Type 1 metabotropic glutamate receptors (mGlu1) trigger the gating of GluD2 delta glutamate receptors (original) (raw)
Abstract
The orphan GluD2 receptor belongs to the ionotropic glutamate receptor family but does not bind glutamate. Ligand-gated GluD2 currents have never been evidenced, and whether GluD2 operates as an ion channel has been a long-standing question. Here, we show that GluD2 gating is triggered by type 1 metabotropic glutamate receptors, both in a heterologous expression system and in Purkinje cells. Thus, GluD2 is not only an adhesion molecule at synapses but also works as a channel. This gating mechanism reveals new properties of glutamate receptors that emerge from their interaction and opens unexpected perspectives regarding synaptic transmission and plasticity.
Keywords: GluD2, GluRdelta2, GPCR, ionotropic glutamate receptors, mGlu1, synaptic transmission
Introduction
GluD2 is an ionotropic glutamate receptor (iGluR) family member 1 almost exclusively expressed by cerebellar Purkinje cells 2. Its absence or mutation causes ataxia, deficits in motor learning 3, and cognitive disorders in rodents 4 5 6 7. Its N-and C-terminal domains respectively control parallel fiber (PF) synapse development/maintenance and long-term depression (LTD) 3 7 8 9 10. Evidence that the channel-pore of GluD2 is functional exists 1 10 11, but this point is still debated since no gating ligand has ever been identified 12. Interestingly, the subtype 1 metabotropic glutamate receptor (mGlu1) associates with GluD2 and transient receptor potential cation channel TRPC3 in Purkinje cells 13. Moreover, some mGlu1-activated conductances share properties with GluD2 14. The occurrence of a functional crosstalk between mGlu1 and iGluRs 15 16 17 and the report of a mGlu1-mediated slow current carried by TRPCs 18 19 20 prompted us to investigate if a similar functional coupling occurs between mGlu1 and GluD2 receptors. Here we show that mGlu1 indeed triggers the opening of the GluD2 channel.
Results
mGlu1 triggers the opening of GluD2 channels in HEK293 cells
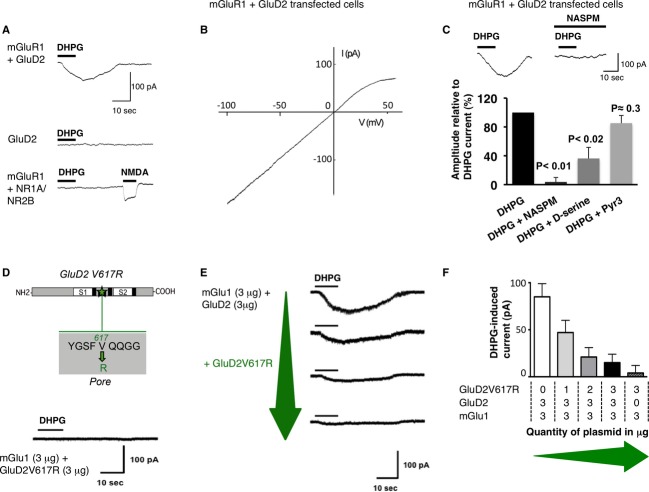
To examine the coupling between mGlu1 and GluD2 receptors, we first used HEK293 cells and the mGlu1alpha variant that we activated with 3,5-dihydroxyphenylglycine (DHPG, 100 μM). Membrane expression of the various constructs was verified (supplementary Figs S1–S3). In cells transfected with GluD2 or mGlu1 separately, DHPG did not induce any current (Fig1A). Conversely, in cells co-transfected with mGlu1 and GluD2 receptors, DHPG induced a slow inward current (81 ± 12 pA; n = 27, Fig1A). This current relied on GluD2 as DHPG elicited no current in cells co-transfected with mGlu1s and NMDA receptors (Fig1A). In cells co-expressing mGlu1 and GluD2, the current-voltage (I–V) relationship of the DHPG-induced current was similar to that of the constitutively opened Lurcher-or chimeric-GluD2s channels, with a characteristic inward rectification around + 20 mV 11 21 (Fig1B, n = 7). Two inhibitors of GluD2, the calcium-permeable AMPA receptor (AMPA-R) blocker NASPM (100 μM) and D-serine (10 mM) 1 11 22 23, both reduced the slow DHPG-induced current (Fig1C), by 95 ± 8% (n = 7, P < 0.01) and by 64 ± 15% (n = 8, P < 0.02). On the contrary, the TRPC3 inhibitor Pyr3 did not change it (n = 5, P = 0.31, Fig1C). These data indicate that mGlu1 triggers GluD2 opening, resulting in a slow current with biophysical and pharmacological features typical of GluD2 currents.
