Midlife measurements of white matter microstructure predict subsequent regional white matter atrophy in healthy adults (original) (raw)
Abstract
Objectives: Although age‐related brain changes are becoming better understood, midlife patterns of change are still in need of characterization, and longitudinal studies are lacking. The aim of this study was to determine if baseline fractional anisotropy (FA), obtained from diffusion tensor imaging (DTI) predicts volume change over a 4‐year interval. Experimental design: Forty‐four cognitively healthy middle‐age adults underwent baseline DTI and longitudinal T1‐weighted magnetic resonance imaging. Tensor‐based morphometry methods were used to evaluate volume change over time. FA values were extracted from regions of interest that included the cingulum, entorhinal white matter, and the genu and splenium of the corpus callosum. Baseline FA was used as a predictor variable, whereas gray and white matter atrophy rates as indexed by Tensor‐based morphometry were the dependent variables. Principal observations: Over a 4‐year period, participants showed significant contraction of white matter, especially in frontal, temporal, and cerebellar regions (P < 0.05, corrected for multiple comparisons). Baseline FA in entorhinal white matter, genu, and splenium was associated with longitudinal rates of atrophy in regions that included the superior longitudinal fasciculus, anterior corona radiata, temporal stem, and white matter of the inferior temporal gyrus (P < 0.001, uncorrected for multiple comparisons). Conclusions: Brain change with aging is characterized by extensive shrinkage of white matter. Baseline white matter microstructure as indexed by DTI was associated with some of the observed regional volume loss. The findings suggest that both white matter volume loss and microstructural alterations should be considered more prominently in models of aging and neurodegenerative diseases. Hum Brain Mapp 35:2044–2054, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: aging, fractional anisotropy, microstructure, atrophy, longitudinal, diffusion tensor imaging, tensor‐based morphometry
INTRODUCTION
Normal aging is accompanied by a progressive cognitive decline and neural degeneration; however, the mechanisms for these changes are not fully understood. Histological studies have established that there is a decrease in the number and dendritic extent of cortical neurons [Coleman and Flood, 1987] and shrinkage of neurons [Terry et al., 1987], with consequent cerebral atrophy. Although gray matter loss is evident in aging, several studies suggest that the age‐related structural deterioration of white matter [Tang et al., 1997] is central in the brain aging process [O'Sullivan et al., 2001; Pfefferbaum et al., 2005] and may be involved in disruption of neural networks underlying normal cognitive function [Grady, 2008; Greenwood, 2007]. Moreover, human white matter development is thought to be heterochronic and regionally heterogeneous; specifically, axons from the prefrontal and other association areas continue to myelinate temporally longer than, for example, sensory or motor areas [Bartzokis et al., 2004; Benes, 2004]. Fundamental questions still remain, however, about the temporal relationship between white and gray matter changes in normal aging. Recently, it has been proposed that white matter alterations may precede gray matter changes [Bartzokis, 2004; Bartzokis et al., 2004].
Longitudinal in vivo brain imaging of white and gray matter may help define the temporal relationship of brain tissue change. Diffusion tensor imaging (DTI), in particular, has allowed investigators to examine white matter in a way that was previously not possible [Basser, 1995; Basser and Pierpaoli, 1996b]. Derived from DTI, fractional anisotropy (FA) [Giorgio et al., 2010] is a quantitative index of the directionality of water diffusion, reflecting the integrity of the brain tissue [Basser and Pierpaoli, 1996a]. Alterations in the microstructure environment, such as demyelination of axons and loss of axonal structure, reduce directional water diffusion and thus reduce FA [Englund, 1998]. Several DTI studies of healthy aging have shown widespread age‐related reductions in FA and elevations in diffusivity in white matter [Ardekani et al., 2007; Benedetti et al., 2006; Charlton et al., 2008; Grieve et al., 2007]; however, across studies there is regional variability, suggesting that the effect of aging on white matter is still in need of clarification. Decreases in FA have been consistently reported in large cerebral white matter regions such as the centrum semiovale, corona radiata, frontal and parietal pericallosal areas, and periventricular regions, whereas less consistent findings have been detected in the splenium of the corpus callosum, parietal white matter, and limbs of the internal capsule [Hugenschmidt et al., 2008; Madden et al., 2004; Pfefferbaum et al., 2005; Salat et al., 2005a].
Although it is well established that white matter integrity, as indexed by FA, decreases with age, the relationship between these microstructural changes and volume loss remains to be fully elucidated. In a review article evaluating voxel‐based morphometry (VBM) and DTI studies of prefrontal white matter [Salat et al., 2005b], a positive correlation between FA and volume was observed only in participants of >40 years. Although another study [Fjell et al., 2008] found moderately correlated regional white matter volume and FA in a similar cohort, Salat et al.'s result is consistent with the previous research [Bartzokis et al., 2001; Courchesne et al., 2000; Raz et al., 2004], showing that anterior white matter myelination peaks during middle age and subsequently declines. The authors [Salat et al., 2005b] suggest that FA may be a microstructural marker of volumetric measures and thus reduced FA may reflect decreased white matter volume. In contrast, a previous study [Benedetti et al., 2006] used whole‐brain histograms and found no correlation between mean diffusivity and volume, as measured through magnetization transfer magnetic resonance imaging (MRI). However, this inconclusive result may be owing to averaging whole‐brain FA and volume, a method that may overlook regional variability. Using DTI and VBM, Hugenschmidt et al. [2008] showed that regions exhibiting decreased FA in middle age were the same areas that exhibit white matter volume loss in older age, suggesting that microstructural FA changes may precede and predict white matter atrophy although proving temporal ordering is difficult.
