Intelligent design of multifunctional lipid-coated nanoparticle platforms for cancer therapy (original) (raw)
. Author manuscript; available in PMC: 2013 Oct 1.
Published in final edited form as: Ther Deliv. 2012 Dec;3(12):1429–1445. doi: 10.4155/tde.12.127
Abstract
Nanotechnology is rapidly evolving and dramatically changing the paradigms of drug delivery. The small sizes, unique chemical properties, large surface areas, structural diversity and multifunctionality of nanoparticles prove to be greatly advantageous for combating notoriously therapeutically evasive diseases such as cancer. Multifunctional nanoparticles have been designed to enhance tumor uptake through either passive or active targeting, while also avoiding reticuloendothelial system uptake through the incorporation of PEG onto the surface. First-generation nanoparticle systems, such as liposomes, are good carriers for drugs and nucleic acid therapeutics, although they have some limitations. These lipid bilayers are now being utilized as excellent carriers for drug-loaded, solid core particles such as iron oxide, mesoporus silica and calcium phosphate. In this article, their design, as well as their multifunctional role in cancer therapy are discussed.
Key Terms: Multidrug resistance, State where cancer cells become resistant to drugs, Mononuclear phagocyte system, Previously called a reticuloendothelial system and is a part of immune system, Enhanced permeability and retention, Process of nanoparticle accumulation into tumor tissue due to leaky vasculature, Matrix metalloproteinase, Enzyme expressed exclusively in tumor tissue, Cancer hyperthermia treatment, Applying high temperatures (up to 113°F) to kill cancer cells, STAT protiens, Signal Transducer and activator of transcription protein, which regulates cell survival and proliferation
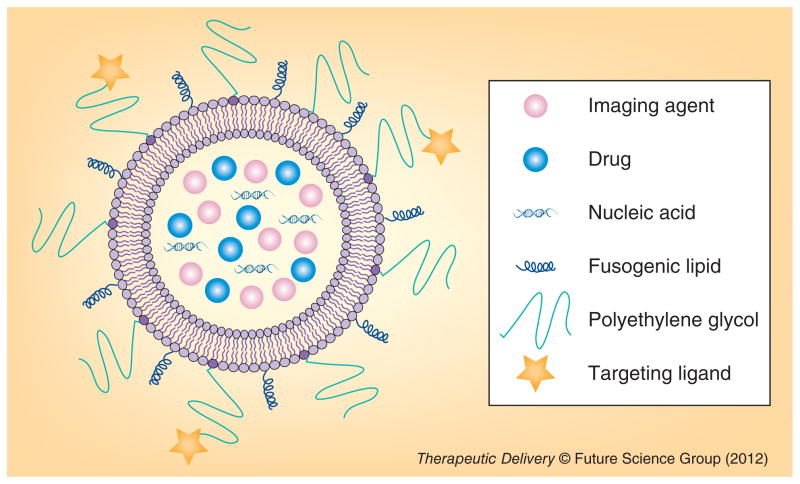
In the past 25 years, there has been significant progress in the prevention, detection and treatment of cancer, yet complete eradication for most cancers has not been accomplished. Cancer research must overcome difficulties in early tumor detection, multidrug resistance (MDR), subtleties and variation in cell-specific treatment and immunogenicity of introduced therapies [1,2]. For example, most anticancer agents, including doxorubicin (DOX), paclitaxel and other small-molecule drugs, not only kill cancer cells but also induce toxicity in normal cells. These anticancer drugs cause many side effects including immunosuppression, hair loss and nausea [3,4]. These challenges create a need for a new fundamental strategy for cancer therapeutic designs. Nanoparticles, led by liposomes, began to infiltrate cancer research nearly three decades ago. Preliminary studies evaluated the ability of liposomes to deliver cancer drugs and act as imaging agents for tumor detection [5]. Since then, nanoparticles for bio-medical applications have continued to prove effective drug-delivery agents [6,7]. In particular, the diversity of nanoparticle delivery platforms highlights their promise for producing tailor-made cancer therapies. Liposomes, pure drug nanoparticles, polymeric micelles, dendrimers and inorganic nanoparticles (e.g., calcium phosphate [CP], silica, iron oxide and gold) have gained attention as potential candidates to address the challenges of treating resilient cancer disease [8–11]. These engineered, multifunctional nanoparticles can simultaneously treat and monitor cancer through the inclusion of many different diagnostic and therapeutic agents within a single formulation (Figure 1). Furthermore, multifunctional nanoparticles can also greatly improve therapeutic efficacy and reduce nonspecific toxicity of anticancer drugs, enabling safe and effective cancer treatments. In this review, the design and multifunctional properties of lipid bilayer-coated, inorganic nanoparticles such as iron oxide, mesoporus silica and CP, as well as polycation-based solid nanoparticles for cancer therapy is discussed.
Figure 1.
Multifunctional nanoparticle.
Properties of nanoparticle conveying unique advantages in cancer therapy
The biodistribution of nanoparticles in circulation is an important factor that affects treatment. Properties of nanoparticles such as size, shape, surface chemistry and hydrophilicity determine their therapeutic effect.
Nanoparticle size & shape
The size of nanoparticles plays a major role in facilitating their in vitro and in vivo biodistribution. Nanoparticles possess characteristics that are similar to those of small biomolecules found naturally inside the body, enabling efficient uptake and drug delivery into the cell cytoplasm [12–15]. In general, nanoparticles with sizes of 10–100 nm are ideal for cancer therapy [16]; very small particles (~5 nm) are cleared rapidly from circulation by kidney filtration [17,18], while larger particles (nanometer to micrometer in size) are easily cleared by the mononuclear phagocyte system (MPS) [19,20]. Clearance by the MPS is due to the fact that accumulation of larger particles occurs primarily in the liver and spleen. Spleen sinusoids trap the nanoparticles via mechanical filtration, and then particles are cleared by the MPS. On the other hand, in one study, engineered nanoparticles prepared from polymer micelle assemblies, named filomicells, with a size of 18 μm (single dimension) and a half-life in circulation of 7 days [21]. Typically, studies have focused on nanoparticles that are spherical in shape, but recent research has determined that the shape of the nanoparticle will also impact their biodistribution [13]. For example, in one study, nonspherical particles with dimensions of up to 3 μm were observed to be taken up by HeLa cells. In this example, nanoparticles were prepared with different shapes using PRINT® technology [12]. The role of particle shape in macrophage uptake has also been examined. Macrophage uptake is mainly influenced by particle shape, not by particle size [22]. The fate of silica-based particles with different shapes and sizes following intravascular administration was examined in a recent study. The accumulation of spherical particles in organs, other than the mononuclear phagocyte system (MPS), decreased, with increased particle size. Discoidal particles were also observed in most of the organs, while cylindrical particles primarily accumulated in the liver [23].
Nanoparticle surface chemistry
The surface properties of nanoparticles also direct the journey of nanoparticles in circulation [24]. Opsonization and MPS clearance are the two factors most highly influenced by nanoparticle surface properties. Nanoparticles have a high surface-area-to-volume ratio compared with larger particles, allowing the nanoparticle to interact with more drug particles, which leads to a high loading capacity [25,26]. In general, positively charged particles are internalized by cells through an electrostatic interaction with the negatively charged cell membrane by non-specific endocytosis. Negatively charged particles, in comparison, tend to exhibit more selective internalization because of a slight charge repulsion effect. In either case, as the surface charge increases either positively or negatively, the macrophage surveillance increases, followed by MPS clearance [21]. In a recent study, gold nanorods of different charges, with and without active targeting, were examined for macrophage and dendritic cell uptake. Interestingly, the surface charge influenced both the macrophage and dendritic cell uptake; the nanoparticles with targeting ligands RGD (specific ligand for integrins) and GLF (a tripeptide with an affinity for macrophages) altered cell functions [27]. In another report, PEG–oligocholic acid dendrimers with positively (using lysine residues) and negatively (using aspartic acid residues) charged surface modifications were examined for MPS cell uptake. The in vivo biodistribution studies illustrated that highly positively or highly negatively charged nanoparticles accumulated in the liver due to MPS cells uptake, whereas slightly negatively charged particles accumulated in the tumor tissue and were excluded by the MPS system [28]. However, optimal surface charge and charge density vary for different nanoparticle systems; an earlier report demonstrated that positively charged or neutral liposomes reduced MPS clearance more than negatively charged liposomes [29].
PEGylation of nanoparticles & challenges
The surface hydrophobicity/hydrophilicity also influences MPS uptake. Due to hydrophobicity on the nanoparticle surface, blood proteins adsorb to the surface of nanoparticles (also known as opsonization). Nanoparticles that have been adsorbed by blood proteins are cleared by the MPS. Hydrophobic nanoparticles are more quickly opsonized than hydrophilic ones [30]. To reduce the hydrophobic interaction with plasma proteins and MPS clearance, hydrophilic shielding was introduced. One method of hydrophilic shielding is PEGylation [19,31]. PEGylation is a process of introducing a polyethylene glycol moiety onto the surface of a particle through adsorption or covalent binding. The benefits of PEGylation were demonstrated in systems as early as the first-generation liposomal systems. These systems can carry cancer therapeutics and deliver them more efficiently to the tumor than their free counterparts. Unfortunately, first-generation liposomal delivery systems can also accumulate in the liver and spleen, where they will be cleared by the MPS system [32]. However, after incorporation of hydrophilic PEG–phosphatidylethanolamine or PEG-stearate on the surface of conventional liposomes, the half-life in circulation increases and MPS uptake is reduced [19,31]. PEGylation has become a common method for prolonging nanoparticle circulation and reducing MPS uptake. An optimal PEG chain length is necessary for avoiding MPS clearance. PEG moieties with a molecular weight of 2000 Da or more were able to reduce MPS uptake [33]. In a recent study, the effect of the PEG chain length on the biodistribution of quantum dot (QD) nanoparticles has been reported. PEG chain lengths from 2 to 22 (units?) using dihydrolipoic acid spacers on QDs. Short PEG-containing QDs of length 2, 3 and 8–14 accumulated mainly in the liver, kidneys and pancreas, respectively. However, PEG with a length of 22 remained in the bloodstream for 4 h post injection [34]. In another report, 3H-labeled polylysine dendrimers with small PEG chains were eliminated rapidly by the kidneys, while those with larger PEG chains reduced MPS uptake and had little renal clearance [35]. An increase in particle accumulation in the spleen also leads to increase a MPS clearance. Unfortunately, such an increase can be caused by the prolonged circulation of PEG nanoparticles. Interestingly PEG-coated polycyanoacrylate nanoparticles with a 14C radiolabel were observed to have a reduced liver uptake, but a significant increase in spleen uptake [36]. All of these observations indicate that although the PEG coating on the nanoparticles cannot prevent uptake by the MPS, it can reduce the rate of uptake [37]. In summary, even though PEGylation is transient, it facilitates prolonged circulation, favorable biodistribution and tumor uptake [38].