Figure 1. mGlu1 triggers GluD2 currents in HEK293 cells.
- DHPG induced current at −60 mV in cells expressing mGlu1, GluD2 or NR1-NR2B subunits, alone or in combination.
- The I–V relationship of the DHPG current in a cell expressing mGlu1 and GluD2.
- NASPM, D-serine but not Pyr3 inhibit the DHPG current in cells transfected with GluD2 and mGlu1.
- The GluD2V617R dominant-negative construct (top, S1, S2: LBD). Lack of DHPG current in cells expressing mGlu1 and GluD2V617R (bottom).
- GluD2V617R reduces the DHPG current. Representative traces from cells transfected with mGlu1 and GluD2 together with increasing amounts of GluD2V617R plasmid (green arrow).
- Averaged amplitude of DHPG currents (± s.e.m.) as a function of the quantity of plasmids co-transfected as indicated below.
To verify that it is carried by GluD2 but not by other conductances, we tested the mGlu1-mediated slow current in cells transfected with a dominant-negative GluD2 subunit. Introducing an arginine near the Q/R editing site of GluD2 disrupts the channel pore and turns it into a dominant-negative subunit as for AMPA/KA-Rs 12 24. We generated the GluD2V617R subunits by replacing a valine at position 617 by an arginine in the GluD2 amino-acid sequence (Fig1D). DHPG elicited no current in cells transfected with mGlu1 and GluD2V617R (Fig1D, n = 7). Thus, this current requires functional GluD2 channels. In cells transfected with mGlu1, wild-type (WT) GluD2 and GluD2V617R, the DHPG current decreased as the quantity of GluD2V617R plasmids co-transfected increases (Fig1E and F). It was almost abolished when WT and dominant-negative GluD2s were transfected in equal proportions (Fig1E and F). Thus, GluD2 channels themselves carry the slow current triggered by mGlu1s. This supports the view that they are functional 5 25, and designates mGlu1 as their physiological activator.
mGlu1 triggers GluD2 opening in native conditions
In cerebellar Purkinje cells, repetitive stimulations of PFs induce a mGlu1-mediated slow excitatory post-synaptic current (PF-slow EPSC) carried by several conductances, notably TRPC3s 18 19 20. As mGlu1s, TRPC3s and GluD2s associate and regulate the mGlu1-dependent current at PF-Purkinje cell synapses 13 26 27, we tested whether GluD2 pore contributes to the mGlu1-dependent PF-slow EPSC.
We induced this slow mGlu1-mediated EPSC with 8 pulses of 100 Hz PF-stimulation (Fig2A), in the presence of GABA-A, AMPA and NMDA receptor inhibitors (bicuculline, NBQX, D-APV, resp. 20, 10, 50 μM). Its intensity was 281 ± 26 pA (n = 46, Fig2B), and it was blocked by the mGluR1/5 antagonist AIDA (150 μM, supplementary Fig S4A). As expected 18 19 20, it was reduced by 35 ± 9% by the TRPC3s inhibitor Pyr3 (10 μM, n = 8, P < 0.01). The GluD2 blocker NASPM (100 μM) further decreased it to 78.3 ± 3% of the control (Fig2B). D-serine (10 mM) also reduced this slow current by 58.5 ± 4.3% when considering its NASPM-sensitive fraction (n = 7, Fig2C). Thus, GluD2 channels likely contribute to the mGlu1-dependent slow EPSC in Purkinje cells. If so, the dominant-negative GluD2V617R mutant should reduce it. To test this, we transduced cerebella of WT mice with recombinant Sindbis virus carrying the sequences of GluD2V617R and the marker green fluorescent protein (GFP) (Fig2D). In all the Purkinje cells expressing GluD2V617R tested, the PF-slow EPSC was reduced (87 ± 36 pA, n = 8, Fig2E) as compared to cells transfected with GFP only (265 ± 23 pA, n = 5; P < 0.01, Fig2E) or to those not transfected (281 ± 26 pA; n = 46; P < 0.01). In the GFP alone condition, the averaged trace displayed slower kinetics, two of the five cells recorded here having slow mGlu1 currents. However, this could not be attributed to the presence of GFP, but simply reflected a cell-to-cell variability that we observed in all the experiments of the study (see another example in supplementary Fig S4A), whatever the genotype or manipulation. In our hands, a quarter of the Purkinje cells had such slow kinetics, however we can provide any explanation for this.
Figure 2. GluD2 channels contribute to mGlu1 slow EPSC in cerebellar Purkinje cells.
