Archaeal Elp3 catalyzes tRNA wobble uridine modification at C5 via a radical mechanism (original) (raw)
. Author manuscript; available in PMC: 2015 Jun 24.
Published in final edited form as: Nat Chem Biol. 2014 Aug 24;10(10):810–812. doi: 10.1038/nchembio.1610
Abstract
Approximately 25% of cytoplasmic tRNAs in eukaryotic organisms have the wobble uridine (U34) modified at C5 through a process that, according to genetic studies, is carried out by the eukaryotic Elongator complex. Here we show that a single archaeal protein, the homolog of the third subunit of the eukaryotic Elongator complex (Elp3), is able to catalyze the same reaction. The mechanism of action by Elp3 described here represents unprecedented chemistry performed on acetyl-CoA.
The eukaryotic Elongator complex is a large protein complex consisting of six subunits, the Elongator proteins (Elp1–Elp6; Supplementary Results, Supplementary Fig. 1a)1–3. Among these, Elp3 is generally regarded as the catalytic subunit, and it has two putative enzymatic domains: a radical _S_-adenosylmethionine (SAM) domain near the N terminus4,5 and a histone acetyltransferase (HAT) domain at the C terminus (Supplementary Fig. 1a)6. Nonetheless, genetic studies in five different eukaryotic organisms have shown that all six subunits are required for cellular functions of the Elongator complex7–11. The complex has been implicated in two biochemical activities, histone acetylation and tRNA U34 modification at C5. However, the biochemical activity responsible for the in vivo functions of the Elongator complex has been hotly debated and remains unresolved.
To shed light on the biochemical functions of the Elongator complex, we performed a bioinformatic search of all six subunits of human Elongator. We found that homologs of Elp3, but not other subunits, are present in essentially all archaea, a small number of bacteria and two viruses (Supplementary Fig. 2). Furthermore, all the archaeal and bacterial organisms possessing Elp3 lack genes encoding MnmE and MnmG, which are responsible for (bacteria) or implicated in (archaea) tRNA modification at C5 of U34 (ref. 12,13). These revelations, together with the fact that Elp3 possesses a radical SAM and a HAT domain, led us to propose a simple mechanism of tRNA U34 modification at C5 that requires only Elp3 for catalysis: a 5′-deoxyadenosyl radical (5′-dA•) generated in the radical SAM domain first abstracts the hydrogen atom attached to C5 of U34 in tRNA, which reacts with the methyl group of acetyl-CoA in the HAT domain to form a C-C bond; hydrolysis of CoA then results in formation of 5′-carboxymethyluridine (cm5U).
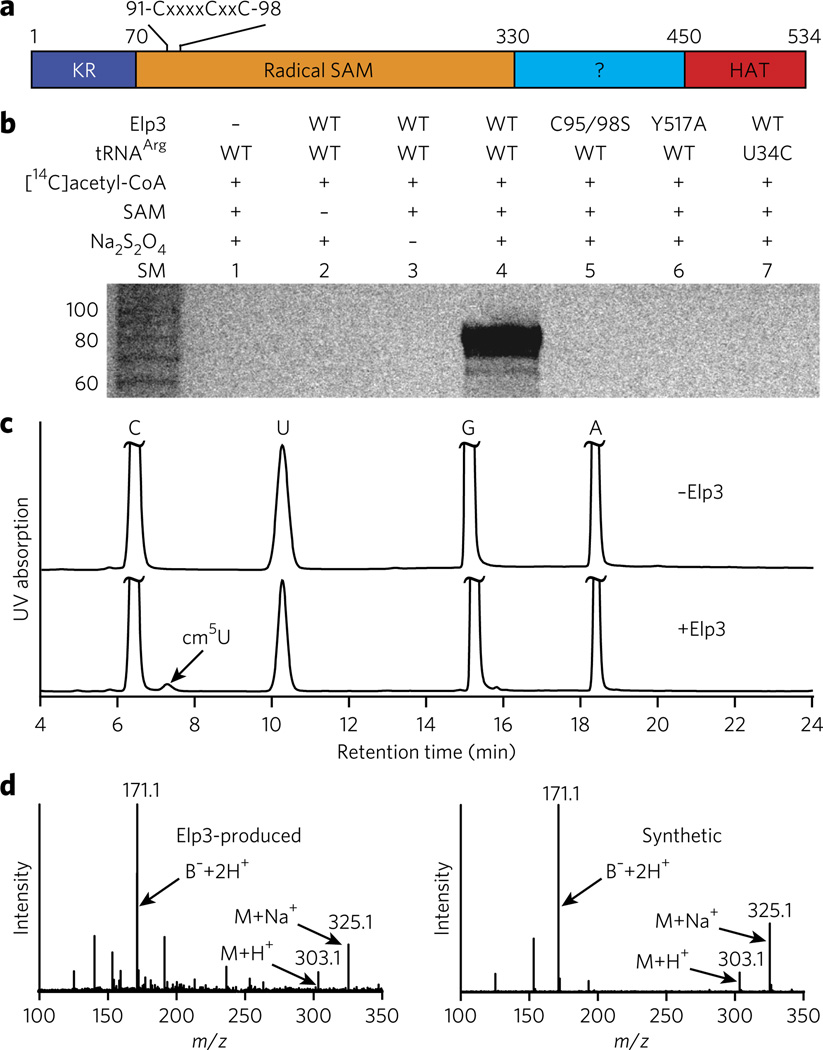