Testing the extent to which microstructural alterations precede volume loss requires a within‐subject longitudinal approach and was a primary focus of this study. Using imaging data acquired in a sample of healthy middle‐aged adults, the aim of this study was to understand if microstructural alterations, as indexed by FA, were related to gray and white matter volume change over time. Baseline white matter health was assessed in regions of interest (ROIs) within multiple white matter tracts where age‐related declines in tissue integrity have been found previously. The selected white matter regions included the cingulum adjacent to hippocampus, entorhinal white matter, and the cingulum subjacent to the posterior cingulate, all association fibers observed to be susceptible to age‐related deterioration in comparison to projection fibers [Stadlbauer et al., 2008]. The ROIs also included the genu and splenium of the corpus callosum, both of which have been reported to have age‐related FA decreases [Bhagat and Beaulieu, 2004; Head et al., 2004; Ota et al., 2006; Pfefferbaum et al., 2000, 2005; Sullivan et al., 2006]. FA was chosen based on its reliable relationship with age‐related white matter alterations [Pfefferbaum et al., 2000; Salat et al., 2005a; Westlye et al., 2010]. The relationship between FA and white matter alteration has been observed to be stronger in adults of >40 years [Salat et al., 2005a], similar in age range to the present sample. Thus, we expected that the FA signal from the preselected white matter tracts would predict volume loss in related white matter regions and gray matter structures, including frontal, parietal, basal temporal, and parahippocampal regions.
METHODS
Participants
Forty‐four cognitively healthy participants underwent two MRI sessions (baseline and follow‐up) as part of the previous functional MRI studies of memory and aging. All participants were from the Wisconsin Registry for Alzheimer's Prevention [Sager et al., 2005], which is a registry of healthy middle‐aged adults who have at least one parent with late onset Alzheimer's disease (AD) or no parental family history of AD. The sample included participants with parental family history and genetic risk for AD, specifically, positive Apolipoprotein E ε4 (APOE4) status. All participants underwent a baseline MRI and a follow‐up MRI approximately 4 years later. In addition to MRI, participants received a neuropsychological assessment. Demographics and cognitive performance scores are listed in Table 1.
Table 1.
Demographic features and cognitive performance
| Total N | 44 |
|---|---|
| Female: N (%) | 27 (61%) |
| Parental family history AD: N (%) | 25 (56%) |
| APOE4 positive: N (%) | 20 (45%) |
| Baseline age: years, SD (range) | 56.3 ± 6.9 (42–75) |
| Baseline education: years, SD (range) | 15.9 ± 2.4 (12–20) |
| Time from baseline scan to follow‐up: years, SD (range) | 3.48 ± 0.88 (2.17–4.92) |
| MMSE: mean, SD | 29.6 ± 0.66 |
| WRAT‐III reading: mean, SD | 52.2 ± 3.5 |
| BVMT‐R total: mean, SD | 24.9 ± 7.1 |
| BVMT‐R delayed recall: mean, SD | 9.7 ± 2.0 |
| RAVLT delayed recall: mean, SD | 10.7 ± 2.9 |
| Digit span: mean, SD | 18.0 ± 3.4 |
| TMT A: mean, SD | 28.4 ± 8.5 |
| TMT B: mean, SD | 58.7 ± 20.9 |
| BNT: mean, SD | 56.0 ± 7.8 |
Inclusion criteria for all subjects consisted of the following: normal cognitive function determined by neuropsychological evaluation, no current diagnosis of major psychiatric disease or other major medical conditions (e.g., diabetes, myocardial infarction, or recent history of cancer), no history of head trauma, and no contraindications for a MRI scan. Study procedures were approved by the University of Wisconsin Health Sciences Institutional Review Board and were in accordance with U.S. federal regulations. All participants provided written informed consent.
MRI Acquisition
Participants were imaged on a General Electric 3.0 Tesla SIGNA (Waukesha, WI) MRI system with a quadrature birdcage head coil at baseline and after 4 years. At baseline, cardiac‐gated diffusion‐weighted echo planar magnetic resonance images were acquired using 12 optimum noncollinear encoding directions (obtained by minimum energy numerical optimization) with a diffusion weighting of 1,114 s/mm2 and a non‐DWT2‐weighted reference image. The effective TR was 10–13 heartbeats (∼10–15 s) dependent on the subject's heart rate. Other imaging parameters were echo time (TE) = 78.2 ms, three averages (NEX: magnitude averaging), and an image acquisition matrix of 120 × 120 over a field of view (FOV) of 240 × 240 mm2. Three averages were acquired and the cerebrum was covered using 39 contiguous 3‐mm thick axial slices. The acquired voxel size of 2 × 2 × 3 mm was interpolated to 0.9375‐mm isotropic dimensions (256 × 256 in plane image matrix). The total acquisition time was between 6.5 and 8 min dependent on the heart rate. High order shimming was performed prior to the DTI acquisition to optimize the homogeneity of the magnetic field across the brain and to minimize EPI distortions.
3D T1‐weighted volumes were obtained at baseline and follow‐up using an inversion recovery prepared fast gradient echo pulse sequence. The whole brain was imaged in the axial plane with the following parameters: TI = 600 ms; repetition time (TR) = 9 ms; TE = 1.8 ms; NEX = 1; flip angle = 20°; acquisition matrix = 256 × 192 × 124, interpolated to 256 × 256 × 124; FOV = 240 mm; slice thickness = 1.2 mm (124 slices), receiver bandwidth = ±16 kHz; acquisition time, ∼7.5 min.
