Long noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and interacts with miR-205 (original) (raw)
. Author manuscript; available in PMC: 2018 Apr 5.
Abstract
Recently long non-coding RNAs (lncRNA) have emerged as new gene regulators and prognostic markers in several cancers including renal cell carcinoma (RCC). In this study, we investigated the contributions of the lncRNA MALAT1 in RCC with a specific focus on its transcriptional regulation and its interactions with Ezh2 and miR-205. We found that MALAT1 expression was higher in human RCC tissues where it was associated with reduced patient survival. MALAT1 silencing decreased RCC cell proliferation and invasion and increased apoptosis. Mechanistic investigations showed that MALAT1 was transcriptionally activated by c-Fos and that it interacted with Ezh2. After MALAT1 silencing, E-cadherin expression was increased while beta-catenin expression was decreased through Ezh2. Reciprocal interaction between MALAT1 and miR-205 was also observed. Lastly, MALAT1 bound Ezh2 and oncogenesis facilitated by MALAT1 was inhibited by Ezh2 depletion, thereby blocking epithelial-mesenchyme transition via E-cadherin recovery and beta-catenin downregulation. Overall, our findings illuminate how overexpression of MALAT1 confers an oncogenic function in RCC that may offer a novel theranostic marker in this disease.
Keywords: long non-coding RNA, MALAT1, RCC, real-time RT-PCR, MTS assay, invasion assay, apoptosis, FACS, Western blot, TOPflash, ChIP (Chromatin immunoprecipitation), RIP (RNA Immunoprecipitation), Immunohistochemistry, miR-205
INTRODUCTION
Renal cell carcinoma (RCC) is one of the 10 most common cancers and it is estimated that there were 63,920 new cases of RCC in the United States in 2013 (1). The incidence of renal cell carcinoma has increased for over two decades (2) and patients with advanced RCC (stage IV) have a significantly reduced five-year survival rate (less than 30%) (3). Compared to other cancers, there are very few tumor and biomarkers for renal cancer (4). Also, renal cancer patients respond poorly to conventional chemotherapy and radiation treatment (5), thus early detection and treatment are very important for RCC patients.
Human genome sequence data indicates that more than 90 % of the DNA sequence is actively transcribed but only 2% of it encodes protein, thus the majority of transcripts are referred to as non-coding RNAs (ncRNAs) (6, 7). Based on size, non-coding RNAs are divided into two groups, small non-coding RNAs and long non-coding RNAs greater than 200 nt (lncRNA). Small non-coding RNAs such as microRNAs have been studied extensively and their roles in gene regulation and cell function have been elucidated in numerous cancers (7). Recent studies have shown that lncRNAs play important roles in both normal development and diseases including cancer (8). LncRNAs participate in several different biological processes including epigenetic regulation, nuclear import, cell cycle control, nuclear and cytoplasmic trafficking, imprinting, cell differentiation, alternative splicing, RNA decay, transcription and translation (9, 10). Thus long non-coding RNAs have emerged as new players in cancer research and several studies have shown that some lncRNAs function as oncogenes, tumor suppressor genes or both, depending on the circumstance (11).
Several lncRNAs [GAS5, MEG3 (=GTL2), HIF-1alpha-AS1, H19, KCQN1OT1, MALAT1 (alpha-gene), and HOTAIR] have been reported to be involved in renal cancer (12–19). MALAT1 (alternative name “alpha gene”) is located on chromosome 11q13 and the expression of MALAT1 has been reported to be up-regulated in several cancers including lung, breast, pancreas, liver, colon, uterus, cervix and prostate (10). Previous papers showed that MALAT1 expression is an independent prognostic parameter for survival in several cancers (10). Davis et al has reported that chromosomal translocation of t (6; 11) (p21; q13) was observed in pediatric renal cancer, resulting in the fusion of the alpha gene (MALAT1) with TFEB, a basic helix-loop-helix leucine zipper (bHLH-LZ) transcription factor (17). Kuniper et al has reported that the bHLH-LZ transcription factor TFEB gene on chromosome 6 is fused to the anonymous non protein encoding Alpha gene (MALAT1) on chromosome 11 through chromosomal translocation, t(6;11)(p21;q13) in papillary RCC (18).
In this study we focused on lncRNA MALAT1 in clear cell RCC since the majority of adult renal cancers are of the clear cell type and many studies have focused on clear cell RCC. The aim of this study was to: 1) determine the role of MALAT1 in renal cancer, 2) determine the reason for MALAT1 overexpression in renal cancer tissues, 3) determine the downstream effects of MALAT1 in renal cancer and 4) determine the association between MALAT1 and microRNA-205 in renal cancer.
MATERIALS AND METHODS
Clinical Samples
A total of 50 patients (34 male and 16 female) with pathologically confirmed clear cell RCC (cc-RCC) were enrolled in this study (Toho University Hospital, Tokyo, Japan). The mean age of the patients was 60.2 (range 37–77). They were classified according to the WHO criteria and staged according to the tumor-node-metastasis (TNM) classification. Information about the patients is shown in Table S1. Samples were obtained from the patients after written informed consent was obtained at Toho University hospital.
Cell culture
Normal renal epithelial cells (HK-2; ATCC number: CRL-2190) and renal cancer cell lines (A-498; ATCC number: HTB-44, 786-O; ATCC number: CRL-1932, Caki-2; ATCC number: HTB-47, Caki-1: ATCC number HTB-46) were purchased from the American Type Culture Collection (Manassas, VA). The cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum. HK-2 cells were cultured in keratinocyte-SFM (GIBCO/Invitrogen, Carlsbad, CA, USA). When purchased, permanent stocks of cells were prepared and all cells were stored at −80 degree until use. Cells were used for experiments within 6 months.
Total RNA, DNA and protein extraction
RNA (microRNA and total RNA) was extracted from formalin-fixed, paraffin-embedded (FFPE) human renal cancer and adjacent non-cancerous normal kidney tissues using a miRNeasy FFPE kit (QIAGEN, Valencia, CA, USA) after micro-dissection based on a pathologist’s reviews. To digest DNA, the Qiagen RNase-Free DNase kit was used. RNA (microRNA and total RNA) was also extracted from human cell lines using a miRNeasy mini kit (QIAGEN). Genomic DNA was extracted from cell lines using a QIAamp DNA mini kit (QIAGEN). Cells were lysed with RIPA buffer (Thermo Fisher Scientific, Rockford, IL, USA) containing protease inhibitors (Sigma-Aldrich, St. Louis, MO). Protein quantification was done using a BCA protein assay kit (Thermo Fisher Scientific).
