Peritoneal Cavity Regulatory B Cells (B10 Cells) Modulate IFN-γ+CD4+ T Cell Numbers During Colitis Development in Mice (original) (raw)
. Author manuscript; available in PMC: 2014 Sep 1.
Published in final edited form as: J Immunol. 2013 Aug 5;191(5):2780–2795. doi: 10.4049/jimmunol.1300649
Abstract
The spleen regulatory B cell subset with the functional capacity to express IL-10 (B10 cells) modulates both immune responses and autoimmune disease severity. However, the peritoneal cavity also contains relatively high frequencies of functionally-defined IL-10-competent B10 cells. In this study, peritoneal cavity B10 cells shared similar cell surface phenotypes with their spleen counterparts. However, peritoneal cavity B10 cells were 10-fold more frequent among B cells than occurred within the spleen, intestinal track or mesenteric lymph nodes and were present at higher proportions among the phenotypically-defined peritoneal B1a>B1b>B2 cell subpopulations. The development or localization of B10 cells within the peritoneal cavity was not dependent on the presence of commensal microbiota, T cells, IL-10 or B10 cell IL-10 production, or differences between their fetal liver or adult bone marrow progenitor cell origins. The BCR repertoire of peritoneal cavity B10 cells was diverse, as occurs in the spleen, and predominantly included germline-encoded VH and VL regions commonly found in either the conventional or B1 B cell compartments. Thereby, the capacity to produce IL-10 appears to be an intrinsic functional property acquired by clonally diverse B cells. Importantly, IL-10 production by peritoneal cavity B cells significantly reduced disease severity in spontaneous and induced models of colitis by regulating neutrophil infiltration, colitogenic CD4+ T cell activation and pro-inflammatory cytokine production during colitis onset. Thus, the numerically small B10 cell subset within the peritoneal cavity has regulatory function and is important for maintaining homeostasis within gastrointestinal tissues and the immune system.
Introduction
Chronic inflammatory disorders of the intestine are collectively referred to as inflammatory bowel disease (IBD), with ulcerative colitis and Crohn's disease being the most prevalent in humans (1). Various effector T cell subsets are pathogenic in IBD, with different subsets playing different roles in each mouse model. Th1 and Th17 cells are major disease contributors in both the IL-10-deficient (IL-10−/−) mouse model of spontaneous disease and the CD4+ T cell-induced model of colitis, with IFN-γ– and IL-17-competent T cells detectable at all stages of disease in mice and humans (1-4). Mice deficient in IL-10, a potent immunoregulatory cytokine with anti-inflammatory properties (5), are highly susceptible to chronic enterocolitis that is spontaneously triggered by intestinal microbiota (6, 7). IL-10-deficiency in regulatory Foxp3+CD4+ T cells (Tregs) alone can also lead to colitis (8). Continuous recombinant IL-10 treatment attenuates pathology in the T cell transfer model of colitis following the adoptive transfer of CD25−CD45RBhiCD4+ T cells into lymphocyte-deficient _Rag2_−/− mice (4, 9, 10). Human IBD is also genetically linked with Il10 locus polymorphisms or altered serum IL-10 concentrations (11, 12). T cells, B cells, monocytes, macrophages, mast cells, and eosinophils can all secret IL-10 that suppresses inflammatory cytokine production, Th1/Th2 polarization, and antigen presentation (5, 13, 14). Thereby, IL-10 production protects intestinal integrity and controls gut inflammation.
Mature B cell depletion in humans with ulcerative colitis using CD20 mAb was ineffective in a placebo-controlled study (15), and has even been suggested to exacerbate colonic inflammation in some patients (16, 17). B cell deficiency also increases the severity of chronic autoimmune inflammatory colitis in _TCR_α−/− mice (18). Even though _TCR_α−/− mice lack most T cell populations and have B cell populations with altered phenotypes, B cell IL-10 production normally suppresses their inflammatory colitis (19, 20). Spleen B cells exposed to enterobacterial components can also acquire IL-10-dependent suppressive functions and inhibit experimental colitis (21). A mouse regulatory B cell subset (B10 cells) in the spleen can significantly reduce dextran sodium sulfate (DSS)-induced colon inflammation (22). B10 cells are functionally defined in humans and mice by their ability to express IL-10 following 5 h of ex vivo phorbol ester and ionomycin stimulation (23-25), which distinguishes them from regulatory B cells that modulate immune responses through other mechanisms (26, 27). Human and mouse B10 cell IL-10 production is central to their ability to negatively regulate innate and Ag-specific adaptive immune responses as well as inflammation and autoimmune disease (23-25, 28-33). B10 cell effector function during autoimmunity and infections is regulated through cognate interactions with CD4+ T cells and IL-21 receptor signals that induce B10 cells to become IL-10-secreting B10 effector cells (32, 33). B10 cells are found at low frequencies (1-5%) among spleen B cells in naïve mice but expand with autoimmunity (28). Spleen B10 cells are predominantly found within the minor CD1dhiCD5+ B cell subpopulation along with B10 progenitor (B10pro) cells that are induced to acquire IL-10-competence during in vitro culture with agonistic CD40 mAb or LPS (28, 30, 32). Despite the predominant expression of CD5 by spleen B10 and B10pro cells, B10 cells generally represent only a fraction of the CD5+ B cell pool, and B10 and CD5+ B cell frequencies are not linearly correlated (28, 34). There are currently no specific cell surface markers that exclusively distinguish the B10 or B10pro cell subsets as not all CD5+ or CD1dhi B cells are B10 or B10pro cells and not all B10 cells express CD5 or are CD1dhi (28, 35). Regardless of their small numbers or phenotype, spleen B10 cells play important inhibitory roles during T cell-mediated inflammation and autoimmune disease.
In contrast to the spleen, a large fraction of peritoneal cavity B cells are competent to express IL-10 (24, 28). Peritoneal B1 B cells that are identified by CD5 expression also secrete large amounts of IL-10 (36). Peritoneal B1 cells can also reverse the prolonged contact hypersensitivity reactions observed in CD22-deficient mice, an effect that is blocked by anti-IL-10 receptor antibodies (37). Considering the proximity of peritoneal cavity B10 and B1 cells to the intestinal tract and the regulatory role of IL-10 in autoimmune disease and inflammation, their abilities to express IL-10 during intestinal inflammation and modulate T cell activation was examined during spontaneous colitis in IL-10−/− mice and induced colitis in the T cell transfer model.
Materials and Methods
Mice
Tiger (B6.129S6-Il10 tm1Flv/J) (38), C57BL/6, and _Il10_−/− (B6.129P2-Il10 tmlCgn/J) mice were from The Jackson Laboratory (Bar Harbor, ME). _Rag2_−/− and 10BiT reporter mice were as described (39, 40). All mice were bred in a specific pathogen-free barrier facility and were used at 8-20 weeks of age unless indicated otherwise. Peritoneal cavity cells were obtained from 12-14 week-old mice. Gnotobiotic and specific pathogen-free mice (129S6/SvEv) were a gift of Dr. R. Balfour Sartor from the National Gnotobiotic Rodent Resource Center funded by the NCRR resource grant (P40 RR018603) and the University of North Carolina at Chapel Hill Center for Gastrointestinal Biology and Disease (P30 DK034987). The Duke University Animal Care and Use Committee approved all studies.
Cell preparation and immunofluorescence analysis
Single-cell spleen, inguinal lymph node, and mesenteric lymph node suspensions were generated by gentle dissection. To isolate peritoneal cavity leukocytes, 5 ml of ice-cold RPMI 1640 medium (Cellgro, Manassas, VA) containing 5% FBS was injected into the peritoneal cavity of euthanized mice followed by gentle massage of the abdomen and recovery of the fluid with a large-gauge needle. Intraepithelial lymphocytes (IELs) were recovered from PBS-flushed intestinal tissues (small intestine or colon pieces) washed with PBS for 20 min at 37 °C after surgical removal of Peyer's patches. Lamina propria lymphocytes (LPLs) were purified from IEL-free intestinal tissue pieces after collagenase D digestion (Roche, Indianapolis, IN) with subsequent mononuclear cell isolation using a Ficoll (GE Healthcare Biosciences, Piscataway, NJ) density gradient, as described (40). Viable cells were counted using a hemocytometer with relative lymphocyte percentages determined by flow cytometry analysis.
For immunofluorescence analysis, single-cell leukocyte suspensions (1 × 106 cells) were stained on ice for 20-30 minutes using predetermined optimal concentrations of mAbs as described (41). Washed cells were resuspended in PBS containing 1.5% paraformaldehyde and were kept at 4 °C in the dark until final analysis. Cells with light scatter properties of singlet lymphocytes were analyzed for 4-5 color immunofluorescence staining using a FACS Canto II flow cytometer (Becton Dickinson, San Jose, CA). Background staining was determined using unreactive isotype-matched control mAbs (Caltag Laboratories, San Francisco, CA).
The mAbs used in this study are as follows: CD21/35 (7G6), CD23 (B3B4), CD24 (M1/69), CD25 (PC61), CD38 (90/CD38), CD43 (S7), CD44 (IM7), CD138 (281-2), and CD11b (M1/70) from BD PharMingen (San Diego, CA); IgM (11/41), CD4 (GK1.5), CD8 (53-6.7), CD19 (6D5), CD1d (1B1), CD62L (MEL-14), CD5 (53-7.3), CD80 (16-10A1), MHC class II (I-A, I-E; clone 114.15.2), CD45RB (C363-16A), IL-17 (TC11-18H10.1), and IFN-γ (XNG1.2) from BioLegend (San Diego, CA); CD45.1 (A20), CD90.1 (HIS51), and CD86 (GL1) from eBioscience (San Diego, CA); and anti-IgM and -IgD (11-26) from Southern Biotechnology Associates (Birmingham, AL).
IL-10 staining was as described (42). Purified mononuclear cells were resuspended (2 × 106 cells/ml) in complete medium [RPMI 1640 media, 10% (v/v) FCS (Sigma-Aldrich, St. Louis, MO), 200 μg/ml penicillin, 200 U/ml streptomycin, 4 mM L-Glutamine (all Cellgro), and 55 μM 2-mercaptoethanol (Life Technologies, Grand Island, NY)] containing LPS (10 μg/ml, Escherichia coli serotype 0111: B4, Sigma), phorbol myristate acetate (PMA, 50 ng/ml; Sigma), ionomycin (500 ng/ml; Sigma), and monensin (2 μM; eBioscience) for 5 h. B10pro cells were induced to mature and acquire IL-10 competence in vitro by culturing the cells with agonistic CD40 mAb (2 μg/ml, HM40-3, BD Biosciences, San Jose, CA) or LPS (10 μg/ml) for 48 h at 37 °C in a tissue culture incubator with 5% CO2 atmosphere with the addition of monensin, PMA, and ionomycin for the last 5 h of culture. Before cell surface staining, Fc receptors were blocked using Fc receptor mAb (2.4G2; BD Biosciences), and dead cells were labeled using the LIVE/DEAD® Fixable Violet Dead Cell Stain Kit (Invitrogen-Molecular Probes). Stained cells were fixed and permeabilized using a Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions prior to staining with anti-IL-10 (JES5-16E3, eBioscience) mAb. Isotype-matched mAb or splenocytes from IL-10−/− mice served as negative controls for IL-10 staining to demonstrate specificity and to establish background IL-10 staining levels.
T cell cytoplasmic cytokine production was measured after culturing the indicated cells in complete media with anti-CD3ε (1 μg/ml; 500A2, BD Pharmingen) and anti-CD28 (5 μg/ml; 37.51, Biolegend) mAbs in the presence of Brefeldin A (1 μl; Biolegend) for 4 hours prior to cytoplasmic staining with anti-IFN-γ or anti-IL-17 mAbs. Isotype-matched mAbs or cells cultured with Brefeldin A served as negative controls.
Cell isolation
B or T cells were enriched by positive selection using CD19 or CD4 mAb-coupled microbeads (Miltenyi Biotech, Auburn, CA), respectively, according to the manufacturer's instructions with obtained purities ≥95%. Where indicated, subsets of CD19+ B cells were further purified following immunofluorescence staining using a FACS Diva flow cytometer (BD Biosciences) with >98% purities. Spleen B cells were predominantly isolated based on CD1d and CD5 expression, whereas peritoneal cavity B cells were isolated based on CD11b and CD5 expression.
In vitro cultures and cell activation
To measure IL-10 secretion, 2 × 105 CD19+ purified B cells were cultured either with LPS (10 μg/ml), polyclonal goat anti-mouse IgM-specific F(ab’)2 antibody (5 μg/ml, Jackson Immunoresearch, West Grove, PA), or CD40 mAb (2 μg/ml) in 0.2 ml complete medium in a 96-well flat-bottom plate for 48 h. Tissue culture supernatant was then collected to assess cytokine production and the corresponding cells were recovered for additional in vitro stimulation, immunofluorescence staining, and flow cytometry analysis.
Adoptive transfer experiments
For syngeneic adoptive transfer experiments, enriched CD19+ spleen or peritoneal cavity B cells were washed with PBS and labeled using the Vybrant CFDA SE Cell Tracer Kit (Invitrogen) according to the manufacturer's instructions. Briefly, cells were incubated in 0.5 μM CFSE for 20 min at 37 °C. Labeled cells were then washed twice with cold PBS, counted for viability, and resuspended in cold PBS prior to i.p. injection of 1 × 106 viable cells into each untreated recipient. Spleens or peritoneal cavity fluids of recipient mice were harvested for analysis 72 h post-transfer.