DTI and tract‐based spatial statistics preprocessing
Diffusion‐weighted DICOM images acquired at baseline were converted into NIFTI format using AFNI (http://afni.nimh.nih.gov/). FA maps were generated via the FMRIB Software Library (FSL) (http://www.fmrib.ox.ac.uk/fsl/fdt/index.html, Behrens et al., 2003) using the following procedures: (1) image distortions in the DTI data caused by eddy currents were corrected; (2) estimation of diffusion tensors was achieved using DTIFIT; (3) three‐dimensional maps of FA images were computed from the tensors from Step 2. The FA maps were then aligned using registration methods based on the tract‐based spatial statistics (TBSS: http://www.fmrib.ox.ac.uk/fsl/tbss/index.html) processing scheme. TBSS methods were employed because the method is known to provide accurate registration of FA maps, the method allowed us to confidently position ROIs for the extraction of FA values, and this method of registration reduces the inclusion of CSF voxels in the final extracted FA estimates. TBSS performs alignment of all FA data by projecting the original FA maps onto a mean FA skeleton. The main steps of the procedure we employed were as follows: (a) FA images were eroded slightly and the end slices were zeroed to remove outliers from the diffusion tensor fitting. (b) A nonlinear registration was estimated to align the FA images to a 1 × 1 × 1 mm standard space. The target image was affine transformed to Montreal Neurological Institute (MNI) space and each subject's FA image had its nonlinear transform to the target and an affine transform to MNI space applied, resulting in a transformation of the original FA image into MNI space. (c) The mean of all FA images was created and the image was skeletonized. (d) The mean FA skeleton was then thresholded to produce a binary skeleton mask that defined the set of voxels used in all subsequent processing. (e) A “distance map” was then created from the skeleton mask. This was used in the projection of the subjects' FA maps onto the skeleton. (f) All of the subjects' aligned FA data were projected onto the mean FA skeleton using warping methods that are based on free‐form deformations and B‐Splines [Rueckert et al., 1999]. The process is achieved by filling the skeleton with FA values from the nearest relevant tract center. This was performed for each skeleton voxel, by searching perpendicular to the local skeleton structure for the maximum value in the subject's FA image. (g) After projection onto the mean FA skeleton, the skeletonized data in standard space were used for the ROI analyses.
Regions of Interest
Each ROI was drawn on a common space skeleton mask in FSLview (http://www.fmrib.ox.ac.uk/fsl/fslview/index.html) and was then applied to the normalized individual maps. ROIs drawn on the template were individually checked to ensure correct placement on the single‐subject‐normalized FA maps. Individual FA values for each ROI were extracted by acquiring the mean value across the tract labels of interest. ROIs were drawn bilaterally and included the cingulum subjacent to the posterior cingulate, cingulum adjacent to hippocampus, entorhinal white matter, and the genu and splenium of the corpus callosum (Fig. 1). Corticospinal tract was included as a control ROI based on the literature, suggesting these tracts, along with primary sensory and motor cortices, are preserved during aging relative to association cortices.
Figure 1.
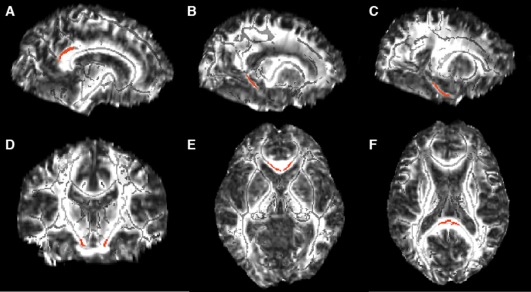
White matter ROIs (shown in red) overlayed on the FA template image (skeletonized FA underlayed in light gray). The bilateral ROIs included: (A) cingulum bundle subjacent to posterior cingulate (119 voxels, MNI coordinates: ±11,−45, 28), (B) cingulum adjacent to hippocampus (60 voxels, MNI coordinates: ±20, −42, −2), (C) entorhinal white matter (96 voxels, MNI coordinates: ±24, −26, −19), (D) corticospinal tract (31 voxels, MNI coordinates: ±10, −20, −24), (E) splenium (49 voxels, MNI coordinates: ±1, −35, 14), and (F) genu (48 voxels, MNI coordinates: ±4, 23, −1) of the corpus callosum. (A–C) Sagittal view, (D) coronal view, and (E, F) an axial view.
Tensor‐Based Morphometry
To produce estimates of volume change from baseline to follow‐up, we employed TBM methods implemented in the SPM5 software package (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). First, bias correction with eight iterations, a FWHM of Gaussian smoothing set at a 60 mm cutoff, and a medium level of regularization was applied to both the baseline and the follow‐up scans to correct for intensity nonuniformity. TBM procedures followed those described by Kipps et al. [2005]. Briefly, a high‐dimensional deformation field was used to warp the corrected late image to match the baseline scan within subject [Ashburner and Friston, 2000]. The amount of volume change was indexed by the determinant of the gradient of deformation at a single‐voxel level (Jacobian determinants). The Jacobian image represented a measure of the brain‐specific volume change between the first and the second scan. The maps were converted to annual rate of change maps using the formula: Annual Rate = ([Jacobian determinant]^[1/Interscan duration] − 1), where “Interscan duration” was the number of years between baseline and follow‐up scans. To warp the final TBM maps to template space, normalization parameters were estimated by matching the brain‐volume images from the baseline scan with the MNI brain‐volume template, these were then applied to the Jacobian image [Ashburner and Friston, 1999]. Finally, the normalized TBM maps were smoothed using an 8‐mm isotropic Gaussian kernel.
