Systematic analysis of rat 12/15-lipoxygenase enzymes reveals critical role for spinal eLOX3 hepoxilin synthase activity in inflammatory hyperalgesia (original) (raw)
Abstract
Previously, we observed significant increases in spinal 12-lipoxygenase (LOX) metabolites, in particular, hepoxilins, which contribute to peripheral inflammation-induced tactile allodynia. However, the enzymatic sources of hepoxilin synthase (HXS) activity in rats remain elusive. Therefore, we overexpressed each of the 6 rat 12/15-LOX enzymes in HEK-293T cells and measured by LC-MS/MS the formation of HXB3, 12-HETE, 8-HETE, and 15-HETE from arachidonic acid (AA) at baseline and in the presence of LOX inhibitors (NDGA, AA-861, CDC, baicalein, and PD146176) vs. vehicle-treated and mock-transfected controls. We detected the following primary intrinsic activities: 12-LOX (Alox12, Alox15), 15-LOX (Alox15b), and HXS (Alox12, Alox15). Similar to human and mouse orthologs, proteins encoded by rat Alox12b and Alox12e possessed minimal 12-LOX activity with AA as substrate, while eLOX3 (encoded by Aloxe3) exhibited HXS without 12-LOX activity when coexpressed with Alox12b or supplemented with 12-HpETE. CDC potently inhibited HXS and 12-LOX activity in vitro (relative IC50s: CDC, ∼0.5 and 0.8 μM, respectively) and carrageenan-evoked tactile allodynia in vivo. Notably, peripheral inflammation significantly increased spinal eLOX3; intrathecal pretreatment with either siRNA targeting Aloxe3 or an eLOX3-selective antibody attenuated the associated allodynia. These findings implicate spinal eLOX3-mediated hepoxilin synthesis in inflammatory hyperesthesia and underscore the importance of developing more selective 12-LOX/HXS inhibitors.—Gregus, A. M., Dumlao, D. S., Wei, S. C., Norris, P. C., Catella, L. C., Meyerstein, F. G., Buczynski, M. W., Steinauer, J. J., Fitzsimmons, B. L., Yaksh, T. L., Dennis, E. A. Systematic analysis of rat 12/15-lipoxygenase enzymes reveals critical role for spinal eLOX3 hepoxilin synthase activity in inflammatory hyperalgesia.
Keywords: pain, eicosanoid, Aloxe3
Lipoxygenases (LOXs) are nonheme iron-containing metalloproteins that catalyze insertion of O2 stereospecifically in polyunsaturated fatty acids (PUFAs). Historically, LOX enzymes were classified according to their activity of stereoselective oxygenation to the corresponding hydroperoxy derivative; e.g., 5-LOX, 12-LOX, and 15-LOX (1–3). More recently, it has been appreciated that this principle applies to 5-LOX but not necessarily to the 12/15-LOX family, which also includes isozymes that exhibit hepoxilin synthase (HXS) and/or 15-LOX activities (Fig. 1), as well as 8-LOX activity in mice (1, 3). In addition to Alox5 (which encodes 5-LOX), 6 different rat genes with putative 12-LOX, 15-LOX, or HXS activities have been identified to date and are named according to the cells in which they were initially discovered (Table 1): platelet-type (Alox12), epidermal-type (Alox12b, Alox12e, Aloxe3), and leukocyte-type (Alox15, Alox15b) (1). In contrast, mice possess Alox8 in lieu of Alox15b, while the human ortholog of Alox12e (ALOX12P2) encodes a pseudogene. Notably, their specific activities appear to depend on species, as well as substrate, and have been examined only partially in rats (1, 3).
Figure 1.
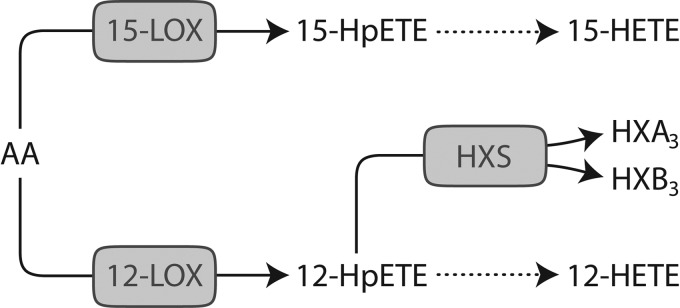
Putative activities of the rat 12/15-LOX enzyme family using arachidonic acid (AA) as substrate. AA can be metabolized by each of 6 rat 12-LOX family enzymes (see Table 1), which exhibit one or more of the following activities depicted above: 15-LOX, 12-LOX, or HXS.
Table 1.
Activities of overexpressed rat 12/15-LOX enzymes
While 12/15-LOX expression has been reported in brain, spinal cord, and cervical ganglia (4–6), these accounts precede the discovery of all 6 cloned isozymes (1). As a result, the relative functional contributions of this family of enzymes and their metabolites in central (spinal) nociceptive processing have remained largely undefined. Several groups have reported increased 12-LOX activity in inflammatory hyperalgesia through the use of commercially available inhibitors or direct administration of metabolites (7–10). Recently, we described a critical role for spinal 12-LOX enzymes in the development of mechanical hypersensitivity (tactile allodynia) following peripheral inflammation evoked by intraplantar (IPLT) injection of carrageenan (11). We found that IPLT carrageenan significantly increases levels of hepoxilin B3 (HXB3) in rat lumbar spinal cord and that either systemic or intrathecal (IT) pretreatment with the general LOX inhibitor nordihydroguaiaretic acid (NDGA) attenuates both the increase in spinal HXB3, as well as the associated hyperesthesia (11–12). Similarly, IT pretreatment with inhibitors of 12-LOX [cinnamyl-3,4-dihydroxy-α-cyanocinnamate (CDC) and baicalein] also reduced carrageenan-induced tactile allodynia, while there was no significant effect of spinal administration of the 5-LOX active site inhibitor zileuton, consistent with the observation that spinal 5-LOX metabolites are either not present or are below the limit of detection (11). Given that the selectivity of these 12-LOX inhibitors is unknown, we demonstrated that spinal delivery of 12-LOX metabolites of arachidonic acid (AA), including hepoxilins and their parent molecule 12-HpETE, directly produces persistent tactile allodynia via activation of transient receptor potential ankyrin 1 (TRPA1) and transient receptor potential vanilloid 1 (TRPV1) receptors on primary afferent central terminals (11). These novel results point to the unexpected role of the 12-LOX enzymes and their metabolites in neuraxial pain processing.
However, several important issues are unresolved. First, it remains unknown which of the 6 rat 12/15-LOX isoenzymes are responsible for production of hepoxilins that contribute to spinal facilitated states. Second, the isozyme selectivity (if any) of the existing inhibitors is undefined. Finally, the selectivity of available antibodies targeting specific 12/15-LOX isozymes has not been established. Accordingly, the development of heterologous overexpression systems of these enzymes would be of great value in the verification of their activity, as well as positive controls for validation of selective primers and antisera in the subsequent characterization of their expression and distribution in tissue at the spinal level. Such information would allow for targeted disruption of those enzymes or the downstream targets of metabolites to reduce hyperalgesia with greater selectivity. Thus, we overexpressed each of the rat 12/15-LOX enzymes in HEK-293T cells and proceeded to characterize their activities, responsiveness to inhibitors, and expression in spinal cord by quantitative polymerase chain reaction (qPCR) and immunoblot. Our findings suggest that agents targeting the primary sources of HXS and 12-LOX activity exhibit potent antihyperalgesic effects in vivo and that spinal epidermal lipoxygenase 3 (eLOX3) is a crucial source of HXS activity mediating tactile allodynia following peripheral inflammation.
MATERIALS AND METHODS
Animals
Male Holtzman Sprague-Dawley rats (300–350 g; Harlan, Indianapolis, IN, USA) were maintained on a 12-h light-dark cycle with free access to food and water. All experiments were conducted according to protocols approved by the Institutional Animal Care Committee of University of California–San Diego.
Expression plasmids
Complementary DNAs (cDNAs) encoding each of the 6 rat 12-LOX enzymes [_Alox12_, source accession no. BC168184.1 (complete cds); Alox12b, NM_001039377.1; Alox12e, sNM_001107014.1; Aloxe3, NM_001105793.1, Alox15, NM_031010.2, Alox15b, NM_153301.2] were cloned and inserted into pcDNA3.1(+) vector (Invitrogen, Carlsbad, CA, USA) by Genscript (Piscataway, NJ, USA). Quality control-verified constructs were reconfirmed after amplification and purification by restriction digest followed by agarose gel electrophoresis. Monster Green Fluorescent Protein (phMGFP) vector was purchased from Promega (Madison, WI, USA).