To test our hypothesis, we cloned, overexpressed and purified the recombinant Elp3 from archaeal Methanocaldococcus infernus (_Min_Elp3, Fig. 1a and Supplementary Fig. 3) and performed in vitro reconstitution of the modification reaction using synthetic _Min_tRNAArg and [14C]acetyl-CoA. In the presence of all components required for the reaction, tRNA became radioactive (Fig. 1b, lane 4, and Supplementary Fig. 4), demonstrating the covalent attachment of the [14C]acetyl group (or the entire [14C]acetyl-CoA) to tRNA. tRNA was not radiolabeled in the absence of Elp3 (Fig. 1b, lane 1), indicating that the reaction requires catalysis by Elp3. When we omitted either SAM (the source of 5′-dA•) or Na2S2O4 (the reducing agent that provides the electron to generate 5′-dA•) from the reaction, the tRNA product was not formed (Fig. 1b, lanes 2 and 3), indicating that the reaction occurs via a radical mechanism. Elp3 with a C95S C98S double mutation or a Y517A mutation was also inactive (Fig. 1b, lanes 5 and 6). In yeast Elp3, mutation to alanine of residues equivalent to _Min_Elp3 Cys95, Cys98 or Tyr517 abolishes tRNA U34 modification at C5 in vivo14 (Supplementary Fig. 5). Finally, tRNA with a U34C mutation was not an effective substrate for the reaction (Fig. 1b, lane 7), indicating that U34 is the likely site of modification.
Figure 1. In vitro reconstitution of _Min_Elp3 activity.
(a) Schematic view of the domain structure of _Min_Elp3, similar to the structure for human Elp3 shown in Supplementary Figure 1a. The CxxxxCxxC motif that coordinates a [4Fe-4S] cluster is highlighted. (b) Denaturing PAGE analysis of tRNA products from the Elp3-catalyzed reactions. SM, size markers. (c) RP-HPLC chromatograms of the digests of tRNA products from the reactions without (top) and with (bottom) Elp3. The Elp3-produced product cm5U is indicated by an arrow. (d) ESI-LC/MS analyses of cm5U from the Elp3-catalyzed reaction (left) and the synthetic compound (right).
To gain further insight into the chemical nature of the Elp3-catalyzed reaction, we carried out the reaction on a preparative scale and then characterized the tRNA product using RP-HPLC and ESI-LC/MS. RP-HPLC analysis of the digested tRNA showed the presence of a new peak in addition to the ones belonging to C, U, G and A (Fig. 1c, bottom). The new peak was absent in tRNA from the reaction without Elp3 (Fig. 1c, top), indicating that it is the product of the Elp3-catalyzed reaction. Quantification of the peaks indicated that approximately 50% of tRNA substrate was converted to product. ESI-LC/MS of the material from the new peak (Fig. 1d, left) as compared to the synthetic compound (Fig. 1d, right) identified the modified nucleoside as cm5U. This conclusion is further supported by the fact that the Elp3-produced product co-eluted with the synthetic compound in an RP-HPLC analysis (Supplementary Fig. 6).