Statistical Analyses
To test the extent to which participants showed tissue change over a 4‐year period, tissue maps were thresholded to either (1) values above zero to reflect tissue atrophy, or (2) values below zero to reflect contraction. One‐way _t_‐tests were used to determine significant regions of change. To test the extent to which FA obtained at baseline predicted volume change, individual FA estimates from each ROI were entered into a multiple regression analysis, where the independent predictor variable was baseline FA, and the dependent variable (volume change) was the participant's TBM map thresholded above zero (reflecting tissue atrophy). For all models, the covariates were baseline age and gender. Results were considered significant at P 0.001 (uncorrected).
RESULTS
Participants with parental family history of AD or positive APOE4 status did not differ with respect to demographic characteristics or neuropsychological test performance compared to those with no AD risk factors.
There was a significant change in volume for more than 4 years. As summarized in Table 2 and shown in Figure 2, significant volume contraction was observed predominantly in frontal, temporal, and cerebellar regions. These results survived family wise error correction (FWE) P 0.05. In contrast, a voxel‐wise analysis revealed no areas of significant expansion (P 0.001, uncorrected).
Table 2.
Regions of tissue contraction for more than 4 years (P 0.05, FWE corrected)
| Location | Cluster size | MNI coordinates of peak voxel | Peak‐level _T_‐statistic | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R Medial orbital gyrus | 12,841 | 16 | 32 | −28 | 11.10 |
| R cingulate gyrus | 1,263 | 6 | −22 | 48 | 9.22 |
| R superior frontal gyrus WM | 498 | 8 | −4 | 66 | 8.56 |
| L posterior orbital gyrus WM | 502 | −32 | 38 | −20 | 8.37 |
| L posterior corona radiata | 492 | −18 | −48 | 30 | 8.20 |
| R cerebellar hemisphere | 318 | 20 | −26 | −22 | 8.06 |
| L superior frontal gyrus WM | 346 | −10 | 4 | 62 | 7.84 |
| CSF space ventral to L inferior temporal gyrus | 299 | −50 | −30 | −32 | 7.81 |
| R cuneus | 99 | 4 | −78 | 40 | 7.11 |
| R precentral gyrus | 69 | 6 | −30 | 72 | 7.01 |
| CSF space ventral to R fusiform gyrus | 35 | 18 | 4 | −46 | 6.77 |
| L fusiform gyrus WM | 55 | −32 | −42 | −16 | 6.77 |
| L lingual gyrus WM | 179 | −16 | −68 | −8 | 6.58 |
| L cerebellar hemisphere | 94 | −26 | −72 | −26 | 6.45 |
| R precentral gyrus WM | 32 | 14 | −18 | 64 | 6.38 |
| R middle temporal gyrus | 67 | 62 | −48 | −4 | 6.19 |
| R fusiform gyrus | 21 | 60 | −60 | −20 | 6.17 |
| R supramarginal gyrus | 76 | 62 | −40 | 38 | 6.16 |
| CSF space anterior to L cerebellar hemisphere | 58 | −20 | −30 | −46 | 6.16 |
| R middle frontal gyrus | 71 | 46 | 38 | 24 | 6.07 |
| R middle frontal gyrus | 34 | 28 | 62 | 20 | 5.93 |
Figure 2.
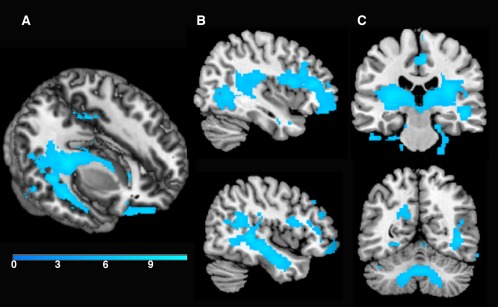
Regions of tissue contraction for more than 4 years (P < 0.05, FWE corrected). As shown in the 3D render (A) and sagittal cross‐section (B), there was significant contraction in temporal stem white matter for more than 4 years. Additionally, as shown in the coronal sections (C), there was significant contraction in large portions of bilateral subcortical white matter and the cerebellum. The color bar represents _T_‐values.
With regard to FA, there was no significant effect of AD risk factors. The mean FA values extracted from bilateral ROIs were as follows: 0.70 (±0.06) from the corticospinal tract, 0.71 (±0.03) from the genu, 0.54 (±0.06) from the entorhinal white matter, 0.53 (±0.03) from the cingulum subjacent to the posterior cingulate bundle, 0.51 (±0.04) from the cingulum adjacent to hippocampus, and 0.87 (±0.03) from the splenium. No significant differences were found between the left and the right hemisphere per each of the investigated ROIs and thus left and right were averaged for the remaining analyses.
As summarized in Table 3, baseline FA in entorhinal white matter, and genu and splenium of the corpus callosum predicted atrophy (P 0.001, uncorrected) as indexed by the TBM maps (Fig. 3). With the exception of the right cerebellar hemisphere volume loss predicted by splenium FA, the majority of tissue contraction was primarily in white matter regions, specifically within the superior longitudinal fasciculus (SLF), anterior corona radiata, and the temporal stem, all regions predicted by baseline entorhinal FA. In addition, atrophy in the white matter of the inferior temporal gyrus was predicted by baseline genu FA. Baseline FA in the corticospinal tract, cingulum adjacent to hippocampus, and cingulum subjacent to the posterior cingulate did not predict any regions of volume loss.
Table 3.
