SAR405, a PIK3C3/Vps34 inhibitor that prevents autophagy and synergizes with MTOR inhibition in tumor cells (original) (raw)
Abstract
Autophagy plays an important role in cancer and it has been suggested that it functions not only as a tumor suppressor pathway to prevent tumor initiation, but also as a prosurvival pathway that helps tumor cells endure metabolic stress and resist death triggered by chemotherapeutic agents. We recently described the discovery of inhibitors of PIK3C3/Vps34 (phosphatidylinositol 3-kinase, catalytic subunit type 3), the lipid kinase component of the class III phosphatidylinositol 3-kinase (PtdIns3K). This PtdIns3K isoform has attracted significant attention in recent years because of its role in autophagy. Following chemical optimization we identified SAR405, a low molecular mass kinase inhibitor of PIK3C3, highly potent and selective with regard to other lipid and protein kinases. We demonstrated that inhibiting the catalytic activity of PIK3C3 disrupts vesicle trafficking from late endosomes to lysosomes. SAR405 treatment also inhibits autophagy induced either by starvation or by MTOR (mechanistic target of rapamycin) inhibition. Finally our results show that combining SAR405 with everolimus, the FDA-approved MTOR inhibitor, results in a significant synergy on the reduction of cell proliferation using renal tumor cells. This result indicates a potential therapeutic application for PIK3C3 inhibitors in cancer.
Keywords: autophagy, MTOR, PIK3C3/Vps34, renal cell carcinoma, vesicle trafficking
Macroautophagy (hereafter referred to as autophagy) is a lysosome-dependent mechanism of intracellular degradation. Autophagy can be seen as a housekeeper process as it occurs naturally in most cells in order to eliminate protein aggregates and damaged organelles. Under stress conditions, this mechanism can be upregulated allowing cell survival via the degradation and recycling of proteins, carbohydrates, and lipids. Dysregulation of autophagy was described in many diseases such as cancer and infectious or neurodegenerative diseases. Because of the lack of specific inhibitors of the autophagy machinery, the precise role of this cellular process in physiology and diseases remains a matter of debate.
With the aim to develop autophagy inhibitors we decided to perform a high-throughput phenotypic screen (HTS) using starved GFP-LC3 HeLa cells and our proprietary chemical compounds collection. After chemogenomic deconvolution we identified compounds that inhibit PIK3C3. This lipid kinase is one of the druggable targets in the signaling pathways that regulate the autophagy machinery (Fig. 1A). The fact that we obtained chemical compounds inhibiting PIK3C3 validated our phenotypic screening approach. However, it is important to note that PIK3C3 was also described as regulating intracellular vesicle trafficking during endocytosis.
Figure 1.
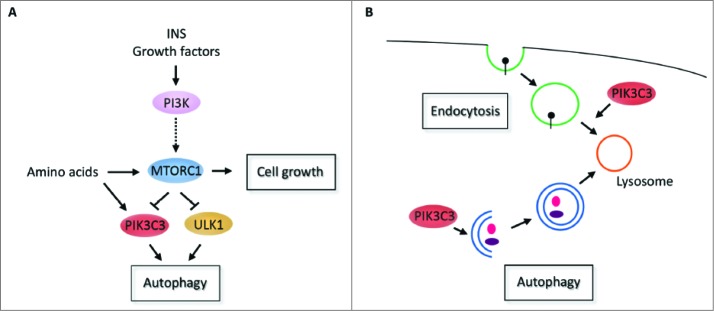
(A) Signaling pathways that regulate autophagy. In fed conditions, insulin (INS) and/or growth factors, MTOR complex 1 (MTORC1) is activated via a known signaling pathway including class I PI3K and negatively regulates downstream kinases such as PIK3C3 and ULK1 (unc-51 like autophagy activating kinase 1) leading to a prevention of autophagy. In this condition, MTORC1 is also a key regulator of cell growth. Under starvation, the activity of MTORC1 is suppressed releasing the inhibition of PIK3C3 and ULK1 and allowing the induction of the autophagy machinery. (B) Cellular functions of PIK3C3. The process of autophagy starts with the generation of autophagosomes that then fuse with the lysosome allowing the degradation of the autophagosome contents. Our results using SAR405 demonstrated that PIK3C3 has a dual role during the initial steps of autophagy, with the generation of autophagosomes, and also during endocytosis between late endosomes to lysosomes.