Knockdown of MALAT1 mRNA in renal cancer cells
Of four renal cancer cell lines, the expression of MALAT1 was higher in 786-O and A-498 than Caki-2 and Caki-1 cells. Thus we used 786-O and A-498 cells for further experiments in this study. Renal cancer cells (786-O and A-498) were transfected with two MALAT1 siRNAs [si-MALAT1 (No.1 and No.2); Life Technologies, Carlsbad, CA, USA] or negative control siRNA [si-negative control (si-NC); Life Technologies, Carlsbad, CA, USA] according to the manufacturer’s instructions. Briefly, cells were grown in six-well plates and transfected individually with two si-MALAT1 siRNAs [no. 1 (custom order) and no.2 (product#n272234)] at a concentration of 50 pmol/well. Knock down effect of si-MALAT1 was examined by real-time RT-PCR using RNA extracted 48 hours after transfection (si-NC, si-MALAT) in two RCC cell lines (A-498 and 786-O). Cell viability (24hr, 48hr, 72hr- after transfection), invasion (48hr after transfection) and apoptosis analysis (48hr after transfection) was performed using si-NC or si-MALAT1 transfected RCC cells. The two siRNA sequences are shown in Table S2. Transfection was performed with Lipofectamine™ RNAiMAX Transfection Reagent (Life Technologies) accordingly.
Cell viability, cell invasion and apoptosis assay
Cell viability was measured 72 hours after transfection with MTS (CellTiter 96 Aqueous One Solution Cell Proliferation Assay, Promega, Madison, WI, USA). Cell invasion assays were performed with the CytoSelect 24-well cell invasion assay kit (Cell BioLab, San Diego, CA, USA) according to the manufacturer’s instructions. FACS analysis for apoptosis was done using Annexin V-FITC solution and 7-AAD kit after 48 hours transfection according to the manufacturer’s protocol (Beckman Coulter, Fullerton, CA). All experiments were performed in triplicate.
Search for transcription factors binding to the MALAT1 promoter
In order to determine why MALAT1 is overexpressed in renal cancer tissues, we focused on transcription factors binding to the MALAT1 promoter. Based on several computer algorithms (PROMO; http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3, ChIPbase: http://deepbase.sysu.edu.cn/chipbase, and TFSEARCH: http://www.cbrc.jp/research/db/TFSEARCH.html), we identified 22 transcription factors involved in cancer pathways as potential transcription activators for MALAT1 (Table S3). Of the 22 transcription factors, we selected c-Fos since An et al found that inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene induces activation of c-Jun-NH2-kinase (JNK) via phosphorylation of members of the AP1 family of transcription factors such as c-Jun and c-Fos and Twist expression is upregulated, resulting in induced EMT in RCC (20). Clear cell RCC is the major histologic subtype (about 80% of RCC cases) and inactivation of the VHL tumor suppressor gene is typical in ccRCC (20). We also found that high c-Fos overexpression was associated with shorter overall survival of RCC patients (data not shown). Thus we focused on c-Fos as a potentially important transcription factor in MALAT1 activation.
Construction of luciferase expressing plasmid vector containing the MALAT1 promoter region
Based on several computer algorithms as described above, we identified potential binding sites for transcription factor c-Fos in the promoter region of MALAT1 gene. Using 786-O genomic DNA, the identified MALAT1 promoter DNA region was amplified and the polymerase chain reaction products were cloned into the pGEM-T easy vector System (Promega) (Table S2). Then the MALAT1 promoter DNA region was incorporated into the pGL4 luciferase expression vector (Promega). Luciferase activity was assessed using the Dual-Luciferase® Reporter Assay System (Promega) (48 hours after their transfection) and the ratio of Firefly/Renilla luciferase activity was determined.
Overexpression of transcription factor genes (c-Fos)
In order to construct transcription factor gene (c-Fos) over expressing plasmids, the gene was amplified with total RNA from human adult normal kidney tissues (catalog#: R1234142-50, Biochain Institute, Newark, CA, USA) by transcription–polymerase chain reaction (RT-PCR) as described previously (21). The sequences of primers for cloning are shown in Table S2.
TCF/LEF reporter assay (TOPflash luciferase assay)
TOPflash luciferase assays were performed to assess the knock down effect of MALAT1 on the Wnt-beta catenin signaling pathway as described previously (21).
RNA immunoprecipitation (RIP) Assay
RNA immunoprecipitation (RIP) was performed to investigate whether ribonucleoprotein (RNP) complex contained lncRNA MALAT1 and its potential binding protein (Ezh2) in renal cancer cells. We used an Imprint RNA Immunoprecipitation (RIP) kit according to the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO, USA). The RIP RNA fraction was digested by DNase and cDNA was generated using PrimeScript 1 st strand cDNA Synthesis Kit (Takara Bio Company, Shiga, Japan). Final analysis was performed using RT-qPCR and shown as fold enrichment of MALAT1. The RIP RNA fraction Ct value was normalized to the input RNA fraction Ct value.
ChIP assay
ChIP assays were performed on cell line DNA using an Imprint Chromatin Immunoprecipitation Kit (Sigma-Aldrich). Antibody for trimethyl-Histone H3 (Lys27) (H3K27-me3) (catalog#07-449, EMD Millipore, Billerica, MA, USA) was obtained from vendors. The immunoprecipitated DNA was analyzed using quantitative real-time PCR with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Quantitative PCR was performed in triplicate with an Applied Biosystems Prism 7500 Fast Sequence Detection System (Applied Biosystems) using the following conditions: 10 min, 95°C; followed by 40 cycles of 30 sec; 95°C, 60 sec; 60°C. Delta-delta cycle threshold (Ct) method was used to calculate real-time PCR results. The fold changes related to 10% input delta Ct were calculated as 2−(delta-delta Ct). All experiments were performed in triplicate. The primer pairs used for ChIP assays are shown in Supporting Information Table S2.
Immunohistochemistry study
Immunostaining of E-Cadherin was performed on formalin-fixed, paraffin-embedded (FFPE) specimens using rabbit polyclonal antibody against human E-Cadherin (#3195, Cell Signaling Technology, Beverly, MA). The staining procedure was according to a commercial kit (Lab vision, Fremont, CA). The sections were counterstained with Harris’ hematoxylin. Immunohistochemical staining was evaluated by visually assessing staining intensity (0–3) using a microscope at 200x. The criteria of intensity are as follows: 0, negative expression; 1+, weakly positive expression; 2+, moderately positive expression; 3+, strongly positive expression. All specimens were scored blindly by two observers.
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR was performed in triplicate with an Applied Biosystems Prism 7500 Fast Sequence Detection System using TaqMan universal PCR master mix according to the manufacture’s protocol (Applied Biosystems Inc., Foster City, CA, USA). The TaqMan probes and primers were purchased from Applied Biosystems. Human GAPDH and RNU48 were used as endogenous controls. Levels of RNA expression were determined using the 7500 Fast System SDS software version 1.3.1 (Applied Biosystems).