PEGylation may contribute to the success of drug delivery through nanoparticles, but it also presents its own set of challenges. For example, while the Harishima group observed that PEGylated MEND nanoparticles displayed a longer half-life in circulation and a superior enhanced permeability and retention (EPR) accumulation in tumor cells in vivo than un-PEGylated nanoparticles, they also observed that the PEGylated particles induced a lower transgene expression in tumor cells in vitro. Because of steric hindrance caused by PEG chains, nanoparticle interaction with cellular membranes and endosomal membranes was reduced. Collectively, these difficulties are termed the ‘PEG dilemma’ [39,40]. After nanoparticles accumulate in the tumor, however, PEG chains become unnecessary. Once at the tumor site, nanoparticles need to be unmasked to expose their positive/negative surface charge in order to be internalized into the tumor cells. To address this issue, attempts have been made to ‘intelligently’ design the nanoparticles; for example, modifying PEG conjugation with lipids using the matrix metalloproteinase cleavable peptide [41] as well as other groups reporting pH-sensitive linkers [42]. Ishida et al shown administration of PEGylated nanoparticles repeatedly, reduced the stealthiness and increased the liver accumulation [43]. They found that the spleen is responsible for rapid clearance of PEGylated liposomes, and after the first injection of PEGylated nanoparticles, anti-PEG IgM will be produced from the spleen, and upon the second injection of the same particles, the IgM will interact with the nanoparticles, leading to MPS clearance [44]. This is called accelerated blood clearance. Recently in another study, prostaglandin E1 encapsulated in PEG–PLA nanoparticles is rapidly cleared in the second injection [45]. In a separate study, cleavable PEG–cholesterol lipid derivatives were used to prevent the accelerated blood clearance phenomena [46]. Other hydrophilic polymers such as polyvinyl alcohol [47], poly-_N-_vinylpyrrolidines [48], poly(N-(2-hydroxypropyl) methacrylamide) [49] and L-amino acid-based biodegradable polymer [50] were also reported for prolonged circulation.
Passive & active targeting
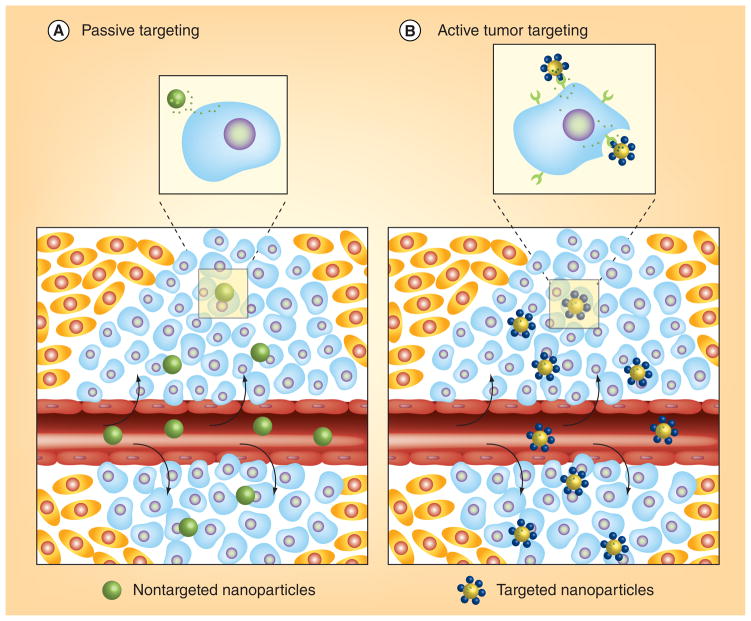
The blood vessels of tumors have abnormal morphological characteristics compared with the vasculature of normal tissues. Tumor blood vessels exhibit an incomplete endothelial lining, which results in irregular blood flow, a characteristic that is known as a ‘leaky’ vasculature. This defective architecture facilitates the entry of nutrients and oxygen into the tumor site, allowing the tumor’s rapid growth. The leaky pores in tumor vasculature vary in dimension based on tumor type; openings range from 200 nm to 2 μm. These same pores also allow nanoparticles to readily enter the tumor site. Nanoparticles are then accumulated and retained within the tumor because of the absence of a lymphatic drainage system (Figure 2A). The combination of the irregular vasculature and the absence of a drainage system constitute the EPR effect [51]. The EPR effect results in a high therapeutic index where drug-loaded nanoparticles deliver high therapeutic concentrations at the tumor site, reducing systemic toxicity [52,53]. The EPR effect is considered to be a landmark principle in tumor-targeting chemotherapy, but there are some limitations to the accumulation of nanoparticles in the tumor site, as the phenomenon is highly dependent on tumor vasculature [54]. In addition to variable pore size in different types of tumors, [55] many tumors have a high level of interstitial pressure, which leads to the inhibition of uptake and distribution of nanoparticles [56]. Details of the EPR effect and its limitations are well reviewed elsewhere [51]. After the accumulation of nanoparticles via the EPR effect, it is necessary to release the entrapped therapeutics into the cancer cells. This can be achieved by using target-specific moieties on the surface of nanoparticles. Although passive targeting can be successful in exploiting the EPR effect, the fact that the ‘leaky’ nature is not the same for all types of tumors makes the creation of applicable therapeutics difficult or impossible.
Figure 2. Passive and active targeting.
(A) Nanoparticles enter the tumor environment via the enhanced permeability and retention effect; (B) target-specific uptake by tumor cells is facilitated by receptor-specific ligands on the surface of nanoparticles.
Reproduced and modified with permission from [57] © American Chemical Society (2009).
Passive targeting of tumor cells within the tumor is not always feasible. In addition, the tumor environment can affect the nanoparticle interaction with tumor cells, leading to drug expulsion and induction of MDR [57,58]. Surface modification with targeting moieties specific for receptors expressed on tumor cells and tumor neovasculature can help to overcome this problem (Figure 2B). However, in a recent report, immunoliposomes with anti-HER2 did not exhibit significant tumor accumulation compared with nontargeted liposomes. Immunoliposomes with anti-HER2 have shown significant uptake by HER2-overexpressed breast cancer cells in vitro compared with nontargeted liposomes, but their pharmacokinetic profiles for targeted and untargeted liposomes are identical [59]. In another study, liposomes with five different ligands were tested for in vivo brain accumulation, however, the brain was poorly targeted [60]. Although targeting ligands do not increase the tumor accumulation, they can facilitate enhanced cellular internalization [61]. If the drugs are cell permeable, release of drugs into the tumor interstitium is enough, but therapeutics such as DNA, siRNA or other impermeable drugs are difficult to internalize into tumor cells. Nanoparticles with targeted ligands can efficiently deliver drugs and therapeutics inside the tumor cell for effective therapy and reduce the drug expulsion from the tumor. The degree of expression of receptors is also important for targeted delivery [62,63]. A variety of receptors expressed on tumor cells and neovasculature can be utilized for the targeted delivery of therapeutics, including folate receptors, sigma receptors, transferrin receptors and integrins (Table 1).
Table 1.
Examples for targeting ligands used in active targeting.
| Targeting ligand | Targeting receptor | Ref. |
|---|---|---|
| Monoclonal antibodies | ||
| HER-2 | EGF receptor 2 express on breast cancer cells | [164–166] |
| Aptamers | ||
| A10RNA | Prostate-specific membrane antigen | [167,168] |
| Proteins | ||
| Transferrin | Overexpressed on most of the malignant tissues | [169,170] |
| EGF | Overexpressed on most of the malignant tissues | [171] |
| Peptides | ||
| RGD | Tumor neovasculature (integrins) | [172,173] |
| HAIYPRH | Transferrin | [174] |
| SP 94 | Hepatocellular carcinoma | [175] |
| Small molecules | ||
| Folic acid | Folate | [176] |
| SW 107, SW 43, SKF 10047 and anisamide | Sigma receptors | [151, 177–179] |
| Galactose | Hepatocytes | [180] |
Inorganic solid nanoparticles with supported bilayers
Liposome formation was first observed by Bangham four decades ago as, “bilayered structures of phospholipids formed in an aqueous system” [64]. Liposomes exhibit a spherical shape and a size that varies based on the number of enclosed lipid layers. Multilamellar vesicles are 500 nm–5 μm. Small, unilamellar vesicles are 100–200 nm and are formed by a single bilayer, whereas large, unilamellar vesicles can be 200–800 nm and formed by multiple bilayers [65]. Since their discovery, liposomes have been extensively studied as potential delivery systems in pharmaceutical research [66,67]. Liposomes can entrap water soluble drugs (hydrophilic) in their aqueous interior and water insoluble drugs (hydrophobic) into the membrane. Lipoplexes, composed of cationic liposomes complexed with DNA, have been studied extensively in gene therapy applications. Recent studies show that these cationic liposomes are very useful in vaccine formulations to treat cancer [68–70]. Ceramides, a lipid molecule active in regulating cell-proliferation, differentiation, and apoptosis, have also been examined using a liposome delivery system [71]. Liposomes with a size greater than 100 nm are also considered as nanoparticles, because they can extravasate from circulation into the interstitial space of their site of action. Liposomes are capable of passively or actively targeting specific tissues, but due to their large size, liposomes will be quickly eliminated from circulation and cleared by MPS in the liver. Modifying the drugloaded liposomes with hydrophilic polymers such as PEG can enhance the circulation time of the particles in the blood and allow them to avoid MPS clearance [19]. This surface modification of liposomes with PEG changes the fate of the liposomes in vivo. Doxil®, a PEGylated liposomal formulation with DOX, was approved by the US FDA for the treatment of breast and ovarian cancer [62]. Combination therapy with liposomal DOX and paclitaxel, or Caelyx® (liposomal DOX available in Europe) and carboplatin are undergoing clinical trials [72,73]. The leakiness, aggregation, MPS clearance and limited loading of therapeutics of liposomes have led to the exploration of newer nanoparticles. Although liposomes have several drawbacks, the lipid bilayer plays a major role in several successful delivery systems by protecting drug-loaded, engineered inorganic nanoparticles.
