Phylogenetic and chemical studies in the potential psychotropic species complex of Psilocybe atrobrunnea with taxonomic and nomenclatural notes (original) (raw)
Abstract
Five Psilocybe species with unresolved systematic position (P. atrobrunnea, P. laetissima, P. medullosa, P. pelliculosa, and P. silvatica) were investigated using four molecular markers (EF1-α, ITS, LSU, and IGS). Phylogenetic analysis revealed that with the exception of P. laetissima, which is now rightfully classified in the genus Leratiomyces, all investigated species belong to Psilocybe sect. Psilocybe. For the first time, psychotropic compounds psilocin and psilocybin were detected in P. medullosa using gas chromatography-mass spectrometry. On the contrary, neither psilocin, nor psilocybin was detected in P. atrobrunnea and negative results were also obtained from mycelia grown in vitro on tryptamine/tryptophan-amended media. These results strongly suggest that biosynthesis of these alkaloids was lost in P. atrobrunnea. With the exception of minor differences detected in EF1-α marker, all sequences of American and European collections of P. atrobrunnea were identical. On the other hand, a thorough nomenclatural study revealed that the name P. atrobrunnea must be considered dubious; the oldest available candidate name, P. fuscofulva, was therefore adopted. The molecular data suggests that morphologically identical American P. silvatica and European P. medullosa likely represent distinct species; epitypes of both taxa were therefore designated.
Keywords: hallucinogenic fungi, Leratiomyces, phylogeny, psilocin, psilocybin, Strophariaceae
INTRODUCTION
As recently demonstrated, the European wood-rotting psychotropic Psilocybe species (_P. cyanescens_-complex) include P. cyanescens, P. azurescens, and the highly variable P. serbica (Borovička et al. 2011). Furthermore, in Europe there are another related and possibly psychotropic Psilocybe species with unresolved systematic position: P. atrobrunnea (Lasch: Fr.) Gillet and P. medullosa (Bres.) Borov.
Psilocybe atrobrunnea was originally described from Europe by Lasch (1828; as Agaricus atrobrunneus) from material collected in a region named 'Marchiae Brandenburgicae', an area currently corresponding to Eastern Germany and Western Poland. Collections of this species have also been reported from other European countries (Singer 1986, Borovička 2006, Noordeloos 2011), Canada, and the USA (Guzmán 1983, Borovička 2006). Psilocybe atrobrunnea is found in peat bogs growing in Sphagnum, rarely also on moist, decaying wood in peaty habitats. No bluing reaction, typical for psychotropic species, has been reported for this species but according to Stamets (1996), it is 'possibly active'. Various European authors (Noordeloos 1999, Horak 2005, Knudsen & Vesterholt 2008) have preferred the name Psilocybe turficola J. Favre (Favre 1939). However, despite being cited as legitimate by Mycobank (MB255229), P. turficola was invalidly published (Art. 39.1, no Latin diagnosis). Noordeloos (2011) has therefore adopted the name P. atrobrunnea, which has been used by both American and European mycologists (Guzmán 1983, 1995, Singer 1986, Smith 1940, Borovička 2006).
Psilocybe medullosa (Borovička 2007) was originally described from Italy (Bresadola 1898, as Naucoria medullosa). In recent publications (Knudsen & Vesterholt 2008, Borovička 2011), it was synonymized with Psilocybe silvatica (Peck) Singer & A.H. Sm. described from North America (Peck 1889, as Psathyra silvatica). It grows on woody debris and detritus (mostly under conifers – usually Picea, but also under Fagus) and is apparently rare. According to Borovička (2011), the microcharacters of P. medullosa observed in the holotype and recent European collections match well with those in the holotype of P. silvatica. But molecular data, now included in this paper, was not available in order to clarify the relationship of both taxa. According to Stamets (1996), P. silvatica is a psychotropic species ('weakly to moderately active') but to our best knowledge, chemical analyses have never been published. However, a very similar American species, P. pelliculosa (A.H. Sm.) Singer & A.H. Sm., is known to contain psychotropic compounds (Repke et al. 1977, Beug & Bigwood 1982). Both, P. silvatica and P. pelliculosa were placed in Psilocybe sect. Semilanceatae by Guzmán (1983, 1995).
