Analgesic effects of stem bark extracts of Trichilia monadelpha (Thonn.) JJ De Wilde (original) (raw)
Abstract
Objectives:
Various parts of Trichilia monadelpha (Thonn) JJ De Wilde (Fam. Meliaceae) are used in Ghanaian traditional medicine for the treatment of painful and inflammatory conditions. The present study examined the analgesic properties of the petroleum ether (PEE), ethyl acetate (EAE), and the hydro-ethanolic (HAE) extract of the stem bark of the plant in murine models.
Materials and Methods:
PEE, EAE, and HAE were assessed in chemical (acetic acid-induced abdominal writhing and formalin tests), thermal (hot plate test), and mechanical (Randall-Selitto paw pressure test) pain models. The possible mechanisms of the antinociceptive action were also examined with various antagonists in the formalin test.
Results:
HAE, EAE, and PEE, each at doses of 10–100 mg/kg orally, and the positive controls (morphine and diclofenac) elicited significant dose-dependent antinociceptive activity in the chemical (acetic acid abdominal writhing and formalin tests), thermal (hot plate test), and mechanical (Randall-Selitto paw pressure test) pain models in rodents. The antinociceptive effect of HAE was partly or wholly reversed by systemic administration of atropine, naloxone, and glibenclamide. The antinociceptive effects of EAE and PEE were inhibited by atropine.
Conclusion:
The extracts HAE, EAE, and PEE caused dose-related antinociception in chemical, thermal, and mechanical models of pain in animals. The mechanism of action of HAE involves an interaction with muscarinic cholinergic, adenosinergic, opioidergic pathways, and ATP-sensitive K+ channels while that of EAE and PEE involve the muscarinic cholinergic system.
KEY WORDS: Formalin test, hot plate, pain, randall-selitto test, writhing test
Introduction
Trichilia monadelpha (Thonn) JJ De Wilde (Family: Meliaceae), known locally as Otanduro (Twi) or Tenuba (Nzema), is a tree that grows 12–20 m high and establishes itself well in the lowland high forest and evergreen semideciduous secondary jungles, often near river banks.[1] Preparations (decoctions, infusions and tinctures) of the stem bark of the plant have been used in Ghanaian traditional medicine to treat pain, psychoses, epilepsy, and inflammation for many years and their efficacies are widely acclaimed in different communities in Ghana.[1,2] The ethanolic stem bark extract of T. monadelpha has been found to have antitrypanosomal activity and as well antiplasmodial activity against chloroquine and pyrimethamine-resistant Plasmodium falciparum strains.[3] Oral administration of the aqueous stem bark extract (400 mg/kg/day for 4 weeks) significantly reduced serum testosterone levels and increased both sperm motility and viability with no significant difference in the sperm counts in adult male albino rats.[4] Ainooson et al.[5] have also shown recently that the stem bark extracts of the plant inhibit carrageenan-induced foot-oedema in the 7-day-old chick and the oedema associated with adjuvant-induced arthritis in rats. No study has been reported on the analgesic properties of T. monadelpha.
Currently, pain treatment is far from perfect and there is a need for new, efficacious analgesics with minimum adverse effects. In the present study, therefore, we evaluated the analgesic potential of stem bark extracts (petroleum ether, ethyl acetate, and hydroethanolic extracts) of T. monadelpha in animal models. We also assessed some possible mechanisms by which the analgesic action is achieved.
Materials and Methods
Plant Collection and Extraction
The stem bark of T. monadelpha was collected from Bomaa, Brong-Ahafo region, Ghana (7°05′06.60′′N, 2°10′01.66′′W), in the month of September, 2009 and authenticated by Mr. G.H. Sam of the Department of Herbal Medicine, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana. A voucher specimen (KNUST/FPP/079/10) was kept at the herbarium of the faculty.
The stem bark was chopped into pieces and sun-dried for a week after which it pulverized through a 2 mm screen using a hammer-mill. Fifteen kilograms of the powdered plant bark was serially extracted with 40–60 °C petroleum ether, ethyl acetate, and 70% ethanol over a 24-hour period using the Soxhlet apparatus. Resulting extracts were each concentrated into a syrupy mass under reduced temperature (60 °C for petroleum ether and ethyl acetate extracts and 70 °C for the ethanol extract) and pressure in a rotary evaporator. Extracts were further dried over a water bath and then kept in a desiccator for use. Yields obtained for the extraction were 0.9% w/w (petroleum ether extract, PEE), 0.7% w/w (ethyl acetate extract, EAE), and 9.6% w/w (hydro-ethanol extract, HAE).