Magnetoliposomes
Superparamagnetic iron oxide nanocrystals (SPIONs) are well known contrast agents used in the clinical applications of T2-weighted magnetic imaging resonance [74,75] in the treatment of hyperthermia, and to enable drug delivery and magnetofection [76–80]. SPIONs are also a key component of magnetoliposomes (MLs), which consist of these SPIONs surrounded by lipid bilayers. These particles were first prepared by De Cuyper et al. with phosphatidylglycerols [81]. Classic MLs have an overall size of 20 nm with a 14 nm inner magnetic core. Extruded MLs consist of large unilamellar vesicles with the size of 200 nm. Iron oxide particles with a 1–10 nm size have been incorporated in the aqueous phase of liposomes [82–84]. The latter has the advantage of the ability to incorporate hydrophilic drugs into the aqueous phase of the liposome [85]. These MLs can reduce the agglomeration and toxicity of iron oxide particles. PEGylation can increase the circulation half-life and aid the ML’s avoidance of MPS uptake in vivo [86]. Further nanoparticle modification with tumor-specific-targeting ligands and incorporation of a therapeutic agent in the MLs proves to increase their multifunctional properties for cancer research. In a recent study, a thermo-sensitive liposome was used to encapsulate both iron oxide particles and DOX with an external, folate-receptor targeting ligand. The drug release from MLs is dependent on the temperature induced by the AC magnetic field, which is used in cancer hyperthermia treatment. In vivo administration of these targeted liposomes can be internalized by tumor cells via folate mediated endocytosis; drug release is then modulated at the tumor site using an alternate magnetic field. The resulting effect is an improved therapeutic ratio for tumor cells versus system toxicity, especially compared with Doxil [87–89]. In another study, folate-targeted, worm-like polymeric vesicles loaded with SPIONs and the anticancer drug DOX were utilized for multifaceted cancer treatment. These worm-like particles are formed by amphiphilic triblock co-polymers (PEG114-PLAx-PEG46-acrylate). The long PEG chain contains a folate segregated outer side to facilitate active tumor targeting, and smaller PEG acrylate groups make up a segregated inner layer to cross-link the PEG layers via free radical polymerization for enhanced in vivo stability. These multifunctional magnetic nanoparticles release the attached drug at endosomal pH value faster than at a physiological pH for cross-linked and noncross-linked worm-like vesicles. Moreover, these worm-like vesicles loaded with SPIONs/DOX show higher T2 relaxitivity value than commercially available Feridex® [90]. Researchers have reported that the SPIONs/DOX is co-encapsulated in poly(lactic-co-glycolic acid)/polymeric liposome nanocarriers. This core-shell structure is formed through the self-assembly of the hydrophobic poly(lactic-co-glycolic acid) core and a hydrophilic folate modified PEG lipid shell [91]. Several different types of functionalized and drug-loaded SPIONs have been reported and discussed in recent reviews [8,92].
Lipid, polycation-based nanoparticle systems
Cationic lipids contribute to the encapsulation of negatively charged nucleic acids through electrostatic interaction. Particles resulting from these interactions are not only sensitive to the ionic strength of the external solution, but are also challenged by DNA aggregation and the subsequent decrease in transfection ability [93,94]. To address these problems, one laboratory established the lipid–DNA–protamine (LPD) nanoparticle system [95,96]. The LPD nanoparticles are prepared by condensing DNA with protamine and coating the resulting products with cationic lipids. This nanoparticle system has many advantages over other liposomal delivery systems, including increased long-term storage and stability, protection of the nucleic acids from degradation and a higher level of gene expression after cell uptake [95–98]. Furthermore, these LPD nanoparticles are modified with PEG on the surface to avoid the MPS system and promote a long half-life in circulation [99–101]. Most solid tumors overexpress the sigma receptor [102–105]. This fact is explored in the creation of the LPD nanoparticle system by conjugating anisamide to the DSPE–PEG, enabling sigma receptor specific internalization of the cancer therapeutics to tumor cells [106–108].
Even the most successful cancer therapies can be hindered by the development of MDR. P-glycoprotein plays a key role in the resiliency of cancer cells against introduced therapeutics [2]. Chen et al. delivered siRNA and DOX simultaneously using the LPD nanoparticle system to overcome MDR [109]. Two nanoparticle systems that possess different characteristics, enabling them to circumvent MDR, have been documented. One of these LPD nanoparticle systems contains a guanidinylated cationic lipid, siRNA and DOX. Cationic lipids on the nanoparticle can induce reactive oxygen species and down-regulate MDR transporters, increasing the DOX penetration. A second nanoparticle system involves a modified LPD, termed LPD-II, in which the cationic lipid is replaced with a DOPA (1,2-dioleoyl-sn-glycero-3-phosphate) anionic lipid, c-myc siRNA and DOX. Anionic DOPA was used in the LPD-II nanoparticle system to enable a higher encapsulation of DOX compared with the LPD system with cationic lipid. Both LPD and LPD-II systems provoked significant tumor growth inhibition compared with untargeted nanoparticle or free drug treatment in an NCI/ADR reticuloendothelial system xenograft tumor model in vivo [109]. In another study, a successful combination therapy using combined siRNA and miRNA with a GC4 single chain antibody fragment (scFv; a tumor targeting monoclonal antibody) was reported. In this study, an LPH nanoparticle system was used, where a calf thymus DNA of LPD system was replaced with hyaluronic acid. Combined siRNA (c-myc/MDM2/VEGF) delivered with LPH nanoparticles downregulates targeted genes in this antibody-anchored LPH nanoparticle system. Indeed, the LPH combination particle encapsulating miRNA-34 induced apoptosis and downregulated the MAPK pathway, while further combination with siRNA and miRNA enhanced tumor growth inhibition in vivo against B16F10 lung metastasis in a syngeneic mouse model [110].
This nanoparticle system not only delivers drugs and nucleic acids, but can also deliver peptides to block the signaling cascades that cause cancer cell proliferation and survival. Among those signaling cascades are STAT proteins; the control of cancer cell proliferation and survival [111]. For example, Stat5b, is a protein activated by the phosphorylated Y845 kinase domain of EGFR in EGF-stimulated tumor cells where loss of Stat5b can induce apoptosis through the Apaf-1/caspase-9 pathway [112,113]. Kim et al. [114] delivered a therapeutic peptide using a lipid–protamine–heparin nanoparticle system. In this work, EV peptide (EEEEpYFELV), a mimic sequence of the Y845 kinase domain of the EGF receptor kinase for inhibition of Stat5b phosphorylation and activation, was successfully delivered into H460 non-small-cell lung cancer cells. The nanoparticles facilitated EV-mediated inhibition of Stat5b activation and stimulated H460 cell apoptosis in vitro. When a nanoparticle system modified with a sigma receptor-specific targeting ligand was used to deliver the EV peptide in an H460 xenograft mouse model, a significant dose-dependent tumor inhibition was observed in vivo, as compared with delivery of nanoparticles formulated with a scrambled peptide in an H460 xenograft mouse model [114].
MEND nanoparticles, as developed by the Harashima group, make up another novel multifunctional nanoparticle delivery system that possesses superior functionality over liposomes [115]. Similar to the LPD nanoparticle, the MEND nanoparticle involves condensing nucleic acids with polycations and formulating the resulting particle in a lipid envelope composed of cationic lipid and DSPE–PEG with functional groups and cell-penetrating peptides. They modified the lipid envelope with sterylated R8, a modified octa-arginine with a steryl hydrophobic chain, to enhance cellular uptake and avoid the lysosomal degradation of the particles compared with octalysine modified liposome [116,117]. They also reported that octa-arginine modified lipid (R8-lip) acts as an efficient cancer vaccine. These R8-lip-containing MEND nanoparticles deliver the attached antigen to antigen presenting cells, which present the antigen to T-cells via their MHC-I complex [118]. MEND nanoparticles have successfully delivered Mycobacterium bovis bacillus Calmette-Guerin cell wall (BCG-CW) as an immunotherapeutic agent. In addition, MEND nanoparticles formulated with the R8-lip/BCG-CW completely inhibit tumor growth in murine MBT-2 tumor model [119]. As aforementioned, nanoparticle interaction with cell membranes, as well as internalization of the particle into the tumor cell, is required to achieve therapeutic effect. Modification of MEND nanoparticles containing siRNA with pH-sensitive GALA peptides enhances their cellular uptake [120–122]. The GALA peptide has no effect on physiological pH, but at an acidic pH (~5.5), the carboxylic groups of glutamic acid deplete the electric field repulsion, causing a structural change of the peptide and inducing membrane fusion [123,124]. In a previous study, the GALA-modified MEND nanoparticles formulated with siRNA provoked effective gene silencing in tumor tissues, yet the introduced particles were eliminated rapidly [121,122]. To overcome this problem, a shorter version of GALA (shGALA) has been introduced, and shown more efficient gene silencing in vitro and in vivo [125].