Reconstitution experiments
For the reconstitution experiments described in figure 3A, tibia and femur bone marrow cells from three 8 week-old C57BL/6 CD45.1+ mice were isolated by flushing with complete media, pooled and strained using 70 μM mesh. Liver cells from E14 stage fetuses of C57BL/6 CD45.1+ mice were isolated by gentle mashing, subsequent red blood cell lysis, and filtration. Single-cell suspensions of viable fetal liver or bone marrow cells were resuspended in PBS prior to i.v. injection (4 × 106 cells) into each adult _Rag2_−/− recipient mouse, with spleens or peritoneal cavity fluids harvested from recipient mice six weeks post-transfer. For the reconstitution experiments described in figure 4C, viable, flow cytometry-purified CD19+CD11b+ peritoneal cavity B cells or CD19+CD1dhighCD5+ spleen B cells were isolated, washed and resuspended in cold PBS prior to i.v. injection (1 × 106 cells) into each _Rag2_−/− recipient. Spleens or peritoneal cavity fluids of recipient mice were harvested for analysis 14 days post-transfer.
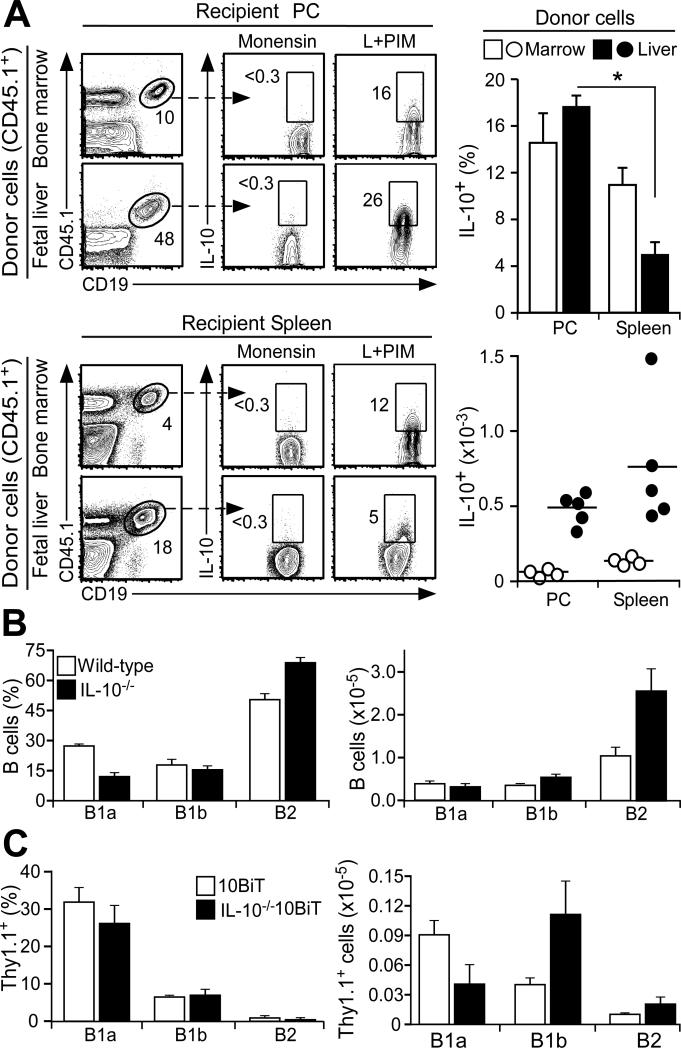
Figure 3. IL-10 competence is independent of B cell precursor populations and IL-10.
(A) IL-10 competence is independent of precursor B cell origin. Bone marrow and fetal liver hematopoietic cells from congenic CD45.1 transgenic donor mice were transferred i.v. into recipient _Rag2_−/− mice. Six weeks later, CD45.1+CD19+ B10 cells within the peritoneal cavity and spleen of recipient mice were quantified. Bar graphs show mean (±SEM) B10 cell frequencies and numbers from four recipient _Rag2_−/− mice in two experiments. Significant differences between sample means are indicated: * p<0.05. (B) Peritoneal cavity B cell subsets develop normally in IL-10−/− mice. B cells isolated from 10-12 week-old wild type or IL-10−/− littermates were stimulated with L+PIM for 5 h before immunofluorescence staining with flow cytometry analysis. Bar graphs indicate mean (±SEM) frequencies or numbers of B cells with B1a, B1b or B2 phenotypes (n=8 mice in two experiments). (C) IL-10 is not required for peritoneal cavity B10 cell development in 10BiT mice. B cells isolated from 10-12 week-old 10BiT or IL-10−/−10BiT littermates were stimulated with L+PIM for 5 h before immunofluorescence staining with flow cytometry analysis. Bar graphs indicate mean (±SEM) frequencies or numbers of IL-10+ cells among B cells with B1a, B1b or B2 phenotypes (n=3-4 mice/group).
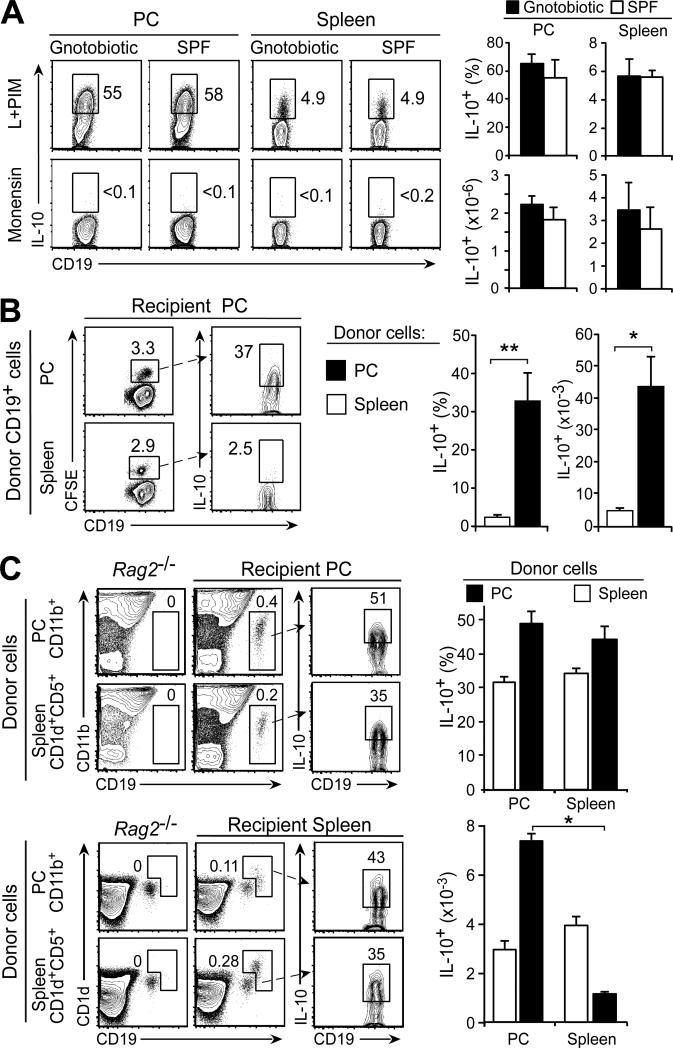
Figure 4. B cell IL-10 production is not regulated by microbiota or anatomic location.
(A) B10 cell development in gnotobiotic and specific pathogen-free (SPF) mice (6 mo-old; 129S6/SvEv strain). Representative flow cytometry histograms of peritoneal cavity and spleen B cells are shown. Bar graphs show mean (±SEM) B10 cell frequencies and numbers (n=3 mice/group). (B) The peritoneal cavity environment does not induce B10 cell expansion in C57BL/6 mice. Purified peritoneal cavity and spleen CD19+ B cells were CFSE-labeled and transferred i.p. into untreated recipient mice. After 72 h, peritoneal cavity B10 cell frequencies among CFSE+CD19+ B cells and numbers were quantified. Representative contour plots and percentages of CFSE+ B cells are shown. Bar graphs show mean (±SEM) B10 cell frequencies and numbers (n=4 mice) from one of two experiments with similar results. (C) IL-10-competent B cells reconstitute both the spleen and peritoneal cavity of _Rag2_−/− mice given PBS (control), or equal numbers of purified peritoneal cavity CD11b+CD19+ or spleen CD5+CD1dhiCD19+ B cells i.v. Two weeks later, IL-10-competent B cell frequencies and numbers among transferred B cells within the peritoneal cavity and spleen of recipient mice were quantified. Representative contour plots and percentages of cells within the indicated gates are shown. Bar graphs show mean (±SEM) B10 cell frequencies and numbers from three recipient _Rag2_−/− mice in two experiments. (B-C) Significant differences between sample means are shown; *p<0.05, **p<0.01.
Cytokine ELISAs
IL-10 concentrations within tissue culture supernatant fluid (diluted 1:2 in PBS) were quantified in triplicate using an OptEIA Mouse IL-10 ELISA Set (BD Pharmingen) according to the manufacturer's instructions.
Ig sequences
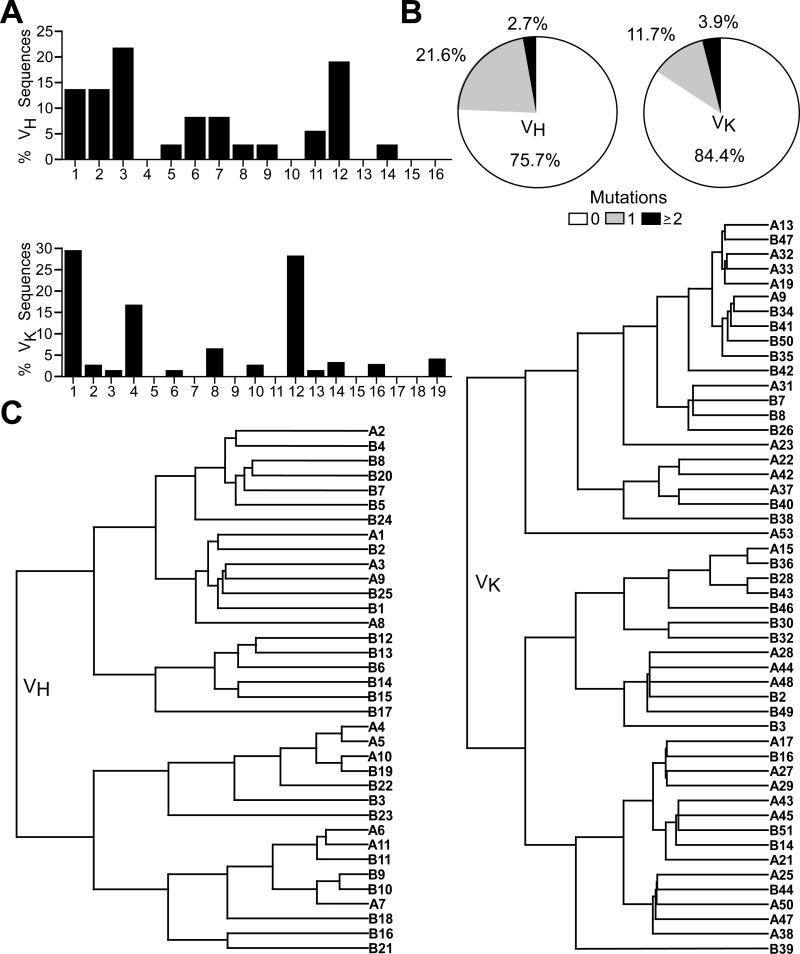
In two individual experiments, enriched peritoneal cavity CD19+ B cells from three individual wild type C57BL/6 mice were stimulated with LPS (10 μg/ml), PMA (50 ng/ml), and ionomycin (1 μg/ml) for 5 h. Individual IL-10+ λ −CD19+ cells were identified using the Mouse IL-10 Secretion Assay Kit (Miltenyi Biotech) according to the manufacturer's instructions and sorted into single wells of 96-well PCR plates using a FACSAria II cell sorter (BD Biosciences). cDNA was synthesized and Ig heavy (H) and light (L) chain transcripts were amplified using nested PCR primers as described (43). PCR products were purified (QIAquick PCR Purification Kit, Qiagen, Valencia, CA) and cloned (StrataClone PCR Cloning Kit, Agilent Technologies, La Jolla, CA) before sequencing (Duke University DNA Analysis Facility). Productive Ig rearrangements were compared against germline Ig sequences according to the Ig Basic Local Alignment Search Tool (IgBLAST) database (National Center for Biotechnology Information, Bethesda, MD) and analyzed using the Immunogenetics V-query and Standardization tool (44) to determine V(D)J gene family usage. Mutation frequencies were determined using germline V, D, and J sequences from IgBLAST. VH-D-JH and VK-JK transcript alignments and phylogenetic trees based on average percent identity were constructed using ClustalW2 (45). In one case, identical sequences were obtained from adjacent wells so only one sequence is reported.
Intestinal injury and colitis models and histopathological scoring
Intestinal inflammation was induced in male Tiger mice by the continuous provision of freshly-prepared 3% DSS (MP Biomedicals, Solon, OH) in autoclaved drinking water on days 0 and 3 as described (22). Mice were weighed daily and euthanized if they lost >20% of their initial body weight. Lymphocytes were isolated on days 3 or 7 and assayed for reporter protein expression as described (46). In the spontaneous model of colitis, enriched CD19+ B cells were pooled from the peritoneal cavities of wild type or IL-10−/− littermates and were adoptively transferred (0.5-1.0 × 106, i.p.) into IL-10−/− mice. In the induced model of colitis, single-cell suspensions of purified spleen CD25−CD45RBhiCD4+ T cells (4 × 105) were transferred i.p. into 10-12 week-old _Rag2_−/− mice along with pooled CD19+-enriched peritoneal cavity B cells from wild type or IL-10−/− littermates (0.5-1.0 × 106). Mice were monitored for weight loss, fur appearance, and the severity of diarrhea/wasting. Mice were euthanized if they lost ≥20% of their original body weight. Histological colitis scores from representative mice of each group were obtained 16 weeks after the adoptive transfers in the spontaneous model of colitis and 7 weeks after transfer in the induced colitis model.