Psilocybe laetissima Hauskn. & Singer, only known from Europe, is another species with unclear systematic placement. Described from Austria (Hausknecht & Singer 1986), it grows in dry grasslands and has never been considered to contain psychotropic compounds. Whereas the original authors considered it related to P. merdaria (Fr.: Fr.) Ricken in Psilocybe sect. Merdariae (Hausknecht & Singer 1986), Guzmán (1995) and Noordeloos (1999) classified it in Psilocybe sect. Atrobrunneae.
The aim of our study was:
- i.
to explore the relationships among P. atrobrunnea, P. laetissima, P. medullosa, P. pelliculosa, and P. silvatica by the use of molecular markers; - ii.
to verify possible occurrence of the psychotropic compounds psilocin and psilocybin in P. atrobrunnea and P. medullosa; - iii.
to inspect the assumed conspecifity of the European and American collections (P. atrobrunnea; P. silvatica vs P. medullosa).
MATERIALS AND METHODS
Collections used
Collections of Psilocybe species used for the molecular study are listed in Table 1. Available sequences of related species, including those from our previous studies (Borovička et al. 2011, 2012) were downloaded from public databases. Collections of P. atrobrunnea, P. pelliculosa, and P. silvatica were identified according to Guzmán (1983, 1995), Noordeloos (1999), Borovička (2006, 2011), and Guzmán et al. (2008). Canadian specimens of P. silvatica kept at DAOM could not be used for DNA extraction, but template DNA extracted from a strain (isolated from the collection DAOM 187848) was kindly donated by Scott A. Redhead. The sequenced collection of P. laetissima was identified by its original co-author Anton Hausknecht (conf. J. Borovička); a detailed description of this collection was reported by Antonín & Dvořák (2010). Herbarium specimens of sequenced collections are available at BRNM, DAOM, IB, PRM, and UBC (Table 1); herbarium acronyms are used according to Thiers (2012). Samples used for testing of psychotropic compounds were collected and identified by J. Borovička (Table 2); the sample of P. atrobrunnea from NY, USA was kindly donated by Eric Smith.
Table 1.
Species under molecular study.
Table 2.
Samples for analysis of psilocin and psilocybin.
| Species | Collection |
|---|---|
| Hypholoma marginatum** | PRM 921867 |
| Inocybe corydalina* | PRM 899248 |
| Psilocybe atrobrunnea (Czech Rep.) | PRM 922256 |
| Psilocybe atrobrunnea (USA) | PRM 922257 |
| Psilocybe medullosa (Czech Rep.) | PRM 909630 |
| Psilocybe medullosa (Czech Rep.) | PRM 922258 |
| Psilocybe serbica var. arcana* | PRM 899282 |
| Stropharia caerulea** | PRM 921876 |
| Stropharia aeruginosa** | PRM 858114 |
In-vitro production of psychotropic compounds
Isolates of P. atrobrunnea and P. serbica were obtained from explants of basidiomata and their identity was verified using the comparison of their ITS rDNA sequences with our data. The production of psilocin (PS) and psilocybin (PSB) was then tested in liquid cultures. The mycelium was first pre-cultivated on potato dextrose agar. The colony margin was cut to 5×5×3 mm blocks, which were used as inoculum for stationary liquid cultures (50 ml liquid medium per 250 ml Erlenmeyer flask). Three inoculum blocks were used per flask. The cultivation medium contained 20 g glucose, 20 g fructose, 9 g glycine, 1 g ammonium succinate, 1 g yeast extract, 500 mg MgSO4.7H2O, 100 mg KH2PO4, 2.5 mg ZnSO4.7H2O and 5 mg FeSO4.5H2O per litre (pH 5.5, before autoclaving). After autoclaving, a volume of 0.5 ml filter-sterilized solution of tryptamine hydrochloride (246557 Aldrich) and DL-tryptophan (T3300 Sigma) in dimethylsulfoxide was added to 50 ml medium to reach 2 mM final concentration of both compounds. Control treatments received 0.5 ml of pure solvent.
