Pre-administration of G9a/GLP Inhibitor during Synaptogenesis Prevents Postnatal Ethanol-induced LTP Deficits and Neurobehavioral Abnormalities in Adult Mice (original) (raw)
. Author manuscript; available in PMC: 2015 Nov 1.
Abstract
It has been widely accepted that deficits in neuronal plasticity underlie the cognitive abnormalities observed in fetal alcohol spectrum disorder (FASD). Exposure of rodents to acute ethanol on postnatal day 7 (P7), which is equivalent to the third trimester of fetal development in human, induces long-term potentiation (LTP) and memory deficits in adult animals. However, the molecular mechanisms underlying these deficits are not well understood. Recently, we found that histone H3 dimethylation (H3K9me2), which is mediated by G9a (lysine dimethyltransferase), is responsible for the neurodegeneration caused by ethanol exposure in P7 mice. In addition, pharmacological inhibition of G9a prior to ethanol treatment at P7 normalized H3K9me2 proteins to basal levels and prevented neurodegeneration in neonatal mice. Here, we tested the hypothesis that pre-administration of G9a/GLP inhibitor (Bix-01294, Bix) in conditions in which ethanol induces neurodegeneration would be neuroprotective against P7 ethanol-induced deficits in LTP, memory and social recognition behavior in adult mice. Ethanol treatment at P7 induces deficits in LTP, memory and social recognition in adult mice and these deficits were prevented by Bix pretreatment at P7. Together, these findings provide physiological and behavioral evidence that the long-term harmful consequences on brain function after ethanol exposure with a third trimester equivalent have an epigenetic origin.
Keywords: Synaptic plasticity, FASD, methyltransferase, memory loss, epigenetics, histones, Bix, postnatal development, ethanol, H3K9
Introduction
Ethanol exposure during pregnancy causes birth defects (Jones and Smith, 1973) and can lead to fetal alcohol spectrum disorders (FASDs) (Streissguth, et al., 1990). FASD symptoms generally include growth deficiency and brain damage. FASD is one of the major contributors to intellectual disability in the Western world (Mattson, et al., 2011). Some of the most persistent deficits are neurobehavioral hallmarks, such as learning and memory deficits (Goodman, et al., 1999, Mattson, et al., 1999). As many as 1 in 100 children born in the United States and Canada (Chudley, et al., 2005, May and Gossage, 2001) are estimated to be diagnosed with FASD, whereas heavily afflicted areas of South Africa exhibit the most pervasive diagnoses of FASD in around 10.9 per 100 children (May, et al., 2000, May, et al., 2007, Urban, et al., 2008). The developing brain is so sensitive to ethanol exposure that even a single exposure can produce massive losses of neurons in several brain regions (Ikonomidou, et al., 2000) during the first few postnatal days in neonatal mice (postnatal days 4–10 [P4–10]), a developmental period which corresponds with the third trimester pregnancy in humans (Bayer, et al., 1993). Excessive acute ethanol intoxication in P7 mice prompts neurodegeneration in vital brain regions including the hippocampus and cortex (Ikonomidou, et al., 2000, Sadrian, et al., 2012, Subbanna, et al., 2014, Subbanna, et al., 2013a, Subbanna, et al., 2013b, Wilson, et al., 2011), as well as impairments in LTP (Izumi, et al., 2005, Sadrian, et al., 2012, Subbanna, et al., 2013a, Wilson, et al., 2011) and spatial memory task performance in adult mice (Subbanna, et al., 2013a). Similarly, the local and interregional brain circuitry of the olfacto-hippocampal pathway in adult mice is compromised when P7 mice are exposed to acute ethanol (Sadrian, et al., 2012, Wilson, et al., 2011).
Increasing evidence suggests that ethanol exposure during brain development induces chromatin dysregulation in numerous brain regions (Bekdash, et al., 2013, Kaminen-Ahola, et al., 2010a, Kaminen-Ahola, et al., 2010b, Perkins, et al., 2013, Subbanna, et al., 2014, Subbanna, et al., 2013b), which may be responsible for the development of ethanol associated brain disorders (Mattson, et al., 2011, Mattson, et al., 2010). Recent studies focus on the importance of post-translational modification of histone proteins on the regulation of normal brain function and the development of several human developmental disorders (Campuzano, et al., 1996, Gavin and Sharma, 2010,Makedonski, et al., 2005, Petronis, 2003, Ryu, et al., 2006, Warren, 2007). In addition to acetylation and phosphorylation, histone methylation is one of the most extensively investigated histone modification mechanism in the central nervous system (CNS) (Tsankova, et al., 2006). Histone H3K9 dimethylation is correlated with transcriptional inhibition, whereas histone H3 trimethylation at lysine 4 (H3K4me3) is linked to active transcription (Schneider, et al., 2004). The dimethylation of histone H3K9 is catalyzed by the euchromatic histone methyltransferases (EHMTases), including G9a (Tachibana, et al., 2002) and the G9a-related protein (GLP) (Ogawa, et al., 2002); these can repress gene expression by inducing local dimethylation of H3K9 at target promoters. Consequentially, G9a/GLP regulate neuronal function during brain development (Schaefer, et al., 2009). Recently, we reported that histone H3K9 dimethylation by G9a was responsible for postnatal ethanol-induced neurodegeneration (Subbanna, et al., 2013b). In addition, in the presence of ethanol, the G9a exon itself is regulated by epigenetic modification of histone proteins during early brain development (Subbanna, et al., 2014). The present study evaluated the neuroprotective role of G9a inhibition on postnatal ethanol-induced long-lasting neurobehavioral deficits in adult mice.
Materials and methods
Animals and treatment
Animal care and handling procedures followed Institutional (NKI IACUC) and National Institutes of Health guidelines. C57BL/6J mice were housed in groups under standard laboratory conditions (12 hr light / 12 hr dark cycle) with food and water available ad libitum. An ethanol treatment paradigm, which has been previously shown to induce robust apoptotic neurodegeneration in P7 mice (Olney, et al., 2002) and causes no lethality, was used in the current study. Litters of mice were culled to four to six pups per litter, and on the day of treatment, half of the pups (male) in each litter were treated subcutaneously (s. c.) with saline and the other half with ethanol at P7 (based on the day of birth) (2.5 g/kg s. c. at 0 h and again at 2 h) in their home cage with the dam as described previously by our laboratory (Subbanna, et al., 2013a,Subbanna, et al., 2013b). For blood ethanol levels (BEL), pups were euthanized by decapitation ; truncal blood was collected at 3 and 9 hr following the first ethanol injection. The concentrations of ethanol in pup serum were then determined using a standard alcohol dehydrogenase-based method (Lundquist, 1959). For the Bix experiments, Bix-01294 (2-(Hexahydro-4-methyl-1_H_-1,4-diazepin-1-yl)-6,7-dimethoxy-_N_-[1-(phenylmethyl)-4-piperidinyl]-4-quinazolinamine trihydrochloride) (Cayman, Michigan, USA) was dissolved in 10µl of ethanol followed by 2–3 drops of Tween 80 (10 µl) and then volume was made up with sterile saline solution. The Bix solution was administered by s. c. injection at a volume of 5 µl/g body weight 30 min before ethanol treatment. The Bix vehicle solution was injected as a control. Bix treatment did not alter P7 ethanol induced intoxication (sleeping time) and Bix alone treated P7 mice were similar like saline treated mice and did not cause any inflammation or bleeding in any of the organs (Subbanna, et al., 2014, Subbanna, et al., 2013b). Mice were kept with the dams until they were weaned. Three months old mice derived from different litters after P7 treatment [saline + vehicle (S + V), ethanol + vehicle (E + V), saline + Bix (S + Bix) and ethanol + Bix (E + Bix)] were used for several analyses, as described below. Five to 8 animals were used for each data point. In the current study, male mice were used for behavioral analysis to avoid the hormonal fluctuation that occurs during the estrous cycle; this could potentially affect animal behavior, thus complicating the data interpretation . Separate sets of animals were subjected to each behavioral study (n=8/group).
Immunohistochemistry
Mice were anesthetized and perfused with a solution containing 4% paraformaldehyde and 4% sucrose in 0.05 M cacodylate buffer (pH 7.2), 8 h after the first ethanol dose treatment . It has been shown that this time point is optimal to induce maximum caspase-3 activation in one or more brain regions (Ikonomidou, et al., 2000, Subbanna, et al., 2013b, Wilson, et al., 2011). The brains were further processed according to our previously described protocols (Subbanna, et al., 2014, Subbanna, et al., 2013a, Subbanna, et al., 2013b). Free-floating sections were obtained from ethanol- and saline-exposed brains (8 h of exposure) and immunostained using an antibody against anti-rabbit cleaved caspase-3 (Asp175) (CC3) (# 9661 , Cell Signaling Technology, Danvers, MA, USA) with ABC reagents (Vectastain ABC Elite Kit, Vector Labs, Burlingame, CA, USA) and a peroxidase substrate (DAB) kit (Vector Labs) to label neurodegenerating neurons. The primary antibodies were omitted from the reactions as a control for secondary antibody specificity. In addition, pre-incubation with blocking peptides for the anti-CC3 (GenScript, Piscataway, NJ, USA) completely blocked the immunostaining of CC3 antibody. All photomicrographs were taken through a 2.5X, or 40X objective with a Nikon Eclipse TE2000 inverted microscope attached to a digital camera (DXM1200F, Morrell Instrument Company, Melville, NY, USA).
Electrophoresis and immunoblot
For Western blot analysis, homogenates from the hippocampus and cortex of the pups were processed 4–24 h after saline or ethanol ( first ethanol dose ) injection as described previously (Lubin and Sweatt, 2007, Subbanna, et al., 2013a, Subbanna, et al., 2013b). Cytosolic and nuclear fractions from tissue homogenates were prepared as described in our recent publications (Basavarajappa, et al., 2014, Basavarajappa and Subbanna, 2014). The samples were prepared in a sample buffer as previously described by our laboratory (Basavarajappa, et al., 2008). The blots were incubated with the following primary antibodies: anti-rabbit-CC3 (Asp175) (polyclonal, #9661, 1:1000), anti-mouse-β-actin (monoclonal, #3700, 1:1,000), anti-rabbit-H3K9me2 (monoclonal, # 4658, 1: 1000) and anti-rabbit-H3 (polyclonal, # 9715, 1:1000) (Cell Signaling Technology, Danvers, MA, USA). The blots were incubated with the primary antibodies for 3 h at room temperature or overnight at 4 °C and processed as previously described by our laboratory (Basavarajappa, et al., 2008). Incubation with a secondary antibody alone did not produce any bands (data not shown).
