A Comprehensive Review on the Phytochemical Constituents and Pharmacological Activities of Pogostemon cablin Benth.: An Aromatic Medicinal Plant of Industrial Importance (original) (raw)
Abstract
Pogostemon cablin Benth. (patchouli) is an important herb which possesses many therapeutic properties and is widely used in the fragrance industries. In traditional medicinal practices, it is used to treat colds, headaches, fever, nausea, vomiting, diarrhea, abdominal pain, insect and snake bites. In aromatherapy, patchouli oil is used to relieve depression, stress, calm nerves, control appetite and to improve sexual interest. Till now more than 140 compounds, including terpenoids, phytosterols, flavonoids, organic acids, lignins, alkaloids, glycosides, alcohols, aldehydes have been isolated and identified from patchouli. The main phytochemical compounds are patchouli alcohol, α-patchoulene, β-patchoulene, α-bulnesene, seychellene, norpatchoulenol, pogostone, eugenol and pogostol. Modern studies have revealed several biological activities such as antioxidant, analgesic, anti-inflammatory, antiplatelet, antithrombotic, aphrodisiac, antidepressant, antimutagenic, antiemetic, fibrinolytic and cytotoxic activities. However, some of the traditional uses need to be verified and may require standardizing and authenticating the bioactivity of purified compounds through scientific methods. The aim of the present review is to provide comprehensive knowledge on the phytochemistry and pharmacological activities of essential oil and different plant extracts of patchouli based on the available scientific literature. This information will provide a potential guide in exploring the use of main active compounds of patchouli in various medical fields.
Keywords: Pogostemon cablin, biological activities, phytomedicine, essential oil
1. Introduction
A major segment of the flora includes medicinal and aromatic plants which are the source of raw materials used in the pharmaceutical, fragrance, cosmetic, flavor and perfumery industries. In spite of much progress made in synthetic drug research, plants and their products are still considered to be the major sources of medicaments and have extensive use in the pharma industry [1,2]. Most modern medicines are derived from plants and their products obtained by applying modern technologies to traditional practices [3]. The use of plants is customary in Indian systems of medicine like Ayurveda, Unani, Sidda and many other indigenous and folk practices [4]. Pogostemon cablin Benth. (patchouli), belonging to the family Lamiaceae is an aromatic herb having immense commercial importance. Patchouli plants have been widely used in traditional medicinal practices in India and China to treat many medical ailments. In Chinese medicine, it has been used to remove dampness, relieve summer heat and exterior syndrome, and as an anti-emetic and appetite stimulant [5]. Patchouli plant is a component of the traditional Chinese medicine, Pogostemoni Herba which is widely used in the cosmetic and hygiene industries. Chinese traditional formulae, such as Baoji Pill and Houdan Pill containing patchouli herb are used in treating inflammatory diseases [6,7]. The plant is included in the preparations of Indian Ayurvedic treatments such as Rasa, Guna and Virya. In China, Japan and Malaysia, it is used to treat colds, headaches, nausea, vomiting, diarrhea, abdominal pain, insect and snake bites [5]. The commercial importance of patchouli is due to its oil, which can be obtained by steam distillation of the shade dried leaves. It possesses a powerful sweet, herbaceous aromatic, spicy fragrance. [8]. Among all the essential oil yielding plants, patchouli is considered to have tremendous business potential in the global market because of its unique flavor and fragrance properties, and also biological activities [9,10,11]. Though it is not a dominant source of fragrance, its ability to blend with other essential oils gives a strong base, lasting character, fixative properties and aids in preventing evaporation thus promoting tenacity. Hence, the oil is widely used in the manufacturing of soaps, scents, body lotions and detergents [12]. Patchouli oil is used in aromatherapy to relieve depression, stress, calm nerves, control appetite and to improve sexual interest. Patchouli also possesses insecticidal, antibacterial and antifungal properties [13,14,15]. Some of its other biological activities include antimicrobial, antioxidant, analgesic, anti-inflammatory, antiplatelet, antithrombotic, aphrodisiac, antidepressant, antimutagenic, antiemetic, fibrinolytic and cytotoxic activities [13,16,17]. Therefore, in this review, a compilation of literature is made in order to produce a comprehensive report related to phytochemistry of different plant parts of P. cablin and various biological properties exhibited by purified compounds as well as by the crude extracts. An updated research survey was carried out by using various search engines like Google Scholar, Scopus, PubMed and ScienceDirect.
2. Botanical Description
Patchouli is a native of the Philippines and grows wild in many South Asian countries, and is presently cultivated on a commercial scale in India, Indonesia, Malaysia, China, Singapore, West Africa and Vietnam [18,19]. The word ‘cablin’ is derived from ‘cabalam’ which is also a local name for the patchouli plant in the Philippines and these are synonymous [20]. About 25 species of Pogostemon are reported to occur in India. Patchouli is also known as patchouly, tamala patra in Sanskrit, patcholi in Hindi, patche tene in Kannada, pacchilai in Tamil, patchilla in Malayalam, patchapan or patcha in Marathi and guang hou xiang in Chinese, nilam in Malaysia and Indonesia, phimsen in Thailand. Morphologically, patchouli is a hardy perennial herb adapted to hot and humid climatic conditions. It grows up to 1 to 1.2 m height with an erect stem and broad leaves (0.85 inches). The margins of the leaves are lobed and abundant hairs are present on its dorsal surface [21]. The leaves are known to accumulate essential oil in the glandular trichomes [22]. The plant bears small pale pink-white flowers [13].
3. Phytochemistry
The potential benefits of P. cablin have been well explored in recent years by many scientific communities. Various studies on its chemistry and biological activities are well documented and more recent research studies are focused on isolating individual compounds to understand their mechanism involved in various pharmacological activities. The title plant contains various phytochemicals including many monoterpenoids, triterpenoids, sesquiterpenoids, phytosterols, flavonoids, organic acids, lignins, glycosides, alcohols and aldehydes.
3.1. Volatile Chemical Composition of P. cablin
A major constituent obtained from the leaves of P. cablin is the essential oil (patchouli oil) which possesses many pharmacological properties. The curative action of patchouli oil is directly related to its qualitative as well as quantitative chemical constituents. The known volatile chemical components of patchouli oil are presented in Table 1, while the structures of some major and important compounds are depicted in Figure 1. Generally, steam or fractional distillation is used to obtain patchouli oil [23,24], although these processes cause the thermal degradation of a few of the chemical constituents present in the oil. Therefore, Donelian et al. [25] established the supercritical carbon dioxide extraction method to obtain the maximum yield of patchouli oil with better extraction quality. Molecular distillation has also been reported by Hu et al. and Chen et al. [26,27]. However, microwave assisted extraction using ethanol as solvent improves oil extraction compared to other conventional methods as it employs a precise computer controlled high temperature in a closed vessel equipped with a magnetic stirrer [28].
Table 1.
The volatile constituents of P. cablin.
| Compound Name | Formula | Analytical Method | References |
|---|---|---|---|
| Aciphyllene * | C15H24 | GCMS | [37,38] |
| Alloaromadendrene | C15H24 | GC | [39] |
| Aromadendrene | C15H24 | GC | [39] |
| β-Bourbonene | C15H24 | GC×GC–TOF MS | [40] |
| α- and β-Bulnesene | C15H24 | GCMS;NMR | [31,32,41,42,43,44,45,46] |
| (+)-Camphene | C10H16 | GC | [39] |
| (−)-Camphor | C10H16O | GC×GC–TOF MS | [40] |
| δ-Cardinene | C15H24 | GC;GCMS;NMR | [40,41] |
| α-Caryophyllene (α-Humulene) | C15H24 | GCMS;NMR | [11,31,32,37,38,39,40,41,42,44,45,47] |
| β-Caryophyllene * | C15H24 | GCMS | [37,48] |
| _trans_-Caryophyllene * | C15H24 | GCMS | [37,44,49] |
| Caryophyllene oxide | C15H24O | GC×GC–TOF MS;GCMS | [36,38,40] |
| Copaene | C15H24 | GC | [11,38] |
| β-Cubebene | C15H24 | GCMS | [45,46] |
| β-Copaen-4-α-ol | C15H24O | GCMS | [44] |
| Cycloseychellene | C15H24 | GC;GCMS | [31,32,38] |
| Diidro-aromadendrane | C15H26 | GCMS | [44] |
| α-, β- and δ-Elemene | C15H24 | GC;GCMS; NMR | [11,38,39,40,41,44,45] |
| Elemol | C15H26O | GC×GC–TOF MS | [40] |
| Epiglobulol | C15H26O | GC×GC–TOF MS | [40] |
| Eucalyptol | C10H18O | GC×GC–TOF MS | [40] |
| α-Elemenone | C15H22O | GC×GC–TOF MS | [40] |
| Epifriedelinol | C30H52O | NMR;IR;MS;UV | [50] |
| 7-Epi-α-selinene | C15H24 | GCMS | [40,47] |
| _cis_-Farnesol | C15H24O | GC×GC–TOF MS | [40] |
| Friedelin | C30H50O | NMR;IR;MS;UV | [11] |
| Germacrene- A, B, D | C15H24 | GC/GCMS | [11,38,45,46] |
| Globulol | C15H26O | GC×GC–TOF MS | [40,46] |
| α-, β- and δ-Guaiene | C15H24 | GC;GCMS; NMR | [11,31,32,36,37,38,39,40,41,42,45,47] |
| α-, γ-Gurjunene | C15H24 | GC; GCMS | [38,50] |
| Heptanal | C7H14O | GC×GC–TOF MS | [40] |
| Limonene | C10H16 | GC;GCMS; GC×GC–TOF MS | [38,39,40] |
| Longipinanol | C15H26O | GCMS | [47] |
| Myrtenol | C10H16O | GC×GC–TOF MS | [40] |
| Nonanal | C9H18O | GC×GC–TOF MS | [40] |
| Norpatchoulenol | C14H22O | GCMS | [11,31,32,40] |
| 1-Octen-3-ol | C8H16O | GCMS | [43,47] |
| 3-Octanol | C8H18O | GC×GC–TOF MS | [36,39] |
| Oleanolic acid | C30H48O3 | NMR;IR;MS;UV | [39] |
| Patchouli alcohol ** | C15H26O | GC;GCMS; NMR | [11,31,32,38,39,40,41,43,44,46,47] |
| α-,β-,γ- and δ-Patchoulene | C15H24 | GCMS; GC;GCMS; NMR | [31,32,36,37,39,40,41,42,43,44,46,47,50] |
| β-Phellandrene | C10H16 | GC×GC–TOF MS | [40] |
| α- and β-Pinene | C10H16 | GC;GCMS | [36,39,40,47,50] |
| Pogostol | C15H26O | GC;GCMS; NMR | [31,32,39,40,41,50] |
| Pogostone | C12H16O4 | NMR;IR;MS;UV | [11,36,40,41,43,44,47,49,50,51] |
| α- and β-Selinene | C15H24 | GC×GC–TOF MS | [36,40,45,46,47] |
| Seychellene | C15H24 | GC;GCMS; NMR | [31,32,37,39,40,41,42,44,46,47,50] |
| Spathulenol | C15H24O | GCMS;GC×GC–TOF MS | [40,44] |
| (−)-α-Terpineol | C10H18O | GC×GC–TOF MS | [40] |
| Valencene | C15H24 | GCMS | [47] |
| Viridiflorene | C15H26O | GCMS | [47] |
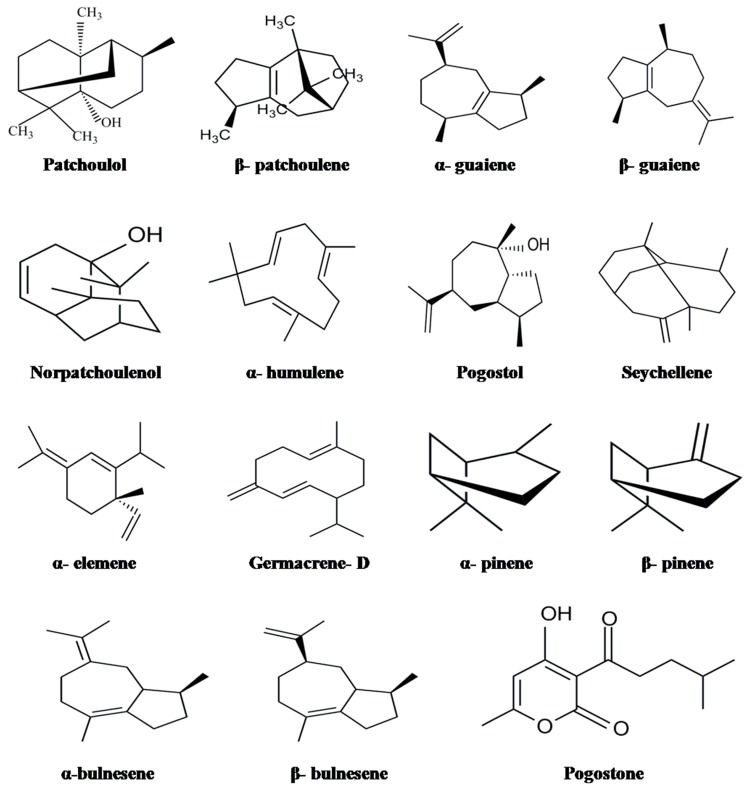
Figure 1.