Drugs and Chemicals
The following drugs and chemicals were used: acetic acid, formalin, and theophylline (British Drug Houses Ltd., Poole, UK); diclofenac (KRKA®, Novo Mesto, Slovenia); diazepam (INTAS®, Gujarat, India); morphine (PhytoRiker®, Accra, Ghana); λ-carrageenan, atropine, naloxone, yohimbine pentylenetetrazole (Sigma-Aldich Inc., St. Louis, MO, USA) and glibenclamide (Daonil®, Sanofi-Aventis, Guildford, UK). The extracts were suspended in 2% tragacanth.
Animals
Male Sprague–Dawley rats (100–180 g), ICR mice (20–25 g), and C3 H mice (20–25 g) were used in the experiments. The animals were purchased from Noguchi Memorial Institute for Medical Research, Accra, Ghana and kept at the animal house of the Department of Pharmacology, KNUST, Kumasi. Each group consisted of at least five animals, housed in stainless cages (34×47×18 cm) with soft wood shavings as bedding. The animals were maintained under laboratory conditions (temperature 24–25°C, 12-hour light-dark cycle) with free access to pellet diet (GAFCO, Tema, Ghana) and water. All experiments were conducted in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1985, revised 1996). All protocols used were approved by the departmental Ethics Committee.
Phytochemical Screening
Preliminary phytochemical tests were performed on the extracts using methods described by Trease and Evans.[6]
Acetic Acid-induced Writhing Test
The test was performed as described earlier by Woode et al.[7] Briefly, male ICR mice (n = 5) were pretreated with the test preparations [HAE, EAE or PEE (10, 30 and 100 mg/kg p.o.); diclofenac (3, 10 or 30 mg/kg, i.p.) or vehicle (10 ml/kg, p.o.)] 30 minutes (i.p.) or 1 h (p.o.) before administration of acetic acid 0.6% (10 ml/kg, i.p.).
Formalin-induced Nociception
The formalin test was carried out as described earlier by Woode and Abotsi.[8] Male rats were pretreated with the test preparations, as described for the writhing test above, and then given intraplantar injection of 10 μl of 5% formalin.
Hot Plate Test
The method described by Pietrovski et al.[9] was used. Briefly, male C3 H mice (n = 5) were placed on a hot plate (Model 7280, Ugo Basile Inc., Milan, Italy) heated to 55 ± 0.5 °C and the baseline reaction time of the animals to nociceptive responses (licking/shaking of the paws, jumping) were recorded. They were then treated with the test preparations (similar to that described for the writhing test above) and the reaction times were taken again at 0.5, 1, and 2 hours intervals after a latency period of 30 minutes (i.p.) or 1 hour (p.o.) following the administration of the vehicle, drugs or extract. A cut-off reaction time was set at 30 seconds to prevent damage to tissues of the foot.
Carrageenan-induced Mechanical Hyperalgesia
The test was carried out as described earlier by Murase et al.[10] with modifications. Hyperalgesia was induced by the injection of λ-carrageenan (100 μl, 20 mg/ml) into the right hind paw of the rat. Rats were treated orally with the test preparations (similar to that described for the writhing test above) 3 hours postcarrageenan administration. Paw withdrawal thresholds (PWTs) were determined at 0.5 hours before and 2.5, 3.5, 4, 4.5, 5, 5.5, 6, and 6.5 hours postcarrageenan injection using an analgesimeter (IITC Life Science Inc., Woodland Hills, CA, USA). Mechanical pressure was increased until vocalization or withdrawal reflex occurred while rats were lightly restrained. A change in the hyperalgesic state was calculated as a percentage of the maximum possible effect (% MPE) from the formula: [(P2-P1)/(P0-P1) × 100], where P1 and P2 were the pre- and postdrug paw withdrawal thresholds respectively, and P0 was the cut-off (250 g).
Analysis of the Mechanism of Action of the Extracts in the Formalin Test
The effects of HAE (100 mg/kg, p.o.), EAE( 100 mg/kg, p.o.), PEE (100 mg/kg, p.o.), morphine (1 mg/kg, i.p.) or vehicle (10 ml/kg, p.o.) were evaluated in the presence of naloxone (2 mg/kg, i.p.), theophylline (10 mg/kg, i.p.), glibenclamide (8 mg/kg, p.o.), atropine (5/mg/kg, i.p.), and yohimbine (3 mg/kg, p.o.) to determine the involvement of the opioidergic, adenosinergic, muscarinic cholinergic systems as well as ATP-sensitive K+ channels and α2-adrenoceptors respectively in their antinociceptive activity. Doses of antagonists and other drugs were selected on the basis of previous literature data and in experiments in our laboratory.[8]
Rotarod Test
The effect of the extracts on motor coordination was assessed using the rotarod apparatus (Model 7600, Ugo Basile, Cormerio, Italy) rotating at a speed of 25 rpm. Mice (preselected) were randomly divided into 13 groups (n = 6) and received HAE, EAE or PEE (10-100 mg/kg, p.o.), diazepam (0.1-1.0 mg/kg, i.p.) or vehicle (10 ml/kg, p.o.). Thirty minutes (i.p.) or 1 hour (p.o.) after the treatments, the latencies to fall from the rod were measured. Mice that stayed on the rotarod for more than 120 seconds were given the maximum score, 120 seconds.