LTP
Three-month-old male mice, treated at P7 with S + V, E + V, S + Bix, or E + Bix (n=5/group), were sacrificed by cervical dislocation followed by decapitation . Hippocampi were quickly removed. Transverse hippocampal slices (400 µm) were cut and recorded according to standard procedures (Basavarajappa and Subbanna, 2014, Sadrian, et al., 2012, Subbanna, et al., 2013a, Vitolo, et al., 2002). Following cutting, hippocampal slices were transferred to a recording chamber where they were maintained at 29° C and perfused with artificial cerebrospinal fluid (ACSF) continuously bubbled with 95% O2 and 5% CO2. The ACSF composition in mM was: 124.0 NaCl, 4.4 KCl, 1.0 Na2HPO4, 25.0 NaHCO3, 2.0 CaCl2, 2.0 MgSO4, 10.0 glucose, osmolarity 290–300. CA1 fEPSPs were recorded by placing both the stimulating and the recording electrodes in CA1 stratum radiatum . Basal synaptic transmission (BST) was determined by plotting the fiber volley amplitude against slopes of field-excitatory-post-synaptic potential (fEPSP). For LTP experiments, a 10 min baseline was recorded every min at an intensity that evokes a response ~35% of the maximum evoked response. LTP was induced using theta-burst stimulation (4 pulses at 100 Hz, with the bursts repeated at 5 Hz, and each tetanus including 3× 10-burst trains separated by 15 seconds). Responses were recorded for 2 hrs after and measured as fEPSP slope expressed as percentage of baseline.
Object recognition memory
Novel object recognition memory was evaluated as described before (Basavarajappa and Subbanna, 2014, Ennaceur and Delacour, 1988, Subbanna, et al., 2013a), which is based on the natural tendency of rodents to explore a novel object more than a familiar one. In brief, three month-old male mice, treated at P7 with S + V, E + V, S + Bix and E + Bix (n=8/group) were submitted individually to a habituation session where they were allowed to freely explore the open field for 3 min twice a day for two days . No objects were placed in the box during the habituation trial. Twenty-four hours after habituation, training (T1) was conducted by placing individual mice for 3 min in the open field, in which two identical objects (objects a1 and a2) were positioned in two adjacent corners at 10 cm distance from the walls. In a short-term recognition memory test given at 1 or 4 h (retention) after the training (T2), the mice explored the open field for 3 min in the presence of one familiar (a1) and one novel (b1, 1h; b2, 4h) object. In a long-term recognition memory test given at 24h (retention) after training (T2), the mice explored the open field for 5min in the presence of one familiar (a1) and one novel object (b3; different from b1 and b2). All objects had similar textures and sizes but had distinctive shapes and colors. Between trials, the objects were washed with 10% ethanol solution. Exploration was defined as directing the nose to the object at a distance of no more than 2 cm and/or touching the object with the nose. Sitting on the object was not considered as exploratory behavior. e1 and e2 are measures of the total exploration time of both objects during T1 and T2, respectively. d2 was considered as index measures of discrimination between the new and the familiar objects. d2 is a relative measure of discrimination which corrects the difference between exploring the old and the novel object for exploration activity (e2) and appears to be independent of the total exploration times (Sik, et al., 2003).
Spontaneous alternation Y maze task
Spontaneous alternation was tested as described previously (Basavarajappa and Subbanna, 2014, Holcomb, et al., 1998) to assess spatial working memory that is dependent upon the hippocampus. In brief, three month old male mice, treated at P7 with S + V, E + V, S + Bix and E + Bix (n=8/group) were used in this test. Each mouse was placed in the center of the Y maze and was allowed to explore freely through the maze during an 8 min session. The sequence and total number of arms entered was recorded. Arm entry was considered to be completed when the hind paws of the mouse had been completely placed in the arm. Percentage alternation is the number of triads containing entries into all three arms divided by the maximum possible alternations (the total number of arms entered minus 2) × 100.
Spatial recognition memory using Y maze
Spatial recognition memory using Y maze was tested as described previously (Basavarajappa and Subbanna, 2014, Sarnyai, et al., 2000). Briefly, three-month-old male mice, treated at P7 with S + V, E + V, S + Bix and E + Bix (n=8/group) were used in this test. Briefly, each mouse was placed into one of the arms of the Y maze (start arm) and allowed to explore the maze with one of the arms closed for 10 min (training trial). After a 1h intertrial interval, mice were returned to the Y maze by placing them in the start arm. Then, the mice were allowed to explore freely all three arms of the maze for 3 min (test trial). The number of entries into and the time spent in each arm, and the first choice of entry were registered manually and from video recordings by an observer blind to the treatment or genotype of the mice.
Social recognition memory
The social recognition test was performed as previously described (Kogan, et al., 2000, Thor, et al., 1982). Three-month-old male mice were treated on P7 with S + V, E + V, S + Bix and E + Bix (n=8/group). Each mouse was placed in an individual cage in an observation room with dim light immediately prior to the experimental sessions. The mice were allowed to habituate to the new environment for 15 min. The experimental cages were identical to those in which the animals were normally housed (plastic, 27 cm long × 16 cm wide × 12 cm high). A male, juvenile mouse (3–4 weeks old) was placed into a cage with an adult for an initial interaction trial (2 min duration). Following the intertrial delay, the same juvenile mouse was placed back into the adult’s cage for a 2-min test trial. The animals were returned to their home cages during the interim between the initial and the test trials. A trained observer continuously timed the duration of the social investigation (with a hand-held stopwatch). The scored social investigative behaviors have been previously described and include the following: direct contact with the juvenile while inspecting any part of the body surface (including grooming, licking, and pawing); sniffing of the mouth, ears, tail, ano-genital area; and close following (within 1 cm) of the juvenile (Kogan, et al., 2000, Thor, et al., 1982). If the adult mouse did not investigate the juvenile mouse for a minimum of 24 s during the initial trial (i.e., 20% of trial time), they were retested once with another juvenile. The trials with initial investigation times less than 24 s were excluded from the analysis. Any aggressive encounter between animals was an immediate cause for termination of the experiment, and these data were excluded from the analysis. The percentage of social investigation was calculated by dividing the investigation time during the second exposure by the initial investigation time × 100.
Statistical analysis
All of the data are presented as the mean ± SEM. A statistical comparison of the data was performed by either a student’s_t_ test or one-way analysis of variance (ANOVA) or a two-way ANOVA with Bonferroni’s post hoc test. In all of the comparisons, p < 0.05 was considered to indicate statistical significance. The statistical analyses were performed using the Prism software (GraphPad, San Diego, CA).
Results
Pre-administration of G9a/GLP inhibitor before ethanol treatment in P7 mice prevents neurodegeneration in neonatal mice and LTP deficits in adult mice
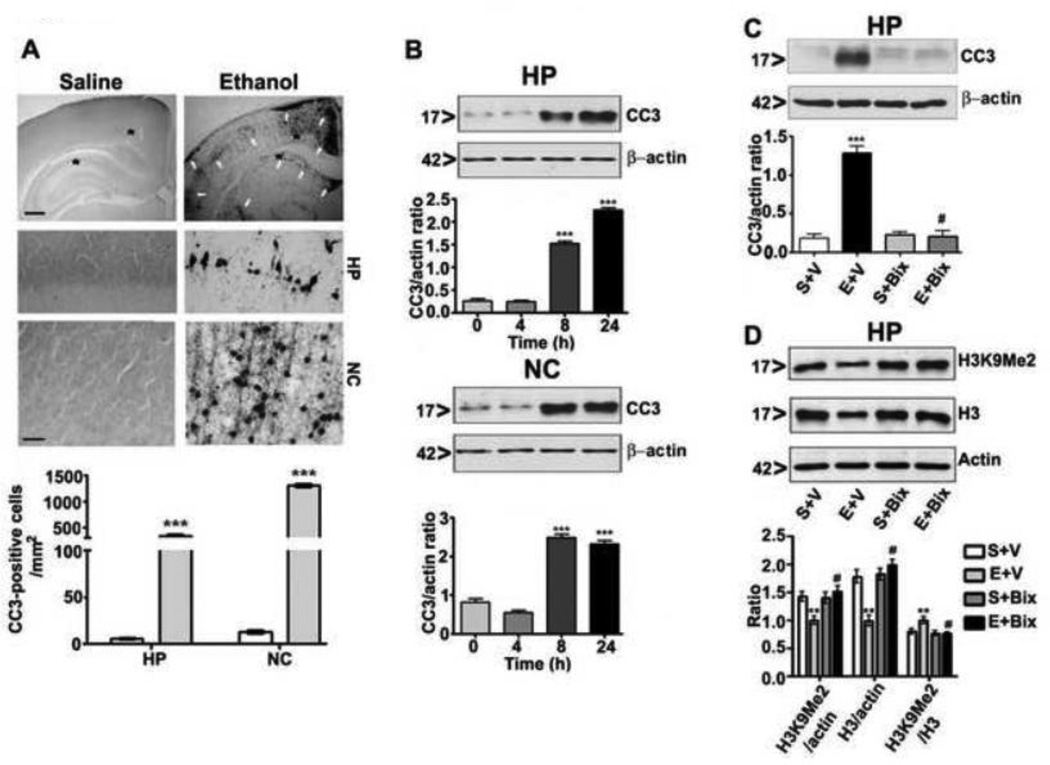
Ethanol administration (2.5 g/kg s. c. at 0 h and again at 2 h) to mouse pups at P7 resulted in a blood ethanol level (BEL) of ~0.46 ± 0.22 g/dL at 3 h (first ethanol dose) that gradually decreased to 0.28 ± 0.05 g/dL at 9 h (first ethanol dose) . This ethanol paradigm produced a widespread pattern of neurodegeneration throughout the forebrain [hippocampus (F1, 11 = 60, p<0.001) and cortex (F1, 11 = 100, p<0.001) regions] (one-way ANOVA). The neurodegeneration was measured by caspase-3 activation [formation of cleaved caspase-3 (CC3)] in the ethanol-exposed brains (Fig. 1A). We also evaluated neurodegeneration in the hippocampal and neocortical protein extracts by Western blot analysis. Comparisons with a one-way ANOVA and Bonferroni’s post hoc tests indicated that the 4 h (first ethanol dose) ethanol-treated group was not significantly different compared to saline (0 h) group. The 8 and 24 h ethanol-treated (first ethanol dose) groups exhibited significantly greater proportions of neuronal death in both the hippocampus (F3, 28 = 55, p < 0.001) and neocortex (F3, 28 = 60, p < 0.001) (Fig. 1B) compared to saline (0 h) group . Taken together, our experimental conditions demonstrated the apoptotic patterns and severity that has been previously described for this ethanol treatment paradigm in the developing brain (Ikonomidou, et al., 2000, Olney, et al., 2002,Subbanna, et al., 2013a, Subbanna, et al., 2013b).
