Increased Water Activity Reduces the Thermal Resistance of Salmonella enterica in Peanut Butter (original) (raw)
Abstract
Increased water activity in peanut butter significantly (P < 0.05) reduced the heat resistance of desiccation-stressed Salmonella enterica serotypes treated at 90°C. The difference in thermal resistance was less notable when strains were treated at 126°C. Using scanning electron microscopy, we observed minor morphological changes of S. enterica cells resulting from desiccation and rehydration processes in peanut oil.
TEXT
Salmonellosis outbreaks linked to contaminated peanut butter products have brought worldwide attention to the microbial safety of these popular food items. Salmonella enterica serotype Tennessee caused a salmonellosis outbreak in 2006–2007 that was linked to peanut butter; it sickened 425 persons and resulted in 71 (20%) hospitalizations in 44 states in the United States (1). This and other food-borne outbreaks (2, 3) have highlighted the need for a reexamination of S. enterica behavior in low-water-activity (aw) peanut butter products.
The water activity of peanut butter is typically 0.35 or less (2, 4–9), which precludes the growth of spoilage and pathogenic microorganisms. When present in peanut butter, S. enterica becomes heat resistant, possibly due to adaptation to the desiccation stress and the protective effects of the fat content in the product (2, 4–7, 10–14). We recently demonstrated that heat treatment at 72°C for 1 hour resulted in a less-than-2-log reduction of desiccation-stressed S. enterica in artificially contaminated peanut butter with an aw of 0.4 (15). In this study, we evaluated the effects of desiccation and subsequent rehydration on the relative heat resistance of three S. enterica serotypes: S. Tennessee K4643 (a human isolate from the 2006–2007 peanut butter outbreak in the United States) (1), S. Enteritidis BSS-1045 (an isolate from the 2000–2001 raw almonds outbreak in the United States and Canada) (16–18), and S. Typhimurium LT2 (19, 20). We compared two commercial peanut butter formulations (regular and low fat) to assess the influence of carbohydrate and fat contents on the heat resistance of S. enterica. Most published thermal challenge studies of S. enterica in peanut butter have focused on heat treatments at either 72°C or 90°C (11, 21, 22) and not at the higher temperatures commonly used in commercial peanut butter processing, such as dry roasting at 126°C (22). In this study, we thermally challenged S. enterica serotypes in artificially contaminated peanut butter at both 90°C and 126°C.
Individual serotypes and a three-serotype cocktail were grown separately as previously described (15), followed by suspension in 5 ml of peanut oil prior to inoculation of peanut butter samples (aw, 0.2). Bacterial cell suspensions were transferred to 500 g of peanut butter and vigorously stirred for 20 min by using a sampler spatula. Homogenous distribution of the cells was verified as previously described (15). Inoculated samples were stored at 25°C for 4 weeks, then serially diluted and plated on brain heart infusion (BHI) agar for calculating bacterial death rates. The storage simulated the stress that S. enterica may typically encounter during peanut butter processing (15). The bacterial population in low-fat formulation A (33% fat and 42% carbohydrate) decreased by an average of 0.6 to 0.8 log, compared to an average of 0.9 to 1.2 logs in the regular formulation, E (49% fat and 24% carbohydrate) (see Fig. S1 in the supplemental material). This observation was consistent with our previous finding that S. enterica survived better in peanut butter with lower fat but higher carbohydrate content during an extended storage period (15).
After the 4-week incubation, select volumes of phosphate-buffered saline (PBS) were mixed into the spiked peanut butter samples to adjust the aw to 0.4, 0.6, or 0.8 in order to evaluate the effects of an increased aw on S. enterica heat resistance. The samples were incubated at 25°C for 24 h before thermal treatment. Each inoculated sample (20 g) was transferred into an aluminum foil bag, which was sealed, compressed to a thickness of 1 mm, and submerged in an oil bath for heat treatment at 90°C or 126°C. The come-up time to reach the final treatment temperature was less than 10 s. The heat-treated samples were taken at 30 s, 90 s, 5 min, 10 min, and 20 min and immediately cooled on ice for 1 min. Viable cell counts were determined as previously described (15). D values were calculated using the Bigelow model (23). Each data set was analyzed using the Weibull model (24, 25). Statistical analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC) and Matlab 7.10.0.499 (MathWorks, Inc., Natick, MA). A P value of <0.05 was considered statistically significant.