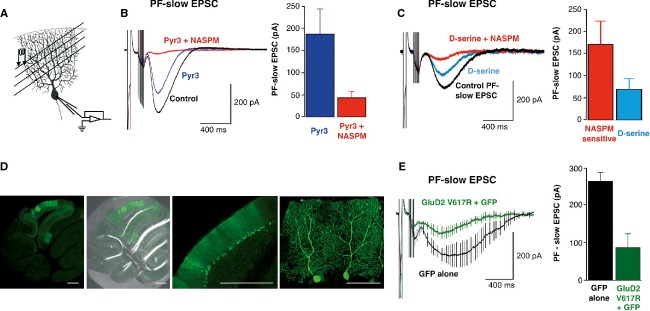
- A Experimental arrangement for the mGlu1 synaptic activation.
- B,C mGlu1-slow ESPCs are inhibited by Pyr3, NASPM (B) and D-serine (C). Representative sweeps. Histograms show the PF-slow EPSC averaged peak amplitudes.
- D A slice from WT cerebellum infected with Sinbis carrying GFP and GluD2V617R. Scale bars: 500 μm (three left images), 100 μm (right).
- E Averaged PF-slow EPSC from V617RGluD2 (green) or GFP (black) expressing cells. Histograms: Corresponding PF-slow EPSCs averaged peak amplitudes.
Thus, altogether, both pharmacological blockade and genetic disruption of GluD2 channel pore decrease the mGlu1-dependent slow EPSC, indicating that mGlu1s trigger GluD2s gating in native conditions.
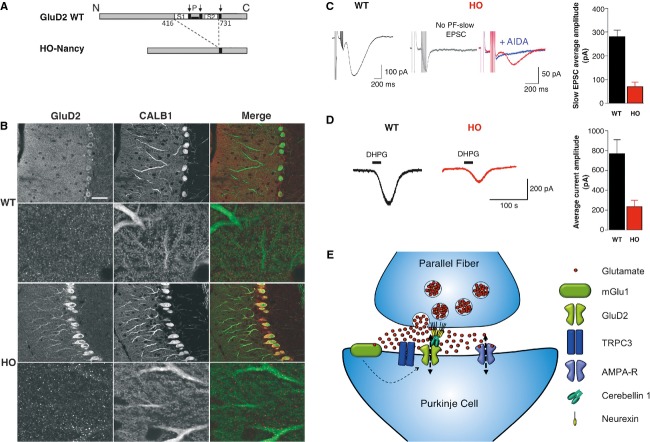
The PF-slow EPSC is reduced in mutant mice lacking GluD2 channels
Mice from the Hotfoot (HO) family bear deletions in the GluD2 encoding gene Grid2. The HO-Nancy GluD2 proteins lack their channel and ligand-binding domain (LBD) (28 6, Fig3A), providing a model to test mGlu1 currents in the absence of GluD2 pore. Immunohistochemistry in WT mice confirmed that GluD2s locate in thin dendrites of Purkinje cells (7, Fig3B). In HO-Nancy, although a fraction of GluD2 proteins remained trapped at the level of Purkinje cells somata (Fig3B), their dendrites, including proximal ones, were also labeled, supporting the previous observation that HO-Nancy GluD2s reach the membrane 8. An anti-GluD1/2 antibody gave similar results (supplementary Fig S5). Then, we quantified the mGluR1-mediated PF-slow EPSC in HO-Nancy Purkinje cells using calibrated PFs stimulation to compare with WT (see supplementary Fig S4B–C). Half of the HO-Nancy cells display no PF-slow EPSC (n = 19/37, Fig3C, left trace on right panel, versus n = 4/72 in WT). In the 18 others, a small slow EPSC was recorded (71 ± 18 pA), which was blocked by AIDA (Fig3C, right trace on right panel). In the HO-4j mice, extrasynaptic mGlu1 currents were shown to increase 13. To record both synaptic and extrasynaptic mGlu1-currents, we used bath applications of DHPG (50 μM, 30 s) in the presence of TTX, NBQX, D-APV and bicuculline (resp. 1, 10, 50, 20 μM). The DHPG current was detectable in all the HO-Nancy Purkinje cells but was smaller than in WT cells, (respectively 234 ± 60 pA, n = 13 versus 773 pA ± 136 pA, n = 9, P < 0.01; Fig3D). Thus, in Ho-Nancy Purkinje cells, mGlu1 currents are smaller than in WT cells, the reduction being more pronounced at synapses than outside. Importantly, this does not result from a reduced number of mGlu1s at the membrane since their amount does not change in the absence of GluD2 3 13. Together, these data suggest that GluD2 channels participate to the mGlu1-activated currents in Purkinje cells, and support our previous conclusion that GluD2 gating is triggered by mGlu1s.
Figure 3. The mGlu1 current is strongly reduced in HO-Nancy Purkinje cells.