Fig. 1.
Ethanol induces apoptotic neurodegeneration in the P7 mouse brain and pharmacological inhibition of G9a rescues P7 ethanol-induced neurodegeneration and H3K9me2 in the neonatal mouse brain. (A) Coronal brain sections (hippocampus and retrosplenial cortex) from saline- and ethanol-treated animals were immunostained with an anti-rabbit CC3 antibody. The white arrows indicate the CC3-positive neurons in the hippocampus and retrosplenial cortex. Scale bars = 200 µm. The respective images were enlarged to show the CC3-positive cells (*). The scale bars represent 50 µm. CC3-positive cells were quantified in the hippocampus and retrosplenial cortex (n = 10 pups/group). Student’s t test: ***p < 0.001 vs. respective saline group . Each point is presented as the mean ± SEM. (B) Western blot analysis of CC3 using cytosolic extracts (20 µg) of hippocampal and cortical samples from the saline- and ethanol-treated groups (n = 8 pups/group). The graphs represent the ratio of the proteins normalized to the expression of β-actin. ***p < 0.001 vs. 0 h (respective saline control) . Each point is presented as the mean ± SEM. (C and D) Mice pre-treated (30 min) with Bix (1 mg/kg) or vehicle were exposed to ethanol, and hippocampal extracts from S + V, E + V, S + Bix and E + Bix (n = 6 pups/group) were collected 8 h after first dose of ethanol treatment and processed for Western blotting to analyze CC3, H3K9me2 and H3 levels. β-actin was used as a loading control. Representative blots are shown for the hippocampal cytosolic (CC3) and nuclear (H3K9 and H3) extracts. HP, hippocampus; NC, neocortex. Each point is presented as the mean ± SEM. One-way ANOVA with Bonferroni’s post hoc tests; ***p < 0.001, **p < 0.01 vs. S + V; #p < 0.001 vs. E + V .
In our previous studies, we found that Bix pretreatment inhibited ethanol-induced caspase 3 activation in the neocortex in a dose-dependent manner (0.25, 0.5, and 1 mg/kg) (Subbanna, et al., 2013b). The administration of Bix at the maximum dose (1 mg/kg) 30 min before ethanol treatment (first ethanol dose) was more effective in inhibiting G9a-mediated ethanol-induced caspase-3 activation (Fig. 1C) than co-treatment (ethanol and Bix together) or post-treatment [administration of Bix 1 h after ethanol treatment (first ethanol dose) ] (Subbanna, et al., 2013b). Pre-administration of Bix also rescued ethanol-induced loss of H3K9me2 and H3 as observed in the previous study (p < 0.001) (Fig. 1D) (Subbanna, et al., 2013b). Furthermore, the administration of Bix (1 mg/kg) before ethanol treatment does not alter the BELs (Subbanna, et al., 2013b) [BEL peaked at 3 h (first ethanol dose) at 0.43 ± 0.20 g/dl and was gradually reduced to 0.23 ± 0.09 g/dl at 9 h (first ethanol dose)], which indicates that Bix pretreatment inhibited neurodegeneration without modulating the ethanol metabolism.
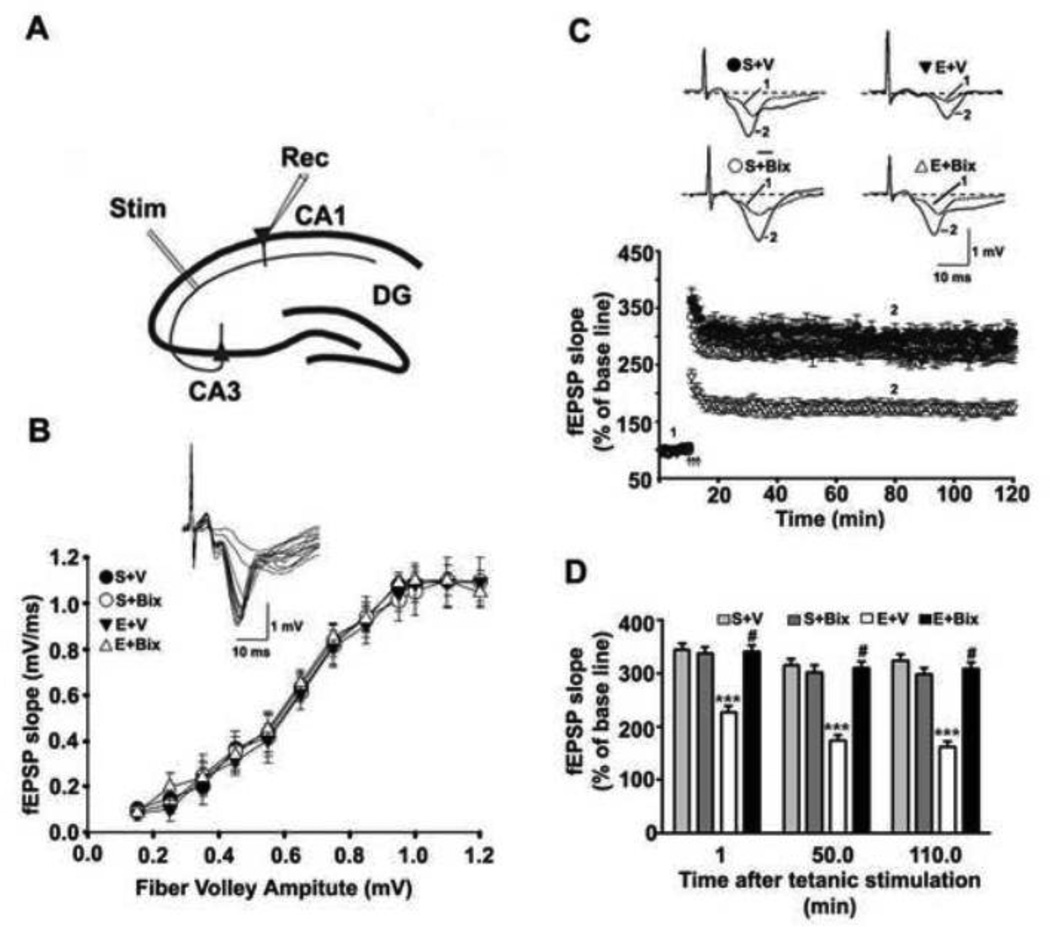
We first determined the input/output (I/O) responses and the LTP in the Schaffer collateral pathway of hippocampal slices (Fig. 2A) prepared from the adult animals treated with S + V, E + V, S + Bix and E + Bix at P7. The robust I/O responses were evoked by increasing the stimulus intensity in all groups. The I/O curve was not altered by S + V, E + V, S + Bix and E + Bix treatment (p>0.05) (Fig. 2B). Prior to tetanic stimulations, the baseline fEPSP was recorded at 60 s intervals with a stimulation intensity equivalent to ~35% of the maximum evoked response. The tetanic stimulation evoked typical LTP (Fig. 2C) in slices from adult mice treated on P7 with S + V, E + V, S + Bix and E + Bix . These responses were stable over 120 min. However, the tetanic stimulation evoked a significantly reduced LTP in slices (n = 10 slices/5 mice/group) prepared from P7 E + V animals compared to S + V animals (p < 0.001) with a significant group interaction (two-way ANOVA) (F1, 36 = 26, p< 0.001; _post-hoc_ test): S + V vs. E + V significantly different at all post-tetanic stimulation time intervals) (p < 0.001). The LTP in slices prepared from S + Bix-treated animals did not differ significantly from those in S + V-treated mice (p > 0.05). Bix pre-treatment completely rescued the P7 ethanol-induced LTP defects (p < 0.001) (Fig. 2D).
Fig. 2.
Inhibition of G9a before ethanol treatment in P7 pups prevents long-lasting synaptic deficits in adult mice. (A) A schematic diagram showing the stimulating and recording electrode positions in the CA1 region of the hippocampus. (B) A summary graph showing the field input/output relationships for P7 treated S + V, E + V, S + Bix and E + Bix adult mice.Insert: An example of traces taken from representative experiments from input/output relationships for S + V. Although not shown other groups also exhibited similar pattern . (C) Time course of the averages of the fEPSP slopes in slices obtained from S+V, E+V, S + Bix- and E + Bix-treated mice. The fEPSP slopes were normalized to the average value 10 min before stimulation in each experiment. Arrows denote the time of tetanic stimulation (4 pulses at 100 Hz, with bursts repeated at 5 Hz, and each tetanus including three 10-burst trains separated by 15 s). Representative traces of fEPSPs before (trace 1) and after (trace 2) induction of LTP in hippocampal slices from S +V, E + V, S + Bix and E + Bix mice . (D) A combined plot of the averages of the fEPSP slopes at several time points. Each point is presented as the mean ± SEM (n= 5 mice/group; 10 slices/group). Two-way ANOVA with Bonferroni’s post hoc tests; ***p < 0.001 vs. S + V; #p < 0.001 vs. E + V .
