The Deleterious Effect of Red Blood Cell Storage on Microvascular Response to Transfusion (original) (raw)
. Author manuscript; available in PMC: 2014 Nov 1.
Published in final edited form as: J Trauma Acute Care Surg. 2013 Nov;75(5):807–812. doi: 10.1097/TA.0b013e3182a74a9b
Abstract
Background
The transfusion of relatively older red blood cells (RBCs) has been associated with both morbidity and mortality in trauma patients in observational studies. Although the mechanisms responsible for this phenomenon remain unclear, alterations in the microcirculation as a result of the transfusion of relatively older blood may be a causative factor. To assess this hypothesis, we evaluated microvascular perfusion in trauma patients during RBC transfusion.
Methods
Anemic but otherwise stable trauma ICU patients with orders for transfusion were identified. Thenar muscle tissue oxygen saturation (StO2) was measured continuously by near infrared spectroscopy during the course of transfusion of one RBC unit. Sublingual microcirculation was observed by sidestream dark field illumination microscopy before and after transfusion of one RBC unit. Thenar muscle StO2 was recorded over the course of transfusion. Pre- and post-transfusion perfused capillary vascular density (PCD) was determined by semi-quantitative image analysis. Changes in StO2 and PCD relative to age of RBC unit were evaluated using mixed models that adjusted for baseline StO2 and Spearman's correlation, respectively.
Results
Overall, 93 patients were recruited for study participation, 69% were male and average Injury Severity Score was 26.4. Average pre-transfusion hemoglobin was 7.5 mg/dL and the average age of RBC unit transfused was 29.4 days. Average peri-transfusion StO2 was negatively associated with increasing RBC age (slope -0.11, p = 0.0014). Change in PCD from pre- to post-transfusion was found to correlate negatively with RBC storage age (Spearman correlation = -0.27, p = 0.037).
Conclusions
The transfusion of relatively older RBC units was associated with a decline in both StO2 and PCD. Collectively, these observations demonstrate that transfusions of older RBC units are associated with the inhibition of regional microvascular perfusion. In patients requiring multiple units of RBCs, alteration of the microcirculation by relatively older units could potentially contribute to adverse outcomes.
Level of Evidence: II, prognostic study
Introduction
Over the past century, advances in blood storage have culminated in the capacity to store red blood cells (RBCs) for up to six weeks prior to transfusion. Nevertheless, it is well known that changes occur during RBC storage, resulting in structural and functional defects collectively referred to as the red cell storage lesion.1 The 42-day expiration date for RBC units is based on regulations that specify only two requirements: (1) 75% of the RBCs remain in the circulation at 24 hours after transfusion and (2) hemolysis in the storage bag does not exceed 1%. Although 42 day-old RBCs meet this standard, a significant number of laboratory and clinical studies have raised concerns that relatively older, but not expired RBCs may not be as safe for transfusion as RBCs stored for a shorter duration.2-9
Although some clinical studies suggest that there is no harm associated with the transfusion of relatively older RBC units10-12, an expanding body of clinical research suggests otherwise, demonstrating associations between the transfusion of older RBC units and both morbidity and mortality.5,13 Among trauma patients, death, pneumonia, renal failure, and extended length of stay have been demonstrated to be associated with the transfusion of older RBCs.14
The corresponding pathophysiologic mechanisms to explain the deleterious effect of older RBC transfusions have yet to be clearly defined. We theorize that the transfusion of relatively older RBCs may be injurious to patients as a result of a negative effect on microvascular perfusion. Observation of the sublingual microcirculation during transfusion may be performed at the bedside with sidestream dark field (SDF) microscopy.15,16 Tissue oxygen saturation (StO2), as measured by near-infrared spectroscopy, is also measurable at the bedside, and has been demonstrated in a multi-center study to identify poor perfusion and predict multi- organ dysfunction and death in trauma patients.17 Using these technologies, we evaluated the microcirculation in trauma patients to determine if, in fact, transfusion of relatively older RBCs was associated with a decrease in perfusion.
Methods
A prospective cohort study was carried out between September 2009 and July 2012 at two level I academic trauma centers. Study approval was obtained from the Institutional Review Boards of the University of Alabama at Birmingham (UAB) and the University of Tennessee Health Science Center (UT).
