Bioenhancers from mother nature and their applicability in modern medicine (original) (raw)
Abstract
Concept of bioenhancers or biopotentiators was first time reported in 1929 by Bose. A bioenhancer is an agent capable of enhancing bioavailability and efficacy of a drug with which it is co-administered, without any pharmacological activity of its own at therapeutic dose used. Development and consequent isolation of these molecules, such as piperine and quercetin, is considered as scientific breakthrough. A fixed drug combination (Risorine) of rifampicin, isoniazid, and piperine is the result of this research. It contains almost 60% less dose of rifampicin because of its increased bioavailability and it also prevents resistance. This concept is mentioned as yogvahi in ayurveda and was used to increase the effect of medicines by increasing oral bioavailability, decreasing adverse effects and to circumvent parenteral routes of drug administration. More such useful and economically viable drug combinations can be developed by integrating knowledge of time tested ayurveda with modern methods of research. This review is an account of these bioenhancers, available from the natural resources.
Keywords: Biopotentiators, piperine, yogvahi
INTRODUCTION
The concept of bioenhancers or biopotentiators is new to the modern science. It was first time reported by Bose in 1929, who described the increase in the antiasthmatic effects of vasaka (Adhatoda vasica) leaves by the addition of long pepper to it.[1] The development and consequent isolation of these molecules is considered as a scientific breakthrough. A bioenhancer is an agent capable of enhancing the bioavailability and efficacy of a drug with which it is co-administered, without any pharmacological activity of its own at the therapeutic dose used. They tend to decrease the dose of active drug required for the optimal endpoint of the treatment strategy, bypassing the need to use injectable routes of drug administration to a larger extent, might help in overcoming the resistance to antimicrobials and saving the precious raw materials for the manufacturing of medicines. Such fixed drug combinations (FDCs) are economically viable too.
The concept of bioenhancer is called Yogvahi in Ayurveda. Synergism, that is, increase in the action of one biomolecule by another unrelated chemical is the hallmark of polyherbal formulations of ayurveda. Yogvahi is used to enhance the bioavailability, tissue distribution, and efficacy of drugs, especially with poor oral bioavailability and decreasing the adverse effects in the process. Specific yogvahis or bioenhancers are termed as Anupaan and Sehpaan. Anupaan means food concomitantly given with the medicament to increase the effect of the medicament, such as “Amrit Dhara” drops used for gastrointestinal diseases are ingested after putting the drops over sugar, to increase their potency. Sehpaan means that the vehicle, which is used during the manufacturing of the medicament increases the effect of the medicament, like for panchgavya ghrit and brahmi ghrit, clarified butter/ghee/ghrit is used. General yogvahis routinely used in many ayurvedic preparations are trikatu [Piper longum (long pepper/ pippali), Piper nigrum (black pepper/ kali mirch) and Zingiber officinale (ginger/ adrak)],[2] sesame/til, gold/ swarn bhasam, and heerak bhasm[3] and cow urine distillate.[4]
Modern researchers are increasingly showing interest toward the improvement of bioavailability of a large number of drugs by addition of various herbs with bioenhancing properties. Of the promising approaches being used are absorption enhancers, prodrugs, micronization, and manufacturing of delayed release, timed release, sustained release capsules and spansules, and permeability-enhancing dosage forms, such as liposomes and emulsions. Recently, the application of P-glycoprotein (P-gp) inhibitors in improving oral drug delivery has gained special interest.[5,6]
In oral drug delivery system, the co-administration of therapeutic agents with natural compounds possessing absorption improving activities, has also garnered great interest. Active components of these natural compounds with bioenhancing properties, such as piperine, quercetin, genistein, naringin, niazeridine, lysergols, capmul, Callistemon rigidus, Carum carvi, sinomenine, glycyrrhizin, and nitrile glycoside, are being isolated for their possible use along with modern medicines.
Exhaustive ayurvedic literature search led to the identification and isolation of piperine, an alkaloid, from _Piper longum_—the world's first purified bioenhancer molecule.[7] Piperine obtained from botanical sources is about 98% pure. The Ayurvedic Materia Medica mentions trikatu as an essential ingredient either in combination or alone of many formulations used for a wide range of diseases.[8] Recently Risorine, an FDC of rifampicin, isoniazid, and piperine was marketed for the management of tuberculosis, resulting in a decrease of rifampicin dose from 450 to 200 mg, with 60% improvement in its bioavailability.