Drugs
The nonselective LOX inhibitor NDGA, as well as inhibitors of 5-LOX (AA-861), platelet-type 12-LOX (baicalein and CDC) and leukocyte-type 12/15-LOX (PD-146176) were purchased as follows: baicalein and PD-146176 from Cayman Chemical (Ann Arbor, MI, USA), AA-861 from Sigma (St. Louis, MO, USA), and CDC from Enzo Life Sciences (Plymouth Meeting, PA, USA). AA, 12(±)-hydroperoxyeicosatetraenoic acid (HpETE) and 12(S)-HpETE were obtained from Cayman Chemical (Ann Arbor, MI, USA). For cell culture, LOX inhibitors were prepared fresh immediately before use by dissolving in DMSO, followed by serial dilution in serum-free DMEM minus phenol red to a final maximum DMSO concentration of 0.1%. AA and 12(±)-HpETE were rapidly prepared from stocks stored at −80°C under argon by dilution in serum-free DMEM without phenol red (Invitrogen). For in vivo studies, LOX inhibitors AA-861 and PD-146176 were prepared in 3% DMSO/3% Cremaphor-EL in saline to obtain doses of 1–10 μg/10 μl for IT delivery, followed by a 10-μl saline flush.
Cell culture and transfection
HEK-293T cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in high-glucose DMEM (Invitrogen) supplemented with 10% FBS, 2 mM glutamine, and 100 U/ml penicillin G sodium + 100 μg/ml streptomycin. At 1 d prior to transfection, cells were seeded at a density of 1 × 106 cells/well in DMEM without antibiotics on 6-well plates coated with γ-irradiated poly-d-lysine hydrobromide (Sigma). Cells were transfected using Lipofectamine 2000 reagent (Invitrogen) with one of each of the 6 recombinant pcDNA3.1/rat 12/15-LOX constructs (3 μg) plus the phMGFP vector at a 3:1 ratio or cotransfected with Aloxe3 at a 1:1 ratio (total 3 μg) or with phMGFP alone as a negative control. The presence of each 12/15-LOX mRNA and protein was measured by qPCR and immunoblot, respectively, with maximal expression of each enzyme protein observed at 48 h post-transfection, as described previously (13).
Treatments
Transfected HEK-293T cells were serum starved for 1 h, then pretreated with vehicle (0.1% DMSO) or LOX inhibitors (AA-861, CDC, baicalein, NDGA, or PD-146176; final concentrations of 0.1–10 μM) for 30 min prior to either supplementation with the ω-6 fatty acid substrate AA (70 μM). In separate studies, transfected cells were supplemented with hydroperoxy derivative 12(S)-HpETE (5 μM) or 12(±)-HpETE (10 μM) for 30 min. Untransfected cells in the presence or absence of lipid substrate served as negative controls. These treatment conditions were described previously as optimal for the quantification of 5-, 8-, 12-, or 15-hydroxyeicosatetraenoic acid (HETE) generated from heterologously expressed forms of human and mouse LOXs in HEK-293 cells (13–18). Medium was harvested, supplemented with deuterated internal standard, and then stored at −80°C prior to lipid extraction for liquid chromatography–tandem mass spectrometry (LC-MS/MS), as outlined below.
Real-time quantitative reverse transcriptase-PCR (qRT-PCR)
Lumbar spinal cord (n_=6 rats) was rapidly dissected under RNase-free conditions, flash-frozen, and then stored at −80°C prior to extraction. Total RNA was isolated using the RNeasy lipid tissue mini kit and RNase-free DNase kit (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions. The concentration and purity of RNA were assessed with a nanodrop spectrophotometer (Implen, Westlake Village, CA, USA). cDNA templates were synthesized for all cords in the same session from equal amounts of total RNA using the RT2 first-strand cDNA kit (SA Biosciences, Valencia, CA, USA). Spinal cord samples were prepared for real-time qPCR with RT2 SYBR Green/ROX kit as directed, using the following prevalidated rat primer sets: Alox12, Alox12b, Alox12e, Aloxe3, Alox15, Alox15b, and Actb (SA Biosciences). Samples were analyzed in triplicate by qPCR on an ABI Prism 7500 (Applied Biosystems, Carlsbad, CA, USA) using the following parameters: 95°C for 10 min, then (95°C for 15 s; 60°C for 60 s) for 40 cycles. The specificity of PCR products was verified further by the detection of a single peak at the predicted melting temperature (Tm) using a dissociation curve. The cycle threshold (CT) value for each amplified PCR product was calculated by the ABI Prism SDS software as the intensity of SYBR green fluorescence that exceeded 10 times the sd of the baseline fluorescence. The comparative CT method was used for relative quantification (19), in which the target CT for each sample was subtracted from that of β-actin and then normalized to untransfected HEK cells (for 12/15-LOX overexpression) or to HEK cells transfected with the corresponding 12/15-LOX isozyme (for spinal tissue) in order to obtain a ΔΔ_CT value. The 2−ΔΔ_CT_ values were calculated and averaged for each target.
Immunoblot
HEK-293T cells (control or 12/15-LOX transfected) were washed 2 times in cold phosphate-buffered saline (PBS) and then harvested in cold modified RIPA buffer (50 mM Tris-HCl, pH 7.4; 1% Nonidet P-40; 150 mM NaCl; and 1 mM EDTA) containing protease inhibitor cocktail and phosphatase inhibitor cocktails II and III (Sigma) supplemented with 2 mM PMSF. Cell lysates were sonicated on ice and then centrifuged at 14,000 g for 20 min at 4°C to obtain the supernatant. Rats (_n_=8–10/group) were euthanized under isoflurane anesthesia, and the spinal cords were removed by saline hydroextrusion. The L4/L5 segment was rapidly dissected, flash-frozen, and then lysed in RIPA buffer, as above. Following the determination of protein concentrations of homogenates using a bicinchoninic acid(BCA) assay (Thermo Scientific, San Diego, CA, USA), samples were diluted to equal concentrations in NuPAGE LDS sample buffer supplemented with DTT and then heat denatured. Equal amounts of total protein (10 or 30 μg) were subjected to gel electrophoresis using 4–12% Bis-Tris gradient gels (Invitrogen) and then transferred to nitrocellulose membranes, which were subsequently blocked in 5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h at room temperature. Blots were incubated in primary antibodies generated in mice for 12-LOX-p/Alox12 (pan 12-LOX antibody, 239-M01; Abnova Taiwan, Taipei, Taiwan), eLOX3/Aloxe3 (ab73091; Abcam, Cambridge, MA, USA), 12/15-LOX-l/Alox15 (246-M04; Abnova Taiwan) or 12/15-LOX-2/Alox15b (239-A01; Abnova Taiwan) at 1:1000 overnight at 4°C, followed by horseradish peroxidase (HRP)-conjugated anti-mouse secondary antibodies at 1:10,000 (Cell Signaling, Danvers, MA, USA) for 1 h at room temperature. Blots were reprobed for β-actin as a loading control using a mouse monoclonal antibody at 1:10000 (Sigma). Membranes were developed using chemiluminescent reagents SuperSignal West Pico or Femto (Thermo Scientific) and then exposed to X-ray film. Exposures yielding signal intensity in the linear range without saturation were used for densitometric analysis with ImageJ [U.S. National Institutes of Health (NIH), Bethesda, MD, USA].
LC-MS/MS
Sample preparation and LC-MS were conducted as described previously (12). Briefly, eicosanoids were separated by a 25-min reverse-phase LC gradient using solvent A (water/acetonitrile/acetic acid, 70:30:0.02; v/v/v) and solvent B (acetonitrile/isopropyl alcohol, 50:50; v/v). Eicosanoids were subsequently analyzed using a tandem quadrupole mass spectrometer (ABI 4000 Q-Trap; Applied Biosystems) via multiple-reaction monitoring (MRM) in negative-ion mode. Eicosanoids were identified in samples by matching their MRM signal and LC retention time with those of a pure standard. Adaptations to the LC-MS/MS method include using the scheduled MRM mode (Analyst 1.5 software; Applied Biosystems) to increase the total MRM transitions monitored to 174 (20). Multiquant 1.1 software (Applied Biosystems) was used in the quantitation of each metabolite.