Study population
Over the period of study, patients in the trauma intensive care unit with orders to receive RBC transfusion were screened for participation. Study inclusion criteria included admission to the trauma intensive care unit from the emergency department (directly or by way of the operating room or angiography suite) following blunt or penetrating injury. Patients who were receiving transfusion in the context of volume resuscitation were excluded from participation. Patients who were receiving vasopressors or were otherwise hemodynamically unstable were also excluded from participation. Additional exclusion criteria included pregnancy, age less than 19 years, and burn injuries. Patients were enrolled in the study following obtainment of informed consent from the patient or patient's legally authorized representative.
The decision to transfuse was made by the attending physician prior to and independently of the patient's participation in the study. There was no protocol in place related to the indication for transfusion that was specific to the study. During the study period, all RBC units transfused had undergone pre-storage leukoreduction within 24 hours of collection by high-efficiency filters. The storage duration (days) for each unit transfused was recorded.
Variables of interest
Enrolled patients underwent evaluation of the microcirculation by way of sublingual video microscopy and near infrared spectroscopy of the thenar muscle bed. Sublingual microscopy was performed with a handheld video microscope (MicroScan BV, Amsterdam, The Netherlands). This device captures video images of the sublingual microcirculation with SDF illumination technology. The details regarding this technique have been reported elsewhere.18 The device was applied to the sublingual capillary bed, and three to five separate images of approximately 20 seconds duration each were recorded at three time points: immediately prior to transfusion of a single red blood cell unit, immediately following transfusion of the RBC unit, and one hour following completion of the transfusion.
Video images were later evaluated by an observer blinded to both study patient and image sequence (i.e. pre- vs. post-transfusion). Perfused capillary density (PCD) as described by De Backer et al was determined.19 In brief, a grid consisting of three equidistant horizontal and vertical lines was placed over the video image. Capillary vascular density was discerned by counting the number of small vessels (< 20 micrometers) that cross the lines divided by the total length of the lines. PCD was calculated by determining the fraction of the number of small vessels with visualized flow that cross the lines.
A near infrared tissue spectrometer was used to record StO2 values (Hutchinson Technology, Hutchinson, MN). The spectrometer was placed on either the left or right thenar eminence (if a radial arterial line was present, the opposite hand was used for StO2 monitoring). StO2 monitoring was initiated approximately 10 minutes prior to starting RBC transfusion, and was discontinued one hour following the completion of the transfusion. StO2 measurements were recorded at two-second intervals and were averaged for every two-minutes of observation from the beginning of transfusion until 1-hour post-transfusion, approximately 2 hours of observation.
Other variables included demographic (age, gender), injury (blunt versus penetrating mechanism, and injury severity score (ISS)), and hospitalization (hospital days, ICU Days, ventilator support days, and in-hospital mortality) characteristics. In addition, pre-transfusion clinical data (hemoglobin, serum lactate, base excess, and mean arterial pressure) measured on the day of study participation was recorded.
Statistical analysis
Differences in PCD between transfusion time points were evaluated with nonparametric measures of comparison. Specifically, correlations between RBC storage age and changes in PCD were assessed by Spearman – rho, a correlation comparison based on ranks. The repeated measures analysis of StO2 utilized mixed models and evaluated the association between RBC storage age and StO2 over the duration of the transfusion period. The mixed models included subjects' baseline StO2 values, adjusting for pre-transfusion StO2. Resulting beta parameters represent the effect of the predictor variables, i.e., baseline StO2 and blood age, on StO2. Finally, the association between StO2 and a number of potential confounders, including time of StO2 measurment, institution (UAB versus UT), sex, age, injury mechanism, (blunt versus penetrating), ISS, and pre-transfusion mean arterial pressure and hemoglobin, were evaluated similarly with mixed models that adjusted for patients' baseline StO2. SAS version 9.2 (Cary, NC) was used for the statistical analysis.
Results
Overall, 93 patients were enrolled in the study. The characteristics of the study group are demonstrated in Table 1. All patients were in an intensive care unit at the time of study enrollment and all had severe injuries (mean Injury Severity Score of 26.4). In-hospital mortality for the cohort was 5.4%.
Table 1.