Piperine act by suppressing P-gp and cytochrome P450 enzymes, which counteract the metabolism of rifampicin via these proteins, thus enhancing the oral bioavailability of rifampicin. It also decreases the intestinal production of glucuronic acid, thus allowing more substances to enter the body in active form. It was found to increase the bioavailability of various drugs from 30% to 200%.[25]
PIPERINE AS BIOENHANCER
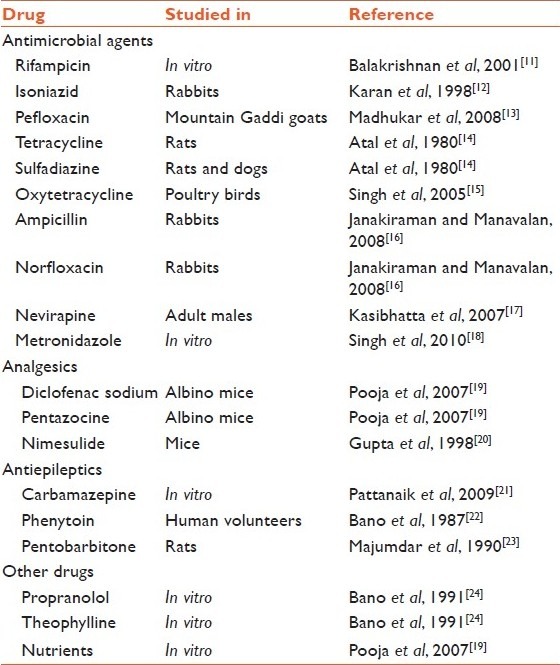
Piperine, obtained from the oleoresin in the peppercorns is by far the most studied and researched bioenhancer. It improves the bioavailability of other nutritive substances, including β-carotene, curcumin, selenium, pyroxidine, glucose, and amino acids[9] and coenzyme Q10, and gallic acid. Piperine increases area under the curve (AUC) of phenytoin, propranolol, and theophylline in healthy volunteers and plasma concentrations of rifamipicin in patients with pulmonary tuberculosis.[10] A lot of research is being carried out on piperine and its bioenhancing effect on various modern medicines [Table 1].
Table 1.
Published research on bioenhancer effect of piperine with various medicines
Piperine and antitubercular treatment
Risorine is a formulation developed by Indian Institute of Integrative Medicine, Jammu, and marketed in India in November 2009 in public–private partnership with Cadila Pharmaceutical Ltd, Ahmedabad. Risorine has been approved for marketing by Drug Controller General of India, after successful completion of all the phased clinical trials. It contains rifampicin (200 mg), isoniazid (300 mg), and piperine (10 mg). It has been found to be bioequivalent with commercially available rifampicin preparations. This is due to enhanced uptake of the drug by body cells, and also because the drug remains available in blood for longer durations. Combining piperine with rifampicin decreases the dose of rifampicin from 450 to 200 mg.[25]
Interestingly, in a multicentric clinical trial conducted across India in patients with radiologically confirmed diagnosis of pulmonary tuberculosis, more than 90% of the patients treated with Risorine were cured of tuberculosis with lesser side effects.[25] A formulation, containing rifampicin, isoniazid, pyrazinamide, and piperine have been tested in human volunteers (Indian Patent No.1232/DEL/89). In the majority of cases, the comparative levels and peak concentration of the drugs in the presence of piperine were higher.[26]
A 24:1 (w/w) mixture of rifampicin and piperine showed remarkable growth inhibition, which was higher than that of rifampicin alone. This combination acted by completely abolishing the transcriptional activity of rifampicin-resistant RNA polymerase. Interestingly, piperine alone, even at higher concentration, did not inhibit the growth of mycobacteria.[11] This combination may also reduce the emergence of multiple drug—resistant strains of mycobacterium.
In a conflicting report, rabbits treated with a single dose of trikatu (500 mg/kg × 7 days, p.o.) showed a significant decrease in the peak plasma concentration (_C_max) of rifampicin (24 mg/kg, p.o.) (P < 0.05). Multiple doses of trikatu also reduced the _C_max and delayed the _T_max of rifampicin, although not to a statistically significant level.[27] It could be due to the higher dose (500 mg/kg) of piperine used in this study, whereas in other studies the dose used was much lower (10 mg).