Protocells
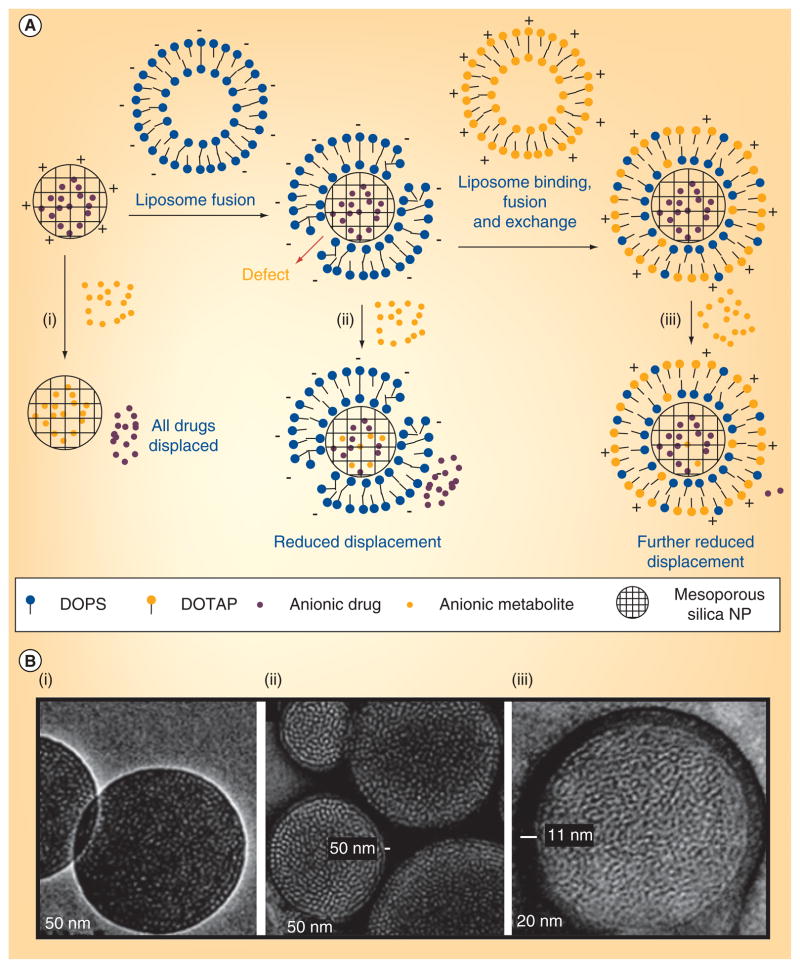
Inorganic mesoporous silica nanoparticles have emerged as important delivery vehicles due to their biocompatibility, efficient encapsulation and specific nanoporosity [126–128]. These silica nanoparticles have larger surface areas, variable pore sizes, which can be manipulated, and different pore surface chemistry. In addition, the nanoparticles can encapsulate a wide range of therapeutic agents and moderate controlled drug release [129–131]. The surface modification of silica nanoparticles with organic molecules for long circulation and targeted delivery has already been used in biological applications [131–134]. For example, supported lipid bilayers act as models of the cell membrane and provide several advantages for drug-delivery applications [66,135,136]. Recently, supported lipid bilayers were used to coat mesoporous silica nanoparticles [130,131,136,137]. In one study, a combined system called a ‘protocell’ was shown to use liposome fusion on silica nanoparticles to simultaneously load a drug for intracellular delivery. These lipid bilayer-coated, silica nanoparticles can combine the features of liposomes and silica nanoparticles. In another report, they have used positively charged mesoporous silica nanoparticles to deliver the negatively charged calcein, however, calcein cell uptake was not observed [138]. To solve this problem, positively charged silica nanoparticles were fused with a negatively charged DOPS bilayer. The negatively charged cell membrane, however, repelled the negatively charged nanoparticles and formulations of the purified DOPS-coated silica nanoparticles with positively charged DOTAP still producing defective protocells (Figure 3). Finally, the fusion of mesoporous silica nanoparticles on lipid bilayer was achieved by successive addition of three liposomes (DOTAP/DOPS/DOTAP), producing particles 100–150 nm in diameter and that successfully delivered encapsulated DOX to CHO cells in vitro [138]. In a recent study, the same group illustrated the multifunctional application with these silica nanoparticle supported lipid bilayers via modification with PEG, fusion peptides and targeting agents (Figure 4). SP94 peptide was used to target the hepatocellular carcinoma and a histidine-rich fusogenic peptide (H5WYG) was utilized to promote endosomal escape. These nanoporous silica nanoparticles were wrapped with different liposomal bilayers such as cationic DOTAP, zwitterionic DPPC and DOPC. This modification resulted in enhanced bilayer fluidity, greater stability and drug leakage over conventional liposomes (Figure 4). The lipid-supported silica particles produced, with a minimal amount of targeting peptide, achieved a 100-fold greater specificity than liposomes in Hep3B target cells. Because of their high surface area and porosity, the lipid-supported silica particles have 1000-times higher capacity for encapsulation of the anticancer drug DOX than liposomes. Protocells produced with DOPC lipid were very effective and safe, provoking a 90% cell viability in normal hepatocytes and 97% killing in vitro in MDR1 and hepatocellular carcinoma cells (Hep3B) [136]. In vivo biodistribution studies and tumor-specific delivery of therapeutics using these lipid-supported silica nanoparticles will help to validate their clinical relevance.
Figure 3. Supported bilayer coating of silica nanoparticles.
(A) A negatively charged drug is adsorbed into the pores of a cationic mesoporous silica nanoparticle. (i) Other anions that are adsorbed more strongly can displace the loaded drugs. (ii) Fusion with a negatively charged liposome reduces the displacement, (iii) and further lipid exchange/fusion with cationic liposomes reduces it more. (B) Representative transmission electron microscopy images of (i) bare anionic mesoporous silica cores and protocells with (ii) single or (iii) dual supported bilayers formed after successive DOTAP and DOPS fusion/exchange steps (lipid-fixed and negative-stained).
NP: Nanoparticle.
Modified and reprinted with permission from [138] © American Chemical Society (2009).
Figure 4. Lipid bilayer supported multifunctional silica nanoparticle (protocell).
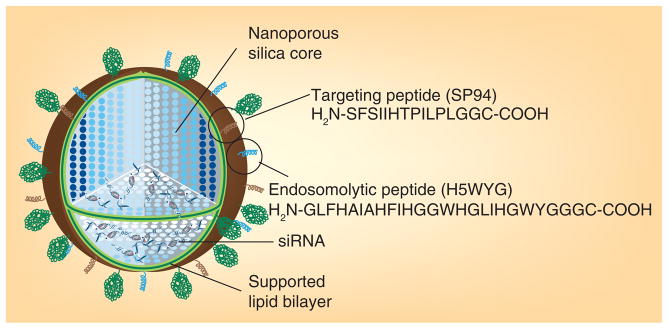
Reproduced with permission from [181] © American Chemical Society (2012).
Lipid-coated CP nanoparticle system
CP is commonly found in the body as the principal component of human bone and teeth. CP nanoparticles are emerging candidates in the field of drug-delivery systems, because they are nontoxic, biodegradable and capable of delivering therapeutics and imaging agents. CP was first used in the gene therapy context in 1973 [139]. Subsequently, it has been used as a nonviral transfecting agent and been denoted the second generation of nonviral vectors [140–143]. Currently, these CP nanoparticles are utilized for drug-delivery and imaging agents in cancer treatment [144]. The CP carrier is prepared by simple precipitation method of calcium chloride with DNA and a phosphate buffer solution, where the solution pH, calcium chloride concentration, time between precipitation and transfection efficiency are the key parameters [139,145]. In drug delivery, releasing the cargo at the target site is critical to the drug’s therapeutic effect. The advantage of using a CP nanoparticle delivery system is that it is destabilized at an endosomal pH (~5.0). After endocytosis, the CP nanoparticle destabilizes and triggers the release of the cargo. CP nanoparticles are very efficient in transfecting cells in vitro, but in vivo applications are limited because of their instability in circulation, their size, their tendency for agglomeration and their surface functionalization. In an attempt to overcome these problems, a micro-emulsion method for the preparation of CP nanoparticles is especially warranted to define the nanoparticle size, shape and surface properties [145–147].
In order to further improve the prolonged circulation, stability and targeted delivery in systemic administration, our group developed a new class of CP nanoparticle with lipid coating. These lipid calcium phosphate (LCP) nanoparticles prepared using a common micro emulsion method. An anionic lipid DOPA was used along with the sodium phosphate fraction to form a solid lipid core with calcium. The lipid protects the CP/nucleic acid or drug complex in the core. The LCP core measures approximately 20–25 nm in size with 8–12 nm of this dimension acting as an inner cavity[148].
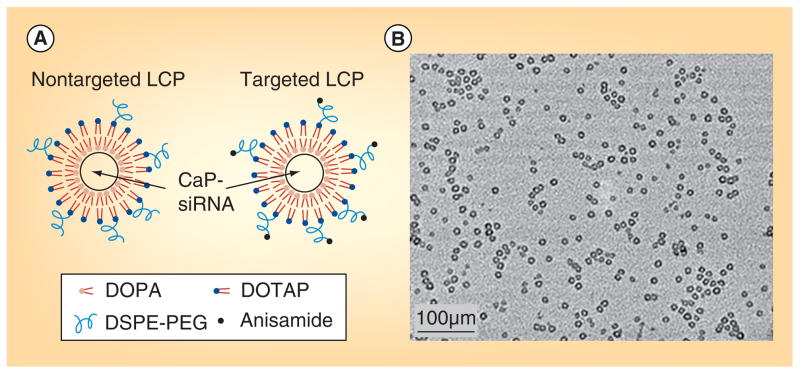
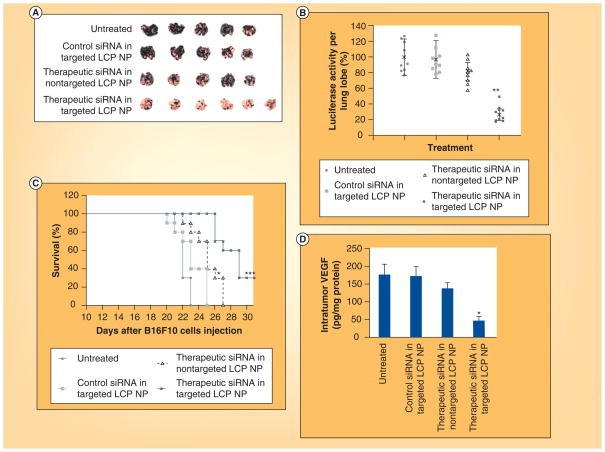
The LCP’s signature hollow structure is due to the smaller size of the inner CP compared with water in the micro emulsion; the DOPA forms a surface layer on the CP core due to the insolubility in water. DOPA-layered core particles are encapsulated by an external lipid layer functionalized with DSPE–PEG modified with anisamide, to enable prolonged circulation and targeted delivery to tumor cells (Figure 5) [101,148,149]. Systemic administration of these functionalized LCPs resulted in sigma receptor-specific uptake in H460 tumor-bearing mice without any inflammatory cytokine elevation [145]. A study utilizing LCP nanoparticles formulated with cy5.5-fluorophore-labeled siRNA showed that enhanced tumor uptake of the nanoparticles is PEG-density dependent. The result suggests that nanoparticles can evade the MPS at high PEG density because nanoparticles produced with a high amount of PEG have a curved surface, supporting a ‘brush-type’ structure compared with the ‘mushroom-type’ structure observed in LCP nanoparticles with low PEG surface densities [150]. LCP nanoparticles can encapsulate multiple nucleic acids in one formulation, allowing for the specific inhibition of multiple cancer signaling pathways. In a recent study, LCP nanoparticles formulated with siRNAs against MDM2, c-myc and VEGF showed a significant therapeutic effect in a murine B16F10 lung metastasis model [149]. Altogether, these multifunctional nanoparticles target several major cancers attributes, including p53 function and apoptosis (MDM2), proliferation and cell differentiation (c-myc) and angiogenesis (VEGF). Further sigma receptor-specific delivery of these nanoparticles to B16F10 lung metastasis model adds to the inhibition of tumor growth compared with untargeted nanoparticles treatment (Figure 6). The LCP nanoparticle system is also known for low immunogenicity and toxicity [151]. In another recent study, sigma receptor-targeted LCPs formulated with pooled siRNA inhibited the growth of A549 and H460 xenografts in a murine non-small cell lung cancer tumor model. Targeted LCPs encapsulating fluorescent Texas red-labeled siRNA also showed significant tumor accumulation [149].
Figure 5. Lipid-coated calcium phosphate nanoparticles.
(A) Nontargeted and targeted LCP; (B) transmission electron microscopy images of LCP coated with DOTAP and DSPE–PEG.
LCP: Lipid calcium phosphate.
Reproduced with permission from [149] © American Chemical Society (2012).
Figure 6. Therapeutic effect of siRNA in liposomal calcium phosphate nanoparticles.
(A) Photographs of lungs excised from tumor-bearing mice; (B) quantification of luciferase activity in lung metastasis; (C) survival analysis of B16F10 lung metastases bearing mice on day 19 after four treatments; and (D) intratumoral expression of VEGF after treatment.
LCP: Lipid calcium phosphate; NP: Nanoparticle.