Demographic, injury, and hospitalization characteristics of study subjects (n=93), as well as single unit RBC age. Values reported as mean (SD) unless otherwise stated.
| Demographic | |
|---|---|
| Age, years | 46.5 (20.07) |
| Male (%) | 64 (68.8) |
| Institution | |
| UAB | 36 (38.7) |
| UT | 57 (61.3) |
| Injury | |
| Mechanism (%) | |
| Blunt | 71 (76.3) |
| Penetrating | 22 (23.7) |
| Injury Severity Score | 26.4 (14.23) |
| Hospitalization | |
| Hospital Days | 37.3 (30.79) |
| ICU Days | 26.3 (28.24) |
| Ventilator support Days | 21.9 (28.68) |
| In-hospital Mortality (%) | 5 (5.4) |
Table 2 demonstrates clinical data pertaining to the day of study participation. All patients were hemodynamically stable without the support of any vasopressors, and all were anemic (average hemoglobin, 7.5 g/dL). The average base excess was 3.2 mmol/L. No patient had received any transfusions on the day of study before study enrollment. The age of RBC unit transfused ranged from 7 to 42 days, with an average age of 29.
Table 2.
Clinical characteristics of study cohort at time of study participation (n=97). Values reported as mean (SD).
| Pre-transfusion Hemoglobin (g/dL) | 7.5 (0.86) |
|---|---|
| Serum Lactate (mmol/L) | 1.1 (0.60) |
| Base Excess (mmol/L) | 3.2 (4.54) |
| Mean Arterial Pressure (mm Hg) | 85.6 (12.28) |
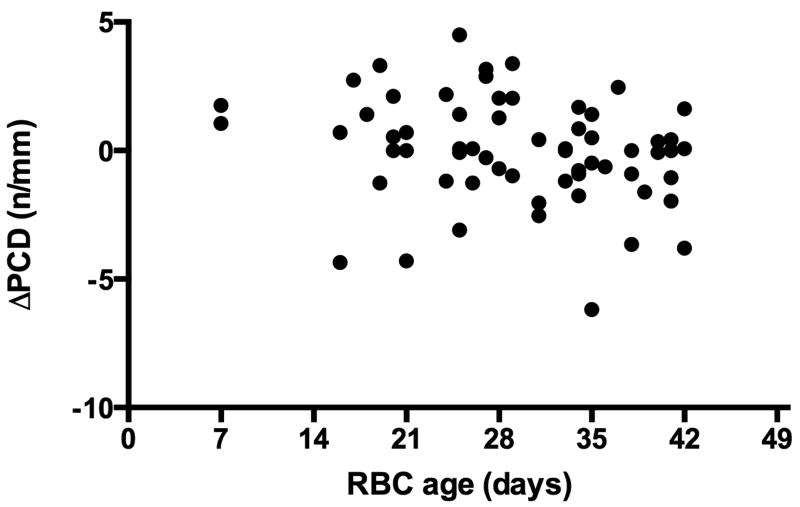
Sublingual microcirculation was observed in 62 patients. Adequate video microscopy images were either not obtainable or of sufficient quality secondary to patient cooperation in the remaining patients. Pre-transfusion PCD ranged from 1.8 to 18.4 n/mm, with a median of 9.2 n/mm. Immediately post-transfusion, the median PCD was 8.3 n/mm (range 3.9 to 18.7 n/mm), and 1-hour post-transfusion, the median PCD was 12.9 n/mm (range 5.8 to 22.2 n/mm). The change in PCD from pre-transfusion to immediately post-transfusion ranged from -6.2 to 4.5 n/mm and was found to correlate negatively (Spearman correlation = -0.266, p = 0.564).with RBC storage age (Figure 1). The change in PCD from pre-transfusion to 1-hour post-transfusion ranged from -6.3 to 7.6 n/mm and was not significantly correlated with RBC storage age (Spearman correlation = -0.076, p = 0.564).
Figure 1.
Scatter plot representing RBC storage age (days, x-axis) and the change in PCD (n/mm, y-axis) immediately post-transfusion. Spearman correlation = -0.266, p = 0.037.