Statistical Analysis
All data are presented as mean±S.E.M. The time-course curves were subjected to two-way (treatment × time) repeated measures analysis of variance (ANOVA) with Bonferroni's post hoc test. Total nociceptive score for each treatment was calculated in an arbitrary unit as the area under the curve (AUC). Differences in AUCs were analyzed using one-way ANOVA, with drug treatment as a between-subjects factor, followed by the Newman–Keuls test. GraphPad Prism for Windows, Version 5 (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses and ED50 determinations. P <0.05 was considered statistically significant in all analyses.
Results
Phytochemical Screening
Preliminary phytochemical screening indicated the presence of alkaloids, flavonoids, glycosides, saponins, sterols, tannins, and terpenoids in HAE. EAE had alkaloids, glycosides, tannins, and terpenoids while PEE showed the presence of alkaloids, sterols, and terpenoids.
Acetic Acid-induced Writhing Test
Figure 1 shows the effect of the extracts and diclofenac on acetic acid-induced writhing during the 30-minute observation period. HAE, EAE or PEE (10-100 mg/kg, p.o.) dose dependently and significantly (all P < 0.0001) reduced the number of abdominal writhes over 30 minutes [Figure 1b, d, f]. Diclofenac, a nonsteroidal antiinflammatory drug (NSAID) (3–30 mg/kg, i.p.), similarly inhibited (P < 0.0001) the acetic acid-induced abdominal writhing [Figure 1g]. From the ED50 values obtained by nonlinear regression, PEE (2.11 ± 0.46 mg/ kg) was comparable in potency to HAE (ED50: 2.54 ± 1.22 mg/kg) but more potent than EAE (6.29 ± 3.65 mg/kg). However, diclofenac was the most potent (1.02 ± 0.26 mg/kg).
Figure 1.
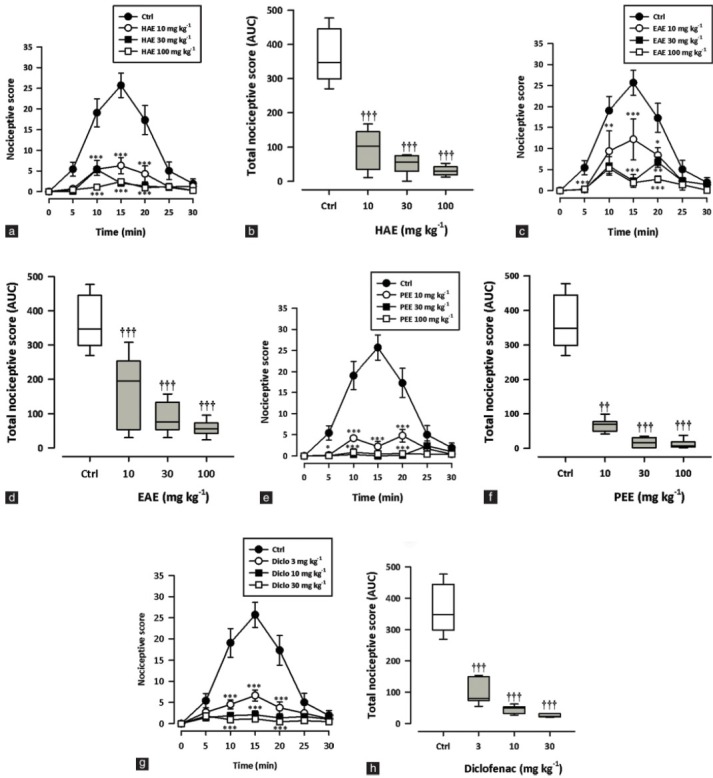
Effect of HAE (10–100 mg/kg), EAE (10–100 mg/kg), PEE (10–100 mg/kg) and diclofenac (3-30 mg/kg) on the time course curves (a, c, e, g) and the total nociceptive score (calc. as AUCs) (b, d, f, h) of acetic acid-induced writhing in mice. Nociceptive scores are shown in 5-minute time blocks up to 30 min for the time course curves. Data are presented as mean ± SEM (n = 5). The lower and upper margins of the boxes (b, d, f, h) represent the 25th and 75th percentiles, with the extended arms representing the 10th and 90th percentiles, respectively. The median is shown as the horizontal line within the box.*P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group (ctrl) (two-way repeated measures ANOVA followed by Bonferroni's post hoc). †P < 0.05, ††P < 0.01, †††P < 0.001 compared to the control group (ctrl) (one-way ANOVA followed by Newman–Keuls post hoc)