The structures of some of the volatile chemical constituents.
Patchouli oil is rich in sesquiterpenes, mainly the patchouli alcohol (patchoulol), a tricyclic sesquiterpene which is widely used in perfumery products, soaps and other cosmetic goods [29]. Patchoulenes, guaiene, seychellene are few other sesquiterpene hydrocarbons also characterize the aroma of patchouli oil. According to many researchers, patchoulol and α-patchoulene are the major constituents which regulate and control patchouli oil quality [25,29,30]. Several other minor sesquiterpenes, caryophyllene, pogostol, α-, β-, γ- and δ-patchoulene, seychellene, cycloseychellene, α- and β-bulnesene, α- and β-guaiene and norpatchoulenol are also reported in patchouli oil [31,32]. The biological activities of patchouli oil are strongly associated with the chemical constituents such as pogostone, patchoulol, α- and β-patchoulene. Previous studies have stated that pogostone as one of the major chemical constituent of patchouli oil largely responsible for the intense aromatic odor and more recently this compound has been demonstrated to exert many pharmaceutical activities [33,34,35]. Later on, the chemical structure of pogostone present in the patchouli oil was revealed by Zheng et al. [36].
The chemical composition of patchouli oil varies among samples collected from different geographic locations. Li et al. [52] revealed the significant effect of different habitats, collection periods, and processing methods on the volatile oil yield and its main constituents. Similarly, the oil yield is also influenced by different collection times. It is reported that contents of volatile oil obtained from leaves harvested from June to August and cultivated in Hainan, China were 0.8%, 0.7% and 0.6%, respectively, while the patchouli alcohol content was highest in the month of June [53]. Gas chromatography (GC), Gas chromatography/mass spectroscopy (GC/MS) and nuclear magnetic resonance (NMR) were used for studying the chemical composition of patchouli oil from Vietnam. The major compounds identified were α-, β- and δ-patchoulene, β-elemene, β-caryophyllene, α- and δ-guaiene, seychellene, α-bulnesene, δ-cardinene, pogostol and patchouli alcohol. The presence of 32%–37% patchouli alcohol content was found to be more odor intensive component of the essential oil [41]. However, in the Philippines, it was predicted that a distinct aroma is due to the occurrence of germacrene-B, a new sesquiterpene identified as the major component of the patchouli oil [11]. Later, Rakotonirainy et al. [42] used 1- and 2-dimensional NMR and GC/MS to derive the structural formulae of the compounds, including α-patchoulene, α-bulnesene, α-guaiene, and seychellene.
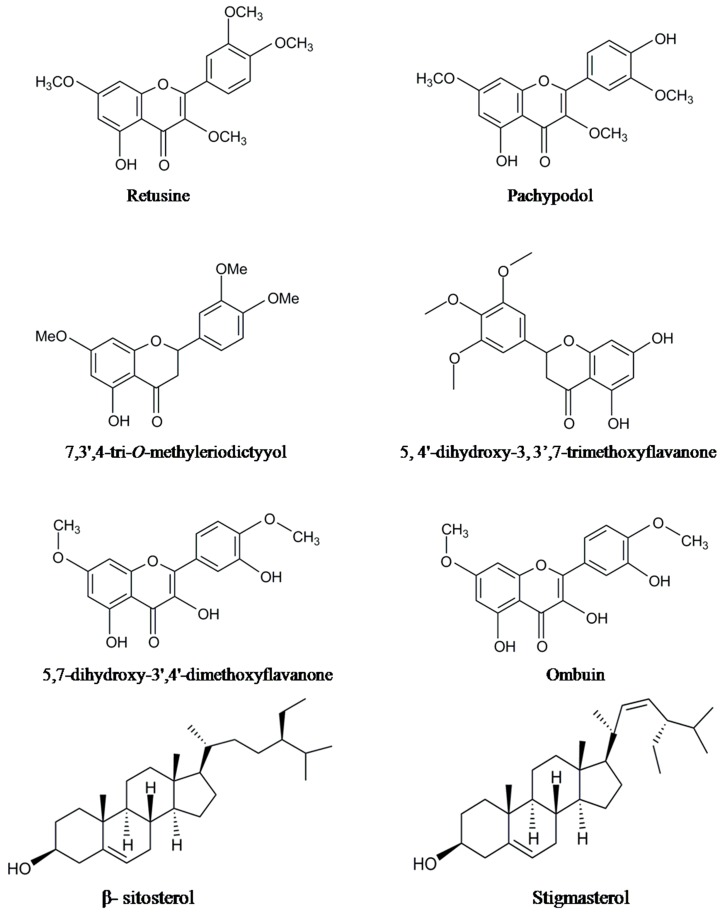
The essential oil from P. cablin plants collected from China (Gaoyao County, Guangdong Province) and its volatile chemical compositions were analyzed by GC/MS. The study revealed the presence of pogostone (30.99% in stems, 21.31% in leaves), patchouli alcohol (10.26% in stems, 37.53% in leaves), _trans_-caryophyllene (4.92% in stems, 6.75% in leaves), α-guaiene (2.27% in stems, 6.18% in leaves) and seychellene (1.56% in stems, 1.99% in leaves) as the main constituents [49]. Similarly, GC/MS analysis of essential oil extracted from both leaves and stems of Patchouli plants collected from the Leizhou County of China revealed sesquiterpenes such as patchouli alcohol, α-guaiene, δ-guaiene, α-patchoulene, seychellene, aciphyllene and _trans_-caryophyllene [37]. Guan et al. [50] have identified nine sesquiterpene compounds, namely patchouli alcohol, pogostone, frieddelin, epifriedelinol, pachypodol, retusine, oleanolic acid, β-sitosterol and daucosterol on the basis of spectral data. In the same year, Kang et al., developed an efficient method to isolate and determine the patchoulol constituent quantitatively from P. cablin [39].
Patchouli oil analyzed by comprehensive two–dimensional gas chromatography time-of-flight mass spectrometry contained mainly monoterpenes and sesquiterpenes [40]. The identified compounds were; (−)-β-pinene, β-pinene, β-phellandrene, δ-elemene, limonene, eucalyptol, 3-octanol, heptanal, nonanal, (−)-camphor, β-bourbonene, β-elemene, α-guaiene, 4-terpinenol, (−)-α-terpineol, α-patchoulene, β-patchoulene, α-caryophyllene, δ-guaiene, β-selinene, δ-cadinene, myrtenol, caryophyllene oxide, elemol, α-elemenone, globulol, epiglobulol, patchoulol, (−)-spathulenol, ledene oxide-(I), _cis_-farnesol and pogostone. A GC and GC/MS study of Indonesian patchouli oil indicated the presence of the following compounds; α-pinene, δ-patchoulene, β-pinene, aciphyllene, limonene, δ-guaiene, δ-elemene, 7-_epi_-α-selinene, α-copaene, norpatchoulenol, α-patchoulene, 1,10-epoxy-11-bulnesene, β-elemene, caryophyllene oxide, cycloseychellene, nortetrapatchoulol, β-caryophyllene, patchouli alcohol, α-guaiene, patchoulenone, seychellene, 9-oxopatchoulol, α-humulene, pogostol, α-patchoulene, isopatchoulenone, γ-gurjunene, and germacrene D [38]. A successful GC/MS method for determining patchoulol content in the dried samples of patchouli was developed and demonstrated by Zhao et al. [54].
Patchouli plants collected from different cultivation regions and harvested at different times showed differences in their volatile oil compositions [43,44,47]. Thus, P. cablin is differentiated into 2-chemotypes namely, pogostone type and patchouliol type on the basis of chemical differences in their volatile oil composition [43]. The GC/MS profile of the patchouli oils collected at different time periods during 24 h showed the presence of 1-octen-3-ol, β-patchoulene, β-elemene, _trans_-caryophyllene, α-guaiene, γ-patchoulene, α-hmulene, α-patchoulene, dihydroaromadendrane, _trans_-β-guaiene, α-bulnesene, β-copaen-4-α-ol, and patchouli alcohol. However, they did not present any significant difference as a function of the harvest time [44]. The chemical profile of patchouli oil collected from different regions of China was identified with nine compounds, namely α-guaiene, β-guaiene, δ-guaiene, β-patchoulene, caryophyllene, seychellene, spathulenol, patchouliol and pogostone. Based on the GC profiles, 18 samples were categorized into three major groups, patchouliol type, pogostone type and interim type [47]. Among the various compounds identified from patchouli oil using GC/MS, patchoulol, germacrene-A, α-guaiene, α-bulnesene, β-patchoulene and patchouli alcohol were the major components while β-pinene, β-elemene, β-caryophyllene, α-patchoulene, β-cubebene and α-selinene were the minor components [45,46].
Zeng et al. [36] studied and compared the volatile chemical components of P. cablin from different culture varieties to produce a chromatographic fingerprint and identified the structure of pogostone. They isolated eight new compounds, including _trans_-famisol, 1-(propen-2-yl)-4-methylspiro-(4,5)-decan-7-one, 1,2,3,4-tetrahydro-1,6-dimethyl-4-(1-methyl)ethylnapthalene, 1,2,3,4,4a,5,6,8a-octa- hydro-4a,8-dimethyl-2-(1-methylethenyl)-2_R_-(2α,4a,α,8a,β)-napthalene, 1,8,8,10-tetramethylcyclo-decene, aristolone, 2,4,5-trimethoxy-1-propenylbenzene and 1,2,4,6-tetramethyltricyclo[5,4,0,0(3,9) undecan-2-antiol. The essential oil of _P. cablin_ contained 11 compounds while essential oils of _P. travancoricus_ contained 13 compounds. The components such as α-patchoulene and β-patchoulene, patchoulol, β-caryophyllene, α-guaiene, seychellene and selinene were common to both species. However, _P. travancoricus_ had a relatively lesser quantity [48]. It was found that lab-scale produced oil had a maximum patchoulol content compared to other commercial patchouli samples [55]. More recently, Li et al. [51] developed a more sensitive, precise and accurate HPLC-DAD method to quantify the volatile compound, pogostone and eight other non-volatile flavonoids such as apigenin, rhamnetin, ombuine, 5-hydroxy-7,3ʹ,4ʹ-trimethoxyflavanone, 4ʹ,5-dihydroxy-3,3ʹ,7-trimethoxyflavone, 3,5-dihydroxy-7,4ʹ-dimethoxyflavone, 5-hydroxy-3,3ʹ,4ʹ,7-tetramethoxyflavone and 5-hydroxy-3,4ʹ,7-trimethoxyflavone.