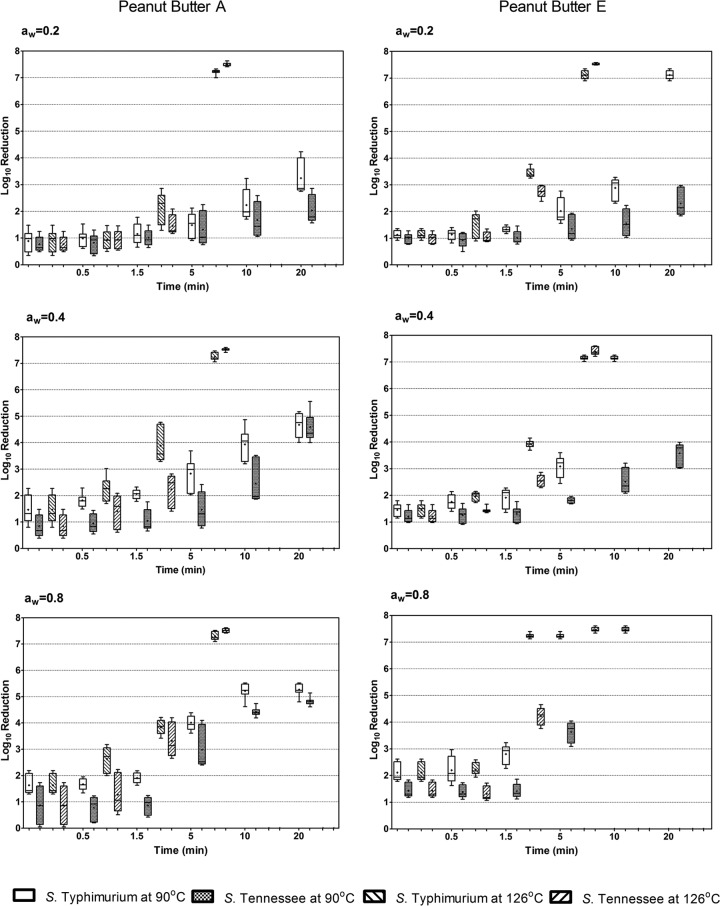
Figure 1 shows the overall S. enterica population changes after treatment at 90°C and 126°C over 20 min in both peanut butter formulations with adjusted water activities. More detailed population dynamics are shown in Fig. S2 and S3 in the supplemental material. At an aw of 0.2, 90°C treatment for 20 min resulted in a less-than-3-log reduction of S. Tennessee, whereas S. Typhimurium showed 3.4 and 7.2 log reductions in peanut butter A and E, respectively. At an aw of 0.4, 20 min of heating at 90°C resulted in 4- to 5-log reductions of both S. Typhimurium and S. Tennessee in peanut butter A, compared to no detectable levels of S. Typhimurium and a 3- to 4-log reduction of S. Tennessee in peanut butter E. At an aw of 0.8, the same thermal treatment resulted in 4.8- to 5.2-log reductions of S. Typhimurium and S. Tennessee in peanut butter A, in contrast to no detectable levels in peanut butter E. These results suggest that an increase in the aw in peanut butter formulation A had less of an impact on S. enterica thermal resistance than in peanut butter E, which contained a higher percentage of fat but lower carbohydrate levels. At 126°C, regardless of the adjusted water activities, an approximately 7- to 8-log reduction was achieved after 5 min, and at 10 min S. enterica could not be detected in either peanut butter formulation.
Fig 1.
Box plots showing log reductions of S. Typhimurium and S. Tennessee at 90°C and 126°C in peanut butters A (low fat) and E (regular fat) with adjusted water activities. The horizontal bars and stars in boxes represent median and mean values, respectively; box edges represent the upper and lower hinges of the H spread.