In vivo procedures
IT catheterization
IT catheter implantation was performed in rats as described previously (21), with modifications (22). Animals exhibiting motor or postural deficits after surgery (<5%) were immediately euthanized.
Models of inflammatory hyperalgesia
After brief induction with isoflurane leading to a transient loss in the righting response, 100 μl of degraded λ-carrageenan (Wako Pure Chemicals Industries, Osaka, Japan) dissolved at 2% (w/v) in physiological saline was injected subcutaneously into the plantar surface (IPLT) of the left hind paw (_n_=6–8 rats/group). Local swelling was evaluated at 4 h postinjection by measurement of paw thickness using calipers. The development of tactile allodynia was assessed at defined intervals after 30 min IT pretreatment with vehicle (3% DMSO and 3% Cremaphor-EL in saline) or inhibitors of 5-LOX (AA-861) or 15-LOX (PD-146176), as we described previously for NDGA, CDC, baicalein, and zileuton (11). In separate sets of rats, we examined the effect of 2 h IT pretreatment with eLOX3 antibody (5 μg; validated in Fig. 3) vs. saline or mouse IgG isotype control antibody (5 μg); or the effect of IT pretreatment with siRNA targeting Aloxe3 vs. control nontargeting siRNA (2 μg, 1×/d for 3 d) on carrageenan-induced tactile allodynia. Prevalidated On-Target Plus siRNAs (Thermo Scientific) were mixed with i-Fect (Neuromics, Edina, MN, USA) and administered according to the manufacturer's instructions and as described previously (23).
Figure 3.
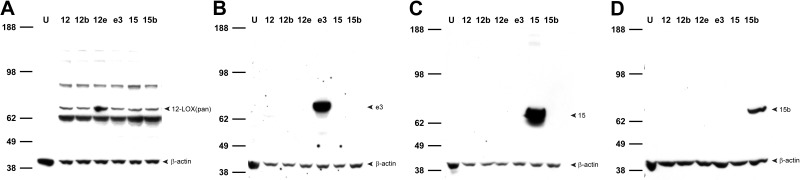
Experimental validation of rat 12/15-LOX antibodies. Antibodies detecting all six 12/15-LOX isozymes 12-LOX (pan; A) or those selectively targeting e3 (Aloxe3; B), 15 (Alox15; C), or 15b (Alox15b; D), as revealed by immunoblot of lysates from HEK-293T cells overexpressing each of the 6 rat 12/15-LOX enzymes. Samples were run with β-actin as a loading control in each lane as follows: U, untransfected control; 12, Alox12; 12b, Alox12b; 12e, Alox12e; e3, Aloxe3; 15, Alox15; 15b, Alox15b.
Assessment of tactile allodynia
Rats were acclimated to each apparatus for ≥30 min prior to testing. Measurements of tactile paw withdrawal thresholds were conducted by an observer blinded to the treatment conditions at baseline and following IPLT carrageenan and/or IT drug delivery. Tactile thresholds were measured using von Frey filaments with buckling forces between 0.4 and 15 g (Stoelting, Wood Dale, IL, USA) by the up-down method of Dixon, as described previously (24). Any rat with a basal 50% paw withdrawal threshold < 10 g was excluded from the study.
Statistics
Normalized metabolite, mRNA, or protein levels and tactile thresholds are expressed as means ± se. P values were determined using SPSS PASW 18 software (SPSS Inc., Chicago, IL, USA) as follows: unpaired t test for 2-group analysis of 12/15-LOX immunoblots and siRNA-mediated knockdown of Aloxe3; unpaired t test or standard ANOVA followed post hoc by Bonferroni for area under the curve (AUC) or Dunnett's for multiple-group analysis of individual metabolites; repeated measures ANOVA with Bonferroni post hoc for time course behavior. A value of P < 0.05 was considered significant.
RESULTS
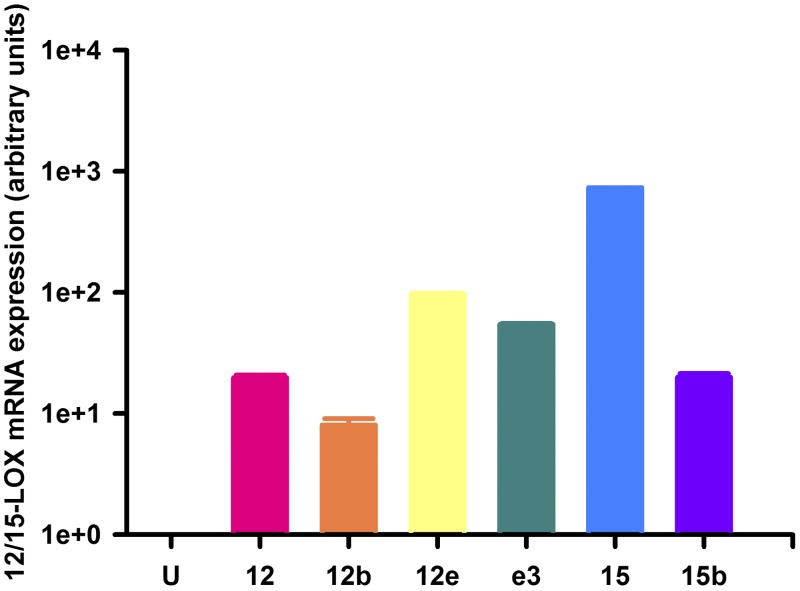
First, we confirmed overexpression of each of the rat 12/15-LOX isozymes in HEK-293T cells. The selectivity of each prevalidated PCR primer set was verified by screening against cDNA libraries obtained from all 6 HEK-12/15-LOX lines, in which no significant cross-reactivity was observed (data not shown). Quantitative RT-PCR revealed that mRNAs of rat 12/15-LOX enzymes were detected in their corresponding transfected cell lines as compared with untransfected HEK-293T cells, in which 12/15-LOX isozymes were not present or were below the limit of detection (Fig. 2). Next, we examined the specificity of commercially available antibodies that target 12/15-LOX isozymes by immunoblot analysis of HEK-293T and HEK-12/15-LOX cell lysates. For simplicity, throughout this work, we refer to each 12/15-LOX protein by its corresponding gene name (see Table 1 for nomenclature). Although the majority of antisera tested failed to exhibit specific binding, we found one pan antibody that recognized all 6 rat 12/15-LOX isozymes (Fig. 3A), as well as three others selective for their respective targets (Aloxe3, Alox15, and Alox15b; Fig. 3B–D). It should be noted that extra bands migrating at ∼85 kDa and ∼62 kDa were also detected by the 12-LOX (pan) antibody in HEK-12/15-LOX cell lysates. The exact identity of these products is unclear, but the upper band may represent complexes or other proteins with similar epitopes, while the lower band may correspond to a proteolytic fragment. Despite extensive screening, we were unable to identify commercially available selective antibodies unique for Alox12, Alox12b, or Alox12e.
Figure 2.
Experimental validation of rat 12/15-LOX overexpression systems. mRNA expression of rat 12/15-LOX enzymes overexpressed in HEK-293T cells (HEK-12/15-LOX) as detected by qPCR. Transient transfection: U, untransfected control; 12, Alox12; 12b, Alox12b; 12e, Alox12e; e3, Aloxe3; 15, Alox15; 15b, Alox15b.