3.2. Non-Volatile Chemical Composition of P. cablin
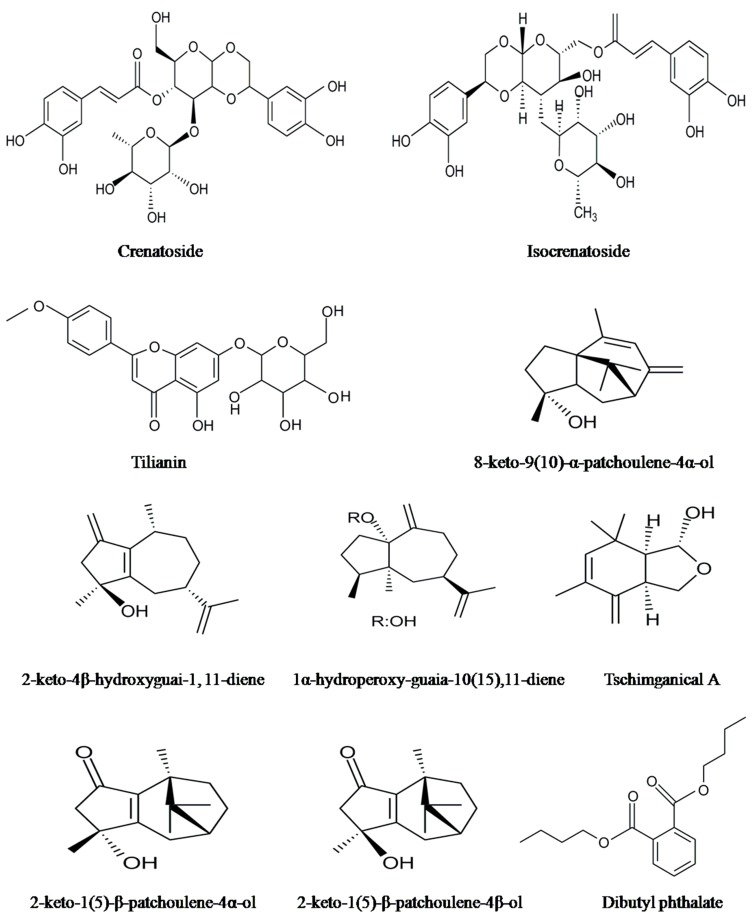
Various phytochemicals belonging to flavonoids, glycosides, triterpenes, sesquiterpenes, lignins, aldehydes, and organic acids along with few other constituents were found as major and minor non-volatile components of P. cablin (Table 2). The structures of some major and important compounds are illustrated in the Figure 2. Park et al. [56] isolated and identified three flavonoids by using cytotoxicity-guided fractionation and spectral analysis. The identified non-volatile compounds were licochalcone A, ombuin and 5,7-dihydroxy-3ʹ,4ʹ-dimethoxyflavanone. Using a TLC and HPLC technique, Amakura et al. [57] assessed the quality of Pogostemoni Herba, a crude drug used in Chinese Kampo medicine and developed a potential chemical marker from methanol extracts of dried leaves of P. cablin. They isolated the first time three phenylethanoids (isoacteoside, acteoside and crenatoside) from this plant. Using a column chromatography technique and spectral data, nine non-volatile chemical compounds were isolated from methanol leaf extracts of patchouli and identified as epifriedelinol, 5-hydroxymethyl-2-furfural, succinic acid, β-sitosterol, daucosterol, 3ʹʹʹ-_O_-methyl-crenatoside, crenatoside, isocrenatoside, and apigenin-7-_O_-β-d-(6ʺ-_p_-coumaryl)-glucoside [58]. Among these compounds, 5-hydroxymethyl-2-furfural, succinic acid, crenatoside, 3ʹʹʹ-_O_-methylcrenatoside and isocrenatoside were identified for the first time in the genus Pogostemon. Five target compounds, including pogostone and four flavonoids 4ʹ,5-dihydroxy-3ʹ,7-dimethoxyflavanone, 5,4ʹ-hihydroxy-3,7,3ʹ-trimethoxy-flavanone, 5-hydroxy-7,3ʹ,4ʹ-trimethoxyflavanone, 5-hydroxy-3,7,3ʹ,4ʹ-tetrmethoxyflavanone were obtained from the ethanol and hexane extracts of P. cablin by using high speed countercurrent chromatography and preparative HPLC techniques [59].
Table 2.
The non-volatile constituents of P. cablin.
| Compound Name | Formula | Plant Part | Analytical Method | References |
|---|---|---|---|---|
| Flavonoids | ||||
| Acacetin | C16H12O5 | Stem (Ethanol extract) | IR; HR; NMR | [60] |
| Apigenin | C15H10O5 | Air dried root (Ethanol extract) | HPLC-DAD | [51,58,60] |
| Diosmetin-7-_O_-β-d-gluco-pyranoside | C22H22O11 | Stem (Ethanol extract) | IR; HR; NMR | [60] |
| 4ʹ,5-Dihydroxy-3,3ʹ,7-trimethoxyflavone | C18H16O7 | Stem/Root (Ethanol extract) | IR; HR; NMR; HPLC-DAD | [51,60] |
| 4ʹ,5-Dihydroxy-3ʹ,7-dimethoxyflavanone | C17H16O6 | Whole plant (Ethanol & hexane extracts) | HSCCC; prep-HPLC;NMR | [59] |
| 5,4ʹ-Dihydroxy-3,3ʹ,7-trimethoxyflavanone | C18H18O7 | Whole plant/Root (Ethanol & hexane extracts) | HSCCC; prep-HPLC; NMR; HPLC-DAD | [51,59] |
| 3,5-Dihydroxy-7,4ʹ-dimethoxyflavanone | C29H36O15 | Whole plant/Root (Ethanol & hexane extracts) | HSCCC; prep-HPLC; NMR; HPLC-DAD | [51] |
| 5,7-Dihydroxy-3ʹ,4ʹ-dimethoxyflavanone | C29H36O15 | Aerial parts (Methanol extract) | UV; IR; MS; NMR | [56] |
| 5-Hydroxy-3,3ʹ,4ʹ,7-tetramethoxyflavone | C19H18O7 | Stem (Ethanol extract) | IR; HR; NMR | [51,60] |
| 5-Hydroxy-7,3ʹ,4ʹ-trimethoxyflavanone | C19H20O7 | Whole plant/Root (Ethanol & hexane extracts) | HSCCC; prep-HPLC; NMR; HPLC-DAD | [51,59] |
| 5-Hydroxy-3,7,3ʹ,4ʹ-tetrmethoxyflavanone | C19H18O6 | Whole plant/Root (Ethanol & hexane extracts) | HSCCC; prep-HPLC; NMR; HPLC-DAD | [51,59] |
| Licochalcone A | C21H2204 | Aerial parts (Methanol extract) | UV; IR; MS; NMR | [56] |
| Ombuin | C17H14O7 | Air dried aerial parts/Root (Ethanol extract) | HPLC-DAD; IR; MS; NMR | [51,56] |
| Rhamnetin | C16H12O7 | Air dried root(Ethanol extract) | HPLC-DAD | [51] |
| Retusine | C19H18O7 | Leaves | NMR; IR; MS; UV | [61] |
| Phytosterols | ||||
| 5α-Stigmast-3,6-dione | C29H48O2 | Aerial parts | Spectroscopy | [61] |
| Daucosterol | C35H60O6 | Leaves | NMR; IR; MS; UV | [58] |
| β-Sitosterol | C29H50O | Dried leaves | NMR; IR; MS; UV | [58,61,62] |
| Stigmasterol | C29H48O | Dried leaves (Hexane extract) | GCMS | [62] |
| Stigmast-4-ene-3-one | C29H48O | Aerial parts | Spectroscopy | [61] |
| Glycosides | ||||
| Acteoside | C29H36O15 | Arial parts (Methanol extract) | TLC; HPLC | [57] |
| Agastachoside | C24H24O11 | Stem (Ethanol extract) | IR; HR; NMR | [60] |
| Apigenin-7-_O_-(3ʺ,6ʺ-di-(E)-_p_-coumaroyl)-β-d-galacto-pyranoside | C30H26O12 | Leaf/Stem (Ethanol extract) | IR; HR; NMR | [58,60] |
| 3α-Hydroxypatchoulol 3-_O_-β-d-glucopyranoside | C21H36O7 | Air dried whole plant (Ethanol extract) | NMR | [63] |
| 15-Hydroxypatchoulol 15-_O_-β-d-glucopyranoside | C21H36O7 | Air dried whole plant (Ethanol extract) | NMR | [63] |
| Isocrenatoside | C29H34O15 | Arial parts/Stem (Methanol/Ethanol extract) | TLC;HPLC; IR;HR;NMR | [58,59,60] |
| 3ʺ-_O_-Methylcrenatoside | C29H36O15 | Stem (Ethanol extract) | IR; HR; NMR | [58,59,60] |
| Soya-cerebroside I and II | C40H75NO9 | Stem (Ethanol extract) | IR; HR; NMR | [60] |
| Tilianin | C22H22O10 | Stem (Ethanol extract) | IR; HR-ESI-MS; NMR | [60] |
| Rubusoside | C32H50O13 | Air dried whole plant (Ethanol extract) | NMR | [63] |
| Triterpenes | ||||
| Epifriedelinol | C30H52O | Leaves | Spectroscopy | [61] |
| Methyl oleanolate | C31H50O3 | Aerial parts | Spectroscopy | [61] |
| Sesquiterpenes | ||||
| 8α,9α-Dihydroxypatchoulol | C15H26O3 | Arial parts (Methanol extract) | IR;NMR | [64] |
| 3α,8α-Dihydroxypatchoulol | C15H26O3 | Arial parts (Methanol extract) | IR;NMR | [64] |
| 2β,12-Dihydroxypathoulol | C15H26O3 | Arial parts (Methanol extract) | IR;NMR | [64] |
| 10 α-Hydroperoxyguaia-1,11-diene | C15H25O2 | Dried whole herb (Acetone extract) | IR; NMR | [65] |
| 1 α-Hydroperoxyguaia-10(15),11-diene | C15H25O2 | Dried whole herb (Acetone extract) | IR; NMR | [65] |
| 15 α-Hydroperoxyguaia-1(10),11-diene | C15H24O2 | Dried whole herb (Acetone extract) | IR; NMR | [65] |
| 2-Keto-4β-hydroxyguai-1, 11-diene | C15H22O2 | Air dried stem (Ethanol extract) | UV; IR; NMR; MS | [66] |
| 4-Hydroxy-10-epi-rotundone | C15H22O2 | Air dried stem (Ethanol extract) | UV; IR; NMR; MS | [66] |
| 10 α-Hydroperoxyguaia-1,11-diene | C15H25O2 | Dried whole herb (Acetone extract) | IR; NMR | [65] |
| 1 α-Hydroperoxyguaia-10(15),11-diene | C15H25O2 | Dried whole herb (Acetone extract) | IR; NMR | [65] |
| 15 α-Hydroperoxyguaia-1(10),11-diene | C15H24O2 | Dried whole herb (Acetone extract) | IR; NMR | [65] |
| 6-Hydroxypatchoulol | C15H26O2 | Arial parts (Methanol extract) | IR; NMR | [64] |
| Patchouli alcohol | C15H26O | Leaf (n-hexane extract) | TLC; HPLC; HRMS; EIMS | [61,62] |
| Organic Acids | ||||
| Dibutyl phthalate | C16H22O4 | Aerial parts | Spectroscopy | [61] |
| Succinic acid | C4H6O4 | Aerial parts | Spectroscopy | [58,59] |
| Others | ||||
| Tschimganical A | C11H16O3 | Air dried stem (Ethanol extract) | UV; IR; NMR; MS | [62] |
| Uracil | C4H4N2O2 | Stem (Ethanol extract) | IR; HR; NMR | [60] |
Figure 2.
The structures of some of the non-volatile chemical constituents.
