Mitochondrial Protein Quality Control: The Mechanisms Guarding Mitochondrial Health (original) (raw)
Abstract
Significance: Mitochondria are complex dynamic organelles pivotal for cellular physiology and human health. Failure to maintain mitochondrial health leads to numerous maladies that include late-onset neurodegenerative diseases and cardiovascular disorders. Furthermore, a decline in mitochondrial health is prevalent with aging. A set of evolutionary conserved mechanisms known as mitochondrial quality control (MQC) is involved in recognition and correction of the mitochondrial proteome. Recent Advances: Here, we review current knowledge and latest developments in MQC. We particularly focus on the proteolytic aspect of MQC and its impact on health and aging. Critical Issues: While our knowledge about MQC is steadily growing, critical gaps remain in the mechanistic understanding of how MQC modules sense damage and preserve mitochondrial welfare, particularly in higher organisms. Future Directions: Delineating how coordinated action of the MQC modules orchestrates physiological responses on both organellar and cellular levels will further elucidate the current picture of MQC's role and function in health, cellular stress, and degenerative diseases. Antioxid. Redox Signal. 22, 977–994.
Introduction
Mitochondria are dynamic semiautonomous organelles present in virtually all eukaryotic cells and play fundamentally important roles in various aspects of cellular physiology. The vital functions of mitochondria include generation of ATP through respiration, integration of several key metabolic and cofactor-generating pathways, and regulation of ion homeostasis and apoptosis (141, 179). Perturbations of mitochondrial homeostasis and integrity lead to severe pathophysiological consequences and the onset of disease. Numerous studies implicate mitochondrial dysfunction as an underlying factor of multiple pathologies in humans, including cardiovascular disorders, myopathies, certain cancers, type II diabetes, and neurological and neurodegenerative diseases (23, 36, 50, 95, 104, 115, 130, 141, 149, 157, 179, 188). These maladies become particularly prevalent as people age and have been linked with age-associated decline in mitochondrial health (18, 95, 115, 157). Given the fundamental biological roles that mitochondria play in cellular physiology, it is crucial to sustain their welfare. To this end, several interdependent mechanisms exist, from the molecular to the organellar level, to ensure mitochondrial homeostasis. These conserved mitochondrial quality control (MQC) mechanisms maintain the mitochondrial proteome to promote the organelle's normal function and thus cellular survival (7, 18, 21, 95, 157, 175).
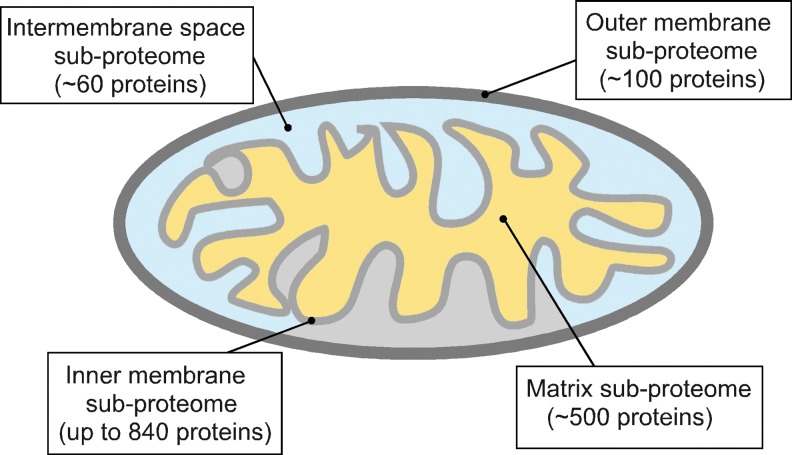
The endosymbiotic origin of mitochondria defines several distinctive hallmarks of these organelles. Mitochondria, whose proteome accounts for 900–1500 polypeptides (146, 165), retained two membranes, the outer mitochondrial membrane (OM) and the inner mitochondrial membrane (IM), respectively. These phospholipid bilayers segregate two mitochondrial compartments—the matrix and the intermembrane space (IMS) (Fig. 1). The large matrix compartment houses multiple metabolic enzymes and the mitochondrion's own small circular genome (mtDNA) and the machineries necessary for its replication and expression. A recent study showed that ∼500 proteins reside in the matrix of mammalian mitochondrion (156). The IMS compartment is smaller and contains fewer (∼60) proteins (82, 183). The large portion of mitochondrial proteins—including the electron transport chain and F1FO ATPase multiprotein complexes (collectively known as the oxidative phosphorylation [OXPHOS] system)—localizes to the IM. The IM proteome is particularly enriched in the specific invaginations of the membrane termed cristae. The OM subproteome is estimated to contain ∼100 polypeptides (198). Another hallmark of the mitochondrial proteome is its bigenomic nature. The vast majority of proteins comprising the mitochondrial proteome are encoded by nuclear genes, synthesized in the cytosol, and subsequently imported into the organelle, while only 13 of the ∼1500 mitochondrial proteins are derived from mtDNA (18, 147, 179), These unique properties of mitochondrial architecture impose several significant challenges to mitochondrial functions and necessitate constant monitoring by MQC (Fig. 2). One challenge stems from the inherent generation of reactive oxygen species (ROS) by the electron transport chain complexes of OXPHOS (2, 9, 61) (Fig. 2A). During this process, some electrons may leak from the electron transport chain and rapidly react with molecular oxygen. Various reports estimate that 0.3%–2% of the mitochondrial O2 consumption may be diverted toward ROS formation (2, 9, 61). Incomplete reduction of O2 by escaping electrons leads to the formation of highly reactive superoxide anion, which can further promote formation of other ROS and reactive nitrogen species (RNS) radicals (161). While free radical-scavenging mechanisms are in place (82), they are not always sufficient to eliminate harmful ROS/RNS that are highly damaging to nucleic acids, proteins, and lipids (61, 161). In addition, many ROS are now recognized as important signaling molecules essential for intracellular communication and stress response (161). Therefore, as exemplified by a number of failed trials aiming to correct mitochondrial damage by antioxidant treatment (30, 29), massive neutralization of ROS may be equally detrimental for cells.
FIG. 1.
Distribution of mitochondrial proteome throughout the organelle. The vast majority of mitochondrial proteins reside in the matrix and inner mitochondrial membrane (IM) subcompartments. The approximate numbers of polypeptides in each subproteome are calculated based on available data (82, 146, 156, 165, 185, 198). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
FIG. 2.
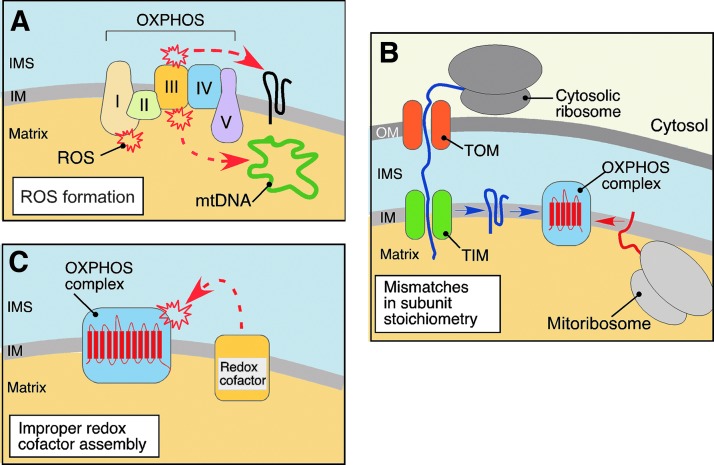
Biochemical stresses that challenge normal mitochondrial function. Mitochondrial respiration is inherently linked to reactive oxygen species (ROS) production due to incomplete reduction of molecular oxygen by electron transport components of the oxidative phosphorylation system (OXPHOS system) (A). Stalling the high-energy electrons at respiratory complexes I and III leads to generation of superoxide anion which—either directly or via subsequent ROS radicals—can damage biological molecules like mtDNA and propel additional damage. The biogenesis of OXPHOS complexes requires tight coordination between synthesis and assembly of the mitochondrial- and nuclear-coded proteins (B). Polypeptides derived from the nuclear genome are translated on cytosolic ribosomes and imported in an unfolded state into the mitochondrion via presequence translocases of the outer (TOM) and inner (TIM) membranes. Imported polypeptides are inserted into the IM where they are joined with mitochondria-synthesized subunits. Mismatches in subunit stoichiometry can lead to accumulation of unfolded or unassembled proteins that can affect functional integrity of mitochondria. In addition, the electron transport chain units of OXPHOS contain redox-active cofactors poised for rapid electron exchange reactions (C). When improperly assembled, these prosthetic groups can act as pro-oxidants through their inherent ability to generate ROS via Fenton-like reactions (61, 161). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The other challenge arises from the dual genetic origin of several OXPHOS complexes (complexes I, III, IV, and V) (Fig. 2B). Biogenesis of these respiratory units requires tightly coordinated expression, sorting and folding of both mtDNA- and nuclear-coded polypeptides, and their subsequent assembly into stoichiometric complexes within the IM (18, 128). If such coordination fails, unfolded or orphaned subunits that are prone to misfolding and/or aggregation may accumulate. Finally, the electron transport chain complexes contain multiple redox cofactors, which when improperly assembled or stalled in the assembly process, can act as pro-oxidants and further contribute to the challenging biochemical environment in the organelle (31, 99, 100, 132) (Fig. 2C). If unopposed, these challenges can distort mitochondrial protein homeostasis and propel progressive mitochondrial failure. The acute failure at one of the aforementioned risk sites may trigger a so-called vicious cycle—a series of deleterious events leading to the gradual increase of mitochondrial damage. For instance, accumulation of unassembled or misfolded polypeptides can impede biogenesis and/or function of the OXPHOS, which in turn will produce excessive ROS. These can further disrupt the mitochondrial proteome through induction of mutations in mtDNA and additional perturbation of protein folding, leading to more ROS and ROS-induced damage and ultimately, to the demise of the organelles (18, 57).
In this review, we summarize the current knowledge about the MQC mechanisms by which cells cope with biochemical stresses arising from the unique functional and organizational properties of mitochondria, and how these mechanisms sustain normal mitochondrial function and integrity. We will primarily focus on the proteolytic facet of MQC and outline recent advances and current concepts in the field.