Pre-administration of G9a/GLP inhibitor at P7 rescues P7 ethanol-induced memory loss in adult mice
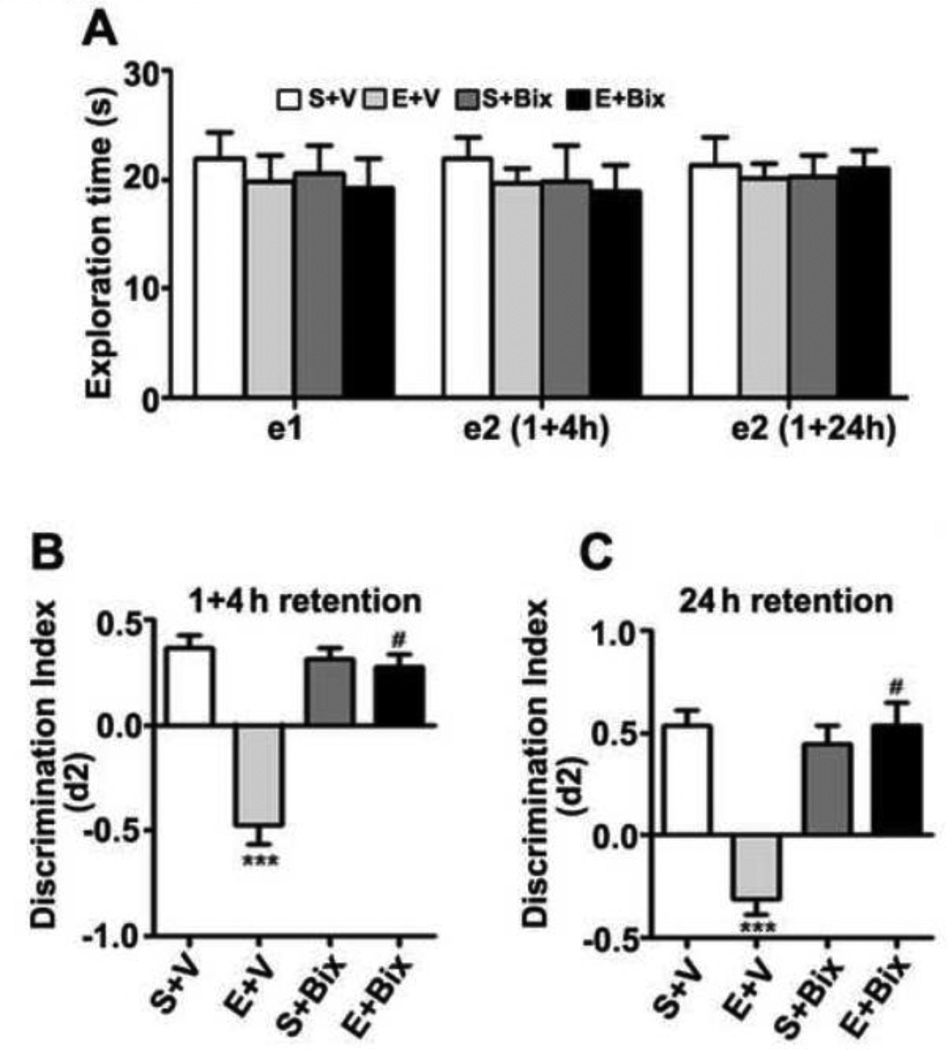
First, we investigated object recognition memory (ORM) to examine whether G9a/GLP inhibitor , which protects neurons from P7 ethanol-induced neurodegeneration (Subbanna, et al., 2013b) and LTP deficits, also rescues ethanol-induced memory impairments. The results indicate that P7 S + V, E + V or S + Bix, E + Bix treatment has no significant effect on exploration times (e1 or e2) in the ORM task (e1; F3, 28 = 1.2, p > 0.05; e2; F3, 28 = 1.2, p > 0.05; one-way ANOVA) (Fig. 3A). The ethanol (E + V) treatment at P7 impaired both short- (1+ 4 h retention, combined) (Fig. 3B) (F3, 45 = 43, p < 0.001) and long- (24 h retention) (Fig. 3C) (F3, 21 = 21, p < 0.001) (Two-way ANOVA) term ORM performance. Bix treatment before P7 ethanol administration (E + Bix) rescued ORM performance as observed with LTP. The ORM performance by S + Bix-treated animals was not significantly different from saline-treated mice (p > 0.05). Therefore, blocking G9a function before P7 ethanol exposure prevents ORM deficits in the adult mice.
Fig. 3.
Inactivation of G9a activity before ethanol treatment at P7 prevents object recognition memory loss in adult mice. (A) Level of exploration was measured at el and e2, respectively: the time spent exploring the two objects in T1 and T2 (at 1+4 and 24 h) by S+V, E+V, S+ Bix, and E+ Bix-treated mice. (B) Discrimination indices (d2) obtained from the S+V, E+V, S+ Bix, and E+ Bix-treated mice after 1and 4 h retention intervals. (C) Discrimination indices (d2) obtained from the S+V, E+V, S+ Bix, and E+ Bix-treated mice after 24 h retention intervals. Each point is the mean + SEM (n= 8 mice/group). One-way ANOVA with Bonferroni’s post hoc test; ***p < 0.001 vs. S + V; #p < 0.001 vs. E + V .
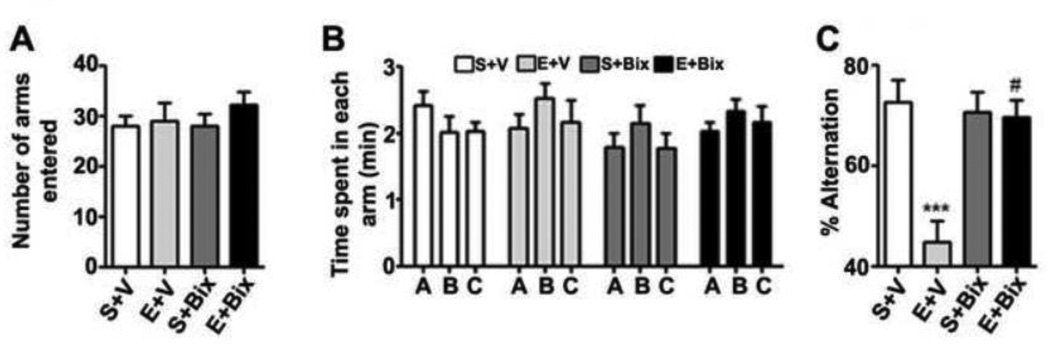
In our second behavioral test, the adult mice treated with P7 S + V, E + V, S + Bix and E + Bix were tested with a spontaneous alternation in the Y maze (Lalonde, 2002). P7 S + V, E + V, S + Bix and E + Bix treatment had no significant effect on the exploratory activities as assessed by the number of arm entries (Fig. 4A) and time spent (Fig. 4B) in each arm during the Y-maze test. Consistent with the ORM performance, two-way ANOVA revealed that the ethanol-treated mice (E + V) exhibited significantly reduced spontaneous alternation performance compared to saline-treated mice (S + V), and Bix treatment (E + Bix) rescued these deficits [F3,21 = 10, p < 0.001] (Fig. 4C). P7 mice treated with Bix alone (S + Bix) showed no significant difference in their spontaneous alternation performance (p > 0.05). Taken together, these findings suggest that Bix pretreatment rescues P7 ethanol-induced (E + Bix) deficits in spontaneous alteration performance in adult mice.
Fig. 4.
P7 ethanol treatment impairs and prior administration of G9a/GLP inhibitor prevents the spontaneous alternation performance deficit in adult mice. (A) Total number of arm entries reflecting exploratory activities of mice in the Y-maze does not differ between the four groups (p > 0.05). (B) The time spent in each arm was not different between four groups (p > 0.05). (C) The spontaneous alternation performance was reduced in by ethanol (E+V) and was rescued by Bix treatment (E+ Bix). Alternation performance was not affected by saline (S+V) and Bix (S+Bix) treatment. Each point is the mean ± SEM (n= 8 mice/group). (***p < 0.001 vs. S+V; #p < 0.001 vs. E+V) . One-way ANOVA with Bonferroni’s post hoc test.
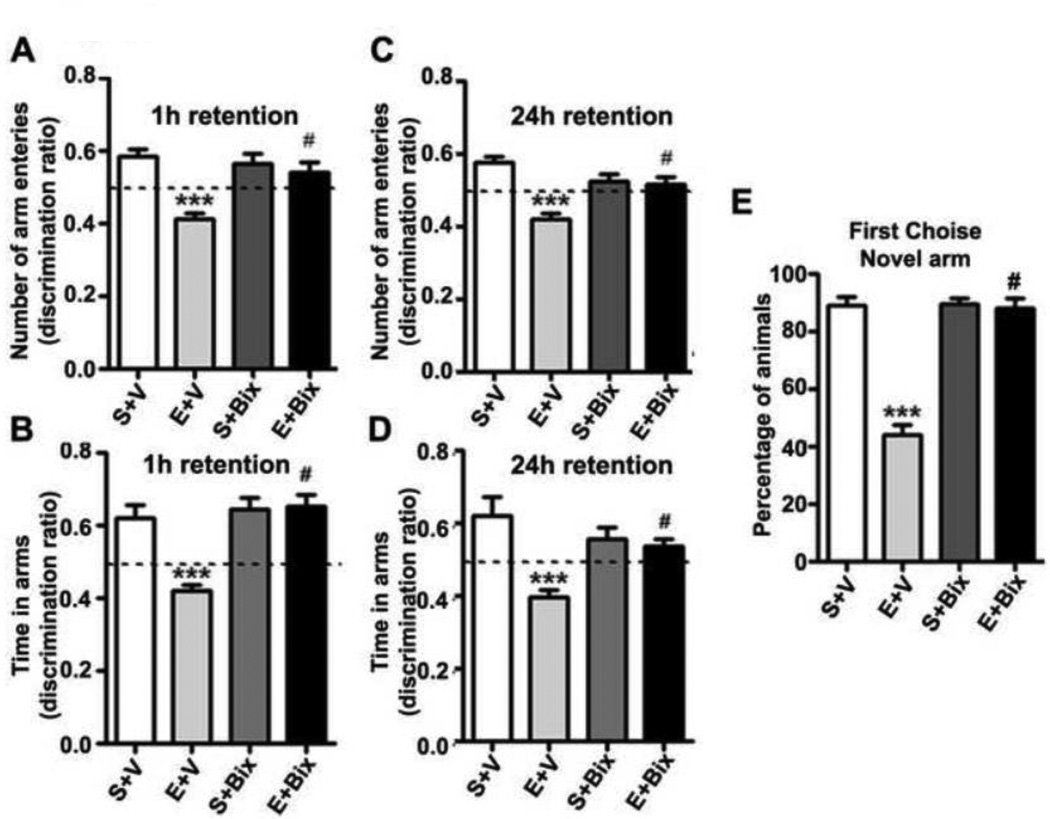
We tested spatial recognition memory using a Y-maze in our third behavioral test. A two-way ANOVA revealed that S + V and S + Bix-treated mice entered more frequently into (Arm Entry: 1 h [F3,21 = 18, p < 0.01; 24 h [F3,21 = 23, p < 0.01]) (Fig. 5 A and B) and spent more time in (Dwell Time: 1 h [F3,21 = 51, p < 0.01; 24 h [F3,21 = 25, p < 0.01]) (Fig. 5 C and D) the novel, previously unvisited arm of the maze. In contrast, P7 E + V-treated mice showed a reduced preference toward the novel arm (p<0.01) and spent less time (Dwell time) (p<0.01) in the novel arm compared to the P7 S + V mice in both 1 h (Fig. 5 A and B) and 24 h (Fig. 5 C and D) retention. Bix pretreatment rescued ethanol-induced impairments (E + Bix) with a preference toward exploration of the novel arm (p<0.01) and time spent (p<0.01) in the novel arm in both 1 h and 24 h retention. Although all S + V, S + Bix and E + Bix-treated mice (combined 1 and 24 h) selected the novel arm as the first choice, E + V-treated animals showed a reduced preference for the novel arm (Fig. 5E), and this was prevented by Bix pretreatment (E + Bix) [F3,45 = 50, p < 0.01]). These findings suggest that G9a inhibition before P7 ethanol treatment rescues the spatial recognition memory loss in adult mice.