Formalin Test
Intraplantar injection of the right hind paw with formalin (5%, 10 μl) evoked a characteristic biphasic response in rats – an initial intense response to pain (0–10 minutes; first phase/neurogenic phase) followed by a slowly rising but longer lasting response (10–60 minutes; second phase/inflammatory phase). Oral administration of HAE, EAE or PEE (10–100 mg/kg) 1 hour before formalin injection significantly and dose dependently inhibited both the neurogenic (all P < 0.0001; [Figure 2b, d, f] and inflammatory (all P < 0.0001; Figure 2b, d, f) phases in the formalin test. Morphine (0.3–3.0 mg/kg, i.p.) also significantly and dose dependently inhibited both the neurogenic (P < 0.0001; Figure 2h) and inflammatory (P < 0.0001; Figure 2h) phases in the formalin test. The ED50 values obtained were 8.15 ± 2.30 mg/kg, 3.23 ± 0.94 mg/kg (PEE); 6.43 ± 1.14 mg/kg, 3.16 ± 0.37 mg/kg (HAE), and 7.19 ± 1.45 mg/kg, 2.61 ± 0.83 mg/kg (EAE). Morphine was more potent in both phases (0.26 ± 0.05 mg/kg, 0.18 ± 0.02 mg/kg) compared to the extracts.
Figure 2.
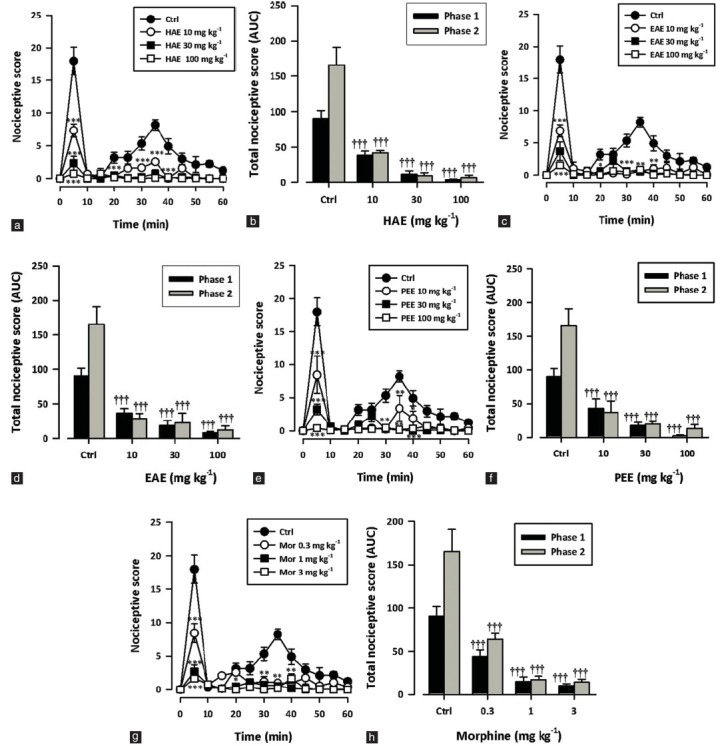
Effect of HAE (10–100 mg/kg), EAE (10–100 mg/kg), PEE (10–100 mg/kg) and morphine (0.3-3 mg/kg) on the time course curves (a, c, e) and the total nociceptive score (calc. as AUCs) (b, d, f) of formalin-induced nociception in mice. Nociceptive scores are shown in 5-minute time blocks up to 60 min for the time course curves. Data are presented as mean ± SEM (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group (ctrl) (two-way repeated measures ANOVA followed by Bonferroni's post hoc). †P < 0.05, ††P < 0.01, †††P < 0.001 compared to the control group (ctrl) (one-way ANOVA followed by Newman–Keuls post hoc)
Hot-plate Test
Figure 3 shows the effect of various treatments on paw withdrawal latency, calculated as % MPE. HAE, EAE or PEE (10–100 mg/kg, p.o.) produced significant (HAE: P = 0.0034; EAE: P = 0.0028; PEE: P = 0.0005; Figure 3a–f) and dose-dependent increase in the paw withdrawal latencies with a maximum effect at 100 mg/kg. Morphine (0.3–3 mg/kg, i.p.) also exhibited similar effects (P < 0.0001; Figure 3g, h). The ED50 values obtained were 3.68 ± 2.82 mg/kg, 4.92 ± 3.60 mg/kg, and 2.91 ± 2.93 mg/kg for PEE, HAE, and EAE, respectively. Morphine was the most potent (0.16 ± 0.09 mg/kg).