Western analysis
Western blot was performed as described previously. The antibodies used were specific for c-Myc (#551101, BD Biosciences, San Jose, CA, USA), Ezh2 (#5246, Cell Signaling Technology, Beverly, MA, USA), E-Cadherin (#3195, Cell Signaling Technology), beta-catenin (#9562, Cell Signaling Technology), PCNA (#13110, Cell Signaling Technology), and beta-tubulin (#2128, Cell Signaling Technology). Proteins were enhanced by chemiluminescence (Amersham ECL plus Western Blotting detection system, Fairfield, CT, USA or SuperSignal West Pico chemiluminescent substrate, Thermo Scientific) for visualization. The ChemiDoc XRS+ system was used for chemiluminescent detection (BIO-RAD Laboratories, Hercules, CA, USA). The protein expression levels were expressed relative to beta-tubulin or PCNA levels.
Statistical analysis
All statistical analyses were performed using StatView (version 5; SAS Institute Inc., NC). Error bars in figures represent S.D. (Standard Deviation). Mann-Whitney U test was used to compare median value between two groups. Fisher’s exact test or chi-square test was used to determine whether there is significant difference between two groups. Correlation between cFos mRNA expression and MALAT1 mRNA or MALAT1 mRNA and Ezh2 mRNA in human renal cancer tissues was examined with two sided Pearson’s correlation (22). Statistical significance was determined using the Students t-test or Analysis of Variance (ANOVA) for functional analysis. A _p_-value of < 0.05 was regarded as statistically significant.
RESULTS
Relationship between MALAT1 expression and clinical characteristics
Initially we compared MALAT1 expression in 16 matched normal kidney and renal cancer tissues (all cc-RCC) using real-time RT-PCR. MALAT1 expression was significantly higher in all renal cancer tissues compared to matched normal kidney tissues (Fig. 1A). We then used 50 renal cancer samples and divided the RCC patients into two groups using a median MALAT1 2^-delta Ct value of 0.1485 (Fig. 1B). Next we investigated the association of MALAT1 expression with several clinical parameters including grade, pathologic tumor classification (pT), pathologic lymph node status (pN), pathologic metastatic status (pM), stage and outcome (overall survival). The expression of MALAT1 was significantly higher in pT (pT3+pT4), pN (+), pM (+), and high stage (3+4) patients compared to lower stage patients (Table S1). Over-all survival was also significantly shorter in the high MALAT1 expression group compared to the low MALAT1 group (Fig. 1C).
Figure 1. MALAT1 expression and association with clinical parameters in renal cancer tissues.
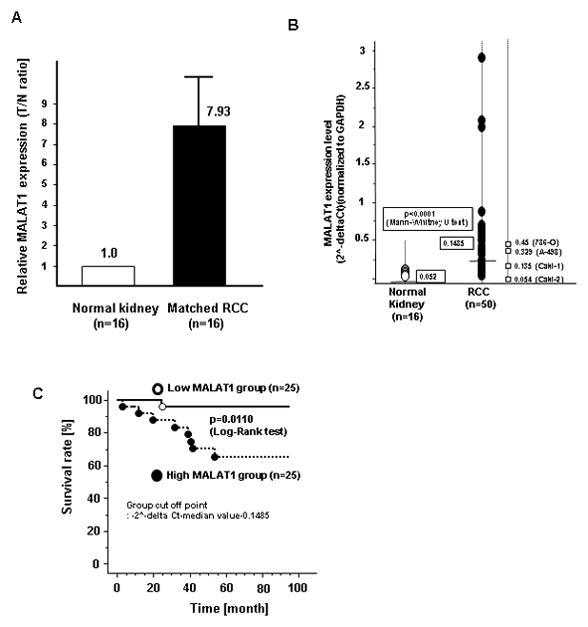
A. Comparison of MALAT1 expression in paired human clinical samples (each normal expression is “1”. B. MALAT1 expression in clinical samples and renal cancer cell lines (786-O, A-498, Caki-1, Caki-2), C. Association of MALAT1 expression with overall survival (Kaplan Meier plot).
Effect of MALAT1 on renal cancer cell viability, invasion and apoptosis
As shown in Figure 2A, the knock down effect was best using si-MALAT1 (No.1) compared to si-MALAT1 (No.2). Thus we used si-MALAT1 (No.1) for further experiments. As shown in Figure 2, we observed significantly decreased cell viability (Fig. 2B) and invasion (Fig. 2C) in si-MALAT1 transfected renal cancer cells compared to si-NC transfected cells. The percent of apoptotic cells was significantly increased in si-MALAT1 transfected cells (Fig. 2D).
Figure 2. Effect of MALAT1 knock down on renal cancer cell function (A-498, 786-O).
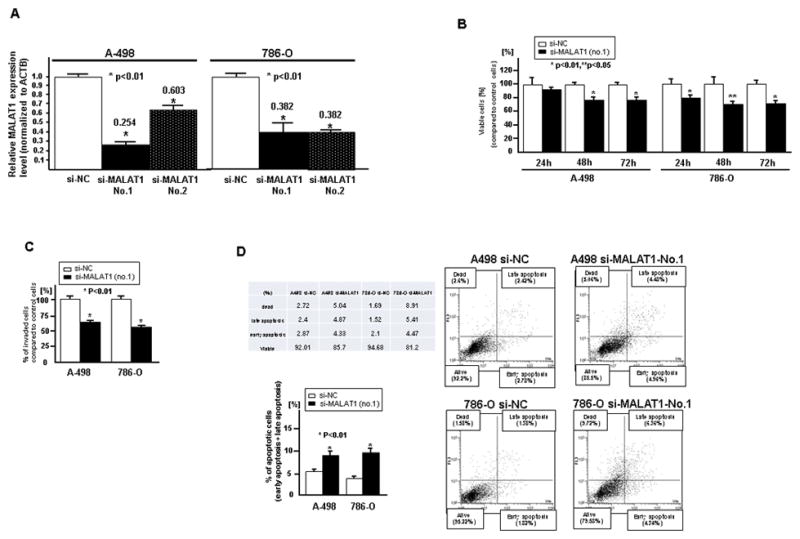
Two renal cancer cell lines (A-498 and 786-O) were transiently transfected with either si-MALAT1 (No.1, No.2) or control (si-NC). A. Validation of si-MALAT1 knock down in RCC cell lines, B. Cell viability assay, C. Invasion assay, D. Flow cytometric analysis of apoptosis in si-NC or si-MALAT1 transfected renal cancer cells. Error bars represent ±S.D. (standard deviation).
c-Fos expression in normal kidney and renal cancer tissues
Expression of transcription factor c-Fos mRNA was significantly higher in renal cancer tissues compared to those in matched normal kidney tissues (Fig. 3A).