Eight flasks were established in total: 2 fungal species × 2 replicates × 2 treatments (control medium and the medium with tryptamine and tryptophan). Inoculated flasks were incubated for 4 wk at 25 °C in the dark. The pelleted mycelium was shortly dried using paper towel, lyophilized, and subjected to analysis of PS/PSB as described below.
Determination of psilocin and psilocybin
Basidiomata of specimens selected for determination of PS and PSB (Table 2) were cleaned of substrate debris, frozen shortly after harvest and later lyophilized. Based on previously published analytical approaches (Keller et al. 1999, Stříbrný et al. 2003), dry basidiomata were then ground in a mortar and 10 mg of the powdered biomass were extracted with 0.5 ml of methanol (puriss p.a.) in ultrasonic bath for 30 min. The mixture was then centrifuged; 100 μl of the supernatant was separated into a glass vial and evaporated under a stream of nitrogen at room temperature. The dry residue was silylated by 50 μl of the derivatization agent N-methyl-N-trimethylsilyltrifluoroacetamide (MSTFA, puriss p.a., for GC, Fluka) at 100 °C for 30 min. After cooling to room temperature, 1 μl of the reaction mixture was analysed by gas chromatography-mass spectrometry (GC-MS). The lyophilized mycelium was processed in a similar way.
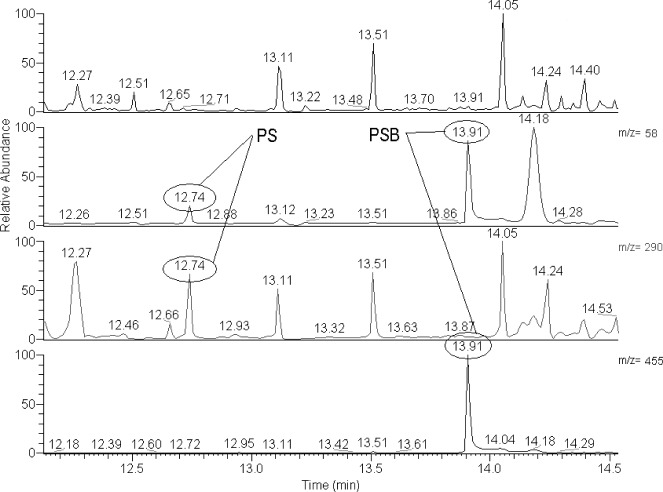
GC-MS analysis was performed with Thermo DSQ equipped with a fused-silica capillary column SLB-5ms (15 m, 0.25 mm, 0.25 μm). The temperature of the injector was 220 °C, splitless time 30 s, the temperature of the transfer line was 250 °C. The oven temperature program: 70 °C for 1 min, then ramp 10 °C/min to 170 °C and final ramp 30 °C/min to 300 °C. Helium was used as carrier gas (1.5 ml/min). The mass spectrometer operated in full scan mode (FS, m/z 40-530) together with selected ions monitoring mode (SIM, m/z 58, m/z 290 and m/z 455 – characteristic ions of silylated PS and PSB). The proof of the presence of PS/PSB in the tested samples was carried out by comparing the mass spectra and retention times (RT) of the relevant peaks with the mass spectra and RT of the PS and PSB standards (QUICK-CHEK Solution, Alltech). The retention time of PS was 12:74 and that of PSB 13:91 min.
In order to verify the obtained results, species already known to contain PS/PSB, Inocybe corydalina and P. serbica var. arcana (Stijve et al. 1985, Stříbrný et al. 2003), and negative controls (Stropharia aeruginosa, S. caerulea, and Hypholoma marginatum), were analysed and compared with the tested samples. We were not able to quantify the amounts of PS/PSB in the biomass since only a few micrograms of PS/PSB standards were available. GC-MS chromatogram of silylated extract of P. medullosa with SIM chromatograms of ions m/z 58, 290 and 455 are in Fig. 1. Mass spectra of silylated PS and PSB are given in Fig. 2, 3.