Figure 3.
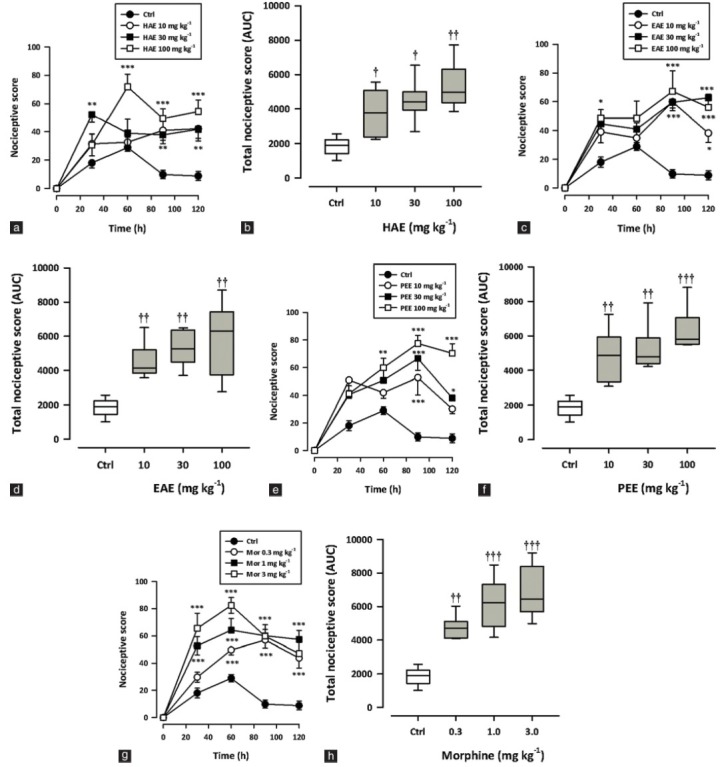
Effect of HAE (10–100 mg/kg), EAE (10–100 mg/kg), PEE (10–100 mg/kg) and morphine (0.3-3 mg/kg) on the time course curves (a, c, e) and the total antinociceptive effect (calc. as AUCs) (b, c, f) in the hot plate test in mice. Data are presented as mean ± S.E.M. (n = 5). The lower and upper margins of the boxes (b, d, f) represent the 25th and 75th percentiles, with the extended arms representing the 10th and 90th percentiles, respectively. The median is shown as the horizontal line within the box..*P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group (ctrl) (two-way repeated measures ANOVA followed by Bonferroni's post hoc). †P < 0.05, ††P < 0.01, †††P < 0.001 compared to the control group (ctrl) (one-way ANOVA followed by Newman–Keuls post hoc).
Carrageenan-induced Mechanical Hyperalgesia Test
HAE, EAE or PEE (30–300 mg/kg, p.o.) administered 3 hours after carrageenan administration produced significant (HAE: P < 0.0001; EAE: P = 0.0009; PEE: P < 0.0001; Figure 4a–f) and dose-dependent increase in the paw withdrawal thresholds with maximal effect at 300 mg/kg. Morphine (0.3–3 mg/kg, i.p.) also profoundly and significantly (P < 0.0001) reversed the mechanical hyperalgesia with the highest effect at 3 mg/kg [Figure 4g–h]. The ED50 values obtained were 57.13 ± 25.44 mg/kg, 74.63 ± 21.75 mg/kg, and 46.97 ± 25.33 mg/kg for PEE, HAE, and EAE, respectively.
Figure 4.