Three new sesquiterpene hydroperoxides such as 10α-hydroperoxyguaia-1,11-diene, 1α-hydroperoxyguaia-10(15),11-diene and 15α-hydroperoxyguaia-1(10),11-diene were determined using chromatographic separation of an acetone extract of dried patchouli herb [65]. Similarly, Kongkathip et al. [62] obtained 0.05% dry weight of patchoulol, 0.09% dry weight of phytosterols such as β-sitosterol and stigmasterol and 0.04% dry weight of a flavonoid, 7,3ʹ,4-tri-_O_-methyl-eriodictyol. Wang et al. [60] identified 12 non-volatile compounds, including flavonoids and glycosides from the ethanol extract of P. cablin stems. The identified compounds were tilianin, diosmetin-7-_O_-β-d-glucopyranoside, 3ʺ-_O_-methylcrenatoside, uracil, soya-cerebroside I and II, agastachoside, apigenin-7-_O_-(3ʺ,6ʺ-di-(E)-_p_-coumaroyl)-β-d-galactopyranoside, 5-hydroxy-3,3ʹ,4ʹ,7-tetramethoxyflavone, 4ʹ,5-dihydroxy-3,3ʹ,7-trimethoxyflavone, acacetin, crenatoside and isocrenatoside. The compounds tilianin, diosmetin-7-_O_-β-d-glucopyranoside, uracil, soya-cerebroside I and II, agastachoside, apigenin-7-_O_-(3ʺ,6ʺ-di-(E)-_p_-coumaroyl)-β-d-galactopyranoside and acacetin were isolated for the first time. From ethanolic extracts of complete patchouli plants, the compounds 3α-hydroxypatchoulol 3-_O_-β-d-glucopyranoside, rubusoside (diterpene glycoside) and 15-hydroxy-patchoulol 15-_O_-β-d-glucopyranoside (sesquiterpene glycosides) were isolated for the first time [63]. Using 1D- and 2D-NMR techniques, Zhou et al. [64] isolated and elucidated the structures of four new patchoulol derivatives, namely 8α,9α-dihydroxypatchoulol, 3α,8α-dihydroxypatchoulol, 6-hydroxypatchoulol, and 2β,12-dihydroxypathoulol from the aerial parts of P. cablin. Four new sesquiterpenes, namely 8-keto-9(10)-α-patchoulene-4α-ol, 2-keto-1(5)-β-patchoulene-4β-ol, 2-keto-1(5)-β-patchoulene-4α-ol and 2-keto-4β-hydroxyguai-1,11-diene were isolated from the air dried powdered stems of P. cablin [66].
Zhou et al. [61] investigated the chemical constituents of the aerial parts of P. cablin and isolated 13 compounds using column chromatography and then used spectroscopic data to identify the following compounds; patchouliol, pogostone, friedelin, epifriedelinol, oleanolic acid, methyl oleanolate, 5α-stigmast-3,6-dione, stigmast-4-ene-3-one, β-sitosterol, pachypodol, retusin, (−)-guaiacylglycerol and dibutyl phthalate. The compounds, methyl oleanolate, 5α-stigmast-3,6-dione, stigmast-4-ene-3-one, (−)-guaiacylglycerol and dibutyl phthalate were isolated for the first time in this genus. A new HPLC-DAD method was developed to quantify both volatile and non-volatile components from patchouli [51]. Using this method pogostone (volatile) and eight flavonoids (non-volatile) such as apigenin, rhamnetin, ombuine, 4ʹ,5-dihydroxy-3,3ʹ,7-trimethoxyflavone, 5-hydroxy-7,3ʹ,4ʹ-trimethoxyflavanone, 5-hydroxy-3,3ʹ,4ʹ,7-tetramethoxyflavone, 3,5-dihydroxy-7,4ʹ-dimethoxy-flavone and 5-hydroxy-3,4ʹ,7-trimethoxyflavone can be determined simultaneously.
4. Pharmacological Activities
4.1. Antimicrobial Activities
4.1.1. Antibacterial Activities
In traditional medicine, patchouli plants are used for treating common cold and fungal infections [67]. Among 10 essential oils studied for antibacterial and antifungal activity, patchouli oil was found to be more effective in inhibiting 20 bacterial strains and all 12 fungi [68]. The essential oils of P. cablin from three different geographic regions (China, India and Indonesia) were assessed in vitro against 17 pathogenic fungi and 16 commensal bacteria (from the skin, mucous membrane, nail, foot and armpit). The results revealed a clear antifungal and antibacterial activity of patchouli oil [69]. The essential oil of patchouli was effective in inhibiting Acenitobacter baumanii, Aeromonas veronii, Candida albicans, Enterococcus faecalis, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Salmonella enteric and Staphyllococcus aureus [70]. Patchouli oil, tea tree oil, geranium oil, lavender oils and CitricidalTM (seed extract of grapefruit) were used against bacteria. They also used patchouli oil for treating epidemic methicillin-resistant S. aureus infection and when compared to a blend of β-sitosterol and stigmasterol and 7,3ʹ,4-tri-_O_-methyleriodictyol, patchoulol and hexane extract of dried patchouli leaves evidenced higher antibacterial activity against Staphylococcus aureus and Bacillus subtilis [60,71]. The antimicrobial activity of patchouli oil was studied by using molecular docking technology and in vitro antimicrobial assay [72]. The study confirmed the strong antimicrobial effects. Mainly the constituents, pogostone and (−)-patchoulol had broader therapeutic prospects in bacterial infection. The aqueous and organic extracts of patchouli and geranium leaves showed significant antimicrobial properties against E. coli, B. subtilis, S. aureus and E. aerogenes [73]. Patchouli oil was effective in preventing biofilm formation by food borne pathogens, Staphylococcus aureus [74]. In recent times, green synthesis of nanoparticles was performed by using the essential oils of vanilla, patchouli and ylang-ylang. These nanobiosystems were very effective against adherence and biofilm formation by clinical strains of Staphylococcus aureus and Klebsiella pneumonia [75]. Patchouli oil also exhibited antimicrobial activity against the isolated enteric bacteria from common house lizard faecal droppings [76]. Selective antibacterial activity against Helicobacter pylori was exhibited by patchoulol without affecting the normal flora of the gastrointestinal tract. Patchoulol also possessed urease inhibitory potential and thus it can be used as a promising biomolecule to cure H. pylori infections [77]. The gram-positive bacteria such as Staphylococcus, Bacillus, and Streptococcus species were successfully inhibited by using patchouli oil [78].
4.1.2. Antifungal Activities
The medium having 100 µg/mL of an oil mixture (lemongrass, thyme, patchouli and cedarwood oils inhibited mycelial growth of C. albicans [79]. Patchouli alcohol (44.52%) of the plant essential oil showed antifungal activity against a population of Aspergillus species [80]. The potential of pogostone, a natural product from P. cablin effective in treatment of Candida infections, particularly for vulvovaginal candidiasis, was evidenced by Li et al. [34]. Similarly, patchouli oil effectively inhibited C. albicans [81]. Further, pogostone and its synthesized analogues showed effective activities against Gram-positive, Gram-negative bacteria and C. albicans. Based on molecular docking studies, it was suggestd that a promising antifungal agent can be obtained by appropriate structural modifications of pogostone analogues [33].
4.1.3. Antiviral Activities
Traditional medicines from herbs, including patchouli were screened for ant-influenza viral activity. It was shown that about 10 μg/mL concentration of methanol extract of patchouli leaves could inhibit influenza virus A/PR/8/34 (H1N1) up to 99.8% and the IC50 values was estimated to be 2.6 μM [82]. Similarly, the results presented by Wu et al. [83] suggested anti-influenza A (H2N2) viral activities of patchoulol with an IC50 of 4.0 μM and thus making it a potent anti-influenza viral agent to be used by pharma industries. The in vivo anti-influenza virus effect of patchouli alcohol was studied using mouse as a model and the study confirmed that oral administration of patchouli alcohol (20 mg/kg to 80 mg/kg) augmented protection against influenza virus infection by improving the immune response and attenuating systemic as well as pulmonary inflammatory responses [84]. More recently, Wu et al. [85] observed anti-influenza viral properties using patchoulol (10 μg/mL) under in vitro condition and found that patchoulol enhanced the ability of innate immune recognition and response, and restrained the expression of IFN-α inflammatory factor to attenuate the inflammatory responses.
4.1.4. Treatment of HIV/AIDS and Opportunistic Infections
In French hospitals, essential oils were used as suitable antimicrobial agents to treat specific opportunistic infections caused by Candida albicans, Cryptococcus neoformans, methicillin-resistant Staphylococcus aureus and Herpes simplex type I and II found in Acquired Immunodeficiency Syndrome patients [86].
4.2. Gastrointestinal Protective Activity
Water extract of P. cablin was shown to protect and maintain the membrane fluidity of intestinal epithelial cells by regulating the levels of nitric oxide and tumor necrosis factor in serum. This study provides an experimental basis for gastrointestinal protection against trauma or surgical operation [87].
4.3. Antiemetic Activity
Hexane extracts of patchouli plant leaves showed anti-emetic activity in young chicks. Patchoulol, pachypodol, pogostol, retusin and stigmast-4-en-3-one demonstrated anti-emetic properties at doses of 50–70, 10–50, 20–50 and 50 mg/kg, respectively [88].
4.4. Defaecation and Constipation
Mikuriya et al. [89] carried out an experiment to study the influence of patchouli oil on defecation and constipation using two mouse models; one having flaccid constipation and other with constipation because of lower fibrous food intake. It was found that in both models the number of faeces and its dry weight increased after smelling the patchouli oil aroma. Also, it was proved that olfactory neurotransmission systems responsible for overcoming constipation in mice.
4.5. Blood Coagulation and Fibrinolytic Activities
Blood coagulation and fibrinolytic activity of various essential oils including patchouli was studied using in vitro enzymatic reactions such as fibrin formation from fibrinogen by thrombin and fibrin resolution by urokinase. The study revealed that both coagulation and fibrinolytic activities were demonstrated by chamomile, eucalyptus and neroli oils while an efficient hyperfibrinolysis was revealed by the oils of citrus, pine, patchouli and frankincense [90].
4.6. Antithrombotic Activities
Park et al. [91] have examined in vitro, ex vitro and in vivo use of the herbal medicine Sunghyangjunggisan and its ingredients (P. cablin, Perilla frutescens, Arisaema amurensa, Aucklandia lappa, Atractylodes macrocephala, Citrus unshiu, and Ziziphus jujuba) as a novel antithrombotic agent. In vitro adenosine 5ʹ-diphosphate (ADP) and collagen induced rat platelet aggregations were not inhibited by Sunghyangjunggisan. However, Sunghyangjunggisan significantly inhibited ex vitro rat platelet aggregation. It also showed significant protection from death due to pulmonary thrombosis in mice.
4.7. Antioxidant Activities
Patchouli oil showed an efficient free radical scavenging activity and inhibited the oxidation of hexanal to hexanoic acid [92]. Reactive oxygen species induced brain cell injury can be treated by using patchouli herb [93]. P. cablin protected from cell death due to necrosis and apoptosis induced by hydrogen peroxide in human neuroglioma cell line A172, thus suggesting its use for treating many neurodegenerative disorders_._ Patchouli oil prevented photoaging by exhibiting antioxidative property and maintained skin structural integrity caused by UV irradiation [94]. Similarly, the use of patchoulol increased the revitalization of UV-induced skin lesions through antioxidant and anti-inflammatory actions together with down-regulating the expression of matrix metalloproteinases (MMP-1 and MMP-3) [95]. Lipopolysaccharides present on the outer membrane of Gram-negative bacteria are responsible for causing mastitis and the study conducted by Li et al. [96] showed that patchouli alcohol was efficient in inhibiting TNF-α, IL-6, and IL-1β productions. It was also observed that patchouli alcohol attenuated mammary histopathologic changes, therefore patchouli alcohol can be used as a potent therapeutic reagent for preventing mastitis.