Fig. 5.
P7 ethanol treatment impairs and Bix pretreatment rescues impaired spatial memory performance as measured by Y maze. (A–D) Discrimination ratio [preference for the Novel arm over the familiar Other arm (Novel/Novel + Other)] for arm entries (A and C, 1 h and 24 h) and dwell time (B and D, 1 h and 24 h) of S+V and E+V mice treated with or without Bix (S+Bix and E+Bix), 1 or 24 h after the first encounter with the partially opened maze. The dashed line indicates chance performance (0.5). (E) The percentage of animals selecting the novel arm as the first choice is shown for S+V and E+V mice treated with or without Bix (S+Bix and E+Bix), 1 and 24 h after the first encounter with the partially opened maze. Each point is the mean + SEM (n= 8 mice/group). Two-way ANOVA with Bonferroni’s post hoc test; ***p < 0.001 vs. S + V; # p < 0.01 vs. E + V .
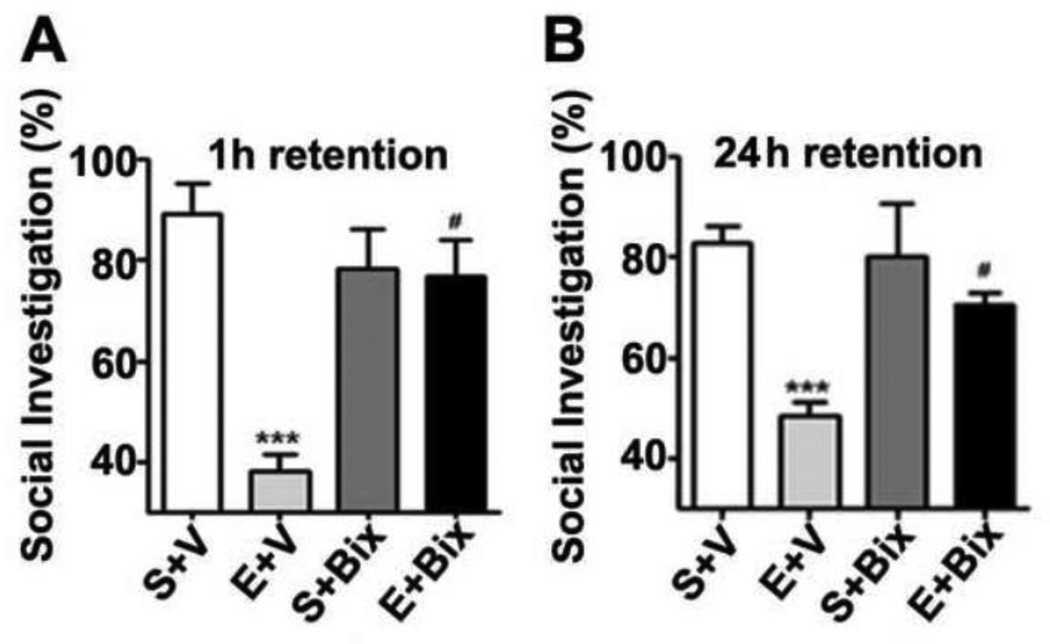
The initial experiments evaluated the social investigation of the adult mouse with 1, 4 and 24 h intervals between the initial and test trials. The social investigation was higher with a 1 h intertrial delay compared to a 4 and 24 h intertrial delay (data not shown). The social investigation results revealed that E + V-treated mice exhibited significantly reduced short (Fig. 6 A) and long-term (Fig. 6 B) social recognition memory performance compared to the S + V-treated mice. A two-way ANOVA revealed that Bix pretreatment rescued the ethanol-induced (E + Bix) short- [F3,21 = 18, p < 0.01] and long-term [F3,21 = 14, p < 0.01] social recognition memory deficits compared to the ethanol (E + Bix) treated mice. In addition, S + Bix alone had no significant effect (p > 0.05) on the social recognition memory and these mice exhibited normal social recognition memory. These findings suggest that G9a inhibition by Bix before P7 ethanol treatment rescues social recognition memory loss in adult mice.
Fig. 6.
Bix pretreatment rescues P7 ethanol-induced social recognition memory loss in the adult mice. (A and B) Percent of social investigation is shown for S+V, E+V, S+ Bix, and E+ Bix-treated mice, 1 (A) and 24 h (B) after the first encounter with same juvenile mice. Each point is the mean ± SEM (n= 8 mice/group). Two-way ANOVA with Bonferroni’s _post hoc_test. ***p < 0.001 vs. S + V; # p < 0.01 vs. E + V .
Discussion
Our current research suggests that blocking the loss of H3K9me2 by pre-administration of G9a/GLP inhibitor before P7 ethanol treatment rescues long-lasting synaptic dysfunction in adult mice . Most importantly, we have shown that the pre-administration of G9a/GLP inhibitor before ethanol exposure in P7 mice, which rescues H3K9me2 degradation, was adequate to prevent LTP deficits in adult mice. Although the molecular mechanism is not clear, there is growing evidence that neuronal plasticity is persistently impaired in animal models of FASD [for references see (Subbanna, et al., 2013a)]. It is possible that ethanol-induced activation of G9a and histone H3 modification during development disrupts the specific process involved in refinement of neuronal circuits, which leads to persistent synaptic dysfunction in adulthood. This could explain why some cortical maps (Margret, et al., 2006, Medina, et al., 2005, Powrozek and Zhou, 2005, Zhou, et al., 2005) and olfacto-hippocampal networks (Sadrian, et al., 2012, Wilson, et al., 2011) are altered in FASD models. Moreover, G9a-deficient mice display signs of severe developmental growth retardation and generally die between the embryonic days 9.5 and 12.5 (Tachibana, et al., 2002, Tachibana, et al., 2005). In a recent study, conditional inactivation of G9a/GLP in large numbers of functionally diverse neurons in the postnatal forebrain significantly erased euchromatic H3K9 dimethylation (Schaefer, et al., 2009). This lack of euchromatic H3K9me2 led to an intellectual-disability-like phenotype in adult mice (Schaefer, et al., 2009). In humans, genetic alterations of the GLP gene are associated with a severe intellectual disability syndrome that is further characterized by craniofacial abnormalities and a gradual decline in goal-directed cognition and behavior (Kleefstra, et al., 2006, Kleefstra, et al., 2009, Kramer and van Bokhoven, 2009). Intellectual disability in humans is distinguished by decreased cognitive function, as well as impaired adaptive behaviors in response to environmental triggers including perinatal trauma or intra-uterine infections, maternal and early childhood nutritional deficits, and maternal alcohol abuse (APA., 2000). Consistent with this notion, the deficiency of G9a/GLP leads to loss of H3K9me2 in postnatal neurons causing severe defects in learning and memory (Schaefer, et al., 2009). Furthermore, our study is also consistent with other study which suggest that loss of H3K9me2 due to genetic ablation of G9a leads to reduced higher order dendrite branching and impaired learning and memory in Drosophila (Kramer, et al., 2011). In another study, loss of H3K9me2 due to partial ablation of G9a leads to the developmental delay, hypotonia, and cranial abnormalities, which are three of the core features of specific intellectual disability syndrome called Kleefstra syndrome (Balemans, et al., 2014) . Thus, it seems possible that genetically predetermined or environmentally (postnatal ethanol) induced epigenetic changes of the enzymes controlling H3K9 dimethylation (Subbanna, et al., 2014, Subbanna, et al., 2013b) may induce long-lasting learning and memory deficits as found in many postnatal ethanol studies (Izumi, et al., 2005, Sadrian, et al., 2012, Subbanna, et al., 2013a, Wilson, et al., 2011). Although more studies are warranted, in P7 ethanol model, the loss of H3K9me2 was initiated with enhanced G9a expression and dimethylation sensitive degradation of H3K9me2 protein (Subbanna, et al., 2013b) and suggest the G9a function is dependent on the level of expression, context and its H3K9 dimethylation activity. The observed loss of dimethylated H3K9 due to proteolytic degradation (Subbanna, et al., 2013b) during the synaptogenic period may partially explain the observed neuronal dysfunction in the present study as well as in several animal models of postnatal ethanol (Izumi, et al., 2005, Sadrian, et al., 2012, Subbanna, et al., 2013a, Wilson, et al., 2011) .