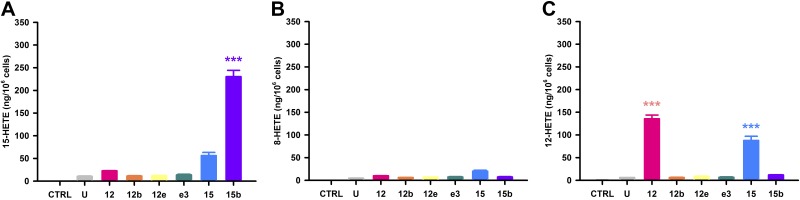
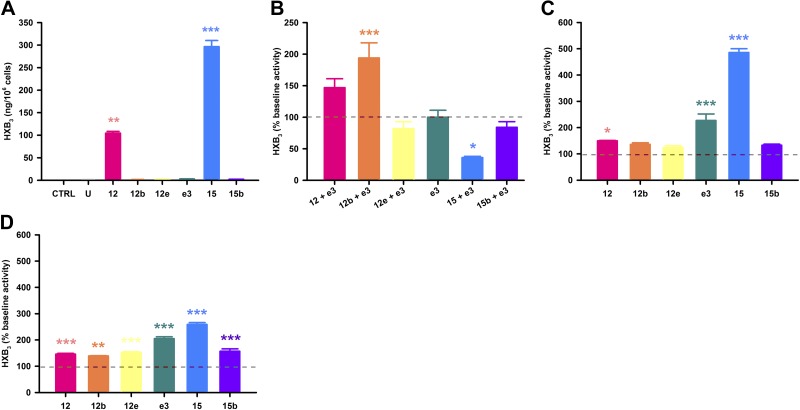
Once the HEK-12/15-LOX overexpression systems were validated, we performed systematic characterization of 8-, 12-, and 15-LOX, as well as HXS activities of the rat 12/15-LOX enzymes using quantitative LC-MS/MS. Using AA as a substrate, we observed that Alox15b is the principal 15-LOX, yielding significant production of 15-HETE vs. control untransfected HEK-293T cells in the presence or absence of substrate (Fig. 4A). _Alox15_-mediated production of 15-HETE was not significant, similar to a previous report (25). Unlike in mice, rat 12/15-LOX isozymes showed no significant turnover of AA to 8-HETE, particularly from Alox15b (Fig. 4B). Alox12 and Alox15 are the primary 12-LOXs, as demonstrated by significant formation of 12-HETE from AA (Fig. 4C). In contrast, much lower levels of 12-HETE were released from cells expressing Alox12b, Alox12e, and Aloxe3 despite detectable mRNA expression post-transfection (see Fig. 2). With regard to hepoxilin synthesis, we measured both HXA3 and HXB3 formation, yet we report levels of the more stable HXB3, as we have described previously (11). Both Alox12 and Alox15 produced highly significant levels of HXB3 when AA was used as a substrate (Fig. 5A). While Aloxe3 alone does not possess intrinsic hepoxilin synthase activity, we observed increased HXB3 synthesis from AA when Aloxe3 was coexpressed with Alox12b at a 1:1 ratio, keeping total DNA constant (Fig. 5B). There was a strong trend toward increased HXB3 levels when Aloxe3 was coexpressed with Alox12, yet it was not quite significant compared with baseline under these conditions. Hepoxilins also were generated via Alox12, Aloxe3, or Alox15 from 12(S)-HpETE or from racemic 12(R,S)-HpETE (Fig. 5C, D, respectively). There was no significant production of 12-HETE, 15-HETE, or HXB3 in untransfected cells, demonstrating that these metabolites were formed enzymatically in cells transfected with the appropriate constructs. Thus, the 6 enzymes may be classified as follows: 12-LOXs, Alox12, Alox12b (low activity), Alox12e (low activity), and Alox15; hepoxilin synthases, Alox12, Alox15, and Aloxe3 (when coexpressed with Alox12 or Alox12b); and 15-LOXs, Alox15 (low activity) and Alox15b (Table 1).
Figure 4.
Activities of overexpressed rat 12/15-LOX enzymes. 15-LOX, 8-LOX, or 12-LOX activity with exposure of HEK-12/15-LOX cells to AA substrate as revealed by the release of 15-HETE (A), 8-HETE (B), or 12-HETE (C) from HEK-293T cells either untreated (ctrl) or treated with AA (70 μM), as follows: U, untransfected control; 12, Alox12; 12b, Alox12b; 12e, Alox12e; e3, Aloxe3; 15, Alox15; 15b, Alox15b. ***P < 0.001 vs. untransfected control; n = 4 wells/treatment.
Figure 5.
Formation of hepoxilins observed from rat 12(S)- and (R)-type LOXs and Aloxe3/eLOX3 using AA or 12-HpETE as substrate. Hepoxilins (HXB3) are synthesized directly from AA (70 μM) by Alox12 or Alox15 (A); by Aloxe3 when it is coexpressed at a 1:1 ratio with Alox12b (B); from 12(S)-HpETE (5 μM) by Alox12, Aloxe3, or Alox15 (C); or from racemic 12(R,S)-HpETE (10 μM; D). U, untransfected control; 12, Alox12; 12b, Alox12b; 12e, Alox12e; e3, Aloxe3; 15, Alox15; 15b, Alox15b. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control or baseline; n = 4 wells/treatment.
Next, we investigated the effect of commercially available LOX inhibitors on 12-LOX, 15-LOX, and HXS activity using AA as substrate (Table 2). As expected, high concentrations of the general LOX inhibitor NDGA (30–100 μM) significantly attenuated 12-HETE or HXB3 production from all six 12/15-LOX enzymes, including the epidermal types that possess relatively minor basal release activity (Alox12b, Alox12e, and Aloxe3). Paradoxically, lower concentrations of NDGA (0.1–10 μM) actually increased synthesis of 12-LOX metabolites (some data not shown, see Supplemental Table S1); however, this effect of NDGA was reported previously for human ALOXE3 (26). Unexpectedly, the putative 5-LOX inhibitor AA-861 (10 μM) also reduced 12-HETE and HXB3 levels, in particular, from the primary sources Alox12 and Alox15. Pretreatment with platelet-type 12-LOX inhibitors CDC or baicalein (10 μM) resulted in a concentration-dependent decrease in levels of both 12-HETE and HXB3 from all six 12/15-LOX enzymes, with CDC exhibiting greater apparent potency. In contrast, while the 15-LOX inhibitor PD146176 (10 μM) profoundly reduced 15-HETE generated by Alox15b in a concentration-dependent manner (Supplemental Table S1), it produced only a small decrease in 12-HETE and HXB3 up to >10-fold of its published IC50.
Table 2.
Selectivity of commercially available LOX inhibitors for rat 12/15-LOX enzymes
| Drug | Target | Conc (μM) | IC50 (μM) | HXB3 inhibition (%) | 12-HETE inhibition (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 | 12b | 12e | e3 | 15 | 15b | 12 | 15 | 12 | 15 | |||
| NDGA | ++ | + | + | −, # | ++ | + | 30 | 0.20–30.0 | 80 ± 1*** | 90 ± 1*** | 53 ± 2*** | 85 ± 1*** |
| AA861 | ++ | − | + | + | + | + | 10 | 0.80 | 38 ± 2** | 25 ± 10 | 61 ± 1*** | 55 ± 4** |
| CDC | ++ | ++ | ++ | ++ | ++ | ++ | 10 | 0.06–3.33 | 86 ± 1*** | 77 ± 2*** | 48 ± 5*** | 71 ± 1*** |
| BAIC | + | ++ | + | ++ | ++ | + | 10 | 0.12–5.00 | 77 ± 1*** | 38 ± 7** | 11 ± 3 | 48 ± 7** |
| PD | + | + | ++ | ++ | − | ++ | 10 | 0.50–0.80 | 33 ± 5** | 8 ± 4 | 5 ± 6 | 3 ± 6 |
Recently, we demonstrated that systemic or IT pretreatment with the general LOX inhibitor NDGA attenuates both the increase in spinal HXB3 as well as the associated hyperesthesia (11, 12). In addition, we showed that spinal delivery of inhibitors of 12-LOX (CDC and baicalein), but not an active site inhibitor of 5-LOX (zileuton), also reduced IPLT carrageenan-evoked tactile allodynia (11). For the purpose of facile comparison to in vivo studies utilizing other LOX inhibitors in the current work, these data have been converted to percentage inhibition of carrageenan-induced allodynia vs. the respective vehicle control (Table 3). Surprisingly, IT administration of the 5-LOX inhibitor AA-861 also significantly attenuated tactile allodynia in a dose-dependent manner (Supplemental Fig. S1_A_, B and Table 3), which could reflect, at least in part, its nonselective actions on 12/15-LOX (Table 2). In contrast, spinal delivery of the 15-LOX inhibitor PD146176 did not alter nociceptive tactile thresholds following peripheral inflammation, even at the maximum soluble dose of 10 μg (Supplemental Fig. S1_C_, D and Table 3). We also tested a higher dose of 30 μg with similar results; however, as the drug precipitated out of solution even at 37°C we cannot draw appropriate conclusions from this experiment (data not shown).
Table 3.