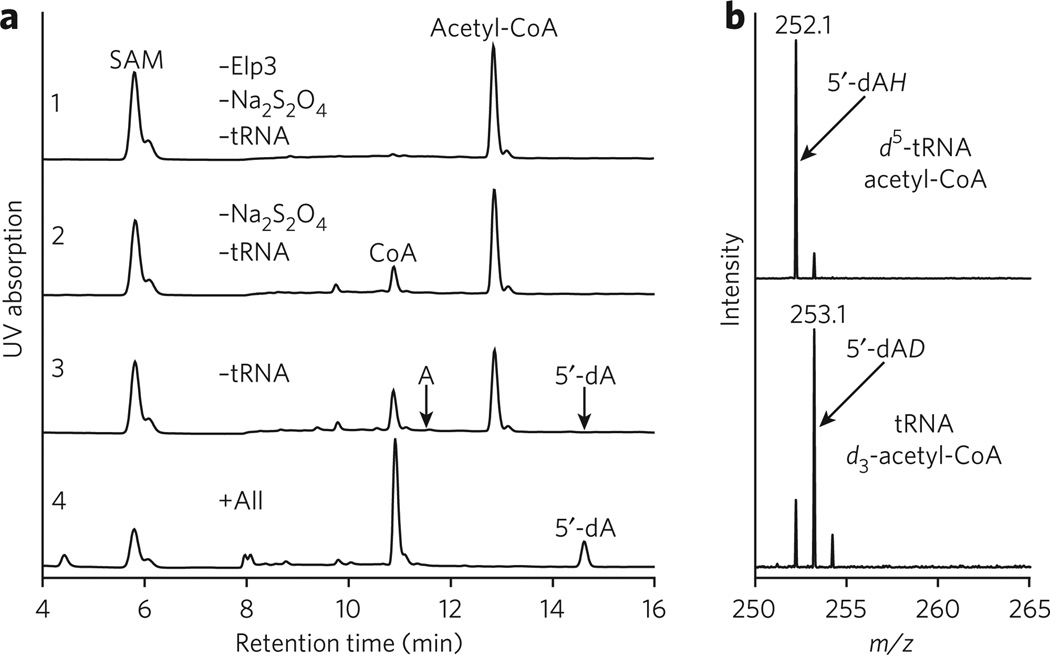
To identify whether tRNA or acetyl-CoA is the reacting target of 5′-dA•, we first examined the formation of 5′-dA by RP-HPLC (Fig. 2a) and then determined the molecular mass (m/z) of 5′-dA in the presence of tRNA labeled with deuterium at C5 of U (_d_5-tRNA) or deuterium-labeled acetyl-CoA (_d_3-acetyl-CoA) (Fig. 2b). Surprisingly, the deuterium was found in 5′-dA only from the reaction employing d_3-acetyl-CoA (Fig. 2b, bottom), not the reaction employing d_5-tRNA (Fig. 2b, top), demonstrating that 5′-dA• abstracts a hydrogen atom from acetyl-CoA, rather than from tRNA as we initially envisioned. In addition to 5′-dA_D, a small but meaningful amount of 5′-dA_H was also formed in the presence of _d_3-acetyl-CoA (Fig. 2b, bottom). The presence of hydrogen atoms is not due to impurity of acetyl-CoA in the _d_3-acetyl-CoA sample because this was at least 96% pure as judged by ESI-LC/MS (Supplementary Fig. 7, bottom). We speculate that a small amount of 5′-dA• might have carried out an abortive reaction by abstracting a hydrogen atom from the solvent or from amino acids of Elp3 near the SAM-binding pocket.
Figure 2. Chromatographic and spectrometric analyses of the consumption of cofactors and the production of 5′-dA.
(a) RP-HPLC chromatograms of the reactions differing in components, starting with the one containing only the cofactors (SAM and acetyl-CoA) in the reaction buffer (panel 1). Missing components were added one at a time (panels 2 and 3) until all the components required for the reaction were included (panel 4). (b) ESI-LC/MS analyses of the resulting 5′-dA from the reactions in the presence of the deuterated tRNA (top) and the deuterated acetyl-CoA (bottom).