Figure 3. Transcriptional activation of MALAT1 by c-Fos in 786-O cells.
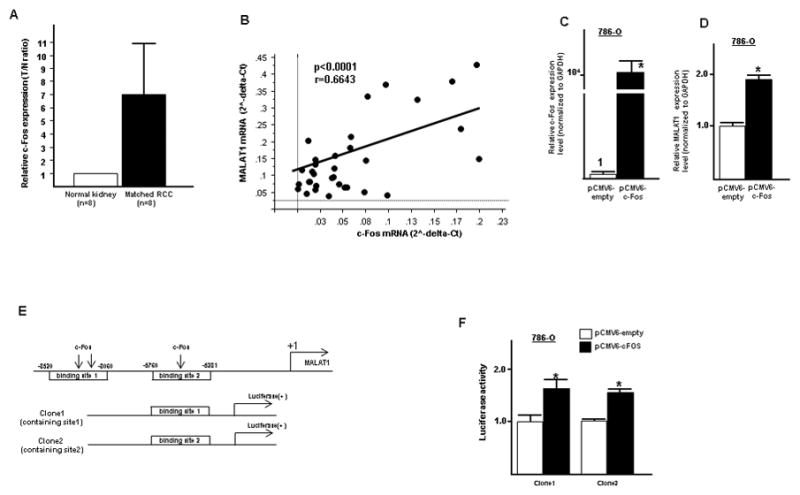
A. c-Fos expression level in renal cancer and matched normal kidney tissues, B. Pearson’s correlation between c-Fos mRNA and MALAT1 mRNA, C. Validation of c-Fos overexpression in 786-O cells after transfection, D. MALAT1 mRNA expression level after transfection of c-Fos overexpressing vector plasmid and control, E. Potential c-Fos binding region in the promoter region of MALAT1 used for construction of luciferase vector containing the binding region, F. luciferase reporter assay (pCMV6-empty vs pCMV6-c-Fos), Error bars represent ±S.D. (standard deviation).
Positive correlation between c-Fos and MALAT1 expression
As shown, there was significant positive correlation between c-Fos mRNA and MALAT1 mRNA expression (Two sided Pearson’s correlation, r=0.6643, p<0.0001) (Fig. 3B). MALAT1 expression was also significantly increased after c-Fos overexpression in 786-O cells (Fig. 3C and Fig. 3D). To investigate the direct binding of c-Fos to the MALAT1 promoter, we constructed luciferase vectors containing potential c-Fos binding sites (clone 1; clone 2) (Fig. 3E). The clone 1 luciferase vector contained two potential c-Fos binding sites and clone 2 contains one binding site. As shown in Figure 3F, luciferase activity was significantly increased in c-Fos transfected cells compared to control vector with the two constructs (Fig. 3F).
Signaling cross-contribution of transcription factors, MALAT1 and Ezh2 in RCC
Some lncRNAs are activated transcriptionally (23–26) and several reports have shown that lncRNA HOTAIR binds to Polycomb repressive complex2 (PRC2) (Ezh2, Suz12, EED) enhancing methylation of H3K27me3, resulting in gene silencing (27). Several long non-coding RNAs such as H19 and EBIC have been found to bind to Ezh2 in other cancers (28, 29). MALAT1 has been reported to bind to Suz12, a component of PRC2 in bladder cancer cell lines (30). These papers indicated that the interaction between MALAT1 and Ezh2 might be important for this study. We have made a schematic diagram illustrating MALAT1 signaling, transcription factor activator and downstream effectors via Ezh2 in promoting renal cancer growth (Fig. 4A).
Figure 4. MALAT1 interacts with Ezh2 in renal cancer.
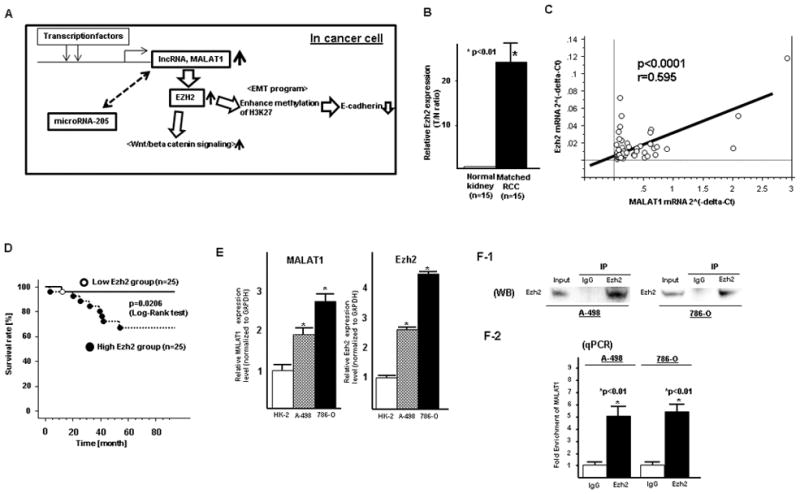
A. Schematic diagram illustrating signaling of MALAT1 and its upstream activator and its downstream effectors in RCC. B. Ezh2 mRNA expression in normal kidney and matched renal cancer tissues, C. Pearson’s correlation between MALAT1 mRNA and Ezh2 mRNA, D. High Ezh2 was associated with shorter overall survival of RCC patients, E. Expression of MALAT1 and Ezh2 mRNA in cell lines (HK-2, A-498, 786-O), F. RNA immunoprecipitation (A-498 and 786-O), (F-1) Western blot (input, rabbit IgG, anti-Ezh2 antibody; immunoprecipitation followed by Western blot with Ezh2 antibody), (F-2) qPCR showing MALAT1 is significantly enriched with the Ezh2 antibody compared to IgG (control antibody) in two renal cancer cell lines (A-498 and 786-O)
Ezh2 expression in renal cancer and normal kidney tissues
Ezh2 mRNA expression was significantly higher in renal cancer tissues compared to normal kidney tissues (Fig. 4B).
Positive correlation between MALAT1 mRNA and Ezh2 mRNA expression
We compared the mRNA expression levels for both MALAT1 and Ezh2 in renal cancer tissues and found that there was significant positive correlation between the two groups (Two sided Pearson’s correlation, r=0.595, p<0.0001) (Fig. 4C).
Association of Ezh2 with clinicopathological parameters in RCC tissues
The expression of Ezh2 was significantly higher in pT (pT3+pT4) and pN (+) patients compared to those in pT1+pT2 and pN (−) patients (Table S4). Over-all survival was also significantly shorter in the high Ezh2 expression group compared to the low Ezh2 group (Fig. 4D).