Fig. 1.
Total (first line) and single ion (second, third, and fourth line) GC–MS chromatograms of the silylated extract of Psilocybe medullosa (PRM 909630). Ions typical for silylated psilocin are m/z 58 and 290, ions typical for silylated psilocybin are m/z 58 and 455. Retention time of silylated psilocin (PS) is 12:74, retention time of silylated psilocybin (PSB) is 13:91.
Fig. 2.
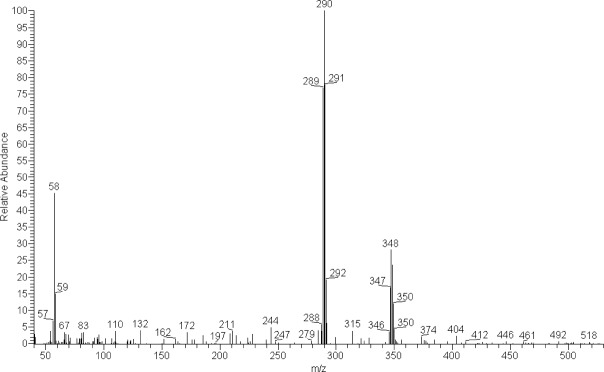
Mass spectrum of the silylated psilocin found in the extract of Psilocybe medullosa (PRM 909630).
Fig. 3.

Mass spectrum of the silylated psilocybin found in the extract of Psilocybe medullosa (PRM 909630).
Molecular phylogeny
Nuclear DNA was extracted from a small piece of dried or frozen fungal biomass (a piece of basidiome) using the Nucleo-Spin® Plant II extraction kit (Macherey-Nagel) according to the manufacturer’s instructions. Three distinct molecular markers were used to study phylogeny of selected Psilocybe species: partial sequences of the divergent D1-D2 domain at the 5’ end of the nuclear LSU rRNA gene (Maruyama et al. 2003, 2006), ITS rDNA region containing ITS1, 5.8S, and ITS2 sequences (Pornpakakul et al. 2009, Kallifatidis et al. 2014), and part of the elongation factor 1-α (EF1-α) (Antonín et al. 2009, 2013, Tomšovský et al. 2010). Oligonucleotide primers and PCR conditions were used as published previously (Borovička et al. 2011). Furthermore, partial intergenic spacer (IGS) sequences were obtained for P. atrobrunnea, P. medullosa, and P. silvatica using the primer pair NL11-CNS1 according to Aoki et al. (2003); IGS region was used to assess the similarity among the sequences of these species. Obtained amplicons were purified by isopropanol precipitation and both strands were sequenced at Macrogen Inc., Korea and sequences edited in BioEdit (Hall 1999).
Newly obtained sequences of EF1-α, ITS, and LSU rRNA gene were aligned together with appropriate homologues downloaded from public databases (see Table 1 for details): nucleotide sequences coding for rRNA genes (LSU and ITS rDNA) were aligned using Kalign (Lassmann & Sonnhammer 2005) at http://www.ebi.ac.uk/Tools/msa/kalign/; sequences coding for EF1-α were translated to amino acids, aligned using ClustalW algorithm as implemented in BioEdit, and retranslated back to nucleotides. Introns were aligned separately in nucleotides and were included into the dataset; nucleotide alignment of EF1-α was used to infer the tree. Alignments were manually edited in BioEdit, gaps and ambiguously aligned positions (2 positions from the primary LSU alignment, 61 positions from the EF-1α, and 80 positions from the ITS) were excluded from further analysis; final alignments used for computing trees consisted of 477 nt (EF-1α), 586 nt (ITS), and 575 nt (LSU). Alignments were analysed separately as well as in a combined dataset (1601 nt).