- A WT and HO-Nancy schematic proteins. Amino-acid numbers flanking the deletion are indicated. Arrows: transmembrane domains. P: channel pore.
- B Confocal images of calbindin and GluD2 immunolabelings in WT (top) and HO-Nancy (bottom) cerebella. Scale bar in top left image represents 45 μm.
- C,D Representative PF-slow ESPCs (C) and DHPG currents (D) from WT (left) and HO-Nancy (right) Purkinje cells. Histograms: averaged peak amplitude of PF-slow ESPCs (C) or DHPG currents (D) from all the cells (± s.e.m.).
- E Schematic representation of PF synapses. GluD2s are not directly activated by glutamate (red spots) but require mGlu1 activation and thus presynaptic PF bursts.
Discussion
Here, we demonstrate that mGlu1s activation triggers a current carried by GluD2 channels, showing for the first time that WT GluD2 have an ionotropic nature and that their gating can be, at least indirectly, triggered by glutamate.
An important consequence of this coupling is that GluD2s as well as TRPCs contribute to the mGlu1-activated currents in Purkinje cells 29 30. This unexpected finding may explain why several previous studies disagreed on the nature of mGlu1 currents, or more recently, on the effects of TRPCs inhibitors on them 14 31 32 33. The respective contribution of TRPC1/3 and GluD2 may depend on the experimental conditions and/or on the splicing variants of mGlu1, as these latter vary among cerebellar regions 34. These conditions remain to be clarified.
The presence of the dominant-negative or the HO-Nancy GluD2s could have changed the number of mGlu1s or TRPC3 13 35 thereby explaining the decrease of the mGlu1 current. This is very unlikely. GluD2s do not seem to behave as scaffold or auxiliary proteins 3 13. Moreover, the existence of a GluD2-dependent mGlu1 current in HEK293 cells that is inhibited by D-serine, NASPM and GluD2V617R but not Pyr3 shows that the mGlu1 current flows through GluD2s, and not through some other interacting channel.
Even if the coupling between mGlu1 and GluD2 is reminiscent of other metabotropic-ionotropic receptor crosstalks 15 36, it appears unique in that mGlu1 triggers, rather than modulates, the gating of GluD2. However, none of our data suggest that it involves a direct activation of GluD2 by mGlu1. Intermediate steps cannot be excluded. Remarkably, the GluD2s gating mechanism supports the view that their LBD works differently from that of other iGluRs 11 25 22. It also makes glutamate their indirect activator, which eventually brings these orphans back to their original family of ionotropic glutamate receptors. As such, GluD2s have some permeation, regulation and trafficking properties of AMPA/KA-R, but they also require mGlu1 for their activation. Thus, GluD2 currents display chimeric properties that derive from both mGlu1 and AMPA/KA-Rs.
Some of our results seem to contradict previous ones showing no reduction of mGlu1 currents and a redistribution of mGlu1s and TRPC3s in the absence of GluD2 13. However, these studies have been made in HO-4j mice, where truncated GluD2s are retained in the endoplasmic reticulum, which is not the case for HO-Nancy. Thus, the two models are different.
Some studies question the ionotropic nature of GluD2, based on the fact that PF LTD and the establishment of normal climbing fiber connection do not to depend on GluD2 channels 12 37. However, this is not enough to refute the existence of GluD2 currents. Such currents could be necessary for other aspects of cerebellar physiology. As GluD2s are much less permeable to calcium than TRPC3 21 38, the GluD2/TRPC3 ratio could set the permeability of the mGlu1 conductance to calcium which could, for example, determine the polarity (LTP/LTD) of synaptic plasticity.
In contrast to conventional fast AMPA-Rs, the mGlu1/GluD2 duo converts high frequency input into a slow current. This duo is expressed in the vast majority of PF synapses 27 whereas more than 80% of these synapses are silent 39, and thus have no or few AMPA-Rs. This suggests that most PF synapses act as a highpass filter and that Purkinje cells relay high versus low frequency PF inputs with very different dynamics.
The other delta family member GluD1 has 60% sequence homology with GluD2 40 and is similarly endowed with a functional channel pore domain 25 41. We suggest that GluD1 might also be activated by metabotropic receptors, which would provide synapses with differential dynamical response to low and high frequency inputs.
Finally, the metabotropic-dependent gating of GluDelta receptors enriches the computational repertoire of synapses. It makes these former orphans prodigal children of the glutamate receptor family.