Piperine and antimicrobial agents
The pharmacokinetics of orally administered pefloxacin was evaluated in 6 mountain Gaddi goats for the bioenhancing effect of the herbal bioenhancer, trikatu. Overall, higher values for the AUC, the area under the first moment of the plasma drug concentration time curve, the mean residential time, the total duration of pharmacological action and bioavailability were observed for pefloxacin. Co-administration of trikatu, however, significantly reduced the elimination half-life. The apparent volume of distribution was significantly higher in trikatu-treated animals, indicating a better penetration of the drug.[13]
The oral bioavailability of ampicillin is 62%±17% and that of norfloxacin is 30%–40% alone. When piperine is administered concomitantly, AUC was observed to be increased by 338% with ampicillin and by 174.6% with norfloxacin in rabbits.[16]
Piperine and analgesics
Piper nigrum extract (10 mg/kg orally) significantly enhanced the analgesic activity of diclofenac sodium (5 mg/kg) and pentazocine (5 mg/kg). P. nigrum extract alone did not show any significant analgesic activity in tail flick and writhing methods in albino mice. P. nigrum extract and diclofenac sodium combination produced significant decrease in writhes, which was much higher (78.43%) than diclofenac sodium alone (54.90%). P. nigrum extract combined with pentazocine showed a significant increase (P < 0.05) in tail flick latency in comparison with pentazocine alone and control group.[19]
In another study by Lala et al, it was found that the anti-inflammatory effect of trikatu (1:1:1 ratio of P. nigrum, P. longum, and Z. officinale) alone and in combination with diclofenac sodium was similar in a carragenin-induced rat paw edema model in rabbits.[28] Piperine showed a dose-dependent synergistic effect on nimesulide-induced antinociception in the acetic acid-induced writhing test in mice. Piperine significantly (P < 0.001) increased the analgesic activity of nimesulide.[20]
Piperine and other drugs
Piperine (20 mg p.o.) significantly increased the mean plasma concentrations of carbamazepine (300 or 500 mg twice daily) in both dose groups. There was a significant increase in AUC (0–12 h) (P < 0.001), average C (ss) (P < 0.001), t (1\2el) (P < 0.05) and a decrease in K (el) (P < 0.05), in both the dose groups. Piperine could significantly enhance the oral bioavailability of carbamazepine, phenytoin, and pentobarbitone possibly by decreasing the elimination and/or by increasing its absorption.[21–23]
A highly significant increase in the systemic availability and AUC of propranolol was observed when administered along with piperine, while its elimination kinetics was not changed. A higher _C_max, a longer elimination half-life and a larger AUC were observed when theophylline was administered after piperine.[24]
In a study on New Zealand rabbits, _C_max value of 3.9 ± 2.38 mg/mL with metronidazole alone and 6.0 ± 3.4 mg/mL with a combination of metronidazole and piperine was obtained. This represents an increase of 57% in peak plasma levels of metronidazole. Plasma t1/2 of metronidazole increased from 11.48 to 12.24 h when it was administered with piperine. This combination may result in a reduced strength dosage form and also reduced dose-dependent side effects.[18]
LD50 of piperine has been found to be 330 and 514 mg/kg in mice and rats, respectively. In subacute toxicity tests, piperine in a dosage of 100 mg/kg was found to be nontoxic.[29]
OTHER BIOENHANCERS
Bioflavonoids
Bioflavonoids were first discovered by the Nobel Prize laureate, Albert Szent Gyorgyi in 1930. They have been extensively studied by researchers for the past 30 years. Three bioflavonoids, namely quercetin, genistein, and naringin are known to enhance the activity of certain drugs.
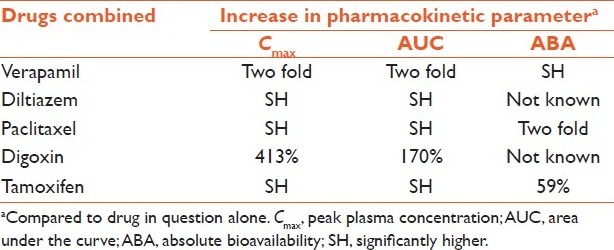
Quercetin is found in citrus fruits and is a dual inhibitor of cytochrome P 3A4 (CYP3A4) and P-gp. Quercetin has exhibited a wide range of beneficial biological activities, including antioxidant, radical scavenging, anti-inflammatory, antiatherosclerotic, antitumor, and antiviral effects.[30] In a study, pretreatment of quercetin (5.0 and 15 mg/kg) half an hour before verapamil (10 mg/kg) administration significantly altered the pharmacokinetics of verapamil. Compared with verapamil alone group, the _C_max and AUC of verapamil increased approximately two times in the rabbits pretreated with quercetin. Absolute and relative bioavailability values of verapamil in the rabbits pretreated with quercetin were significantly higher (P < 0.05) than verapamil alone group.[31] Similar results have been reported with pretreatment/co-treatment of quercetin with diltiazem,[32] paclitaxel,[33] digoxin,[34] doxorubicin,[35] and tamoxifen[36] Table 2.