The statistical differences among the D values of the three serotypes (Table 1) were most notable in peanut butter E at an aw of 0.2, where S. Tennessee displayed the highest D value (8.35 min) and S. Typhimurium the lowest (2.61 min). These observations suggest that S. Typhimurium was considerably less heat resistant than the other two serotypes in the peanut butter formulations tested. Interestingly, however, as the water activities in both formulations increased from 0.2 to 0.8, the differences in D values at 90°C among the three serotypes were not statistically significant. In addition, no statistical difference in D values was found among the three serotypes after treatment at 126°C in either formulation.
Table 1.
D values, calculated based on first-order kinetics, for S. enterica serotypes in peanut butter samples with adjusted water activities at 90°C and 126°C
| Peanut butter | Temp (°C) | aw | Mean ± SD D value (_r_2)a | |||
|---|---|---|---|---|---|---|
| S. Enteritidis | S. Typhimurium | S. Tennessee | Three-serotype cocktail | |||
| A | 90 | 0.20 | 7.05 ± 1.12 (0.93) Aa | 3.71 ± 0.74 (0.99) Ab | 6.41 ± 1.36 (0.93) Aa | 6.95 ± 1.69 (0.97) Aa |
| A | 90 | 0.40 | 2.64 ± 0.36 (0.99) BCa | 2.43 ± 0.41 (0.93) Ba | 2.44 ± 0.19 (0.98) BCa | 3.13 ± 0.72 (0.95) Ba |
| A | 90 | 0.60 | 3.00 ± 0.69 (0.97) Bab | 2.07 ± 0.30 (0.89) Ba | 2.96 ± 0.75 (0.97) Bab | 3.14 ± 0.94 (0.96) Bb |
| A | 90 | 0.80 | 1.91 ± 0.35 (0.90) Ca | 1.95 ± 0.37 (0.87) Ba | 1.89 ± 0.37 (0.89) Ca | 2.06 ± 0.42 (0.90) Ca |
| A | 126 | 0.20 | 1.20 ± 0.52 (0.95) Aa | 0.59 ± 0.09 (0.91) Aa | 1.00 ± 0.15 (0.96) Aa | 1.19 ± 0.46 (0.94) Aa |
| A | 126 | 0.40 | 0.55 ± 0.32 (0.99) Aa | 0.28 ± 0.05 (0.99) Aa | 0.62 ± 0.33 (0.92) Aa | 0.44 ± 0.19 (0.97) Aa |
| A | 126 | 0.60 | 0.48 ± 0.17 (0.96) Aa | 0.76 ± 0.50 (0.96) Aa | 0.70 ± 0.29 (0.91) Aa | 0.54 ± 0.18 (0.95) Aa |
| A | 126 | 0.80 | 0.27 ± 0.07 (0.98) Aa | 0.30 ± 0.06 (0.98) Aa | 0.29 ± 0.06 (0.97) Aa | 0.22 ± 0.02 (0.95) Aa |
| E | 90 | 0.20 | 4.81 ± 1.58 (0.97) Aa | 2.61 ± 0.59 (0.97) Ab | 8.35 ± 4.09 (0.88) Ac | 4.84 ± 0.95 (0.96) Aa |
| E | 90 | 0.40 | 3.43 ± 0.51 (0.98) ABa | 1.35 ± 0.20 (0.98) Bb | 3.64 ± 0.45 (0.98) Ba | 3.67 ± 0.18 (0.97) Aa |
| E | 90 | 0.60 | 2.10 ± 0.27 (0.96) BCa | 1.24 ± 0.05 (0.99) Ba | 1.79 ± 0.14 (0.98) Ca | 1.94 ± 0.36 (0.96) Ba |
| E | 90 | 0.80 | 0.94 ± 0.22 (0.89) Ca | 1.15 ± 0.44 (0.88) Ba | 1.12 ± 0.19 (0.90) Ca | 1.15 ± 0.07 (0.90) Ba |
| E | 126 | 0.20 | 0.43 ± 0.05 (0.87) Aa | 0.31 ± 0.06 (0.92) Aa | 0.42 ± 0.04 (0.91) Aa | 0.54 ± 0.08 (0.91) Aa |
