesis in cultured rat calvaria. J Clin Invest 66:709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, Mendell JT. 2008. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 40:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. 2008. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA 105:13027–13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Garzon R, Sun H, Ladner KJ, Singh R, Dahlman J, Cheng A, Hall BM, Qualman SJ, Chandler DS, Croce CM, Guttridge DC. 2008. NF-κB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell 14:369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park SY, Lee JH, Ha M, Nam JW, Kim VN. 2009. miR-29 miRNAs activate p53 by targeting p85α and CDC42. Nat Struct Mol Biol 16:23–29 [DOI] [PubMed] [Google Scholar]
- 47.Zhao JJ, Lin J, Lwin T, Yang H, Guo J, Kong W, Dessureault S, Moscinski LC, Rezania D, Dalton WS, Sotomayor E, Tao J, Cheng JQ. 2010. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood 115:2630–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ugalde AP, Ramsay AJ, de la Rosa J, Varela I, Mariño G, Cadiñanos J, Lu J, Freije JM, López-Otín C. 2011. Aging and chronic DNA damage response activate a regulatory pathway involving miR-29 and p53. EMBO J 30:2219–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nie J, Liu L, Zheng W, Chen L, Wu X, Xu Y, Du X, Han W. 2012. microRNA-365, down-regulated in colon cancer, inhibits cell cycle progression and promotes apoptosis of colon cancer cells by probably targeting cyclin D1 and Bcl-2. Carcinogenesis 33:220–225 [DOI] [PubMed] [Google Scholar]
- 50.Xu Z, Xiao SB, Xu P, Xie Q, Cao L, Wang D, Luo R, Zhong Y, Chen HC, Fang LR. 2011. miR-365, a novel negative regulator of interleukin-6 gene expression, is cooperatively regulated by Sp1 and NF-κB. J Biol Chem 286:21401–21412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peruzzi B, Cappariello A, Del Fattore A, Rucci N, De Benedetti F, Teti A. 2012. c-Src and IL-6 inhibit osteoblast differentiation and integrate IGFBP-5 signaling. Nat Commun 3:630. [DOI] [PubMed] [Google Scholar]
- 52.Guan YJ, Yang X, Wei L, Chen Q. 2011. miR-365: a mechanosensitive micro RNA stimulates chondrocyte differentiation through targeting histone deacetylase 4. FASEB J 25:4457–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reinecke M, Schmid AC, Heyberger-Meyer B, Hunziker EB, Zapf J. 2000. Effect of growth hormone and insulin-like growth factor I (IGF-1) on the expression of IGF-1 messenger ribonucleic acid and peptide in rat tibial growth plate and articular chondrocytes in vivo. Endocrinology 141:2847–2853 [DOI] [PubMed] [Google Scholar]
- 54.Kang JS, Alliston T, Delston R, Derynck R. 2005. Repression of Runx2 function by TGF-β through recruitment of class II histone deacetylases. EMBO J 24:2543–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun L, Xie H, Mori MA, Alexander R, Yaun B, Hattangadi SM, Liu Q, Khan CR, Lodish HF. 2011. Mir-193b-365 is essential for brown fat differentiation. Nat Cell Biol 13:958–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong HL, Koh WP, Probst-Hensch NM, Van den Berg D, Yu MC, Ingles SA. 2008. Insulin-like growth factor-1 promoter polymorphisms and colorectal cancer: a functional genomics approach. Gut 57:1090–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosen CJ, Kurland ES, Vereault D, Adler RA, Rackoff PJ, Craig WY, Witte S, Rogers J, Bilezikian JP. 1998. Association between serum insulin growth factor-I (IGF-1) and a simple sequence repeat in IGF-1 gene: implications for genetic studies of bone mineral density. J Clin Endocrinol Metab 83:2286–2290 [DOI] [PubMed] [Google Scholar]
- 58.Bågeman E, Ingvar C, Rose C, Jernström H. 2007. Absence of the common Insulin-like growth factor-1 19-repeat allele is associated with early age at breast cancer diagnosis in multiparous women. Br J Cancer 96:712–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosskopf D, Schwahn C, Neumann F, Bornhorst A, Rimmbach C, Mischke M, Wolf S, Geissler I, Kocher T, Grabe HJ, Nauck M, Hebebrand J, Kroemer HK, Friedrich N, Völzke H, Wallaschofski H. 2011. The growth hormone-IGF-1 axis as a mediator for the association between FTO variants and body mass index: results of the study of health in Pomerania. Int J Obes (Lond) 35:364–372 [DOI] [PubMed] [Google Scholar]
- 60.Rivadeneira F, Houwing-Duistermaat JJ, Beck TJ, Janssen JA, Hofman A, Pols HA, Van Duijn CM, Uitterlinden AG. 2004. The influence of an insulin-like growth factor I gene promoter polymorphism on hip bone geometry and the risk of nonvertebral fracture in the elderly: the Rotterdam study. J Bone Miner Res 19:1280–1290 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data