4.8. Analgesic and Anti-Inflammatory Activities
The methanol extract of patchouli plants was demonstrated to have analgesic and anti-inflammatory activity in mice. This validates the good acceptance of the patchouli herb in traditional medicinal practices [97]. Results of an analgesic study indicated that acetic acid induced writhing responses were decreased by using methanol extracts of patchouli (1.0 g/kg). The extracts of Chrysanthemum indicum, P. cablin and Curcuma wenyujin herbs, the components of the traditional medication recipe CPZ used in China, exhibited a strong anti-inflammatory response by regulating interleukin-1β (IL-1β) and prostaglandin E(2). Likewise, patchoulol effectively regulated the expression of TNF-α, IL-1β, IL-6, iNOS and COX-2 mRNAs in RAW264.7 cells due lipopolysaccharide induced inflammation [98]. Similarly, patchouli oil and ethanol extract of its root and rhizome exhibited strong in vivo anti-inflammatory properties [99,100]. The mechanism involved in anti-inflammatory property of patchoulol was investigated in mouse macrophage and human colorectal cancer cells [101]. More recently, Li et al. [35] revealed that pogostone possessed anti-inflammatory effect and suggested its use for development of a pharmaceutical drug to treat septic shock. Water extracts of patchouli herb suppressed colon inflammation through suppressing the expression of pro-inflammatory cytokines [102].
4.9. Antimutagenic Activity
In Salmonella typhimurium TA1535/pSK1002, umu gene expression due to SOS response stimulated due to mutagenic agent, 2-(2-furyl)-3-(5-nitro-2-furyl)acrylamide was suppressed by patchouli plant methanolic extract [103].
4.10. Effects on Tumors/Cancer Cells and the Immune System
The compounds 5,7-dihydroxy-3ʹ,4ʹ-dimethoxyflavanone, ombuin and licochalcone A were shown to exhibit cytotoxicity. The compound, licochalcone A showed PI- PLC gamma 1 inhibition activity. When licochalcone A was applied to promyelocyti leukaemia cells (HL-60), terminal differentiation along with the production of monocytes was observed [56]. However, patchoulol inhibited HeLa cell proliferation, suppressed cell differentiation and enhanced apoptosis in human colorectal cancer cell lines, HCT116 and SW480 [104,105]. According to these authors, patchouli alcohol inhibited histone deacetylase 2 expressions and histone deacetylase enzyme activity, down regulating c-myc and activating NF-κB pathway. A patchouli sesquiterpenoid, α-bulnesene was effective in inhibiting platelet-activating factor [106]. Anti-platelet activity was due to induction of intracellular signal transduction by α-bulnesene which interferes with activities of cyclooxygenase resulting in decreased thromboxane production. Now α-bulnesene is considered as a potent anti-platelet aggregation agent. The investigation on immunomodulatory potential of patchouli alcohol in Kumming mice revealed that patchouli alcohol activates the mononuclear phagocytic system and improves humoral immune response by suppressing the cellular immune response [107]. Xinxiang granule (XXG) is prepared from Biond magnolia flowers (Magnolia biondii), patchouli herb and small centipeda herb (Centipeda minima) and is used to treat allergic rhinitis and passive skin irritability [108]. Recently, the potential uses of patchouli oil for its antinociceptive and anti-allergic activities were confirmed in mouse models [109].
4.11. Effects against Skin Diseases
The essential oils, including patchouli oil at 12% concentration, effectively controlled skin infections and odor in patients suffering ulcers, torn skin, skin abrasions and pressure sores. The healing duration was considerably reduced by the use of essential oils [110]. The structural integrity of UV irradiated skin was maintained by the application of patchouli essential oil and prevented photoaging and increased revival of skin lesions [97,98].
4.12. Pharmacokintetic Activities
A simple and sensitive liquid chromatography tandem mass spectroscopy (LC-MS) method was developed to analyze pogostone in the plasma of rat after intravenous and oral administration [111]. This method is useful in pharmacokinetic studies of preclinical investigations. Lately, Li et al. [112] investigated the metabolic profile of pogostone in vitro and in vivo using LC-MS. Patchouli oil exhibited significant gastroprotective effects against gastric ulceration. According to them, the possible mechanism of anti-ulcerogenic potential of patchouli oil might be due to stimulation of COX-mediated PGE2, improvement of antioxidant and anti-inflammatory status, preservation of GBF and NP-SH, as well as boost of gastric mucus production [113].
4.13. Insecticidal Activity
Cymbopogon nardus and P. cablin leaf ashes at 1% (w/w) each caused pest mortality and were found to be effective repellents against Stegobium paniceum with 78% and a 64% repellency, respectively [114]. High fumigation insecticidal activity against four museum insect pests (Lasioderma serricorne, Sitophilus zeamais, Tribolium confusum and Falsogastrallus sauteri) by geranium oil, spikenard oil, muskmelon oil and patchouli oil was reported by Chun et al. [115]. Both patchouli oil and its main constituent, patchoulol showed toxicity and repellency towards Formosan subterranean termites (Coptotermes formosanus Shiraki). Topical application of patchoulol to the dorsum portion showed unusual tissue damage inside the exoskeleton of the termite [116]. Similarly, insecticidal activity against Trypanosoma cruzi was exhibited by the sesquiterpene hydroperoxides such as 10 α-hydroperoxyguaia-1,11-diene, 1α-hydroperoxyguaia-10(15),11-diene and 15α-hydro-peroxyguaia-1(10),11-diene [65]. Thirty eight essential oils were screened against the mosquito under laboratory conditions using human subjects. From the report it was noticed that the use of undiluted Patchouli oil was effective in providing two hours of complete mosquito repellency [117].
An eco-friendly, biodegradable insecticidal agent, consisting of petroleum ether extracts of C. cassia, P. cablin and E. caryophyllata can be used to control the house dust mite (Dermatophagoides farina) [118]. Patchouli oil showed strong repellency to three species of urban ants and possessed strong bio-insecticidal activity and particularly, patchouli alcohol had the most effective repellent activity against Ades aegypti, Anopheles stephensi and Culex quinquefasciatus mosquito bites [119,120]. Likewise, Phal et al. [121] evaluated herbal mosquito coils against Aedes aegypti mosquito and found a combination of patchouli and valamus (75:25) exhibiting significant knockdown activity at 7.5% concentration with all the coils (rice husk, corn cob and sawdust-based coils). In recent times, strong larvicidal activity, antifeedant activity, pupicidal activity and growth inhibition property were exhibited by progostone against Spodoptera litura and Spodoptera exigua thus making it a promising candidate to control various insects which destroy agricultural plants [122].
4.14. Aromatherapy
In aromatherapy, patchouli oil is used to help reduce tension, insomnia and anxiety. It’s wine-like intoxicating aroma functions as an aphrodisiac and helps to sharpen intelligence, improve concentration, and provide insight. Spiritually, it is used in incense sticks as it helps to create a calming atmosphere. In normal adult subjects, the fragrance inhalation effects on sympathetic activities was studied by measuring the amount of catecholamine content in the serum and monitoring fluctuations in blood pressure. There was a decreased sympathetic activity after inhalation of fragrance in normal subjects [123]. Aqueous cream containing lavender oil, sweet marjoram oil, patchouli oil and vetiver oil was applied five times a day onto the bodies and limbs of care facility residents and the results indicated a decreased frequency of dementia-associated behavior. Mental alertness and awareness in participants was improved by the use oils. The problems related to stress due to exhaustion and anxiety can be addressed by using essential oils as they stimulate adrenal hormone secretion [124]. The anti-inflammatory and cooling oils such as P. cablin and Citrus limonum were used for treating symptoms of menopause such as hot flashes and sweating. An aromatherapy oil blend of patchouli, with jasmine, ylang-ylang, sandalwood, rose and vetiver can definitely inspire clarity and a harmonious flow of energy that interacts with thyroid glands and balances hormonal secretion [125]. Various psychiatric disorders can be treated by aromatherapy. Patchouli oil influences cerebral functions including calming, sedative and uplifting [126]. The vaporized mixtures of essential oils (patchouli, orange, ylang ylang, rosemary, basil, peppermint, geranium, bergamot, rosewood, chamomile and jasmine) reduced disturbed behavior among 10 patients with dementia [127]. The physiological responses such as blood pressure, pulse rate, stress index and brain waves were reduced after sniffing patchouli oil odor. This suggests that patchouli oil odor improves mood and possess curative effects when its fragrance is inhaled by humans. The influence of odor was due to its main compound, patchouli alcohol [128]. Aromatherapy (inhalation, compresses, baths and massages) can help to manage stress and reduce perceptions of chronic pain [86].
5. Conclusions and Recommendations
P. cablin is being explored globally to obtain key chemical compounds for making new drug molecules with great therapeutic potential as well as in the fragrance industry. This review summarizes the updated research studies on the phytochemisty and pharmacological effects of P. cablin. The present information helps in bridging the gap between modern scientific studies and available traditional medical reports on this plant. As chemical constituents of P. cablin vary depending on the origin and plant parts, a protocol needs to be standardized to isolate and obtain pure compounds. Though various chemical compounds have been isolated, purified and characterized, many compounds are yet to be studied in detail. Till now, limited efforts are made in the pharmacokinetics investigations related to the mechanism of action of the individual isolated compounds under in vivo conditions. Also, research should be emphasized on the toxicity and safety aspects of P. cablin compounds using animal models. Further, therapeutic prospects of many new chemical compounds from P. cablin under in vitro and in vivo conditions should be explored in detail.
Acknowledgments
The authors are grateful to the Department of Crop Science, Faculty of Agriculture, Universiti Putra Malaysia for supporting this study.