MALAT1 and Ezh2expression in renal cancer cells and a normal kidney cell line
MALAT1 expression was significantly higher in renal cancer cell lines (A-498 and 786-O) compared to that in normal kidney cell line HK-2 (Fig. 4E). Ezh2 mRNA expression was also significantly higher in renal cancer cell lines (Fig. 4E) compared to normal kidney cells.
RNA immunoprecipitation (RNA-IP)
Some lncRNAs affect expression of protein coding genes by interacting with Polycomb repressive complex2 subunit Ezh2 (27). To investigate potential interaction between lncRNA MALAT1 and Ezh2, RNA-IP was performed. We found that MALAT1 was significantly enriched with the Ezh2 antibody compared to IgG (control antibody) in two renal cancer cell lines (A-498 and 786-O) (Fig. 4F).
Effect of MALAT1 on downstream effectors in renal cancer cell
After knock down of MALAT1, expression of Ezh2, beta-catenin and c-Myc protein was significantly decreased in RCC cell lines while in contrast E-Cadherin protein expression was significantly increased (Fig. 5A). E-Cadherin mRNA expression was low in renal cancer cells (A-498 and 786-O) compared to HK-2 cells (Fig. S1) and E-Cadherin protein expression was also significantly lower in renal cancer tissues (Fig. 5B). There was also inverse correlation between E-Cadherin protein and MALAT1 mRNA expression in renal cancer tissues (Fig. 5C). Since Ezh2 is an enhancer of H3K27 methylation, we used ChIP analysis using anti-H3K27 me3 antibody to determine the effect of si-MALAT1 on histone modification in the E-Cadherin gene promoter. The histone associated DNAs that were immunoprecipitated with antibody against H3K27-me3 were individually amplified with primers sets covering the E-Cadherin gene promoter regions. The level of H3K27-me3 was significantly decreased by MALAT1 knock down compared to control cells (Fig. 5D). Since total beta-catenin expression was significantly decreased by knock down of MALAT1, we looked at the nuclear fraction and found that nuclear beta-catenin was also significantly decreased (Fig. 5E). TCF reporter activity (TOPflash) was also significantly decreased by MALAT1 knock down in both renal cancer cell lines (Fig. 5F).
Figure 5. Downstream Effect of MALAT1 on Ezh2 mediated pathways in renal cancer.
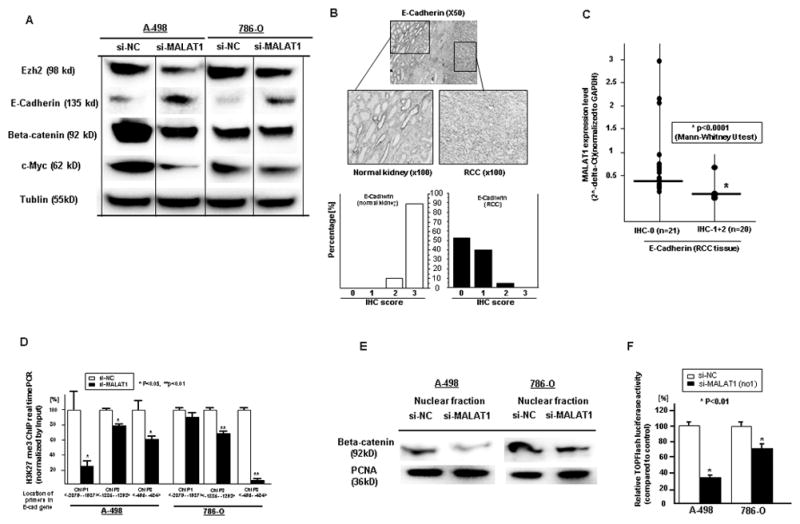
A. Western blot results (si-NC vs si-MALAT1), B. Immunohistochemistry of E-Cadherin in renal cancer and normal kidney tissues. C. Association of MALAT1 mRNA with E-Cadherin protein expression, D. Quantification by ChIP real-time PCR of H3K27me3 in the E-Cadherin gene promoter region using si-NC and si-MALAT1 transfected cells (A-498, 786-O). E. Beta-catenin protein expression (Western blot) in the cell nuclear fraction, F. TOPflash luciferase assay
Interaction between MALAT1 and miRNA-205 in renal cancer
We also investigated the potential relationship between lncRNA MALAT1 and microRNA. Using miRcode (http://www.mircode.org/mircode), a computer algorithm to identify miRNA target genes including lncRNAs (31), we identified several miRNAs MALAT1 binding sites. Of these miRNAs, our lab has previously reported that miR-205 functions as tumor suppressor gene in renal cancer (32). Thus we focused on miR-205 and investigated the interaction between MALAT1 and miR-205 in renal cancer cell lines. While the expression of MALAT1 was high in renal cancer cell lines compared to normal HK-2 cells, miR-205 expression was significantly lower in renal cancer cell lines (Fig. 6A). Knock down of MALAT1 significantly increased miR-205 expression in renal cancer cells (Fig. 6B), however after overexpression of miR-205 (Fig. 6C), MALAT1 expression was significantly decreased (Fig. 6D). These results suggest that interaction between MALAT1 and miRNA-205 has reciprocal effects.
Figure 6. Interaction between MALAT1 and miR-205 in A-498 and 786-O cells.
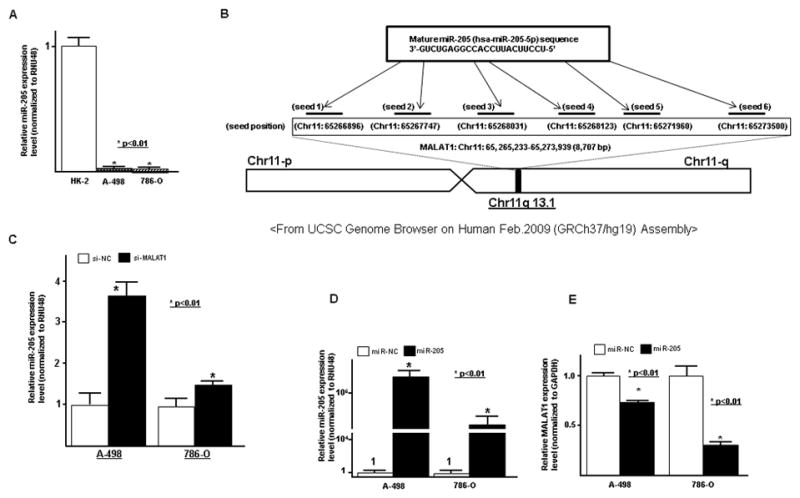
A. miR-205 expression in cell lines (HK-2, A-498, 786-O), B. representation of the miR-205 binding sites in MALAT1 based on miRcode (http://www.mircode.org/mircode/), B. Potential miR-205 binding sites in MALAT1 based on miRcode and UCSC Genome Brower. C. MALAT1 knock down and effect on miR-205 expression (qPCR) in RCC cells (A-498 and 786-O), D. Overexpression of miR-205 in A-498 and 786-O transfected cells (qPCR), E. Effect of miR-205 overexpression on MALAT1 in transfected RCC cell lines (A-498 and 786-O)(qPCR)
Discussion
LncRNA MALAT1 (metastasis associated in lung adenocarcinoma transcript 1) has been reported to be up-regulated in many cancers (33). Initially we looked at MALAT1 mRNA expression in matched normal human kidney and renal cancer tissues and found that MALAT1 expression was significantly higher in renal cancer tissues (all samples: ccRCC). High MALAT1 expression was also associated with high stage, metastasis and shorter overall survival after radical nephrectomy in RCC patients. After siRNA knock down of MALAT1, cell proliferation and invasion were significantly decreased while the percentage of apoptotic cells was significantly increased in renal cancer cells. These results suggest that MALAT1 functions as an oncogene in renal cancer.