For histopathological analysis, proximal and distal colon pieces of ~4 mm length were washed, fixed in 10% formalin in PBS and embedded in paraffin. Hematoxylin-Eosin and Alcian Blue (to identify goblet cell abundance) staining were performed on 5-μm thick slices of contiguous colon. A modified scoring system was used to evaluate histological changes based on three parameters of disease as described (47). Scores of 0-3 for three parameters in each sample were determined as follows: goblet cell loss (0 - no loss relative to wild type mice, 1 – focal loss of goblet cells from <5% of the total colonic tissue section area, 2 - loss of goblet cells from 5-20%, and 3 – diffuse loss of goblet cells from >20%); mononuclear cell infiltration into the lamina propria (0 - no cellular infiltration, 1 - <5 foci, 2 - 5-10 foci, and 3 - >10 foci in the total colonic tissue section); and epithelial erosion (0 - no epithelial erosions, 1 - epithelial erosion in <10% of the total colonic tissue section area, 2 - epithelial erosion in 10-30%, and 3 - epithelial erosions in >30%). Thereby, each sample received a cumulative score of 0 to 9.
Statistical analysis
All data are shown as means (±SEM). Significant differences between sample means were determined using a 2-tailed Student's t test for homoscedastic or heteroscedastic paired sample groups or an unpaired 1-tailed Fisher exact probability test.
Results
IL-10-competent B cells within gut-associated lymphoid tissues
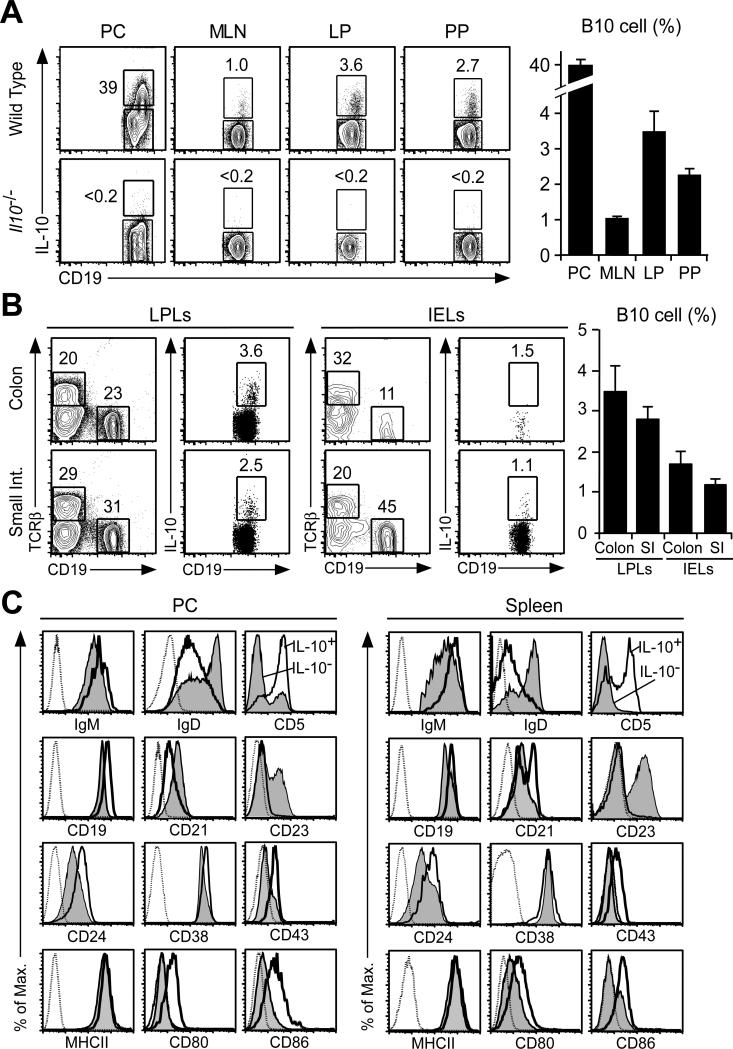
Cytoplasmic IL-10 expression by peritoneal cavity, mesenteric lymph node, colonic lamina propria, and Peyer's patch B cells was quantified to enumerate B10 cells. Cytoplasmic IL-10 staining reveals the frequency and numbers of B cells that are competent to express IL-10 and the level of IL-10 synthesis once induced as described for spleen B cells (24). Few, if any, B cells expressed IL-10 following ex vivo culture with monensin for 5 h when compared with control cells from IL-10−/− mice (data not shown). However, B cells with the capacity to express IL-10 (B10 cells) were readily identified following 5 h of in vitro stimulation with LPS, PMA, ionomycin, and monensin (L+PIM) to induce cytoplasmic IL-10 accumulation visualized by immunofluorescence staining with flow cytometry analysis (Fig. 1A), as described (24, 42). LPS was added to the 5 h PIM stimulated cultures as it marginally enhances B10 cell enumeration (28). B10 cell frequencies were highest (~40%) within the peritoneal cavity, while mesenteric lymph node, lamina propria, and Peyer's patch B10 cell frequencies were similar to those in the peripheral lymph nodes and spleen (24). Rare B10 cells were also found among the lamina propria and intraepithelial regions of the colon and small intestine (Fig. 1B). Thus, B10 cell frequencies are low except within the peritoneal cavity.
Figure 1. Peritoneal B cell IL-10 production.
(A) IL-10 expression by CD19+ B cells within the peritoneal cavity (PC), mesenteric lymph nodes (MLN), colonic lamina propria (LP), and Peyer's patches (PP). B cells isolated from either wild type or IL-10−/− mice were stimulated ex vivo with LPS, PMA, ionomycin and monensin (L+PIM) for 5 h before cell surface CD19 and cytoplasmic IL-10 immunofluorescence staining with flow cytometry analysis. Positive IL-10 gating was established using IL-10−/− or wild type B cells that were incubated with monensin alone with similar results. Numbers indicate cell frequencies within the indicated gates. Bar graphs show mean (±SEM) IL-10-competent B cell frequencies (n=4-8 mice per group). (B) The lamina propria (LP) and intraepithelial lymphocyte (IEL) subsets include B10 cells. IL-10+CD19+ B cells were identified as in (A) among B cell subsets isolated from the colon and small intestine. Bar graphs show mean (±SEM) IL-10-competent B cell frequencies (n=4 mice per group). Differences between tissues were not significant. (C) Peritoneal cavity and spleen IL-10+ B cell phenotypes. IL-10+CD19+ B cells identified as in (A) are shown (thick empty lines) in comparison with IL-10−CD19+ B cells (thin shaded histograms) and background control mAb staining (dotted lines). MHC class II (MHCII). Histograms represent results from ≥3 mice.
Peritoneal cavity B10 cells expressed high levels of IgM, CD5, CD19, CD24, CD43 and MHC class II relative to non-B10 cells, while their IgD and CD23 levels were low as with spleen B10 cells (Fig. 1C). Peritoneal cavity and spleen B10 cells also expressed CD80 and CD86 at higher levels than their IL-10− counterparts. Peritoneal cavity B10 cells expressed CD21 at low levels, which is in contrast to their intermediate and high level CD21 expression in the spleen. CD38 levels were variable, but were generally similar between B10 and non-B10 cells. CD1dhi or CD21hi B cells are not found within the peritoneal cavity (24). Importantly, IL-10 induction by L+PIM stimulation does not affect ex vivo expression of these cell surface molecules due to the presence of monensin and the short 5 h stimulation period (24). Thus, IL-10-competent B10 cells within the peritoneal cavity and spleen shared similar phenotypes.
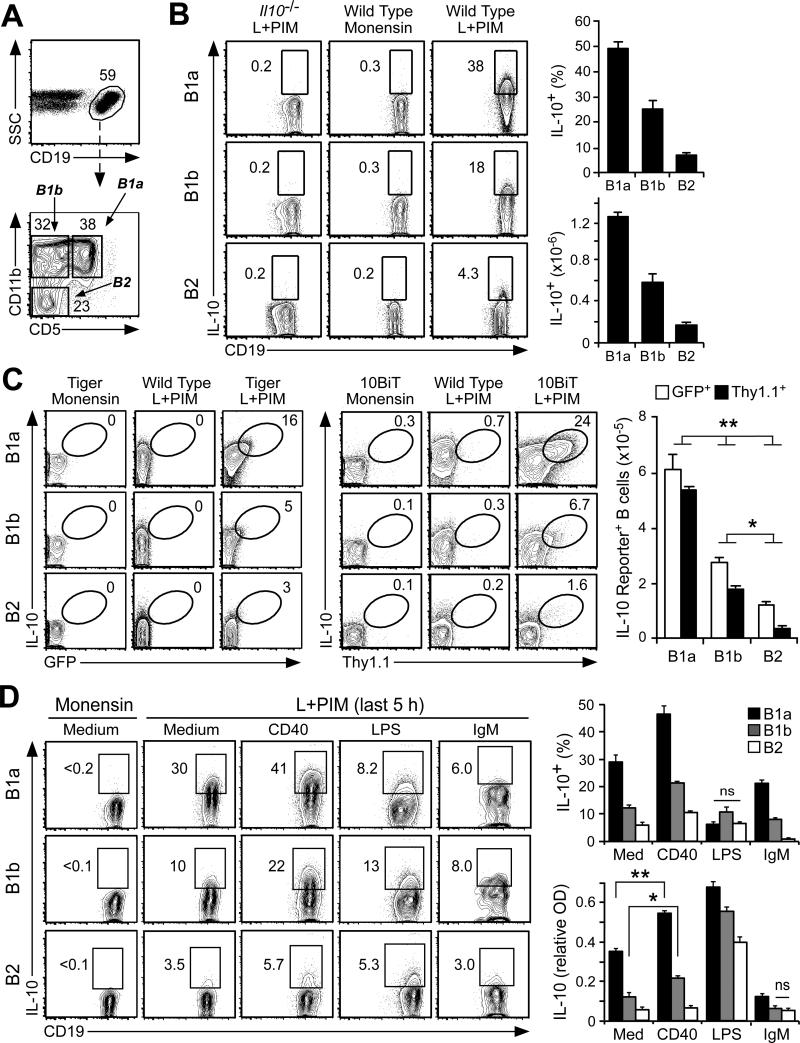
Within the peritoneal cavity, IL-10-competent B10 cells identified after L+PIM stimulation were present among the phenotypically-defined B1a (CD5+CD11b+, 49.2±2.2%, n=9) and B1b (CD5−CD11b+, 24±3.4%) cell subsets (Fig. 2A-B) that are functionally distinct (48). Significantly fewer B cells with a conventional CD5−CD11b− B2 cell phenotype were competent to express IL-10 (6.9±0.7%). Similar results were obtained using two lines of IL-10 reporter mice. Tiger mice have an IRES-GFP element inserted following the il10 locus (38). GFP expression mimics IL-10 induction but GFP has a longer half-life in B cells that have expressed IL-10 (46). By contrast, 10BiT mice have multiple bacterial artificial chromosome transgene insertions that drive Thy1.1 expression under the control of IL-10 regulatory elements (40). Cell surface Thy1.1 expression is delayed relative to B cell IL-10 expression, but persists on the cell surface following the termination of IL-10 expression (46). Following L+PIM stimulation, simultaneous IL-10 and reporter protein expression were detected in peritoneal B cells from both Tiger and 10BiT mice (Fig. 2C). B cells with a B1a cell phenotype contained the highest proportion of IL-10 reporter+ cells (16.6±1.9% for Tiger mice and 18.5±1.9% for 10BiT mice), followed by cells with a B1b phenotype (7.5±1.7% Tiger, 4.3±0.7% 10BiT), and finally cells with a B2 cell phenotype (3.2±0.4% Tiger, 2.1±0.6% 10BiT). Wild type B cells cultured with L+PIM served as negative controls for both GFP and Thy1.1 reporter expression and were essentially identical to B cells from reporter mice that were cultured with monensin alone. Thereby, peritoneal cavity B cells were competent to express IL-10 in the following hierarchical phenotype-dependent manner; B1a>B1b>B2.
Figure 2. IL-10 expression by phenotypically-defined peritoneal cavity B cell subsets.
(A) Representative gating used for the identification of CD19+ B cells with B1a (CD5+CD11b+), B1b (CD5+CD11b−), or B2 (CD5−CD11b−) cell phenotypes during flow cytometry analysis. Cell frequencies within the indicated gates are shown. Histograms represent results from ≥3 mice. (B) IL-10 expression by B cells with B1a, B1b or B2 cell phenotypes. IL-10-competent B cell frequencies and numbers were determined following L+PIM stimulation for 5 h, followed by cell surface molecule and cytoplasmic IL-10 staining before flow cytometry analysis. B cells from IL-10−/− mice or B cells cultured in monensin alone were used as controls for background IL-10 staining. Bar graphs show mean (±SEM) frequencies and numbers of IL-10-competent B cells (n=10 mice). (C) Representative IL-10 expression by B cells with B1a, B1b or B2 cell phenotypes in IL-10 reporter Tiger (eGFP reporter) and 10BiT (Thy1.1 reporter) mice in comparison with wild type mice shown as negative controls for reporter expression. B cells were stained for intracellular IL-10 and GFP for Tiger mice or Thy1.1 for 10BiT mice following 5 h of culture with monensin only or L+PIM. Bar graphs show mean (±SEM) reporter-positive cell numbers (n=8 mice). Significant differences between sample means are shown; *p <0.05, **p<0.01. (D) Influence of CD40, TLR, and BCR signaling on IL-10 expression. Purified B cells with B1a, B1b or B2 cell phenotypes were cultured in medium alone or containing agonistic CD40 mAb, LPS, or anti-IgM antibody for 43 h. The culture supernatant fluid was harvested for ELISA and the cells were then cultured with monensin or L+PIM for an additional 5 h. Representative contour plots for intracellular IL-10 expression by CD19+ B cells are shown. Bar graphs show IL-10-competent B cell frequencies after monensin or L+PIM stimulation and IL-10 concentrations in tissue culture supernatant fluid. Data are pooled from two experiments using three to four mice/group. Differences between values for each group are significant (p<0.05), except where noted as non-significant (n.s.).