Author Contributions
M.K.S. and U.R.S. together initiated and designed the work. M.K.S. contributed to literatures collection and drew the chemical compound structures. U.R.S. drafted the manuscript. Both M.K.S. and U.R.S. finalized and critically edited the manuscript before submission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Harvey A.L. Natural products in drug discovery. Drug Discov. Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Meena A.K., Bansal P., Kumar S. Plants-herbal wealth as a potential source of ayurvedic drugs. Asian J. Tradit. Med. 2009;4:152–170. [Google Scholar]
- 3.Sucher N.J., Carles M.C. Genome-based approaches to the authentication of medicinal plants. Planta Med. 2008;74:603–623. doi: 10.1055/s-2008-1074517. [DOI] [PubMed] [Google Scholar]
- 4.Kumara S.M., Sudipta K.M., Lokesh P., Neeki A., Rashmi W., Bhaumik H., Darshil H., Vijay R., Kashyap S.S.N. Phytochemical screening and in vitro antimicrobial activity of Bougainvillea spectabilis flower extracts. Int. J. Phytomedicine. 2012;4:375–379. [Google Scholar]
- 5.China Pharmacopoeia Commission . Pharmacopoeia of the People’s Republic of China. Volume 1. Chemical Industry Press; Beijing, China: 2010. pp. 42–46. [Google Scholar]
- 6.Zhang D., Xiao L.Y., Cheng Y.W., Li H.L., Feng Z.M., Lin P.Y., Wu W.Y., Huang K.R. Pharmacological action of Baoji Pill. Tradit. Chin. Drug. Res. Clin. Pharmacol. 1998;9:212–214. [Google Scholar]
- 7.Xian Y.F., Suo J., Huang X.D., Hou S.Z., Chen J.N., Ye M.R., Su Z.R. A pharmacological study on anti-inflammatory effects of refined Huodan recipe. Chin. J. Exp. Tradit. Med. Formul. 2007;13:54–56. [Google Scholar]
- 8.Vijayakumar K. Patchouli and India—A great leap forward; Proceedings of the National Seminar of Prospectus and Potentials of Medicinal and Aromatic Crops; Bangalore, India. 18–19 June 2004. [Google Scholar]
- 9.Bizzo H.R., Hovell A.M.C., Rezende C.M. Óleos essenciais no Brasil: Aspectos gerais, desenvolvimento e perspectivas. Quím. Nova. 2009;32:588–594. doi: 10.1590/S0100-40422009000300005. [DOI] [Google Scholar]
- 10.Varshney S.C. Vision 2005: Essential oil industry of India. Indian Perfum. 2000;44:101–118. [Google Scholar]
- 11.Hasegawa Y., Tajima K., Toi N., Sugimura Y. An additional constituent occurring in the oil from a Patchouli cultivar. Flavor Fragr. J. 1992;7:333–335. doi: 10.1002/ffj.2730070608. [DOI] [Google Scholar]
- 12.Kumaraswamy M., Anuradha M. Micropropagation of Pogostemon cablin Benth. through direct regeneration for production of true-to-type plants. Plant. Tissue Cult. Biotechnol. 2010;20:81–89. doi: 10.3329/ptcb.v20i1.5971. [DOI] [Google Scholar]
- 13.Chakrapani P., Venkatesh K., Singh B.C.S., Jyothi B.A., Kumar P., Amareshwari P., Roja A.R. Phytochemical, pharmacological importance of Patchouli (Pogostemon cablin (Blanco) Benth) an aromatic medicinal plant. Int. J. Pharm. Sci. Rev. Res. 2013;21:7–15. [Google Scholar]
- 14.Kalra A., Prakasa Rao E.V.S., Khanuja S.P.S. Cultivation and processing technologies of Patchouli (Pogostemon cablin) J. Med. Arom. Plants Sci. 2006;28:414–419. [Google Scholar]
- 15.Kumara S.M., Anuradha M. Analysis of genetic variability in Patchouli cultivars (Pogostemon cablin Benth.) using RAPD Markers. Res. Biotechnol. 2011;2:64–71. [Google Scholar]
- 16.Liu X.R., Fan R., Zhang Y.Y., Zhu M.J. Study on antimicrobial activities of extracts from Pogestemon cablin (Blanco) Benth. Food Sci. Technol. 2009;24:220–227. [Google Scholar]
- 17.Priya D., Swati D., Vilasrao D.K. A Review on Pogostemon patchouli. Res. J. Pharmacogn. Phytochem. 2014;691:41–47. [Google Scholar]
- 18.Swamy M.K., Balasubramanya S., Anuradha M. In vitro multiplication of Pogostemon cablin Benth through direct regeneration. Afr. J. Biotechnol. 2010;9:2069–2075. [Google Scholar]
- 19.Maheswari M.L., Vasantha Kumar T., Sharma N., Chandel K.P.S. Patchouli—An Indian perspective. Indian Perf. 1993;37:9–11. [Google Scholar]
- 20.Bhaskar S., Vasantha Kumar T. Agronomic bottlenecks, genetic barriers and marketing impediments in Patchouli production. J. Med. Aromat. Plant Sci. 2000;22:396–403. [Google Scholar]
- 21.Angadi S.P., Vasanthakumar T. Patchouli. In: Chadha K.L., Gupta R., editors. Advances in Horticulture: Medicinal and Aromatic Plants. Volume 11. Malhotra Publishing House; New Delhi, India: 1995. pp. 751–771. [Google Scholar]
- 22.Guo J., Yuan Y., Liu Z., Zhu J. Development and structure of internal glands and external glandular trichomes in Pogostemon cablin. PLoS ONE. 2013;8:e77862. doi: 10.1371/journal.pone.0077862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z.H., Zhou D.Y. Chemical component comparison of essential oil extracted from Pogostemon cablin by supercritical CO2 extraction and stream distillation. J. Anhui Agric. Sci. 2009;37:8816–8817. [Google Scholar]
- 24.Su Z.Q., Wu X.L., Bao M.J., Li C.W., Kong S.Z., Su Z.R., Lai X.P., Li Y.C., Chen J.N. Isolation of (−)-Patchouli alcohol from Patchouli oil by fractional distillation and crystallization. Trop. J. Pharm. Res. 2014;13:359–363. doi: 10.4314/tjpr.v13i3.7. [DOI] [Google Scholar]
- 25.Donelian A., Carlson L.H.C., Lopes T.J., Machado R.A.F. Comparison of extraction of patchouli (Pogostemon cablin) essential oil with supercritical CO2 and by steam distillation. J. Supercrit. Fluids. 2009;48:15–20. doi: 10.1016/j.supflu.2008.09.020. [DOI] [Google Scholar]
- 26.Hu H.Y., Peng J.F., Huang S.L., Wu L.H., Zhu B.Z., Xuan Y.M., Yang D.P. Study on purification technology of Patchouli oil with molecular distillation. Chin. J. Chin. Mater. Med. 2004;29:320–322. [PubMed] [Google Scholar]
- 27.Chen H., Zhang J.W., Zhu H.W., Song Z.L. Purification of patchouli alcohol in volatile oil of Pogostemon cablin by molecular distillation. Chin. Tradit. Herb. Drugs. 2009;40:60–63. [Google Scholar]
- 28.Ahmad Kamal M. Bachelor’s Thesis. Universiti Putra Malaysia; Serdang, Malaysia: Apr 14, 2010. Microwave Assisted Extraction of Patchouli Essential Oil Using Ethanol as Solvent. [Google Scholar]
- 29.Bauer K., Garbe D., Surburg H. Preparation, Properties and Used Common Fragrance and Flavour Materials. 3rd ed. Wiley-VCH; Weinheim, Germany: 1997. p. 205. [Google Scholar]
- 30.Ramya H.G., Palanimuthu V., Rachna S. An introduction to Patchouli (Pogostemon cablin Benth.)—A medicinal and aromatic plant: It’s importance to mankind. Agric. Eng. Int. CIGR J. 2013;15:243–250. [Google Scholar]
- 31.Akhila A., Nigam M.C. Gas chromatography–mass spectroscopy analysis of the essential oil of Pogostemon cablin (Patchouli oil) Fitotherapia. 1984;55:363–365. [Google Scholar]
- 32.Akhila A.K., Sharma P.K., Thakur R.S. Biosynthetic relationships of patchouli alcohol, seychellene and cycloseychellene in Pogostemon cablin. Phytochemistry. 1988;27:2105–2108. doi: 10.1016/0031-9422(88)80105-5. [DOI] [Google Scholar]
- 33.Yi Y.Y., He J.J., Su J.Q., Kong S.Z., Su J.Y., Li Y.C., Huang S.H., Li C.W., Lai X.P., Su Z.R. Synthesis and antimicrobial evaluation of pogostone and its analogues. Fitotherapia. 2013;84:135–139. doi: 10.1016/j.fitote.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Li Y.C., Liang H.C., Chen H.M., Tan L.R., Yi Y.Y., Qin Z., Zhang W.M., Wu D.W., Li C.W., Lin R.F., et al. Anti-candida albicans activity and pharmacokinetics of pogostone isolated from Pogostemonis Herba. Phytomedicine. 2012;20:77–83. doi: 10.1016/j.phymed.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Li Y.C., Xian Y.F., Su Z.R., Ip S.P., Xie J.H., Liao J.B., Wu D.W., Li C.W., Chen J.N., Lin Z.X., et al. Pogostone suppresses proinflammatory mediator production and protects against endotoxic shock in mice. J. Ethnopharmacol. 2014;157:212–221. doi: 10.1016/j.jep.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 36.Zeng Z., Tan L., Meng S., Zhang H. Study on the chemical constituents and fingerprint of Pogostemon. cablin from three culture varieties. Chin. J. Anal. Chem. 2006;34:1249–1254. doi: 10.1016/S1872-2040(07)60014-0. [DOI] [Google Scholar]
- 37.Feng Y., Guo F., Luo J. GC–MS analysis of volatile oil of Herba Pogostemonis collected from Leizhou county. Zhong Yao Cai. 1999;22:241–243. [PubMed] [Google Scholar]
- 38.Bure C.M., Sellier N.M. Analysis of the essential oil of Indonesian Patchouli (Pogostemon cablin Benth) using GC/MS (EI/CI) J. Essent. Oil Res. 2004;16:17–19. doi: 10.1080/10412905.2004.9698638. [DOI] [Google Scholar]
- 39.Kang S.S., Kim J.S., Chi H.J., Won D.H. Isolation and quantitative determination of patchouli alcohol from Pogostemon cablin Benth. Korean J. Pharmacogn. 1998;29:18–21. [Google Scholar]
- 40.Wu J.F., Lu X., Tang W.Y., Kong H.W., Zhou S.F., Xu G.W. Application of comprehensive two–dimensional gas chromatography time of flight mass spectrometry in the analysis of volatile oil of traditional Chinese medicines. J. Chromatogr. A. 2004;1034:199–205. doi: 10.1016/j.chroma.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 41.Dung N.X., Leclercq P.A., Thai T.H., Moi L.D. Chemical composition of Patchouli oil of Vietnam; Proceedings of the International Congress of Essential Oils, Fragrances and Flavours; New Delhi, India. 12–16 November 1989. [Google Scholar]
- 42.Rakotonirainy O., Gaydou E.M., Faure R., Bombarda I. Sesquiterpenes from Patchouli (Pogostemon cablin) essential oil. Assignment of the proton and carbon–13 NMR spectra. J. Essent. Oil Res. 1997;9:321–327. doi: 10.1080/10412905.1997.10554251. [DOI] [Google Scholar]
- 43.Luo J.P., Liu Y.P., Feng Y.F., Guo X.L., Cao H. Two chemotypes of Pogostemon cablin and influence of region of cultivation and harvesting time on volatile oil composition. Acta Pharmacol. Sin. 2003;38:307–310. [PubMed] [Google Scholar]
- 44.Silva M.A.S., Ehlert P.A.D., Ming L.C., Marques M.O.M. Composition and chemical variation during daytime of constituents of the essential oil of Pogostemon pachouli Pellet. leaves. Acta Hortic. 2004;629:145–147. [Google Scholar]
- 45.Bunrathep S., Lockwood G.B., Songsak T., Ruangrungsi N. Chemical constituents from leaves and cell cultures of Pogostemon cablin and use of precursor feeding to improve patchouli alcohol level. Sci. Asia. 2006;32:293–296. doi: 10.2306/scienceasia1513-1874.2006.32.293. [DOI] [Google Scholar]