| E | 126 | 0.40 | 0.39 ± 0.03 (0.93) Aa | 0.29 ± 0.05 (0.96) Aa | 0.51 ± 0.05 (0.96) Aa | 0.64 ± 0.18 (0.98) Aa |
| E | 126 | 0.60 | 0.67 ± 0.15 (0.96) Aa | 0.35 ± 0.04 (1.00) Aa | 0.59 ± 0.17 (0.89) Aa | 0.43 ± 0.06 (0.93) Aa |
| E | 126 | 0.80 | 0.26 ± 0.01 (0.89) Aa | 0.95 ± 0.55 (1.00) Aa | 0.26 ± 0.03 (0.89) Aa | 0.30 ± 0.05 (0.89) Aa |
To achieve a 5-log reduction in peanut butter A, significantly more time (108.08 min) was required for S. Tennessee than for S. Typhimurium (48.14 min) or S. Enteritidis (66.69 min), indicating that S. Tennessee was the most heat-resistant serotype tested (Table 2). To achieve the same 5-log reduction at an aw of 0.8, less heating time was required for all serotypes; therefore, increased water activity diminished the difference in thermal resistance among the different serotypes. In peanut butter E at an aw of 0.2, similar patterns of heat resistance were observed; however, when heated at an aw of 0.8, all serotypes decreased to below detection limits, suggesting that the higher fat and lower carbohydrate contents may lead to reduced heat resistance of S. enterica.
Table 2.
Calculated minimum times to achieve 1- to 7-log reductions of S. enterica serotypes at 90°C in peanut butter, based on the Weibull model
| Peanut butter | aw | Bacterial serotype | Calculated minimum time (min) to reach growth reduction ofa: | _r_2 | |||
|---|---|---|---|---|---|---|---|
| 1 log | 3 logs | 5 logs | 7 logs | ||||
| A | 0.2 | S. Enteritidis | 15.12 ± 2.81A | 41.12 ± 18.18AB | 66.69 ± 37.72A | 92.38 ± 59.82A | 0.95 |
| S. Typhimurium | 8.22 ± 2.39B | 27.36 ± 7.37A | 48.14 ± 13.97A | 70.12 ± 21.92A | 0.98 | ||
| S. Tennessee | 13.44 ± 7.14A | 55.16 ± 43.63BC | 108.08 ± 97.44B | 169.49 ± 164.46B | 0.93 | ||
| Three-serotype cocktail | 17.97 ± 3.16C | 68.74 ± 11.68C | 131.91 ± 41.30B | 204.91 ± 83.37B | 0.98 | ||
| A | 0.4 | S. Enteritidis | 5.27 ± 1.34A | 24.84 ± 3.40A | 51.72 ± 9.23A | 84.41 ± 19.89A | 0.97 |
| S. Typhimurium | 2.44 ± 1.66BC | 16.52 ± 6.60A | 43.09 ± 17.69A | 82.59 ± 35.13A | 0.95 | ||
| S. Tennessee | 6.29 ± 1.07A | 19.46 ± 4.58A | 33.03 ± 9.18A | 46.92 ± 14.41A | 0.96 | ||
| Three-serotype cocktail | 5.05 ± 2.00AC | 25.53 ± 13.35A | 57.01 ± 37.43A | 98.31 ± 72.60A | 0.92 | ||
| A | 0.6 | S. Enteritidis | 8.73 ± 0.75A | 25.55 ± 4.77A | 42.81 ± 12.27A | 60.53 ± 21.03A | 0.93 |
| S. Typhimurium | 3.91 ± 0.45B | 12.50 ± 2.9A | 21.80 ± 7.22A | 31.66 ± 12.51A | 0.93 | ||