How SAM and acetyl-CoA were consumed also provided insight into the mechanism of the Elp3-catalyzed reaction (Fig. 2a). In the absence of Elp3, Na2S2O4 and tRNA, both SAM and acetyl-CoA were stable over the course of the reaction (Fig. 2a, panel 1). Addition of Elp3 to the reaction mixture resulted in the consumption of a small but meaningful amount of acetyl-CoA, whereas SAM remained intact (Fig. 2a, panel 2). Additional experiments shown in Supplementary Figure 8a further support the conclusion that Elp3 reacts with acetyl-CoA. These observations may have mechanistic implications, as Elp3 may first react with acetyl-CoA to form an acetyl-Elp3 covalent intermediate (Supplementary Fig. 9). Addition of Na2S2O4 caused further consumption of acetyl-CoA, but still without consumption of SAM (Fig. 2a, panel 3). Failure to detect either 5′-dA or A (Fig. 2a, panel 3) indicates that 5′-dA• is not able to form in the absence of tRNA despite the presence of all of the chemical ingredients required for the production of 5′-dA•. This was confirmed by the detection of 5-dA when tRNA was added to the reaction (Fig. 2a, panel 4). Surprisingly, addition of tRNA also resulted in complete conversion of acetyl-CoA to CoA (Fig. 2a, panel 4), indicating that tRNA promotes hydrolysis of acetyl-CoA. This is supported by additional experiments showing that only a catalytic amount of tRNA is required to rapidly hydrolyze acetyl-CoA (Supplementary Fig. 8b).
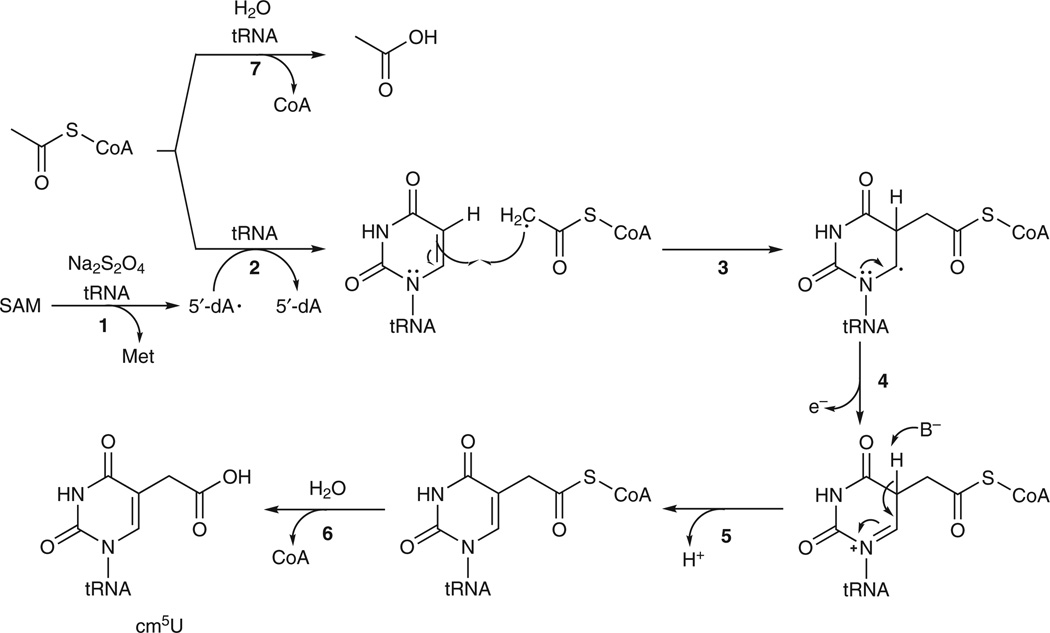
On the basis of the experimental data presented here, we propose a mechanism of the Elp3-catalyzed reaction as shown in Figure 3. Association of tRNA with Elp3 allows 5′-dA• to be generated from SAM in the radical SAM domain of Elp3 (Fig. 3, step 1). 5′-dA• then abstracts a hydrogen atom from the methyl group of acetyl-CoA bound in the HAT domain of Elp3 (Fig. 3, step 2). The acetyl radical adds to C5 of U34 in tRNA to form a C-C bond (Fig. 3, step 3). Loss of an electron from U34 (Fig. 3, step 4), followed by abstraction of the proton at C5 (Fig. 3, step 5), results in formation of the tRNA–acetyl-CoA conjugate. Hydrolysis of CoA produces the modified nucleotide cm5U at the wobble position of tRNA (Fig. 3, step 6). In the absence of tRNA, the Elp3-bound acetyl-CoA is stable; association of tRNA with Elp3 results in hydrolysis of acetyl-CoA (Fig. 3, step 7). Unresolved issues include the identity of the general base that abstracts the proton at C5, the molecular mechanism by which tRNA promotes generation of 5′-dA• and the molecular mechanism by which tRNA catalyzes hydrolysis of CoA from either acetyl-CoA or the tRNA–acetyl-CoA conjugate. In addition, some of our experimental data (Supplementary Fig. 8 is an example) appeared to indicate the formation of an acetyl-Elp3 covalent intermediate during the Elp3-catalyzed reaction, but we were not able to obtain convincing evidence to verify this. Therefore, a reaction mechanism involving an acetyl-Elp3 covalent intermediate, as shown in Supplementary Figure 9, cannot be ruled out. In such a mechanism, hydrolysis of CoA would be the first rather than the last step of the reaction, and an Elp3-tRNA covalent intermediate would be formed over the course of the reaction.