Maximum likelihood (ML), maximum parsimony (MP), and Bayesian inference were used to estimate phylogenetic relationships among investigated fungi using individual gene datasets and the combined dataset. ML analysis was performed using the PhyML program (Guindon & Gascuel 2003) with a gamma corrected GTR model of nucleotide substitution with estimated proportion of invariable sites (GTR+I+G). MP trees were computed using PAUP* 4b10 (Swofford 2000). Robustness of MP and ML trees was calculated by bootstrap analyses in 1 000 replicates. MrBayes 3.2 (Ronquist et al. 2012) was used to assess Bayesian topologies and posterior probabilities (PP) using the gamma corrected GTR substitutional matrix with estimated proportion of invariable sites (GTR+I+G). Two independent Monte-Carlo Markov chains were run (under the default settings) for 3 million generations; due to the low complexity of datasets, the chains reached steady state within the first few thousands of generations. Therefore, we used 10 000 generations as a burn-in and omitted them from topology reconstruction and posterior probability (PP) calculation. The trees were rooted using Hypholoma marginatum sequences as outgroup. Sequences were accessioned into EMBL-Bank (see Table 1 for numbers) and the alignments made available in TreeBASE (study ID: S15446).
RESULTS
Psychotropic compounds
PS and PSB were detected in positive controls of Inocybe corydalina and P. serbica var. arcana (including mycelia from the in vitro experiment), but also in all tested collections of P. medullosa (Fig. 1–3). On the other hand, collections of P. atrobrunnea were PS/PSB negative, similar to negative controls (data not shown). Furthermore, negative results were obtained also from cultivated mycelia of P. atrobrunnea, both from control and tryptamine/tryptophan-amended media (data not shown). Psilocybe laetissima was not tested due to the lack of material; however, its systematic position (see below) and the lack of the bluing reaction indicate PS/PSB absence.
Molecular phylogeny
We have performed phylogenetic analyses based on three different molecular markers: EF1-α, ITS, and LSU rDNA sequences. We analysed those genes individually and also in a combined dataset. Separate analyses of individual genes produced different topologies, likely due to different informational content of particular markers and gene specific taxon sampling.
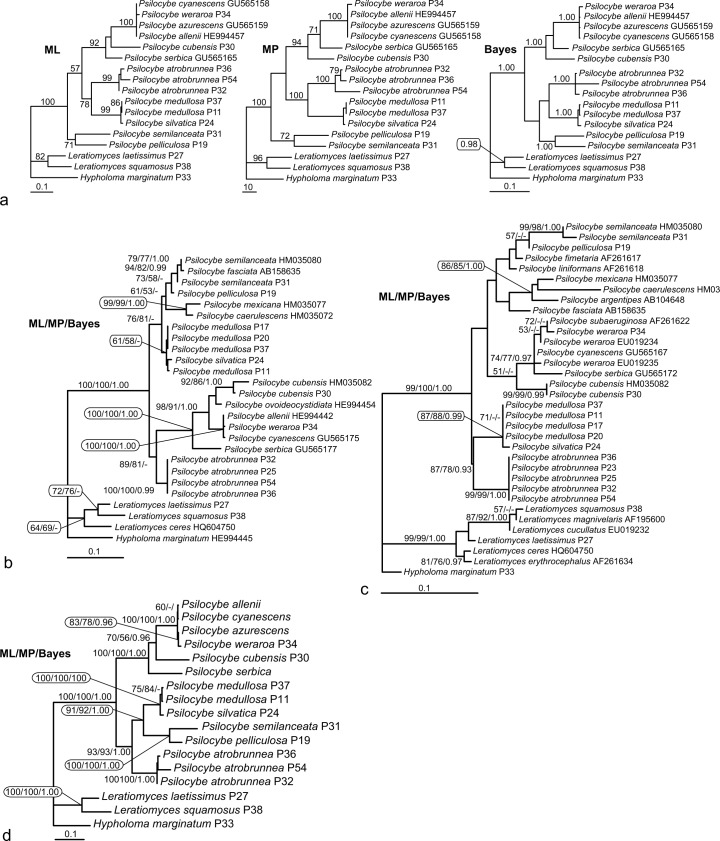
Maximum likelihood (ML) and maximum parsimony (MP) phylogenetic analysis based on the EF1-α nucleotide sequences (Fig. 4a) shows P. semilanceata forming together with P. pelliculosa the most basal lineage in the frame of the studied species of the genus Psilocybe. The rest are divided into two clades: the first clade is composed of P. atrobrunnea and its sister group containing P. medulosa and P. silvatica. The second clade contains P. cubensis and P. serbica paraphyletically branching on its root complemented by sequentially almost identical isolates classified as P. allenii, P. azurescens, P. cyanescens, and P. weraroa. It should be noted that not all the nodes of the tree are well supported. Also, the positions of P. serbica and P. cubensis are opposite in ML and MP trees. In MP analysis, six equally parsimonious trees were obtained; however, they show only variation in the polytomic clade of the very closely related species P. allenii, P. azurescens, P. cyanescens, and P. weraroa (Borovička et al. 2011). Bayesian tree shows different but unsupported topology placing P. pelliculosa and P. semilanceata on the root of the clade containing P. atrobrunnea, P. medulosa, and P. silvatica.