Materials and Methods
Plasmids and virus production
The plasmids encoding mGlu1a and NR1A/NR2B subunits under the control of a cytomegalovirus promoter, have been described previously (pRK5-HA-mGlu1a 15 42). GluD2 coding sequence, obtained by PCR from cerebellar cDNA, was amplified and assembled in pcDNA3.0 (Invitrogen, Carlsbad, CA, USA). The V617R mutation was verified with sequencing. For Sindbis virus production, WT and mutant coding sequences of GluD2 were amplified using Phusion high-fidelity DNA polymerase (Finnzyme, Vantaa, Finland) and inserted in pSinEGdsp 43. Two distinct promoters upstream GluD2 and GFP sequences allowed the 2 proteins to express separately 43. Recombinant viruses were produced as described in 44.
HEK293 cell culture and transfection
HEK293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin and streptomycin, 2 mM l-glutamine (all from Wisent, St Bruno, Canada). Transient transfections were performed using calcium phosphate precipitation method 15 on cells seeded at a density of 2 × 106 cells per 100 mm dish and cultured for 24 h. DMEM was renewed 24 h after transfection and cells cultured for an additional 24 h.
Animals
Animal breeding and euthanasia were performed in accordance to European and French legislation (NOR ATEN0090478A, code rural art. 276). HO-Nancy mice were raised in the lab 28 45. WT mice came from Janvier Laboratory (Le Genest-St-Isle, France). All had C57BL/6 background.
Electrophysiology
Solutions are detailed in supplementary methods. Whole-cell voltage-clamp recordings of HEK293 cells were made at room temperature using 3–5 Ω pipettes filled with a cesium chloride-based solution (20 mM EGTA). Cells were superfused with a HEPES buffered solution containing 10 mM glycine and 300 nM tetrodotoxin (TTX). Currents were recorded with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA), filtered at 1 kHz, digitized at 3 kHz and analyzed with the pClamp 9 software (Molecular Devices).
Patch-clamped recordings of Purkinje cells were made in whole-cell at −70 mV or in current-clamp (supplementary information and 46). The C57BL/6 mice were 2–6 months old. Recording pipettes (2–4 MΩ) contained a K-gluconate (144 mM)/KCl (6 mM) based internal solution (1 mM EGTA). Liquid junction potentials were not corrected. We used Axopatch-200A and Multiclamp 700B (Molecular Devices) amplifiers and ACQUIS1 (Bio Logic, Claix, France), P-Clamp9 (Molecular Devices) and Igor Pro 6.1 (WaveMetrics, Lake Oswego, OR, USA) softwares for acquisition and analysis. Drugs came from Sigma-Aldrich (Saint-Quentin Fallavier, France) except NBQX, D-APV, DHPG and AIDA that came from Tocris Cookson and R&D Systems (Lille, France).
Immunohistochemistry
Mice under deep pentobarbital anesthesia were fixed transcardially with 4% PFA in phosphate-buffered saline. Primary antibodies (dilution; provider; ref.) used: anti-calbindin (CALB1, 1/5000; Swant, Marly, Switzerland, 300), anti-GluD1/2 (1/150; Millipore, Billerica, Maryland, USA, AB1514) and anti-GluD2 (1/200; Frontier Institute, Hokkaido, Japan; GluRd2C-Rb-Af500). They were incubated with 60 μm thick free-floating cerebellar slices overnight. Secondary antibodies were AlexaFluor-488 and-546 (Invitrogen). Confocal images were acquired with a Leica SP5 confocal microscope and processed with the ImageJ software (http://rsbweb.nih.gov/ij/).
Virus injection
Two-month-old C57BL/6 male mice were anesthetized with ketamine and xylazine, then placed on a Kopf stereotaxic apparatus (Harvard Apparatus, Les Ulis, France). Viruses were injected (one injection, 2 μl, over 8 min) in lobule VI of the vermis. Three animals received the virus carrying GluD2V617R + GFP and 3 received the control virus carrying GFP alone. Electrophysiological studies were performed 24 or 48 h later.
Statistical analysis
Data are provided as the mean currents peak amplitude ± s.e.m. calculated for n different cells, details available in supplementary methods. We used Mann–Whitney or Wilcoxon tests, respectively for unpaired and paired groups. The P value is the probability of the null hypothesis.
Acknowledgments
We thank Dr. Claude Meunier for his invaluable scientific contribution, the members of the Neurophysics and Physiology Lab. for their comments, the Central Service for Microscopie (SCM) of Paris Descartes University, Jean-Maurice Petit for technical assistance. This work was supported by a 1-year fellowship from the “Fondation pour la Recherche Médicale (FRM)” to C.P. and L.T. and by the grant ANR # BLAN-SVSE4-LS-110624.