Overview of Mitochondrial Protein Quality Control
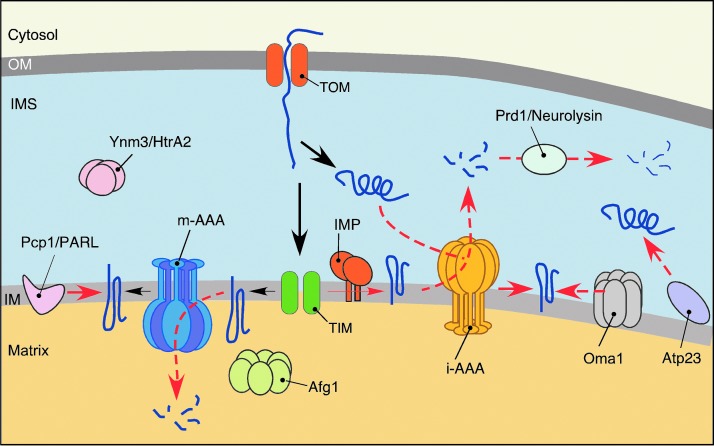
Because of inherent susceptibility of mitochondria to biochemical stresses, elaborate multilayer quality control mechanisms have evolved that survey, repair, or eliminate damaged mitochondria. Depending on the extent of damage, MQC mechanisms can engage at several levels (Fig. 3). The molecular level of MQC (referred hereafter as protein MQC [PMQC]) includes a network of evolutionary conserved mitochondrial proteases and chaperones distributed across mitochondrial compartments (Fig. 3A), as well as cytosolic proteolytic systems like the ubiquitin–proteasome system (UPS), which can associate with the OM (79, 96, 116, 175) (Fig. 3B). Although the number of studies addressing PMQC in mammalian cells is steadily growing, most of our current knowledge stems from studies in bacteria and the yeast model. The key molecules involved in PMQC are summarized in Table 1. One layer of PMQC is represented by ATP-dependent chaperones of mtHsp70 and Hsp60 heat-shock protein families, responsible for the sorting, folding, and disaggregation of proteins in the matrix compartment (136, 187). Similarly, the Hsp70-type and Hsp90-type chaperones operate in the cytosol and prevent aggregation and facilitate transport of unfolded newly synthesized or nascent polypeptides into mitochondria (60, 75, 197). The proteolytic facet of PMQC includes multiple conserved proteases (7, 18, 21, 157). The proteases are distributed across mitochondrial subcompartments and generally can be divided into two groups: (i) ATP-dependent, also known as AAA+ (ATPase associated with diverse cellular activities) proteases and (ii) ATP-independent proteolytic enzymes. The former group includes ClpXP and Lon/Pim1 proteases that reside in the matrix and degrade oxidatively damaged or aggregated polypeptides in the compartment (24, 25, 26, 34, 94, 77, 171, 181). Another two AAA+ proteases localize to the IM. The m-AAA (matrix AAA) protease has its active site exposed to the matrix side of the IM where it performs its quality control functions (11). The active site of i-AAA (intermembrane space AAA) peptidase faces the IMS (113, 190). These proteases appear to be important for removal of dysfunctional or orphaned proteins that are intrinsic to or associated with the IM (11, 105, 112, 113, 190). The AAA+ proteases typically exist as homo-oligomeric (Lon/Pim1, i-AAA) or hetero-oligomeric (m-AAA, ClpXP) complexes, and their proteolytic activity is coupled to ATP hydrolysis (13, 22, 159, 181). Such molecular architecture also permits chaperone-like functions of AAA+ proteases and appears to confer the ability to recognize misfolded or unassembled proteins and refold, and/or extract these polypeptides from phospholipid bilayers (159). The second group of proteases is more heterogeneous and includes the following: (i) processing peptidases (MPP, Oct1, Icp55, Cym1, and IMP) involved in sequential removal and/or degradation of mitochondrial targeting sequences (MTS) and thus proper biogenesis, sorting, and stabilization of matrix-and IM-targeted proteins (3, 73, 92, 131, 133, 176, 185); (ii) soluble peptidases (Prd1/Neurolysin, Atp23, and Ynm3/Omi) that seemingly contribute to MQC in the IMS (92, 143, 145, 180, 199); and (iii) IM-bound proteases (Pcp1/PARL, Oma1) implicated in the quality control of the IM proteome and regulation of mitochondrial dynamics (8, 19, 54, 58, 78, 83, 97, 127).
FIG. 3.
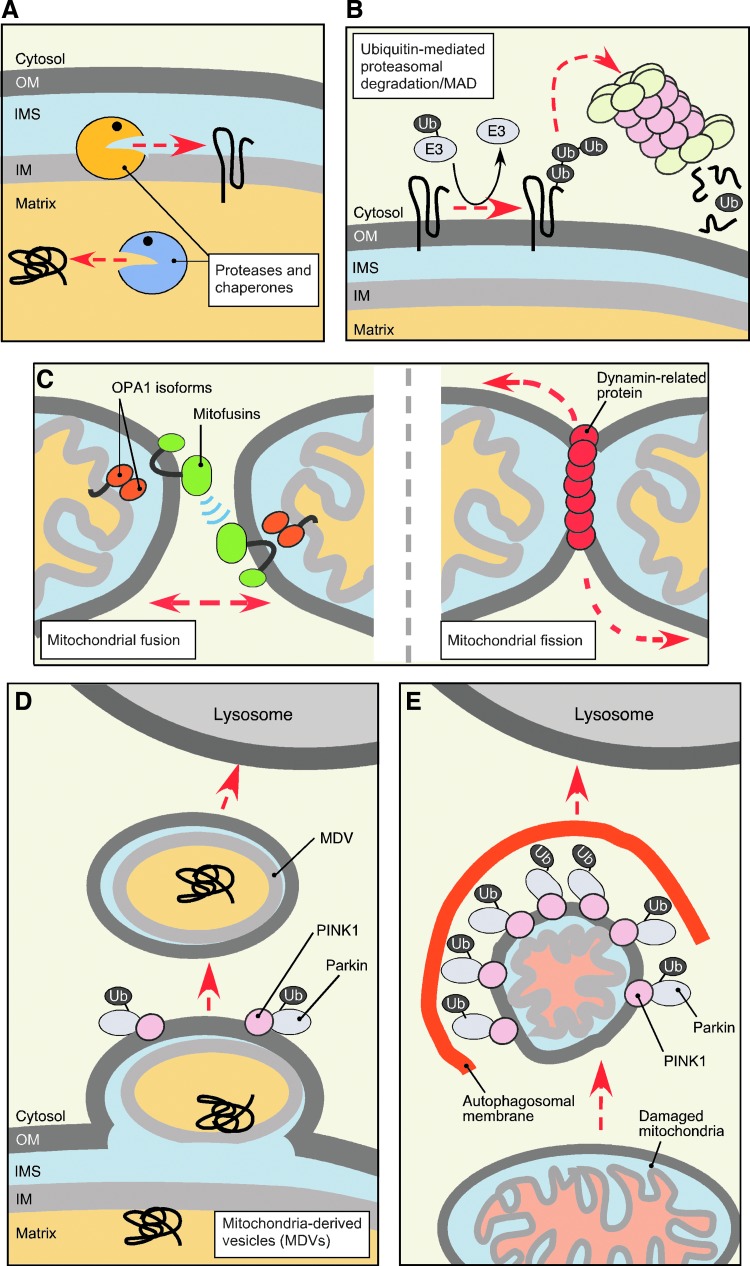
Branches of the mitochondrial quality control (MQC) system. Multiple interdependent mechanisms exist at both molecular and organellar/cellular levels to sustain mitochondrial health. Conserved mitochondrial proteases and chaperones distributed across mitochondrial compartments represent one layer of MQC (A). Removal of the proteins localized to the outer mitochondrial membrane (OM) and potentially other mitochondrial subproteomes, termed mitochondria-associated degradation (MAD), is mediated by the cytosolic ubiquitin–proteasome system (UPS) and assisted by several E3 ubiquitin ligases (B). At the organellar level, MQC is provided through mitochondrial fusion (left panel C) and fission (right panel C) events, necessary for exchange and mixing of mitochondrial content and thus damage dilution, and segregation of damaged mitochondria from the network, respectively. Mitochondrial fusion is mediated by conserved GTPases in the OM (Mitofusins/Fzo1) and the IM (long and short isoforms of OPA1/Mgm1). Another OM-associated GTPase–Dynamin-related protein/Dnm1 is a key mediator of mitochondrial fission. Mitochondria-derived vesicles (MDVs), destined for lysosome, appear to represent yet another facet of organellar MQC (D). This mechanism allows selective removal of fragments of mitochondria without affecting the entire organelle. Reportedly, the MDVs contain oxidized cargo and lipids and their formation in mammalian cells depends on the function of PINK1 kinase and E3 ubiquitin ligase Parkin (see text for details). When mitochondrial damage overwhelms the aforementioned mechanisms, failing organelles are segregated and targeted to autophagosomes, and subsequently to lysosomes where their content is degraded. The PINK1-Parkin functional tandem and UPS play important roles in the initiation of this process known as mitophagy (E). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Table 1.
Proteins Involved in Protein Mitochondrial Quality Control
| Protein or component | Role | Localization | Yeast | Mammals |
|---|---|---|---|---|
| ClpX | ATP-dependent specificity component of ClpXP complex | Matrix | Mcx1 | CLPX |
| ClpP | ATP-dependent protease component of ClpXP complex | Matrix | — | CLPP |
| Lon | ATP-dependent AAA+ serine protease | Matrix | Pim1 | LONP1 |
| MPP | Mitochondrial processing peptidase | Matrix | Mas1; Mas2 | βMPP; αMPP |
| MIP | Mitochondrial intermediate peptidase | Matrix | Oct1 | MIPEP |
| Icp55 | Intermediate cleaving peptidase | Matrix | Icp55 | XPNPEP3 |
| PreP | Presequence metallopeptidase | Matrix | Cym1 (Mop112) | PreP |
| mtHsp70 | Mitochondrial heat shock 70 kDa protein; molecular chaperone | Matrix | Ssc1 | HSPA9 (Grp75) |
| Hsp78 | Heat shock 78 kDa protein; ClpB-type AAA+ chaperone | Matrix | Hsp78 | — |
| Hsp60 | Heat shock 60 kDa protein; ATP-dependent chaperonin | Matrix | Hsp60 (Mna2) | HSPD1 |
| Hsp10 | Heat shock 10 kDa protein; Hsp60 cochaperonin | Matrix | Hsp10 (Cpn2) | HSPE1 |
| Afg1 | ATPase family gene 1; AAA+ protein | Matrix | Afg1 | LACE1 |
| m-AAA | Matrix-facing ATP-dependent AAA+ metalloprotease | IM | Yta10 (Afg3); Yta12 (Rca1) | AFG3L2; AFG3L1; SPG7/Paraplegin |
| i-AAA | IMS-facing ATP-dependent AAA+ metalloprotease | IM | Yme1 (Osd1) | YME1L |
| IMP | Intermediate mitochondrial protease | IM | Imp1; Imp2 | IMMP1L; IMMP2L |
| PARL | Presenilin-associated rhomboid-like serine protease | IM | Pcp1 (Rbd1) | PARL |
| Oma1 | Overlapping with m-AAA 1; ATP-independent metalloprotease | IM | Oma1 | OMA1 |
| Mgr1 | Mitochondrial genome required 1; likely adaptor for i-AAA protease | IM | Mgr1 | — |
| Mgr3 | Mitochondrial genome required 3; likely adaptor for i-AAA protease | IM | Mgr3 | C10orf118 |
| OPA1 | Optic atrophy 1; dynamin-related GTPase | IM | Mgm1 | OPA1 |
| Prd1 | Proteinase yscD 1; zinc metallopeptidase | IMS | Prd1 | Neurolysin |
| Atp23 | ATP synthase 23; ATP-independent metalloprotease | IMS | Atp23 | XRCC6BP1 |
| HtrA2 | High-temperature required A2; serine protease | IMS | Ynm3 | HtrA2 (Omi) |
| PINK1 | Phosphate and tensin homolog-induced putative kinase 1 | IM/OM | — | PINK1 |
| Msp1 | Mitochondrial sorting of proteins 1; AAA+ protein | OM | Msp1 | ATAD1 |
| MFN | Mitofusin; transmembrane GTPase | OM | Fzo1 | Mfn1; Mfn2 |
| Mdm30 | Mitochondrial distribution and morphology 30; ubiquitin ligase | OM | Mdm30 | — |
| MARCH-V | Membrane-associated ring finger (C3HC4) 5; ubiquitin ligase | OM | — | MARCH5 (MITOL) |
| Parkin | Autosomal recessive Parkinson's disease 2; ubiquitin ligase | Cyto./OM | — | Parkin (PARK2) |
| MULAN | Mitochondrial ubiquitin ligase activator of NFKB1; ubiquitin ligase | Cyto./OM | — | MUL1 |
| Vms1 | VCP/Cdc48-associated mitochondrial stress responsive 1 | Mult. | Vms1 | VMS1 |
| VCP | Valosin-containing peptide; AAA+ protein | Mult. | Cdc48 | VCP (p97) |
| Ubiquitin | Small modifying protein; component of ubiquitin–proteasome system | Mult. | Ubi4 | UBC; UBD |
| DRP | Dynamin-related protein; GTPase | Mult. | Dnm1 | DRP1 |
| Hsp70 | Heat shock 70 kDa protein; molecular chaperone | Cyto. | Ssb1; Ssb2 | HSPA1A |
| Hsp90 | Heat shock 90 kDa protein; molecular chaperone | Cyto. | Hsc82; Hsp82 | HSP90AA1; AB1 |
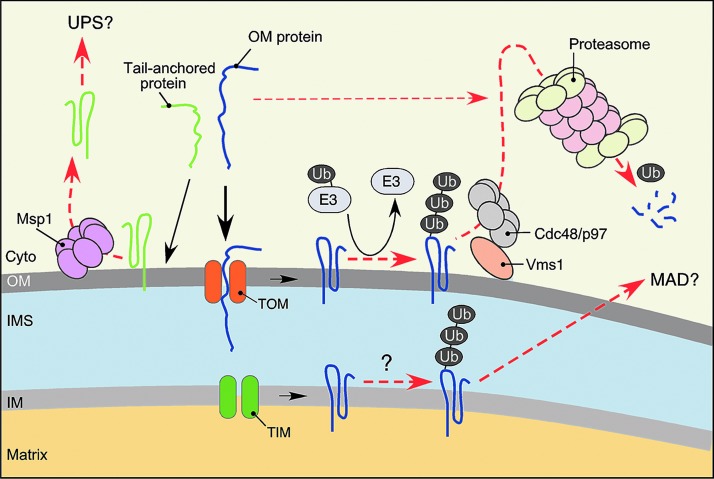
Growing evidence indicates that the cytosolic UPS also represents an important facet of PMQC. First, UPS has been shown to participate in quality control and removal of several mitochondria-targeted proteins before or during their import into the organelle (7, 35, 116). Moreover, UPS can access the OM subproteome and mediate retrotranslocation and degradation of OM resident proteins—a process termed mitochondria-associated degradation (MAD) (79, 96, 116). MAD apparently relies on p97/Cdc48 AAA+ protein (79, 193), which is also involved in a well-described extraction of ubiquitylated proteins from the endoplasmic reticulum (ER) (37, 88).