Figure 3. Proposed mechanism of the Elp3-catalyzed reaction.
B−, unidentified general base.
It is interesting to compare the Elp3-catalyzed reaction with the one catalyzed by RlmN and Cfr, which are responsible for the methylation of A2503 at C2 and C8 in 23S ribosomal RNA15. Both Elp3 and RlmN and Cfr employ a radical mechanism, and in both cases 5′-dA• reacts first with the donors of the modification (acetyl and methyl groups, respectively) despite having the opportunity to first react with the RNA base. Thus, it is indeed conceivable that 5′-dA• might not be able to generate a radical on a carbon atom with sp2 hybridization, which has not yet been observed so far15. In addition, if Elp3 catalyzes tRNA modification through an acetyl-Elp3 covalent intermediate (Supplementary Fig. 9), the overall sequence of reactions carried out by Elp3 is essentially the same as the one carried out by RlmN and Cfr15 despite the drastic difference in how the donors of the modifications are loaded.
It is also instructive to compare the chemistry carried out by Elp3 with the chemistry carried out by CmoA and CmoB, which also perform tRNA wobble uridine modification at C5 in Gram-negative bacteria16. Both systems transfer a carboxymethyl group from a cofactor to U34, but they employ different chemistry. A comparison of the components required for the two systems demonstrates the greater efficiency of the Elp3-catalyzed reaction, which requires only one protein and two of the most common cofactors (SAM and acetyl-CoA).
Because Elp3 is highly conserved between archaea and eukaryota (Supplementary Fig. 5; _Min_Elp3 is 44% and 45% identical to human and yeast Elp3, respectively), Elp3 in the eukaryotic Elongator complex is likely to be solely responsible for the chemistry of the tRNA modification reaction. If this is the case, the functional characterization of Elp3 reported here, together with the evidence that the Elongator complex is present mainly in the cytoplasm17–20, supports the view that tRNA U34 modification at C5 is the ancient and primary biochemical function of the Elongator complex. In addition, the mechanistic insight provided here into Elp3’s mode of action indicates that the primary role of the C-terminal HAT domain of Elp3 is to provide an acetyl-CoA for tRNA modification, not for histone acetylation.
ONLINE METHODS
Cloning, expression and purification of Elp3
The gene encoding M. infernus Elp3 (NCBI Protein accession code: YP_003616086) was amplified by PCR from M. infernus genomic DNA and inserted into the petDuet-1 vector (Novagen). The plasmid was transformed into the Escherichia coli expression strain BL21 (DE3) (Invitrogen). A single colony was used to inoculate a starter culture, which was grown overnight at 37 °C. This was used to inoculate 1 liter of lysogeny broth containing 50 µg/ml ampicillin. Cells were grown at 37 °C to _A_600nm of approximately 0.4 and cooled to 18 °C. Expression was induced with 0.5 mM isopropylthiogalactoside (IPTG). Cells were grown overnight at 18 °C and collected by centrifugation. The recombinant protein (_Min_Elp3) was purified to homogeneity using DEAE ion exchange, heparin affinity, Mono-Q ion exchange and Superdex 75 size-exclusion chromatography. The C95/98S and Y517A mutants were generated by QuikChange (Stratagene) using the plasmid of M. infernus Elp3 as the template. Purification of the mutants was similar to that of wild-type _Min_Elp3.