Reproduced with permission from [151] © Macmillan Publishers Ltd. Molecular Therapy (2012).
Lipid nanocapsules
The Benoit group developed another tool for drug delivery known as lipid nanocapsules using a phase inversion temperature method. In this method, all components (oil phase, aqueous phase and surfactant) are mixed and heated above the phase inversion temperature, then cooled down to below it [152]. After several cycles of heating and cooling, a sudden addition of cold water induces an irreversible shock and leads to the formation of stable nanocapsules. They have used capric and caprylic acid triglycerides as oil phase, hydrpophilic surfactant Solutol® composed of PEG 660 and PEG 660 hydroxy state. Lipoid is another hydrophobic surfactant added in small portion for better stability [152]. Hydropholic surfactants such as Solutol can also inhibit the P-glycoprotein function, adding to their desirability for use as stabilizers [153,154]. These lipid nanocapsules have greater advantage over liposomes in terms of leakage and drug loading. Lipid nanocapsules have been shown to be effective carriers for delivering hydrophobic drugs [155]. However, Morille et al. reported that hydrophilic DNA can be formulated in nanocapsules. They have reported that DNA–lipid nanocapsules with PEG enhances the gene expression 84-times higher in tumors after intravenous injection [156]. DNA may also be complexed with DOTAP–DOPE liposomes and encapsulated using nanocapsules. The size and stability of the lipoplex in the nanocapsules is then well controlled [157]. DNA lipid nanocapsules such as these have been used in suicide gene-based anticancer therapy also called gene-directed enzyme prodrug therapy [158]. In these cases, fluorescent-labeled nanocapsules may be utilized for imaging purposes simultaneously [159].
Conclusion & future perspective
The diversity of nanoscale particles creates a new paradigm of flexible, effective therapies to fight even the most resilient cancer diseases. Flexibility in nanoparticle shape and size adds to the advantage, as nanoparticle therapies can be custom-built to target and release drugs to the tumor site based on their physical characteristics. Along with these properties, surface modification with targeting ligands and PEGylation makes nanoparticles even more durable in circulation and specific to cancerous cells, increasing the therapeutic ratio of effectiveness- to-systemic toxicity. Liposomal drugs such as Doxil, Ambisome® and Daunoxome® have already been approved by the FDA, and more nanoparticle formulations continue to be tested in clinical trials [66]. The multifunctional property of nanoparticles attracts researchers towards developing new methods for simultaneous detection and therapy. We have discussed the intelligent design and multifunctional role of several important lipid-based nanoparticle platforms used for cancer therapy. However, there are many other types of nanoparticles including surface-modified glyceryl monooleate/chitosan nanostructures [160], lipid-coated polymeric nanoparticles [161], polymer-based metallic nanoparticles [162] and pure drug nanoparticles [8,163]. There have been tremendous developments in the refinement of nanoparticle delivery systems, however significant challenges remain. It is always challenging for an in vitro success to translate into a safe in vivo therapy. One of the major issues nanoparticles face involves opsonization followed by rapid MPS-facilitated clearance in vivo. Modification with PEG is widely employed to overcome this problem, yet PEGylation is also transient and will not stop MPS clearance completely. There is a need to develop new hydrophilic polymers to escape the MPS system. The size of the nanoparticle is also an important issue in opsonization. It is important to continue to develop targeting ligands to facilitate tumor site-specific treatment, saving normal tissue from exposure to toxic agents. Therapeutic release kinetics also needs to be improved to encourage sustained release of drugs. Despite the challenges, nanoparticles continue to encourage optimism that a new wave of therapies aims to fix the discouraging obstacles inherent to cancer treatment.
Executive summary.
Background
- Nanoparticles are an emerging field in pharmaceutical and drug-delivery applications.
- The first generation of nanoparticles involves liposomes. Although a promising delivery system, they have limitations for in vivo applications.
PEGylation & drawbacks
- PEGylation of nanoparticles reduces the uptake of mononuclear phagocyte system and facilitates longer blood circulation time and subsequent accumulation in tumors through the enhanced permeability and retention effect.
- Although PEGylation is transient, it facilitates favorable biodistribution. Receptor-specific modification by PEGylation enhances the tumor-cell internalization of nanoparticles.
- PEGylation of nanoparticles causes steric hindrance that prevents interactions between the nanoparticle and the cellular membranes.
- Upon repeated administration of PEGylated nanoparticles, accelerated blood clearance is generated.
Multifunctional nanoparticles
- PEGylated, multifunctional nanoparticles, composed of target-specific ligands, were developed to provide prolonged circulation in the body and to deliver multiple agents for therapy and imaging.
- Inorganic nanoparticles such as calcium phosphate, mesoporous silica and superparamagnetic iron oxide nanoparticles are being refined; further modification with liposomal-bilayer systems enhances their ability in multifunctional applications for cancer therapy.
Acknowledgments
The authors would like to thank K Racette, A Satterlee and M Foote for their helpful suggestions.
Footnotes
Financial & competing interest disclosure
The original work from this laboratory was supported by NIH grants CA149363, CA129835, CA129421, CA151455 and CA151652. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Sinha R, Kim GJ, Nie S, Shin DM. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther. 2006;5(8):1909–1917. doi: 10.1158/1535-7163.MCT-06-0141. [DOI] [PubMed] [Google Scholar]
- 2.Jabr-Milane LS, Van Vlerken LE, Yadav S, Amiji MM. Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treat Rev. 2008;34(7):592–602. doi: 10.1016/j.ctrv.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 4.Marshall E. Cancer research and the $90 billion metaphor. Science. 2011;331(6024):1540–1541. doi: 10.1126/science.331.6024.1540-a. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 6.Kim BY, Rutka JT, Chan WC. Nanomedicine. N Engl J Med. 2010;363(25):2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 7.Buse J, El-Aneed A. Properties, engineering and applications of lipid-based nanoparticle drug-delivery systems: current research and advances. Nanomedicine (Lond ) 2010;5(8):1237–1260. doi: 10.2217/nnm.10.107. [DOI] [PubMed] [Google Scholar]
- 8.Yu MK, Park J, Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012;2(1):3–44. doi: 10.7150/thno.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrell D, Ptak K, Panaro NJ, Grodzinski P. Nanotechnology-based cancer therapeutics – promise and challenge – lessons learned through the NCI alliance for nanotechnology in cancer. Pharm Res. 2011;28(2):273–278. doi: 10.1007/s11095-010-0214-7. [DOI] [PubMed] [Google Scholar]
- 10.Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Nanomedicine – challenge and perspectives. Angew Chem Int Ed Engl. 2009;48(5):872–897. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokolova V, Epple M. Inorganic nanoparticles as carriers of nucleic acids into cells. Angew Chem Int Ed Engl. 2008;47(8):1382–1395. doi: 10.1002/anie.200703039. [DOI] [PubMed] [Google Scholar]
- 12.Gratton SE, Ropp PA, Pohlhaus PD, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA. 2008;105(33):11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res. 2009;26(1):235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 14.Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26(1):244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith BR, Kempen P, Bouley D, et al. Shape matters: intravital microscopy reveals surprising geometrical dependence for nanoparticles in tumor models of extravasation. Nano Letts. 2012;12(7):3369–3377. doi: 10.1021/nl204175t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 17.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond ) 2008;3(5):703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 20.Barreto JA, O’Malley W, Kubeil M, Graham B, Stephan H, Spiccia L. Nanomaterials: applications in cancer imaging and therapy. Adv Mater. 2011;23(12):18–40. doi: 10.1002/adma.201100140. [DOI] [PubMed] [Google Scholar]
- 21.Geng Y, Dalhaimer P, Cai S, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103(13):4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decuzzi P, Godin B, Tanaka T, et al. Size and shape effects in the biodistribution of intravascularly injected particles. J Control Release. 2010;141(3):320–327. doi: 10.1016/j.jconrel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Schipper ML, Iyer G, Koh AL, et al. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small. 2009;5(1):126–134. doi: 10.1002/smll.200800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartlett DW, Davis ME. Physicochemical and biological characterization of targeted, nucleic acid-containing nanoparticles. Bioconjug Chem. 2007;18(2):456–468. doi: 10.1021/bc0603539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, Lee K, Park TG. Hyaluronic acidpaclitaxel conjugate micelles: synthesis, characterization, and antitumor activity. Bioconjug Chem. 2008;19(6):1319–1325. doi: 10.1021/bc8000485. [DOI] [PubMed] [Google Scholar]
- 27.Bartneck M, Keul HA, Wambach M, et al. Effects of nanoparticle surface-coupled peptides, functional endgroups, and charge on intracellular distribution and functionality of human primary reticuloendothelial cells. Nanomedicine. 2012 doi: 10.1016/j. nano.2012.02.012. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 28.Xiao K, Li Y, Luo J, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32(13):3435–3446. doi: 10.1016/j.biomaterials.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juliano RL, Stamp D. The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem Biophys Res Commun. 1975;63(3):651–658. doi: 10.1016/s0006-291x(75)80433-5. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal P, Hall JB, Mcleland CB, Dobrovolskaia MA, Mcneil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61(6):428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen TM, Hansen C. Pharmacokinetics of stealth versus conventional liposomes: effect of dose. Biochim Biophys Acta. 1991;1068(2):133–141. doi: 10.1016/0005-2736(91)90201-i. [DOI] [PubMed] [Google Scholar]