| S. Tennessee | 7.32 ± 1.18AC | 22.32 ± 6.96A | 37.88 ± 14.71A | 53.93 ± 23.54A | 0.98 | ||
| Three-serotype cocktail | 6.06 ± 1.74BC | 16.70 ± 1.10A | 27.21 ± 2.44A | 37.81 ± 6.16A | 0.97 | ||
| A | 0.8 | S. Enteritidis | 3.90 ± 0.87A | 10.89 ± 2.01A | 17.55 ± 2.96A | 24.06 ± 3.85A | 0.93 |
| S. Typhimurium | 2.96 ± 0.74A | 9.41 ± 0.62A | 16.29 ± 1.28A | 23.49 ± 2.86A | 0.91 | ||
| S. Tennessee | 4.18 ± 0.98A | 10.37 ± 1.69A | 15.85 ± 2.14A | 21.01 ± 2.56A | 0.93 | ||
| Three-serotype cocktail | 3.73 ± 0.60A | 11.50 ± 2.70A | 19.93 ± 7.12A | 28.90 ± 12.59A | 0.93 | ||
| E | 0.2 | S. Enteritidis | 8.26 ± 3.35A | 31.18 ± 1.86A | 59.20 ± 4.93AC | 91.05 ± 13.58AC | 0.95 |
| S. Typhimurium | 6.32 ± 1.84A | 20.06 ± 6.48B | 34.39 ± 11.93B | 49.13 ± 17.96B | 0.98 | ||
| S. Tennessee | 12.08 ± 2.63B | 38.35 ± 10.70C | 67.60 ± 29.48A | 99.50 ± 55.43A | 0.96 | ||
| Three-serotype cocktail | 10.98 ± 3.17B | 31.34 ± 4.53A | 52.12 ± 10.19C | 73.44 ± 18.33C | 0.98 | ||
| E | 0.4 | S. Enteritidis | 6.62 ± 2.07A | 24.82 ± 5.91A | 46.14 ± 10.38A | 69.66 ± 15.73A | 0.98 |
| S. Typhimurium | 2.69 ± 0.44B | 11.80 ± 3.31B | 23.83 ± 8.77B | 38.13 ± 16.29B | 0.93 | ||
| S. Tennessee | 7.32 ± 1.04A | 31.92 ± 2.76C | 63.35 ± 4.57C | 99.70 ± 7.05C | 0.97 | ||
| Three-serotype cocktail | 8.08 ± 0.75A | 25.08 ± 1.08A | 42.59 ± 3.46A | 60.50 ± 6.84AB | 0.94 | ||
| E | 0.6 | S. Enteritidis | 5.96 ± 1.09A | 19.81 ± 4.37A | 34.92 ± 9.65A | 50.97 ± 16.13A | 0.92 |
| S. Typhimurium | 2.94 ± 0.34B | 10.90 ± 0.49B | 20.09 ± 1.16B | 30.11 ± 2.46A | 0.94 | ||
| S. Tennessee | 5.40 ± 0.50A | 14.83 ± 2.88AB | 24.06 ± 7.06AB | 33.28 ± 11.96A | 0.89 | ||
| Three-serotype cocktail | 5.94 ± 1.63A | 14.91 ± 4.20AB | 22.93 ± 6.71AB | 30.51 ± 9.23A | 0.91 | ||
| E | 0.8 | S. Enteritidis | NA | NA | NA | NA | NA |
| S. Typhimurium | NA | NA | NA | NA | NA | ||
| S. Tennessee | NA | NA | NA | NA | NA | ||
| Three-serotype cocktail | NA | NA | NA | NA | NA |
Statistical comparisons of the minimum times for achieving a 5-log reduction and correpsonding D values indicated that S. Typhimurium and S. Tennessee were the least and the most heat-resistant S. enterica serotypes, respectively, in both peanut butter formulations tested. The serotype-specific difference in heat resistance was most significant when S. enterica was treated at 90°C in peanut butter with an aw of 0.2. When subjected to a higher temperature (126°C) or an increased aw (0.8), no significant difference in heat resistance was detected among the three serotypes.