Preparation of tRNA substrate
M. infernus tRNAArg was in vitro transcribed using T7 polymerase. Synthetic DNA oligonucleotides (template strand and a shorter strand complementary to the T7 promoter) were purified using denaturing PAGE followed by ethanol precipitation. A partially double-stranded DNA template was formed by annealing both oligonucleotides together by heating at 95 °C for 3 min and cooling slowly. Reaction was performed in 4 mL scale with 0.2 µM template, 1.25 mM each NTP, 5 mM DTT and 1 µM T7 polymerase in an in vitro transcription buffer containing 40 mM Tris-HCl, pH 7.9, 6 mM MgCl2 and 2 mM spermidine. The reaction was incubated at 37 °C overnight, and the transcribed tRNA was purified using denaturing PAGE and ethanol precipitation. The recovered tRNA was dried using a SpeedVac and stored at −80 °C.
Anaerobic reconstitution of Elp3 activity
The [4Fe-4S] cluster of the purified recombinant _Min_Elp3 was chemically reconstituted in an anaerobic chamber. Specifically, the purified _Min_Elp3 was incubated with 3 mM DTT, 300 µM (NH4)2Fe(SO4)2 and 300 µM Na2S in Reconstitution Buffer (50 mM HEPES (pH 7.5), 150 mM KCl, 150 mM NaCl and 1% glycerol). The following day, _Min_Elp3 was desalted using a NAP-5 column (GE Healthcare). A 10 kDa Ultra-0.5 filter (Amicon) was used to concentrate the protein and perform buffer exchange with Reaction Buffer (50 mM HEPES (pH 7.5), 150 mM KCl, 300 mM NaCl, 5 mM MgCl2 and 1% glycerol).
The in vitro reconstitution reaction was carried out in a 50 µL scale inside the anaerobic chamber. tRNAArg was annealed in Reaction Buffer by heating at 90 °C for 3 min and quickly cooling on ice. 5 µM _Min_Elp3 was incubated with 5 µM tRNAArg, 50 µM SAM, 50 µM [14C]acetyl-CoA and 0.5 mM Na2S2O4 in Reaction Buffer at room temperature for 1 h. After the reaction, the samples were moved out of the anaerobic chamber, and the tRNA product was recovered by phenol extraction followed by ethanol precipitation. The recovered tRNA was dissolved in 10 µL of TE buffer. After the addition of gel loading buffer, the samples were analyzed on a 15% denaturing polyacrylamide gel. The gel was dried, and the dried gel was exposed to a Phosphor Imaging screen (GE Healthcare). The radioactivity was detected by STORM 840 PhosphorImager (GE Healthcare). ImageQuant software (GE Healthcare) was used to quantify the radioactive signals.
To prepare tRNA products for the characterization of the modified nucleoside shown in Figure 1, a truncated tRNAArg (with both D and T loops deleted) was employed and the in vitro reconstitution reaction described above was scaled up 10 times using unlabeled acetyl-CoA. After the reaction, tRNA was recovered with phenol extraction followed by ethanol precipitation.
To prepare samples for RP-HPLC and ESI-LC/MS analyses shown in Figure 2, the in vitro reconstitution reaction was carried out on a 50 µL scale with 100 µM SAM, 100 µM acetyl-CoA, 50 µM _Min_Elp3 (if necessary), 0.5 mM Na2S2O4 (if necessary) and 50 µM tRNAArg (if necessary) in Reaction Buffer at room temperature for 1 h. After the reaction, the sample was filtered by a 10 kDa Ultra-0.5 filter (Amicon), and the flow-through was analyzed by RP-HPLC or ESI-LC/MS. The samples for the experiments shown in Supplementary Figure 8 were prepared similarly except that no Na2S2O4 was added and the reaction time and the concentrations of Elp3 and tRNA were varied according to the graphs shown in Supplementary Figure 8.