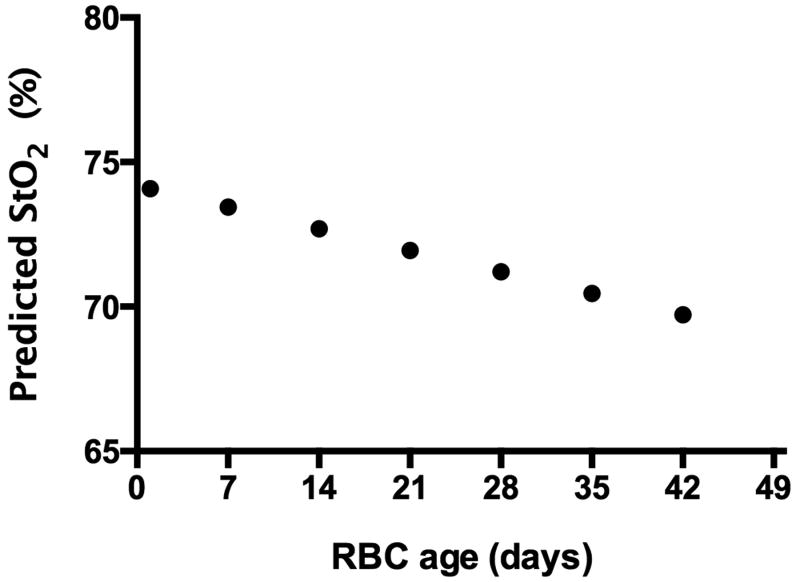
Continuous StO2 measurements were recorded for 81 patients over the peri-transfusion period. In the remaining patients, monitoring was hindered by inadequate sensor adherence to the patient's hand during the entire observation period or similar technical problems. Results of the mixed model used to examine the association between StO2 and RBC age over the transfusion period, i.e., from the beginning of transfusion to one-hour post-transfusion (Table 3) demonstrates that with each increase in day of blood storage age, the StO2 decreased by 0.1064 (p=0.0012). The model predicts a progressive decline in overall StO2 of approximately 0.11% for each day of blood age (Figure 2). Table 3 also presents results for potential confounders that were evaluated similarly; no significant association was found for any of the variables examined.
Table 3.
Associations between peri-transfusion StO2 and predictor variables; adjusting for patient baseline StO2.1
| Adjusted for baseline StO2 | ||
|---|---|---|
| Predictors | Beta | p-value |
| Time | 0.0254 | 0.9229 |
| Center (UAB versus UT) | 0.6551 | 0.2526 |
| Blood age (days) | -0.1064 | 0.0012 |
| Female versus male | 0.6306 | 0.2974 |
| Age (years) | -0.0049 | 0.7353 |
| Blunt versus penetrating mechanism | 0.8344 | 0.2089 |
| Injury severity score | 0.0333 | 0.0929 |
| MAP | -0.0089 | 0.3239 |
| Pre Hemoglobin | 0.0449 | 0.7657 |
| Base deficit | -0.1329 | 0.2345 |
Figure 2.
Predicted average StO2 over the duration of transfusion as related to RBC age (based on pre-transfusion StO2 of 74%).
Discussion
In clinical practice, the underlying goal of RBC transfusion is the maintenance of tissue oxygen delivery. Nonetheless, there is a notable lack of evidence for this desired effect, and, in fact, a growing body of work suggests that stored RBCs may have adverse effects on microcirculatory flow and oxygen utilization. Raat et al. demonstrated that RBCs stored for longer periods of time (5 weeks) resulted in diminished oxygen delivery to the gut microcirculation of anemic oxygen-supply dependent rats. In contrast, relatively fresh RBCs, stored only several days, and intermediate-aged RBCs, stored several weeks, both improved oxygen delivery.20 Similarly, Fitzgerald et al. demonstrated that RBCs stored for 28 days did not improve oxygen delivery and consumption when transfused to rats while transfusion of blood stored only 3 days did, in fact, improve oxygen delivery.21
In human studies, observations have been less conclusive. Marik et al. observed a decreased gastric pH, a surrogate measure of gastric mucosal oxygenation status, in patients receiving blood that had been stored beyond 15 days.22 Walsh et al., however, were unable to replicate the results of Marik et al, which were identified in the course of post hoc analysis. In a prospective, double-blind trial of critically ill intensive care unit patients randomized to receive leukodepleted red cells stored either ≤ 5 days or ≥ 20 days, Walsh et al. observed no significant differences in gastric pH measurements or other indices of global tissue oxygenation.23 More recently, Kiraly et al. evaluated peripheral tissue oxygenation as measured by near infrared spectroscopy during the course of red cell transfusion.24 The authors observed that patients transfused with blood stored 21 days or longer had a statistically significant decline in tissue oxygen saturation compared with those transfused with blood less than 21 days old.