Author contributions
VA/CL/CP and JP/SD performed and analyzed the patch-clamp experiments, respectively in acute slices and HEK293 cells. LT and BL designed, produced and tested the plasmids and viruses encoding WT-and V617R-GluD2s. ID and XC made the injections of recombinant viruses. ID and VA did the immunochemistry. CL designed and supervised the study and wrote the manuscript with the contribution of VA, BL, JP, LF and the other authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting Information
Supplementary information for this article is available online: http://embor.embopress.org
Supplementary Data S1
Review Process File
References
- Kohda K, Wang Y, Yuzaki M. Mutation of a glutamate receptor motif reveals its role in gating and delta2 receptor channel properties. Nat Neurosci. 2000;3:315–322. doi: 10.1038/73877. [DOI] [PubMed] [Google Scholar]
- Lomeli H, Sprengel R, Laurie DJ, Kohr G, Herb A, Seeburg PH, Wisden W. The rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor family. FEBS Lett. 1993;315:318–322. doi: 10.1016/0014-5793(93)81186-4. [DOI] [PubMed] [Google Scholar]
- Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Takeshi Yagi T, Kang Y, Aizawa S, Mishina M. Impairement of motor coordination, Purkinje cell synapses formation, and cerebellar long-term depression in GluR-delta-2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- Filali M, Lalonde R, Bensoula AN, Guastavino JM, Lestienne F. Spontaneous alternation, motor activity, and spatial learning in hot-foot mutant mice. J Comp Physiol A. 1996;178:101–104. doi: 10.1007/BF00189594. [DOI] [PubMed] [Google Scholar]
- Martin LA, Goldowitz D, Mittleman G. Repetitive behavior and increased activity in mice with Purkinje cell loss: a model for understanding the role of cerebellar pathology in autism. Eur J Neurosci. 2010;31:544–555. doi: 10.1111/j.1460-9568.2009.07073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J, Pevet P, Felder-Schmittbuhl MP, Bailly Y, Challet E. The cerebellum harbors a circadian oscillator involved in food anticipation. J Neurosci. 2010;30:1894–1904. doi: 10.1523/JNEUROSCI.5855-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W, Miyazaki T, Emi K, Matsuda K, Kohda K, Motohashi J, Mishina M, Kawahara S, Watanabe M, Yuzaki M. Differential regulation of synaptic plasticity and cerebellar motor learning by the C-terminal PDZ-binding motif of GluRdelta2. J Neurosci. 2008;28:1460–1468. doi: 10.1523/JNEUROSCI.2553-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Miura E, Miyazaki T, Kakegawa W, Emi K, Narumi S, Fukazawa Y, Ito-Ishida A, Kondo T, Shigemoto R, Watanabe M, Yuzaki M. Cbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizer. Science. 2010;328:363–368. doi: 10.1126/science.1185152. [DOI] [PubMed] [Google Scholar]
- Uemura T, Lee SJ, Yasumura M, Takeuchi T, Yoshida T, Ra M, Taguchi R, Sakimura K, Mishina M. Trans-synaptic interaction of GluRdelta2 and Neurexin through Cbln1 mediates synapse formation in the cerebellum. Cell. 2010;141:1068–1079. doi: 10.1016/j.cell.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Schmid SM, Hollmann M. To gate or not to gate: are the delta subunits in the glutamate receptor family functional ion channels? Mol Neurobiol. 2008;37:126–141. doi: 10.1007/s12035-008-8025-0. [DOI] [PubMed] [Google Scholar]
- Schmid SM, Kott S, Sager C, Huelsken T, Hollmann M. The glutamate receptor subunit delta2 is capable of gating its intrinsic ion channel as revealed by ligand binding domain transplantation. Proc Natl Acad Sci USA. 2009;106:10320–10325. doi: 10.1073/pnas.0900329106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakegawa W, Kohda K, Yuzaki M. The delta2 ‘ionotropic’ glutamate receptor functions as a non-ionotropic receptor to control cerebellar synaptic plasticity. J Physiol. 2007;584:89–96. doi: 10.1113/jphysiol.2007.141291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato AS, Knierman MD, Siuda ER, Isaac JT, Nisenbaum ES, Bredt DS. Glutamate receptor delta2 associates with metabotropic glutamate receptor 1 (mGluR1), protein kinase Cgamma, and canonical transient receptor potential 3 and regulates mGluR1-mediated synaptic transmission in cerebellar Purkinje neurons. J Neurosci. 