B10pro cell development and maturation within the peritoneal cavity
Stimulating spleen B10pro cells through CD40 ligation induces them to acquire IL-10 competence and then express IL-10 following L+PIM stimulation as occurs with B10 cells (28). Because functionally-matured B10pro cells and ex vivo B10 cells both express IL-10 after these cultures, B10+B10pro cell frequencies are measured (42). Peritoneal cavity B cells with B1a, B1b, and B2 phenotypes were therefore purified and cultured with agonistic CD40 mAb or media alone for 48 h, a time point before measurable B cell division occurs (28). B cells cultured in medium alone were not induced to express IL-10 (Fig. 2D). However, ~30% of B cells with a B1a phenotype were B10 cells because they were inherently competent to express IL-10 after L+PIM stimulation without a requirement for CD40-induced maturation, while the B cell subsets with B1b and B2 phenotypes contained significantly fewer B10 cells. B10pro cells were also present within each of the B cell subsets with B1a, B1b, and B2 phenotypes as CD40 ligation before L+PIM stimulation induced more B cells to express IL-10 than did L+PIM stimulation alone. For example, almost half of peritoneal cavity cells with a B1a phenotype were competent to express IL-10 following in vitro maturation and stimulation. Thereby, peritoneal cavity B10+B10pro cell frequencies were highest within the B1a>B1b>B2 cell subsets.
LPS induces spleen B10pro cells to functionally mature into B10 cells and induces B10 cell IL-10 secretion (28), although spleen B10 cells appear to only secrete IL-10 transiently in vivo and following in vitro stimulation (46). Culturing peritoneal cavity B cells of all phenotypes with LPS appeared to induce both B10pro cell maturation and B10 cell IL-10 secretion, with most peritoneal cavity B cells having already ceased to express cytoplasmic IL-10 by the time they were stimulated with PIM at the end of the 48-hour culture period (Fig. 2D). Consistent with this, LPS induced high levels of IL-10 secretion into the culture supernatant fluid during the 48-hour cultures (Fig. 2D, bottom right graph). In unstimulated cultures, purified B cells with a B1a phenotype secreted more IL-10 than did cells with B1b or B2 phenotypes. CD40 stimulation alone enhanced IL-10 production by cells with B1a and B1b phenotypes in comparison with media alone (p<0.05). By contrast, potent IgM crosslinking significantly reduced both B10+B10pro cell development and IL-10 secretion as occurs with spleen B10+B10pro cells (28). Thus, 30-50% of peritoneal cavity CD5+ B cells could be induced to express IL-10, whereas B cells with B1b and B2 phenotypes were more similar to spleen B cells in their relative B10pro cell frequencies.
Precursor B10 cells are found in both fetal liver and adult bone marrow
Whether fetal liver or adult bone marrow precursor cells preferentially give rise to IL-10-competent B10 cells in vivo was assessed in adoptive transfer experiments. Precursor cells from congenic CD45.1+ donor mice were used to reconstitute untreated _Rag2_−/− mice with spleen and peritoneal cavity B10 cell development assessed six weeks later. Spleen B cells from mice that received CD45.1+ bone marrow cells contained 10.9±1.6% B10 cells, while peritoneal cavity B cells contained 14.5±1.6% B10 cells (Fig. 3A). Mice that received fetal liver cells had significantly different proportions of B10 cells among their spleen (4.9±1.1%) and peritoneal cavity (17.5±1.0%) B cells, although total B10 cell numbers were similar in both tissues (Fig. 3A, lower right graph). There were five-times more B cells and B10 cells generated within the spleens and peritoneal cavities of recipient mice given fetal liver cells than in those given bone marrow cells (data not shown). Thereby, B10 cells do not have a strict ontogeny-restricted origin, and both the fetal liver and adult bone marrow compartments provide a source for IL-10-competent spleen and peritoneal cavity B cells.
Peritoneal cavity B10 cells develop independent of IL-10 expression
A role for IL-10 in peritoneal cavity B cell development was assessed using young (4 week-old) IL-10−/− mice before their development of colitis or other autoimmune manifestations. B cells with B1a, B1b, and B2 phenotypes developed to similar frequencies and numbers in IL-10−/− and wild type control littermates (Fig. 3B). Whether autocrine IL-10 influences peritoneal cavity B10 cell development was assessed using IL-10−/−10BiT mice as described (46), where Thy1.1 is used as a surrogate marker for IL-10 production because IL-10 is not produced. Remarkably, Thy1.1+ B cells with B1a, B1b, and B2 phenotypes were found at similar frequencies and numbers in both 10BiT and IL-10−/−10BiT littermates (Fig. 3C). Thus, neither IL-10 nor B10 cell IL-10 production appear to be significant factors driving B cell subset or B10 cell development within the peritoneal cavity.
Peritoneal cavity B10 cell development is independent of commensal microbiota
Whether commensal microbiota sensitize peritoneal cavity B cells towards IL-10 production was examined using gnotobiotic mice. Peritoneal cavity and spleen IL-10-competent B cell frequencies were essentially identical in gnotobiotic and specific pathogen-free mice (Fig. 4A). Thereby, gastrointestinal bacteria do not appear to influence the ability of peritoneal cavity B cells to produce IL-10 under non-pathogenic homeostatic conditions.
B10 cell IL-10 production is independent of anatomic location
Whether the peritoneal microenvironment influences B cell IL-10-competence was examined by the adoptive transfer of spleen B cells into the peritoneal cavity under otherwise unaltered physiological conditions. Purified spleen and peritoneal cavity B cells were CFSE-labeled and independently transferred into the peritoneal cavities of untreated wild type mice. After 3 days, transferred CFSE+CD19+ spleen (2.2±0.1%) and peritoneal cavity (2.9±0.1%) B cells were present within the peritoneal cavity at similar proportions (Fig. 4B). However, B10 cell frequencies (32.8±7.2%) among peritoneal cavity donor cells were ~10-fold greater when compared to transferred spleen B10 cell frequencies (2.5±0.1%). These IL-10+ B10 cell frequencies closely resemble those of spleen and peritoneal cavity B cells in untreated mice.
To further determine whether IL-10 competence is directed by anatomic location, B10 cell-enriched spleen CD1dhiCD5+CD19+ and peritoneal cavity CD11b+CD19+ B cell subsets were CFSE-labeled and transferred i.v. into untreated _Rag2_−/− mice. Two weeks later, the proportion of transferred B10 cells within tissues was quantified. Lymphopenic _Rag2_−/− mice that did not receive transferred cells were used as controls (left contour plots, Fig. 4C). Remarkably, B10 cell frequencies were equivalent among transferred donor spleen B cells found within the spleen and peritoneal cavity. Similarly, B10 cell frequencies among transferred donor peritoneal cavity B cells were similar within the peritoneal cavity and spleen. As expected, transferred peritoneal cavity B cells and B10 cells had a tendency to localize within the peritoneal cavity. Regardless, the peritoneal cavity or spleen microenvironments alone did not regulate IL-10 competence among resident B cells under these disease-free conditions and T cells were not required for the maintenance of B10 cell IL-10 competence.
Peritoneal cavity B10 cells express diverse Ag receptors
The BCRs of individual peritoneal cavity IL-10+λ−CD19+ B cells of wild type mice were sequenced to obtain an unbiased representation of their IgH and IgL repertoires. Both H and L chain transcripts from single cells revealed the utilization of diverse VH and VK family members (Fig. 5A, Tables I-II). VH3 and VH12 were the most frequently observed VH families, consistent with the predominance of these families within the B1 cell repertoire (49, 50). Germline sequences without mutations encoded 76% of 39 representative VH-D-JH sequences and 84% of 77 representative VK-JK sequences (Fig. 5B). Thereby, peritoneal cavity B10 cells expressed clonally diverse BCRs that were predominantly germline-encoded, as has been observed for splenic B10 cells (46).
Figure 5. Peritoneal cavity B10 cells utilize diverse V genes that are largely unmutated.
(A) VH family gene usage by 36 IL-10+ B cells and VK family gene usage by 81 IL-10+ B cells from two individual mice. (B) Mutation frequencies within the VH-D-JH and VK-JK gene sequences. (C) Phylogenetic trees showing relationships between the VH-D-JH (n=36) or VK-JK (n=50) amino acid sequences of individual B cells from individual mice named A or B with numbers indicating different B cells. Branches indicate the average distance between two sequences based on percent identity.