Several MQC mechanisms are available on the organellar level (Fig. 3C–E). Fusion and fission events (Fig. 3C) mediate organellar dynamics and facilitate mitochondrial biogenesis, and even redistribution of mtDNA and proteome throughout the mitochondrial network (39, 41, 64, 196). Such redistribution due to fusion of several mitochondria permits the dilution of damaged molecules and/or replenishment of depleted components in malfunctioning organelles (41, 196). A phenomenon known as stress-induced mitochondrial hyperfusion (164, 177) represents an example of coupling between mitochondrial fusion and cellular stress response and highlights the significance of mitochondrial dynamics in stress management. Upon homeostatic insults like oxidative stress or starvation, mitochondria in the stressed cells form highly interconnected networks thereby increasing content mixing, ATP production, and protecting mitochondria from autophagic removal (68, 138, 155, 164, 177). When transient stress protection via mitochondrial hyperfusion is not sufficient, damaged organelles are removed from the network through fragmentation (fission) events (178). Mitochondrial fission serves to increase the number of mitochondria in the cell before mitochondrial biogenesis or cellular division, as well as to segregate dysfunctional or depolarized mitochondria away from the healthy network (144, 178, 196). Once malfunctioning (e.g., severely depolarized) mitochondria have been segregated, the components of their OM subproteome that are involved in establishing contact/tethering sites with other mitochondria are ubiquitylated and proteolyzed by UPS to prevent their rejoining with healthy mitochondria (56, 66, 172, 189). Then, damaged organelles are removed via another facet of organellar MQC (Fig. 3E)—a mitochondria-specific type of autophagy known as mitophagy (see Refs. 119, 195 for detailed review). An acute overwhelming stress such as treatment with oxidants causes massive fragmentation of the mitochondrial network followed by initiation of apoptosis (194). Conversely, genetic inhibition of mitochondrial fission increases apoptotic resistance and cell survival (109, 154).
Finally, a novel MQC mechanism has recently been described by the McBride laboratory (Fig. 3D). Mitochondria-derived vesicles (MDVs), which carry selected oxidized cargo and deliver this cargo to lysosomes, have been reported to facilitate MQC (137, 170). The MDV route appears to function at both normal and oxidative stress conditions and is independent of mitochondrial dynamics and mitophagy (166, 170). Although the identity of the vesicle cargo has not been fully characterized, this mechanism offers a potential strategy to remove segments of mitochondrial membranes containing damaged, hard to dissociate protein complexes and/or reactive prosthetic groups such as heme, which cannot be catabolized within the mitochondrion.
Mitochondrial Subproteomes and Their Regulation by PMQC
Matrix subproteome
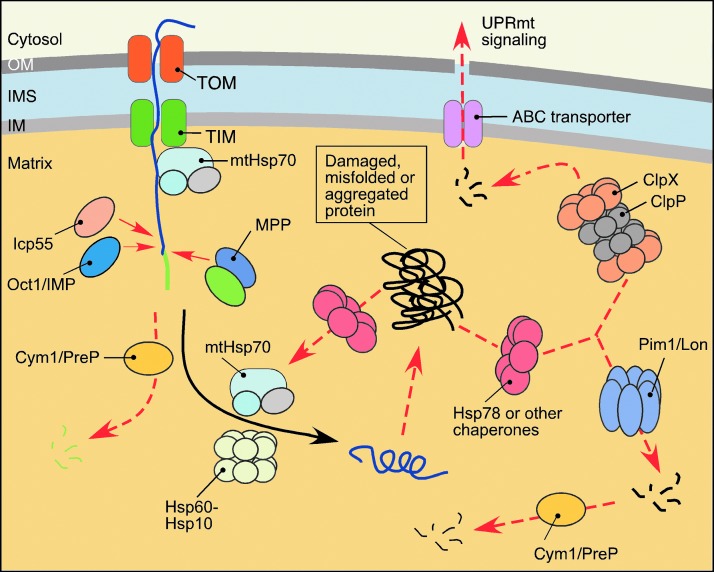
Virtually all proteins of the dense matrix subproteome are synthesized in the cytosol and imported into the compartment as precursors in an unfolded state (136). Proper maturation and folding of these proteins in the matrix are facilitated by several PMQC factors (Fig. 4). First, mtHsp70 and its J-type cochaperones participate in the import of precursor proteins through the TIM23 IM translocase complex and later, in conjunction with the Hsp60-Hsp10 chaperone system, they promote folding of the imported polypeptides (136, 187). Second, proteolytic removal of the N-terminal MTS is mediated through the action of the two-subunit MPP processing metallopeptidase complex (73, 131, 176). Some proteins undergo an additional processing by the mitochondrial intermediate peptidase (MIP/Oct1), which removes additional residues following the MTS (131, 176). Recent studies identified an additional intermediate cleaving aminopeptidase Icp55 that stabilizes multiple matrix proteins via the removal of a single, potentially destabilizing N-terminal amino acid residue following the MTS (131, 133, 185). Interestingly, Oct1 processing also appears to contribute to such stabilization (184). This situation resembles the N-end rule protein stabilization pathway described for the cytoplasm (173); however, the downstream protease(s) degrading destabilized polypeptides remain(s) to be identified.
FIG. 4.
PMQC in the matrix. Multiple proteases and molecular chaperones regulate the matrix subproteome. The regulation involves control of protein maturation and accumulation and degradation of poly- and oligopeptides. Proper maturation of the precursor proteins transported via the TIM23 translocase complex requires removal of mitochondrial targeting sequence by MPP processing metallopeptidase complex and, in certain cases, additional stabilizing processing by intermediate peptidases MIP/Oct1 and Icp55. Resulting free targeting peptides, as well as other small oligopeptides, are removed by mitochondrial presequence peptidase Cym1/PreP. Subsequent protein folding is facilitated by Hsp family chaperones. Stress-damaged, misfolded, and/or aggregated proteins are recognized and cleaved by AAA+ proteases Lon/Pim1 and ClpXP. Peptides produced by these proteolytic events are either subjected to additional processing by oligopeptidases or extruded through ATP-binding cassette (ABC)-type transporters into the cytosol where they activate mitochondrial unfolded protein response (UPRmt). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The mitochondrial presequence peptidase, Cym1/PreP, also appears to contribute to the matrix MQC. Originally misidentified as an IMS-localized enzyme, this conserved metallopeptidase has been implicated in the clearance of free targeting peptides generated by MPP and MIP, as well as small (up to 65 amino acid residues), unstructured oligopeptides (92, 176), which upon accumulation may impair mitochondrial integrity (85, 139). Also, studies on mammalian PreP showed that the peptidase is required to prevent mitochondrial accumulation of the amyloid-β (Aβ) peptide, which upon accumulation can cause mitochondrial dysfunction (59).
Finally, two conserved AAA+ serine proteases, Lon/Pim1 and ClpXP, are found in the mitochondrial matrix (94, 181) (Fig. 4). The Lon/Pim1 protease exists as a large homo-oligomeric complex with each subunit containing both ATPase and serine protease motifs (167, 181). It preferentially targets heat-damaged or oxidatively damaged proteins (24, 25, 26, 34). While protein misfolding and/or loss of prosthetic groups appear to contribute to substrate recognition by mitochondrial Lon (72, 121, 186), the exact recognition determinants remain to be identified. Studies in yeast indicate that Lon/Pim1 cooperates with ClpB-type AAA+ chaperone Hsp78 (24, 186) and mtHsp70 (160) to accelerate disaggregation/degradation of aggregated protein. The significance of this cooperation in higher eukaryotes remains unclear, as metazoans lack an apparent Hsp78 ortholog. In addition, Lon plays an important role in regulation of stability and expression of mtDNA via proteolytic control of the abundance of mitochondrial transcription factor TFAM (117, 124).
Unlike Lon, the ClpXP protease is a hetero-oligomeric complex consisting of two stacked ClpP serine protease heptamers topped with two hexameric rings formed by AAA+ ClpX subunits (22, 94). Reportedly, the ClpX component participates in recognition of misfolded polypeptides and channels them into a proteolytic chamber formed by the ClpP subunits (12, 22). Although multiple studies implicate bacterial ClpXP as a protein quality control protease (22), its exact role within the mitochondrion remains elusive. Of particular interest is the recently postulated function of ClpXP in the mitochondrial unfolded protein response (UPRmt) in nematodes (76, 77). Peptides generated via ClpXP-mediated proteolysis of unfolded proteins in the matrix are extruded from mitochondria into the cytosol, whereby they trigger a specific transcriptional response that promotes expression and synthesis of nuclear-borne mitochondrial chaperones and proteases to restore mitochondrial proteostasis (76). This mechanism serves to sense and correct imbalances between the proteins of nuclear and mitochondrial origin, particularly subunits of the OXPHOS complexes. Recently, a Lon-regulated facet of UPRmt was reported in a roundworm model. The bZip transcriptional factor ATFS-1 required for UPRmt signaling is destined to the mitochondrial matrix where Lon degrades it; however, under stress conditions, ATFS-1 is stabilized and trafficked to the nucleus where it initiates the transcriptional response (76, 135). Although UPRmt is conserved among worms, mice, and humans (191), it remains to be determined if stress-sensing and signaling mechanisms in mammals are identical to the ones described for nematodes.
Inner membrane subproteome
The mitochondrial IM is among the most proteinaceous biological membranes and houses a significant portion of mitochondrial proteome, including the OXPHOS complexes. Assembly and function of the reactive respiratory complexes create major challenges for mitochondrial protein homeostasis (Fig. 2). Several mechanisms are in place to assure normal biogenesis and maintenance of the proteins residing in the IM. First, a large number of dedicated chaperones and chaperone-like assembly factors assist and regulate biogenesis and maintenance of the respiratory complexes (63, 125, 128). The second set of mechanisms controlling IM proteostasis involves proteolytic enzymes (Fig. 5). The two-subunit, IM-bound IMP proteolytic complex—similar to Oct1 and Icp55 peptidases—was shown to stabilize its substrate proteins (131). For instance, processing of the Mgr2 subunit of TIM23 translocase by IMP stabilizes Mgr2 and promotes TIM23 assembly (86).
FIG. 5.