Preparation of deuterated substrates
To prepare tRNA substrate with a deuterium at C5 of uridine, _d_5-uridine-5′-monophosphate (_d_5-UMP) was synthesized using a protocol for the synthesis of _d_5-uridine21. In a 1 mL reaction scale, 2.5 mM of _d_5-UMP, 2.5 mM adenosine-5′-triphosphate (ATP), 10 mM phospho(enol)pyruvate (PEP), 0.1 unit Nucleoside Monophosphate Kinase (Roche) and 5 units Pyruvate Kinase (Sigma) were incubated in Kinase Buffer (80 mM Tris-HCl (pH 7.6), 20 mM MgCl2 and 50 mM KCl) at room temperature for 2 h to produce _d_5-uridine-5′-triphosphate (_d_5-UTP) and the recovered ATP. After reaction, 2.5 mM cytidine-5′-triphosphate (CTP) and 2.5 mM guanosine-5′-triphosphate (GTP) were added, and the mixture was directly used for in vitro transcription of the deuterated tRNAArg as described previously.
The deuterated acetyl-CoA (_d_3-acetyl-CoA) was synthesized according to a published procedure22, and _d_3-acetyl-CoA was further purified by RP-HPLC.
Analysis of reaction products by RP-HPLC and ESI-LC/MS
To analyze the modified nucleoside, tRNA product was first digested by P1 nuclease in a P1 buffer (10 mM sodium acetate (pH 5.1), 0.5 mM ZnCl2). This is followed by digestion with Calf Alkaline Phosphatase (CAP) in a CAP buffer (20 mM Tris-HCl (pH 8.5), 20 mM NaCl, 1 mM MgCl2). RP-HPLC analyses were carried out in house with a Waters 1525 HPLC system on a C18(2) Luna column (250 mm × 2 mm, Phenomenex) with a flow rate of 0.3 mL/min. For analysis of the digest of tRNA shown in Figure 1c, solvent A was 5 mM ammonium acetate (pH 5.3), solvent B was 40% acetonitrile in water and the following gradient was used: 0.0 min, 0% B; 0.5 min, 0% B; 15 min, 20% B; 25 min, 22% B; 35 min, 30% B; 43 min, 50% B; 48 min, 50% B; 53 min, 0% B; 60 min, 0% B. For analysis of SAM, acetyl-CoA, and 5′-dA as shown in Figure 2a, solvent A was 50 mM sodium phosphate (pH 6.5), solvent B was 40% acetonitrile in water, and the following gradient was used: 0.0 min, 0% B; 5 min, 20% B; 15 min, 28% B; 19 min, 50% B; 22 min, 50% B; 26 min, 0% B; 30 min, 0% B.
ESI-LC/MS experiments were carried out at the School of Chemical Sciences Mass Spectrometry Laboratory of the University of Illinois with a Waters 2795 HPLC system and a Waters Q-TOF Ultima API Mass Spectrometer. The absorption was monitored by UV (220–310 nm), and the mass was monitored in positive mode. The same column and gradients described above were used for ESI-LC/MS. The solvents for ESI-LC/MS were 5 mM ammonium acetate (pH 5.3) (solvent A) and 40% acetonitrile in water (solvent B). The synthetic cm5U used for comparison via RP-HPLC and ESI-LC/MS was a generous gift from Y. Fu and C. He23.
Continuous-wave EPR experiment
EPR samples were prepared with 150 µM reconstituted _Min_Elp3, with or without 0.5 mM sodium dithionite, in Reconstitution Buffer (50 mM HEPES (pH 7.5), 150 mM KCl, 150 mM NaCl and 1% glycerol). CW-EPR experiments were performed on a Varian E-line 122 X-band spectrometer with an Air Products helium cryostat. Data acquisition parameters were: microwave frequency = 9.24 GHz, field center = 350.0 mT, field sweep = 100.0 mT, scan time = 60 s, modulation frequency = 100 kHz, modulation amplitude = 0.5 mT, time constant = 32 ms, temperature = 8 K, 16 scans for each spectrum.
Supplementary Material
Acknowledgments
The study was supported by US National Institute of Health (GM107533 to R.H.H.). We thank Y. Fu and C. He (University of Chicago) for providing us with the synthetic cm5U, F. Sun for assistance with ESI-LC/MS, J. Li and E. Oldfield for assistance with EPR and J. Imlay for the initial use of an anaerobic chamber.