- 46.Tsai Y.C., Hsu H.C., Yang W.C., Tsai W.J., Chen C.C., Watanabe T. Alpha-Bulnesene, a PAF inhibitor isolated from the essential oil of Pogostemon Cablin. Fitoterapia. 2007;78:7–11. doi: 10.1016/j.fitote.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Hu L.F., Li S.P., Cao H., Liu J.J., Gao J.L., Yang F.Q., Wang Y.T. GC–MS fingerprint of Pogostemon cablin in China. J. Pharm. Biomed. Anal. 2006;42:200–206. doi: 10.1016/j.jpba.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Sundaresan V., Singh S.P., Mishra A.N. Composition and comparison of essential oils of Pogostemon cablin (Blanco) Benth. (Patchouli) and Pogostemon travancoricus Bedd. var. travancoricus. J. Essent. Oil Res. 2009;21:220–222. doi: 10.1080/10412905.2009.9700152. [DOI] [Google Scholar]
- 49.Luo J., Feng Y., Guo X., Li X. GC-MS analysis of volatile oil of Herba Pogostemonis. collected from Gaoyao county. Zhong Yao Cai. 1999;22:25–28. [PubMed] [Google Scholar]
- 50.Guan L., Quan L.H., Xu L.Z., Cong P.Z. Chemical constituents of Pogostemon cablin (Blanco) Benth. Planta Med. 1998;64:464–466. doi: 10.1055/s-2006-957485. [DOI] [PubMed] [Google Scholar]
- 51.Li P., Yin Z.Q., Li S.L., Huang X.J., Ye W.C., Zhang Q.W. Simultaneous determination of eight flavonoids and pogostone in Pogostemon cablin by high performance liquid chromatography. J. Liquid Chromatogr. Relat. Technol. 2014;37:1771–1784. doi: 10.1080/10826076.2013.809545. [DOI] [Google Scholar]
- 52.Li W., Wei G., Pan C.M., Liu X.X., Huang S., Xiu H.H. Investigation on the influential factors of the volatile oil and main constituent content in Pogostemon cablin. Zhongguo Zhong Yao Za Zhi. 2004;29:28–31. [PubMed] [Google Scholar]
- 53.Luo J., Guo X., Feng Y. Constituents analysis on volatile oil of Pogostemon cablin from different collection time cultivated in Hainan. Zhong Yao Cai. 2002;25:21–30. [PubMed] [Google Scholar]
- 54.Zhao Z., Lu J., Leung K., Chan C.L., Jiang Z.H. Determination of patchouli alcohol in Herba Pogostemonis by GC–MS–MS. Phytother. Res. 2005;19:303–309. doi: 10.1002/ptr.1637. [DOI] [PubMed] [Google Scholar]
- 55.Hussin N., Mondello L., Costa R., Dugo P., Yusoff N.I., Yarmo M.A., AbWahab A., Said M. Quantitative and physical evaluation of Patchouli essential oils obtained from different sources of Pogostemon cablin. Nat. Prod. Commun. 2012;7:927–930. [PubMed] [Google Scholar]
- 56.Park E.J., Park H.R., Lee J.S., Kim J.W. Licochalcone A: An inducer of cell differentiation and cytotoxic agent from Pogostemon cablin. Planta Med. 1998;64:464–466. doi: 10.1055/s-2006-957485. [DOI] [PubMed] [Google Scholar]
- 57.Amakura Y., Yoshimura M., Mouri C., Mikage M., Kawahara N., Goda Y., Yoshida T., Yakugaku Z. Convenient TLC-based identification test for the crude drug “Pogostemoni Herba”. Yakugaku Zasshi. 2008;128:1833–1837. doi: 10.1248/yakushi.128.1833. [DOI] [PubMed] [Google Scholar]
- 58.Huang L., Mu S., Zhang J., Deng B., Song Z., Hao X. Chemical constituents from involatile moiety of Pogostemon cablin. Zhongguo Zhong Yao Za Zhi. 2009;34:410–413. [PubMed] [Google Scholar]
- 59.Li K., Zhang H., Xie H., Liang Y., Wang X., Ito Y. Preparative isolation and purification of five flavonoids from Pogostemon cablin Benth. by high-speed countercurrent chromatography and preparative high-performance liquid chromatography. J. Liquid Chromatogr. Relat. Technol. 2011;34:1617–1629. doi: 10.1080/10826076.2011.580486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang D., Yin Z., Zhang Q., Ye W., Zhang X., Zhang J. Non-volatile chemical constituents from Pogostemon cablin. Zhongguo Zhong Yao Za Zhi. 2010;35:704–707. [PubMed] [Google Scholar]
- 61.Zhou Q.M., Peng C., Li X.H., Guo L., Xiong L., Lin D.S. Study on constituents of the aerial parts of Pogostemon cablin. J. Chin. Med. Mater. 2013;36:915–8. [PubMed] [Google Scholar]
- 62.Kongkathip N., Sam-ang P., Kongkathip B., Pankaew Y., Tanasombat M., Udomkusonsri P. Development of Patchouli extraction with quality control and isolation of active compounds with antibacterial activity. Kasetsart. J. (Nat. Sci.) 2009;43:519–525. [Google Scholar]
- 63.Ding W.B., Lin L.D., Liu M.F., Wei X.Y. Two new sesquiterpene glycosides from Pogostemon cablin. J. Asian Nat. Prod. Res. 2011;13:599–603. doi: 10.1080/10286020.2011.577424. [DOI] [Google Scholar]
- 64.Zhou L., Xu M., Yang C.R., Wang Y.F., Wang Y.F., Zhang Y.J. New patchoulol-type sesquiterpenoids from Pogostemon cablin. Helv. Chim. Acta. 2011;94:218–223. doi: 10.1002/hlca.201000151. [DOI] [Google Scholar]
- 65.Kiuchi F., Matsuo K., Ito M., Qui T.K., Honda G. New sesquiterpene hydroperoxides with trypanocidal activity from Pogostemon cablin. Chem. Pharm. Bull. 2004;52:1495–1496. doi: 10.1248/cpb.52.1495. [DOI] [PubMed] [Google Scholar]
- 66.Li F., Li C.J., Ma J., Yang J.Z., Chen H., Liu X.M., Li Y., Zhang D.M. Four new sesquiterpenes from the stems of Pogostemon cablin. Fitoterapia. 2013;86:183–187. doi: 10.1016/j.fitote.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 67.Ichikawa K.T., Kinoshita T., Sankawa U. The screening of Chinese crude drugs for calcium antagonist activity: Identification of active principles from the aerial part of Pogostemon cablin and the fruits of Prunus mume. Chem. Pharm. Bull. 1989;37:345–348. doi: 10.1248/cpb.37.345. [DOI] [PubMed] [Google Scholar]
- 68.Pattnaik S., Subramanyam V.R., Kole C. Antibacterial and antifungal activity of ten essential oils in vitro. Microbios. 1996;86:237–246. [PubMed] [Google Scholar]
- 69.Yang D., Michel D., Mandin D., Andriamboavonjy H., Poitry P., Chaumont J.P., Millet Clerc J. Antifungal and antibacterial properties in vitro of three Patchouli oils from different origins. Acta Bot. Gallica. 1996;143:29–35. doi: 10.1080/12538078.1996.10515316. [DOI] [Google Scholar]
- 70.Hammer K.A., Carson C.F., Riley T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 71.Edwards J.V., Buck R., Shawcross S.G., Dawson M.M., Dunn K. The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns. 2004;30:772–777. doi: 10.1016/j.burns.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Yang X., Zhang X., Yang S.P., Liu W.Q. Evaluation of the antibacterial activity of Patchouli oil. Iran. J. Pharm. Res. 2013;12:307–316. [PMC free article] [PubMed] [Google Scholar]
- 73.Pullagummi C., Rao N.B., Singh B.C.S., Bheemagani A.J., Kumar P., Venkatesh K., Rani A.R. Comparitive studies on antibacterial activity of Patchouli [Pogostemon cablin (Blanco) Benth] and Geranium (Pelargonium graveolens) aromatic medicinal plants. Afr. J. Biotechnol. 2014;13:2379–2384. doi: 10.5897/AJB12.1369. [DOI] [Google Scholar]
- 74.Vázquez-Sánchez D., Cabo M.L., Rodríguez-Herrera J.J. Antimicrobial activity of essential oils against Staphylococcus aureus biofilms. Food Sci. Technol. Int. 2014;3 doi: 10.1177/1082013214553996. [DOI] [PubMed] [Google Scholar]
- 75.Bilcu M., Grumezescu A.M., Oprea A.E., Popescu R.C., Mogoșanu G.D., Hristu R., Stanciu G.A., Mihailescu D.F., Lazar V., Bezirtzoglou E., et al. Efficiency of Vanilla, Patchouli and Ylang Ylang essential oils stabilized by Iron Oxide@C14 Nanostructures against bacterial adherence and biofilms Fformed by Staphylococcus aureus and Klebsiella pneumoniae Clinical Strains. Molecules. 2014;19:17943–17956. doi: 10.3390/molecules191117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh B.R., Singh V., Ebibeni N., Singh R.K. Antimicrobial and herbal drug resistance in Enteric bacteria isolated from faecal droppings of common house Lizard/Gecko (Hemidactylus frenatus) Int. J. Microbiol. 2013 doi: 10.1155/2013/340848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu X.D., Xie J.H., Wang Y.H., Li Y.C., Mo Z.Z., Zheng Y.F., Su J.Y., Liang Y.E., Liang J.Z., Su Z.R., et al. Selective antibacterial activity of patchouli alcohol against Helicobacter pylori based on inhibition of Urease. Phytother. Res. 2015;29:67–72. doi: 10.1002/ptr.5227. [DOI] [PubMed] [Google Scholar]
- 78.Karimi A. Characterization and antimicrobial activity of Patchouli essential oil extracted from Pogostemon. cablin [Blanco] Benth [Lamiaceae] Adv. Env. Biol. 2014;8:2301–2309. [Google Scholar]
- 79.Abe S., Sato Y., Inoue S., Ishibashi H., Maruyama N.A., Takizawa T., Oshima H., Yamaguchi H. Anti-Candida albicans activity of essential oils including lemongrass (Cymbopogon citratus) oil and its component citral. Jpn. J. Med. Mycol. 2003;44:285–291. doi: 10.3314/jjmm.44.285. [DOI] [PubMed] [Google Scholar]
- 80.Kocevski D., Du M., Kan J., Jing C., Lačanin I., Pavlović H. Antifungal effect of Allium tuberosum, Cinnamomum cassia, and Pogostemon cablin essential oils and their components against population of Aspergillus species. J. Food Sci. 2013;78:M731–M737. doi: 10.1111/1750-3841.12118. [DOI] [PubMed] [Google Scholar]
- 81.Liu Q., Luyten W., Pellens K., Wang Y., Wang W., Thevissen K., Liang Q., Cammue B.P., Schoofs L., Luo G. Antifungal activity in plants from Chinese traditional and folk medicine. J. Ethnopharmacol. 2012;143:772–778. doi: 10.1016/j.jep.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 82.Kiyohara H., Ichino C., Kawamura Y., Nagai T., Sato N., Yamada H. Patchouli alcohol: In vitro direct anti-influenza virus sesquiterpene in Pogostemon cablin Benth. J. Nat. Med. 2012;66:55–61. doi: 10.1007/s11418-011-0550-x. [DOI] [PubMed] [Google Scholar]
- 83.Wu H., Li B., Wang X., Jin M., Wang G. Inhibitory effect and possible mechanism of action of patchouli alcohol against Influenza A (H2N2) virus. Molecules. 2011;16:6489–6501. doi: 10.3390/molecules16086489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y.C., Peng S.Z., Chen H.M., Zhang F.X., Xu P.P., Xie J.H., He J.J., Chen J.N., Lai X.P., Su Z.R. Oral administration of patchouli alcohol isolated from Pogostemonis Herba augments protection against influenza viral infection in mice. Int. Immunopharmacol. 2012;12:294–301. doi: 10.1016/j.intimp.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 85.Wu X.L., Ju D.H., Chen J., Yu B., Liu K.L., He J.X., Dai C.Q., Wu S., Chang Z., Wang Y.P., et al. Immunologic mechanism of patchouli alcohol anti-H1N1 influenza virus may through regulation of the RLH signal pathway in vitro. Curr. Microbiol. 2013;67:431–436. doi: 10.1007/s00284-013-0381-y. [DOI] [PubMed] [Google Scholar]
- 86.Buckle J. Clinical aromatherapy and AIDS. J. Assoc. Nurses Aids Care. 2002;13:81–99. doi: 10.1177/10529002013003006. [DOI] [PubMed] [Google Scholar]