2012;32:15296–15308. doi: 10.1523/JNEUROSCI.0705-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepari M, Papageorgiou G, Corrie JE, Watkins C, Ogden D. The conductance underlying the parallel fibre slow EPSP in rat cerebellar Purkinje neurones studied with photolytic release of L-glutamate. J Physiol. 2001;533:765–772. doi: 10.1111/j.1469-7793.2001.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroy J, Raynaud F, Homburger V, Rousset MC, Telley L, Bockaert J, Fagni L. Direct interaction enables cross-talk between ionotropic and group I metabotropic glutamate receptors. J Biol Chem. 2008;283:6799–6805. doi: 10.1074/jbc.M705661200. [DOI] [PubMed] [Google Scholar]
- Moutin E, Raynaud F, Roger J, Pellegrino E, Homburger V, Bertaso F, Ollendorff V, Bockaert J, Fagni L, Perroy J. Dynamic remodeling of scaffold interactions in dendritic spines controls synaptic excitability. J Cell Biol. 2012;198:251–263. doi: 10.1083/jcb.201110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Fagni L, Perroy J. Functional crosstalk between group I metabotropic glutamate receptors and ionotropic glutamate receptors controls synaptic transmission. RSC Drug Discovery Series. 2011;8:269–283. [Google Scholar]
- Nelson C, Glitsch MD. Lack of kinase regulation of canonical transient receptor potential 3 (TRPC3) channel-dependent currents in cerebellar Purkinje cells. J Biol Chem. 2012;287:6326–6335. doi: 10.1074/jbc.M111.246553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Henning HA, Konnerth A. mGluR1/TRPC3-mediated synaptic transmission and calcium signaling in mammalian central neurons. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WC, Young JS, Glitsch MD. Changes in TRPC channel expression during postnatal development of cerebellar neurons. Cell Calcium. 2007;42:1–10. doi: 10.1016/j.ceca.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Wollmuth LP, Kuner T, Jatzke C, Seeburg PH, Heintz N, Zuo J. The Lurcher mutation identifies delta 2 as an AMPA/kainate receptor-like channel that is potentiated by Ca(2 + ) J Neurosci. 2000;20:5973–5980. doi: 10.1523/JNEUROSCI.20-16-05973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Naur P, Kurtkaya NL, Kristensen AS, Gajhede M, Kastrup JS, Traynelis SF. Modulation of the dimer interface at ionotropic glutamate-like receptor delta2 by D-serine and extracellular calcium. J Neurosci. 2009;29:907–917. doi: 10.1523/JNEUROSCI.4081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naur P, Hansen KB, Kristensen AS, Dravid SM, Pickering DS, Olsen L, Vestergaard B, Egebjerg J, Gajhede M, Traynelis SF, Kastrup JS. Ionotropic glutamate-like receptor delta2 binds D-serine and glycine. Proc Natl Acad Sci USA. 2007;104:14116–14121. doi: 10.1073/pnas.0703718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Hume RI, Heinemann SF. Structural determinants of barium permeation and rectification in non-NMDA glutamate receptor channels. J Neurosci. 1992;12:4080–4087. doi: 10.1523/JNEUROSCI.12-10-04080.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth A, Tapken D, Hollmann M. The delta subfamily of glutamate receptors: characterization of receptor chimeras and mutants. Eur J Neurosci. 2013;37:1620–1630. doi: 10.1111/ejn.12193. [DOI] [PubMed] [Google Scholar]
- Grandes P, Mateos JM, Ruegg D, Kuhn R, Knopfel T. Differential cellular localization of three splice variants of the mGluR1 metabotropic glutamate receptor in rat cerebellum. NeuroReport. 1994;5:2249–2252. doi: 10.1097/00001756-199411000-00011. [DOI] [PubMed] [Google Scholar]
- Zhao HM, Wenthold RJ, Wang YX, Petralia RS. Delta-glutamate receptors are differentially distributed at parallel and climbing fiber synapses on Purkinje cells. J Neurochem. 1997;68:1041–1052. doi: 10.1046/j.1471-4159.1997.68031041.x. [DOI] [PubMed] [Google Scholar]
- Lalouette A, Lohof A, Sotelo C, Guenet J, Mariani J. Neurobiological effects of a null mutation depend on genetic context: comparison between two hotfoot alleles of the delta-2 ionotropic glutamate receptor. Neuroscience. 2001;105:443–455. doi: 10.1016/s0306-4522(01)00193-2. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepari M, Auger C, Ogden D. Ca2+ ion permeability and single-channel properties of the metabotropic slow EPSC of rat Purkinje neurons. J Neurosci. 2004;24:3563–3573. doi: 10.1523/JNEUROSCI.5374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch MD. Activation of native TRPC3 cation channels by phospholipase D. FASEB J. 2010;24:318–325. doi: 10.1096/fj.09-134973. [DOI] [PubMed] [Google Scholar]