Footnotes
Author contributions
R.H.H. conceived the project. K.S. and R.H.H. designed the experiments. K.S. expressed and purified proteins, in vitro transcribed and purified tRNA substrate and performed in vitro reconstitution. K.S. and R.H.H. performed RP-HPLC and ESI-LC/MS analyses. P.W. and J.S. assisted K.S. in the purification of Elp3 and tRNA. R.H.H. wrote the manuscript with input from other authors.
Competing financial interests
The authors declare no competing financial interests.
Additional information
Supplementary information is available in the online version of the paper.
References
- 1.Otero G, et al. Mol. Cell. 1999;3:109–118. doi: 10.1016/s1097-2765(00)80179-3. [DOI] [PubMed] [Google Scholar]
- 2.Krogan NJ, Greenblatt JF. Mol. Cell. Biol. 2001;21:8203–8212. doi: 10.1128/MCB.21.23.8203-8212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winkler GS, et al. J. Biol. Chem. 2001;276:32743–32749. doi: 10.1074/jbc.M105303200. [DOI] [PubMed] [Google Scholar]
- 4.Chinenov Y. Trends Biochem. Sci. 2002;27:115–117. doi: 10.1016/s0968-0004(02)02058-3. [DOI] [PubMed] [Google Scholar]
- 5.Sofia HJ, Chen G, Hetzler BG, Reyes-Spindola JF, Miller NE. Nucleic Acids Res. 2001;29:1097–1106. doi: 10.1093/nar/29.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wittschieben BO, et al. Mol. Cell. 1999;4:123–128. doi: 10.1016/s1097-2765(00)80194-x. [DOI] [PubMed] [Google Scholar]
- 7.Huang B, Johansson MJ, Bystrom AS. RNA. 2005;11:424–436. doi: 10.1261/rna.7247705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehlgarten C, et al. Mol. Microbiol. 2010;76:1082–1094. doi: 10.1111/j.1365-2958.2010.07163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Tuck S, Bystrom AS. PLoS Genet. 2009;5:e1000561. doi: 10.1371/journal.pgen.1000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh N, Lorbeck MT, Zervos A, Zimmerman J, Elefant F. J. Neurochem. 2010;115:493–504. doi: 10.1111/j.1471-4159.2010.06892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer F, et al. Cell Reports. 2012;1:424–433. doi: 10.1016/j.celrep.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moukadiri I, et al. Nucleic Acids Res. 2009;37:7177–7193. doi: 10.1093/nar/gkp762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCloskey JA, et al. Nucleic Acids Res. 2001;29:4699–4706. doi: 10.1093/nar/29.22.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Huang B, Eliasson M, Ryden P, Bystrom AS. PLoS Genet. 2011;7:e1002258. doi: 10.1371/journal.pgen.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grove TL, et al. Science. 2011;332:604–607. doi: 10.1126/science.1200877. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, et al. Nature. 2013;498:123–126. doi: 10.1038/nature12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pokholok DK, Hannett NM, Young RA. Mol. Cell. 2002;9:799–809. doi: 10.1016/s1097-2765(02)00502-6. [DOI] [PubMed] [Google Scholar]
- 18.Fichtner L, Frohloff F, Jablonowski D, Stark MJ, Schaffrath R. Mol. Microbiol. 2002;45:817–826. doi: 10.1046/j.1365-2958.2002.03055.x. [DOI] [PubMed] [Google Scholar]
- 19.Holmberg C, et al. J. Biol. Chem. 2002;277:31918–31928. doi: 10.1074/jbc.M200719200. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Lane WS, Reinberg D. Proc. Natl. Acad. Sci. USA. 2002;99:1241–1246. doi: 10.1073/pnas.251672198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ragab AE, Gruschow S, Tromans DR, Goss RJ. J. Am. Chem. Soc. 2011;133:15288–15291. doi: 10.1021/ja206163j. [DOI] [PubMed] [Google Scholar]
- 22.Broussard TC, Price AE, Laborde SM, Waldrop GL. Biochemistry. 2013;52:3346–3357. doi: 10.1021/bi4000707. [DOI] [PubMed] [Google Scholar]
- 23.Fu Y, et al. Angew. Chem. Int. Edn Engl. 2010;49:8885–8888. doi: 10.1002/anie.201001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.