Regions of tissue contraction (P 0.001, uncorrected) predicted by baseline FA
| Baseline ROI where FA was extracted | Cluster size | Location | MNI coordinates of peak voxel | Peak‐level _T_‐statistic | R 2 | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Entorhinal | 36 | R anterior corona radiata | 28 | 18 | 12 | 4.0 | 0.25 |
| Entorhinal | 22 | R temporal stem | 32 | 4 | −14 | 3.9 | 0.21 |
| Entorhinal | 27 | L superior longitudinal fasciculus | −30 | −46 | 30 | 3.66 | 0.21 |
| Genu | 91 | R inferior temporal gyrus WM | 36 | 4 | −28 | 4.59 | 0.33 |
| Splenium | 42 | R cerebellar hemisphere | 16 | −72 | −36 | 4.6 | 0.25 |
Figure 3.
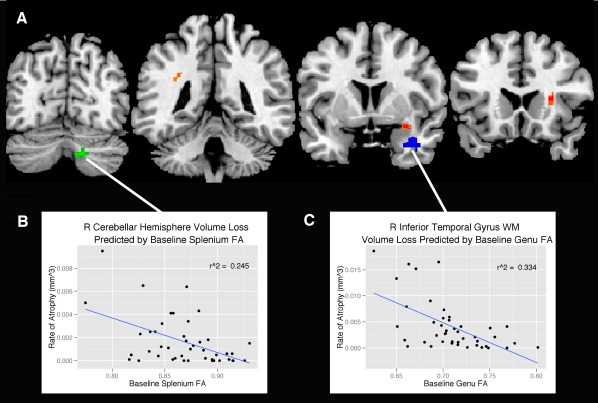
Regions (A) where baseline FA from the splenium (green), entorhinal white matter (orange), and genu (blue) predict volume loss from baseline to follow‐up (P < 0.001, uncorrected). The statistical map is overlaid on coronal sections of the “CH2” template available in MRIcron (Rorden, 2007). The correlation between (B) baseline splenium FA and cerebellar hemisphere volume loss was r 2 = 0.25, P < 0.001 and (C) baseline genu FA and inferior temporal gyrus WM volume loss was r 2 = 0.33, P < 0.001. Data points represent individual participants. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
The results of this study indicate that for more than a 4‐year period, middle‐aged adults show significant shrinkage of white matter. Further, midlife measures of FA—a putative marker of white matter integrity—predict longitudinal rates of white matter atrophy. Although T1‐weighted imaging in this study and other studies appears to be sensitive to gross volume loss, techniques such as DTI are sensitive to microstructural alterations, and the results suggest that they may, in fact, be predictive of subsequent volume loss.
One of the largest areas of atrophy was observed in the white matter of the inferior temporal gyrus, as predicted by genu FA. Additionally, entorhinal FA was associated with atrophy in temporal stem. This is in accordance with prior findings, with both volume [Raz et al., 2004] and anisotropy [Head et al., 2004; Salat et al., 2005a] in the temporal lobes showing moderate decline with age. This decline is second to the frontal cortices and is followed by smaller decreases in the parietal and occipital lobes, suggesting an anterior to posterior gradient. A volumetric cross‐sectional study in men showed a quadratic relationship between age and white matter volume in the temporal lobes, with white matter volume increasing to the age of 47 years and declining subsequently [Bartzokis et al., 2001]. The mean age of the current cohort was 56 years at baseline, suggesting that the majority of the cohort had crossed over the peak of myelination in this brain region, and was on a downward trajectory over the subsequent 4 years. The nonlinear nature of white matter development over the life‐span contrasts with the linear decline of gray matter volume throughout most of adulthood, and this is important to take into consideration in the studies of midlife white matter change.
Another significant region of atrophy, predicted by splenium FA, was located in the right cerebellar hemisphere, which is consistent with several findings of age‐related decreases in total cerebellar volume, cerebellar white matter, and other cerebellar structures [Jernigan et al., 2001; Liu et al., 2003; Luft et al., 1999; Raz et al., 2001; Sullivan et al., 2000; Walhovd et al., 2005]. Moreover, longitudinally, the cerebellum shows pronounced longitudinal shrinkage with advancing age [Raz et al., 2005, 2010;], possibly beginning to decline during the fifth decade of life, reflecting an exponential fit (Luft, 1999).
We observed a region of volume loss in the SLF, predicted by baseline entorhinal FA. This tract is a heavily myelinated white matter bundle that connects the anterior and posterior regions of the cerebrum, sending projections to the temporal lobes [Wakana et al., 2004]. Findings of longitudinal changes in the SLF converge with a longitudinal DTI study showing reduced FA in the SLF in healthy elderly subjects [Teipel et al., 2010], as well as in older individuals with mild cognitive impairment [Cho et al., 2008]. Age‐related decreases in FA of the SLF has been shown to be associated with poorer performance in a number of cognitive tasks involved in set‐shifting [Perry et al., 2009], episodic memory [Lockhart et al., 2012], executive function [Sasson et al., 2012], and word finding [Stamatakis et al., 2011]. In addition, late‐life‐depressed individuals exhibited greater white matter hyperintensity burden in this region [Sheline et al., 2008]. Although atrophy reflected by the TBM maps was not correlated with neuropsychological performance (likely owing to limited variability in this cognitively healthy sample), this volume loss may predict cognitive changes as the cohort ages, and will be tested at future follow‐ups. In addition to the SLF, entorhinal FA was associated with atrophy in anterior corona radiata. These results replicate findings in a multimodal imaging study in younger individuals where significant quadratic relationships between white matter volume and age were observed in the superior corona radiata bilaterally and in the left SLF [Giorgio et al., 2010]. Overall, baseline entorhinal FA was associated with the majority of regions of longitudinal atrophy observed in this study. Althoough specific pathways in humans have not been well characterized, study in nonhuman primates suggest that the entorhinal region is widely connected with association cortices [Insausti et al., 1987], linking the hippocampus to the association areas of the frontal, parietal, temporal, and occipital lobes [Van Hoesen and Pandya, 1975].