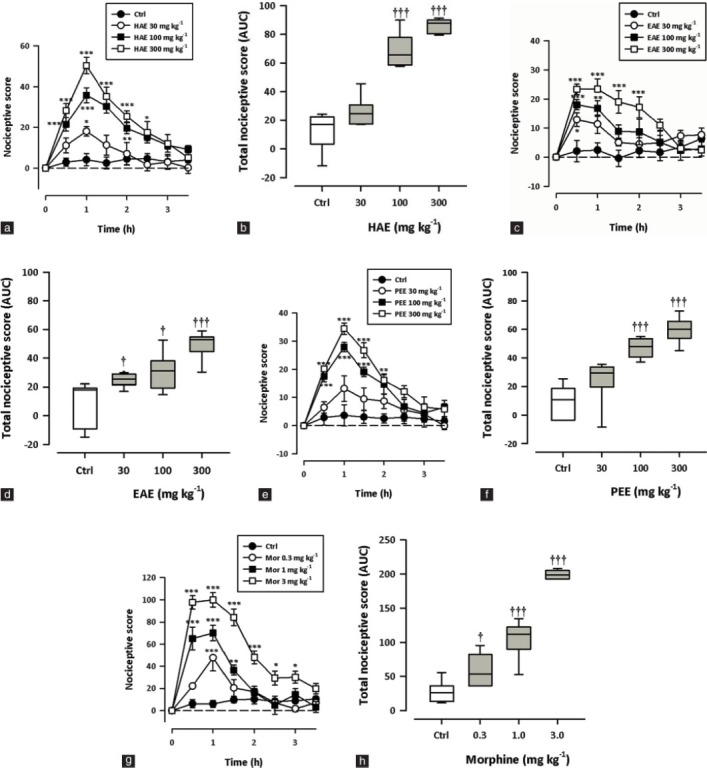
Effect of HAE (10–100 mg/kg), EAE (10–100 mg/kg), PEE (10–100 mg/kg) and morphine (0.3-3 mg/kg) on the time course curves (a, c, e, g) and the total nociceptive score (AUCs) (b, d, f, h) of carrageenan-induced mechanical hyperalgesia in rats. Data are presented as mean ± S.E.M. (n = 5). The lower and upper margins of the boxes (b, d, f, h) represent the 25th and 75th percentiles, with the extended arms representing the 10th and 90th percentiles, respectively. The median is shown as the horizontal line within the box.*P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group (ctrl) (two-way repeated measures ANOVA followed by Bonferroni's post hoc). †P < 0.05, ††P < 0.01, †††P < 0.001 compared to the control group (ctrl) (one-way ANOVA followed by Newman–Keuls post hoc).
Assessment of the Possible Mechanism of Action of HAE, EAE, and PEE
Pretreatment of rats with naloxone (2 mg/kg, i.p.), theophylline (10 mg/kg, i.p.) or glibenclamide (8 mg/kg, p.o.) reversed the antinociceptive effect of HAE and morphine in the formalin test [Figure 5a, d]. There was, however, no inhibition of the antinociception caused by EAE or PEE [Figure 5b, c] in the formalin test.
Figure 5.
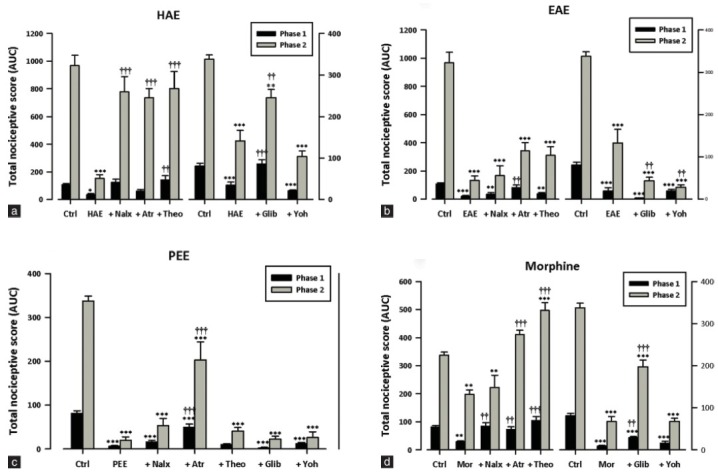
Effects of pretreatment of rats with atropine (5 mg/kg, i.p.), naloxone (2 mg/kg, i.p.), theophylline (10 mg/kg, i.p.); glibenclamide (8 mg/kg, p.o.) and yohimbine (3 mg/kg, p.o.) on the antinociceptive effects of (a) HAE (100 mg/kg, p.o.), (b) EAE (100 mg/kg, p.o.), (c) PEE (100 mg/kg, p.o.) or (d) morphine (1 mg/kg, i.p.) in the formalin test. Each column represents mean ± S.E.M. (n = 5). *P < 0.05; **P < 0.001; ***P < 0.0001 compared to the vehicle-treated group. (two-way ANOVA followed by Bonferroni's post hoc test). ††P < 0.01; †††P < 0.0001 compared to the HAE-, EAE- or PEE-treated group (one-way ANOVA followed by Newman=Keuls post hoc).
Atropine (5 mg/kg, i.p.) reversed the antinociceptive effect of HAE and EAE in the second and first phases respectively [Figure 5a, b] of the formalin test. It also significantly reversed the antinociceptive effects of PEE and morphine in both phases [Figure 5c, d].