PMQC in the IM and intermembrane space (IMS). Complexity of mitochondrial IM anticipates vastly efficient systems to maintain protein homeostasis. These include two tightly coordinated proteases matrix-facing AAA metalloprotease (m-AAA) and intermembrane space-facing AAA metalloprotease (i-AAA), which along with other regulatory functions recognize excessive, misassembled, and damaged subunits of OXPHOS complexes associated with the IM. Another IM protease complex Oma1, with m-AAA-overlapping functions, is also proposed to play a major role in mitochondrial dynamics and homeostasis upon stress conditions. Rhomboid-like Pcp1/PARL protease is implicated in the intramembrane proteolysis of several IM proteins in yeast, whereas in mammalian cells, it also contributes to regulation of mitochondrial turnover. The IMS PMQC is less studied. In addition to the i-AAA, which exerts both proteolytic and chaperone functions toward IMS-localized proteins, the IMS subproteome appears to be regulated by oligopeptidase Prd1/Neurolysin and serine protease Ymn3/HtrA2. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The membrane-bound m-AAA and i-AAA metalloprotease complexes—surveying the matrix and the IMS sides of IM, respectively—are two major factors that provide quality control of the IM subproteome (7, 18, 21, 112, 113). The importance of coordinated actions between the IM AAA proteases is highlighted by the observation that simultaneous loss of these molecules in yeast is lethal (111, 112). The m-AAA protease is a large hetero-hexameric complex typically formed by Yta10/AFG3L2 and Yta12/SPG7/Paraplegin subunits in yeast and humans (11, 103). An additional rodent-specific subunit AFG3L1 that can substitute for AFG3L2 has been described (107). Also, studies on mammalian m-AAAs showed that other forms of the enzyme consisting of AFG3L2 subunits only (in humans) or AFGL32 and AFG3L1 (in rodents) do exist (103). The cryo-EM reconstruction of the yeast m-AAA revealed that the protease complex is ring shaped and contains a central pore, which may be restricted to unfolded polypeptide segments (108). This finding provides insights into the substrate recognition by mitochondrial AAA metallopeptidases, however, the detailed mechanism is yet to be determined. The molecular architecture of the i-AAA protease is similar to one of the m-AAA complex, except that the former proteolytic machine is always a homo-oligomer formed by six copies of the Yme1/YME1L peptidase (69, 70). Known substrates of the AAA metalloproteases include surplus, misassembled, and/or damaged subunits of the OXPHOS complexes that are intrinsic or peripheral to the IM (10, 45, 84, 105, 112, 113, 134, 148, 168, 190). In addition, via its involvement in maturation of the nuclear-borne constituent of the large mitoribosomal subunit MrpL32, the m-AAA is critical for synthesis of mtDNA-coded polypeptides (33, 140). The i-AAA complex is an important regulator of the IM's functional integrity and dynamics via several proteolytic events. One such event is constituent regulatory proteolysis of the PRELI protein family members Ups1 and Ups2 involved in transport, synthesis, and accumulation of mitochondrial phospholipids (46, 152). Second, the i-AAA is involved in biogenesis of the short isoform of the optic atrophy 1 (OPA1) dynamin-related GTPase, which is central to mitochondrial dynamics and mtDNA maintenance (8, 71). The versatility of the IM AAA proteases is impressive and remains to be understood. A possible explanation is provided by cooperation of these proteolytic machines with other proteases and adaptor-like proteins. For instance, the conserved IM-associated peptidase Atp23 involved in maturation of the respiratory complex V (143, 199) reportedly cooperates with the Yme1 proteolytic complex to degrade Ups1 (152). Likewise, two adaptor-like proteins Mgr1 and Mgr3 have been shown to facilitate degradation of model substrates by the i-AAA in yeast (51, 52). The evolutionary conservation of Mgr3 suggests that the YME1L complex may also operate in a similar manner. Similarly, overexpression of the matrix AAA+ protein Afg1/LACE1 (1, 110) has been shown to facilitate degradation of several m-AAA protease substrates in a series of respiratory-deficient yeast mutants (99). However, it remains to be determined if Afg1 is a bona fide adaptor/cooperating partner of the m-AAA complex. In addition, the m-AAA protease exerts overlapping activity with the ATP-independent membrane-bound metallopeptidase Oma1 complex (97, 100). Studies by our group established that Oma1 per se is also an important stress-activated protease required for cell survival (32). In mammalian cells, Oma1 mediates rapid proteolytic processing of the long membrane-anchored form of OPA1 (L-OPA1), thereby promoting IM fragmentation and subsequent cellular MQC actions in response to homeostatic insults (8, 19, 200). Conversely, the mitochondrial network remains intact or even hyperfused in stressed Oma1-deficient mouse embryonic fibroblasts (19, 152). While the exact mechanism of stress sensing by Oma1 only begins to emerge (19, 32, 120, 200), it is clear that Oma1 activation is central to the regulation of IM proteostasis and dynamics upon various stresses and/or pathological states.
Pcp1/PARL is yet another proteolytic module intrinsic to the IM. It belongs to the rhomboid family of serine proteases and mediates intramembrane cleavage of several IM polypeptides in yeast, including cytochrome c peroxidase Ccp1 (processed in conjunction with m-AAA protease) (174) and yeast OPA1 ortholog Mgm1 (81). Interestingly, the latter polypeptide does not appear to be an Oma1 substrate either under normal or under stress conditions (53, 114), thereby suggesting partial evolutionary repurposing of Oma1 function. Reciprocally, PARL does not seem to cleave OPA1 in vivo (53). Instead, recent studies have implicated mammalian PARL in constituent proteolytic removal of the phosphate and tensin homolog-induced putative kinase 1 (PINK1) —a crucial regulator of mitophagic (whole mitochondria) and nonmitophagic (respiratory chain complexes) mitochondrial turnover (48, 89, 182, 189). PINK1 is translated in the cytosol and imported into the mitochondria. Under normal conditions, the protein is sorted to the IM where it is degraded in a PARL-dependent manner (48). This pathway is blocked in severely depolarized or ATP-depleted mitochondria, whereby PINK1 is stabilized and accumulates on the OM subsequently recruiting and activating E3 ubiquitin ligase Parkin/PARK2, which in turn triggers segregation and mitophagic removal of malfunctioning mitochondria (87, 93, 106, 119, 195). Interestingly, a recent study demonstrated the important role of the PINK1-Parkin pathway in the formation of MDVs under mitochondrial stress conditions (126).
IMS subproteome
Relatively little is known about the IMS MQC (Fig. 5). Recent studies identified the i-AAA protease as an important regulator of the IMS subproteome through its involvement in the folding and degradation of unassembled and/or misfolded small TIM proteins (20, 162). Of note, small TIM proteins themselves have been postulated to chaperone several folding reactions in the IMS (82). Similarly to Cym1/PreP, the IMS-localized oligopeptidase Prd1/Neurolysin appears to degrade cleaved presequences and small oligopeptides (92, 131, 176), thereby preventing their accumulation in the IMS. The high-temperature requirement A2 (HtrA2/Omi/Ynm3) serine protease is a highly conserved enzyme, whose functions and mechanism of action are understood primarily via studies on its bacterial orthologs—stress-inducible quality control peptidases DegP and DegS (44). HtrA2/Omi is a homotrimer and appears to be the only mitochondrial protease containing a PDZ-domain required for recognition of exposed hydrophobic stretches of misfolded proteins. The postulated modulation of Htr2A via PARL-assisted processing (40) and PINK1-mediated phosphorylation suggests its involvement in regulation of mitophagy (151). However, while studies in yeast and mammalian models indicated the role of Htr2A in thermotolerance (145), prevention of accumulation of aberrant respiratory chain subunits (123, 129), and even identified several substrates of the protease (91), its precise role in the IMS MQC awaits clarification.
Outer membrane subproteome
Recent findings provide several important insights into the PMQC mechanisms controlling OM proteostasis (Fig. 6). In addition to the aforementioned cytosolic Hsp70 and Hsp90 chaperones assuring proper delivery and likely the removal of nascent and/or newly synthesized unfolded polypeptides to be inserted into the OM (60, 122, 197), additional MQC modules have been characterized recently. The role of UPS in OM proteostasis becomes increasingly evident. Although it has been initially thought that the UPS may only intercept misfolded or damaged proteins en route to the mitochondrion (7, 35), numerous studies have suggested that mitochondria-localized polypeptides can be ubiquitylated and subsequently removed by the UPS in the process known as MAD (96, 116, 175). Consistently, several E3 ubiquitin ligases such as Mdm30, MITOL/MARCH-V, and MULAN (56) were found to associate with the cytosolic side of the OM. Likewise, the ubiquitin ligase Parkin is recruited to depolarized mitochondria (56, 66, 119, 172, 189, 195). Additional mitochondria-associated modifiers like SUMO ligases have also been described (56). Subsequently, multiple OM proteins have been identified as targets of these ligases and/or UPS (56, 66, 158). The discovery of VCP/p97/Cdc48-associated mitochondria stress responsive 1 (Vms1) protein in yeast that translocates from the cytosol to the mitochondrial OM upon stress and recruits the Cdc48-Npl4 complex, provided further mechanistic understanding of MAD (79, 80). The AAA+ protein VCP/p97/Cdc48 complex is involved in multiple cellular processes but plays a key role in the extraction of ubiquitylated ER- and OM-anchored proteins for proteasomal degradation (37, 88, 193). Reciprocally, loss of Vms1 results in accumulation of ubiquitylated OM proteins and mitochondrial damage (79). Vms1 apparently is not the only VCP/p97/Cdc48-Npl4 recruiting factor, as a fraction of the complex can still associate with dysfunctional mitochondria in the absence of Vms1 (55, 79). Therefore, additional UPS recruitment factors are likely to exist and remain to be identified. Another intriguing line of findings suggests the involvement of MAD in retrotranslocation and removal of several IM- and matrix-localized proteins (14, 15, 122). However, more work is needed to corroborate this postulate.
FIG. 6.
PMQC in the OM. In addition to interception of mitochondria-destined proteins en route, the UPS provides an additional level of OM PMQC. It removes misfolded, damaged, or surplus proteins in the OM via the MAD process. MAD involves ubiquitylation by E3 ubiquitin ligases that tag proteins to be degraded and extraction of the peptides by the AAA+ protein VCP/Cdc48/p97 complex, which is, in turn, recruited to the OM through several mechanisms, including targeting by stress-responsive factor Vms1 or PINK1-Parkin functional tandem. Several reports (80, 123, 158) suggest that some IM proteins might also be subject of MAD. Another AAA+ protein Msp1/ATAD1 targets and removes tail-anchored proteins mislocalized to the OM. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Recent studies identified yet another OM-associated AAA+ protein Msp1/ATAD1 required to prevent accumulation of mislocalized ER-destined tail-anchored proteins in the OM, thereby maintaining proper mitochondrial function and morphology (43, 142). It has been proposed that Msp1 represents a novel PMQC component involved in the extraction of mistargeted tail-anchored polypeptides. It remains to be determined if Msp1 constitutes an additional branch of MAD or is an independent facet of the OM MQC.
PMQC in Disease and Aging
Over time, a combination of increasing oxidative damage, failing protein homeostasis, and mitochondrial and organellar QC capacity can contribute to cellular aging and instability of mtDNA and mitochondrial proteome (27, 41). The resulting decline in mitochondrial health can primarily affect organs and tissues with high energetic demands and contribute to the onset of cardiovascular, neurodegenerative, and complex metabolic diseases like cancer.
Reduced activity/abundance of Lon/Pim1 and ClpP proteases has been demonstrated in cells from a patient with late-onset autosomal dominant hereditary spastic paraplegia (type SPG13) and aged rat hepatocytes (17, 74). In line with these findings, overexpression of Lon in the Podospora anserina fungal model of aging significantly extended the lifespan of the organism (118). Interestingly, the same effect is achieved through the depletion of P. anserina ClpP (62). The prospective role of Lon in cancer stems from its regulation by hypoxia-inducible factor HIF1α and Lon's role in hypoxia-induced remodeling of the respiratory chain (65, 153). Indeed, a recent study identified Lon as a prospective anticancer target (28).
The ability of the oligopeptidase PreP to prevent accumulation of mitochondria-targeted Aβ peptides (59) indicates its protective role against Alzheimer's disease (AD). Consistently, the activity but not abundance of PreP is attenuated in AD patients' brain mitochondria and in AD transgenic mouse models (4). The exact role of PreP in the onset of AD is yet to be determined.
Mutations in genes encoding the subunits of m-AAA protease are known with both familial and sporadic forms of autosomal recessive hereditary spastic paraplegia (Paraplegin) (38, 140), spinocerebellar ataxia type 28 (AFG3L2) (49), and spastic ataxia-neuropathy syndrome (150) in humans. Studies in mice also indicate that the AFG3L2 subunit of m-AAA is important for the survival of Purkinje cells (5) and anterograde mitochondrial transport in murine cortical neurons (102), thereby highlighting the role of failing m-AAA function in late-onset neurodegeneration. In addition, a recent report identified a variant of Paraplegin, which is linked to enhanced ROS generation and several clinical phenotypes, including type 2 diabetes mellitus and coronary artery disease (6).