- 87.Xie Y.C., Tang F. Protective effect of Pogostemon cablin on membrane fluidity of intestinal epithelia cell in ischemia/reperfusion rats after ischemia/reperfusion. Chin. J. Integr. Traditional West. Med. 2009;29:639–641. [PubMed] [Google Scholar]
- 88.Yang Y., Kinoshita K., Koyama K., Takahashi K., Tai T., Nunoura Y., Watanabe K. Anti–emetic principles of Pogostemon cablin (Blanco) Benth. Phytomedicine. 1999;6:89–93. doi: 10.1016/S0944-7113(99)80041-5. [DOI] [PubMed] [Google Scholar]
- 89.Mikuriya N., Kim Y., Fujimura K. The effect of the aroma of Patchouli essential oil on defecation and constipation. Aroma Res. 2004;5:70–75. [Google Scholar]
- 90.Sumi H. Fibrinolysis–accelerating activity of the essential oils and Shochu aroma. Aroma Res. 2003;4:264–267. [Google Scholar]
- 91.Park E.K., Yoon H.K., Kim D.H. Antithrombotic activity of Sunghyangjunggisan. Nat. Prod. Sci. 2002;8:71–75. [Google Scholar]
- 92.Wei A., Shibamoto T. Antioxidant activities and volatile constituents of various essential oils. J. Agric. Food Chem. 2007;55:1737–1742. doi: 10.1021/jf062959x. [DOI] [PubMed] [Google Scholar]
- 93.Kim H.W., Cho S.J., Kim B.Y., Cho S.I., Kim Y.K. Pogostemon cablin as ROS scavenger in oxidant-induced cell death of human neuroglioma cells. Evid. Based Complement. Alternat. Med. 2010;7:239–247. doi: 10.1093/ecam/nem176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin R.F., Feng X.X., Li C.W., Zhang X.J., Yu X.T., Zhou J.Y., Zhang X., Xie Y.L., Su Z.R., Zhan J.Y. Prevention of UV radiation-induced cutaneous photoaging in mice by topical administration of patchouli oil. J. Ethnopharmacol. 2014;154:408–418. doi: 10.1016/j.jep.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 95.Feng X.X., Yu X.T., Li W.J., Kong S.Z., Liu Y.H., Zhang X., Xian Y.F., Zhang X.J., Su Z.R., Lin Z.X. Effects of topical application of patchouli alcohol on the UV-induced skin photoaging in mice. Eur. J. Pharm. Sci. 2014;63:113–123. doi: 10.1016/j.ejps.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 96.Li Y.P., Yuan S.F., Cai G.H., Wang H., Wang L., Yu L., Ling R., Yun J. Patchouli alcohol dampens lipopolysaccharide induced mastitis in mice. Inflammation. 2014;37:1757–1762. doi: 10.1007/s10753-014-9905-2. [DOI] [PubMed] [Google Scholar]
- 97.Lu T.C., Liao J.C., Huang T.H., Lin Y.C., Liu C.Y., Chiu Y.J., Peng W.H. Analgesic and anti-inflammatory activities of the methanol extract from Pogostemon cablin. Evid. Based Complement. Alternat. Med. 2011;2011:671741. doi: 10.1093/ecam/nep183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xian Y.F., Li Y.C., Ip S.P., Lin Z.X., Lai X.P., Su Z.R. Anti-inflammatory effect of patchouli alcohol isolated from Pogostemonis Herba in LPS-stimulated RAW264.7 macrophages. Exp. Ther. Med. 2011;2:545–550. doi: 10.3892/etm.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y.C., Xian Y.F., Ip S.P., Su Z.R., Su J.Y., He J.J., Xie Q.F., Lai X.P., Lin Z.X. Anti-inflammatory activity of patchouli alcohol isolated from Pogostemonis Herba in animal models. Fitoterapia. 2011;82:1295–1301. doi: 10.1016/j.fitote.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 100.Li C.W., Wu X.L., Zhao X.N., Su Z.Q., Chen H.M., Wang X.F., Zhang X.J., Zeng H.F., Chen J.N., Li Y.C., et al. Anti-inflammatory property of the ethanol extract of the root and rhizome of Pogostemon cablin (Blanco) Benth. Sci. World J. 2013 doi: 10.1155/2013/434151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jeong J.B., Shin Y.K., Lee S.H. Anti-inflammatory activity of patchouli alcohol in RAW264.7 and HT-29 cells. Food Chem. Toxicol. 2013;55:229–233. doi: 10.1016/j.fct.2012.12.062. [DOI] [PubMed] [Google Scholar]
- 102.Park S.Y., Neupane G.P., Lee S.O., Lee J.S., Kim M.Y., Kim S.Y., Park B.C., Park Y.J., Kim J.A. Protective effects of Pogostemon cablin Bentham water extract on inflammatory cytokine expression in TNBS-induced colitis in rats. Arch. Pharmacal. Res. 2014;37:253–262. doi: 10.1007/s12272-013-0260-x. [DOI] [PubMed] [Google Scholar]
- 103.Miyazawa M., Okuno Y., Nakamura S.I., Kosaka H. Antimutagenic activity of flavonoids from Pogostemon cablin. J. Agric. Food Chem. 2000;48:642–647. doi: 10.1021/jf990160y. [DOI] [PubMed] [Google Scholar]
- 104.Yu J., Qi Y., Luo G., Duan H.Q., Zhou J. Extraction and analysis of the essential oil in Pogostemon cablin by enzymatic hydrolysis and inhibitory activity against HeLa cell proliferation. J. Chin. Med. Mater. 2012;35:796–799. [PubMed] [Google Scholar]
- 105.Jeong J.B., Choi J., Lou Z., Jiang X., Lee S.H. Patchouli alcohol, an essential oil of Pogostemon cablin, exhibits anti-tumorigenic activity in human colorectal cancer cells. Int. Immunopharmacol. 2013;16:184–190. doi: 10.1016/j.intimp.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 106.Hsu H.C., Yang W.C., Tsai W.J., Chen C.C., Huang H.U., Tsai Y.C. Alpha–bulnesene, a novel PAF receptor antagonist isolated from Pogostemon cablin. Biochem. Biophys. Res. Commun. 2006;345:1033–1038. doi: 10.1016/j.bbrc.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 107.Liao J.B., Wu D.W., Peng S.Z., Xie J.H., Li Y.C., Su J.Y., Chen J.N., Su Z.R. Immunomodulatory potential of patchouli alcohol isolated from Pogostemon cablin (Blanco) Benth (Lamiaceae) in mice. Trop. J. Pharm. Res. 2013;12:559–565. [Google Scholar]
- 108.Dang H., Huang Q.Y. Preliminary investigation into the pharmacodynamics of Xinxiang granule. Chin. J. Inform. Traditional Chin. Med. 2002;9:20–23. [Google Scholar]
- 109.He J.J., Chen H.M., Li C.W., Wu D.W., Wu X.L., Shi S.J., Lai X.P. Experimental study on antinociceptive and anti-allergy effects of patchouli oil. J. Essent. Oil Res. 2013;25:488–496. doi: 10.1080/10412905.2013.809319. [DOI] [Google Scholar]
- 110.Kerr J. The use of essential oils in healing wounds. Int. J. Aromather. 2002;12:202–206. doi: 10.1016/S0962-4562(03)00004-3. [DOI] [Google Scholar]
- 111.Chen H., Li Y., Wu X., Li C., Li Q., Qin Z., Yi Y., Chen J., Lai X., Su Z. LC-MS/MS determination of pogostone in rat plasma and its application in pharmacokinetic studies. Biomed. Chromatogr. 2013;27:1092–1099. doi: 10.1002/bmc.2897. [DOI] [PubMed] [Google Scholar]
- 112.Li Y., Su Z., Lin S., Li C., Zhao Y., Gao X., Lai Y., Wu X., Wu H., Cai Z., Lai X. Characterisation of the metabolism of pogostone in vitro and in vivo using liquid chromatography with mass spectrometry. Phytochem. Anal. 2014;25:97–105. doi: 10.1002/pca.2471. [DOI] [PubMed] [Google Scholar]
- 113.Zheng Y.F., Xie J.H., Xu Y.F., Liang Y.Z., Mo Z.Z., Jiang W.W., Chen X.Y., Liu Y.H., Yu X.D., Huang P., et al. Gastroprotective effect and mechanism of patchouli alcohol against ethanol, indomethacin and stress-induced ulcer in rats. Chem.-Biol. Interact. 2014;222:27–36. doi: 10.1016/j.cbi.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 114.Kardinan A., Wikardi E.A. The prospect of botanical insecticides on stored food insects management. Biotrop. Spec. Publ. 1997;59:199–208. [Google Scholar]
- 115.Chun W., DeJun Y., ShiLin H., Qun T. Determination of toxicity of plant essential oils to museum insect pests. J. Southwest Agric. Univ. 2000;22:494–495. [Google Scholar]
- 116.Betty C.R.Z., Gregg H., Ying Y., Roger A.L. Toxicity and repellency of Patchouli oil and patchouli alcohol against Formosan subterranean termites Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae) J. Agric. Food Chem. 2003;51:4585–4588. doi: 10.1021/jf0301495. [DOI] [PubMed] [Google Scholar]
- 117.Trongtokit Y., Rongsriyam Y., Komalamisra N., Apiwathnasorn C. Comparative repellency of 38 essential oils against mosquito bites. Phytother. Res. 2005;19:303–309. doi: 10.1002/ptr.1637. [DOI] [PubMed] [Google Scholar]
- 118.Wu H.Q., Li J., He Z.D., Liu Z.G. Acaricidal activities of traditional Chinese medicine against the house dust mite, Dermatophagoides farinae. Parasitology. 2010;137:975–983. doi: 10.1017/S0031182009991879. [DOI] [PubMed] [Google Scholar]
- 119.Albuquerque E.L., Lima J.K., Souza F.H., Silva I.M., Santos A.A., Araújo A.P., Blank A.F., Lima R.N., Alves P.B., Bacci L. Insecticidal and repellence activity of the essential oil of Pogostemon cablin against urban ants species. Acta Trop. 2013;127:181–186. doi: 10.1016/j.actatropica.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 120.Gokulakrishnan J., Kuppusamy E., Shanmugam D., Appavu A., Kaliyamoorthi K. Pupicidal and repellent activities of Pogostemon cablin essential oil chemical compounds against medically important human vector mosquitoes. Asian Pac. J. Trop. Dis. 2013;3:26–31. doi: 10.1016/S2222-1808(13)60006-7. [DOI] [Google Scholar]
- 121.Phal D., Naik R., Deobhankar K., Vitonde S., Ghatpande N. Laboratory evaluation of herbal mosquito coils against Aedes aegypti mosquito. Bull. Environ. Pharmacol. Life Sci. 2012;1:16–20. [Google Scholar]
- 122.Huang S.H., Xian J.D., Kong S.Z., Li Y.C., Xie J.H., Lin J., Chen J.N., Wang H.F., Su Z.R. Insecticidal activity of pogostone against Spodoptera litura and Spodoptera exigua (Lepidoptera: Noctuidae) Pest. Manag. Sci. 2014;70:510–516. doi: 10.1002/ps.3635. [DOI] [PubMed] [Google Scholar]
- 123.Haze S., Sakai K., Gozu Y. Effects of fragrance inhalation on sympathetic activity in normal adults. Jpn. J. Pharmacol. 2002;90:247–253. doi: 10.1254/jjp.90.247. [DOI] [PubMed] [Google Scholar]
- 124.Bowles E.J., Griffiths D.M., Quirk L., Brownrigg A., Croot K. Effects of essential oils and touch on resistance to nursing care procedures and other dementia–related behaviours in a residential care facility. Int. J. Aromather. 2002;12:22–29. doi: 10.1054/ijar.2001.0128. [DOI] [Google Scholar]
- 125.Smith M. Aromatherapy care for the caregivers research paper. [(accessed on 14 January 2015)]. Available online: essentialoiltechniques.com/docs/Aromatherapy-for-the-care-giver.PDF.
- 126.Perry N., Perry E. Aromatherapy in the management of psychiatric disorders-Clinical and neuropharmacological perspectives. CNS Drugs. 2006;20:257–280. doi: 10.2165/00023210-200620040-00001. [DOI] [PubMed] [Google Scholar]
- 127.Beshara M.C., Giddings D. Use of plant essential oils in treating agitation in a dementia unit: 10 case studies. Int. J. Aromather. 2003;12:207–212. doi: 10.1016/S0962-4562(02)00085-1. [DOI] [Google Scholar]
- 128.Pujiarti R., Ohtani Y., Widowati T.B., Wahyudi, Kasmudjo, Herath N.K., Wang C.N. Effect of Melaleuca leucadendron, Cananga odorata and Pogostemon cablin oil odors on Human physiological responses. Wood Res. J. 2012;3:100–105. [Google Scholar]