Interestingly, baseline corticospinal FA, the control ROI, did not predict any areas of atrophy. The results are in accordance with the observations that primary motor and sensory cortices are relatively spared during aging. A recent study [Jang, 2011] has shown corticospinal tract FA decreases with age, whereby participants who are 50 years and older show lower FA compared to participants in the third decade of life. The results of our study suggest that changes in microstructural parameters of the corticospinal tract may occur in the absence of volume loss and may not predict downstream volume change, at least during a 4‐year interval in middle‐aged years. Moreover, baseline FA within the cingulum adjacent to hippocampus and cingulum subjacent to the posterior cingulate did not predict any volume loss. These negative findings may suggest that the microstructural integrity of the cingulum does not decline as rapidly during middle age.
The largest areas of atrophy were primarily in frontal and temporal white matter, findings which complement a cross‐sectional study from Hugenschmidt et al. (2008), where FA had significant relationships with several areas of white matter volume loss, including temporal and parietal regions of the corona radiata, the length of the corpus callosum, and centrum semiovale [Hugenschmidt et al., 2008]. In addition, our results are consistent with the convergence of research showing cerebral white matter to have an anterior–posterior gradient of decline [Buckner, 2004; Head et al., 2004; Raz, 2000]. This decline in prefrontal white matter follows an inverted‐U trajectory, with a linear increase in young adulthood, a plateau in middle age and significant contraction starting in the fifth decade of life [Bartzokis et al., 2001; Courchesne et al., 2000; Raz et al., 2004], the average age of our participants. This rate of decline increases with age [Raz et al., 2005], which is in line with other age‐related acceleration in other indices of white matter integrity, such as MRI relaxation times [Bartzokis, 2004; Bartzokis et al., 2003] and ratio of small to large myelinated axons [Tang et al., 1997]. These studies suggest that certain brain regions that are late to mature and which contain a high ratio of thinly myelinated fibers (e.g., prefrontal cortex) may be more susceptible to age‐related atrophy.
Several of the white matter changes found in aging are likely to affect the measurements of water diffusion anisotropy. Histopathological studies have shown that aging is associated with white matter deterioration that include myelin pallor [Kemper, 1994], loss of myelinated fibers [Marner et al., 2003; Meier‐Ruge et al., 1992; Pakkenberg and Gundersen, 1997], and in nonhuman primates, malformation of myelin sheaths [Peters and Sethares, 2002]. Further, nonhuman primate studies also show that age is associated with decreases in synapses, dendritic spines, and myelin sheath degradation in the upper layers of neocortex [Peters, 2002a, 2002b]. These histological studies reveal localized splitting of myelin lamellae causing spherical cytoplasmic cavities or “balloons” within the myelin sheath, and continued myelin production constructing double myelin sheaths, where fluid may build up between layers. Furthermore, as suggested by Bartzokis [2004], this later myelination is more vulnerable, and age‐related declines in membrane cholesterol—a hydrophobic molecule—make myelin more water permissive. All of these changes are candidates for influencing measurements of diffusion anisotropy, in addition to being candidates for predicting later volume loss. Additional histopathological studies will be needed to determine how closely microstructural changes link to overt volume loss; however, only brain imaging studies—while limited in their ability to directly measure pathology—are currently the sole approach to mapping out in vivo changes longitudinally.
Our findings must be interpreted in light of several limitations. As our sample incorporates individuals with varying risk for AD, we cannot rule out the possibility that our findings are Alzheimer's risk specific. However, participants carrying risk factors for Alzheimer's did not differ with regards to demographics, neuropsychological scores, or baseline FA measures. Although our results showing white matter contraction for more than 4 years survived correction for multiple comparisons, our models showing the predictive value of FA are reported at an uncorrected threshold and thus, we cannot rule out the possibility of Type 1 error. Despite this, the exploratory analyses converge on white matter volume loss and thus are less likely to be owing to chance. We should also note that while we used DTI measures as the predictor variables in our study design, we cannot definitively conclude that microstructural alterations precede volume loss. The temporal ordering of microstructural and volumetric changes over the lifespan is still in need of further characterization. Finally, although other DTI indices, such as axial or radial diffusion, do inform about axonal morphology and myelin characteristics, respectively, we decided to only use FA owing to its consistency in the normal aging literature and reflection of several factors, such as changes in axon density, myelination, axonal membrane integrity, fiber orientation, and other alterations.
CONCLUSIONS
To our knowledge, this is the first study demonstrating that white matter alterations collected at baseline are associated with future longitudinal white matter volume loss in cognitively normal adults. Following these individuals as they enter the “golden years” will help in further fleshing out the time course of structural brain changes and also determine whether any of the individual variability in atrophy in middle age is owing to preclinical diagnosis of age‐related neurodegenerative disease. Although longitudinal studies on age‐related neurodegenerative diseases including AD are still needed to evaluate patterns of degeneration in pathological processes, this study suggests that DTI may be useful for characterizing the distribution and time course of alterations that occur in the brain with normative aging, improving models of disease progression, and will likely be important for early diagnosis and for monitoring the efficacy of treatments.
Acknowledgments
None of the authors have a conflict of interest to declare. The project was facilitated by the facilities and resources at the Geriatric Research, Education, and Clinical Center (GRECC) of the William S. Middleton Memorial Veterans Hospital, Madison, WI, GRECC MS# 2013‐05. The authors acknowledge the support of researchers and staff at the Waisman Center, University of Wisconsin, Madison, where imaging data were collected. The authors also thank Aparna Sodhi for her helpful technical assistance on the manuscript. Finally, the authors thank their dedicated participants for their valuable time.