Table II.
B10 cell VK-JK sequences
| Cell | V gene | VK | JK | V end | P | N | P | J End | CDR3 | Mutations |
|---|---|---|---|---|---|---|---|---|---|---|
| A9 | bb1 | 1 | 1 | _TGC_TCTCAAAGTACACATGTTCC | G | TGGACG | SQSTHVPWT | |||
| A12 | kf4 | 4 | 5 | _TGC_CAGCAGGGTAGTAGTATACCG | CT | CACG | QQGSSIPLT | |||
| A13 | cr1 | 1 | 1 | _TGC_TTTCAAGGTTCACATGTTCC | G | TGGACG | FQGSHVPWTF | |||
| A14 | 12-46 | 12 | 1 | _TGT_CAACATTTTTGGGGTACTCC | G | TGGACG | QHFWGTPWT | |||
| A15 | by9 | 10 | 1 | _TGT_CAGCAGTATAGTAAGCTTCCTCC | GACG | QQYSKLPPT | ||||
| A16 | ba9 | 14 | 2 | _TGT_CTACAGTATGATGAGTTTCC | G | TACACG | LQYDEFPYT | C206G, FWR3 | ||
| A17 | 12-46 | 12 | 2 | _TGT_CAACATTTTTGGGGTACTCC | G | TACACG | QHFWGTPYT | |||
| A18 | cr1 | 1 | 2 | _TGC_TTTCAAGGTTCACATGTTCC | G | TACACG | FQGSHVPYT | |||
| A19 | cr1 | 1 | 4 | _TGC_TTTCAAGGTTCACATGTTCC | A | TTCACG | FQGSHVPFT | |||
| A20 | bb1 | 1 | 2 | _TGC_TCTCAAAGTACACATGTTCC | G | TACACG | SQSTHVPYT | G272A, JK2 | ||
| A21 | 12-41 | 12 | 1 | _TGT_CAACATTTTTGGAGTACTCCT | C | GGACG | QHFWSTPRT | |||
| A22 | 8-24 | 8 | 2 | _TGT_CAGCAACATTATAGCACTCC | G | TACACG | QQHYSTPYT | |||
| A23 | he24 | 2 | 1 | _TGT_GCTCAAAATCTAGAACT | G | TGGACG | AQNLELWT | |||
| A24 | kf4 | 4 | 4 | _TGC_CAGCAGGGTAGTAGTATACCA | TTCACG | QQGSSIPFT | ||||
| A25 | fl12 | 12 | 1 | _TGT_CAAAATGTGTTAAGTACTCC | G | TGGACG | QNVLSTPWT | |||
| A26 | 12-46 | 12 | 1 | _TGT_CAACATTTTTGGGTTACTCCT | C | GGACGT | QHFWVTPRT | T96C, CDR1; G278T, CDR3 | ||
| A27 | 12-46 | 12 | 1 | _TGT_CAACATTTTTGGGGTACTCC | G | TGGACG | QHFWGTPWT | |||
| A28 | kf4 | 12 | 4 | _TGC_CAGCAGGGTAGTAGTATACCA | TTCACG | QQGSSIPFT | ||||
| A29 | 12-46 | 12 | 1 | _TGT_CAACATTTTTGGGGTACTCC | G | TGGACG | QHFWGTPWT | |||
| A30 | 12-46 | 12 | 1 | _TGT_CAACATTTTTGGGGTACTCC | G | TGGACG | QHFWGTPWT | |||
| A31 | bd2 | 1 | 1 | _TGC_TGGCAAGGTACACATTTTCCT | C | GGACG | WQGTHFPRT | |||
| A32 | cr1 | 1 | 4 | _TGC_TTTCAAGGTTCACATGTTCC | A | TTCACG | FQGSHVPFT | |||
| A33 | cr1 | 1 | 4 | _TGC_TTTCAAGGTTCACATGTTCC | A | TTCACG | FQGSHVPFT | |||
| A34 | bb1 | 1 | 5 | _TGC_TCTCAAAGTACACATGTTCCT | CC | CACG | SQSTHVPPT | |||
| A35 | cr1 | 1 | 4 | _TGC_TTTCAAGGTTCACATGTTCC | A | TTCACG | FQGSHVPFT | |||
| A36 | 8-27 | 8 | 2 | _TGT_CATCAATACCTCTCCT | CG | TACACG | HQYLSSYT | |||
| A37 | 8-21 | 8 | 5 | _TGC_AAGCAATCTTATAATCT | G | CTCACG | KQSYNLLT | |||
| A38 | fl12 | 12 | 4 | _TGT_CAAAATGTGTTAAGTACTCC | A | TTCACG | QNVLSTPFT | |||
| A39 | bb1 | 1 | 4 | _TGC_TCTCAAAGTACACATGTTCCT | ATA | TTCACG | SQSTHVPIFT | |||
| A40 | ai4 | 4 | 4 | _TGC_CACCAGTATCATCGTTCCCCA | TTCACG | HQYHRSPFT | T72C, FWR1 | |||
| A41 | 12-46 | 12 | 2 | _TGT_CAACATTTTTGGGGTACTCC | G | TACACG | QHFWGTPYT | |||
| A42 | 8-24 | 8 | 1 | _TGT_CAGCAACATTATAGCACTCC | G | TGGACG | QQHYSTPWT | |||
| A43 | 12-44 | 12 | 2 | _TGT_CAACATCATTATGGTACTCC | G | TACACG | QHHYGTPYT | |||
| A44 | kf4 | 4 | 4 | _TGC_CAGCAGGGTAGTAGTATACCA | TTCACG | QQGSSIPFT | ||||
| A45 | 12-44 | 12 | 2 | _TGT_CAACATCATTATGGTACTCC | G | TACACG | QHHYGTPYT | |||
| A46 | cr1 | 1 | 5 | _TGC_TTTCAAGGTTCACATGTTCC | G | CTCACG | FQGSHVPLT | |||
| A47 | fl12 | 12 | 2 | _TGT_CAAAATGTGTTAAGTACTCCT | CC | CACG | QNVLSTPPT | |||
| A48 | kf4 | 4 | 4 | _TGC_CAGCAGGGTAGTAGTATACCA | TTCACG | QQGSSIPFT | ||||
| A49 | ap4 | 4 | 4 | _TGC_CAGCAAAGGAGTAGTTACCCA | TTCACG | QQRSSYPFT | ||||
| A50 | fl12 | 12 | 2 | _TGT_CAAAATGTGTTAAGTACTCC | G | TACACG | QNVLSTP | |||
| A51 | ap4 | 4 | 4 | _TGC_CAGCAAAGGAGTAGTTACCCA | TTCACG | QQRSSYPFT | ||||
| A52 | fl12 | 12 | 2 | _TGT_CAAAATGTGTTAAGTACTCC | G | TACACG | QNVLSTPYT | |||
| A53 | 21-2 | 3 | 1 | _TGT_CAGCAAAGTAAGGAGGTTCC | G | TGGACG | QQSKEVPWT | |||
| A54 | fl12 | 12 | 2 | _TGT_CAAAATGTGTTAAGTACTCCT | CC | CACG | QNVLSTPPT | |||
| B2 | kf4 | 4 | 4 | _TGC_CAGCAGGGTAGTAGTATACCA | TTCACG | QQGSSIPFT | ||||
| B3 | kn4 | 4 | 2 | TGCCATCAGCGGAGTAGT(TAC) | ACG | HQRSSYT | ||||
| B7 | bd2 | 1 | 1 | _TGC_TGGCAAGGTACACATTTTCCT | C | GGACG | WQGTHFPRT | |||
| B8 | bd2 | 1 | 1 | _TGC_TGGCAAGGTACACATTTTCCT | C | GGACG | WQGTHFPRT | |||
| B14 | 12-44 | 12 | 1 | _TGT_CAACATCATTATGGTACT | TGGACG | QHHYGTWT | A312G, JK1 | |||
| B16 | 12-46 | 12 | 1 | _TGT_CAACATTTTTGGGGTACTCCT | CC | GACG | QHFWGTPPT | |||
| B23 | kh4 | 4 | 1 | _TGT_CAACAGTGGAGTAGTTACCCACTC | ACG | QQWSSYPLT | ||||
| B26 | bd2 | 1 | 5 | _TGC_TGGCAAGGTACACATTTTCC | G | CTCACG | WQGTHFPLT | A106G, CDR1 | ||
| B27 | cr1 | 1 | 1 | _TGCT_TTCAAGGTTCACATGTTCC | G | TGGACG | FQGSHVPWT | |||
| B28 | gj38c | 19 | 1 | _TGT_CTACAGTATGATAATCT | G | TGGACG | LQYDNLWT | |||
| B29 | 12-44 | 12 | 1 | _TGT_CAACATCATTATGGTACT | TGGACG | QHHYGTWT | T249C, FWR3 | |||
| B30 | RF | 16 | 1 | _TGT_CAACAGCATAATGAATACCCG(T) | GGACG | QQHNEYPWT | ||||
| B31 | gj38c | 19 | 1 | _TGT_CTACAGTATGATAATCT | G | TGGACG | LQYDNLWT | |||
| B32 | RF | 16 | 2 | _TGT_CAACAGCATAATGAATACCCG(TAC) | ACG | QQHNEYPYT | ||||
| B33 | aa4 | 4 | 2 | _TGC_CAGCAGTATCATAGTTACCCACCC | ATG | TACACG | QQYHSYPPMYT | |||
| B34 | bb1 | 1 | 1 | _TGC_TCTCAAAGTACACATGTTCC | G | TGGACG | SQSTHVPWT | |||
| B35 | bb1 | 1 | 5 | _TGC_TCTCAAAGTACACATGTTCC | G | CTCACG | SQSTHVPLT | A106T, CDR1; G150A, FWR2 | ||
| B36 | ce9 | 10 | 1 | _TGC_CAACAGGGTAATACGCTTCC | G | TGGACG | QQGNTLPWT | |||
| B37 | bd2 | 1 | 1 | _TGC_TGGCAAGGTACACATTTT | ACG | TGGACG | WQGTHFTWT | |||
| B38 | 19-17 | 6 | 1 | _TGT_CAGCAACATTATAGTACTCCT | C | GGACG | QQHYSTPRT | |||
| B39 | gn33 | 13 | 5 | _TGT_CAACAGTATTGGAGTACTCC | G | CTCACG | QQYWSTPLT | T311G, JK5 | ||
| B40 | 8-27 | 8 | 1 | _TGT_CATCAATACCTCTCCT | CG | TGGACG | HQYLSSWT | |||
| B41 | bb1 | 1 | 1 | _TGT_TCTCAAAGTACACATGTTCCT | CC | GACG | SQSTHVPPT | G313A, JK1 | ||
| B42 | cv1 | 1 | 2 | _TGT_TTCCAGAGTAACTATCTTCC | G | TACACG | FQSNYLPYT | |||
| B43 | gj38c | 19 | 2 | _TGT_CTACAGTATGATAATCT | G | TACACG | LQYDNLYT | |||
| B44 | fl12 | 12 | 1 | _TGT_CAAAATGTGTTAAGTACTCC | G | TGGACG | QNVLSTPWT | |||
| B45 | he24 | 2 | 1 | _TGT_GCTCAAAATCTAGAACTT | TGGACG | AQNLELWT | ||||
| B46 | ba9 | 14 | 1 | _TGT_CTACAGTATGATGAGTTTCCT | C | GGACG | LQYDEFPRT | |||
| B47 | cr1 | 1 | 2 | _TGC_TTTCAAGGTTCACATGTTCC | G | TACACG | FQGSHVPYT | |||
| B48 | 12-46 | 12 | 1 | _TGT_CAACATTTTTGGGGTACTCCT | CC | GACG | QHFWGTPPT | |||
| B49 | kf4 | 4 | 4 | _TGC_CAGCAGGGTAGTAGTATACCATG(C) | ACG | QQGSSIPCT | C289T, CDR3; A309G, JK4 | |||
| B50 | bb1 | 1 | 2 | _TGC_TCTCAAAGTACACATGTTCC | G | TACACG | SQSTHVPYT | T274A, FWR3 | ||
| B51 | 12-44 | 12 | 2 | _TGT_CAACATCATTATGGTACTCC | G | TACACG | QHHYGTPYT |
Peritoneal cavity B10 cells express IL-10 during DSS-induced intestinal inflammation
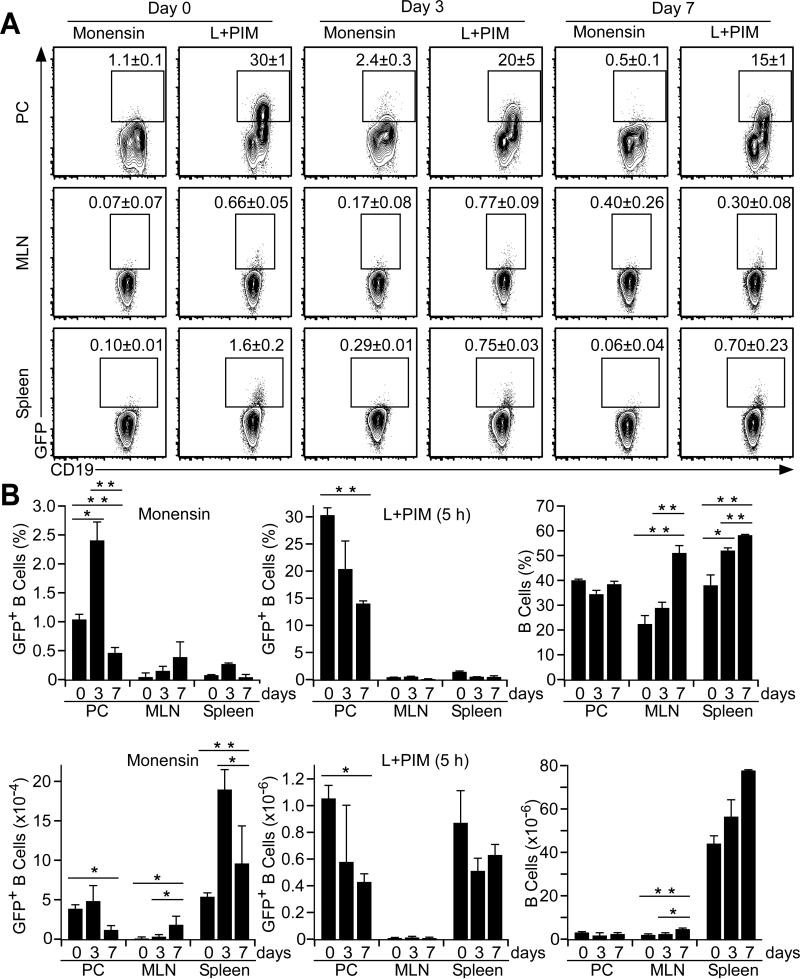
Given the ability of spleen B10 cells to suppress DSS-induced intestinal injury, the regulatory capacity of peritoneal cavity B10 cells was investigated in male Tiger mice by the provision of 3% DSS in drinking water for 3 or 7 days. When visualized directly ex vivo, peritoneal cavity and spleen IL-10+ B10 effector cell frequencies and numbers (monensin only) increased significantly by day 3 relative to day 0 control littermates (Fig. 6A-B). Peritoneal cavity B10 effector cell numbers decreased by day 7, while mesenteric lymph node and spleen B10 effector cell frequencies and numbers remained elevated. Peritoneal cavity B10 cell frequencies and numbers (L+PIM, 5 h) decreased significantly by day 7 relative to day 0 control littermates, while mesenteric lymph node and spleen B10 cell frequencies and numbers did not vary significantly over the course of the experiment. Thereby, DSS-induced gut inflammation induced peritoneal cavity B10 cells to become IL-10 expressing B10 effector cells, with the decrease in B10 effector cell and B10 cell numbers likely to result from the fact that some cells from both populations had already produced IL-10 in vivo and were no longer measurable in vitro following PIM stimulation. Importantly however, peritoneal cavity B10 cells produced IL-10 during periods of acute gut-associated inflammation.
Figure 6. B10 effector cell IL-10 expression during DSS-induced intestinal injury.
(A) B cells in Tiger mice express GFP during acute DSS-induced gut inflammation. Peritoneal cavity, mesenteric lymph node and spleen B cells were isolated from Tiger mice before (day zero) or three or seven days after DSS treatment and were cultured with either monensin or L+PIM for 5 h. Cell surface CD19 and cytoplasmic GFP expression were analyzed by flow cytometry. Mean (± SEM) GFP+ cell frequencies among CD19+ B cells are shown for three mice per group. (B) B10 effector cell frequencies and numbers during DSS-induced intestinal injury as measured in (A). Significant differences between sample means are shown; *p<0.05, **p<0.01.