Depletion of OMA1 in the mouse model reduces energy expenditure and specifically leads to obesity, hepatic steatosis, and altered thermogenesis/cold stress resistance (154). These phenotypes are likely due to defective L-OPA1 processing and inability to initiate fragmentation of the mitochondrial networks in response to metabolic and/or oxidative insults (154, 192). Also, a recent high-throughput sequencing study of patients with familial and sporadic forms of amyotrophic lateral sclerosis (ALS) identified several mutations in the conserved residues of OMA1, thus implicating OMA1 as a prospective ALS-causing gene (47).
The IMS protease HtrA2/Omi plays an important role in protecting neurons from degeneration (90, 91, 123) and has been associated with Parkinson's disease (PD) (98, 169). Likewise, a PD-associated mutation in PARL has been recently identified (163). Although in vitro studies highlight the importance of MAD for prevention of neurodegenerative pathologies such as PD, its physiological role in human health awaits further investigation. Two lines of evidence implicate MAD in mitochondrial disease. First, a murine knockin model of p97 mutations (associated with inclusion body myopathy and Paget's bone disease) presents with mitochondriopathy-like phenotypes (16). Second, the PINK1-Parkin system, which likely represents a facet of the MAD that links molecular and organellar MQC—is clearly associated with neurodegenerative processes. Loss-of-function mutations in Parkin have been described in juvenile PD patients and account for ∼50% of the familial cases of PD (101).
Concluding Remarks and Perspectives
The role of functionally intertwined multilayer MQC mechanisms in assuring mitochondrial health becomes increasingly evident. It is important to note that humans sustain mitochondrial damage not only from age-related decline in mitochondrial function but also from causes that can affect younger populations. For instance, aggressive anticancer or antiviral therapies may enhance the formation of malfunctioning mitochondria in nontarget cells in patients undergoing such treatments (42). Modulation of the activity of certain MQC components can therefore be a promising approach to enhance cellular health and lifespan (62, 118). Reciprocally, proteases like Lon can be prospective targets in anticancer therapies to promote the death of drug-treated malignant cells (28, 67).
A number of important unresolved questions regarding MQC persist. For instance, the mechanisms by which MQC components promptly recognize mitochondrial stress signals remain obscure. Likewise, it remains to be determined how MQC modules in different mitochondrial subcompartments coordinate their actions in response to homeostatic insults. The regulation of nuclear output and physiological responses via function/dysfunction of MQC modules is another exciting question. Answering these questions is likely to yield new insights into MQC in health and disease and may lead to novel or supplementary therapeutic and preventive approaches against mitochondria-related maladies.
Abbreviations Used
AAA+
ATPase associated with diverse cellular activities
ABC
ATP-binding cassette
AD
Alzheimer's disease
AFG
ATPase family gene
ALS
amyotrophic lateral sclerosis
ATAD1
ATPase family AAA domain containing 1
ATFS-1
activating transcription factor associated with stress 1
ATPase
adenosine triphosphate hydrolyzing enzyme
Aβ
amyloid-β peptide
bZip
basic leucine zipper domain
Cdc48
cell division cycle 48, AAA ATPase
cryo-EM
cryoelectron microscopy
Cym1
cytosolic metalloprotease, metallopeptidase of the intermembrane space
Cyto.
cytosol
ER
endoplasmic reticulum
GTPase
guanosine triphosphate hydrolyzing enzyme
Hsp
heat shock protein
HtrA2
high-temperature required A2 (also known as Omi)
i-AAA
intermembrane space-facing AAA metalloprotease
Icp55
intermediate cleaving peptidase 55
IM
inner mitochondrial membrane
IMP
intermediate mitochondrial protease
IMS
intermembrane space
LACE1
lactation elevated 1, AAA ATPase
Lon
long filaments forming, AAA protease
m-AAA
matrix-facing AAA metalloprotease
MAD
mitochondria-associated degradation
MARCH-V
membrane-associated ring finger (C3HC4) 5 ubiquitin ligase
Mdm30
mitochondrial distribution and morphology 30
MDVs
mitochondria-derived vesicles
Mgm1
mitochondrial genome maintenance 1, yeast homolog of OPA1
Mgr
mitochondrial genome required
MIP
mitochondrial intermediate peptidase
MITOL
mitochondrial ubiquitin ligase
MPP
mitochondrial processing peptidase
MQC
mitochondrial quality control
Msp1
mitochondrial sorting of proteins 1
mtDNA
mitochondrial DNA
mtHsp
mitochondrial heat shock protein
MTS
mitochondrial targeting sequence
MULAN
mitochondrial ubiquitin ligase activator of NFKB1
Mult.
multiple cellular locations
Npl4
nuclear protein localization 4, substrate-recruiting cofactor of Cdc48 complex
Oct1
octapeptidyl aminopeptidase 1
OM
outer mitochondrial membrane
Oma1
overlapping with m-AAA 1
OPA1
optic atrophy 1
OXPHOS
oxidative phosphorylation system
PARL
presenilin-associated rhomboid-like
Pcp1
processing of cytochrome c peroxidase 1
PD
Parkinson's disease
PDZ
postsynaptic density protein domain
Pim1
proteolysis in mitochondria 1, yeast homolog of Lon
PINK1
phosphate and tensin homolog-induced putative kinase 1
PMQC
protein mitochondrial quality control
Prd1
proteinase yscD homolog 1
PRELI
protein of relevant evolutionary and lymphoid interest
PreP
presequence peptidase
RNS
reactive nitrogen species
ROS
reactive oxygen species
SPG
spastic paraplegia
SUMO
small ubiquitin-like modifier
TFAM
mitochondrial transcription factor A
TIM
translocase of the inner membrane
TOM
translocase of the outer membrane
UPRmt
mitochondrial unfolded protein response
UPS
ubiquitin–proteasome system
Ups
unprocessed, mitochondrial phosphatidic acid transport protein
VCP
valosin-containing protein (or p97), mammalian homolog of Cdc48
Vms1
VCP/Cdc48-associated mitochondrial stress responsive 1
Yme1
yeast mitochondrial escape 1
Acknowledgments
We apologize to those authors whose work we were unable to cite due to space limitations. This work was supported by NIH grants P30GM103335 (to the Nebraska Redox Biology Center), 1R01 GM108975 (to O.K.), 5P20RR024485-02, and 8 P20 GM103542-02 Redox COBRE subproject award (to S.C.). We thank Drs. Dennis Winge and Rodrigo Franco for critical reading of the article.
References
- 1.Abrahams BS, Mak GM, Berry ML, Palmquist DL, Saionz JR, Tay A, Tan YH, Brenner S, Simpson EM, and Venkatesh B. Novel vertebrate genes and putative regulatory elements identified at kidney disease and NR2E1/fierce loci. Genomics 80: 45–53, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Adam-Vizi V. and Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci 27: 639–645, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Alikhani N, Berglund AK, Engmann T, Spanning E, Vogtle FN, Pavlov P, Meisinger C, Langer T, and Glaser E. Targeting capacity and conservation of PreP homologues localization in mitochondria of different species. J Mol Biol 410: 400–410, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Alikhani N, Guo L, Yan S, Du H, Pinho CM, Chen JX, Glaser E, and Yan SS. Decreased proteolytic activity of the mitochondrial amyloid-beta degrading enzyme, PreP peptidasome in Alzheimer's disease brain mitochondria. J Alzheimers Dis 27: 75–87, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almajan ER, Richter R, Paeger L, Martinelli P, Barth E, Decker T, Larsson NG, Kloppenburg P, Langer T, and Rugarli EI. AFG3L2 supports mitochondrial protein synthesis and Purkinje cell survival. J Clin Invest 122: 4048–4058, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almontashiri NA, Chen HH, Mailloux RJ, Tatsuta T, Teng AC, Mahmoud AB, Ho T, Stewart NA, Rippstein P, Harper ME, Roberts R, Willenborg C, Erdmann J, CARDIoGRAM Consortium, Pastore A, McBride HM, Langer T, and Stewart AF. SPG7 variant escapes phosphorylation-regulated processing by AFG3L2, elevates mitochondrial ROS and is associated with multiple clinical phenotypes. Cell Rep 7: 834–847, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Anand R, Langer T, and Baker MJ. Proteolytic control of mitochondrial function and morphogenesis. Biochim Biophys Acta 1833: 195–204, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Anand R, Wai T, Baker MJ, Kladt N, Schauss AC, Rugarli E, and Langer T. The i-AAA protease YME1L and OMA1 cleave OPA1 to balance mitochondrial fusion and fission. J Cell Biol 33: 578–593, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreyev AY, Kushnareva YE, and Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70: 200–214, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Arlt H, Steglich G, Perryman R, Guiard B, Neupert W, and Langer T. The formation of respiratory chain complexes in mitochondria is under the proteolytic control of the m-AAA protease. EMBO J 17: 4837–4847, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arlt H, Tauer R, Feldmann H, Neupert W, and Langer T. The Yta10-12 complex, an AAA protease with chaperone-like activity in the inner membrane of mitochondria. Cell 85: 875–885, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Aubin-Tam ME, Olivares AO, Sauer RT, Baker TA, and Lang MJ. Single-molecule protein unfolding and translocation by an ATP-fueled proteolytic machine. Cell 145: 257–267, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augustin S, Gerdes F, Lee S, Tsai FT, Langer T, and Tatsuta T. An intersubunit signaling network coordinates ATP hydrolysis by m-AAA proteases. Mol Cell 35: 574–585, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azzu V. and Brand MD. Degradation of an intramitochondrial protein by the cytosolic proteasome. J Cell Sci 123: 578–585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azzu V, Mookerjee SA, and Brand MD. Rapid turnover of mitochondrial uncoupling protein 3. Biochem J 426: 13–17, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badadani M, Nalbandian A, Watts GD, Vesa J, Kitazawa M, Su H, Tanaja J, Dec E, Wallace DC, Mukherjee J, Caiozzo V, Warman M, and Kimonis VE. VCP associated inclusion body myopathy and paget disease of bone knock-in mouse model exhibits tissue pathology typical of human disease. PLoS One 5: e13183, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakala H, Delaval E, Hamelin M, Bismuth J, Borot-Laloi C, Corman B, and Friguet B. Changes in rat liver mitochondria with aging: lon protease-like activity and Ne-caboxymethyllysine accumulation in the matrix. Eur J Biochem 270: 2295–2302, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Baker BM. and Haynes CM. Mitochondrial protein quality control during biogenesis and aging. Trends Biol Sci 36: 254–261, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Baker MJ, Lampe P, Stojanovski D, Korwitz A, Anand R, Tatsuta T, and Langer T. Stress-induced OMA1 activation and autocatalytic turnover regulates OPA1-dependent mitochondrial dynamics. EMBO J 33: 578–593, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker MJ, Mooga VP, Guiard B, Langer T, Ryan MT, and Stojanovski D. Impaired folding of the mitochondrial small TIM chaperones induces clearance by the i-AAA protease. J Mol Biol Cell 424: 227–239, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Baker MJ, Tatsuta T, and Langer T. Quality control of mitochondrial proteostasis. Cold Spring Harb Perspect Biol 3: a007559, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker TA. and Sauer RT. ClpXP, an ATP-powered unfolding and protein degradation machine. Biochim Biophys Acta 1823: 15–28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med 38: 1278–1295, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Bateman JM, Iacovino M, Perlman PS, and Butow RA. Miochondrial DNA instability mutants of the bifunctional protein Ilv5p have altered organization in mitochondria and are targeted for degradation by Hsp78 and the Pim1p protease. J Biol Chem 277: 47946–47953, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Bayot A, Gareil M, Rogowska-Wrzesinska A, Roepstorff P, Friguet B, and Bulteau AL. Identification of novel oxidized protein substrates and physiological partners of the mitochondrial ATP-dependent Lon-like protease Pim1. J Biol Chem 285: 11445–11457, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bender T, Leidhold C, Ruppert T, Franken S, and Voos W. The role of protein quality control in mitochondrial protein homeostasis under oxidative stress. Proteomics 10: 1426–1443, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Ben-Zvi A, Miller EA, and Morimoto RI. Collapse of protostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci U S A 106: 14914–14919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernstein SH, Venkatesh S, Li M, Lee J, Lu B, Hilchey SP, Morse KM, Metcalfe HM, Skalska J, Andreeff M, Brookes PS, and Suzuki CK. The mitochondrial ATP-dependent Lon protease: a novel target in lymphoma death mediated by the synthetic triterpenoid CDDO and its derivatives. Blood 119: 3321–3329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, and Gluud C. Antioxidants supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev 3: CD007176, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, and Gluud C. Mortality in randomized trials of antioxidants supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 297: 842–857, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Bode M, Longen S, Morgan B, Peleh V, Dick TP, Bihlmaier K, and Herrmann JM. Inaccurately assembled cytochrome c oxidase can lead to oxidative stress-induced growth arrest. Antioxid Redox Signal 18: 1597–1612, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohovych I, Donaldson G, Christianson S, Zahayko N, and Khalimonchuk O. Stress-triggered activation of the metalloprotease Oma1 involves its C-terminal region and is important for mitochondrial stress protection in yeast. J Biol Chem 289: 13259–13272, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonn F, Tatsuta T, Petrungaro C, Riemer J, and Langer T. Presequence-dependent folding ensures MrpL32 processing by the m-AAA protease in mitochondria. EMBO J 30: 2545–2556, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bota DA. and Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol 4: 674–680, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Bragoszewski P, Gornicka A, Sztolsztener M, and Chacinska A. The ubiquitin-proteasome system regulates mitochondrial intermembrane space proteins. Mol Cell Biol 33: 2136–2148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandon M, Baldi P, and Wallace DC. Mitochondrial mutations in cancer. Oncogene 25: 4647–4662, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Brodsky JL. Cleaning up: ER-associated degradation to the rescue. Cell 151: 1163–1167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casari G, De-Fusco M, Ciarmatori S, Zeviani M, Mora M, Fernandez P, DeMichele G, Filla A, Cocozza S, Marconi R, Durr A, Fontaine B, and Ballabio A. Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial protease. Cell 93: 973–983, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet 46: 265–287, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Chao JR, Parganas E, Boyd K, Hong CY, Opferman JT, and Ihle JN. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nature 452: 98–102, 2008. 18288109 [Google Scholar]
- 41.Chen H, Vermlust M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, and Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141: 280–289, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Jungsuwadee P, Vore M, Butterfield DA, and St. Clair DK. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv 7: 147–156, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Chen YC, Umanah GK, Dephoure N, Andrabi SA, Gygi SP, Dawson TM, Dawson VL, and Rutter J. Msp1/ATAD maintains mitochondrial function by facilitating the degradation of mislocalized tail-anchored proteins. EMBO J 33: 758–767, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clausen T, Kaiser M, Huber R, and Ehrmann M. HTRA proteases: regulated proteolysis in protein quality control. Nat Rev Mol Cell Biol 12: 152–162, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Claypool SM, Whited K, Srijumnong S, Han X, and Koehler CM. Barth syndrome mutations that cause tafazzin complex lability. J Cell Biol 192: 447–462, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connerth M, Tatsuta T, Haag M, Klecker T, Westermann B, and Langer T. Intramitochondrial transport of phosphatidic acid in yeast by a lipid transfer protein. Science 338: 815–818, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Daoud H, Valdmanis PN, Gros-Louis F, Belzil V, Spiegelman D, Henrion E, Diallo O, Desjarlais A, Gauthier J, Camu W, Dion PA, and Rouleau G. Resequencing of 29 candidate genes in patients with familial and sporadic amyotrophic lateral sclerosis. Arch Neurol 68: 587–593, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Deas E, Plun-Favreau H, Gandhi S, Desmond H, Kjaer S, Loh SH, Renton AE, Harvey RJ, Whitworth AJ, Martins LM, Abramov AY, and Wood NW. PINK1 cleavage at position A103 by the mitochondrial protease PARL. Hum Mol Genet 20: 867–879, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Bella D, Lazzaro F, Brusco A, Plumari M, Battaglia G, Pastore A, Finardi A, Cagnoli C, Tempia F, Frontali M, Veneziano L, Sacco T, Boda E, Brussino A, Bonn F, Castellotti B, Baratta S, Mariotti C, Gellera C, Fracasso V, Magri S, Langer T, Plevani P, Di Donato S, Mauzi-Falconi M, and Taroni F. Mutations in the mitochondrial protease gene AFG3L2 cause dominant hereditary ataxia SCA28. Nat Genet 42: 313–321, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Di Mauro S, Garone C, and Naini A. Metabolic myopathies. Curr Rheumatol Rep 12: 386–393, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Dunn CD, Lee MS, Spencer FA, and Jensen RE. A genome-wide screen for petite-negative yeast strains yields a new subunit of the i-AAA protease complex. Mol Biol Cell 17: 213–226, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunn CD, Tamura Y, Sesaki H, and Jensen RE. Mgr3p and Mgr1p are adaptors for the mitochondrial i-AAA protease complex. Mol Biol Cell 19: 5387–5397, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duvezin-Caubet S, Koppen M, Wagener J, Zick M, Israel L, Bernacchia A, Jagasia R, Rugarli EI, Imhof A, Neupert W, Langer T, and Reichert AS. OPA1 processing reconstituted in yeast depends on the subunit composition of the m-AAA protease in mitochondria. Mol Biol Cell 18: 3582–3590, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, and Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol 187: 1023–1036, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esaki M. and Ogura T. Cdc48p/p97-mediated regulation of mitochondrial morphology is Vms1p-independent. J Struct Biol 179: 112–120, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Escobar-Henriques M. and Langer T. Dynamic survey of mitochondria by ubiquitin. EMBO Rep 15: 231–243, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Esposito LA, Melov S, Panov A, Cottrell BA, and Wallace D. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci U S A 96: 4820–4825, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Esser K, Tursun B, Ingenhoven M, Michaelis G, and Pratje E. A novel two-step mechanism for removal of a mitochondrial signal sequence involves the mAAA complex and the putative rhomboid protease Pcp1. J Mol Biol 323: 835–843, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Falkevall A, Alikhani N, Bhushan S, Pavlov PF, Busch K, Johnson KA, Eneqvist T, Tjernberg L, Ankacrona M, and Glaser E. Degradation of the amyloid beta-protein by the novel mitochondrial peptidasome PreP. J Biol Chem 281: 29096–29104, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Fan AC, Bhangoo MK, and Young JC. Hsp90 functions in the targeting and outer membrane translocation steps of Tom70-mediated mitochondrial import. J Biol Chem 281: 33313–33324, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Figueira TR, Barros MH, Camargo AA, Castilho RF, Ferreira JC, Kowaltowski AJ, Sluse FE, Souza-Pinto NC, and Vercesi AE. Mitochondria as a source of reactive oxygen and nitrogen species: from molecular mechanisms to human health. Antioxid Redox Signal 18: 2029–2074, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Fischer F, Weil A, Hamann A, and Osiewacz HD. Human CLPP reverts the longevity of a fungal ClpP deletion strain. Nat Commun 4: 1397, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fox TD. Mitochondrial protein synthesis, import and assembly. Genetics 192: 1203–1234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedman JR. and Nunnari J. Mitochondrial form and function. Nature 505: 335–343, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukuda R, Zhang H, Kim J, Shimoda L, Dang CV, and Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129: 111–122, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, and Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/Parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 19: 4861–4870, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goard CA. and Schimmer AD. Mitochondrial matrix proteases as novel therapeutic targets in malignancy. Oncogene 33: 2690–2699, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Gomes LC, Di Benedetto G, and Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13: 589–598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Graef M. and Langer T. Substrate specific consequences of central pore mutations in the i-AAA protease Yme1 on substrate engagement. J Mol Biol 156: 101–108, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Graef M, Seewald G, and Langer T. Substrate recognition by AAA+ ATPases: distinct substrate binding modes in ATP-dependent protease Yme1 of the mitochondrial intermembrane space. Mol Cell Biol 27: 2476–2485, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Griparic L, Kanazawa T, and van der Bliek A. Regulation of the dynamin-like mitochondrila protein Opa1 by proteolytic cleavage. J Cell Biol 178: 757–764, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gur E. and Sauer RT. Recognition of misfolded proteins by Lon, a AAA(+) protease. Genes Dev 22: 2267–2277, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Halwitschek G, Schneider H, Schmidt B, Tropschug M, Hartl FU, and Neupert W. Mitochondrial protein import: identification of processing peptidase and of PEP, a processing enhancing protein. Cell 53: 795–806, 1988 [DOI] [PubMed] [Google Scholar]
- 74.Hansen J, Corydon TJ, Palmfeldt J, Durr A, Fontaine B, Nielsen MN, Christensen JH, Gregersen N, and Bross P. Decreased expression of the mitochondrial matrix proteases Lon and ClpP in cells from a patient with hereditary spastic paraplegia (SPG13). Neuroscience 153: 474–482, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Hartl FU, Bracher A, and Hayer-Hartl M. Chaperone-protease networks in protein folding and homeostasis. Nature 475: 324–332, 2011 [DOI] [PubMed] [Google Scholar]
- 76.Haynes CM, Fiorese CJ, and Lin YF. Evaluating and responding to mitochondrial dysfunction: the mitochondrial unfolded protein response and beyond. Trends Cell Biol 23: 311–318, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haynes CM, Yang Y, Blais SP, Neubert TA, and Ron D. The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol Cell 37: 529–540, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Head B, Griparic L, Gandre-Babbe S, and van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol 187: 959–966, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heo JM, Livnat-Levanon N, Taylor EB, Jones KT, Dephoure N, Ring J, Xie J, Brodsky JL, Madeo F, Gygi SP, Ashrafi K, Glickman MH, and Rutter J. A stress-responsive system for mitochondria protein degradation. Mol Cell 40: 465–480, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heo JM, Nielson JR, Dephoure N, Gygi SP, and Rutter J. Intramolecular interactions control Vms1 translocation to damaged mitochondria. Mol Biol Cell 24: 1263–1273, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Herlan M, Vogel F, Bornhovd C, Neupert W, and Reichert A. Processing of Mgm1 by the rhomboid-type protease Pcp1 is required for maintenance of mitochondrial morphology and of mitochondrial DNA. J Biol Chem 278: 27781–27788, 2003 [DOI] [PubMed] [Google Scholar]
- 82.Herrmann JM. and Riemer J. The intermembrane space of mitochondria. Antioxid Redox Signal 13: 1341–1358, 2010 [DOI] [PubMed] [Google Scholar]
- 83.Hill RB. and Pellegrini L. The PARL family of mitochondrial rhomboid proteases. Semin Cell Dev Biol 21: 582–592, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Horing-Do HT, Tatsuta T, Buckermann A, Bust M, Kollberg G, Rotig A, Hellmich M, Nijtmans L, and Wiesner RJ. Nonsense mutations in COX1 subunit impair the stability of respiratory chain complexes rather then their assembly. EMBO J 31: 1293–1307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hugosson M, Andreu D, Boman HG, and Glaser E. Antibacterial peptides and mitochondrial presequences affect mitochondrial coupling, respiration and protein import. Eur J Biochem 223: 1027–1033, 1994 [DOI] [PubMed] [Google Scholar]