The current findings also reveal significant learning and memory impairments in the adult mice exposed to ethanol at P7 compared to controls. These findings go in line with previous literature showing that mice exposed to an acute dose of ethanol at P7 show impaired synaptic plasticity (Izumi, et al., 2005, Sadrian, et al., 2012, Subbanna, et al., 2013a) and olfacto-hippocampal (Sadrian, et al., 2012,Wilson, et al., 2011) and hippocampal memory (Subbanna, et al., 2013a) in adult mice. Most importantly, we have shown that pharmacologically inhibiting G9a via Bix pretreatment before ethanol exposure can rescue ethanol-induced neuronal deficiencies ranging anywhere from neuronal survival (Subbanna, et al., 2013b) to LTP to learning and memory behavior. Several rodent models also show impaired learning and memory in the adult rodents exposed to acute or chronic ethanol at pre- or postnatal stages of development (Christie, et al., 2005, Girard, et al., 2000, Iqbal, et al., 2006, Pick, et al., 1993, Ryan, et al., 2008,Savage, et al., 2002, Thomas, et al., 2008, Thomas, et al., 1997, Zimmerberg, et al., 1991). There is also growing evidence that heavy prenatal alcohol exposure leads to widespread cognitive deficits in children across several domains, including memory, social and adaptive functioning (Mattson, et al., 2011, Mattson, et al., 1998, Norman, et al., 2013). The children exposed to prenatal alcohol showed impaired verbal and nonverbal learning and memory (Mattson, et al., 1996, Mattson and Roebuck, 2002, Roebuck-Spencer and Mattson, 2004), reduced academic accomplishment, and higher rates of learning disabilities than non-exposed children (Howell, et al., 2006).
The findings that ethanol exposure at P7 also caused deficits in social recognition memory in adult mice generally agree with a previous study in which exposure to acute ethanol during neurogenesis at gestation day 12 (G12) caused pronounced and permanent deficits in social behavior throughout ontogeny (Mooney and Varlinskaya, 2011). Similar social recognition deficits were also established in another animal model of FASD (Shirasaka, et al., 2012) as well as a CD38 knockout autism model (Jin, et al., 2007). Using the optimum dose of Bix treatment (recognized for preventing neurodegeneration in neonatal mice) to pharmacologically inhibit G9a prevented the development of ethanol-induced deficits in social recognition memory in adult mice. It is widely accepted that retaining normal social memories throughout ontogeny is crucial for establishing relationships within a group or between partners, along with developing the ability to recognize families [For references see (Cushing and Kramer, 2005)]. Stable social recognition memories in rodents function to facilitate several complex social and reproductive processes, not limited to pair relationship formation in monogamous species (Demas, et al., 1997), as well as selective pregnancy termination in mice (Kaba, et al., 1989, Keverne, 1998). It is well known that two brain regions, the olfactory system (Sanchez-Andrade and Kendrick, 2009) and the limbic system (Baron-Cohen, et al., 1994, Brothers, et al., 1990) underlie social behavior. It possible that improper processing of socially relevant olfactory stimuli might produce the observed deficit in social recognition memory in P7 ethanol treated adult mice. Early ethanol exposure damages olfactory neuroanatomy and physiology in both humans and rodents [For references see (Wilson, et al., 2011)]. Because the olfactory system provides a major input to the hippocampal formation (Wilson and Sullivan, 2011), and this structure is involved in integrating the complex stimuli necessary for the recognition process (Alvarez, et al., 2002, Ross and Eichenbaum, 2006), the hippocampus might be required for social memory (Kogan, et al., 2000). Consistent with this notion, our previous findings suggest that P7 ethanol treatment significantly modifies olfacto-hippocampal system function in adult mice (Sadrian, et al., 2012, Wilson, et al., 2011). Currently, there are no studies that investigate the epigenetic mechanisms leading to deficits in social recognition memory. However, one study has correlated the early social experience with a change in histone acetylation and DNA methylation status at the promoter region of the glucocorticoid receptor gene (Weaver, et al., 2004). While more research must be conducted, our data emphasizes the importance of specific epigenetic changes mediated by G9a due to ethanol exposure during the postnatal period, which is key for establishing adult social behavior expression (Gordon, 1998, Nelson and Luciana, 2001, Stiles, 2000).
The molecular mechanism(s) by which developmental ethanol exposure induces long-lasting behavioral and cognitive deficits are not fully understood. However, evidence suggests that the observed learning and memory deficits may be a result of the well-known toxic effects of ethanol on CNS development through several pathways, which leads to increased apoptotic neurodegeneration (Ikonomidou, et al., 2000, Sadrian, et al., 2012, Subbanna, et al., 2013a, Subbanna, et al., 2013b,West, et al., 1986), decreased cell motility (Carter, et al., 2008), decreased neurogenesis (Nixon and Crews, 2002), decreased pro-survival signaling (Eade, et al., 2010, Young, et al., 2008), and changes in dendritic tree complexity and the spine shape of hippocampal neurons (Abdollah, et al., 1993, Bonthius, et al., 2001, Butters, et al., 2000, Hayward, et al., 2004, Tarelo-Acuna, et al., 2000). Our previous and current observation directly pinpoints the participation of the epigenetically regulated G9a-induced loss of H3K9me2. A recent study suggests that the inhibition of G9a/GLP in adult rats with the infusion of Bix inhibit the formation H3K9me2 in area CA1 of the hippocampus and long-term memory (Gupta-Agarwal, et al., 2012). Although the mechanisms need to be further established, this evidence suggests that H3K9me2 levels regulate the memory process. Collectively, our studies emphasize the importance of epigenetic mechanisms in the regulation of normal brain developmental processes and how changes in these pathways caused by environmental exposures, such as postnatal ethanol, can possibly trigger a cascade of neurological damage and dysfunction that may have immediate effects (Subbanna, et al., 2014, Subbanna, et al., 2013b) or manifest later in life. Furthermore, it remains unclear whether the observed epigenetic modifications of the G9a gene and the upregulation of G9a expression during postnatal development lasts until adulthood, or if it is even inheritable. Such epigenetic studies will help develop G9a based intervention to treat cognitive deficits in ethanol exposed offspring.
In conclusion, our study suggests an expanded epigenetic regulatory model for the neurodegenerative process in postnatal ethanol teratogenesis. Postnatal ethanol exposure leads to the abnormal alteration of the G9a-dependent H3K9me2 epigenetic system. We propose that the global loss of G9a-mediated H3K9me2 affecting neuronal survival in the postnatal ethanol-treated brain. The identification of a role for G9a in the neurodegenerative process, as well as the long-lasting consequence of postnatal ethanol exposure during synaptogenesis, expand our understanding of the dynamic changes underlying histone modifications and suggests new therapeutic targets for treatment. Currently, no pharmacotherapy aimed at counteracting either the neurodevelopmental or the neurodegenerative component of early-life ethanol exposure has been approved. Understanding the complex epigenetics of early-life ethanol exposure will undoubtedly shed new light on the mechanisms of developmental ethanol neurotoxicity. Most importantly, it will help us identify epigenetic mechanisms for early-life ethanol exposure, which will be essential for designing novel therapeutic strategies to improve specific aspects of the symptomatology of ethanol-induced neurobehavioral teratogenicity.
HIGHLIGHTS.
- Ethanol treatment at P7 induces apoptosis, loss of H3K9me2 and LTP deficits as observed in FASD.
- Bix 01294 administration before P7 ethanol treatment rescues LTP and memory deficits in adult mice.
- P7 ethanol induces while Bix pretreatment rescues social recognition memory deficit in adult mice.
- G9a regulated H3K9me2 may serve as an important and potential therapeutic target against FASD.
Acknowledgements
This work in part was supported by NIH/NIAAA grant AA019443 (BSB).
Abbreviations
H3K4me3
histone H3 trimethylation at lysine 4
H3K9me2
histone H3 dimethylation at lysine 9
FASDs
fetal alcohol spectrum disorders
LTP
long-term potentiation
EHMTases
euchromatic histone methyltransferases
GLP
G9a-related protein
BEL
blood ethanol levels
CC3
cleaved caspase-3
fEPSP
field-excitatory-post-synaptic potential
ORM
object recognition memory
G12
gestation day 12
P7
postnatal day 7
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors declare no conflict of interest.