Peritoneal cavity B10 cells regulate T cell responses during spontaneous colitis
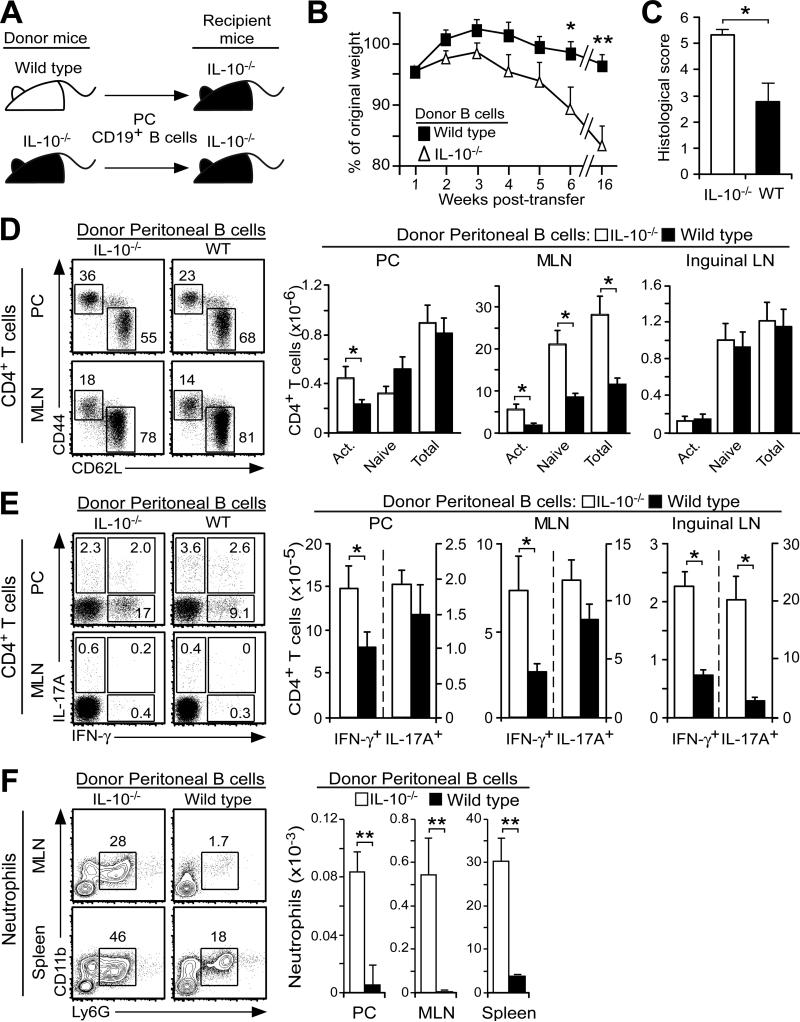
Considering the proximity of peritoneal cavity B10 cells to the intestinal tract and the regulatory role of IL-10 in autoimmune disease and inflammation, the ability of peritoneal cavity B10 cells to modulate T cell activation during spontaneous colitis onset was examined in IL-10−/− mice. Purified peritoneal cavity CD19+ B cells from IL-10−/− or wild type mice were injected i.p. into 10-12 week-old IL-10−/− recipient mice (Fig. 7A). Wasting and weight loss were significantly diminished in IL-10−/− mice given IL-10-competent B cells, although both groups of recipient mice showed signs of colitis during the 4-month period after transfers (Fig. 7B). Anal prolapse was not observed in mice that received IL-10-competent B cells, while 7 of 14 mice that received IL-10-deficient B cells developed anal prolapse during the 4-month period after transfers (p<0.01). The pathological hallmarks of colitis were also reduced in mice that received IL-10-competent B cells (Fig. 7C). IL-10-competent B cell transfers preserved goblet cell numbers and reduced colonic crypt distortion (data not shown). IL-10-competent B cell transfers also significantly reduced activated (CD62L−CD44+) CD4+ T cell numbers by 49% within the peritoneal cavity and naïve and activated CD4+ T cell numbers by 71% within the mesenteric lymph nodes of recipient mice (Fig. 7D). Transferred IL-10-competent B cells also significantly reduced the number of peritoneal and lymph node CD4+ T cells that expressed IFN-γ+ after _in vitro_ stimulation (Fig. 7E). The capacity of CD4+ T cells to produce IL-17 was also reduced by >5-fold within the inguinal lymph nodes of mice that received IL-10-competent B cells. Mice given IL-10-competent B cells also had significantly lower numbers of CD11b+Ly6G+ neutrophils within the peritoneal cavity, mesenteric lymph nodes, and spleen (Fig. 7F). B10 effector cell numbers were not assessed in this model as it was not possible to quantify the small numbers of adoptively transferred B cells present within the peritoneal cavity or gut-associated lymphoid tissues of IL-10−/− mice with colitis. Thus, IL-10 production by adoptively transferred peritoneal cavity B cells significantly reduced colitis onset and disease severity in IL-10−/− mice.
Figure 7. B10 cells regulate spontaneous colitis onset in IL-10−/− mice.
(A) Scheme for the transfer of peritoneal cavity CD19+ B cells from wild type (WT) or IL-10−/− mice into IL-10−/− mice i.p. at 10-12 weeks of age. (B-C) Adoptively transferred peritoneal cavity B cells from wild type mice prevent weight loss and reduce colitis severity in IL-10−/− mice. (B) Weight loss values represent mean (± SEM) results from 20 recipient mice/group during weeks 1-6 and 12 recipient mice/group at week 16 after euthanasia of mice with >20% weight loss. (C) Mean histological scores (± SEM) were obtained 16 weeks after adoptive transfers in four to six representative recipient mice/group from four experiments. (D-E) Adoptively transferred peritoneal cavity B cells from wild type mice reduce CD4+ T cell activation and IFN-γ expression in recipient IL-10−/− mice. (D) Representative peritoneal cavity and inguinal lymph node dot plots and bar graphs show mean frequencies and numbers (± SEM) of total, naïve (CD44−CD62L+) and activated (CD44+CD62L−) CD4+ T cells eight weeks after adoptive transfers with nine recipient mice/group from three experiments. (E) Representative CD4+ T cell cytoplasmic IFN-γ and IL-17A production measured after CD3ε/CD28 mAb stimulation in the presence of Brefeldin A for 4 h. Bar graphs indicate means (±SEM) of eight recipient mice/group from three experiments. (F) Transferred peritoneal cavity B cells from wild type mice reduce neutrophil localization within recipient IL-10−/− mouse tissues. Representative histograms show tissue CD11b+Ly6G+ neutrophil frequencies 16 weeks after adoptive transfers. All CD19+ and TCRβ+ lymphocytes were excluded (“gated out”) from the mononuclear cell preparations for the analysis. Values indicate mean (±SEM) neutrophil frequencies (contour plots) and numbers from seven recipient mice/group in two experiments. (B-F) Significant differences between sample means are indicated; *p<0.05, **p<0.01.
Peritoneal cavity B10 cells regulate colitis induction
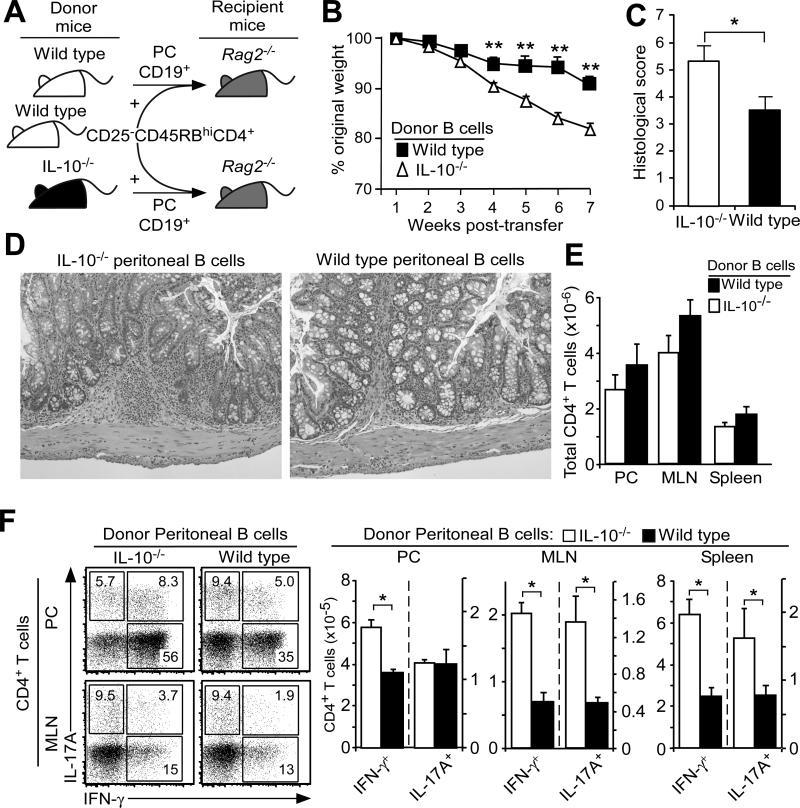
That peritoneal cavity B10 cells can alter the development and severity of colitis was further verified using the inducible T cell transfer model of colitis (10). Spleen CD25−CD45RBhiCD4+ T cells from wild type mice and peritoneal cavity B cells from either wild type or IL-10−/− mice were transferred simultaneously into _Rag2_−/− mice (Fig. 8A). Mice that received IL-10−/− B cells developed obvious wasting and weight loss by 3-4 weeks post-transfer as compared with mice that received IL-10-competent B cells (Fig. 8B). As early as 3 weeks after the adoptive transfer of CD25−CD45RBhiCD4+ T cells, the vast majority of T cells were already CD44hiCD62L− in mice given either _Il10_−/− or IL-10 competent wild type B cell transfers, respectively, within the peritoneal cavity (98±1 vs. 94±1 n=4/group), mesenteric lymph node (97±1 vs. 94±1), inguinal lymph node (97±1 vs. 78±1) and spleen (97±1 vs. 93±2). Colitis scores were significantly higher in mice that received B cells from IL-10−/− mice, mostly due to goblet cell loss and increased numbers of lymphocytic foci (Fig. 8C-D). There were no significant differences in overall CD4+ T cell numbers between groups of mice, but mice that received IL-10−/− B cells contained significantly higher frequencies and numbers of peritoneal cavity IFN-γ-producing CD4+ T cells by weeks 7-8 than did mice receiving IL-10-competent B cells (Fig. 8E-F). Mesenteric lymph nodes and spleens from mice that received IL-10−/− B cells also contained significantly higher numbers of IFN-γ- and IL-17A-producing CD4+ T cells due to significant mesenteric lymph node enlargement and moderate spleen enlargement. B10 or B10 effector cell numbers were not assessed in this model as it was not possible to quantify the small numbers of adoptively transferred B cells present within the peritoneal cavity or gut-associated lymphoid tissues of _Rag2_−/− mice with colitis. These collective results demonstrate that B10 cells from the peritoneal cavity are able to down-regulate T cell activation within intestinal tissues and may also inhibit colitogenic T cell recruitment into the intestinal environment.
Figure 8. B cell IL-10 regulates T cell-induced colitis in _Rag2_−/− mice.
(A) Scheme for the i.p. co-transfer of peritoneal cavity CD19+ B cells from wild type (WT) or IL-10−/− mice along with spleen CD25−CD45RBhiCD4+ T cells from wild type mice into _Rag2_−/− mice. (B-D) Adoptively transferred peritoneal cavity B cells from wild type mice reduce weight loss and colitis severity. (B) Values represent means (± SEM) of 12 recipient mice/group from five experiments. (C) Mean histological scores (± SEM) were obtained seven to eight weeks after adoptive transfers in four to six representative recipient mice/group from four experiments. (D) Representative histologies from mice given either wild type or IL-10−/− B cells as in (C). (E-F) Transferred peritoneal cavity B cells from wild type mice do not significantly affect peritoneal cavity and mesenteric lymph node CD4+ T cell numbers but reduce CD4+ T cell IFN-γ and IL-17A expression in recipient _Rag2_−/− mice. T cell cytoplasmic cytokine production was measured seven to eight weeks after adoptive transfers following CD3ε/CD28 mAb stimulation in the presence of Brefeldin A for 4 h. Representative contour plots are shown. Bar graphs indicate mean (± SEM) CD4+ T cell numbers of eight recipient mice/group from three experiments. (B-D) Significant differences between sample means are indicated; *p<0.05, **p<0.01.
Discussion
These studies demonstrate that IL-10-competent peritoneal cavity B cells are regulatory and functionally comparable to spleen B10 cells. Peritoneal cavity B10 cells were small in total number, but were 10-fold more frequent among B cells than occurs within the intestinal track or mesenteric lymph nodes (Fig. 1) or within the spleen or peripheral lymph nodes (26). Peritoneal cavity B10 cells were present at different proportions among the phenotypically-defined peritoneal B1a>B1b>B2 B cell subpopulations (Fig. 2). B10 cell development or localization within the peritoneal cavity was not dependent on their fetal liver or adult bone marrow progenitor cells of origin (Fig. 3), their production of IL-10 (Fig. 3C) or the presence of T cells (Fig. 4C) or commensal microbiota (Fig. 4A), but appeared to be an intrinsic property of individual B cells that was not dictated by anatomic location (Fig. 4B-C). Importantly, gut-associated inflammation induced B10 effector cell IL-10 production in both the peritoneal cavity and mesenteric lymph nodes (Fig. 6). IL-10 production by adoptively transferred peritoneal cavity B10 cells contributed to significant reductions in disease severity in two different models of colitis by regulating colitogenic CD4+ T cell cytokine production (Figs. 7-8). Thereby, the peritoneal cavity B10 cell subset is important for maintaining homeostasis within gastrointestinal tissues.
B10 cells represent a small and functionally distinct B cell subset that shares some overlapping phenotypic markers with multiple B cell subpopulations, including spleen B1 cells, marginal zone progenitor cells, marginal zone cells, memory cells and plasmablasts (23, 24, 46, 51-53). The developmental relationships among all of these B cell subpopulations are undoubtedly complex, but these results may also reflect functional heterogeneity within these phenotypically-defined B cell subpopulations. Although most B10 cells are CD5+ (Fig. 2), B10 cells only represent a small subset within the spleen CD5+ B cell subpopulation, and CD5+ B cell frequencies do not predict B10 cell frequencies (28). Moreover, all spleen B cells express CD5 following CD40 ligation in vitro, while only a small subset of B cells have the capacity to express IL-10 ex vivo (28, 32). Regardless, IL-10 production remains the essential core of B10 cell regulatory function and thereby serves as a useful and exclusive marker for identifying B10 cells. For all of these reasons, the terms “B10” and “B10pro” cells are used in this manuscript as functional designations for B cells that are competent to express IL-10 ex vivo, regardless of their cell surface phenotypes and potential differences in cellular origins. Given this, the isolation of B1a, B1b, marginal zone progenitor cells, marginal zone cells, memory cells or plasmablasts based on their current phenotypic categorizations will include varying proportions of functionally-defined B10 and B10pro cells.