Table 2.
Effect of quercetin pretreatment/co-treatment on pharmacokinetic parameters of different drugs
The relative bioavailability of paclitaxel after administration of the prodrug to rats pretreated with quercetin was 1.25–2.02-folds higher than the prodrug control. The development of oral paclitaxel preparations as a prodrug or with quercetin is feasible, which is more convenient than the intravenous dosage forms.[33]
Another flavonoid, genistein belongs to the isoflavone class of flavonoids. It is a well-known phytoestrogen. The presence of genistein (10 mg/kg) caused an increase in AUC (54.7%) and a decrease in the total plasma clearance (35.2%) after oral administration of paclitaxel at a dose of 30 mg/kg in rats.[37]
Naringin is the major flavonoid glycoside found in grapefruit and makes grapefruit juice taste bitter. Oral naringin (3.3 and 10 mg/kg) was pretreated 30 min before and after intravenous administration of paclitaxel (3 mg/kg), the AUC was significantly improved (40.8% and 49.1% for naringin doses of 3.3 and 10 mg/kg, respectively).[38]
Callistemon rigidus R.Br
Fraction F5, isolated from crude extract of leaves of C rigidus R.Br. was active against ciprofloxacin-resistant Staphylococcus aureus at a very low concentration (39.06 μg/mL) in combination with the resistant drug. Fraction F5 exhibits powerful in vitro activity against control as well as mutant strain of S. aureus and synergistic interaction with resistant drug (ciprofloxacin).[39]
Carum carvi/Cuminum cyminum (Jeera)
Carum carvi seeds are a prized culinary herb. Extracts of its parts increased significantly (25%–300%), the bioavailability of a number of classes of drugs, such as antibiotics, antifungals, antivirals, anticancer, cardiovascular, anti-inflammatory/antiarthritic, anti-TB, antileprosy, antihistaminic/respiratory disorders, corticosteroids, immunosuppressants, and antiulcers. Such extracts either in the presence or absence of piperine have been found to be highly selective in their bioavailability/bioefficacy-enhancing action.[40]
Capmul
One of the widely used bioenhancers is Capmul MCM C10, a glyceryl monocaprate, produced from edible fats and oils and is commonly used in lip products. In a study in rats, antibiotic ceftriaxone when given concomitantly with capmul, increased the bioavailability of ceftriaxone by 80%.[41]
Nitrile glycoside
Nitrite glycoside is a bioenhancer for drugs and nutrients. Novel bioactive nitrile glycosides, niaziridin and niazirin is obtained from the leaves, pods, and bark of Moringa oleifera.[42] An immunoenhancing polysaccharide and niaziminin, having structural requirement to inhibit tumor promoter-induced Epstein–Barr virus activation have been reported from the leaves of Moringa.[43,44] It enhances the bioactivity of commonly used antibiotics, such as rifampicin, tetracycline, and ampicillin, and also facilitate the absorption of drugs, vitamins, and nutrients through the gastrointestinal membrane, thus increasing their bioavailability.[41] Niazirin is another bioactive nitrile glycoside belonging to M. oleifera.[45,46] Process of isolation of nitrite glycoside from M. oleifera has been patented (US 6858588) by Khanuja et al in 2004–2005.[42]
Cow urine distillate
Cow urine distillate is more effective as bioenhancer than cow urine, to increase the effectiveness of antimicrobial, antifungal, and anticancer drugs.[47] The cow urine has been granted US Patents (No. 6 896 907 and 6 410 059) for its medicinal properties, particularly as a bioenhancer along with antibiotics and antifungal and anticancer drugs. Potency of paclitaxel has been observed to increase against MCF-7, a human breast cancer cell line, in in vitro assays (US Patent No. 6,410,059).[48]
Cow urine distillate increased the activity of rifampicin by about 5–7 times against Escherichia coli and 3–11 times against gram-positive bacteria. It probably acts by enhancing the transport of antibiotics across the membrane of gastrointestinal tract. The enhancement in transport is approximately 2–7 times.[25] The gonadotropin releasing hormone conjugate has a deleterious effect on the reproductive hormones and estrous cycle of female mice; and cow urine distillate acts as a bioenhancer in immunization efficacy to modulate these effects.[49]