- 32.Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30(11):592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Cancer. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Choi HS, Ipe BI, Misra P, Lee JH, Bawendi MG, Frangioni JV. Tissue- and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Letts. 2009;9(6):2354–2359. doi: 10.1021/nl900872r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaminskas LM, Boyd BJ, Karellas P, et al. The impact of molecular weight and PEG chain length on the systemic pharmacokinetics of PEGylated poly l-lysine dendrimers. Mol Pharm. 2008;5(3):449–463. doi: 10.1021/mp7001208. [DOI] [PubMed] [Google Scholar]
- 36.Peracchia MT, Fattal E, Desmaele D, et al. Stealth PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J Control Release. 1999;60(1):121–128. doi: 10.1016/s0168-3659(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 37.Zamboni WC. Liposomal, nanoparticle, and conjugated formulations of anticancer agents. Clin Cancer Res. 2005;11(23):8230–8234. doi: 10.1158/1078-0432.CCR-05-1895. [DOI] [PubMed] [Google Scholar]
- 38.Petros RA, Desimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura T, Akita H, Yamada Y, Hatakeyama H, Harashima H. A multifunctional envelope-type nanodevice for use in nanomedicine: concept and applications. Acc Chem Res. 2012;45(7):1113–1121. doi: 10.1021/ar200254s. [DOI] [PubMed] [Google Scholar]
- 40.Wang T, Upponi JR, Torchilin VP. Design of multifunctional non-viral gene vectors to overcome physiological barriers: dilemmas and strategies. Int J Cancer. 2012;427(1):3–20. doi: 10.1016/j.ijpharm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Hatakeyama H, Akita H, Kogure K, et al. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Ther. 2007;14(1):68–77. doi: 10.1038/sj.gt.3302843. [DOI] [PubMed] [Google Scholar]
- 42.Romberg B, Hennink WE, Storm G. Sheddable coatings for long-circulating nanoparticles. Pharm Res. 2008;25(1):55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishida T, Maeda R, Ichihara M, Irimura K, Kiwada H. Accelerated clearance of PEGylated liposomes in rats after repeated injections. J Control Release. 2003;88(1):35–42. doi: 10.1016/s0168-3659(02)00462-5. [DOI] [PubMed] [Google Scholar]
- 44.Ishida T, Kiwada H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int J Cancer. 2008;354(1–2):56–62. doi: 10.1016/j.ijpharm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Ishihara T, Takeda M, Sakamoto H, et al. Accelerated blood clearance phenomenon upon repeated injection of PEG-modified PLA-nanoparticles. Pharm Res. 2009;26(10):2270–2279. doi: 10.1007/s11095-009-9943-x. [DOI] [PubMed] [Google Scholar]
- 46.Xu H, Wang KQ, Deng YH, Chen Da W. Effects of cleavable PEG-cholesterol derivatives on the accelerated blood clearance of PEGylated liposomes. Biomaterials. 2010;31(17):4757–4763. doi: 10.1016/j.biomaterials.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 47.Takeuchi H, Kojima H, Yamamoto H, Kawashima Y. Evaluation of circulation profiles of liposomes coated with hydrophilic polymers having different molecular weights in rats. J Control Release. 2001;75(1–2):83–91. doi: 10.1016/s0168-3659(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 48.Torchilin VP, Levchenko TS, Whiteman KR, et al. Amphiphilic poly-N-vinylpyrrolidones. synthesis, properties and liposome surface modification. Biomaterials. 2001;22(22):3035–3044. doi: 10.1016/s0142-9612(01)00050-3. [DOI] [PubMed] [Google Scholar]
- 49.Whiteman KR, Subr V, Ulbrich K, Torchilin VP. Poly(Hpma)-coated liposomes demonstrate prolonged circulation in mice. J Liposome Res. 2001;11(2–3):153–164. doi: 10.1081/LPR-100108459. [DOI] [PubMed] [Google Scholar]
- 50.Metselaar JM, Bruin P, De Boer LW, et al. A novel family of l-amino acid-based biodegradable polymer-lipid conjugates for the development of long-circulating liposomes with effective drug-targeting capacity. Bioconjug Chem. 2003;14(6):1156–1164. doi: 10.1021/bc0340363. [DOI] [PubMed] [Google Scholar]
- 51.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54(13):3352–3356. [PubMed] [Google Scholar]
- 53.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 54.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 55.Hobbs SK, Monsky WL, Yuan F, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95(8):4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure – an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806– 813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 57.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 58.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 59.Kirpotin DB, Drummond DC, Shao Y, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66(13):6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 60.Van Rooy I, Mastrobattista E, Storm G, Hennink WE, Schiffelers RM. Comparison of five different targeting ligands to enhance accumulation of liposomes into the brain. J Control Release. 2011;150(1):30–36. doi: 10.1016/j.jconrel.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 61.Kwon IK, Lee SC, Han B, Park K. Analysis on the current status of targeted drug delivery to tumors. J Control Release. 2012 doi: 10.1016/j. jconrel.2012.07.010. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi J, Xiao Z, Kamaly N, Farokhzad OC. Self-assembled targeted nanoparticles: evolution of technologies and bench to bedside translation. Acc Chem Res. 2011;44(10):1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- 63.Kirpotin D, Park JW, Hong K, et al. Sterically stabilized anti-HER2 immunoliposomes: design and targeting to human breast cancer cells in vitro. Biochemistry. 1997;36(1):66–75. doi: 10.1021/bi962148u. [DOI] [PubMed] [Google Scholar]
- 64.Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 65.Srinivas R, Samanta S, Chaudhuri A. Cationic amphiphiles: promising carriers of genetic materials in gene therapy. Chem Soc Rev. 2009;38(12):3326–3338. doi: 10.1039/b813869a. [DOI] [PubMed] [Google Scholar]
- 66.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 67.Puri A, Loomis K, Smith B, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26(6):523–580. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramishetti S, Arup G, Gopikrishna M, Sachin BA, Chaudhuri A. A long-lasting dendritic cell DNA vaccination system using lysinylated amphiphiles with mannose-mimicking head-groups. Biomaterials. 2012;33(26):6220–6229. doi: 10.1016/j.biomaterials.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Vasievich EA, Ramishetti S, Zhang Y, Huang L. Trp2 peptide vaccine adjuvanted with (R)-DOTAP inhibits tumor growth in an advanced melanoma model. Mol Pharm. 2012;9(2):261–268. doi: 10.1021/mp200350n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M. Development of an ultrasound-responsive and mannose-modified gene carrier for DNA vaccine therapy. Biomaterials. 2010;31(30):7813–7826. doi: 10.1016/j.biomaterials.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 71.Stover TC, Sharma A, Robertson GP, Kester M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res. 2005;11(9):3465–3474. doi: 10.1158/1078-0432.CCR-04-1770. [DOI] [PubMed] [Google Scholar]
- 72.Goncalves A, Braud AC, Viret F, et al. Phase I study of pegylated liposomal doxorubicin (Caelyx) in combination with carboplatin in patients with advanced solid tumors. Anticancer Res. 2003;23(4):3543–3548. [PubMed] [Google Scholar]
- 73.Schwonzen M, Kurbacher CM, Mallmann P. Liposomal doxorubicin and weekly paclitaxel in the treatment of metastatic breast cancer. Anticancer Drugs. 2000;11(9):681–685. doi: 10.1097/00001813-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 74.Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci USA. 2004;101(30):10901–10906. doi: 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bulte JW, Zhang S, Van Gelderen P, et al. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: magnetic resonance tracking of cell migration and myelination. Proc Natl Acad Sci USA. 1999;96(26):15256–15261. doi: 10.1073/pnas.96.26.15256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilhelm C, Fortin JP, Gazeau F. Tumour cell toxicity of intracellular hyperthermia mediated by magnetic nanoparticles. J Nanosci Nanotechnol. 2007;7(8):2933–2937. doi: 10.1166/jnn.2007.668. [DOI] [PubMed] [Google Scholar]
- 77.Lin BL, Shen XD, Cui S. Application of nanosized Fe3O4 in anticancer drug carriers with target-orientation and sustained-release properties. Biomed Mater. 2007;2(2):132–134. doi: 10.1088/1748-6041/2/2/011. [DOI] [PubMed] [Google Scholar]
- 78.Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60(11):1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhattarai SR, Kim SY, Jang KY, et al. N-hexanoyl chitosan-stabilized magnetic nanoparticles: enhancement of adenoviral-mediated gene expression both in vitro and in vivo. Nanomedicine. 2008;4(2):146–154. doi: 10.1016/j.nano.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 80.Plank C, Schillinger U, Scherer F, et al. The magnetofection method: using magnetic force to enhance gene delivery. Biol Chem. 2003;384(5):737–747. doi: 10.1515/BC.2003.082. [DOI] [PubMed] [Google Scholar]
- 81.De Cuyper M, Joniau M. Magnetoliposomes. Formation and structural characterization. Eur Biophys J. 1988;15(5):311–319. doi: 10.1007/BF00256482. [DOI] [PubMed] [Google Scholar]
- 82.Cesur H, Rubinstein I, Pai A, Onyuksel H. Self-associated indisulam in phospholipid-based nanomicelles: a potential nanomedicine for cancer. Nanomedicine. 2009;5(2):178–183. doi: 10.1016/j.nano.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sabate R, Barnadas-Rodriguez R, Callejas- Fernandez J, Hidalgo-Alvarez R, Estelrich J. Preparation and characterization of extruded magnetoliposomes. Int J Cancer. 2008;347(1–2):156–162. doi: 10.1016/j.ijpharm.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 84.Martina MS, Fortin JP, Menager C, et al. Generation of superparamagnetic liposomes revealed as highly efficient MRI contrast agents for in vivo imaging. J Am Chem Soc. 2005;127(30):10676–10685. doi: 10.1021/ja0516460. [DOI] [PubMed] [Google Scholar]
- 85.Janib SM, Moses AS, Mackay JA. Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliv Rev. 2010;62(11):1052–1063. doi: 10.1016/j.addr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y, Kohler N, Zhang M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23(7):1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 87.Tai LA, Tsai PJ, Wang YC, Wang YJ, Lo LW, Yang CS. Thermosensitive liposomes entrapping iron oxide nanoparticles for controllable drug release. Nanotechnology. 2009;20(13):135101. doi: 10.1088/0957-4484/20/13/135101. [DOI] [PubMed] [Google Scholar]