Antihyperalgesic effects of LOX inhibitors that reduce 12-LOX or hepoxilin synthase activities
| Drug | Target | IT dose (μg) | Allodynia inhibition (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 12 | 12b | 12e | e3 | 15 | 15b | |||
| NDGA | ++ | + | + | −, # | ++ | + | 60 | 84 ± 13*** |
| AA861 | ++ | − | + | + | + | + | 3 | 80 ± 19*** |
| CDC | ++ | ++ | ++ | ++ | ++ | ++ | 10 | 71 ± 21§§§ |
| Baic | + | ++ | + | ++ | ++ | + | 10 | 59 ± 8§§ |
| PD | + | + | ++ | ++ | − | ++ | 10 | 48 ± 20 |
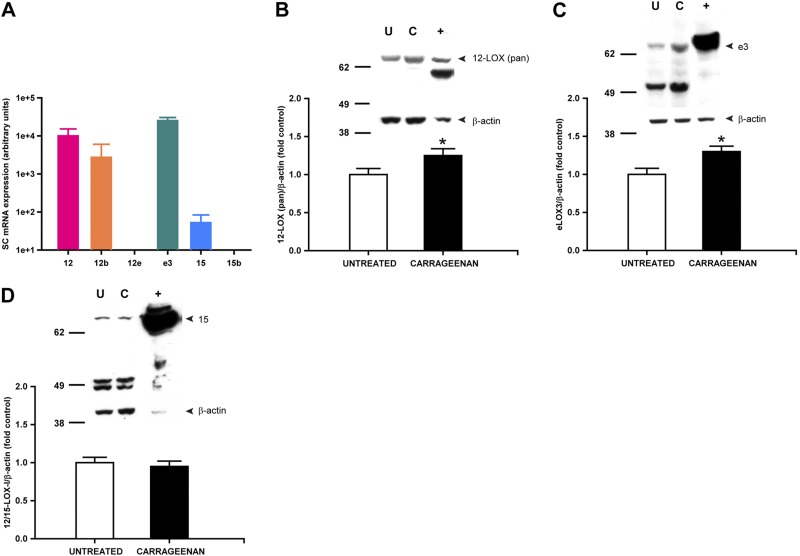
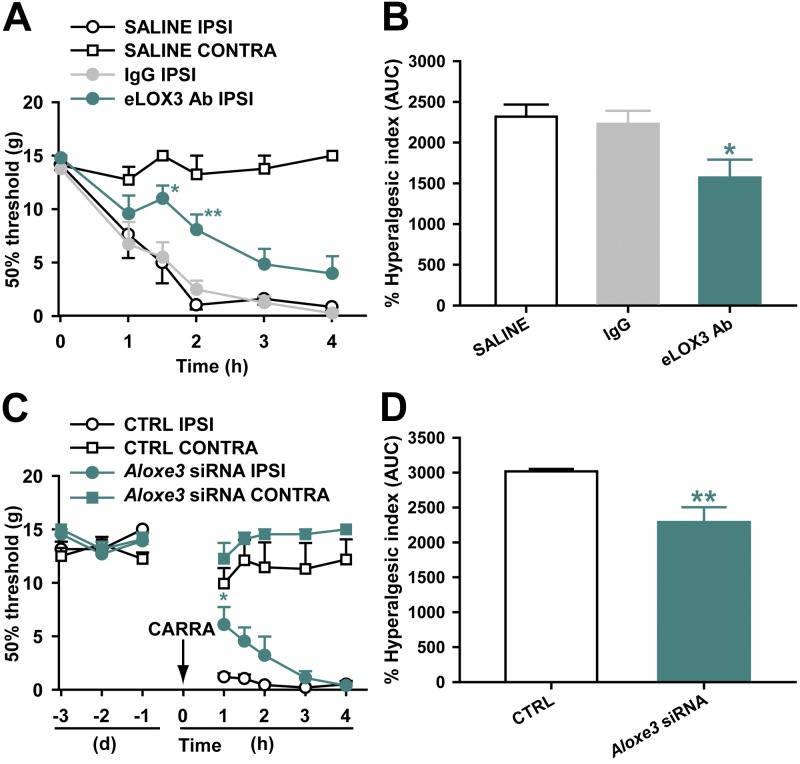
To determine the potential sources of spinal hepoxilins in vivo, we examined expression of the six 12/15-LOX isozymes in rat lumbar spinal cord under basal conditions and during peripheral inflammation, using our validated HEK-12/15-LOX overexpression systems as positive controls. As depicted in Fig. 6A, mRNAs for Alox12, Alox12b, Aloxe3, and Alox15 are present at the spinal level of naive rats in the absence of treatment. Accordingly, we also detected by immunoblot the basal expression of the 12/15-LOX family of proteins, as well as that of _Aloxe3_- and _Alox15_-encoded proteins using antisera prevalidated in Fig. 3 (Fig. 6B–D). Interestingly, the hepoxilin synthase Aloxe3 (as well as total 12/15-LOX with the pan antibody) was increased significantly at 4 h after paw carrageenan, in direct correlation with tactile allodynia, as well as elevated levels of spinal HXB3 (11). In contrast, expression of Alox15 was unchanged with peripheral inflammation. Therefore, we hypothesized that Aloxe3/eLOX3 likely is a critical source of hepoxilins (HXB3) that are generated in the spinal cord and contributes to hyperesthesia during peripheral inflammation. Given that constitutive deletion of genes encoding epidermal LOXs results in early postnatal lethality (27–29) and that none of the commercially available LOX inhibitors tested are selective for eLOX3 at concentrations approaching their reported IC50 (Table 2), we investigated whether neutralization by the eLOX3 antibody validated in Fig. 3 or selective siRNA-mediated knockdown of Aloxe3 at the spinal level would ameliorate carrageenan-induced hyperalgesia. Although carrageenan produces both thermal hyperalgesia and tactile allodynia, we found that spinal administration of hepoxilins elicits tactile allodynia (11); thus, we examined effects of these reagents on the inflammation-induced reduction in tactile thresholds. Quantitative RT-PCR analysis revealed that spinal delivery of siRNA targeting Aloxe3 once daily for 3 d produced significant knockdown of Aloxe3 expression compared with nontargeting control siRNA (control, 1.00±0.23-fold vs. Aloxe3 siRNA, 0.54±0.05-fold; P<0.05). Notably, hyperesthesia was significantly attenuated either by IT pretreatment with eLOX3 antibody (5 μg, Fig. 7A, B) or by Aloxe3 siRNA (2 μg, Fig. 7C, D), as compared with saline vehicle or rabbit IgG isotype control or nontargeting control siRNA, respectively.
Figure 6.
Spinal expression of the hepoxilin synthase eLOX3 is increased following peripheral inflammation. Quantitative PCR and immunoblot reveal isozyme-specific expression of 12/15-LOX in rat spinal cord. Basal expression of Alox12, Alox12b, Aloxe3, and Alox15 mRNA (A) and induction of 12/15-LOX (as detected by pan antibody; B) and eLOX3 (Aloxe3; C) but not 12/15-LOX-l (Alox15) proteins (D) in spinal cord at 4 h after IPLT carrageenan. U, untreated/naive; C, 4 h postintraplantar carrageenan; +, transfected HEK293T cell lysate positive controls for Alox12 (B), Aloxe3 (C), or Alox15 (D). Total protein loaded: spinal cord, 30 μg; positive control, 10 μg. *P < 0.05 vs. control (untreated); n = 6 rats/group.
Figure 7.
Spinal inhibition of eLOX3 or knockdown of Aloxe3 attenuates inflammatory hyperesthesia. IT pretreatment with either prevalidated antibody (5 μg) selectively targeting eLOX3 protein encoded by Aloxe3 (e3; A, B) or siRNA (2 μg; C, D) against Aloxe3 significantly reduced carrageenan (carra)-induced tactile allodynia. *P < 0.05, **P < 0.01 vs. controls (saline vehicle, 5 μg rabbit IgG, or 2 μg nontargeting siRNA); n = 6–10 rats/group.