Similar to the findings of Kiraly et al.24, the results of the present study demonstrate that transfusion of older RBC units was associated with inhibition of microvascular perfusion and/or oxygen delivery as evidenced by degradation in both functional capillary density and tissue oxygen saturation. The clinical relevance of these collective observations is unclear, as the magnitude of the changes observed in PCD in the present study and StO2 in both this study and the work of Kiraly et al., while statistically significant, are relatively small. Nonetheless, these observations do provide some basis for causation with respect to the growing body of observational studies that demonstrate an association between the transfusion of older blood and mortality. Wang et al. recently performed a meta-analyis of 21 studies (409,966 patients) concerning the transfusion of older blood and the risk of death, and found that older blood was associated with a significantly increased risk of death (odds ratio 1.16; 95% confidence interval 1.07 - 1.24).5 In our own observational trauma patient studies, we identified that both storage age and number of units transfused to acutely injured patients were independently associated with mortality.3,4 We speculate that in relatively unstable patients requiring multiple units of blood, the vasoconstrictive effect at the level of the microcirculation potentiated by older stored RBCs is a primary factor related to adverse outcome.
The mechanisms pertaining to storage lesion toxicity continue to be explored by our group and others. It is now appreciated that in addition to transporting oxygen to tissues, the red cell also functions as an oxygen sensor and transducer of vascular tone, mediated by interactions with nitric oxide (NO).25 Aged stored RBCs are thought to interfere with nitric oxide homeostasis.26-28 We have recently demonstrated in competition kinetic analyses that consumption rates of NO increased □ 40-fold and NO-dependent vasodilation was inhibited 2–4-fold comparing 42-day-old with 0-day-old RBCs.6 These results are probably due to the formation of smaller RBCs with increased surface area:volume as a consequence of membrane loss during storage. Associated with this was a significant loss of whole-blood nitrite that was observed in trauma patients after transfusion of 1 RBC unit, with the decrease in nitrite occurring after transfusion with RBCs stored for >25 days, but not with younger RBCs. Other groups have proposed that NO is scavenged by microparticles and free hemoglobin that accumulate during storage. Recently, Solomon et al., utilizing a canine pneumonia experimental model, observed that mortality was significantly higher among the dogs transfused 42 day-old stored RBCs compared with those who received 7 day-old RBCs.2 Other differences between the two groups included higher plasma cell–free hemoglobin and nitric oxide (NO) consumption capability, and lower haptoglobin levels in the older RBC group, suggesting that older RBCs have a propensity to hemolyze in vivo following transfusion, releasing vasoconstrictive cell-free hemoglobin. In our complement studies, we have observed that C5b-9 levels in stored RBC units significantly increased as a function of storage time and were associated with increases in free hemoglobin levels, suggesting the possibility of a complement-mediated storage toxicity.
There are several limitations worth noting concerning the present study. The study cohort comprises relatively few patients that received “younger” blood (only two patients received RBC units less than two weeks old). As previous clinical outcome studies have defined old versus young RBC units with a 14-day cutpoint,3-5 it would have been informative to have younger RBCs better represented in the current study. Nonetheless, our study was strengthened by a repeated measures approach that dramatically increased the number of observations available for estimating a relatively small linear effect of decreasing StO2 with increasing blood age; the potential cumulative effect, however, approaches a 4.5% decrease in oxygen saturation (i.e., -0.1064 × 42 days). Analysis of the sublingual microcirculation by microscopy is semiquantitative by nature. Although both intra- and interobserver variability has been reported to be relatively low in validation studies (around 5-10%)29, it is possible that such variability limits the reliability of the data analysis. Although we evaluated two separate tissue beds, the lack of sufficient paired data as a result of not all patients having data from both StO2 monitoring and sublingual microscopy precluded a sufficient paired-data analysis. In roughly one-third of the study patients, adequate video microscopy images were either not obtainable or of sufficient quality secondary to patient cooperation, and occasionally StO2 monitoring was hindered by inadequate sensor adherence to the patient's hand during the entire observation period or similar technical problems. An additional limitation of the present study pertains to baseline variability in the microcirculation from patient to patient, prior to transfusion. In previous work, we identified the fact that among a cohort of seemingly resuscitated, hemodynamically stable trauma patients, pre-transfusion sublingual perfusion was quite variable, and those patients with relatively poor sublingual perfusion were more likely to respond favorably to transfusion (i.e. a post-transfusion improvement in perfusion), whereby those patients with relatively normal pre-transfusion perfusion tended to demonstrate either no change or, in fact, a deterioration of sublingual perfusion following RBC transfusion.16 To address this concern, a Spearman partial correlation, adjusting for pre-transfusion PCD, was carried out; the resulting correlation between blood age and change in PCD had a magnitude and direction (rho=-0.22316) consistent with the unadjusted result; however, the correlation was no longer statistical significant (p=0.0839), suggesting that a small sample size influenced significance. It is also notable that the change in PCD related to RBC storage age was found to be both of relatively small magnitude and not sustained at one-hour post transfusion. Underlying differences between study patients, including interval from initial injury, presence or absence of infection, and variation in administration of intravenous fluids and medications may contribute to the variability observed in PCD measurements, as routine clinical care was not altered during the observed transfusion. Lastly, it is known that the Inspectra StO2 monitoring device cannot distinguish between hemoglobin and myoglobin.30 A relative muscle perfusion deficit may result in interference with accurate measurement of StO2,. This would not be expected to be a significant problem in this relatively stable study cohort, but could potentially be an underlying issue with respect to accuracy of StO2 measurement.