- 88.Chen Y, Bose A, Bothun GD. Controlled release from bilayer-decorated magnetoliposomes via electromagnetic heating. ACS nano. 2010;4(6):3215–3221. doi: 10.1021/nn100274v. [DOI] [PubMed] [Google Scholar]
- 89.Pradhan P, Giri J, Rieken F, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;142(1):108–121. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Yang X, Grailer JJ, Rowland IJ, et al. Multifunctional SPIO/DOX-loaded wormlike polymer vesicles for cancer therapy and MR imaging. Biomaterials. 2010;31(34):9065–9073. doi: 10.1016/j.biomaterials.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 91.Wang H, Wang S, Liao Z, et al. Folate-targeting magnetic core-shell nanocarriers for selective drug release and imaging. Int J Cancer. 2012;430(1–2):342–349. doi: 10.1016/j.ijpharm.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 92.Fattahi H, Laurent S, Liu F, Arsalani N, Vander Elst L, Muller RN. Magnetoliposomes as multimodal contrast agents for molecular imaging and cancer nanotheragnostics. Nanomedicine (Lond ) 2011;6(3):529–544. doi: 10.2217/nnm.11.14. [DOI] [PubMed] [Google Scholar]
- 93.Felgner PL, Tsai YJ, Sukhu L, et al. Improved cationic lipid formulations for in vivo gene therapy. Ann N Y Acad Sci. 1995;772:126–139. doi: 10.1111/j.1749-6632.1995.tb44738.x. [DOI] [PubMed] [Google Scholar]
- 94.Gustafsson J, Arvidson G, Karlsson G, Almgren M. Complexes between cationic liposomes and DNA visualized by cryo-TEM. Biochim Biophys Acta. 1995;1235(2):305–312. doi: 10.1016/0005-2736(95)80018-b. [DOI] [PubMed] [Google Scholar]
- 95.Li S, Rizzo MA, Bhattacharya S, Huang L. Characterization of cationic lipid–protamine– DNA (LPD) complexes for intravenous gene delivery. Gene Ther. 1998;5(7):930–937. doi: 10.1038/sj.gt.3300683. [DOI] [PubMed] [Google Scholar]
- 96.Li S, Huang L. In vivo gene transfer via intravenous administration of cationic lipid–protamine–DNA (LPD) complexes. Gene Ther. 1997;4(9):891–900. doi: 10.1038/sj.gt.3300482. [DOI] [PubMed] [Google Scholar]
- 97.Li B, Li S, Tan Y, et al. Lyophilization of cationic lipid–protamine–DNA (LPD) complexes. J Pharm Sci. 2000;89(3):355–364. doi: 10.1002/(SICI)1520-6017(200003)89:3<355::AID-JPS7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 98.Gao X, Huang L. Potentiation of cationic liposome-mediated gene delivery by polycations. Biochemistry. 1996;35(3):1027–1036. doi: 10.1021/bi952436a. [DOI] [PubMed] [Google Scholar]
- 99.Li SD, Huang L. Surface-modified LPD nanoparticles for tumor targeting. Ann N Y Acad Sci. 2006;1082:1–8. doi: 10.1196/annals.1348.001. [DOI] [PubMed] [Google Scholar]
- 100.Li SD, Huang L. Nanoparticles evading the reticuloendothelial system: role of the supported bilayer. Biochim Biophys Acta. 2009;1788(10):2259–2266. doi: 10.1016/j.bbamem.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li SD, Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145(3):178–181. doi: 10.1016/j.jconrel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Megalizzi V, Le Mercier M, Decaestecker C. Sigma receptors and their ligands in cancer biology: overview and new perspectives for cancer therapy. Med Res Rev. 2012;32(2):410–427. doi: 10.1002/med.20218. [DOI] [PubMed] [Google Scholar]
- 103.Banerjee R, Tyagi P, Li S, Huang L. Anisamide-targeted stealth liposomes: a potent carrier for targeting doxorubicin to human prostate cancer cells. Int J Cancer. 2004;112(4):693–700. doi: 10.1002/ijc.20452. [DOI] [PubMed] [Google Scholar]
- 104.John CS, Vilner BJ, Geyer BC, Moody T, Bowen WD. Targeting sigma receptor-binding benzamides as in vivo diagnostic and therapeutic agents for human prostate tumors. Cancer Res. 1999;59(18):4578–4583. [PubMed] [Google Scholar]
- 105.John CS, Bowen WD, Fisher SJ, et al. Synthesis, in vitro pharmacologic characterization, and preclinical evaluation of N-[2-(1′-piperidinyl)ethyl]-3-[125I]iodo-4-methoxybenzamide (P[125I]MBA) for imaging breast cancer. Nucl Med Biol. 1999;26(4):377–382. doi: 10.1016/s0969-8051(98)00104-8. [DOI] [PubMed] [Google Scholar]
- 106.Li SD, Chono S, Huang L. Efficient gene silencing in metastatic tumor by siRNA formulated in surface-modified nanoparticles. J Control Release. 2008;126(1):77–84. doi: 10.1016/j.jconrel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li SD, Chen YC, Hackett MJ, Huang L. Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol Ther. 2008;16(1):163–169. doi: 10.1038/sj.mt.6300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Y, Bathula SR, Yang Q, Huang L. Targeted nanoparticles deliver siRNA to melanoma. J Invest Dermatol. 2010;130(12):2790–2798. doi: 10.1038/jid.2010.222. [DOI] [PubMed] [Google Scholar]
- 109.Chen Y, Bathula SR, Li J, Huang L. Multifunctional nanoparticles delivering small interfering RNA and doxorubicin overcome drug resistance in cancer. J Biol Chem. 2010;285(29):22639–22650. doi: 10.1074/jbc.M110.125906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen Y, Zhu X, Zhang X, Liu B, Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol Ther. 2010;18(9):1650–1656. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johnson FM, Saigal B, Tran H, Donato NJ. Abrogation of signal transducer and activator of transcription 3 reactivation after Src kinase inhibition results in synergistic antitumor effects. Clin Cancer Res. 2007;13(14):4233–4244. doi: 10.1158/1078-0432.CCR-06-2981. [DOI] [PubMed] [Google Scholar]
- 112.Kloth MT, Laughlin KK, Biscardi JS, Boerner JL, Parsons SJ, Silva CM. STAT5b, a mediator of synergism between c-Src and the epidermal growth factor receptor. J Biol Chem. 2003;278(3):1671–1679. doi: 10.1074/jbc.M207289200. [DOI] [PubMed] [Google Scholar]
- 113.Shelburne CP, Mccoy ME, Piekorz R, et al. Stat5 expression is critical for mast cell development and survival. Blood. 2003;102(4):1290–1297. doi: 10.1182/blood-2002-11-3490. [DOI] [PubMed] [Google Scholar]
- 114.Kim SK, Huang L. Nanoparticle delivery of a peptide targeting EGFR signaling. J Control Release. 2012;157(2):279–286. doi: 10.1016/j.jconrel.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hatakeyama H, Akita H, Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv Drug Deliv Rev. 2011;63(3):152–160. doi: 10.1016/j.addr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 116.Khalil IA, Kogure K, Futaki S, Harashima H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J Biol Chem. 2006;281(6):3544–3551. doi: 10.1074/jbc.M503202200. [DOI] [PubMed] [Google Scholar]
- 117.El-Sayed A, Khalil IA, Kogure K, Futaki S, Harashima H. Octaarginine- and octalysinemodified nanoparticles have different modes of endosomal escape. J Biol Chem. 2008;283(34):23450–23461. doi: 10.1074/jbc.M709387200. [DOI] [PubMed] [Google Scholar]
- 118.Nakamura T, Moriguchi R, Kogure K, Shastri N, Harashima H. Efficient MHC class I presentation by controlled intracellular trafficking of antigens in octaarginine-modified liposomes. Mol Ther. 2008;16(8):1507–1514. doi: 10.1038/mt.2008.122. [DOI] [PubMed] [Google Scholar]
- 119.Joraku A, Homhuan A, Kawai K, et al. Immunoprotection against murine bladder carcinoma by octaarginine-modified liposomes incorporating cell wall of Mycobacterium bovis bacillus Calmette-Guerin. BJU Int. 2009;103(5):686–693. doi: 10.1111/j.1464-410X.2008.08235.x. [DOI] [PubMed] [Google Scholar]
- 120.Li W, Nicol F, Szoka FC., Jr GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv Drug Deliv Rev. 2004;56(7):967–985. doi: 10.1016/j.addr.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 121.Hatakeyama H, Ito E, Akita H, et al. A pH-sensitive fusogenic peptide facilitates endosomal escape and greatly enhances the gene silencing of siRNA-containing nanoparticles in vitro and in vivo. J Control Release. 2009;139(2):127–132. doi: 10.1016/j.jconrel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 122.Sakurai Y, Hatakeyama H, Akita H, et al. Efficient short interference RNA delivery to tumor cells using a combination of octaarginine, GALA and tumor-specific, cleavable polyethylene glycol system. Biol Pharm Bull. 2009;32(5):928–932. doi: 10.1248/bpb.32.928. [DOI] [PubMed] [Google Scholar]
- 123.Kakudo T, Chaki S, Futaki S, et al. Transferrin-modified liposomes equipped with a pH-sensitive fusogenic peptide: an artificial viral-like delivery system. Biochemistry. 2004;43(19):5618–5628. doi: 10.1021/bi035802w. [DOI] [PubMed] [Google Scholar]
- 124.Subbarao NK, Parente RA, Szoka FC, Jr, Nadasdi L, Pongracz K. pH-dependent bilayer destabilization by an amphipathic peptide. Biochemistry. 1987;26(11):2964–2972. doi: 10.1021/bi00385a002. [DOI] [PubMed] [Google Scholar]
- 125.Sakurai Y, Hatakeyama H, Sato Y, et al. Endosomal escape and the knockdown efficiency of liposomal-siRNA by the fusogenic peptide shGALA. Biomaterials. 2011;32(24):5733–5742. doi: 10.1016/j.biomaterials.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 126.Trewyn BG, Giri S, Slowing Ii, Lin VS. Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems. Chem Commun (Camb) 2007;(31):3236–3245. doi: 10.1039/b701744h. [DOI] [PubMed] [Google Scholar]
- 127.Slowing II, Trewyn BG, Giri S, Lin VS. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv Funct Mater. 2007;17(8):1225–1236. [Google Scholar]
- 128.Rosi NL, Mirkin CA. Nanostructures in biodiagnostics. Chem Rev. 2005;105(4):1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 129.Jiang XM, Brinker CJ. Aerosol-assisted self-assembly of single-crystal core/nanoporous shell particles as model controlled release capsules. J Am Chem Soc. 2006;128(14):4512–4513. doi: 10.1021/ja058260+. [DOI] [PubMed] [Google Scholar]
- 130.Cauda V, Engelke H, Sauer A, et al. Colchicine-loaded lipid bilayer-coated 50 nm mesoporous nanoparticles efficiently induce microtubule depolymerization upon cell uptake. Nano Letts. 2010;10(7):2484–2492. doi: 10.1021/nl100991w. [DOI] [PubMed] [Google Scholar]
- 131.Liu JW, Stace-Naughton A, Jiang XM, Brinker CJ. Porous nanoparticle supported lipid bilayers (protocells) as delivery vehicles. J Am Chem Soc. 2009;131(4):1354–1355. doi: 10.1021/ja808018y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mal NK, Fujiwara M, Tanaka Y. Photocontrolled reversible release of guest molecules from coumarin-modified mesoporous silica. Nature. 2003;421(6921):350–353. doi: 10.1038/nature01362. [DOI] [PubMed] [Google Scholar]
- 133.Angelos S, Choi E, Vogtle F, De Cola L, Zink JI. Photo-driven expulsion of molecules from mesostructured silica nanoparticles. J Phys Chem C. 2007;111(18):6589–6592. [Google Scholar]