Yohimbine (3 mg/kg, p.o.) did not block the antinociception caused by HAE, EAE, PEE or morphine [Figure 5a–d] in both phases of the formalin test.
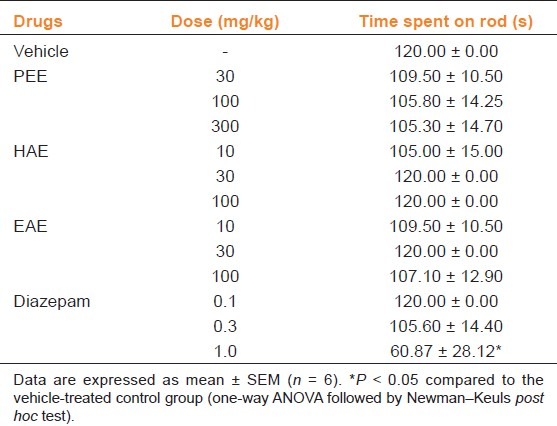
Rotarod Test
Table 1 shows the results of the effects of the extracts and diazepam on motor coordination in the rotarod test. Pretreatment of mice with HAE, EAE or PEE (10–100 mg/kg, p.o.) did not significantly affect (all P > 0.05) the time spent by mice on the rotarod. Diazepam, however, decreased latency to fall off the rotating rod (P = 0.0096) with significant effect at 1 mg/kg [Table 1].
Table 1.
Effect of XAE and XA on motor coordination measured as time spent on the rod
Discussion
The present study has demonstrated that oral administration of the hydroalcoholic, ethyl acetate, and petroleum ether extracts of the stem bark of Trichilia monadelpha have potent antinociceptive activity in the chemical (acetic acid and formalin test), thermal (hot-plate test), and mechanical (Randall–Selitto test) pain models in rodents.
Analgesic activity of the stem bark extracts was first tested in the acetic acid abdominal writhing test since it shows good sensitivity and allows for the detection of the effects of weak analgesics.[11] All the extracts produced marked, dose-related antinociception in this test model. The pain induced by acetic acid is known to be due to the release of nociceptive endogenous mediators including prostaglandins and proinflammatory cytokines.[12–14] It is therefore possible that the extracts exert their antinociceptive effects by inhibiting local synthesis, release and/or action of proinflammatory mediators.
An important limitation of the abdominal writhing test is that it lacks specificity – nonanalgesic drugs, such adrenergic blockers, antihistamines, and muscle relaxants can produce false-positive results.[11] To confirm, therefore, the analgesic properties of HAE, EAE, and PEE, the formalin test was employed. The formalin test produces a distinct biphasic nociceptive response. Centrally acting drugs such as opioids inhibit both early and late phases almost equally.[15] Most NSAIDs and corticosteroids, which are primarily peripherally acting, only inhibit the late phase. Inhibition of both phases by HAE, EAE, and PEE, in this study, implies that both central and peripheral mechanisms are involved in the antinociception. The inhibitory effect in the second phase also suggests antiinflammatory action for the extracts. Indeed, the antiinflammatory properties of the aqueous, alcoholic, and petroleum ether stem bark extract of T. monadelpha have already been shown in our laboratory.[5]
Results from this study also show that all three extracts exhibited significant antinociceptive effect in the hot-plate test. This confirms the involvement of central mechanisms in the antinociceptive effects of the extracts since the two behavioral components that were measured in terms of their reaction times, namely paw licking and jumping, are considered to be supraspinally integrated responses.[11]
The effect of the extracts on mechanical hyperalgesia was assessed with the Randall-Selitto paw pressure test, an inflammatory pain model. All the three extract exhibited significant inhibition of mechanical analgesia, albeit less effectively compared to the effect in other pain models. Results obtained corroborate the observed activity of the extracts in the second phase (inflammatory pain) of the formalin test.
Possible mechanism(s) of analgesic action of the extracts were examined in the formalin test. The antinociceptive effect of the extracts was assessed in the presence of some antagonists including atropine, naloxone, theophylline, glibenclamide, and yohimbine. Naloxone and glibenclamide reversed the antinociceptive effects of only HAE—implicating a possible opioidergic/ATP-sensitive K+ channel involvement in its actions. The fact that the analgesic effects of EAE and PEE were not significantly affected by naloxone suggests their actions are largely independent of the opioid pathway and the ATP-sensitive K+ channel. Our results also show that all extracts interacted with the muscarinic cholinergic pathway to exert antinociception. This is because atropine, a nonselective muscarinic cholinergic antagonist, attenuated the antinociceptive effects of the three extracts. Theophylline also reversed the antinociceptive effects of HAE but not EAE or PEE suggesting a possible adenosinergic pathway involvement in the actions of HAE.