Based on previous reports, many transcription factors are highly expressed in cancer tissues and several (c-Myc, B-MYB, E2F, PU.1) induce upregulation of lncRNAs (23–26). In order to determine the reason for high MALAT1 expression in renal cancer tissues, we focused on the proto-oncogenic transcription factor, c-Fos (34) since inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene induces activation of c-Jun-NH2-kinase (JNK) via phosphorylation of members of the AP1 family of transcription factors such as c-Jun and c-Fos and Twist expression is upregulated, resulting in induced EMT in RCC (20). We also found that c-Fos expression was significantly higher in renal cancer compared to normal kidney tissues and high c-Fos expression was associated with shorter overall survival in RCC patients (data not shown). We observed significant positive correlation between c-Fos and MALAT mRNA expression in renal cancer tissues and found c-Fos binding sites in the MALAT1 promoter region. Luciferase assay showed that c-Fos directly binds to lncRNA MALAT1 in 786-O cells, suggesting that c-Fos regulates MALAT1 expression in renal cancer. Future studies will be essential to understand the role of other transcription factors in the activation of MALAT1.
We sought to determine the underlying molecular mechanisms by which MALAT1 regulated downstream effectors in renal cancer. To examine this, we focused on Ezh2, a Polycomb Repressive Complex 2 (PRC2) subunit, since some lncRNAs regulate downstream effectors via Ezh2 driven H3K27 methylation (27). It was first discovered that lncRNA HOTAIR mediates its effect by interacting with Polycomb Repressive Complex 2 (PRC2), enhancing methylation of histone H3 lysine 27 (H3K27), leading to silencing of tumor suppressor genes (35). Previous reports have shown that Ezh2 expression is high in renal cancer tissues (36) promoting renal cancer progression, invasion and metastasis (37). Ezh2 expression is also associated with metastasis and adverse clinical outcome in RCC patients (37). Thus we investigated Ezh2 expression in renal cancer and normal kidney tissues as well as cell lines (HK-2, A-498, 786-O). Similar to a previous report (38), we observed that Ezh2 mRNA expression was significantly higher in renal cancer cell lines and renal cancer tissues compared to normal cells and tissues. Also Ezh2 expression in kidney cancer patients was associated with higher stage and shorter overall survival. Other lncRNAs (lncRNA EBIC, lncRNA H19) have been shown to interact with Ezh2 in cancer cells based on RNA-IP experiments (28, 29). Our RIP results also showed that MALAT1 interacts with Ezh2 in renal cancer cell lines and there was significant positive correlation between MALAT1 and Ezh2 mRNA in renal cancer tissues. Together these results indicate that MALAT1 regulates downstream effectors through Ezh2 in renal cancer cells.
Ezh2 enhances methylation of H3K27, leading to gene silencing involved in cancer progression and metastasis (35). Of the various metastasis related pathways, epithelial-mesenchymal transition (EMT) has been extensively studied in numerous cancers including renal cancer (39). EMT is an important step in cancer progression and metastasis and downregulation of tumor suppressor gene E-Cadherin (CDH1) is typically observed during EMT in renal cancer (39). Recently Liu et al found that silencing of Ezh2 with sh-Ezh2 inhibited renal cancer cell invasion and migration as well as upregulation of E-Cadherin (37). Their ChIP assay results showed that H3K27me3 binds to the CDH1 gene promoter and knock down of Ezh2 significantly decreased the occupancy of H3K27me3 on the CDH1 promoter in two RCC cell lines (ACHN and 786-O) (37). In the present study, Ezh2 protein expression was significantly decreased while E-Cadherin protein expression was significantly increased after knocked down of MALAT1. Additionally E-Cadherin protein expression was found to be significantly lower in renal cancer cells and renal cancer tissues and there was inverse correlation between E-Cadherin protein expression and MALAT1 mRNA expression. To determine the effect of H3K27me3 on E-Cadherin, we performed ChIP analysis and found that the level of H3K27me3 was significantly decreased in si-MALAT1 transfected cells compared to control cells, suggesting that MALAT1 may decrease E-Cadherin expression through Ezh2 mediated H3K27me3 in renal cancer. Ezh2 also induces Wnt/beta-catenin signaling hyperactivation (40). The Wnt pathway is usually activated in cancer cells causing un-phosphorylated beta-catenin to accumulate in the cytoplasm, move to the nucleus, and bind to TCF/LEF thereby transcriptionally regulating Wnt target genes such as c-Myc that drive tumor formation and promote tumorigenesis (41). Thus we investigated beta-catenin protein expression after knocked down of MALAT1. As expected, beta-catenin expression was significantly decreased in si-MALAT1 treated renal cancer cells. Additionally the expression of c-Myc, a downstream effector in the Wnt-beta catenin signaling pathway was also significantly decreased in si-MALAT1 treated cells. Beta-catenin in the nuclear fraction was significantly decreased as was TCF reporter activity (TOPflash activity) with MALAT1 knock down in RCC cell lines (A-498, 786-O). Thus MALAT1 exerts its oncogenic effect via Ezh2 thereby regulating EMT and beta-catenin signaling pathways in renal cancer cells. Since MALAT1 and beta-catenin exist mainly in nucleus, Ji et al investigated the direct interaction between the two (42). However there was no direct interaction between MALAT1 and beta-catenin based on a RNA-binding protein immunoprecipitation technique in a colon cancer cell line (42). Future study will be needed to elucidate any direct interaction between MALAT1 and beta-catenin.