- 134.Lai CY, Trewyn BG, Jeftinija DM, et al. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J Am Chem Soc. 2003;125(15):4451–4459. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- 135.Bally M, Bailey K, Sugihara K, Grieshaber D, Voros J, Stadler B. Liposome and lipid bilayer arrays towards biosensing applications. Small. 2010;6(22):2481–2497. doi: 10.1002/smll.201000644. [DOI] [PubMed] [Google Scholar]
- 136.Ashley CE, Carnes EC, Phillips GK, et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat Mater. 2011;10(5):389–397. doi: 10.1038/nmat2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mornet S, Lambert O, Duguet E, Brisson A. The formation of supported lipid bilayers on silica nanoparticles revealed by cryoelectron microscopy. Nano Letts. 2005;5(2):281–285. doi: 10.1021/nl048153y. [DOI] [PubMed] [Google Scholar]
- 138.Liu J, Jiang X, Ashley C, Brinker CJ. Electrostatically mediated liposome fusion and lipid exchange with a nanoparticle-supported bilayer for control of surface charge, drug containment, and delivery. J Am Chem Soc. 2009;131(22):7567–7569. doi: 10.1021/ja902039y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Graham FL, Van Der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 140.Kataoka Y, Ka K. Block Copolymer self-assembly into monodispersive nanoparticles with hybrid core of antisense DNA and calcium phosphate. Langmuir. 2002;18(12):4539–4543. [Google Scholar]
- 141.Maitra A. Calcium phosphate nanoparticles: second-generation nonviral vectors in gene therapy. Expert Rev Mol Diagn. 2005;5(6):893–905. doi: 10.1586/14737159.5.6.893. [DOI] [PubMed] [Google Scholar]
- 142.Olton D, Li J, Wilson ME, et al. Nanostructured calcium phosphates (NanoCaPs) for non-viral gene delivery: influence of the synthesis parameters on transfection efficiency. Biomaterials. 2007;28(6):1267–1279. doi: 10.1016/j.biomaterials.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 143.Kakizawa Y, Furukawa S, Ishii A, Kataoka K. Organic-inorganic hybrid-nanocarrier of siRNA constructing through the self-assembly of calcium phosphate and PEG-based block aniomer. J Control Release. 2006;111(3):368–370. doi: 10.1016/j.jconrel.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 144.Tabakovic A, Kester M, Adair JH. Calcium phosphate-based composite nanoparticles in bioimaging and therapeutic delivery applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4(1):96–112. doi: 10.1002/wnan.163. [DOI] [PubMed] [Google Scholar]
- 145.Li J, Chen YC, Tseng YC, Mozumdar S, Huang L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J Control Release. 2010;142(3):416–421. doi: 10.1016/j.jconrel.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Morgan TT, Muddana HS, Altinoglu EI, et al. Encapsulation of organic molecules in calcium phosphate nanocomposite particles for intracellular imaging and drug delivery. Nano Letts. 2008;8(12):4108–4115. doi: 10.1021/nl8019888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorg Med Chem. 2009;17(8):2950–2962. doi: 10.1016/j.bmc.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 148.Li J, Yang Y, Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J Control Release. 2012;158(1):108–114. doi: 10.1016/j.jconrel.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang Y, Hu Y, Wang Y, Li J, Liu F, Huang L. Nanoparticle delivery of pooled siRNA for effective treatment of non-small cell lung cancer. Mol Pharm. 2012 doi: 10.1021/mp300152v. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang L, Liu Y. In vivo delivery of RNAi with lipid-based nanoparticles. Annu Rev Biomed Eng. 2011;13:507–530. doi: 10.1146/annurev-bioeng-071910-124709. [DOI] [PubMed] [Google Scholar]
- 151.Yang Y, Li J, Liu F, Huang L. Systemic delivery of siRNA via LCP nanoparticle efficiently inhibits lung metastasis. Mol Ther. 2012;20(3):609–615. doi: 10.1038/mt.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huynh NT, Passirani C, Saulnier P, Benoit JP. Lipid nanocapsules: a new platform for nanomedicine. Int J Cancer. 2009;379(2):201–209. doi: 10.1016/j.ijpharm.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 153.Coon JS, Knudson W, Clodfelter K, Lu B, Weinstein RS. Solutol HS 15, nontoxic polyoxyethylene esters of 12-hydroxystearic acid, reverses multidrug resistance. Cancer Res. 1991;51(3):897–902. [PubMed] [Google Scholar]
- 154.Lamprecht A, Benoit JP. Etoposide nanocarriers suppress glioma cell growth by intracellular drug delivery and simultaneous P-glycoprotein inhibition. J Control Release. 2006;112(2):208–213. doi: 10.1016/j.jconrel.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 155.Garcion E, Lamprecht A, Heurtault B, et al. A new generation of anticancer, drug-loaded, colloidal vectors reverses multidrug resistance in glioma and reduces tumor progression in rats. Mol Cancer Ther. 2006;5(7):1710–1722. doi: 10.1158/1535-7163.MCT-06-0289. [DOI] [PubMed] [Google Scholar]
- 156.Morille M, Passirani C, Dufort S, et al. Tumor transfection after systemic injection of DNA lipid nanocapsules. Biomaterials. 2011;32(9):2327–2333. doi: 10.1016/j.biomaterials.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 157.Vonarbourg A, Passirani C, Desigaux L, et al. The encapsulation of DNA molecules within biomimetic lipid nanocapsules. Biomaterials. 2009;30(18):3197–3204. doi: 10.1016/j.biomaterials.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 158.Bhaumik S. Advances in imaging gene-directed enzyme prodrug therapy. Curr Pharm Biotechnol. 2011;12(4):497–507. doi: 10.2174/138920111795163896. [DOI] [PubMed] [Google Scholar]
- 159.David S, Carmoy N, Resnier P, et al. In vivo imaging of DNA lipid nanocapsules after systemic administration in a melanoma mouse model. Int J Cancer. 2012;423(1):108–115. doi: 10.1016/j.ijpharm.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 160.Trickler WJ, Munt DJ, Jain N, Joshi SS, Dash AK. Antitumor efficacy, tumor distribution and blood pharmacokinetics of chitosan/glyceryl-monooleate nanostructures containing paclitaxel. Nanomedicine (Lond ) 2011;6(3):437–448. doi: 10.2217/nnm.10.135. [DOI] [PubMed] [Google Scholar]
- 161.Hasan W, Chu K, Gullapalli A, et al. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Letts. 2012;12(1):287–292. doi: 10.1021/nl2035354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li W, Yang Y, Wang C, et al. Carrier-free, functionalized drug nanoparticles for targeted drug delivery. Chem Commun (Camb ) 2012;48(65):8120–8122. doi: 10.1039/c2cc33214k. [DOI] [PubMed] [Google Scholar]
- 163.Khandare J, Calderon M, Dagia NM, Haag R. Multifunctional dendritic polymers in nanomedicine: opportunities and challenges. Chem Soc Rev. 2012;41(7):2824–2848. doi: 10.1039/c1cs15242d. [DOI] [PubMed] [Google Scholar]
- 164.Park JW, Hong K, Kirpotin DB, et al. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8(4):1172–1181. [PubMed] [Google Scholar]
- 165.Sun B, Ranganathan B, Feng SS. Multifunctional poly(D, L-lactide-co-glycolide)/montmorillonite (PLGA/MMT) nanoparticles decorated by Trastuzumab for targeted chemotherapy of breast cancer. Biomaterials. 2008;29(4):475–486. doi: 10.1016/j.biomaterials.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 166.Gao J, Zhong W, He J, et al. Tumor-targeted PE38KDEL delivery via PEGylated anti-HER2 immunoliposomes. Int J Cancer. 2009;374(1–2):145–152. doi: 10.1016/j.ijpharm.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 167.Farokhzad OC, Jon S, Khademhosseini A, Tran TN, Lavan DA, Langer R. Nanoparticle-aptamer bioconjugates: a new approach for targeting prostate cancer cells. Cancer Res. 2004;64(21):7668–7672. doi: 10.1158/0008-5472.CAN-04-2550. [DOI] [PubMed] [Google Scholar]
- 168.Farokhzad OC, Cheng J, Teply BA, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci USA. 2006;103(16):6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sahoo SK, Labhasetwar V. Enhanced antiproliferative activity of transferrin-conjugated paclitaxel-loaded nanoparticles is mediated via sustained intracellular drug retention. Mol Pharm. 2005;2(5):373–383. doi: 10.1021/mp050032z. [DOI] [PubMed] [Google Scholar]
- 170.Li JL, Wang L, Liu XY, et al. In vitro cancer cell imaging and therapy using transferrin-conjugated gold nanoparticles. Cancer Lett. 2009;274(2):319–326. doi: 10.1016/j.canlet.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 171.Thomas TP, Shukla R, Kotlyar A, et al. Dendrimer-epidermal growth factor conjugate displays superagonist activity. Biomacromolecules. 2008;9(2):603–609. doi: 10.1021/bm701185p. [DOI] [PubMed] [Google Scholar]
- 172.Karmali PP, Kotamraju VR, Kastantin M, et al. Targeting of albumin-embedded paclitaxel nanoparticles to tumors. Nanomedicine. 2009;5(1):73–82. doi: 10.1016/j.nano.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sugahara KN, Teesalu T, Karmali PP, et al. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328(5981):1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Oh S, Kim BJ, Singh NP, Lai H, Sasaki T. Synthesis and anti-cancer activity of covalent conjugates of artemisinin and a transferrin-receptor targeting peptide. Cancer Lett. 2009;274(1):33–39. doi: 10.1016/j.canlet.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 175.Lo A, Lin CT, Wu HC. Hepatocellular carcinoma cell-specific peptide ligand for targeted drug delivery. Mol Cancer Ther. 2008;7(3):579–589. doi: 10.1158/1535-7163.MCT-07-2359. [DOI] [PubMed] [Google Scholar]
- 176.Wang S, Low PS. Folate-mediated targeting of antineoplastic drugs, imaging agents, and nucleic acids to cancer cells. J Control Release. 1998;53(1–3):39–48. doi: 10.1016/s0168-3659(97)00236-8. [DOI] [PubMed] [Google Scholar]
- 177.Zeng C, Vangveravong S, Xu J, et al. Subcellular localization of sigma-2 receptors in breast cancer cells using two-photon and confocal microscopy. Cancer Res. 2007;67(14):6708–6716. doi: 10.1158/0008-5472.CAN-06-3803. [DOI] [PubMed] [Google Scholar]
- 178.Hornick JR, Xu J, Vangveravong S, et al. The novel sigma-2 receptor ligand SW43 stabilizes pancreas cancer progression in combination with gemcitabine. Mol Cancer. 2010;9:298. doi: 10.1186/1476-4598-9-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang B, Rouzier R, Albarracin CT, et al. Expression of sigma 1 receptor in human breast cancer. Breast Cancer Res Treat. 2004;87(3):205–214. doi: 10.1007/s10549-004-6590-0. [DOI] [PubMed] [Google Scholar]
- 180.Kim TH, Park IK, Nah JW, Choi YJ, Cho CS. Galactosylated chitosan/DNA nanoparticles prepared using water-soluble chitosan as a gene carrier. Biomaterials. 2004;25(17):3783–3792. doi: 10.1016/j.biomaterials.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 181.Ashley CE, Carnes EC, Epler KE, et al. Delivery of small interfering RNA by peptide-targeted mesoporous silica nanoparticle-supported lipid bilayers. ACS Nano. 2012;6(3):2174–2188. doi: 10.1021/nn204102q. [DOI] [PMC free article] [PubMed] [Google Scholar]