DISCUSSION
While there are several reports examining functions and tissue distribution of human and mouse 12/15-LOX enzymes, there is less published work regarding the rat orthologs (1). Parallels between rat and human spinal pharmacology argue that mechanisms underlying nociceptive spinal sensory processing in these two species have a high likelihood of being similarly congruent. Thus, examination of the specific roles of rat 12/15-LOX enzymes in models of inflammatory pain will help elucidate specific mechanisms underlying nociceptive transmission that may be translatable to humans, and is, therefore, useful for the development of new therapeutics for pain and other diseases featuring increased 12/15-LOX or hepoxilin synthase activity. Recently, our group demonstrated that peripheral inflammation in rats increases 12-LOX-derived hepoxilins in spinal cord that directly produce hyperesthesia (11, 12). However, it was unclear which of the 6 isozymes is responsible for the spinal synthesis of hepoxilins during inflammation. Accordingly, the current study represents the first to systematically examine the lipidomic profiles and spinal expression of all 6 cloned rat 12/15-LOX enzymes in a standard model of inflammatory hyperalgesia. In addition to Alox15, we found that Alox12 also exhibited intrinsic HXS activity, while Aloxe3 was capable of hepoxilin formation from 12-HpETE, in line with previous studies (30, 31).
Unlike mouse Alox15b, which possesses 8-LOX activity (15), but similar to the human ortholog (32), rat Alox15b displayed significant 15-LOX with minor 12-LOX activity. Similar to the mouse (33) and human forms (34), we also observed very low levels of AA conversion by Alox12e, possibly because of the short half-life of the enzyme when overexpressed, as described for the mouse ortholog (33). Using prevalidated primers, we detected mRNAs for the 4 remaining 12/15-LOX genes in naive rat spinal cord: Alox12 (12-LOX-p), Alox12b [12(R)-LOX], Aloxe3 (eLOX3), and Alox15 (12/15-LOX-l). As predicted, we found that rat leukocyte-type Alox15 exhibits both 12-LOX and HXS activities, as described previously for rats (35), as well as mice (18) and humans (32). While the murine platelet-type 12-LOX functions as a putative dioxygenase (18), rat Alox12 has both 12-LOX and HXS activities, as observed for humans (35). Others have shown that the human ALOX12B is capable of efficient conversion of AA to 12(R)-HpETE (17), which can be utilized by Aloxe3 to form hepoxilins (26). In contrast, the rat Alox12b showed very low catalytic activity when AA was used as a substrate, similar to observations made with the mouse ortholog (17, 36). It is possible that rat Alox12b may prefer an alternative substrate to AA, such as arachidonyl ester, as reported for the mouse isozyme (36). AA also was a poor substrate for rat Aloxe3, which lacked appreciable dioxygenase or intrinsic hydroperoxide isomerase activity, similar to both mice and humans (26, 31). Nevertheless, we did observe significant formation of hepoxilins (HXB3) from AA when Alox12b is coexpressed with Aloxe3. Exposure of rat Aloxe3 to exogenous 12(S)-HpETE or racemic 12(R,S)-HpETE also generated hepoxilins, as documented in mice or humans, respectively (31, 37). Collectively, these results corroborate the potential translational relevance of rat inflammatory models in studying 12/15-LOX isozymes, as a number of similarities exist between the rat and human orthologs.
Several of the commercially available LOX inhibitors we tested were initially reported to be selective for their respective targets: e.g., AA-861 for 5-LOX (38, 39), CDC and baicalein for Alox12 (40, 41), and PD146176 for 15-LOX (42). However, emerging evidence suggests that these agents also display concentration-dependent nonselective effects due to their redox or free radical-scavenging properties. Notably, AA-861 (1–10 μM) also inhibits 12/15-LOX (43), in agreement with our observations above. The flavonoid baicalein exerts actions as an antioxidant (44, 45), while CDC inhibits Kv1.5 channel currents (46), as well as 5-LOX at low micromolar concentrations (47). As expected, CDC and baicalein potently blocked Alox12 activity, yet these compounds significantly decreased formation of 12-HETE and hepoxilins from leukocyte-type Alox15 as well. PD146176 was highly specific for 15-LOX (Alox15b), as shown by profound reduction of 15-HETE levels, but also displayed off-target effects on the other enzymes (with the exception of Alox15). The actions of these drugs in vitro, particularly with regard to their ability to block release of hepoxilins and 12-HETE, correlated well with their antihyperalgesic effects in vivo. PD146176 only moderately inhibited platelet-type 12-LOX-mediated synthesis of HXB3, and failed to prevent the development of carrageenan-induced tactile allodynia. In contrast, NDGA and CDC (and to a lesser extent, baicalein) inhibited production of 12-LOX metabolites from all 6 isozymes and potently attenuated hyperesthesia following peripheral inflammation. Surprisingly, the 5-LOX inhibitor AA-861 suppressed both 12-LOX and HXS activity and significantly attenuated the development of tactile allodynia following IPLT carrageenan.
Interestingly, of the 12/15-LOX enzymes that were expressed in spinal cord and for which selective antisera were available, only eLOX3 protein was up-regulated significantly after IPLT carrageenan. Since protein levels do not necessarily correlate with activity, we examined the effect of direct inhibition of eLOX3 on carrageenan-evoked tactile allodynia. The lack of selective inhibitors (Table 2) or viable constitutive deletion mutants (27–29) for epidermal LOXs prompted us to undertake IT delivery of a selective antibody targeting eLOX3 that we validated in this study as well as IT siRNA-mediated knockdown of Aloxe3 mRNA. As expected, spinal inhibition of eLOX3 or knockdown of Aloxe3 significantly attenuated tactile allodynia, indicating that hepoxilins derived, at least in part, from eLOX3 mediate hyperesthesia following peripheral inflammation. Although it is not clear how the eLOX3 antisera enter the cell, others have reported successful use of intrathecal antibody delivery for intracellular targets (48). Given our collective findings, it is likely that in addition to directly forming hepoxilins, Alox12 and/or Alox15 may provide 12(S)-HpETE for hepoxilin synthesis by Aloxe3 (eLOX3) in vivo during inflammation. It is also possible that Aloxe3 may form hepoxilins from 12(R)-HpETE generated by Alox12b. These observations underscore the crucial importance of developing more selective inhibitors of 12/15-LOX isozymes, such as those recently developed at NIH for the human ALOX12 (49) and ALOX15 (50). Constitutive gene deletion has yielded complex and sometimes confounding effects in models of inflammation or neuropathy (51–53), perhaps, in part, due to potential differences in peripheral and central roles of 12/15-LOX enzymes, as well as simultaneous disruption of proresolution metabolites, such as lipoxins and resolvins (54–55). Thus, targeted spinal delivery of selective 12-LOX inhibitors or hepoxilin analogs (56) should be advantageous for evaluating the roles of 12/15-LOX isozymes in central nociceptive processing, and perhaps serve as useful in pain states that are less responsive or even refractory to treatment with COX inhibitors (57, 58). Furthermore, such agents also may offer significant clinical utility for the study and treatment of other diseases in which 12-LOX metabolites may play a role, such as IBD, cancer, hypertension, atherogenesis, psoriasis, and diabetes (2, 56, 59, 60).
Supplementary Material
Supplemental Data
Acknowledgments
The authors thank Dr. Jing Yang for sharing equipment and Drs. Xiao-Ying Hua, Camilla I. Svensson, and Qinghao Xu for helpful discussions.
This work was supported by U.S. National Institutes of Health grants NS16541 and DA02110 (T.L.Y.) and GM064611 and GM069338 (E.A.D.).