Finally, we looked to see if MALAT1 interacts with other non-coding RNAs such as miRNA. As a newly described regulatory mechanism, lncRNA can influence post-transcriptional regulation and interfere with miRNA pathways by competing for shared miRNA response elements (43). In some cases, lncRNAs have miRNA response elements (MRE) and act as a natural miRNA sponge to reduce binding of endogenous miRNAs to target genes. Thus lncRNA may modulate de-repression of miRNA’s target gene expression (10, 43–45). Liu et al have shown that lncRNA HOTAIR functions as a ceRNA to regulate expression of human epithelial growth factor receptor 2 (HER2) through competition for miR-331-3p (46). In contrast miRNA-125b bound to lncRNA MALAT1 and decreased expression of MALAT1 (47). Our results showed reciprocal repression of MALAT1 and miR-205 which may be a new regulatory mechanism between two non-coding RNAs, a finding similar to recent results (23).
In conclusion this is the first report documenting that MALAT1 is highly expressed in clear cell renal cancer tissues and is associated with clinic-pathological parameters including patient outcome. After MALAT1 knock down, cell invasion was decreased and apoptosis increased in RCC cell lines, suggesting that MALAT1 functions as a renal cancer oncogene. Transcriptional activation of MALAT1 by c-Fos contributed to oncogenesis while knock down of MALAT1 inhibited oncogenic function and depleted Ezh2, resulting in inhibition of EMT via recovery of E-Cadherin and downregulation of beta-catenin.
This study shows how overexpression of the long non-coding RNA MALAT1 confers a potent oncogenic signal in renal cancers and may be a novel functional biomarker or therapeutic target in RCC. MALAT1 expression is significantly higher in renal cancer tissues and could be a novel biomarker in blood or urine from RCC patients. Silencing of long non-coding RNAs including MALAT1 via siRNAs could be a useful therapeutically but is complicated because of lncRNAs extensive secondary structure or intracellular localization. Recently Gutschner developed a highly effective silencing method using genomic integration of RNA destabilizing elements (48) which may be useful to silence lncRNA expression in cancer patients. A limitation of the current study is lack of in vivo experiments such as an orthotopic animal model using siRNA or sh-RNA for MALAT1 knock down which may be useful to help the molecular mechanism involved in RCC invasion and metastasis. Future studies using a large cohort of samples from renal cancer patients will be required to establish a strong correlation between MALAT1 expression and renal cancer clinic-pathological characteristics as well as help identify potential clinical application in RCC patients.
Supplementary Material
1
2
3
4
5
6
Acknowledgments
We thank Dr. Roger Erickson for his support and assistance with the preparation of the manuscript. This study was supported by National Center for Research Resources of the National Institutes of Health through Grant Number RO1CA130860, RO1CA138642, RO1CA160079, I01BX001123, VA Merit Review, VA Program Project (PI: R. Dahiya) and Yamada Science Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests were disclosed.
Authors’ Contributions
Conception and design: H. Hirata, R. Dahiya
Development of methodology: H. Hirata
Acquisition of data: K. Nakajima, Z. L. Tabatabai, N. Ishii
Computational analysis: H. Hirata
Writing: H. Hirata
Administrative, technical or material support: V. Shahryari, G. Deng
Study supervision: H. Hirata, Y. Hinoda, R. Dahiya
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Olshan AF, Kuo TM, Meyer AM, Nielsen ME, Purdue MP, Rathmell WK. Racial difference in histologic subtype of renal cell carcinoma. Cancer Med. 2013;2:744–9. doi: 10.1002/cam4.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 4.Linehan WM. Genetic basis of kidney cancer: role of genomics for the development of disease-based therapeutics. Genome Res. 2012;22:2089–100. doi: 10.1101/gr.131110.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh N, Larkin A, Kennedy S, Connolly L, Ballot J, Ooi W, et al. Expression of multidrug resistance markers ABCB1 (MDR-1/P-gp) and ABCC1 (MRP-1) in renal cell carcinoma. BMC Urol. 2009;9:6. doi: 10.1186/1471-2490-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of transcription in human cells. Nature. 2012;489:101–8. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martens-Uzunova ES, Böttcher R, Croce CM, Jenster G, Visakorpi T, Calin GA. Long noncoding RNA in prostate, bladder, and kidney cancer. Eur Urol. 2014;65:1140–51. doi: 10.1016/j.eururo.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–41. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–61. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Shi X, Sun M, Liu H, Yao Y, Song Y. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–66. doi: 10.1016/j.canlet.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Zhou S, Wang J, Zhang Z. An emerging understanding of long noncoding RNAs in kidney cancer. J Cancer Res Clin Oncol. 2014;140:1989–95. doi: 10.1007/s00432-014-1699-y. [DOI] [PubMed] [Google Scholar]
- 12.Qiao HP, Gao WS, Huo JX, Yang ZS. Long non-coding RNA GAS5 functions as a tumor suppressor in renal cell carcinoma. Asian Pac J Cancer Prev. 2013;14:1077–82. doi: 10.7314/apjcp.2013.14.2.1077. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami T, Chano T, Minami K, Okabe H, Okada Y, Okamoto K. Imprinted DLK1 is a putative tumor suppressor gene and inactivated by epimutation at the region upstream of GTL2 in human renal cell carcinoma. Hum Mol Genet. 2006;15:821–30. doi: 10.1093/hmg/ddl001. [DOI] [PubMed] [Google Scholar]
- 14.Bertozzi D, Iurlaro R, Sordet O, Marinello J, Zaffaroni N, Capranico G. Characterization of novel antisense HIF-1α transcripts in human cancers. Cell Cycle. 2011;10:3189–97. doi: 10.4161/cc.10.18.17183. [DOI] [PubMed] [Google Scholar]
- 15.Frevel MA, Sowerby SJ, Petersen GB, Reeve AE. Methylation sequencing analysis refines the region of H19 epimutation in Wilms tumor. J Biol Chem. 1999;274:29331–40. doi: 10.1074/jbc.274.41.29331. [DOI] [PubMed] [Google Scholar]
- 16.Chiesa N, De Crescenzo A, Mishra K, Perone L, Carella M, Palumbo O, et al. The KCNQ1OT1 imprinting control region and non-coding RNA: new properties derived from the study of Beckwith-Wiedemann syndrome and Silver-Russell syndrome cases. Hum Mol Genet. 2012;21:10–25. doi: 10.1093/hmg/ddr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis IJ, Hsi BL, Arroyo JD, Vargas SO, Yeh YA, Motyckova G, et al. Cloning of an Alpha-TFEB fusion in renal tumors harboring the t(6;11)(p21;q13) chromosome translocation. Proc Natl Acad Sci U S A. 2003;100(10):6051–6. doi: 10.1073/pnas.0931430100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuiper RP, Schepens M, Thijssen J, van Asseldonk M, van den Berg E, Bridge J, et al. Upregulation of the transcription factor TFEB in t(6;11)(p21;q13)-positive renal cell carcinomas due to promoter substitution. Hum Mol Genet. 2003;12:1661–9. doi: 10.1093/hmg/ddg178. [DOI] [PubMed] [Google Scholar]