BCR specificity dramatically influences spleen B10 cell development and Ag-specific regulatory function (24, 28, 32). Receptors or pathways that positively or negatively regulate BCR signaling such as CD19, CD22 and CD40 can also significantly modulate B10 cell numbers (28, 54-57). By contrast, potent BCR crosslinking by anti-IgM antibodies significantly reduced the number of peritoneal B cells that expressed or secreted IL-10 (Fig. 2D), as also occurs with spleen B cells (28). Appropriate BCR signals are thereby thought to induce a select subset of B cells to become B10pro and then IL-10-competent B10 cells, while strong BCR signals may divert B cells into a different functional program (26). Consistent with their in vivo Ag stimulation, spleen B10 cells are hyper-responsive to mitogens (23, 28) and can give rise to Ag-specific, self-reactive or natural antibodies (46). The BCR repertoire of peritoneal cavity B10 cells was also predominantly germline-encoded with no somatic mutations in most clones (Fig. 5, Tables I-II). Despite this, the BCR repertoire of peritoneal cavity B10 cells was remarkably diverse, involving a wide spectrum of VH, D, and JH elements, normal frequencies of noncoded nucleotide (N) insertions, as well as considerable CDR3 diversity. Peritoneal cavity B10 cell VH utilization was thereby similar to that observed for spleen B10 cells (46) and conventional B cells (49), but also included sequences commonly associated with peritoneal cavity B1 cells (58, 59). The B10 cell BCR repertoire thus appears to be generated in response to diverse foreign and/or self Ags, which may explain B10 cell enrichment within the peritoneal cavity relative to other tissues.
Spontaneous ex vivo secretion of IL-10 by peritoneal cavity B cells has been well described (36, 60), with B1a, B1b, and B2 cells variably contributing to IL-10 secretion in vitro (Fig. 2D). CD40 stimulation enhanced spontaneous IL-10 secretion by peritoneal cells with B1a and B1b phenotypes. However, peritoneal B2 cells were like spleen B10 cells in that CD40 ligation did not induce IL-10 expression or secretion by these cells but did promote peritoneal (Fig. 2D) and spleen (28) B10pro cell maturation into IL-10-competent B10 cells. Cognate CD40-dependent interactions with CD4+ T cells are also required to induce spleen B10 cell IL-10 production in the mouse model of experimental autoimmune encephalomyelitis and during Listeria infections (32, 33). Thus, spontaneous ex vivo IL-10 production by peritoneal cavity B10 cells may reflect their prior in vivo interactions with T cells, although it remains possible that innate- or pathogen-induced signals may also induce B10 cell IL-10 production in vivo (46, 61). LPS induces spleen B10pro cell maturation and transient B10 cell IL-10 expression and secretion in vitro and in vivo (46). Likewise, LPS stimulation induced peritoneal cavity B10pro cell maturation and transient B10 cell IL-10 secretion in vitro (Fig. 2D). Thereby, BCR specificity drives B10pro and B10 cell development (28, 32), but TLRs, other innate immune signals and/or cytokines may also influence peritoneal B10 effector cell development and IL-10 production.
The cellular origin of B10 cells is unknown, while the origin of CD5+ B1 cells has been a matter of debate for over 30 years (62, 63). Although the fetal liver was once thought to be the exclusive source of B1 cells, adult bone marrow precursor cells can become fully functional CD5+ cells in adult mice (64). Similarly, both fetal liver and adult bone marrow contained precursor cells that reconstituted B10 cells in _Rag2_−/− mice (Fig. 3A). Adoptively transferred peritoneal cavity B10 cells preferentially migrated into and localized within the peritoneal cavity in comparison with transferred spleen B10 cells that migrated into both compartments at similar frequencies (Fig. 4B-C). However, peritoneal B cells can also migrate into the periphery under certain conditions or following stimulation, particularly through TLRs (65). Adoptively transferred peritoneal cavity B10 cells can also regulate skin inflammation during contact hypersensitivity responses (37), and B10 cell numbers expand significantly within the central nervous system during experimental autoimmune encephalomyelitis (29). It is thereby likely that factors other than precursor cell origin result in the high frequency of B10 cells within the peritoneal cavity of adult mice.
Peritoneal cavity B10 cells expressed IL-10 and effectively modulated T cell responses during both spontaneous and induced colitis. DSS-induced intestinal injury in Tiger mice increased the frequency of peritoneal GFP+ B10 effector cells expressing IL-10 acutely in parallel with gut inflammation (Fig. 6). The adoptive transfer of spleen B10 cells dramatically reduces DSS-induced colon inflammation in CD19−/− mice, which are B10 cell-deficient (22). Similarly, the transfer of peritoneal cavity B cells from wild type but not IL-10−/− mice significantly delayed colitis development in the IL-10−/− mouse model of spontaneous colitis, with decreased histological scores, an absence of anal prolapse, and the prevention of weight loss (Fig. 7A-C). IL-10 production by peritoneal cavity B cells also significantly delayed weight loss and reduced histological scores in the T cell transfer model of colitis (Fig. 8). In both mouse models, peritoneal cavity B10 cells were particularly effective at reducing the numbers of activated and IFN-γ producing CD4+ T cells in the peritoneal cavity and mesenteric lymph nodes. Peritoneal cavity B10 cell transfers also reduced Th17 cell numbers and/or function as well as neutrophil recruitment into the peritoneal cavity, lymph nodes and spleen. Thereby, the adoptive transfer of peritoneal cavity B10 cells delayed colitis development and reduced intestinal tissue damage, CD4+ T cell activation and activation of innate immunity through IL-10 production. Thus, B10 cells isolated from the both the peritoneal cavity and spleen demonstrate IL-10-dependent regulatory activities.
Mizoguchi and colleagues first demonstrated that B cells from mesenteric lymph nodes of _TCR_-α−/− mice and their antibody products suppress colitis by affecting the clearance of apoptotic cells (18). Antibody blockade of the CD40 or B7-2 co-stimulatory molecules on the adoptively transferred B cells eliminated their suppressive effects on pathogenic T cells (66). They subsequently coined the term “regulatory B cells” when showing that chronic intestinal inflammation generates IL-10-producing B cells characterized by CD1d upregulation within mesenteric lymph nodes of _TCR_-α−/− mice (20). Mizoguchi et al. recently demonstrated a regulatory role for peritoneal cavity B1 cells from _TCR_α−/− mice during chronic colitis, possibly through generating natural antibodies in response to microbial flora (67). In studies by others, mesenteric lymph node B cells in combination with CD8+ T cells protected mice from colitis induced by _Gαi2_−/− CD4+ T cells through the formation of regulatory T cells (68). Although these diverse regulatory mechanisms have been proposed for B cell amelioration of colitis, the current studies demonstrate that B10 cell IL-10 production is likely to also contribute to the regulatory effects observed in the above studies.
In summary, the current studies demonstrate that peritoneal cavity B10 cells are an important component of the regulatory network that controls gut homeostasis and autoimmunity. Moreover, peritoneal and spleen B10 cells appear to be functionally equivalent, despite their different anatomic locations and the fact that they share cell surface markers with a variety of phenotypically-defined B cell subsets that are often considered to be functionally distinct. Given that B cells can migrate between the spleen and peritoneal cavity (Fig. 3A, ref. 69), B10 cells may be functionally important both centrally within the spleen and within the peritoneal cavity and gut-associated lymphoid tissues during colitis. Given that T cells also play critical regulatory roles during IBD development in mice as well as humans (1, 3), it is likely that B10 and Treg cells share complementary regulatory functions for IBD as previously shown for experimental autoimmune encephalomyelitis (29). Although the induction of colitis requires both spontaneous and Ag-specific T cell proliferation, driven in part by microbiota-derived innate signals (1, 70, 71), the intestinal microbiota also trigger innate immune responses that can modify the T cell compartment and prevent IBD (20, 70, 72). B10 cell regulatory function is likely to have contributed to these regulatory effects. Thereby, B10 cell expansion or future B10 cell-directed therapies may facilitate the control of IBD.
Table 1.
B10 cell VH-DH-JH sequences
| Cell | V Gene | VH | D | JH | V End | P | N | P | D | P | N | P | J End | CDR3 | Mutations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | VH12.1.78 | 12 | DSP2.7 | 2 | _TGT_GCAGGAGACAGA | TCGGG | CTATGGTAACTAC | GACTAC | AGDRSGYGNYDY | ||||||
| A2 | 36-60.6.70 | 3 | DSP2.7 | 4 | _TGT_GCAAGCG (A) | CTATGGTAACTAC | GGTCTAT | ATTACTATGCTATGGACTAC | ASDYGNYGLYYYAMDY | A294C, CDR3 | |||||
| A3 | VH12.1.78 | 12 | DSP2.9 | 1 | _TGT_GCAGGAGAC | CTC | GATGGT (TACTG) | GTACTTCGATGTC | AGDLDGYWYFDV | ||||||
| A4 | J558.52.145 | 1 | DSP2.x | 1 | T_GT_GCAAGA | TACTATAGTAA(CTAC) | TGGTACTTCGATGTC | ARYYSNYWYFDV | |||||||
| A5 | J558.52.145 | 1 | DSP2.x | 1 | _TGT_GCAAGA | TACTATAGTAA (CTACT) | GGTACTTCGATGTC | ARYYSNYWYFDV | |||||||
| A6 | S107.3.62 | 7 | DSP2.8 | 3 | _TGT_GCAAGA | GGGG | ATGGTAACTAC | GT | C C | GGTTTGCTTAC | ARGDGNYVRFAY | ||||
| A7 | J606.1.79 | 6 | DSP2.12 | 3 | _TGC_ACAGG | C | G | ACTATA | TTGCTTAC | TGDYIAY | |||||
| A8 | VH12.1.78 | 12 | DSP2.9 | 1 | _TGT_GC | GGAAACCTC | ATGGT (TACTG) | GTACTTCATGTC | AETSWLLVLHV | G327C, JH1 | |||||
| A9 | VH12.1.78 | 12 | DSP2.9 | 1 | _TGT_GCAGGAGAC | CCC | GATGGT (TACT) | GGTACTTCGATGTC | AGDPDGYWYFDV | ||||||
| A10 | J558.67.166 | 1 | DFL16.1 | 4 | _TGT_GCAAGA | TATCCA | ATTACTACGGTAGTAG | ATTACTATGCTATGGACTAC | ARYPITTVVDYYAMDY | ||||||
| A11 | S107.3.62 | 7 | DSP2.x | 3 | _TGT_GCAAGA | TATAG | AGGCGACGAGG | GGTTTGCTTAC | ARYRGDEGFAY | C260T, FWR3 | |||||
| B1 | VH12.1.78 | 12 | DFL16.1 | 1 | _TGT_GCAGGAG | CTC | TATTACTACGGTAGTAG(CTAC) | TGGTACTTCGATGTC | AGVLYYYGSSYWYFDV | G326T, JH1 | |||||
| B2 | VH12.1.78 | 12 | DST4 | 2 | _TGT_GCAGG (AGACAG) | CTCAGGCTAC | ACCTC | CTTTGACTAC | AGDSSGYTSFDY | ||||||
| B3 | SM7.1.44 | 14 | None | 3 | _TGT_ACTACA | CCCCCC | None | TTTGCTTAC | TTPPFAY | ||||||
| B4 | 36-60.6.70 | 3 | None | 4 | _TGT_GCAAGAGA | GGG | None | CTATGCTATGGACTAC | AREGYAMDY | ||||||
| B5 | 36-60.6.70 | 3 | DST4 | 2 | _TGT_GC | G | ATA | ACAGCTCAGGCTAC | A | TACTTTGACTAC | AITAQATYFDY | ||||
| B6 | Q52.13.40 | 2 | DFL16.1 | 4 | _TGT_GCCAAACA | TGAAG | ACTACGGTAGTAGC | G | ATTACTATGCTATGGACTAC | AKHEDYGSSDYYAMDY | |||||
| B7 | 36-60.6.70 | 3 | DFL16.1 | 2 | _TGT_GCAAGAG (A) | TTACTACGGTAGTA | ACTTTGACTAC | ARDYYGSNFDY | G246A, FWR3 | ||||||
| B8 | 36-60.6.70 | 3 | DFL16.1 | 2 or 3 | _TGT_GCAAGAGA | GGGACCTA | GTAGTAGCTAC | GAGGG | CTAC | AREGPSSSYEGY | |||||
| B9 | J606.4.82 | 6 | DQ52 | 3 | TGT | TCTC | CTAACTGGGAC | GGGG | GGTTTGCTTAC | SPNWDGGFAY | |||||
| B10 | J606.4.82 | 6 | None | 2 | _TGT_ACCAG | C | AACTGG (GAC) | TAC | TSNWDY | ||||||
| B11 | S107.3.62 | 7 | DST4.3 | 2 | _TGT_GCA | C | GGG(AC) | TTTGACTAC | ARDFDY | ||||||
| B12 | Q52.8.22 | 2 | DFL16.1 | 4 | _TGT_GCCAGACA | TC | CTACGGTAGTAG | ATTGG | GCTATGGACTAC | ARHPTVVDWAMDY | |||||
| B13 | Q52.8.22 | 2 | DQ52 | 3 | _TGT_GCCAGACA | AGGGG | AACTGGG | GGT | GTTTGCTTAC | ARQGELGVFAY | |||||
| B14 | Q52.2.4 | 2 | DQ52 | 2 | _TGT_GCCAGA | AACTGG | TAC | TACTTTGACTAC | ARNWYYFDY | ||||||
| B15 | Q52.2.4 | 2 | DQ52 | 2 | _TGT_GCCAGA | AACTGG | TAC | TACTTTGACTAC | ARNWYYFDY | ||||||
| B16 | VH11.2.53 | 11 | DFL16.1 | 1 | _TGT_ATGAGA (TA) | CGGTAGTAG(CTAC) | TGGTACTTCGATGTC | MRYGSSYWYFDV | |||||||
| B17 | 3609.12.174 | 8 | DST4 | 2 | _TGT_GCTCGAAG | G | AGACAGCTCAGGCTA | AGGGGGGAC | TACTTTGACTAC | ARRRQLRLRGDYF DY | G200A, FWR3 | ||||
| B18 | 7183.12.20 | 5 | DFL16.1 | 2 | _TGT_GCAAGAC | ATCT | CTACGGTAGTAGC | C | TTGACTAC | ARHLYGSSLDY | |||||
| B19 | J558.53.146 | 1 | DQ52 | 2 | _TGT_GCAAGA | TGG | ACTGGGAC | GGTTTGC | GACTAC | ARWTGTVCDY | |||||
| B20 | 36-60.6.70 | 3 | DST4.3 | 2 | _TGT_GCAAGAGA | GG | GGGAC | CTG | ACT | TTTGACTAC | AREGDLTFDY | ||||
| B21 | VH11.2.53 | 11 | DFL16.1 | 1 | _TGT_ATGAGA (TA) | CGGTAGTAG(CTAC) | TGGTACTTCGATGTC | MRYGSSYWYFDV | T95C, CDR1; T78C, FWR3 | ||||||
| B22 | J558.6.96 | 1 | DFL16.1 | 2 | _TGT_GCAAGA | GATTGGG | ATTACTACGGTAGTAGCTAC | GG | CTAC | ARDWDYYGSSYGY | A73T, FWR1 | ||||
| B23 | VGAM3.8-3-61 | 9 | DSP2.3 | 2 | _TGT_GCAAGA | GGGGG | TGGTTACGAC | GACTAC | ARGGGYDDY | ||||||
| B24 | 36-60.4.66 | 3 | None | 4 | _TGT_GCAAGAG | G | None | TATGGACTAC | ARGMDY | A88G, FWR1 | |||||
| B25 | VH12.1.78 | 12 | DQ52 | 1 | _TGT_GCAGGAGACA | AACTGGG | T | TACTGGTACTTCGATGTC | AGDKLGYWYFDV |
Acknowledgments
The authors thank Dr. Guglielmo Venturi, Dr. David DiLillo, Dr. Koichi Yanaba and Ms. Jacquelyn Lykken for their input and comments. We also thank Dr. R. Balfour Sartor for providing gnotobiotic mice.