The cellular morphology of S. enterica during desiccation and rehydration was monitored in both peanut oil (aw, 0.2) and in PBS. Peanut oil was used instead of peanut butter because the separation, fixation, and scanning electron microscopy (SEM) imaging of bacteria in peanut butter were technically infeasible. Bacteria were prepared for SEM as previously described (26) with minor modifications, including primary fixation with 2.5% glutaraldehyde in 0.1 M cacodylate buffer at pH 7.2 and final drying with 100% hexamethyldisilazane (EMS, Hatfield, Pennsylvania). Samples were examined using a JSM-6320F field emission scanning electron microscope (JEOL Orion system) at an instrument magnification of ×10,000. A minimum of 30 bacterial cells per serotype per treatment was randomly selected for cell diameter size measurements.
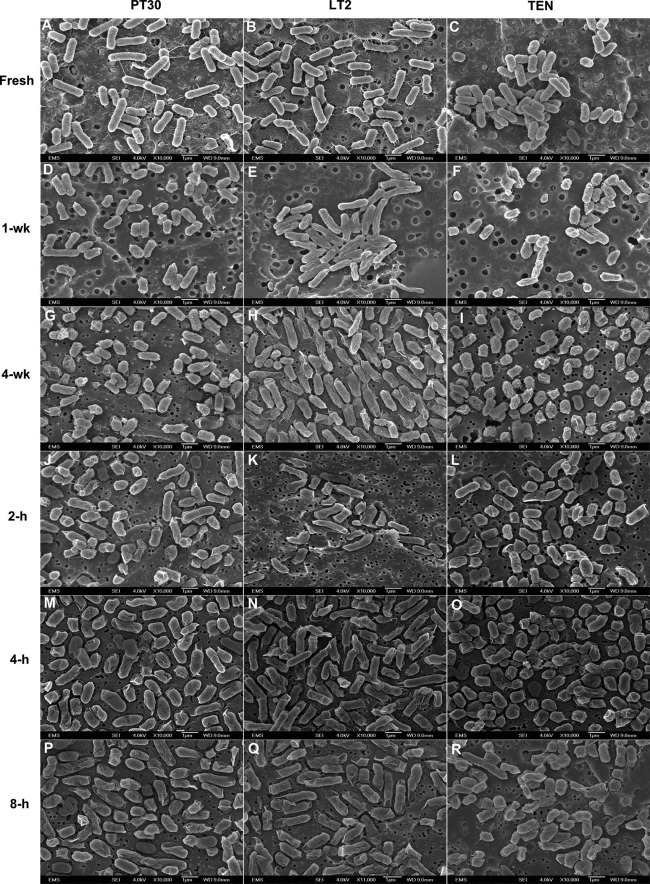
Figure 2 shows the morphological alterations of S. Enteritidis, S. Typhimurium, and S. Tennessee under desiccation stress in low-aw peanut oil over the 4-week storage period and subsequent 8-h rehydration in PBS. Desiccated cell diameters decreased by 21% for S. Enteritidis and by 8.5% for S. Tennessee. The average cell diameters of S. Typhimurium did not change significantly (see Table S1 in the supplemental material). Whether the reduced cell size was a response to the low water activity, which would contribute to the increased heat resistance of S. enterica, is difficult to ascertain without more experimentation. Following rehydration, slight increases in cellular size for all three serotypes were observed. Decreased cell sizes have been reported when S. enterica expresses the rdar morphotype at low temperature and under starvation and desiccation stresses (27–30). Because low moisture is a common environmental stress that S. enterica encounters on peanut shells in preharvest environments, in curing steps, and in finished peanut butter products, this reduced cellular size may constitute an adaptation strategy to the low-aw stress. Such a stress adaptation may subsequently cross-protect the bacteria from other environmental challenges, such as heat, and make the desiccation-stressed S. enterica bacteria more heat resistant.
Fig 2.
Scanning electron micrographs at a magnification of ×10,000 of fresh S. enterica cells in BHI broth (A, B, and C), desiccated S. enterica cells after 1-week (D, E, and F) or 4-week (G, H, and I) incubation in peanut oil (aw, 0.2) at 25°C, or desiccated S. enterica cells after 2-h (J, K, and L), 4-h (M, N, and O), or 8-h (P, Q, and R) rehydration in PBS at 25°C. PT30, S. Enteritidis BSS-1045; LT2, S. Typhimurium LT2; TEN, S. Tennessee K4643.