Fig. 4.
a. Maximum likelihood (ML), maximum parsimony (MP), and Bayesian trees as inferred from EF-1α nucleotide sequences (including introns). Numbers above branches indicate ML bootstrap support, MP bootstrap support, and Bayesian posterior probabilities, respectively; b. ML tree as inferred from ITS nucleotide sequences; c. ML tree as inferred from partial LSU rRNA gene sequences; d. ML tree as inferred from a combined dataset of EF-1α+LSU+ITS. All shown ML trees were constructed using gamma corrected GTR model (GTR+I+G). Numbers above branches in Fig. b, c, and d indicate ML/MP bootstrap support (both from 1 000 replicates)/Bayesian posterior probabilities.
The tree based on ITS rDNA sequences (Fig. 4b) divides the Psilocybe isolates into two clades. The first one shows P. atrobrunnea at the root of the clade composed of P. allenii, P. cubensis, P. cyanescens, P. ovoideocystidiata, P. serbica, and P. weraroa. Similarly to EF1-α, P. allenii, P. cyanescens, and P. weraroa show very low mutual diversity in the sequence of the used marker. The second clade contains P. caerulescens, P. fasciata, P. medullosa, P. mexicana, P. pelliculosa, P. semilanceata, and P. silvatica. Also, contrary with the EF-1α tree, P. semilanceata together with P. pelliculosa do not form an early branch but appear in an advanced position within the clade containing P. caerulescens, P. fasciata, P. medullosa, P. mexicana, and P. silvatica.
A phylogeny based on a part of the nuclear LSU rRNA gene (Fig. 4c) shows, in contrast with the ITS rDNA and similarly to EF-1α trees, a relationship of P. atrobrunnea, P. medulosa, and P. silvatica. The rest of the Psilocybe species are placed in an unsupported clade. Within the first subclade, P. fasciata is not related to P. semilanceata as in the ITS tree, but branches sister to P. argentipes, P. caerulescens, and P. mexicana. This entire lineage is in a sister position to the clade composed of P. fimetaria, P. liniformans, P. pelicullosa, and P. semilanceata but with no bootstrap support. The second subclade contains P. cubensis on the root, with relatively closely related species in the crown: P. cyanescens, P. serbica, P. subaeruginosa, and P. weraroa. According to this analysis, P. weraroa appears to be non-monophyletic. The LSU tree is generally mostly unsupported, obviously due to the low informational content of the DNA marker (from 576 characters, 61 characters are variable and only 48 parsimony informative).