In all the pain models used in this study, the three extracts exhibited significant analgesic activity that cannot be attributed to a decreased motor function. Results from the rotarod test showed clearly that the extracts, at the doses tested, did not have any significant effect on motor performance of the animals used. It can be inferred from this study the presence of multiple compounds with analgesic activity in the stem bark of T. monadelpha. These act via different mechanisms that, together, produce the antinociception seen with the use of the plant extract in traditional medical practice. It is important that the individual compounds in the extracts be isolated and characterized.
Conclusion
In conclusion, the hydroalcoholic, ethyl acetate, and petroleum ether extracts of the stem bark of Trichilia monadelpha have analgesic property in rodents. The hydroalcoholic extract produces analgesic effects through mechanisms that involve an interaction with the opioid, adenosinergic, muscarinic cholinergic pathways, and ATP-sensitive K+ channels while that of the ethyl acetate and petroleum ether extracts involves the muscarinic cholinergic system.
Acknowledgments
We are grateful to the technical staff of the department of Pharmacology for their assistance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Abbiw DK. Useful plants of Ghana. KEW: Intermediate Technology Publications and the Royal Botanic Gardens; 1990. [Google Scholar]
- 2.Dokosi OB. Herbs of Ghana. Accra: Ghana Universities Press; 1998. [Google Scholar]
- 3.Kamanzi Atindehou K, Schmid C, Brun R, Kone MW, Traore D. Antitrypanosomal and antiplasmodial activity of medicinal plants from Cote d’Ivoire. J Ethnopharmacol. 2004;90:221–7. doi: 10.1016/j.jep.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Oyelowo O, Bolarinwa O, Morenikeji O. Assessment of sperm indices and testosterone level on the effect of Trichilia monadelpha extract in male albino rats. Afr J Pharm Pharmacol. 2011;5:1956–8. [Google Scholar]
- 5.Ainooson GK, Owusu G, Woode E, Ansah C, Annan K. Trichilia monadelpha Bark Extracts Inhibit Carrageenan-Induced Foot-Oedema in the 7-Day Old Chick and the Oedema Associated with Adjuvant-Induced Arthritis in Rats. Afr J Tradit Complement Altern Med. 2012;9:8–16. doi: 10.4314/ajtcam.v9i1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trease GE, Evans WC. A Textbook of Pharmacognosy. 13th ed. London: Bailliàre Tindall; 1989. [Google Scholar]
- 7.Woode E, Amoateng P, Ansah C, Duwiejua M. Anti-nociceptive effects of an ethanolic extract of the whole plant of Synedrella nodiflora (L.) Gaertn in Mice: Involvement of adenosinergic mechanisms. J Pharmacol Toxicol. 2009;4:17–29. [Google Scholar]
- 8.Woode E, Abotsi WK. Antinociceptive effect of an ethanolic extract of the aerial parts of Hilleria latifolia (Lam.) H. Walt. (Phytolaccaceae) J Pharm Bioallied Sci. 2011;3:384–96. doi: 10.4103/0975-7406.84445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pietrovski EF, Rosa KA, Facundo VA, Rios K, Marques MC, Santos AR. Antinociceptive properties of the ethanolic extract and of the triterpene 3 beta,6 beta,16 beta-trihidroxilup-20(29)-ene obtained from the flowers of Combretum leprosum in mice. Pharmacol Biochem Behav. 2006;83:90–9. doi: 10.1016/j.pbb.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Murase A, Okumura T, Sakakibara A, Tonai-Kachi H, Nakao K, Takada J. Effect of prostanoid EP4 receptor antagonist, CJ-042,794, in rat models of pain and inflammation. Eur J Pharmacol. 2008;580:116–21. doi: 10.1016/j.ejphar.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 11.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 12.dos Santos DA, Fukui Mde J, Dhammika Nanayakkara NP, Khan SI, Sousa JP, Bastos JK, et al. Anti-inflammatory and antinociceptive effects of Baccharis dracunculifolia DC (Asteraceae) in different experimental models. J Ethnopharmacol. 2010;127:543–50. doi: 10.1016/j.jep.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 13.Deraedt R, Jouquey S, Delevallee F, Flahaut M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol. 1980;61:17–24. doi: 10.1016/0014-2999(80)90377-5. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato AB, Poole S, Ferreira SH, et al. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol. 2000;387:111–8. doi: 10.1016/s0014-2999(99)00790-6. [DOI] [PubMed] [Google Scholar]
- 15.Shibata M, Ohkubo T, Takahashi H, Inoki R. Modified formalin test: characteristic biphasic pain response. Pain. 1989;38:347–52. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]