Supplementary Material
Supplemental material
ACKNOWLEDGMENTS
This work was supported by the Food Research Initiative, grant 2010-65201-20593 from the USDA National Institute of Food Agriculture, Food Safety and Epidemiology: Biological Approaches for Food Safety program (program code 93231).
The sponsor had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 31 May 2013
REFERENCES
- 1.CDC 2007. Multistate outbreak of Salmonella serotype Tennessee infections associated with peanut butter—United States, 2006–2007. MMWR Morb. Mortal. Wkly. Rep. 56:521–524 [PubMed] [Google Scholar]
- 2.CDC 2009. Multistate outbreak of Salmonella infections associated with peanut butter and peanut butter-containing products—United States, 2008–2009. MMWR Morb. Mortal. Wkly. Rep. 58:85–90 [PubMed] [Google Scholar]
- 3.CDC 2013. Notes from the field: Salmonella Bredeney infections linked to a brand of peanut butter—United States, 2012. MMWR Morb. Mortal. Wkly. Rep. 62:107. [PMC free article] [PubMed] [Google Scholar]
- 4.FDA 2009. Guidance for industry. Measures to address the risk for contamination by Salmonella species in food containing a peanut-derived product as an ingredient. Center for Food Safety and Applied Nutrition, FDA, College Park, MD: http://www.fda.gov/Food/GuidanceDocumentsRegulatoryInformation/ProducePlantProducts/ucm115386.htmAccessed 30 March 2009 [Google Scholar]
- 5.Roberson S, Marion JE, Woodroof JG. 1966. Composition of commercial peanut butters. J. Am. Diet. Assoc. 49:208–210 [PubMed] [Google Scholar]
- 6.Hiramatsu R, Matsumoto M, Sakae K, Miyazaki Y. 2005. Ability of Shiga toxin-producing Escherichia coli and Salmonella spp. to survive in a desiccation model system and in dry foods. Appl. Environ. Microbiol. 71:6657–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnett SL, Gehm ER, Weissinger WR, Beuchat LR. 2000. Survival of Salmonella in peanut butter and peanut butter spread. J. Appl. Microbiol. 89:472–477 [DOI] [PubMed] [Google Scholar]
- 8.Scheil W, Cameron S, Dalton C, Murray C, Wilson D. 1998. A South Australian Salmonella Mbandaka outbreak investigation using a database to select controls. Aust. N. Z. J. Public Health 22:536–539 [DOI] [PubMed] [Google Scholar]
- 9.Woodroof JG. 1969. Composition and use of peanuts in the diet. World Rev. Nutr. Diet. 11:142–169 [DOI] [PubMed] [Google Scholar]
- 10.Goepfert JM, Biggie RA. 1968. Heat resistance of Salmonella Typhimurium and Salmonella Senftenberg 775W in milk chocolate. Appl. Microbiol. 16:1939–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shachar D, Yaron S. 2006. Heat tolerance of Salmonella enterica serovars Agona, Enteritidis, and Typhimurium in peanut butter. J. Food Prot. 69:2687–2691 [DOI] [PubMed] [Google Scholar]
- 12.Corry J. 1976. The safety of intermediate moisture foods with respect to Salmonella, p 215–238 Davies R, Birch G, Parker K. (ed), Intermediate moisture foods. Applied Science Publishers Ltd., London, England [Google Scholar]
- 13.D'Aoust J. 1989. Salmonella, p 327–445 Doyle M. (ed), Foodborne bacterial pathogens. Marcel Dekker, New York, NY [Google Scholar]
- 14.Gibson B. 1973. The effect of high sugar concentrations on the heat resistance of vegetative micro-organisms. J. Appl. Bacteriol. 36:365–376 [DOI] [PubMed] [Google Scholar]