- Hirono M, Konishi S, Yoshioka T. Phospholipase C-independent group I metabotropic glutamate receptor-mediated inward current in mouse purkinje cells. Biochem Biophys Res Commun. 1998;251:753–758. doi: 10.1006/bbrc.1998.9465. [DOI] [PubMed] [Google Scholar]
- Mateos JM, Benítez R, Elezgarai I, Azkue JJ, Lázaro E, Osorio A, Bilbao A, Doñate F, Sarría R, Conquet F, Ferraguti F, Kuhn R, Knöpfel T, Grandes P. Immunolocalization of the mGluR1b splice variant of the metabotropic glutamate receptor 1 at parallel fiber-Purkinje cell synapses in the rat cerebellar cortex. J Neurochem. 2000;74:1301–1309. doi: 10.1046/j.1471-4159.2000.741301.x. [DOI] [PubMed] [Google Scholar]
- Kohda K, Kakegawa W, Matsuda S, Yamamoto T, Hirano H, Yuzaki M. The delta2 glutamate receptor gates long-term depression by coordinating interactions between two AMPA receptor phosphorylation sites. Proc Natl Acad Sci USA. 2013;110:E948–E957. doi: 10.1073/pnas.1218380110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benquet P, Gee CE, Gerber U. Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci. 2002;22:9679–9686. doi: 10.1523/JNEUROSCI.22-22-09679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torashima T, Iizuka A, Horiuchi H, Mitsumura K, Yamasaki M, Koyama C, Takayama K, Iino M, Watanabe M, Hirai H. Rescue of abnormal phenotypes in delta2 glutamate receptor-deficient mice by the extracellular N-terminal and intracellular C-terminal domains of the delta2 glutamate receptor. Eur J Neurosci. 2009;3:355–365. doi: 10.1111/j.1460-9568.2009.06841.x. [DOI] [PubMed] [Google Scholar]
- Zitt C, Obukhov AG, Strubing C, Zobel A, Kalkbrenner F, Luckhoff A, Schultz G. Expression of TRPC3 in Chinese hamster ovary cells results in calcium-activated cation currents not related to store depletion. J Cell Biol. 1997;138:1333–1341. doi: 10.1083/jcb.138.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isope P, Barbour B. Properties of unitary granule cell–>Purkinje cell synapses in adult rat cerebellar slices. J Neurosci. 2002;22:9668–9678. doi: 10.1523/JNEUROSCI.22-22-09668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M. Selective expression of the glutamate receptor channel delta 2 subunit in cerebellar Purkinje cells. Biochem Biophys Res Commun. 1993;197:1267–1276. doi: 10.1006/bbrc.1993.2614. [DOI] [PubMed] [Google Scholar]
- Yadav R, Rimerman R, Scofield MA, Dravid SM. Mutations in the transmembrane domain M3 generate spontaneously open orphan glutamate delta1 receptor. Brain Res. 2011;1382:1–8. doi: 10.1016/j.brainres.2010.12.086. [DOI] [PubMed] [Google Scholar]
- Ango F, Albani-Torregrossa S, Joly C, Robbe D, Michel JM, Pin JP, Bockaert J, Fagni L. A simple method to transfer plasmid DNA into neuronal primary cultures: functional expression of the mGlu5 receptor in cerebellar granule cells. Neuropharmacology. 1999;38:793–803. doi: 10.1016/s0028-3908(99)00005-2. [DOI] [PubMed] [Google Scholar]
- Okada T, Yamada N, Kakegawa W, Tsuzuki K, Kawamura M, Nawa H, Iino M, Ozawa S. Sindbis viral-mediated expression of Ca2+-permeable AMPA receptors at hippocampal CA1 synapses and induction of NMDA receptor-independent long-term potentiation. Eur J Neurosci. 2001;13:1635–1643. doi: 10.1046/j.0953-816x.2001.01523.x. [DOI] [PubMed] [Google Scholar]
- Drobac E, Tricoire L, Chaffotte AF, Guiot E, Lambolez B. Calcium imaging in single neurons from brain slices using bioluminescent reporters. J Neurosci Res. 2010;88:695–711. doi: 10.1002/jnr.22249. [DOI] [PubMed] [Google Scholar]
- Guastavino JM, Sotelo C, Damez-Kinselle I. Hot-foot murine mutation: behavioral effects and neuroanatomical alterations. Brain Res. 1990;523:199–210. doi: 10.1016/0006-8993(90)91488-3. [DOI] [PubMed] [Google Scholar]
- Piochon C, Irinopoulou T, Brusciano D, Bailly Y, Mariani J, Levenes C. NMDA receptor contribution to the climbing fiber response in the adult mouse Purkinje cell. J Neurosci. 2007;27:10797–10809. doi: 10.1523/JNEUROSCI.2422-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data S1
Review Process File