Cow urine has antitoxic activity against the cadmium chloride toxicity and it can be used as a bioenhancer of zinc. Mature male mice, Mus musculus, exposed to cadmium chloride only, showed 0% fertility rate. Fertility index increased to 88% in the group treated with cow urine along with cadmium chloride. However, the animals exposed to cadmium chloride + cow urine + zinc sulfate showed 90% fertility rate with 100% viability and lactation indices.[50]
MECHANISM OF ACTION OF BIOENHANCERS
Bioavailability-enhancing activity of natural compounds from the medicinal plants may be attributed to various mechanisms, such as P-gp inhibition activity by flavone, quercetin, and genistein;[51] inhibition of efflux transporters, such as P-gp and breast cancer resistance protein (BCRP),[52,53] by naringin and sinomenine thus preventing drug resistance; DNA receptor binding, modulation of cell signaling transduction, and inhibition of drug efflux pumps[54–56]; by stimulating leucine amino peptidase and glycyl-glycine dipeptidase activity, thus modulating the cell membrane dynamics related to passive transport mechanism as seen with piperine[57]; nonspecific mechanisms, such as increased blood supply to the gastrointestinal tract, decreased hydrochloric acid secretion, preventing breakdown of some drugs[6]; and inhibition of metabolic enzymes participating in the biotransformation of drugs, thus preventing inactivation and elimination of drugs and thereby, increasing their bioavailability.[57–59]
FUTURE PERSPECTIVES
Taking leads from ayurveda and other traditional ways of medicine is nothing new for a modern researcher. Origin of about 75% of antimicrobial and 60% of anticancer drugs approved for clinical use from 1981 to 2002 could be traced back to nature.[60] It has taken lead from the use of “trikatu” as a bioenhancer from ayurveda and successfully applied it to various modern medicines to enhance their bioavailability. The ayurvedic concept of anupaan and sehpaan ought to be incorporated into the modern medicine also. Modern medicine can take the cue from the ayurvedic leads to develop more efficacious and safe medicines with safer routes of drug administration in future also. The underlying mechanisms with their clinical outcomes can also be researched and validated further as per the latest research methodologies.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Varshneya C. Use of herbal bioenhancers in animal health care. [Last accessed on 2010 Nov 22]. Available from:http://www.hillagric.ernet.in/edu/covas/vpharma/winter%20school/lectures/34%20Herbal%20bioenhancers.pdf .
- 2.Sharma PV. Dravyaguna-Vijnana: Vegetable drugs. 2nd ed. Varanasi: Chaukhamba Bharati Academy; 2009. p. 109. (121,331-5,362-5). [Google Scholar]
- 3.Pathak R. Ayurved Sar Sangrah. 12th ed. Kolkatta: Shri Baidyanath Ayurved Bhavan Limited; 2010. pp. 176–9. (212-24). [Google Scholar]
- 4.Randhawa GK. Cow urine distillate as bioenhancer. J Ayur Integ Med. 2010;1:240–1. doi: 10.4103/0975-9476.74089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breedveld P, Beijnen JH, Schellens JH. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27:17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Kang MJ, Cho JY, Shim BH, Kim DK, Lee J. Bioavailability enhancing activities of natural compounds from medicinal plants. J Med Plants Res. 2009;3:1204–11. [Google Scholar]
- 7.Atal CK, Dubey RK, Singh J. Biochemical basis of enhanced drug bioavailability by piperine: Evidence that piperine is a potent inhibitor of drug metabolism. J Pharmacol Exp Ther. 1985;232:58. [PubMed] [Google Scholar]
- 8.Atal N, Bedi KL. Bioenhancers: Revolutionary concept to market. J Ayur Integ Med. 2010;1:96–9. doi: 10.4103/0975-9476.65073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh A, Duggal S. Piperine- Review of Advances in Pharmacology. Int J Pharm Sci Nanotechnol. 2009;2:615–20. [Google Scholar]