This work was supported in part by National Institutes of Health grants AI56363, AI057157, and DK054452 and a grant from The Lymphoma Research Foundation.
Footnotes
Author contributions: D.M., K.M.C., S.H.S., I.K., J.C.P. and T.F.T. designed the experiments; D.M., K.M.C., S.H.S. and I.K. performed the experiments; S.E.P. and C.T.W. provided essential research materials for these studies; all authors reviewed the findings and contributed to writing the manuscript.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Maynard CL, Weaver CT. Intestinal effector T cells in health and disease. Immunity. 2009;31:389–400. doi: 10.1016/j.immuni.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm. Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- 3.Nemoto Y, Kanai T, Kameyama K, Shinohara T, Sakamoto N, Totsuka T, Okamoto R, Tsuchiya K, Nakamura T, Sudo T, Matsumoto S, Watanabe M. Long-lived colitogenic CD4+ memory T cells residing outside the intestine participate in the perpetuation of chronic colitis. J. Immunol. 2009;183:5059–5068. doi: 10.4049/jimmunol.0803684. [DOI] [PubMed] [Google Scholar]
- 4.Asseman C, Read S, Powrie F. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J. Immunol. 2003;171:971–978. doi: 10.4049/jimmunol.171.2.971. [DOI] [PubMed] [Google Scholar]
- 5.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 7.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 10.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D, Sina C, Onnie CM, Weersma RK, Stokkers PC, Wijmenga C, Gazouli M, Strachan D, McArdle WL, Vermeire S, Rutgeerts P, Rosenstiel P, Krawczak M, Vatn MH, Mathew CG, Schreiber S. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat. Genet. 2008;40:1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 12.Aithal GP, Craggs A, Day CP, Welfare M, Daly AK, Mansfield JC, Hudson M. Role of polymorphisms in the interleukin-10 gene in determining disease susceptibility and phenotype in inflamatory bowel disease. Dig. Dis. Sci. 2001;46:1520–1525. doi: 10.1023/a:1010604307776. [DOI] [PubMed] [Google Scholar]
- 13.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacol. Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 15.Leiper K, Martin K, Ellis A, Subramanian S, Watson AJ, Christmas SE, Howarth D, Campbell F, Rhodes JM. Randomised placebo-controlled trial of rituximab (anti-CD20) in active ulcerative colitis. Gut. 2011;60:1520–1526. doi: 10.1136/gut.2010.225482. [DOI] [PubMed] [Google Scholar]
- 16.El Fassi D, Nielsen CH, Kjeldsen J, Clemmensen O, Hegedus L. Ulcerative colitis following B lymphocyte depletion with rituximab in a patient with Graves’ disease. Gut. 2008;57:714–715. doi: 10.1136/gut.2007.138305. [DOI] [PubMed] [Google Scholar]
- 17.Goetz M, Atreya R, Ghalibafian M, Galle PR, Neurath MF. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflamm. Bowel Dis. 2007;13:1365–1368. doi: 10.1002/ibd.20215. [DOI] [PubMed] [Google Scholar]
- 18.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor α mutant mice. J. Exp. Med. 1997;186:1749–1756. doi: 10.1084/jem.186.10.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizoguchi A, Bhan AK. A case for regulatory B cells. J. Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 20.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt EG, Larsen HL, Kristensen NN, Poulsen SS, Claesson MH, Pedersen AE. B cells exposed to enterobacterial components suppress development of experimental colitis. Inflamm. Bowel Dis. 2012;18:284–293. doi: 10.1002/ibd.21769. [DOI] [PubMed] [Google Scholar]
- 22.Yanaba K, Yoshizaki A, Asano Y, Kadono T, Tedder TF, Sato S. IL-10-producing regulatory B10 cells inhibit intestinal injury in a mouse model. Am. J. Pathol. 2011;178:735–743. doi: 10.1016/j.ajpath.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwata Y, Matsushita T, Horikawa M, DiLillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St.Clair EW, Tedder TF. Characterization of a rare IL-10-competent B cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanaba K, Bouaziz J-D, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Matsushita T, Yanaba K, Bouaziz J-D, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann. N. Y. Acad. Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 27.Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J. Immunol. 2012;188:3188–3198. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J. Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling EAE initiation and late-phase immunopathogenesis. J. Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haas KM, Watanabe R, Matsushita T, Nakashima H, Ishiura N, Okochi H, Fujimoto M, Tedder TF. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J. Immunol. 2010;184:4789–4800. doi: 10.4049/jimmunol.0902391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J. Clin. Invest. 2011;121:4268–4280. doi: 10.1172/JCI59266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard WJ, Tedder TF. Regulatory B cells control T cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horikawa M, Weimer ET, DiLillo DJ, Venturi GM, Spolski R, Leonard WJ, Heise MT, Tedder TF. Reglatory B cell (B10 cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J. Immunol. 2013;190:1158–1168. doi: 10.4049/jimmunol.1201427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiu Y, Wong CP, Hamaguchi Y, Wang Y, Pop S, Tisch RM, Tedder TF. B lymphocytes depletion by CD20 monoclonal antibody prevents diabetes in NOD mice despite isotype-specific differences in Fc γ R effector functions. J. Immunol. 2008;180:2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 35.Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res. Ther. 2013;15(Suppl 1):S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur. J. Immunol. 1992;22:711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 37.Nakashima H, Hamaguchi Y, Watanabe R, Ishiura N, Kuwano Y, Okochi H, Takahashi Y, Tamaki K, Sato S, Tedder TF, Fujimoto M. CD22 expression mediates the regulatory functions of peritoneal B-1a cells during the remission phase of contact hypersensitivity reactions. J. Immunol. 2010;184:4637–4645. doi: 10.4049/jimmunol.0901719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, Harhaj E, Flavell RA. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2 deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 40.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat. Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 41.Sato S, Ono N, Steeber DA, Pisetsky DS, Tedder TF. CD19 regulates B lymphocyte signaling thresholds critical for the development of B-1 lineage cells and autoimmunity. J. Immunol. 1996;157:4371–4378. [PubMed] [Google Scholar]
- 42.Matsushita T, Tedder TF. Identifying regulatory B cells (B10 cells) that produce IL-10. Methods Mol. Biol. 2011;677:99–111. doi: 10.1007/978-1-60761-869-0_7. [DOI] [PubMed] [Google Scholar]
- 43.Rohatgi S, Ganju P, Sehgal D. Systematic design and testing of nested (RT-)PCR primers for specific amplification of mouse rearranged/expressed immunoglobulin variable region genes from small number of B cells. J. Immunol. Methods. 2008;339:205–219. doi: 10.1016/j.jim.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 44.Brochet X, Lefranc MP, Giudicelli V. IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res. 2008;36:W503–508. doi: 10.1093/nar/gkn316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maseda D, Smith SH, DiLillo DJ, Bryant JM, Candando KM, Weaver CT, Tedder TF. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J. Immunol. 2012;188:1036–1048. doi: 10.4049/jimmunol.1102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chinen T, Komai K, Muto G, Morita R, Inoue N, Yoshida H, Sekiya T, Yoshida R, Nakamura K, Takayanagi R, Yoshimura A. Prostaglandin E2 and SOCS1 have a role in intestinal immune tolerance. Nat. Commun. 2011;2:190. doi: 10.1038/ncomms1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of VH-D-JH sequences from B1-a, B1-b, and conventional B cells. J. Immunol. 1996;158:1175–1186. [PubMed] [Google Scholar]
- 50.Arnold LW, Pennell CA, McCray SK, Clarke SH. Development of B-1 cells: segregation of phosphatidyl choline-specific B cells to the B-1 population occurs after immunoglobulin gene expression. J. Exp. Med. 1994;179:1585–1595. doi: 10.1084/jem.179.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, Kuhl AA, Loddenkemper C, Haury M, Nedospasov SA, Kaufmann SH, Steinhoff U, Calado DP, Fillatreau S. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity. 2010;33:777–790. doi: 10.1016/j.immuni.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 52.Blair PA, Chavez-Rueda KA, Evans JG, Shlomchik MJ, Eddaoudi A, Isenberg DA, Ehrenstein MR, Mauri C. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J. Immunol. 2009;182:3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Poe JC, Smith S-H, Haas KM, Yanaba K, Tsubata T, Matsushita T, Tedder TF. Amplified B lymphocyte CD40 signaling drives regulatory B10 cell expansion in mice. PLoS One. 2011;6:e22464. doi: 10.1371/journal.pone.0022464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsumoto M, Fujii Y, Baba A, Hikida M, Kurosaki T, Baba Y. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity. 2011;34:703–714. doi: 10.1016/j.immuni.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 56.Scapini P, Lamagna C, Hu Y, Lee K, Tang Q, DeFranco AL, Lowell CA. B cell-derived IL-10 suppresses inflammatory disease in Lyn-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2011;108:16873–16874. doi: 10.1073/pnas.1107913108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J. Clin. Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hardy RR, Carmack CE, Shinton SA, Riblet RJ, Hayakawa K. A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J. Immunol. 1989;142:3643–3651. [PubMed] [Google Scholar]
- 59.Seidl KJ, MacKenzie JD, Wang D, Kantor AB, Kabat EA, Herzenberg LA, Herzenberg LA. Frequent occurrence of identical heavy and light chain Ig rearrangements. Int. Immunol. 1997;9:689–702. doi: 10.1093/intimm/9.5.689. [DOI] [PubMed] [Google Scholar]
- 60.O'Garra A, Howard M. IL-10 production by CD5 B cells. Ann. N. Y. Acad. Sci. 1992;651:182–199. doi: 10.1111/j.1749-6632.1992.tb24615.x. [DOI] [PubMed] [Google Scholar]
- 61.Fillatreau S. Novel regulatory functions for Toll-like receptor-activated B cells during intracellular bacterial infection. Immunol. Rev. 2011;240:52–71. doi: 10.1111/j.1600-065X.2010.00991.x. [DOI] [PubMed] [Google Scholar]
- 62.Hardy RR. B-1 B cell development. J. Immunol. 2006;177:2749–2754. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 63.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat. Rev. Immunol. 2007;7:213–219. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 64.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, Fagarasan S. Regulation of B1 cell migration by signals through Toll-like receptors. J. Exp. Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mizoguchi E, Mizoguchi A, Preffer FI, Bhan AK. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int. Immunol. 2000;12:597–605. doi: 10.1093/intimm/12.5.597. [DOI] [PubMed] [Google Scholar]
- 67.Shimomura Y, Mizoguchi E, Sugimoto K, Kibe R, Benno Y, Mizoguchi A, Bhan AK. Regulatory role of B-1 B cells in chronic colitis. Int. Immunol. 2008;20:729–737. doi: 10.1093/intimm/dxn031. [DOI] [PubMed] [Google Scholar]
- 68.Wei B, Velazquez P, Turovskaya O, Spricher K, Aranda R, Kronenberg M, Birnbaumer L, Braun J. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc. Natl. Acad. Sci. U.S.A. 2005;102:2010–2015. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berberich S, Forster R, Pabst O. The peritoneal micromilieu commits B cells to home to body cavities and the small intestine. Blood. 2007;109:4627–4634. doi: 10.1182/blood-2006-12-064345. [DOI] [PubMed] [Google Scholar]
- 70.Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J. Exp. Med. 2010;207:1321–1332. doi: 10.1084/jem.20092253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 72.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]