Analysis of the combined dataset (Fig. 4d) shows a well-resolved and robust phylogeny (bootstrap supports of the main clades were between 91 and 100 %). We decided to build our taxonomic suggestions (see below) based on this tree. Psilocybe isolates grouped into two well-defined lineages: the first lineage is composed of P. serbica in the most basal position, P. cubensis, and almost identical sequences classified as P. allenii, P. azurescens, P. cyanescens, and P. weraroa. The second lineage contains P. atrobrunnea, P. medullosa, P. pelliculosa, P. semilanceata, and P. silvatica. Within this lineage, P. atrobrunnea forms the earliest branch, P. semilanceata clusters with P. pelliculosa with high support and these two species form a sister group to P. medullosa – P. silvatica subclade. Although the combined tree is well-supported, trees constructed from single genes (EF-1α, ITS, and LSU) based on the limited taxon sampling of the combined tree (Fig. 4d) were not mutually fully congruent. However, the only difference lies in the positions of P. pelliculosa and P. semilanceata in the particular trees. In MP analyses, the EF-1α placed both species on the early branching position shown in Fig. 4a (MP). ITS topology (not shown) was identical with the combined tree with P. pelliculosa and P. semilanceata appearing on the root of the P. medullosa – P. silvatica clade (Fig. 4d), while a weakly supported (bootstrap 52 %) position of P. semilanceata and P. pelliculosa on the root of the P. atrobrunnea, P. medullosa, and P. silvatica clade was obtained for the LSU tree (not shown). It is also obvious that the tree topology is highly affected by taxon sampling, as demonstrated on the case of ITS, which displays different topologies when limited dataset for the combined tree and the larger dataset shown in Fig. 4b is computed.
DISCUSSION
Phylogenetic studies have recently shown that the genus Psilocybe (Fr.: Fr.) P. Kumm. in the sense of Guzmán (1983) and Singer (1986) is polyphyletic (Moncalvo et al. 2002, Ramírez-Cruz et al. 2013). In order to keep the well-known and widely used name Psilocybe for the psychoactive species, it was recently conserved with P. semilanceata (Fr.) P. Kumm. as the conserved type (Redhead et al. 2007), and Psilocybe is now applied to the clade of psychotropic species: Psilocybe sensu stricto (s.str.) (Ramírez-Cruz et al. 2013).
According to our results (Fig. 4d, combined dataset), the phylogenetic placement of P. atrobrunnea clearly indicates its close relationship with P. semilanceata and other psychotropic species. But neither PS nor PSB was detected in its basidiomata. A question has arisen whether this absence pertains to the capability of the fungus to produce those compounds or to environmental stimuli. It could be hypothesized that the absence of PS/PSB might have been induced by the occurrence in peat habitats with limited sources of available nitrogen (Bobbink et al. 1998). In that case, the biosynthesis of PS/PSB can be artificially elicited in axenic culture (Agurell & Nilsson 1968). However, our in vitro experiment, where mycelium of P. atrobrunnea was grown on medium rich in PS/PSB precursors tryptamine and tryptophan, did not lead to detection of PS/PSB in the tissue. This would indicate that the capability of PS/PSB synthesis was lost in P. atrobrunnea and therefore the genus Psilocybe s.str. does not exclusively contain psychotropic species. This is, nevertheless, not a surprise since a similar phenomenon has been recently reported for the family Inocybaceae, where biosynthesis of the alkaloids muscarine and PSB has evolved independently on several occasions, together with several losses (Kosentka et al. 2013). Our negative results confirm the earlier negative report published by Leung et al. (1965). Høiland (1978) reported the occurrence of PSB in P. atrobrunnea from Norway; however, recent revision of his collection revealed it had been misidentified and actually represents P. medullosa (Borovička 2011).
Psychotropic Psilocybe species are well known for their characteristic bluing (or greenish) reaction on stipe/pileus surface including mycelial strands and mycelium – especially when bruised or in age. Despite the fact that P. medullosa has been proven to contain PS/PSB, we have never noted any kind of this reaction on basidiomata in the field, even when damaging the mycelial strands at the stipe base, where the reaction is easily observable in most Psilocybe species. In P. silvatica, which is believed to be psychotropic and very close to P. medullosa (see below), the bluing reaction has not been mentioned by American authors (Smith 1940, Singer & Smith 1958, Stamets 1996). In contrast, Guzmán (1983) reported a slight bluing reaction in the stipe context (on cut) and stipe surface near the base. Judging from our analysis (when compared to our positive controls), it could be hypothesized that the PSB/PS content was very low in the tested specimens. As the bluish product is a consequence of oxidation of PS (Horita & Weber 1961, Levine 1967), we speculate that low PS content might lead to the lack of the characteristic bluing reaction in P. medullosa, similarly as in Panaeolus cinctulus (Stijve 1985, Gerhardt 1996).