AA
arachidonic acid
CDC
cinnamyl-3,4-dihydroxy-α-cyanocinnamate
cDNA
complementary DNA
eLOX3
epidermal lipoxygenase 3
HETE
hydroxyeicosatetraenoic acid
HpETE
hydroperoxyeicosatetraenoic acid
HXA3
hepoxilin A3
HXB3
hepoxilin B3
HXS
hepoxilin synthase
IPLT
intraplantar
IT
intrathecal
LC-MS/MS
liquid chromatography–tandem mass spectrometry
LOX
lipoxygenase
MRM
multiple reaction monitoring
NDGA
nordihydroguaiaretic acid
PBS
phosphate-buffered saline
PUFA
polyunsaturated fatty acid
qPCR
quantitative polymerase chain reaction
qRT-PCR
quantitative reverse transcriptase-polymerase chain reaction
TRPA1
transient receptor potential ankyrin 1
TRPV1
transient receptor potential vanilloid 1
REFERENCES
- 1.Buczynski M. W., Dumlao D. S., Dennis E. A. (2009) Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 50, 1015–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhn H., O'Donnell V. B. (2006) Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid Res. 45, 334–356 [DOI] [PubMed] [Google Scholar]
- 3.Ivanov I., Heydeck D., Hofheinz K., Roffeis J., O'Donnell V. B., Kuhn H., Walther M. (2010) Molecular enzymology of lipoxygenases. Arch. Biochem. Biophys. 503, 161–174 [DOI] [PubMed] [Google Scholar]
- 4.Krieg P., Marks F., Furstenberger G. (2001) A gene cluster encoding human epidermis-type lipoxygenases at chromosome 17p13.1: cloning, physical mapping, and expression. Genomics 73, 323–330 [DOI] [PubMed] [Google Scholar]
- 5.Bendani M. K., Palluy O., Cook-Moreau J., Beneytout J. L., Rigaud M., Vallat J. M. (1995) Localization of 12-lipoxygenase mRNA in cultured oligodendrocytes and astrocytes by in situ reverse transcriptase and polymerase chain reaction. Neurosci. Lett. 189, 159–162 [DOI] [PubMed] [Google Scholar]
- 6.Kawajiri H., Zhuang D., Qiao N., Yoshimoto T., Yamamoto M., Iseki S., Hamaguchi K. (2000) Expression of arachidonate 12-lipoxygenase in rat tissues: a possible role in glucagon secretion. J. Histochem. Cytochem. 48, 1411–1419 [DOI] [PubMed] [Google Scholar]
- 7.Aley O., Levine J. D. (2003) Contribution of 5- and 12-lipoxygenase products to mechanical hyperalgesia induced by prostaglandin E2 and epinephrine in the rat. Exp. Brain Res. 148, 482–487 [DOI] [PubMed] [Google Scholar]
- 8.Shin J., Cho H., Hwang S. W., Jung J., Shin C. Y., Lee S. Y., Kim S. H., Lee M. G., Choi Y. H., Kim J., Haber N. A., Reichling D. B., Khasar S., Levine J. D., Oh U. (2002) Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc. Natl. Acad. Sci. U. S. A. 99, 10150–10155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo S., Han S., Park Y. S., Lee J. H., Oh U., Hwang S. W. (2009) Lipoxygenase inhibitors suppressed carrageenan-induced Fos-expression and inflammatory pain responses in the rat. Mol. Cells 27, 417–422 [DOI] [PubMed] [Google Scholar]
- 10.Trang T., McNaull B., Quirion R., Jhamandas K. (2004) Involvement of spinal lipoxygenase metabolites in hyperalgesia and opioid tolerance. Eur. J. Pharmacol. 491, 21–30 [DOI] [PubMed] [Google Scholar]
- 11.Gregus A. M., Doolen S., Dumlao D. S., Buczynski M. W., Takasusuki T., Fitzsimmons B. L., Hua X. Y., Taylor B. K., Dennis E. A., Yaksh T. L. (2012) Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc. Natl. Acad. Sci. U. S. A. 109, 6721–6726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buczynski M. W., Svensson C. I., Dumlao D. S., Fitzsimmons B. L., Shim J. H., Scherbart T. J., Jacobsen F. E., Hua X. Y., Yaksh T. L., Dennis E. A. (2010) Inflammatory hyperalgesia induces essential bioactive lipid production in the spinal cord. J. Neurochem. 114, 981–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nair D. G., Funk C. D. (2009) A cell-based assay for screening lipoxygenase inhibitors. Prostaglandins Other Lipid Mediat. 90, 98–104 [DOI] [PubMed] [Google Scholar]
- 14.Funk C. D., Keeney D. S., Oliw E. H., Boeglin W. E., Brash A. R. (1996) Functional expression and cellular localization of a mouse epidermal lipoxygenase. J. Biol. Chem. 271, 23338–23344 [DOI] [PubMed] [Google Scholar]
- 15.Jisaka M., Kim R. B., Boeglin W. E., Brash A. R. (2000) Identification of amino acid determinants of the positional specificity of mouse 8S-lipoxygenase and human 15S-lipoxygenase-2. J. Biol. Chem. 275, 1287–1293 [DOI] [PubMed] [Google Scholar]
- 16.Burger F., Krieg P., Marks F., Furstenberger G. (2000) Positional- and stereo-selectivity of fatty acid oxygenation catalysed by mouse (12S)-lipoxygenase isoenzymes. Biochem. J. 348, 329–335 [PMC free article] [PubMed] [Google Scholar]
- 17.Sun D., McDonnell M., Chen X. S., Lakkis M. M., Li H., Isaacs S. N., Elsea S. H., Patel P. I., Funk C. D. (1998) Human 12(R)-lipoxygenase and the mouse ortholog. Molecular cloning, expression, and gene chromosomal assignment. J. Biol. Chem. 273, 33540–33547 [DOI] [PubMed] [Google Scholar]
- 18.Chen X. S., Kurre U., Jenkins N. A., Copeland N. G., Funk C. D. (1994) cDNA cloning, expression, mutagenesis of C-terminal isoleucine, genomic structure, and chromosomal localizations of murine 12-lipoxygenases. J. Biol. Chem. 269, 13979–13987 [PubMed] [Google Scholar]
- 19.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 20.Harmon G. S., Dumlao D. S., Ng D. T., Barrett K. E., Dennis E. A., Dong H., Glass C. K. (2010) Pharmacological correction of a defect in PPAR-γ signaling ameliorates disease severity in Cftr-deficient mice. Nat. Med. 16, 313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaksh T. L., Rudy T. A. (1976) Chronic catheterization of the spinal subarachnoid space. Physiol. Behav. 17, 1031–1036 [DOI] [PubMed] [Google Scholar]
- 22.Malkmus S. A., Yaksh T. L. (2004) Intrathecal catheterization and drug delivery in the rat. Methods Mol. Med. 99, 109–121 [DOI] [PubMed] [Google Scholar]
- 23.Luo M. C., Zhang D. Q., Ma S. W., Huang Y. Y., Shuster S. J., Porreca F., Lai J. (2005) An efficient intrathecal delivery of small interfering RNA to the spinal cord and peripheral neurons. Mol. Pain 1, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaplan S. R., Bach F. W., Pogrel J. W., Chung J. M., Yaksh T. L. (1994) Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T., Haeggstrom J. Z. (1993) Rat 12-lipoxygenase: mutations of amino acids implicated in the positional specificity of 15- and 12-lipoxygenases. Biochem. Biophys. Res. Commun. 192, 1023–1029 [DOI] [PubMed] [Google Scholar]
- 26.Yu Z., Schneider C., Boeglin W. E., Marnett L. J., Brash A. R. (2003) The lipoxygenase gene ALOXE3 implicated in skin differentiation encodes a hydroperoxide isomerase. Proc. Natl. Acad. Sci. U. S. A. 100, 9162–9167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epp N., Furstenberger G., Muller K., de Juanes S., Leitges M., Hausser I., Thieme F., Liebisch G., Schmitz G., Krieg P. (2007) 12R-lipoxygenase deficiency disrupts epidermal barrier function. J. Cell Biol. 177, 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran J. L., Qiu H., Turbe-Doan A., Yun Y., Boeglin W. E., Brash A. R., Beier D. R. (2007) A mouse mutation in the 12R-lipoxygenase, Alox12b, disrupts formation of the epidermal permeability barrier. J. Invest. Dermatol. 127, 1893–1897 [DOI] [PubMed] [Google Scholar]
- 29.Krieg P., Rosenberger S., de Juanes S., Latzko S., Hou J., Dick A., Kloz U., van der Hoeven F., Hausser I., Esposito I., Rauh M., Schneider H. (2012) Aloxe3 knockout mice reveal a function of epidermal lipoxygenase-3 as hepoxilin synthase and its pivotal role in barrier formation. J Invest Dermatol 133, 172–180 [DOI] [PubMed] [Google Scholar]
- 30.Nigam S., Nodes S., Cichon G., Corey E. J., Pace-Asciak C. R. (1990) Receptor-mediated action of hepoxilin A3 releases diacylglycerol and arachidonic acid from human neutrophils. Biochem. Biophys. Res. Commun. 171, 944–948 [DOI] [PubMed] [Google Scholar]