- 19.Chiyomaru T, Fukuhara S, Saini S, Majid S, Deng G, Shahryari V, et al. Long non-coding RNA HOTAIR is targeted and regulated by miR-141 in human cancer cells. J Biol Chem. 2014;289:12550–65. doi: 10.1074/jbc.M113.488593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An J, Liu H, Magyar CE, Guo Y, Veena MS, Srivatsan ES, et al. Hyperactivated JNK is a therapeutic target in pVHL-deficient renal cell carcinoma. Cancer Res. 2013;73:1374–85. doi: 10.1158/0008-5472.CAN-12-2362. [DOI] [PubMed] [Google Scholar]
- 21.Hirata H, Ueno K, Nakajima K, Tabatabai ZL, Hinoda Y, Ishii N, et al. Genistein downregulates onco-miR-1260b and inhibits Wnt-signalling in renal cancer cells. Br J Cancer. 2013;108:2070–8. doi: 10.1038/bjc.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu PY, Erriquez D, Marshall GM, Tee AE, Polly P, Wong M, et al. Effects of a novel long noncoding RNA, lncUSMycN, on N-Myc expression and neuroblastoma progression. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju113. [DOI] [PubMed] [Google Scholar]
- 23.Ma MZ, Li CX, Zhang Y, Weng MZ, Zhang MD, Qin YY, et al. Long non-coding RNA HOTAIR, a c-Myc activated driver of malignancy, negatively regulates miRNA-130a in gallbladder cancer. Mol Cancer. 2014;13:156. doi: 10.1186/1476-4598-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tripathi V, Shen Z, Chakraborty A, Giri S, Freier SM, Wu X, et al. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet. 2013;9:e1003368. doi: 10.1371/journal.pgen.1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldstein O, Nizri T, Doniger T, Jacob J, Rechavi G, Ginsberg D. The long non-coding RNA ERIC is regulated by E2F and modulates the cellular response to DNA damage. Mol Cancer. 2013;12:131. doi: 10.1186/1476-4598-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P, Xue Y, Han Y, Lin L, Wu C, Xu S, et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344:310–3. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- 27.Benetatos L, Voulgaris E, Vartholomatos G, Hatzimichael E. Non-coding RNAs and EZH2 interactions in cancer: long and short tales from the transcriptome. Int J Cancer. 2013;133:267–74. doi: 10.1002/ijc.27859. [DOI] [PubMed] [Google Scholar]
- 28.Sun NX, Ye C, Zhao Q, Zhang Q, Xu C, Wang SB, et al. Long Noncoding RNA-EBIC Promotes Tumor Cell Invasion by Binding to EZH2 and Repressing E-Cadherin in Cervical Cancer. PLoS One. 2014;9:e100340. doi: 10.1371/journal.pone.0100340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett. 2013;333:213–21. doi: 10.1016/j.canlet.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 30.Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, et al. TGF-β-induced upregulation of malat1 promotes bladder cancer metastasis by associating with suz12. Clin Cancer Res. 2014;20:1531–41. doi: 10.1158/1078-0432.CCR-13-1455. [DOI] [PubMed] [Google Scholar]
- 31.Jeggari A, Marks DS, Larsson E. miRcode: a map of putative microRNA target sites in the long non-coding transcriptome. Bioinformatics. 2012;28:2062–3. doi: 10.1093/bioinformatics/bts344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majid S, Saini S, Dar AA, Hirata H, Shahryari V, Tanaka Y, et al. MicroRNA-205 inhibits Src-mediated oncogenic pathways in renal cancer. Cancer Res. 2011;71:2611–21. doi: 10.1158/0008-5472.CAN-10-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutschner T, Hämmerle M, Diederichs S. MALAT1 -- a paradigm for long noncoding RNA function in cancer. J Mol Med (Berl) 2013;91:791–801. doi: 10.1007/s00109-013-1028-y. [DOI] [PubMed] [Google Scholar]
- 34.Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer. 2005;41:2449–61. doi: 10.1016/j.ejca.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–93. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagener N, Holland D, Bulkescher J, Crnković-Mertens I, Hoppe-Seyler K, Zentgraf H, et al. The enhancer of zeste homolog 2 gene contributes to cell proliferation and apoptosis resistance in renal cell carcinoma cells. Int J Cancer. 2008;123:1545–50. doi: 10.1002/ijc.23683. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Xu Z, Zhong L, Wang H, Jiang S, Long Q, et al. EZH2 promotes tumor cell migration and invasion via epigenetic repression of E-cadherin in renal cell carcinoma. BJU Int. 2014 Feb 25; doi: 10.1111/bju.12702. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Xu B, Abourbih S, Sircar K, Kassouf W, Mansure JJ, Aprikian A, et al. Enhancer of zeste homolog 2 expression is associated with metastasis and adverse clinical outcome in clear cell renal cell carcinoma: a comparative study and review of the literature. Arch Pathol Lab Med. 2013;137:1326–36. doi: 10.5858/arpa.2012-0525-OA. [DOI] [PubMed] [Google Scholar]
- 39.He H, Magi-Galluzzi C. Epithelial-to-mesenchymal transition in renal neoplasms. Adv Anat Pathol. 2014;21:174–80. doi: 10.1097/PAP.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 40.Jung HY, Jun S, Lee M, Kim HC, Wang X, Ji H, McCrea PD, et al. PAF and EZH2 induce Wnt/β-catenin signaling hyperactivation. Mol Cell. 2013;52:193–205. doi: 10.1016/j.molcel.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmad I, Morton JP, Singh LB, Radulescu SM, Ridgway RA, Patel S, et al. β-Catenin activation synergizes with PTEN loss to cause bladder cancer formation. Oncogene. 2011;30:178–89. doi: 10.1038/onc.2010.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L, et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PLoS One. 2013;8:e78700. doi: 10.1371/journal.pone.0078700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–8. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–7. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–52. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB, Yin DD, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13:92. doi: 10.1186/1476-4598-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han Y, Liu Y, Zhang H, Wang T, Diao R, Jiang Z, et al. Hsa-miR-125b suppresses bladder cancer development by down-regulating oncogene SIRT7 and oncogenic long noncoding RNA MALAT1. FEBS Lett. 2013;587:3875–82. [PubMed] [Google Scholar]
- 48.Gutschner T. Silencing Long Noncoding RNAs with Genome-Editing Tools. Methods Mol Biol. 2015;1239:241–250. doi: 10.1007/978-1-4939-1862-1_13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3
4
5
6