In summary, we have observed changes in the sublingual microcirculation and in thenar muscle tissue oxygen saturation related to the transfusion of relatively older blood in a hemodynamically stable, but anemic trauma patient cohort. These observations lend pathophysiologic support to clinical studies that demonstrate adverse consequences of RBC transfusion related to storage age. RBC transfusion is often performed in an effort to augment tissue oxygen delivery, but this therapy may in fact be inhibitory to tissue perfusion. Although the transfusion of a single RBC unit of relatively advanced storage age is unlikely to have any negative clinical effect, we speculate that for patients requiring multiple units of RBCs, the compounding impact of older RBCs on microcirculatory dysfunction could contribute to adverse post-injury outcomes.
Acknowledgments
This manuscript was supported by Grant Number R01HL095468 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
AUTHOR CONTRIBUTIONS: J.W., P.M., J.K., L.R., S.B., and R.P. designed this study. Data acquisition was performed by M.V., J.A., C.G., and L.H. Analysis and interpretation of data was performed by P.M. and J.W. Drafting of manuscript was performed by J.W. and P.M. Critical revision was performed by L.M, M.C., T.F., S.B., and R.P.
References
- 1.Hess JR. Red cell storage. Journal of proteomics. 2010;73(3):368–373. doi: 10.1016/j.jprot.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Solomon SB, Wang D, Sun J, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121(9):1663–1672. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberg JA, McGwin G, Jr, Vandromme MJ, et al. Duration of red cell storage influences mortality after trauma. The Journal of trauma. 2010;69(6):1427–1431. doi: 10.1097/TA.0b013e3181fa0019. discussion 1431-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinberg JA, McGwin G, Jr, Marques MB, et al. Transfusions in the less severely injured: does age of transfused blood affect outcomes? The Journal of trauma. 2008;65(4):794–798. doi: 10.1097/TA.0b013e318184aa11. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52(6):1184–1195. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stapley R, Owusu BY, Brandon A, et al. Erythrocyte storage increases rates of NO and nitrite scavenging: implications for transfusion-related toxicity. The Biochemical journal. 2012;446(3):499–508. doi: 10.1042/BJ20120675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. The New England journal of medicine. 2008;358(12):1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 8.Leal-Noval SR, Munoz-Gomez M, Arellano-Orden V, et al. Impact of age of transfused blood on cerebral oxygenation in male patients with severe traumatic brain injury. Critical care medicine. 2008;36(4):1290–1296. doi: 10.1097/CCM.0b013e3181692dfc. [DOI] [PubMed] [Google Scholar]
- 9.Alexander JT, El-Ali AM, Newman JL, et al. Red blood cells stored for increasing periods produce progressive impairments in nitric oxide-mediated vasodilation. Transfusion. 2013 doi: 10.1111/trf.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Straten AH, Soliman Hamad MA, van Zundert AA, et al. Effect of duration of red blood cell storage on early and late mortality after coronary artery bypass grafting. The Journal of thoracic and cardiovascular surgery. 2011;141(1):231–237. doi: 10.1016/j.jtcvs.2010.02.059. [DOI] [PubMed] [Google Scholar]
- 11.Phelan HA, Eastman AL, Aldy K, et al. Prestorage leukoreduction abrogates the detrimental effect of aging on packed red cells transfused after trauma: a prospective cohort study. American journal of surgery. 2012;203(2):198–204. doi: 10.1016/j.amjsurg.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phelan HA, Gonzalez RP, Patel HD, et al. Prestorage leukoreduction ameliorates the effects of aging on banked blood. The Journal of trauma. 