Following chemical optimization of hit compounds from HTS, we identified SAR405 as a new kinase inhibitor of PIK3C3. In order to characterize SAR405, biochemical, biophysical, and cellular assays were developed. We showed that SAR405 has a binding equilibrium constant KD of 1.5 nM and an on-target IC50 activity on GFP-FYVE HeLa cells of 27 nM. The structural analysis of human PIK3C3 in complex with SAR405 showed that SAR405 binds in the ATP-binding cleft of PIK3C3. Because PIK3C3 is phylogenetically related to class I phosphoinositide3-kinase (PI3K), class II PI3K-PtdIns3K and MTOR, we then assessed the off-target activity of SAR405 on these kinases. Our results indicated that SAR405 displays an exquisite selectivity profile on class I and II isoforms of PI3K-PtdIns3K, and on MTOR. When profiled on a large panel of kinases, the inhibitor showed no activity on other lipid and protein kinases, demonstrating the high selectivity of SAR405 for PIK3C3.
We then interrogated the biological effect of SAR405 on vesicle trafficking. Whereas the function of early endosomes was not affected by SAR405, our results showed that inhibiting PIK3C3 kinase activity disrupts the late endosome and lysosome compartments and results in a dysfunction of lysosomes after cell treatment with SAR405.
The effect of SAR405 on autophagy was then investigated. We first used the GFP-LC3 model used for the HTS and confirmed its activity on starved cells (IC50 = 419 nM). The conversion of LC3-I into LC3-II was also analyzed by western blotting on wild-type HeLa and H1299 cells. Treatment of starved cells with SAR405 completely inhibits the conversion to LC3-II in a dose-dependent manner. As MTOR is known as a negative regulator of autophagy upstream of PIK3C3 (Fig. 1A), we assessed if SAR405 could prevent autophagy induced by an MTOR inhibitor. Using the ATP-competitive MTOR inhibitor AZD8055, we showed that SAR405 prevents autophagosome formation with an IC50 of 42 nM.
The allosteric MTOR inhibitors have been approved for the treatment of advanced kidney cancer but the clinical results were, so far, less successful than expected. One hypothesis to explain their limited efficacy is that the induction of autophagy by MTOR inhibitors could mediate the apparent resistance to this chemotherapeutic agent. Evidence for this hypothesis could come from the ongoing clinical trial with hydroxychloroquine, a lysosomotropic drug that is being evaluated for renal cell carcinoma treatment in combination with everolimus. As we showed that 1) SAR405 is a proximal inhibitor of the autophagy machinery, which corresponds to a new mechanism of action for a small molecule drug, and 2) SAR405 displays a 10-fold lower IC50 on autophagy inhibition when induced by an MTOR inhibitor, we were interested to evaluate the potential therapeutic benefit of the FDA-approved MTOR inhibitor everolimus in combination with SAR405. Using a ray design methodology, we were able to measure a significant synergistic antiproliferative activity in vitro with both inhibitors in H1299 and in 2 renal tumor cell lines, ACHN and 786-O, providing a rationale for a potential application in renal cell carcinoma.
Collectively, our results described SAR405 as a new potent and selective PIK3C3 inhibitor. We demonstrated that SAR405 inhibits a key node, not only for vesicle trafficking from late endosomes to lysosomes, but also of autophagosome biogenesis in response to multiple stimuli (Fig. 1B). Our data also strongly support interplay between MTOR and PIK3C3 inhibition. SAR405 is a unique specific kinase inhibitor for further deciphering the autophagy machinery and for clarifying the potential role of autophagy in pathophysiologies.
Disclosure of Potential Conflicts of Interest
BP is a Sanofi employee and also a shareholder of Sanofi stock.