- 86.Ieva R, Heisswolf AK, Gebert M, Vogtle FN, Wollweber F, Mehnert CS, Oeljeklaus S, Warscheid B, Meisinger C, van der Laan M, and Pfanner N. Mitochondrial inner membrane protease promotes assembly of presequence translocase by removing a carboxy-terminal targeting sequence. Nat Commun 4: 2853–2864, 2013 [DOI] [PubMed] [Google Scholar]
- 87.Iguchi M, Kujiro Y, Okatsu K, Koyano F, Kosako S, Kimura M, Suzuki N, Uchiyama S, Tanaka K, and Matsuda N. Parkin-catalyzed ubiquitin esther transfer is triggered by PINK1-dependent phosphorylation. J Biol Chem 288: 22019–22032, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jarosch E, Taxis C, Volkwein C, Bordallo J, Finley D, Wolf DH, and Sommer T. Protein dislocation from the ER requires polyubiqitination and the AAA-ATPase Cdc48. Nat Cell Biol 4: 134–139, 2002 [DOI] [PubMed] [Google Scholar]
- 89.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, and Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 191: 933–942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Johnes JM, Datta P, Sirinivasula SM, Ji W, Gupta S, Zhang Z, Davies E, Hajnoczky G, Saunders TL, Van Keuren ML, Fernandes-Alnemri T, Meisler MH, and Alnemri ES. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature 425: 721–727, 2003 [DOI] [PubMed] [Google Scholar]
- 91.Johnson F. and Kaplitt MG. Novel mitochondrial substrates of omi indicate a new regulatory role in neurodegenerative disorders. PLoS One 4: e7100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kambacheld M, Augustin S, Tatsuta T, Muller S, and Langer T. Role of the novel metallopeptidase Mop112 and saccharolysin for the complete degradation of proteins residing in different subcompartments of mitochondria. J Biol Chem 280: 20132–20139, 2005 [DOI] [PubMed] [Google Scholar]
- 93.Kane LA, Lazarou M, Fogel AI, Li Y, Yamano K, Sarraf SA, Banerjee S, and Youle RJ. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol 205: 143–153, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kang SG, Dimitrova MN, Ortega J, Ginsburg A, and Maurizi MR. Human mitochondrial ClpP is a stable heptamer that assembles into a tetradecamer in the presence of ClpX. J Biol Chem 280: 35424–35432, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Karbowski M. and Neutzner A. Neurodegeneration as a consequence of failed mitochondrial maintenance. Acta Neuropathol 123: 157–171, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Karbowski M. and Youle RJ. Regulating mitochondrial outer membrane proteins by ubiquitination and proteasomal degradation. Curr Opin Cell Biol 23: 476–482, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaser M, Kambacheld M, Kisters-Woike B, and Langer T. Oma1, a novel membrane-bound metallopeptidase in mitochondria with activities overlapping with the m-AAA protease. J Biol Chem 278: 46414–46423, 2003 [DOI] [PubMed] [Google Scholar]
- 98.Kawamoto Y, Kobayashi Y, Suzuki Y, Inoue H, Tomimoto H, Akiguchi I, Budka H, Martins LM, Downward J, and Takahashi R. Accumulation of HtrA2/Omi in neuronal and glial inclusions in brains with alpha-synucleinopathies. J Neuropathol Exp Neurol 67: 984–993, 2008 [DOI] [PubMed] [Google Scholar]
- 99.Khalimonchuk O, Bird A, and Winge DR. Evidence for a pro-oxidant intermediate in the assembly of cytochrome oxidase. J Biol Chem 282: 17442–17449, 2007 [DOI] [PubMed] [Google Scholar]
- 100.Khalimonchuk O, Jeong MY, Watts T, Ferris E, and Winge DR. Selective Oma1-mediated proteolysis of the Cox1 subunit of cytochrome oxidase in assembly mutants. J Biol Chem 287: 7289–7300, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, and Shimizu N. Mutations in parkin gene cause recessive juvenile parkinsonism. Nature 392: 605–608, 1998 [DOI] [PubMed] [Google Scholar]
- 102.Konradi AK, Wang S, Montagner S, Kladt N, Korwitz A, Martinelli P, Herholz D, Baker MJ, Schauss AC, Langer T, and Rugarli EI. Loss of the m-AAA protease subunit AFG3L2 causes mitochondrial transport defects and tau hyperphosphorylation. EMBO J 33: 1011–1026, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koppen M, Metodiev MD, Casari G, Rugarli EI, and Langer T. Variable and tissue-specific subunit composition of mitochondrial m-AAA protease complexes linked to hereditary spastic paraplegia. Mol Cell Biol 27: 758–767, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koppenol WH, Bounds PL, and Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer 11: 325–337, 2011 [DOI] [PubMed] [Google Scholar]
- 105.Korbel D, Wurth S, Kaser M, and Langer T. Membrane protein turnover by the m-AAA protease in mitochondria depends on the transmembrane domains of its subunits. EMBO Rep 5: 698–703, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koyano F, Okatsu K, Kosako S, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, Endo T, Fon EA, Trempe JF, Saeki Y, Tanaka K, and Matsuda N. Ubiquitin is phosphorylated by PINK1 to activate Parkin. Nature 510: 162–166, 2014 [DOI] [PubMed] [Google Scholar]
- 107.Kremmiotidis G, Gardner AE, Settasatian C, Savoia A, Sutherland GR, and Callen DF. Molecular and functional analyses of the human and mouse genes encoding AFG3L1, a mitochondrial metalloprotease homologous to the human spastic paraplegia protein. Genomics 76: 58–65, 2001 [DOI] [PubMed] [Google Scholar]
- 108.Lee S, Augustin S, Tatsuta T, Gerdes F, Langer T, and Tsai FT. Electron cryomicroscopy structure of a membrane-anchored mitochondrial AAA protease. J Biol Chem 286: 4404–4411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee YJ, Jeong SY, Karbowski M, Smith CL, and Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1 and Opa1 in apoptosis. Mol Biol Cell 15: 5001–5011, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee YJ. and Wickner RB. AFG1, a new member of the SEC18-NSF, PAS1, CDC48-VCP, TBP family of ATPases. Yeast 8: 787–790, 1992 [DOI] [PubMed] [Google Scholar]
- 111.Lemaire C, Hamel P, Velours J, and Dujardin G. Absence of mitochondrial AAA protease Yme1p restores Fo-ATPase subunit accumulation in an oxa1 deletion mutant of Saccheromyces cerevisiae. J Biol Chem 275: 23471–23475, 2000 [DOI] [PubMed] [Google Scholar]
- 112.Leonhard K, Guiard B, Pellecchia G, Tzagoloff A, Neupert W, and Langer T. Membrane protein degradation by AAA proteases in mitochondria: extraction of substrates from either membrane surface. Mol Cell 5: 629–638, 2000 [DOI] [PubMed] [Google Scholar]
- 113.Leonhard K, Herrmann JM, Stuart RA, Mannhaupt G, Neupert W, and Langer T. AAA proteases with catalytic sites on opposite membrane surfaces comprise a proteolytic system for the ATP-dependent degradation of inner membrane proteins in mitochondria. EMBO J 15: 4218–4229, 1996 [PMC free article] [PubMed] [Google Scholar]
- 114.Leroy I, Khosrobakhsh F, Diot A, Daloyau M, Arnaune-Pelloquin L, Cavelier C, Emorine EJ, and Belenguer P. Processing of the dynamin Msp1p in S. pombe reveals an evolutionary switch between its orthologs Mgm1 in S. cerevisiae and OPA1 in mammals. FEBS Lett 584: 3153–3157, 2010 [DOI] [PubMed] [Google Scholar]
- 115.Lin MT. and Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443: 787–795, 2006 [DOI] [PubMed] [Google Scholar]
- 116.Livnat-Levanon N. and Glickman MH. Ubiquitin-proteasome system and mitochondria—reciprocity. Biochim Biophys Acta 1809: 480–487, 2011 [DOI] [PubMed] [Google Scholar]
- 117.Lu B, Lee J, Nie X, Li M, Morozov YI, Venkatesh S, Bogenhagen DF, Temiakov D, and Suzuki CK. Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ Lon protease. Mol Cell 49: 18410–18415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luce K. and Osiewacz HD. Increasing organismal healthspan by enhancing mitochondrial protein quality control. Nat Cell Biol 11: 852–858, 2009 [DOI] [PubMed] [Google Scholar]
- 119.MacVicar T. Mitophagy. Essays Biochem 55: 93–104, 2013 [DOI] [PubMed] [Google Scholar]
- 120.MacVicar TD. and Lane JD. Impaired OMA1-dependent cleavage of OPA1and reduced DRP1 fission activity combine to prevent mitophagy in cells that are dependent on oxidative phosphorylation. J Cell Sci 204: 919–929, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Major T, von Janowsky B, Ruppert T, Mogk A, and Voos W. Proteomic analysis of mitochondrial protein turnover: identification of novel substrate proteins of the matrix protease Pim1. Mol Cell Biol 26: 762–776, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Margineantu DH, Emerson CB, Diaz D, and Hockenberry DM. Hsp90 inhibition decreases mitochondrial protein turnover. PLoS One 10: e1066, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P, Abuin A, Grau E, Geppert M, Livi GP, Creasy CL, Martin A, Hargreaves I, Heales SJ, Okada H, Brander S, Schultz JB, Mak T, and Downward J. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol 24: 9848–9862, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matsushima Y, Goto Y, and Kaguni LS. Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM). Proc Natl Acad Sci U S A 107: 18410–18415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McKenzie M. and Ryan MT. Assembly factors of human mitochondrial complex I and their defects in disease. IUBMB Life 62: 497–502, 2010 [DOI] [PubMed] [Google Scholar]
- 126.McLelland GL, Soubannier V, Chen CX, McBride HM, and Fon EA. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J 33: 282–295, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McQuibban GA, Saurya S, and Freeman M. Mitochondrial membrane remodeling regulated by a conserved rhomboid protease. Nature 423: 537–541, 2003 [DOI] [PubMed] [Google Scholar]
- 128.Mick DU, Fox TD, and Rehling P. Inventory control: cytochrome c oxidase assembly regulates mitochondrial translation. Nat Rev Mol Cell Biol 12: 14–20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moisoi N, Klupsch K, Fedele V, East P, Sharma S, Renton A, Plun-Favreau H, Edwaqrds RE, Teismann P, Eposti MD, Morrison AD, Wood NW, Downward J, and Martins LM. Mitochondrial dysfunction triggered by loss of HtrA2 results in the activation of a brain-specific transcriptionsl stress response. Cell Death Differ 16: 449–464, 2009 [DOI] [PubMed] [Google Scholar]
- 130.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, and Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003 [DOI] [PubMed] [Google Scholar]
- 131.Mossmann D, Meisinger C, and Vogtle FN. Processing of mitochondrial presequences. Biochim Biophys Acta 1819: 1098–1106, 2012 [DOI] [PubMed] [Google Scholar]
- 132.Na U, Yu W, Cox J, Bricker DK, Brockmann K, Rutter J, Thummel CS, and Winge DR. The LYR factors SDHAF1 and SDHAF3 mediate maturation of the iron-sulfur subunit of succinate dehydrogenase. Cell Metab 20: 253–266, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Naamati A, Regev-Rudzki N, Galperin S, Lill R, and Pines O. Dual targeting of Nfs1 and discovery of its novel processing enzyme, Icp55. J Biol Chem 284: 30200–302028, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nakai T, Yasuhara T, Fujiki Y, and Ohashi A. Multiple genes, including a member of the AAA family, are essential for degradation of unassembled subunit 2 of cytochrome c oxidase in yeast mitochondria. Mol Cell Biol 15: 4441–4452, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, and Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337: 587–590, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Neupert W. and Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem 76: 723–749, 2007 [DOI] [PubMed] [Google Scholar]
- 137.Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, and McBride HM. Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr Biol 18: 102–108, 2008 [DOI] [PubMed] [Google Scholar]
- 138.Neuspiel M, Zunino R, Gangaraju S, Rippstein P, and McBride HM. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. EMBO Rep 13: 909–915, 2012 [DOI] [PubMed] [Google Scholar]
- 139.Nikolay K, Laterveer FD, and van Heerde WL. Effects of amphipathic peptides, including presequences, on the functional integrity of rat liver mitochondrial membranes. J Bioenerg Biomembr 26: 327–334, 1994 [DOI] [PubMed] [Google Scholar]
- 140.Nolden M, Ehses S, Koppen M, Bernacchia A, Rugarli EI, and Langer T. The m-AAA protease defective in hereditary spastic paraplegia controls ribosome assembly in mitochondria. Cell 123: 277–289, 2005 [DOI] [PubMed] [Google Scholar]