- 31.Yu Z., Schneider C., Boeglin W. E., Brash A. R. (2006) Human and mouse eLOX3 have distinct substrate specificities: implications for their linkage with lipoxygenases in skin. Arch. Biochem. Biophys. 455, 188–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacquot C., Wecksler A. T., McGinley C. M., Segraves E. N., Holman T. R., van der Donk W. A. (2008) Isotope sensitive branching and kinetic isotope effects in the reaction of deuterated arachidonic acids with human 12- and 15-lipoxygenases. Biochemistry 47, 7295–7303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonnell M., Davis W., Li H., Funk C. D. (2001) Characterization of the murine epidermal 12/15-lipoxygenase. Prostaglandins 63, 93–107 [DOI] [PubMed] [Google Scholar]
- 34.Sun D., Elsea S. H., Patel P. I., Funk C. D. (1998) Cloning of a human “epidermal-type” 12-lipoxygenase-related gene and chromosomal localization to 17p13. Cytogenet. Cell Genet. 81, 79–82 [DOI] [PubMed] [Google Scholar]
- 35.Nigam S., Patabhiraman S., Ciccoli R., Ishdorj G., Schwarz K., Petrucev B., Kuhn H., Haeggstrom J. Z. (2004) The rat leukocyte-type 12-lipoxygenase exhibits an intrinsic hepoxilin A3 synthase activity. J. Biol. Chem. 279, 29023–29030 [DOI] [PubMed] [Google Scholar]
- 36.Krieg P., Siebert M., Kinzig A., Bettenhausen R., Marks F., Furstenberger G. (1999) Murine 12(R)-lipoxygenase: functional expression, genomic structure and chromosomal localization. FEBS Lett. 446, 142–148 [DOI] [PubMed] [Google Scholar]
- 37.Hallenborg P., Jorgensen C., Petersen R. K., Feddersen S., Araujo P., Markt P., Langer T., Furstenberger G., Krieg P., Koppen A., Kalkhoven E., Madsen L., Kristiansen K. (2010) Epidermis-type lipoxygenase 3 regulates adipocyte differentiation and peroxisome proliferator-activated receptor gamma activity. Mol. Cell. Biol. 30, 4077–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashida Y., Saijo T., Kuriki H., Makino H., Terao S., Maki Y. (1983) Pharmacological profile of AA-861, a 5-lipoxygenase inhibitor. Prostaglandins 26, 955–972 [DOI] [PubMed] [Google Scholar]
- 39.Mita H., Yui Y., Shida T. (1986) Effect of AA-861, a 5-lipoxygenase inhibitor, on leukotriene synthesis in human polymorphonuclear leukocytes and on cyclooxygenase and 12-lipoxygenase activities in human platelets. Allergy 41, 493–498 [DOI] [PubMed] [Google Scholar]
- 40.Cho H., Ueda M., Tamaoka M., Hamaguchi M., Aisaka K., Kiso Y., Inoue T., Ogino R., Tatsuoka T., Ishihara T. (1991) Novel caffeic acid derivatives: extremely potent inhibitors of 12-lipoxygenase. J. Med. Chem. 34, 1503–1505 [DOI] [PubMed] [Google Scholar]
- 41.Sekiya K., Okuda H. (1982) Selective inhibition of platelet lipoxygenase by baicalein. Biochem. Biophys. Res. Commun. 105, 1090–1095 [DOI] [PubMed] [Google Scholar]
- 42.Cornicelli J. A., Trivedi B. K. (1999) 15-Lipoxygenase and its inhibition: a novel therapeutic target for vascular disease. Curr. Pharm. Des. 5, 11–20 [PubMed] [Google Scholar]
- 43.Wang H., Li J., Follett P. L., Zhang Y., Cotanche D. A., Jensen F. E., Volpe J. J., Rosenberg P. A. (2004) 12-Lipoxygenase plays a key role in cell death caused by glutathione depletion and arachidonic acid in rat oligodendrocytes. Eur. J. Neurosci. 20, 2049–2058 [DOI] [PubMed] [Google Scholar]
- 44.Shen Y. C., Chiou W. F., Chou Y. C., Chen C. F. (2003) Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. Eur. J. Pharmacol. 465, 171–181 [DOI] [PubMed] [Google Scholar]
- 45.Suk K., Lee H., Kang S. S., Cho G. J., Choi W. S. (2003) Flavonoid baicalein attenuates activation-induced cell death of brain microglia. J. Pharmacol. Exp. Ther. 305, 638–645 [DOI] [PubMed] [Google Scholar]
- 46.Gong Y. Z., Ding W. G., Wu J., Tsuji K., Horie M., Matsuura H. (2008) Cinnamyl-3,4-dihydroxy-alpha-cyanocinnamate and nordihydroguaiaretic acid inhibit human Kv1.5 currents independently of lipoxygenase. Eur. J. Pharmacol. 600, 18–25 [DOI] [PubMed] [Google Scholar]
- 47.Pergola C., Jazzar B., Rossi A., Buehring U., Luderer S., Dehm F., Northoff H., Sautebin L., Werz O. (2011) Cinnamyl-3,4-dihydroxy-alpha-cyanocinnamate is a potent inhibitor of 5-lipoxygenase. J. Pharmacol. Exp. Ther. 338, 205–213 [DOI] [PubMed] [Google Scholar]
- 48.Shibasaki M., Sasaki M., Miura M., Mizukoshi K., Ueno H., Hashimoto S., Tanaka Y., Amaya F. (2010) Induction of high mobility group box-1 in dorsal root ganglion contributes to pain hypersensitivity after peripheral nerve injury. Pain 149, 514–521 [DOI] [PubMed] [Google Scholar]
- 49.Kenyon V., Rai G., Jadhav A., Schultz L., Armstrong M., Jameson J. B., 2nd, Perry S., Joshi N., Bougie J. M., Leister W., Taylor-Fishwick D. A., Nadler J. L., Holinstat M., Simeonov A., Maloney D. J., Holman T. R. (2011) Discovery of potent and selective inhibitors of human platelet-type 12- lipoxygenase. J. Med. Chem. 54, 5485–5497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rai G., Kenyon V., Jadhav A., Schultz L., Armstrong M., Jameson J. B., Hoobler E., Leister W., Simeonov A., Holman T. R., Maloney D. J. (2010) Discovery of potent and selective inhibitors of human reticulocyte 15-lipoxygenase-1. J. Med. Chem. 53, 7392–7404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kronke G., Katzenbeisser J., Uderhardt S., Zaiss M. M., Scholtysek C., Schabbauer G., Zarbock A., Koenders M. I., Axmann R., Zwerina J., Baenckler H. W., van den Berg W., Voll R. E., Kuhn H., Joosten L. A., Schett G. (2009) 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. J. Immunol. 183, 3383–3389 [DOI] [PubMed] [Google Scholar]
- 52.Wu M. Y., Lin T. H., Chiu Y. C., Liou H. C., Yang R. S., Fu W. M. (2012) Involvement of 15-lipoxygenase in the inflammatory arthritis. J. Cell. Biochem. 113, 2279–2289 [DOI] [PubMed] [Google Scholar]
- 53.Obrosova I. G., Stavniichuk R., Drel V. R., Shevalye H., Vareniuk I., Nadler J. L., Schmidt R. E. (2010) Different roles of 12/15-lipoxygenase in diabetic large and small fiber peripheral and autonomic neuropathies. Am. J. Pathol. 177, 1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Z. Z., Zhang L., Liu T., Park J. Y., Berta T., Yang R., Serhan C. N., Ji R. R. (2010) Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 16, 592–597, 1 p following 597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Svensson C. I., Zattoni M., Serhan C. N. (2007) Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J. Exp. Med. 204, 245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pace-Asciak C. R. (2009) The hepoxilins and some analogues: a review of their biology. Br. J. Pharmacol. 158, 972–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christianson C. A., Corr M., Firestein G. S., Mobargha A., Yaksh T. L., Svensson C. I. (2010) Characterization of the acute and persistent pain state present in K/BxN serum transfer arthritis. Pain 151, 394–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saito O., Svensson C. I., Buczynski M. W., Wegner K., Hua X. Y., Codeluppi S., Schaloske R. H., Deems R. A., Dennis E. A., Yaksh T. L. (2010) Spinal glial TLR4-mediated nociception and production of prostaglandin E2 and TNF. Br. J. Pharmacol. 160, 1754–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mrsny R. J., Gewirtz A. T., Siccardi D., Savidge T., Hurley B. P., Madara J. L., McCormick B. A. (2004) Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc. Natl. Acad. Sci. U. S. A. 101, 7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dobrian A. D., Lieb D. C., Cole B. K., Taylor-Fishwick D. A., Chakrabarti S. K., Nadler J. L. (2011) Functional and pathological roles of the 12- and 15-lipoxygenases. Prog. Lipid Res. 50, 115–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data