References
- 1.Abdollah S, Catlin MC, Brien JF. Ethanol neuro-behavioural teratogenesis in the guinea pig: behavioural dysfunction and hippocampal morphologic change. Can J Physiol Pharmacol. 1993;71:776–782. doi: 10.1139/y93-116. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez P, Wendelken L, Eichenbaum H. Hippocampal formation lesions impair performance in an odor-odor association task independently of spatial context. Neurobiol Learn Mem. 2002;78:470–476. doi: 10.1006/nlme.2002.4068. [DOI] [PubMed] [Google Scholar]
- 3.APA. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatry Association Press; 2000. [Google Scholar]
- 4.Balemans MC, Ansar M, Oudakker AR, van Caam AP, Bakker B, Vitters EL, van der Kraan PM, de Bruijn DR, Janssen SM, Kuipers AJ, Huibers MM, Maliepaard EM, Walboomers XF, Benevento M, Nadif Kasri N, Kleefstra T, Zhou H, Van der Zee CE, van Bokhoven H. Reduced Euchromatin histone methyltransferase 1 causes developmental delay, hypotonia, and cranial abnormalities associated with increased bone gene expression in Kleefstra syndrome mice. Dev Biol. 2014;386:395–407. doi: 10.1016/j.ydbio.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Baron-Cohen S, Ring H, Moriarty J, Schmitz B, Costa D, Ell P. Recognition of mental state terms. Clinical findings in children with autism and a functional neuroimaging study of normal adults. British Journal of Psychiatry. 1994;165:640–649. doi: 10.1192/bjp.165.5.640. [DOI] [PubMed] [Google Scholar]
- 6.Basavarajappa BS, Nagaraja NN, Xie S, Subbanna S. Elevation of Endogenous Anandamide Impairs LTP, Learning and Memory through CB1 Receptor Signaling in Mice. Hippocampus. 2014 doi: 10.1002/hipo.22272. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basavarajappa BS, Ninan I, Arancio O. Acute Ethanol Suppresses Glutamatergic Neurotransmission through Endocannabinoids in Hippocampal Neurons. J Neurochem. 2008;107:1001–1013. doi: 10.1111/j.1471-4159.2008.05685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basavarajappa BS, Subbanna S. CB1 Receptor-Mediated Signaling Underlies the Hippocampal Synaptic, Learning and Memory Deficits Following Treatment with JWH-081, a New Component of Spice/K2 Preparations. Hippocampus. 2014;24:178–188. doi: 10.1002/hipo.22213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 10.Bekdash RA, Zhang C, Sarkar DK. Gestational Choline Supplementation Normalized Fetal Alcohol-Induced Alterations in Histone Modifications, DNA Methylation, and Proopiomelanocortin (POMC) Gene Expression in beta-Endorphin-Producing POMC Neurons of the Hypothalamus. Alcohol Clin Exp Res. 2013;37:1133–1142. doi: 10.1111/acer.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonthius DJ, Woodhouse J, Bonthius NE, Taggard DA, Lothman EW. Reduced seizure threshold and hippocampal cell loss in rats exposed to alcohol during the brain growth spurt. Alcohol Clin Exp Res. 2001;25:70–82. [PubMed] [Google Scholar]
- 12.Brothers L, Ring B, Kling A. Response of neurons in the macaque amygdala to complex social stimuli. Behavioural Brain Research. 1990;41:199–213. doi: 10.1016/0166-4328(90)90108-q. [DOI] [PubMed] [Google Scholar]
- 13.Butters NS, Gibson MA, Reynolds JN, Brien JF. Effects of chronic prenatal ethanol exposure on hippocampal glutamate release in the postnatal guinea pig. Alcohol. 2000;21:1–9. doi: 10.1016/s0741-8329(99)00096-8. [DOI] [PubMed] [Google Scholar]
- 14.Campuzano V, Montermini L, Molto MD, Pianese L, Cossee M, Cavalcanti F, Monros E, Rodius F, Duclos F, Monticelli A, Zara F, Canizares J, Koutnikova H, Bidichandani SI, Gellera C, Brice A, Trouillas P, De Michele G, Filla A, De Frutos R, Palau F, Patel PI, Di Donato S, Mandel JL, Cocozza S, Koenig M, Pandolfo M. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271:1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- 15.Carter JJ, Tong M, Silbermann E, Lahousse SA, Ding FF, Longato L, Roper N, Wands JR, de la Monte SM. Ethanol impaired neuronal migration is associated with reduced aspartyl-asparaginyl-beta-hydroxylase expression. Acta Neuropathol. 2008;116:303–315. doi: 10.1007/s00401-008-0377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, Webber A. Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur J Neurosci. 2005;21:1719–1726. doi: 10.1111/j.1460-9568.2005.04004.x. [DOI] [PubMed] [Google Scholar]
- 17.Chudley AE, Conry J, Cook JL, Loock C, Rosales T, LeBlanc N. Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. CMAJ. 2005;172:S1–S21. doi: 10.1503/cmaj.1040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cushing BS, Kramer KM. Mechanisms underlying epigenetic effects of early social experience: the role of neuropeptides and steroids. Neurosci Biobehav Rev. 2005;29:1089–1105. doi: 10.1016/j.neubiorev.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Demas GE, Williams JM, Nelson RJ. Amygdala but not hippocampal lesions impair olfactory memory for mate in prairie voles (Microtus ochrogaster) Am J Physiol. 1997;273:R1683–1689. doi: 10.1152/ajpregu.1997.273.5.R1683. [DOI] [PubMed] [Google Scholar]
- 20.Eade AM, Sheehe PR, Youngentob SL. Ontogeny of the enhanced fetal-ethanol-induced behavioral and neurophysiologic olfactory response to ethanol odor. Alcohol Clin Exp Res. 2010;34:206–213. doi: 10.1111/j.1530-0277.2009.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 22.Gavin DP, Sharma RP. Histone modifications, DNA methylation, and schizophrenia. Neurosci Biobehav Rev. 2010;34:882–888. doi: 10.1016/j.neubiorev.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girard TA, Xing HC, Ward GR, Wainwright PE. Early postnatal ethanol exposure has long-term effects on the performance of male rats in a delayed matching-to-place task in the Morris water maze. Alcohol Clin Exp Res. 2000;24:300–306. [PubMed] [Google Scholar]
- 24.Goodman AM, Delis DC, Mattson SN. Normative data for 4-year-old children on the California Verbal Learning Test-Children’s Version. Clin Neuropsychol. 1999;13:274–282. doi: 10.1076/clin.13.3.274.1748. [DOI] [PubMed] [Google Scholar]
- 25.Gordon N. Some influences on cognition in early life: a short review of recent opinions. Eur J Paediatr Neurol. 1998;2:1–5. doi: 10.1016/1090-3798(98)01000-1. [DOI] [PubMed] [Google Scholar]
- 26.Gupta-Agarwal S, Franklin AV, Deramus T, Wheelock M, Davis RL, McMahon LL, Lubin FD. G9a/GLP Histone Lysine Dimethyltransferase Complex Activity in the Hippocampus and the Entorhinal Cortex Is Required for Gene Activation and Silencing during Memory Consolidation. J Neurosci. 2012;32:5440–5453. doi: 10.1523/JNEUROSCI.0147-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayward ML, Martin AE, Brien JF, Dringenberg HC, Olmstead MC, Reynolds JN. Chronic prenatal ethanol exposure impairs conditioned responding and enhances GABA release in the hippocampus of the adult guinea pig. J Pharmacol Exp Ther. 2004;308:644–650. doi: 10.1124/jpet.103.059261. [DOI] [PubMed] [Google Scholar]
- 28.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O’Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 29.Howell KK, Lynch ME, Platzman KA, Smith GH, Coles CD. Prenatal alcohol exposure and ability, academic achievement, and school functioning in adolescence: a longitudinal follow-up. J Pediatr Psychol. 2006;31:116–126. doi: 10.1093/jpepsy/jsj029. [DOI] [PubMed] [Google Scholar]
- 30.Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- 31.Iqbal U, Rikhy S, Dringenberg HC, Brien JF, Reynolds JN. Spatial learning deficits induced by chronic prenatal ethanol exposure can be overcome by non-spatial pre-training. Neurotoxicol Teratol. 2006;28:333–341. doi: 10.1016/j.ntt.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Izumi Y, Kitabayashi R, Funatsu M, Izumi M, Yuede C, Hartman RE, Wozniak DF, Zorumski CF. A single day of ethanol exposure during development has persistent effects on bi-directional plasticity, N-methyl-D-aspartate receptor function and ethanol sensitivity. Neuroscience. 2005;136:269–279. doi: 10.1016/j.neuroscience.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- 34.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- 35.Kaba H, Rosser A, Keverne B. Neural basis of olfactory memory in the context of pregnancy block. Neuroscience. 1989;32:657–662. doi: 10.1016/0306-4522(89)90287-x. [DOI] [PubMed] [Google Scholar]
- 36.Kaminen-Ahola N, Ahola A, Flatscher-Bader T, Wilkins SJ, Anderson GJ, Whitelaw E, Chong S. Postnatal growth restriction and gene expression changes in a mouse model of fetal alcohol syndrome. Birth Defects Res A Clin Mol Teratol. 2010a;88:818–826. doi: 10.1002/bdra.20729. [DOI] [PubMed] [Google Scholar]
- 37.Kaminen-Ahola N, Ahola A, Maga M, Mallitt KA, Fahey P, Cox TC, Whitelaw E, Chong S. Maternal ethanol consumption alters the epigenotype and the phenotype of offspring in a mouse model. PLoS Genet. 2010b;6:e1000811. doi: 10.1371/journal.pgen.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keverne EB. Vomeronasal/accessory olfactory system and pheromonal recognition. Chem Senses. 1998;23:491–494. doi: 10.1093/chemse/23.4.491. [DOI] [PubMed] [Google Scholar]
- 39.Kleefstra T, Brunner HG, Amiel J, Oudakker AR, Nillesen WM, Magee A, Genevieve D, Cormier-Daire V, van Esch H, Fryns JP, Hamel BC, Sistermans EA, de Vries BB, van Bokhoven H. Loss-of-function mutations in euchromatin histone methyl transferase 1 (EHMT1) cause the 9q34 subtelomeric deletion syndrome. Am J Hum Genet. 2006;79:370–377. doi: 10.1086/505693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kleefstra T, van Zelst-Stams WA, Nillesen WM, Cormier-Daire V, Houge G, Foulds N, van Dooren M, Willemsen MH, Pfundt R, Turner A, Wilson M, McGaughran J, Rauch A, Zenker M, Adam MP, Innes M, Davies C, Lopez AG, Casalone R, Weber A, Brueton LA, Navarro AD, Bralo MP, Venselaar H, Stegmann SP, Yntema HG, van Bokhoven H, Brunner HG. Further clinical and molecular delineation of the 9q subtelomeric deletion syndrome supports a major contribution of EHMT1 haploinsufficiency to the core phenotype. J Med Genet. 2009;46:598–606. doi: 10.1136/jmg.2008.062950. [DOI] [PubMed] [Google Scholar]
- 41.Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Kramer JM, Kochinke K, Oortveld MA, Marks H, Kramer D, de Jong EK, Asztalos Z, Westwood JT, Stunnenberg HG, Sokolowski MB, Keleman K, Zhou H, van Bokhoven H, Schenck A. Epigenetic regulation of learning and memory by Drosophila EHMT/G9a. PLoS Biol. 2011;9:e1000569. doi: 10.1371/journal.pbio.1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramer JM, van Bokhoven H. Genetic and epigenetic defects in mental retardation. Int J Biochem Cell Biol. 2009;41:96–107. doi: 10.1016/j.biocel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 45.Lubin FD, Sweatt JD. The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron. 2007;55:942–957. doi: 10.1016/j.neuron.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lundquist F. The determination of ethyl alcohol in blood and tissue. Meth Biochem Analy. 1959;7:217–251. [Google Scholar]
- 47.Makedonski K, Abuhatzira L, Kaufman Y, Razin A, Shemer R. MeCP2 deficiency in Rett syndrome causes epigenetic aberrations at the PWS/AS imprinting center that affects UBE3A expression. Hum Mol Genet. 2005;14:1049–1058. doi: 10.1093/hmg/ddi097. [DOI] [PubMed] [Google Scholar]