- 10.Hu Z, Yang X, Ho PC, Chan SY, Heng PW, Chan E, et al. Herb-drug interactions: A literature review. Drugs. 2005;65:1239–82. doi: 10.2165/00003495-200565090-00005. [DOI] [PubMed] [Google Scholar]
- 11.Balakrishnan V, Varma S, Chatterji D. Piperine augments transcription inhibitory activity of rifampicin by several fold in Mycobacterium smegmatis. Curr Sci. 2001;80:1302–5. [Google Scholar]
- 12.Karan RS, Bhargava VK, Garg SK. Effect of Trikatu (Piperine) on the pharmacokinetic profile of isoniazid in rabbits. Ind J Pharmacol. 1998;30:254–6. [Google Scholar]
- 13.Dama MS, Varshneya C, Dardi MS, Katoch VC. Effect of trikatu pretreatment on the pharmacokinetics of pefloxacin administered orally in mountain Gaddi goats. J Vet Sci. 2008;9:25–9. doi: 10.4142/jvs.2008.9.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atal CK, Manavalan R, Nighojkar R, Sareen AN, Gupta OP. Studies on Piper chaba as a bioavailable agents. Ind drugs. 1980;17:266–8. [Google Scholar]
- 15.Singh M, Varshneya C, Telang RS, Srivastava AK. Alteration of pharmacokinetics of oxytetracycline following oral administration of Piper longum in hens. J Vet Sci. 2005;6:197–200. [PubMed] [Google Scholar]
- 16.Janakiraman K, Manavalan R. Studies on effect of piperine on oral bioavailability of ampicillin and norfloxacin. Afr J Tradit Complement Altern Med. 2008;5:257–62. doi: 10.4314/ajtcam.v5i3.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasibhatta R, Naidu MU. Influence of piperine on the pharmacokinetics of nevirapine under fasting conditions: A randomized, crossover, placebo-controlled study. Drugs R D. 2007;8:383–91. doi: 10.2165/00126839-200708060-00006. [DOI] [PubMed] [Google Scholar]
- 18.Singh A, Pawar VK, Jakhmola V, Parabia MH, Awasthi R, Sharma G. In - Vivo Assessment of Enhanced Bioavailability of Metronidazole with Piperine in Rabbits. Res J Pharm Biol Chem Sci. 2010;1:273–8. [Google Scholar]
- 19.Pooja S, Agrawal RP, Nyati P, Savita V, Phadnis P. Analgesic Activity Of Piper Nigrum Extract Per Se And Its Interaction With Diclofenac Sodium And Pentazocine In Albino Mice. Internet J Pharmacol. 2007;5(1):30. [Google Scholar]
- 20.Gupta SK, Velpandian T, Sengupta TS, Mathur P, Sapra P. Influence of piperine on nimesulide induced antinociception. Phytoth Res. 1998;12:266–9. [Google Scholar]
- 21.Pattanaik S, Hota D, Prabhakar S, Kharbanda P, Pandhi P. Pharmacokinetic interaction of single dose of piperine with steady-state carbamazepine in epilepsy patients. Phytoth Res. 2009;23:1281–6. doi: 10.1002/ptr.2676. [DOI] [PubMed] [Google Scholar]
- 22.Bano G, Amla V, Raina RK, Johri RK, Zutshi U, Chopra CL. The effect of piperine on kinetics of phenytoin in healthy volunteers. Planta Medica. 1987;53:568–9. doi: 10.1055/s-2006-962814. [DOI] [PubMed] [Google Scholar]
- 23.Majumbdar AM, Dhuley JN, Deshmukh VK, Raman PH, Thorat SL, Naik SR. Effect of piperine on pentobarbitone induced hypnosis. Ind J Exp Biol. 1990;28:486–7. [PubMed] [Google Scholar]
- 24.Bano G, Raina RK, Zutshi U, Bedi KL, Johri RK, Sharma SC. Effect of piperine on bioavailability and pharmacokinetics of propranolol and theophylline in healthy volunteers. Eur J Clin Pharmacol. 1991;41:615–7. doi: 10.1007/BF00314996. [DOI] [PubMed] [Google Scholar]
- 25.Chawla PC. Resorine A novel CSIR drug curtails TB treatment. CSIR news. 2010;60:52–4. [Google Scholar]
- 26.Majeed M, Badmaev V, Rajendran R. Use of piperine to increase the bioavailability of nutritional compounds. United States Patent 5536506. 1996 [Google Scholar]
- 27.Karan RS, Bhargava VK, Garg SK. Effect of trikatu, an Ayurvedic prescription, on the pharmacokinetic profile of rifampicin in rabbits. J Ethnopharmacol. 1999;64:259–64. doi: 10.1016/s0378-8741(98)00127-5. [DOI] [PubMed] [Google Scholar]
- 28.Lala LG, D’Mello PM, Naik SR. Pharmacokinetic and pharmacodynamic studies on interaction of “Trikatu” with diclofenac sodium. J Ethnopharmacol. 2004;9:277–80. doi: 10.1016/j.jep.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 29.Piyachaturawat P, Glinsukon T, Toxkulkao C. Acute and subacute toxicity in mice, rats and hamsters. Toxicol Lett. 1983;16:351–9. doi: 10.1016/0378-4274(83)90198-4. [DOI] [PubMed] [Google Scholar]