2010;69(2):330–337. doi: 10.1097/TA.0b013e3181e0b253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aubron C, Nichol A, Cooper DJ, Bellomo R. Age of red blood cells and transfusion in critically ill patients. Annals of intensive care. 2013;3(1):2. doi: 10.1186/2110-5820-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandromme MJ, McGwin G, Jr, Weinberg JA. Blood transfusion in the critically ill: does storage age matter? Scandinavian journal of trauma, resuscitation and emergency medicine. 2009;17:35. doi: 10.1186/1757-7241-17-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakr Y, Chierego M, Piagnerelli M, et al. Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit Care Med. 2007;35(7):1639–44. doi: 10.1097/01.CCM.0000269936.73788.32. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg JA, MacLennan PA, Vandromme-Cusick MJ, et al. Microvascular response to red blood cell transfusion in trauma patients. Shock. 2012;37(3):276–281. doi: 10.1097/SHK.0b013e318241b739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohn SM, Nathens AB, Moore FA, et al. Tissue oxygen saturation predicts the development of organ dysfunction during traumatic shock resuscitation. J Trauma. 62(1):44–54. doi: 10.1097/TA.0b013e31802eb817. [DOI] [PubMed] [Google Scholar]
- 18.De Backer D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: report of a round table conference. Critical care. 2007;11(5):R101. doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. American journal of respiratory and critical care medicine. 2002;166(1):98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 20.Raat NJ, Verhoeven AJ, Mik EG, et al. The effect of storage time of human red cells on intestinal microcirculatory oxygenation in a rat isovolemic exchange model. Critical care medicine. 2005;33(1):39–45. doi: 10.1097/01.ccm.0000150655.75519.02. discussion 238-239. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald RD, Martin CM, Dietz GE, Doig GS, Potter RF, Sibbald WJ. Transfusing red blood cells stored in citrate phosphate dextrose adenine-1 for 28 days fails to improve tissue oxygenation in rats. Critical care medicine. 1997;25(5):726–732. doi: 10.1097/00003246-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Marik PE, Sibbald WJ. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA : the journal of the American Medical Association. 1993;269(23):3024–3029. [PubMed] [Google Scholar]
- 23.Walsh TS, McArdle F, McLellan SA, et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Critical care medicine. 2004;32(2):364–371. doi: 10.1097/01.CCM.0000108878.23703.E0. [DOI] [PubMed] [Google Scholar]
- 24.Kiraly LN, Underwood S, Differding JA, Schreiber MA. Transfusion of aged packed red blood cells results in decreased tissue oxygenation in critically injured trauma patients. The Journal of trauma. 2009;67(1):29–32. doi: 10.1097/TA.0b013e3181af6a8c. [DOI] [PubMed] [Google Scholar]
- 25.Owusu BY, Stapley R, Patel RP. Nitric oxide formation versus scavenging: the red blood cell balancing act. The Journal of physiology. 2012;590(Pt 20):4993–5000. doi: 10.1113/jphysiol.2012.234906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinberg JA, Barnum SR, Patel RP. Red blood cell age and potentiation of transfusion-related pathology in trauma patients. Transfusion. 2011;51(4):867–873. doi: 10.1111/j.1537-2995.2011.03098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51(4):844–851. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roback JD, Neuman RB, Quyyumi A, Sutliff R. Insufficient nitric oxide bioavailability: a hypothesis to explain adverse effects of red blood cell transfusion. Transfusion. 2011;51(4):859–866. doi: 10.1111/j.1537-2995.2011.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Backer D, Ospina-Tascon G, Salgado D, Favory R, Creteur J, Vincent JL. Monitoring the microcirculation in the critically ill patient: current methods and future approaches. Intensive care medicine. 2010;36(11):1813–1825. doi: 10.1007/s00134-010-2005-3. [DOI] [PubMed] [Google Scholar]
- 30.Ward KR, Ivatury RR, Barbee RW, et al. Near infrared spectroscopy for evaluation of the trauma patient: a technology review. Resuscitation. 2006;68(1):27–44. doi: 10.1016/j.resuscitation.2005.06.022. [DOI] [PubMed] [Google Scholar]