REFERENCES
- Ardekani S, Kumar A, Bartzokis G, Sinha U (2007): Exploratory voxel‐based analysis of diffusion indices and hemispheric asymmetry in normal aging. Magn Reson Imaging 25:154–167. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (1999): Nonlinear spatial normalization using basis functions. Hum Brain Mapp 7:254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry—The methods. Neuroimage 11:805–821. [DOI] [PubMed] [Google Scholar]
- Bartzokis G (2004): Age‐related myelin breakdown: A developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging 25:5–18; author reply, 49–62. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J (2001): Age‐related changes in frontal and temporal lobe volumes in men: A magnetic resonance imaging study. Arch Gen Psychiatry 58:461–465. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J (2003): White matter structural integrity in healthy aging adults and patients with Alzheimer disease: A magnetic resonance imaging study. Arch Neurol 60:393–398. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL (2004): Heterogeneous age‐related breakdown of white matter structural integrity: Implications for cortical “disconnection” in aging and Alzheimer's disease. Neurobiol Aging 25:843–851. [DOI] [PubMed] [Google Scholar]
- Basser PJ (1995): Inferring microstructural features and the physiological state of tissues from diffusion‐weighted images. NMR Biomed 8:333–344. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C (1996a): Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. J Magn Reson B 111:209–219. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C (1996b): Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. J Magn Reson B 111:209–219. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen‐Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM (2003): Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med 50:1077–1088. [DOI] [PubMed] [Google Scholar]
- Benedetti B, Charil A, Rovaris M, Judica E, Valsasina P, Sormani MP, Filippi M (2006): Influence of aging on brain gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology 66:535–539. [DOI] [PubMed] [Google Scholar]
- Benedict R (1997): Brief Visuospatial Memory Test‐Revised. Lutz, FL: Psychological Assessment Resources Inc. [Google Scholar]
- Benes FM (2004): A disturbance of late myelination as a trigger for Alzheimer's disease. Neurobiol Aging 25:41–43. [DOI] [PubMed] [Google Scholar]
- Bhagat YA, Beaulieu C (2004): Diffusion anisotropy in subcortical white matter and cortical gray matter: Changes with aging and the role of CSF‐suppression. J Magn Reson Imaging 20:216–227. [DOI] [PubMed] [Google Scholar]
- Buckner RL (2004): Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron 44:195–208. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG (2008): A structural equation modeling investigation of age‐related variance in executive function and DTI measured white matter damage. Neurobiol Aging 29:1547–1555. [DOI] [PubMed] [Google Scholar]
- Cho H, Yang DW, Shon YM, Kim BS, Kim YI, Choi YB, Lee KS, Shim YS, Yoon B, Kim W, Ahn KJ (2008): Abnormal integrity of corticocortical tracts in mild cognitive impairment: A diffusion tensor imaging study. J Korean Med Sci 23:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PD, Flood DG (1987): Neuron numbers and dendritic extent in normal aging and Alzheimer's disease. Neurobiol Aging 8:521–545. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA (2000): Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 216:672–682. [DOI] [PubMed] [Google Scholar]
- Englund E (1998): Neuropathology of white matter changes in Alzheimer's disease and vascular dementia. Dement Geriatr Cogn Disord 9:6–12. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Greve DN, Fischl B, Benner T, van der Kouwe AJ, Salat D, Bjornerud A, Due‐Tonnessen P, Walhovd KB (2008): The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. Neuroimage 42:1654–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen‐Berg H (2010): Age‐related changes in grey and white matter structure throughout adulthood. Neuroimage 51:943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL (2008): Cognitive neuroscience of aging. Ann N Y Acad Sci 1124:127–144. [DOI] [PubMed] [Google Scholar]
- Greenwood PM (2007): Functional plasticity in cognitive aging: Review and hypothesis. Neuropsychology 21:657–673. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E (2007): Cognitive aging, executive function, and fractional anisotropy: A diffusion tensor MR imaging study. Am J Neuroradiol 28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ (2004): Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cereb Cortex 14:410–423. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian JA, Laurienti PJ (2008): Relating imaging indices of white matter integrity and volume in healthy older adults. Cereb Cortex 18:433–442. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM (1987): The entorhinal cortex of the monkey: II. Cortical afferents. J Comp Neurol 264:356–395. [DOI] [PubMed] [Google Scholar]
- Jang SH, Cho SH, Lee MY, Kwon YH, Chang MC (2011): Age‐related changes of the corticospinal tract in the human brain A diffusion tensor imaging study. Neural Regen Res 6:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastak A (1993): Wide Range Achievement Test‐Third Edition. Wilmington: Wide Range Inc. [Google Scholar]
- Jernigan TL, Archibald SL, Fennema‐Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR (2001): Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22:581–594. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S (2001): Boston Naming Test, 2nd ed Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Kemper TL (1994): Neuroanatomical and neuropathological changes during aging and dementia In: Albert ML KJ, editor. Clinical Neurology of Aging. New York: Oxford University Press; pp 3–67. [Google Scholar]
- Kipps CM, Duggins AJ, Mahant N, Gomes L, Ashburner J, McCusker EA (2005): Progression of structural neuropathology in preclinical Huntington's disease: A tensor based morphometry study. J Neurol Neurosurg Psychiatry 76:650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RS, Lemieux L, Bell GS, Sisodiya SM, Shorvon SD, Sander JW, Duncan JS (2003): A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. Neuroimage 20:22–33. [DOI] [PubMed] [Google Scholar]