- 30.Nijveldt RJ, Nood EV, van Hoorn DE, Boelens PG, Norren K, van Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–25. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 31.Choi JS, Han HK. The effect of quercetin on the pharmacokinetics of verapamil and its major metabolite, norverapamil, in rabbits. J Pharm Pharmacol. 2004;56:1537–42. doi: 10.1211/0022357044814. [DOI] [PubMed] [Google Scholar]
- 32.Choi JS, Li X. Enhanced diltiazem bioavailability after oral administration of diltiazem with quercetin to rabbits. Int J Pharm. 2005;13:1–8. doi: 10.1016/j.ijpharm.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Choi JS, Jo BW, Kim YC. Enhanced paclitaxel bioavailability after oral administration of paclitaxel or prodrug to rats pretreated with quercetin. Eur J Pharm Biopharm. 2004;57:313–8. doi: 10.1016/j.ejpb.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Wang YH, Chao PD, Hsiu SL, Wen KC, Hou YC. Lethal quercetin-digoxin interaction in pigs. Life Sci. 2004;74:1191–7. doi: 10.1016/j.lfs.2003.06.044. [DOI] [PubMed] [Google Scholar]
- 35.Lakhanpal P, Rai DK. Quercetin: A Versatile Flavonoid. Internet J Med Update. 2007;2:22–37. [Google Scholar]
- 36.Shin SC, Choi JS, Li X. Enhanced bioavailability of tamoxifen after oral administration of tamoxifen with quercetin in rats. Int J Pharm. 2006;313:144–9. doi: 10.1016/j.ijpharm.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Choi JS. Effect of genistein on the pharmacokinetics of paclitaxel administered orally or intravenously in rats. Int J Pharm. 2007;337:188–93. doi: 10.1016/j.ijpharm.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Lim SC, Choi JS. Effects of naringin on the pharmacokinetics of intravenous paclitaxel in rats. Biopharm Drug Dispos. 2006;27:443–7. doi: 10.1002/bdd.523. [DOI] [PubMed] [Google Scholar]
- 39.Singh G. Thesis. Patiala, Punjab, India: Thapar Institute of Engineering and Technology; 2005. Screening of herbal fractions for antibiotic drug resistance reversal; p. 44. [Google Scholar]
- 40.Qazi GN, Bedi KL, Johri R, Tikoo MK, Tikoo AK, Sharma SC, et al. Bioavailability/bioefficacy enhancing activity of Cuminum cyminum and extracts and fractions thereof. United States Patent 7514105. 2009 [Google Scholar]
- 41.Cho SW, Lee JS, Choi SH. Enhanced oral bioavailability of poorly absorbed drugs. I. Screening of absorption carrier for the ceftriaxone complex. J Pharm Sci. 2004;93:612–20. doi: 10.1002/jps.10563. [DOI] [PubMed] [Google Scholar]
- 42.Khanuja SP, Arya JS, Tiruppadiripuliyur RS, Saikia D, Kaur H, Singh M, et al. Nitrile glycoside useful as a bioenhancer of drugs and nutrients, process of its isolation from Moringa oleifera. United States Patent 6,858,588. 2005 [Google Scholar]
- 43.Mondal S, Chakraborty I, Pramanik M, Rout D, Islamm SS. Structural studies of an immunoenhancing polysaccharide isolated from mature pods (fruits) of Moringa oleifera (Sajina) Med Chem Res. 2004;13:390–400. [Google Scholar]
- 44.Murakami A, Kitazono Y, Jiwajinda S, Koshimzu K, Ohigashi H. Niaziminin, a thiocarbamate from the leaves of Moringa oleifera, holds a strict structural requirement for inhibition of tumor promoter induced Epstein Barr virus activation. Planta Medica. 1998;64:319–23. doi: 10.1055/s-2006-957442. [DOI] [PubMed] [Google Scholar]
- 45.Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani AU. Novel hypotensive agents, niazimin A, niazimin B, niazicin A, and niazicin B from Moringa oleifera: Isolation of first naturally occurring carbamates. J Chem Soc (Perkin Transaction) 1994a;1:3035–40. [Google Scholar]
- 46.Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani AU. Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera. J Nat Prod. 1994b;57:1256–61. doi: 10.1021/np50111a011. [DOI] [PubMed] [Google Scholar]
- 47.Kekuda PT, Nishanth BC, Praveen Kumar SV, Kamal D, Sandeep M, Megharaj HK. Cow Urine Concentrate: A potent agent with Antimicrobial and Anthelmintic activity. J Pharmacy Res. 2010;3:1025–7. [Google Scholar]
- [Last accessed on 2010 Sep 02]. Available from:http://www.patentstorm.us/patents/6896907/description.html .