- 15.He Y, Guo D, Yang J, Tortorello ML, Zhang W. 2011. Survival and heat resistance of Salmonella enterica and Escherichia coli O157:H7 in peanut butter. Appl. Environ. Microbiol. 77:8434–8438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan ES, Aramini J, Ciebin B, Middleton D, Ahmed R, Howes M, Brophy I, Mentis I, Jamieson F, Rodgers F, Nazarowec-White M, Pichette SC, Farrar J, Gutierrez M, Weis WJ, Lior L, Ellis A, Isaacs S. 2002. Natural or raw almonds and an outbreak of a rare phage type of Salmonella Enteritidis infection. Can. Commun. Dis. Rep. 28:97–99 [PubMed] [Google Scholar]
- 17.Isaacs S, Aramini J, Ciebin B, Farrar JA, Ahmed R, Middleton D, Chandran AU, Harris LJ, Howes M, Chan E, Pichette AS, Campbell K, Gupta A, Lior LY, Pearce M, Clark C, Rodgers F, Jamieson F, Brophy I, Ellis A. 2005. An international outbreak of salmonellosis associated with raw almonds contaminated with a rare phage type of Salmonella Enteritidis. J. Food Prot. 68:191–198 [DOI] [PubMed] [Google Scholar]
- 18.Danyluk MD, Nozawa-Inoue M, Hristova KR, Scow KM, Lampinen B, Harris LJ. 2008. Survival and growth of Salmonella Enteritidis PT 30 in almond orchard soils. J. Appl. Microbiol. 104:1391–1399 [DOI] [PubMed] [Google Scholar]
- 19.Neidhardt FC, Curtiss R, III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE. (ed). 1996. Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed ASM Press, Washington DC [Google Scholar]
- 20.McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L, Porwollik S, Ali J, Dante M, Du F, Hou S, Layman D, Leonard S, Nguyen C, Scott K, Holmes A, Grewal N, Mulvaney E, Ryan E, Sun H, Florea L, Miller W, Stoneking T, Nhan M, Waterston R, Wilson RK. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852–856 [DOI] [PubMed] [Google Scholar]
- 21.Ma L, Zhang G, Gerner-Smidt P, Mantripragada V, Ezeoke I, Doyle MP. 2009. Thermal inactivation of Salmonella in peanut butter. J. Food Prot. 72:1596–1601 [DOI] [PubMed] [Google Scholar]
- 22.Woodroof J. 1983. Peanuts: production, processing, products, 3rd ed Avi Publishing Company, Westport, CT [Google Scholar]
- 23.Edwards D, Berry JJ. 1987. The efficiency of simulation-based multiple comparisons. Biometrics 43:913–928 [PubMed] [Google Scholar]
- 24.van Boekel MA. 2002. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Int. J. Food Microbiol. 74:139–159 [DOI] [PubMed] [Google Scholar]
- 25.Corradini MG, Peleg M. 2004. Demonstration of the applicability of the Weibull-log-logistic survival model to the isothermal and nonisothermal inactivation of Escherichia coli K-12 MG1655. J. Food Prot. 67:2617–2621 [DOI] [PubMed] [Google Scholar]
- 26.Wen J, Anantheswaran RC, Knabel SJ. 2009. Changes in barotolerance, thermotolerance, and cellular morphology throughout the life cycle of Listeria monocytogenes. Appl. Environ. Microbiol. 75:1581–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White AP, Gibson DL, Kim W, Kay WW, Surette MG. 2006. Thin aggregative fimbriae and cellulose enhance long-term survival and persistence of Salmonella. J. Bacteriol. 188:3219–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spector MP. 1998. The starvation-stress response (SSR) of Salmonella. Adv. Microb. Physiol. 40:233–279 [DOI] [PubMed] [Google Scholar]
- 29.Römling U, Sierralta WD, Eriksson K, Normark S. 1998. Multicellular and aggregative behaviour of Salmonella Typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol. 28:249–264 [DOI] [PubMed] [Google Scholar]
- 30.Römling U. 2005. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell. Mol. Life Sci. 62:1234–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material