- 141.Nunnari J. and Suomalainen A. Mitochondria: in sickness and in health. Cell 148: 1145–1159, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Okreglak V. and Walter P. The conserved AAA-ATPase Msp1 confers organelle specificity to tail-anchored proteins. Proc Natl Acad Sci U S A 111: 8019–8024, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Osman C, Wilmes C, Tatsuta T, and Langer T. The metalloprotease encoded by ATP23 has a dual function in processing and assembly of subunit 6 of mitochondrial ATPase. Mol Biol Cell 18: 627–635, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Otera H, Ishihara N, and Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta 1833: 1256–1268, 2013 [DOI] [PubMed] [Google Scholar]
- 145.Padmanabhan N, Fichtner L, Dickmans A, Ficner R, Schulz JB, and Braus BH. The yeast HtrA orthologue Ynm3 is a protease with chaprone activity that aids survival under heat stress. Mol Biol Cell 20: 68–77, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, and Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134: 112–123, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Park CB. and Larsson NG. Mitochondrial DNA mutations in disease and aging. J Cell Biol 193: 809–818, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pearce DA. and Sherman F. Degradation of cytochrome oxidase subunits in mutants of yeast lacking cytochrome c and suppression of the degradation by mutation of yme1. J Biol Chem 270: 20879–20882, 1995 [DOI] [PubMed] [Google Scholar]
- 149.Pedersen BL, Baekgaard N, and Quistorff B. Muscle mitochondrial function in patients with Type 2 diabetes and peripheral arterial disease: implications in vascular surgery. Eur J Vasc Endovasc Surg 38: 356–364, 2009 [DOI] [PubMed] [Google Scholar]
- 150.Pierson TM, Adams D, Bonn F, Martinelli P, Cherukuri PF, Teer JK, Hansen NF, Cruz P, Mullicin JC. (for the NISC Comparative Sequencing Program), Blakesley RW, Golas G, Kwan J, Sandler A, Fuentes Fajardo K, Markello T, Tifft C, Blackstone C, Rugarli EI, Langer T, Gahl WA, and Toro C. Whole-exome sequencing identifies homozygous AFG3L2 mutations in a spastic ataxia-neuropathy syndrome linked to mitochondrial m-AAA proteases. PLoS Genet 7: e1002325, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, Harvey K, Deas E, Harvey RJ, McDonald N, Wood NW, Martins LM, and Downward J. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat Cell Biol 9: 1243–1252, 2007 [DOI] [PubMed] [Google Scholar]
- 152.Potting C, Wilmes C, Engmann T, Osman C, and Langer T. Regulation of mitochondrial phospholipids by Ups1/PRELI-like proteins depends on proteolysis and Mdm35. EMBO J 29: 2888–2898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Quiros PM, Espanol Y, Acin-Perez R, Rodriguez F, Barcena C, Watanabe K, Calvo E, Loureiro M, Fernandez-Garcia MS, Fueyo A, Vazquez J, Enriquez JA, and Lopez-Otin C. ATP-dependent Lon protease controls tumor bioenergetics by reprogramming mitochondrial activity. Cell Rep 8: 542–556, 2014 [DOI] [PubMed] [Google Scholar]
- 154.Quiros PM, Ramsay AJ, Sala D, Fernandez-Vizarra E, Rodrigues F, Peinado JR, Fernandez-Garcia MS, Vega JA, Enriques JA, Zorzano A, and Lopez-Otin C. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J 31: 2117–2133, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rambold AS, Kostelecky B, Elia N, and Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A 108: 10190–10195, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rhee HW, Zou P, Udeshi ND, Martell JD, Mootha VK, Carr SA, and Ting AY. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science 339: 1328–1331, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rugarli EI. and Langer T. Mitochondrial quality control: a matter of life and death for neurons. EMBO J 31: 1336–1349, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sarraf SA, Raman M, Guarani-Pereira V, Sowa ME, Huttlin EL, Gygi SP, and Harper JW. Landscape of the PARKIN-dependent ubiquitilome in response to mitochondrial depolarization. Nature 496: 372–376, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sauer RT. and Baker TA. AAA+ proteases: ATP-fueled machines of protein destruction. Annu Rev Biochem 80: 587–612, 2011 [DOI] [PubMed] [Google Scholar]
- 160.Savelev AS, Novikova LA, Kovaleva IE, Luzikov VN, Neupert W, and Langer T. ATP-dependent proteolysis in mitochondria. m-AAA protease and Pim1 protease exert overlapping substrate specificities and cooperate with mtHsp70 system. J Biol Chem 273: 20596–20602, 1998 [DOI] [PubMed] [Google Scholar]
- 161.Schieber M. and Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 24: R453–R462, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schreinr B, Westerburg H, Forne I, Imhof A, Neupert W, and Mokranjac D. Role of the AAA protease Yme1 in folding of protein in the intermembrane space of mitochondria. Mol Biol Cell 23: 143–153, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shi G, Lee JR, Grimes DA, Racacho L, Ye D, Yang H, Ross OA, Farrer M, McQuibban GA, and Bulman DE. Functional alteration of PARL contributes to mitochondrial dysregulation in Parkinson's disease. Hum Mol Genet 20: 1966–1974, 2011 [DOI] [PubMed] [Google Scholar]
- 164.Shutt T, Geoffrion M, Milne R, and McBride HM. The intracellular redox state is a core determinant of mitochondrial fusion. J Biol Chem 280: 25060–25070, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sickmann A, Reinders J, Wagner Y, Joppich C, Zahedi R, Meyer HE, Schonfisch B, Perschil I, Chacinska A, Guiard B, Rehling P, Pfanner N. and Meisinger C. The proteome of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 100: 13207–13212, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Soubannier V, McLelland GL, Zunino R, Braschi E, Rippstein P, Fon EA, and McBride HM. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol 24: 135–141, 2012 [DOI] [PubMed] [Google Scholar]
- 167.Stahlberg H, Kutejova E, Suda K, Wolpensinger B, Lustig A, Schatz G, Engel A, and Suzuki CK. Mitochondrial Lon of Saccharomyces cerevisiae is a ring-shaped protease with seven flexible subunits. Proc Natl Acad Sci U S A 96: 6787–6790, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stiburek L, Cesnekova J, Kostkova O, Fornuskova D, Vinsova K, Wenchich L, Houstek J, and Zeman J. YME1L controls the accumulation of respiratory chain subunits and is required for apoptotic resistance, cristae morphogenesis and cell proliferation. Mol Biol Cell 23: 1010–1023, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, Gasser T, Wszolek Z, Muller T, Bornemann A, Wolburg H, Downward J, Riess O, Schulz JB, and Kruger R. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson's disease. Hum Mol Genet 14: 2099–2111, 2005 [DOI] [PubMed] [Google Scholar]
- 170.Sugiura A, McLelland GL, Fon EA, and McBride HM. A new pathway for mitochondrial quality control: mitochondrial-derived vesicles. EMBO J 33: 2142–2156, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Suzuki CK, Suda K, Wang N, and Schatz G. Requirement for the yeast gene LON in intramitochodnrial proteolysis and maintenance of respiration. Science 264: 273–276, 1994 [DOI] [PubMed] [Google Scholar]
- 172.Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, and Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol 191: 1367–1380, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tasaki T, Sriram SM, Park SO, and Kwon YT. The N-end rule pathway. Annu Rev Biochem 81: 261–289, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tatsuta T, Augustin S, Nolden M, Friedrichs B, and Langer T. m-AAA protease-driven membrane dislocation allows intramembrane cleavage by rhomboid in mitochondria. EMBO J 26: 325–335, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Taylor EB. and Rutter J. Mitochondrial quality control by the ubiquitin-proteasome system. Biochem Soc Trans 39: 1509–1513, 2011 [DOI] [PubMed] [Google Scholar]
- 176.Teixeira PF. and Glaser E. Processing peptidases in mitochondria and chloroplasts. Biochim Biophys Acta 1833: 360–370, 2013 [DOI] [PubMed] [Google Scholar]
- 177.Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, and Martinou JC. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J 33: 578–593, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BC, and Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vafai SB. and Mootha VK. Mitochondrial disorders as windows into an ancient organelle. Nature 491: 374–383, 2012 [DOI] [PubMed] [Google Scholar]
- 180.Vande Walle L, Lamkanfi M, and Vandenabeele P. The mitochondrial serine protease HtrA2/Omi: an overview. Cell Death Differ 15: 453–460, 2008 [DOI] [PubMed] [Google Scholar]
- 181.Venkatesh S, Lee J, Singh K, Lee I, and Suzuki CK. Multitasking in the mitochondrion by the ATP-dependent Lon protease. Biochim Biophys Acta 1823: 56–66, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vincow ES, Merrihew G, Thomas RE, Shulman NJ, Beyer RP, MacCoss MJ, and Pallanck LJ. The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc Natl Acad Sci U S A 110: 5603–6405, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vogtle FN, Burkhart JM, Rao S, Gerbeth C, Hinrichs J, Martinou JC, Chacinska A, Sickmann A, Zahedi R, and Meisinger C. Intermembrane space proteome of yeast mitochondria. Mol Cell Proteomics 11: 1840–1852, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vogtle FN, Prinz C, Kellermann J, Lottspeich F, Pfanner N, and Meisinger C. Mitochondrial protein turnover: role of the precursor intermediate peptidase Oct1 in protein stabilization. Mol Biol Cell 22: 2135–2143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vogtle FN, Wortelkamp S, Zahedi RP, Becker D, Leidhold C, Gevaert K, Kellermann J, Voos W, Sickmann A, Pfanner N, and Meisinger C. Global analysis of the mitochondrial N-proteome identifies a processing peptidase critical for protein stability. Cell 139: 428–439, 2009 [DOI] [PubMed] [Google Scholar]
- 186.von Janowsky B, Knapp K, Major T, Krayl M, Guiard B, and Voos W. Structural properties of substrate proteins determine their proteolysis by the mitochondrial AAA+ protease Pim1. Biol Chem 386: 1307–1317, 2005 [DOI] [PubMed] [Google Scholar]
- 187.Voos W. Chaperone-protease networks in mitochondrial protein homeostasis. Biochim Biophys Acta 1833: 388–399, 2013 [DOI] [PubMed] [Google Scholar]
- 188.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39: 359–407, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, and Schwartz TL. PINK1 and Parkin target Miro for phosphorylationand degradation to arrest mitochondrial motility. Cell 147: 893–906, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Weber ER, Hanekamp T, and Thorsness P. Biochemical and functional analysis of the YME1 gene product, an ATP and zinc-dependent mitochondrial protease from S. cerevisiae. Mol Biol Cell 7: 307–317, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wu Y, Williams EG, Dubuis S, Mottis A, Jovaisaite V, Houten SM, Argmann CA, Faridi P, Wolski W, Kutalik Z, Zamboni N, Auwerx J, and Aebersold R. Multilayered genetic and omics dissection of mitochondrial activity in a mouse reference population. Cell 158: 1415–1430, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xiao X, Hu Y, Quiros PM, Wei Q, Lopez-Otin C, and Dong Z. OMA1 mediates OPA1 proteolysis and mitochondrial fragmentation in experimental models of ischemic kidney injury. Am J Physiol Renal Physiol 306: F1318–F1326, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xu S, Peng G, Wang Y, Fang S, and Karbowski M. The AAA-ATPase p97 is essential for outer mitochondrial membrane protein turnover. Mol Biol Cell 22: 291–300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Youle RJ. and Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol 6: 657–663, 2005 [DOI] [PubMed] [Google Scholar]
- 195.Youle RJ. and Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12: 9–14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Youle RJ. and van der Bliek A. Mitochondrial fission, fusion and stress. Science 337: 1062–1065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Young JC, Hoogenraad NJ, and Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the import receptor Tom70. Cell 112: 41–50, 2003 [DOI] [PubMed] [Google Scholar]
- 198.Zahedi RP, Sickmann A, Boehm AM, Winkler C, Zufall N, Schonfisch B, Guiard B, Pfanner N, and Meisinger C. Proteomic analysis of the yeast mitochondrial outer membrane reveals accumulation of a subclass of preproteins. Mol Biol Cell 17: 1436–14501, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zeng X, Neupert W, and Tzagoloff A. The metalloprotease encoded by ATP23 has a dual function in processing and assembly of subunit 6 of mitochondrial ATPase. Mol Biol Cell 18: 617–626, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang K, Li H, and Song Z. Membrane depolarization activates the mitochondrial protease OMA1 by stimulating self-cleavage. EMBO Rep 15: 576–585, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]