- 49.Ganaie JA, Shrivastava VK. Effects of gonadotropin releasing hormone conjugate immunization and bioenhancing role of Kamdhenu ark on estrous cycle, serum estradiol and progesterone levels in female Mus musculus. Ir J Repro Med. 2010;8:70–5. [Google Scholar]
- 50.Khan A, Srivastava V. Antitoxic and Bioenhancing role of Kamdhenu ark (Cow urine distillate) on fertility rate of male mice (Mus musculus) affected by cadmium Chloride Toxicity. Int J Cow Sci. 2005;1:43–6. [Google Scholar]
- 51.Hayeshi R, Masimirembwa C, Mukanganyama S, Ungell AL. The potential inhibitory effect of antiparasitic drugs and natural products on P-glycoprotein mediated efflux. Eur J Pharm Sci. 2006;29:70–81. doi: 10.1016/j.ejps.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Tsai TH, Lee CH, Yeh PH. Effect of P-glycoprotein modulators on the pharmacokinetics of campothecin using microdialysis. Br J Pharmacol. 2001;134:1245–52. doi: 10.1038/sj.bjp.0704363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan K, Liu XZ, Jiang ZH, Zhou H, Wong YF, Xu HX, et al. The effects of sinomenine on intestinal absorption of paeoniflorin by the everted rat gut sac model. J Ethnopharmacol. 2006;20:425–32. doi: 10.1016/j.jep.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 54.Bajad S, Bedi KL, Singla AK, Johri RK. Piperine inhibits gastric emptying and gastrointestinal transit in rats and mice. Planta Med. 2001;67:176–9. doi: 10.1055/s-2001-11505. [DOI] [PubMed] [Google Scholar]
- 55.Sangwan PL, Koul JL, Koul S, Reddy MV, Thota N, Khan IA, et al. Piperine analogs as Staphylococcus aureus NorA efflux pump inhibitors. Bioorg Med Chem. 2008;15:9847–57. doi: 10.1016/j.bmc.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 56.Kumar A, Khan IA, Koul S, Koul JL, Taneja SC, Aki I, et al. Novel structural analogs of piperine as inhibitors of NorA efflux pump of Staphylococcus aureus. J Antimicrob Chemother. 2008;61:1270–6. doi: 10.1093/jac/dkn088. [DOI] [PubMed] [Google Scholar]
- 57.Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: Influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine. 2002;9:224–31. doi: 10.1078/0944-7113-00114. [DOI] [PubMed] [Google Scholar]
- 58.Goldstein DB. The effect of drugs on membrane fluidity. Ann Rev Pharmacol Toxicol. 1984;24:43–64. doi: 10.1146/annurev.pa.24.040184.000355. [DOI] [PubMed] [Google Scholar]
- 59.Esposito G. Polarity of intestinal epithelial cell: Permeability of border and basolateral membranes. In: Csaky TZ, editor. Pharmacology of Intestinal Permeation. Berlin Heidelberg: Springer-Verlag; 1984. pp. 283–308. [Google Scholar]
- 60.Patwardhan B, Vaidya AD. Natural products discovery: Accelerating the clinical candidate development using reverse pharmacology approaches. Ind J Exp Biol. 2010;48:220–7. [PubMed] [Google Scholar]