- 48.Margret CP, Li CX, Chappell TD, Elberger AJ, Matta SG, Waters RS. Prenatal alcohol exposure delays the development of the cortical barrel field in neonatal rats. Exp Brain Res. 2006;172:1–13. doi: 10.1007/s00221-005-0319-0. [DOI] [PubMed] [Google Scholar]
- 49.Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 1999;23:1808–1815. [PubMed] [Google Scholar]
- 51.Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20:810–816. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- 52.Mattson SN, Riley EP, Gramling L, Delis DC, Jones KL. Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome. Neuropsychology. 1998;12:146–153. doi: 10.1037//0894-4105.12.1.146. [DOI] [PubMed] [Google Scholar]
- 53.Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26:875–882. [PubMed] [Google Scholar]
- 54.Mattson SN, Roesch SC, Fagerlund A, Autti-Ramo I, Jones KL, May PA, Adnams CM, Konovalova V, Riley EP. Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2010;34:1640–1650. doi: 10.1111/j.1530-0277.2010.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.May PA, Brooke L, Gossage JP, Croxford J, Adnams C, Jones KL, Robinson L, Viljoen D. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. Am J Public Health. 2000;90:1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- 57.May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NC, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug Alcohol Depend. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Medina AE, Krahe TE, Ramoa AS. Early alcohol exposure induces persistent alteration of cortical columnar organization and reduced orientation selectivity in the visual cortex. J Neurophysiol. 2005;93:1317–1325. doi: 10.1152/jn.00714.2004. [DOI] [PubMed] [Google Scholar]
- 59.Mooney SM, Varlinskaya EI. Acute prenatal exposure to ethanol and social behavior: effects of age, sex, and timing of exposure. Behav Brain Res. 2011;216:358–364. doi: 10.1016/j.bbr.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nelson CA, Luciana M. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- 61.Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- 62.Norman AL, O’Brien JW, Spadoni AD, Tapert SF, Jones KL, Riley EP, Mattson SN. A functional magnetic resonance imaging study of spatial working memory in children with prenatal alcohol exposure: contribution of familial history of alcohol use disorders. Alcohol Clin Exp Res. 2013;37:132–140. doi: 10.1111/j.1530-0277.2012.01880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ogawa H, Ishiguro K, Gaubatz S, Livingston DM, Nakatani Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science. 2002;296:1132–1136. doi: 10.1126/science.1069861. [DOI] [PubMed] [Google Scholar]
- 64.Olney JW, Tenkova T, Dikranian K, Qin YQ, Labruyere J, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res Dev Brain Res. 2002;133:115–126. doi: 10.1016/s0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- 65.Perkins A, Lehmann C, Lawrence RC, Kelly SJ. Alcohol exposure during development: Impact on the epigenome. Int J Dev Neurosci. 2013 doi: 10.1016/j.ijdevneu.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petronis A. Epigenetics and bipolar disorder: new opportunities and challenges. Am J Med Genet C Semin Med Genet. 2003;123C:65–75. doi: 10.1002/ajmg.c.20015. [DOI] [PubMed] [Google Scholar]
- 67.Pick CG, Cooperman M, Trombka D, Rogel-Fuchs Y, Yanai J. Hippocampal cholinergic alterations and related behavioral deficits after early exposure to ethanol. Int J Dev Neurosci. 1993;11:379–385. doi: 10.1016/0736-5748(93)90009-3. [DOI] [PubMed] [Google Scholar]
- 68.Powrozek TA, Zhou FC. Effects of prenatal alcohol exposure on the development of the vibrissal somatosensory cortical barrel network. Brain Res Dev Brain Res. 2005;155:135–146. doi: 10.1016/j.devbrainres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Roebuck-Spencer TM, Mattson SN. Implicit strategy affects learning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2004;28:1424–1431. doi: 10.1097/01.alc.0000139826.25247.5b. [DOI] [PubMed] [Google Scholar]
- 70.Ross RS, Eichenbaum H. Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory. J Neurosci. 2006;26:4852–4859. doi: 10.1523/JNEUROSCI.0659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryu H, Lee J, Hagerty SW, Soh BY, McAlpin SE, Cormier KA, Smith KM, Ferrante RJ. ESET/SETDB1 gene expression and histone H3 (K9) trimethylation in Huntington's disease. Proc Natl Acad Sci U S A. 2006;103:19176–19181. doi: 10.1073/pnas.0606373103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sadrian B, Subbanna S, Wilson DA, Basavarajappa BS, Saito M. Lithium prevents long-term neural and behavioral pathology induced by early alcohol exposure. Neuroscience. 2012;206:122–135. doi: 10.1016/j.neuroscience.2011.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanchez-Andrade G, Kendrick KM. The main olfactory system and social learning in mammals. Behavioural Brain Research. 2009;200:323–335. doi: 10.1016/j.bbr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 75.Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Toth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci U S A. 2000;97:14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savage DD, Becher M, de la Torre AJ, Sutherland RJ. Dose-dependent effects of prenatal ethanol exposure on synaptic plasticity and learning in mature offspring. Alcohol Clin Exp Res. 2002;26:1752–1758. doi: 10.1097/01.ALC.0000038265.52107.20. [DOI] [PubMed] [Google Scholar]
- 77.Schaefer A, Sampath SC, Intrator A, Min A, Gertler TS, Surmeier DJ, Tarakhovsky A, Greengard P. Control of cognition and adaptive behavior by the GLP/G9a epigenetic suppressor complex. Neuron. 2009;64:678–691. doi: 10.1016/j.neuron.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schneider R, Bannister AJ, Myers FA, Thorne AW, Crane-Robinson C, Kouzarides T. Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat Cell Biol. 2004;6:73–77. doi: 10.1038/ncb1076. [DOI] [PubMed] [Google Scholar]
- 79.Shirasaka T, Hashimoto E, Ukai W, Yoshinaga T, Ishii T, Tateno M, Saito T. Stem cell therapy: social recognition recovery in a FASD model. Transl Psychiatry. 2012;2:e188. doi: 10.1038/tp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sik A, van Nieuwehuyzen P, Prickaerts J, Blokland A. Performance of different mouse strains in an object recognition task. Behav Brain Res. 2003;147:49–54. doi: 10.1016/s0166-4328(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 81.Stiles J. Neural plasticity and cognitive development. Dev Neuropsychol. 2000;18:237–272. doi: 10.1207/S15326942DN1802_5. [DOI] [PubMed] [Google Scholar]
- 82.Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol Clin Exp Res. 1990;14:662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- 83.Subbanna S, Nagre NN, Shivakumar M, Umapathy NS, Psychoyos D, Basavarajappa BS. Ethanol Induced Acetylation of Histone at G9a Exon1 and G9a-Mediated Histone H3 Dimethylation leads to Neurodegeneration in Neonatal Mice. Neuroscience. 2014;258C:422–432. doi: 10.1016/j.neuroscience.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Subbanna S, Shivakumar M, Psychoyos D, Xie S, Basavarajappa BS. Anandamide-CB1 Receptor Signaling Contributes to Postnatal Ethanol-Induced Neonatal Neurodegeneration, Adult Synaptic and Memory Deficits. Journal of neuoscience. 2013a;33:6350–6366. doi: 10.1523/JNEUROSCI.3786-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Subbanna S, Shivakumar M, Umapathy NS, Saito M, Mohan PS, Kumar A, Nixonc RA, Verin AD, Psychoyos D, Basavarajappa BS. G9a-Mediated Histone Methylation Regulates Ethanol-Induced Neurodegeneration in the Neonatal Mouse Brain. Neurobiology of Disease. 2013b;54:475–485. doi: 10.1016/j.nbd.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, Fukuda M, Takeda N, Niida H, Kato H, Shinkai Y. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–1791. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tachibana M, Ueda J, Fukuda M, Takeda N, Ohta T, Iwanari H, Sakihama T, Kodama T, Hamakubo T, Shinkai Y. Histone methyltransferases G9a and GLP form heteromeric complexes and are both crucial for methylation of euchromatin at H3-K9. Genes Dev. 2005;19:815–826. doi: 10.1101/gad.1284005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tarelo-Acuna L, Olvera-Cortes E, Gonzalez-Burgos I. Prenatal and postnatal exposure to ethanol induces changes in the shape of the dendritic spines from hippocampal CA1 pyramidal neurons of the rat. Neurosci Lett. 2000;286:13–16. doi: 10.1016/s0304-3940(00)01075-2. [DOI] [PubMed] [Google Scholar]
- 89.Thomas JD, Sather TM, Whinery LA. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav Neurosci. 2008;122:1264–1273. doi: 10.1037/a0013271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomas JD, Weinert SP, Sharif S, Riley EP. MK-801 administration during ethanol withdrawal in neonatal rat pups attenuates ethanol-induced behavioral deficits. Alcohol Clin Exp Res. 1997;21:1218–1225. [PubMed] [Google Scholar]
- 91.Thor DH, Wainwright KL, Holloway WR. Persistence of attention to a novel conspecific: some developmental variables in laboratory rats. Dev Psychobiol. 1982;15:1–8. doi: 10.1002/dev.420150102. [DOI] [PubMed] [Google Scholar]
- 92.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 93.Urban M, Chersich MF, Fourie LA, Chetty C, Olivier L, Viljoen D. Fetal alcohol syndrome among grade 1 schoolchildren in Northern Cape Province: prevalence and risk factors. S Afr Med J. 2008;98:877–882. [PubMed] [Google Scholar]
- 94.Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Warren ST. The Epigenetics of Fragile X Syndrome. Cell Stem Cell. 2007;1:488–489. doi: 10.1016/j.stem.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 96.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 97.West JR, Hamre KM, Cassell MD. Effects of ethanol exposure during the third trimester equivalent on neuron number in rat hippocampus and dentate gyrus. Alcohol Clin Exp Res. 1986;10:190–197. doi: 10.1111/j.1530-0277.1986.tb05070.x. [DOI] [PubMed] [Google Scholar]
- 98.Wilson DA, Peterson J, Basavaraj BS, Saito M. Local and regional network function in behaviorally relevant cortical circuits of adult mice following postnatal alcohol exposure. Alcoholism Clin and Exp Res. 2011;35:1974–1984. doi: 10.1111/j.1530-0277.2011.01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson DA, Sullivan RM. Cortical processing of odor objects. Neuron. 2011;72:506–519. doi: 10.1016/j.neuron.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Young C, Straiko MM, Johnson SA, Creeley C, Olney JW. Ethanol causes and lithium prevents neuroapoptosis and suppression of pERK in the infant mouse brain. Neurobiol Dis. 2008;31:355–360. doi: 10.1016/j.nbd.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou FC, Sari Y, Powrozek TA. Fetal alcohol exposure reduces serotonin innervation and compromises development of the forebrain along the serotonergic pathway. Alcohol Clin Exp Res. 2005;29:141–149. doi: 10.1097/01.alc.0000150636.19677.6f. [DOI] [PubMed] [Google Scholar]
- 102.Zimmerberg B, Sukel HL, Stekler JD. Spatial learning of adult rats with fetal alcohol exposure: deficits are sex-dependent. Behav Brain Res. 1991;42:49–56. doi: 10.1016/s0166-4328(05)80039-7. [DOI] [PubMed] [Google Scholar]