- Lockhart SN, Mayda AB, Roach AE, Fletcher E, Carmichael O, Maillard P, Schwarz CG, Yonelinas AP, Ranganath C, Decarli C (2012): Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Front Hum Neurosci 6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft AR, Skalej M, Schulz JB, Welte D, Kolb R, Burk K, Klockgether T, Voight K (1999): Patterns of age‐related shrinkage in cerebellum and brainstem observed in vivo using three‐dimensional MRI volumetry. Cereb Cortex 9:712–721. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM (2004): Diffusion tensor imaging of adult age differences in cerebral white matter: Relation to response time. Neuroimage 21:1174–1181. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B (2003): Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol 462:144–152. [DOI] [PubMed] [Google Scholar]
- Meier‐Ruge W, Ulrich J, Bruhlmann M, Meier E (1992): Age‐related white matter atrophy in the human brain. Ann N Y Acad Sci 673:260–269. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS (2001): Evidence for cortical “disconnection” as a mechanism of age‐related cognitive decline. Neurology 57:632–638. [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, Suhara T (2006): Age‐related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage 31:1445–1452. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. (1997): Neocortical neuron number in humans: Effect of sex and age. J Comp Neurol 384:312–320. [PubMed] [Google Scholar]
- Perry ME, McDonald CR, Hagler DJ Jr, Gharapetian L, Kuperman JM, Koyama AK, Dale AM, McEvoy LK (2009): White matter tracts associated with set‐shifting in healthy aging. Neuropsychologia 47:2835–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A (2002a): The effects of normal aging on myelin and nerve fibers: A review. J Neurocytol 31:581–593. [DOI] [PubMed] [Google Scholar]
- Peters A (2002b): Structural changes that occur during normal aging of primate cerebral hemispheres. Neurosci Biobehav Rev 26:733–741. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C (2002): Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol 442:277–291. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M (2000): Age‐related decline in brain white matter anisotropy measured with spatially corrected echo‐planar diffusion tensor imaging. Magn Reson Med 44:259–268. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV (2005): Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage 26:891–899. [DOI] [PubMed] [Google Scholar]
- Raz J (2000): Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings In: Craik F, Salthouse TA, editors. Handbook of Aging and Cognition—II. Mahwah, NJ: Lawrence Erlbaum Associates; pp 1–90. [Google Scholar]
- Raz N, Gunning‐Dixon F, Head D, Williamson A, Acker JD (2001): Age and sex differences in the cerebellum and the ventral pons: A prospective MR study of healthy adults. Am J Neuroradiol 22:1161–1167. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD (2004): Differential aging of the medial temporal lobe: A study of a five‐year change. Neurology 62:433–438. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD (2005): Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U (2010): Trajectories of brain aging in middle‐aged and older adults: Regional and individual differences. Neuroimage 51:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM WD (1993): The Halstead‐Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson: Neuropsychology Press. [Google Scholar]
- Rey A (1964) : L'examen clinique en psychologie. Paris: Presses Universitaires de France. [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ (1999): Nonrigid registration using free‐form deformations: Application to breast MR images. IEEE Trans Med Imaging 18:712–721. [DOI] [PubMed] [Google Scholar]
- Sager MA, Hermann B, La Rue A (2005): Middle‐aged children of persons with Alzheimer's disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer's Prevention. J Geriatr Psychiatry Neurol 18:245–249. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, Dale AM (2005a): Age‐related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging 26:1215–1227. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM (2005b): Age‐related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci 1064:37–49. [DOI] [PubMed] [Google Scholar]
- Sasson E, Doniger GM, Pasternak O, Tarrasch R, Assaf Y (2012): Structural correlates of cognitive domains in normal aging with diffusion tensor imaging. Brain Struct Funct 217:503–515. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, Wilkins CH, Snyder AZ, Couture L, Schechtman K, McKinstry RC (2008): Regional white matter hyperintensity burden in automated segmentation distinguishes late‐life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry 165:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer A, Salomonowitz E, Strunk G, Hammen T, Ganslandt O (2008): Age‐related degradation in the central nervous system: Assessment with diffusion‐tensor imaging and quantitative fiber tracking. Radiology 247:179–188. [DOI] [PubMed] [Google Scholar]
- Stamatakis EA, Shafto MA, Williams G, Tam P, Tyler LK (2011): White matter changes and word finding failures with increasing age. PLoS One 6:e14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A (2000): Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: Relation to ataxia. Neuropsychology 14:341–352. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A (2006): Selective age‐related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex 16:1030–1039. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ (1997): Age‐induced white matter changes in the human brain: A stereological investigation. Neurobiol Aging 18:609–615. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Wagner M, Stieltjes B, Reuter S, Hauenstein KH, Filippi M, Ernemann U, Reiser MF, Hampel H (2010): Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: A DTI follow‐up study. J Alzheimers Dis 22:507–522. [DOI] [PubMed] [Google Scholar]
- Terry RD, DeTeresa R, Hansen LA (1987): Neocortical cell counts in normal human adult aging. Ann Neurol 21:530–539. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN (1975): Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Res 95:39–59. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae‐Poetscher LM, van Zijl PC, Mori S (2004): Fiber tract‐based atlas of human white matter anatomy. Radiology 230:77–87. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B (2005): Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging 26:1261–1270; discussion, 1275–128. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1987): Manual for the Wechsler Memory Scale‐Revised. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due‐Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM (2010): Life‐span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cereb Cortex 20:2055–2068. [DOI] [PubMed] [Google Scholar]