According to Ramírez-Cruz et al. (2013), the species formerly classified in Psilocybe sect. Semilanceateae (Guzmán 1995) are not monophyletic and this was also shown in our analysis (Fig. 4d); the wood rotting species of the so-called P. cyanescens complex are included in a separate lineage together with P. cubensis (Psilocybe sect. Cubensae according to Guzmán 1995) and the secotioid P. weraroa.
While P. silvatica has been traditionally considered to be related to P. semilanceata (Guzmán 1983, 1995), the type species of Psilocybe sect. Psilocybe (Redhead et al. 2007, Norvell 2010), and this has been confirmed by our molecular data, P. atrobrunnea is the type of Psilocybe sect. Atrobrunneae (Singer 1948), which includes 11 non blue staining species (Guzmán 1995). As our phylogenetic analysis shows (Fig. 4d), P. atrobrunnea clearly fits in Psilocybe sect. Psilocybe. We therefore suggest that the Psilocybe sect. Atrobrunnea could be a synonym of the Psilocybe sect. Psilocybe, but this is yet to be tested as P. atrobrunnea was the only species of the section included in our phylogenetic analysis. However not only molecular, but also morphological characters of P. atrobrunnea (cap shape in young basidiomata, shape of basidiospores and cheilocystidia) would suggest close relationship with P. semilanceata; in fact, the only feature not matching Psilocybe sect. Psilocybe (Guzmán 1995, as Psilocybe sect. Semilanceatae) is the lack of the blue staining reaction.
Psilocybe laetissima, also classified in Psilocybe sect. Atrobrunneae, was excluded from Psilocybe s.str., and transferred to Leratiomyces laetissimus (Hauskn. & Singer) Borov. et al. (Noordeloos 2011) and this is confirmed here with the molecular data (Fig. 4d).
Psilocybe medullosa has an interesting taxonomic and nomenclatural history. As shown by Borovička (2011), it used to be classified within various genera by different European authors (Galera, Galerina, Phaeogalera, Psilocybe); it is known from at least 8 European countries. As we demonstrate, it undoubtedly belongs to the genus Psilocybe. Furthermore, P. medullosa has been classified under various names in Europe (Stridvall & Stridvall 1990, Borovička 2011). Agaricus tenax Fr.: Fr. (Fries 1815, Observ. Mycol. 1: 54) and A. temulentus Fr.: Fr. (Fries 1821, Syst. Mycol. 1: 268) which would have priority over Naucoria medullosa Bres. (1898) must be considered ambiguous since they have been interpreted in different senses in specialized literature, very likely due to their equivocal original descriptions and the lack of type or authentic specimens or illustrations.
The microcharacters of the holotype of N. medullosa are practically identical with those in the holotype of Psathyra silvatica (Borovička 2011); both taxa are characterized by relatively small subellipsoid basidiospores and fusiform cheilocystidia with a relatively long neck (more than 10 µm). The only two available illustrations of P. silvatica (Smith 1940, Stamets 1996) known to the authors depict basidiomata very similar to P. medullosa. Despite being sought in various herbaria and within the ‘online community’, no recent collections of P. silvatica have been found so we were unable to investigate genetic variation among American collections as only one collection from the New World could have been sequenced. As revealed from our analysis (Fig. 4d), P. medullosa is closely related to P. silvatica. However, there are appreciable differences in all four investigated regions (Table 3). Consequently, we recommend keeping the European P. medullosa and the American P. silvatica as distinct species. An analogous problem arises in the group of P. cyanescens where only minor differences have been found within P. allenii, P. azurescens, P. cyanescens, P. subaeruginosa, and P. weraroa (Borovička et al. 2011, 2012). On the other hand, DNA regions of European and American collections of P. atrobrunnea were nearly identical, indicating their probable conspecifity (Table 3).
Table 3.
Numbers of nucleotide changes in particular DNA regions of specimens of P. atrobrunnea and P. medullosa vs P. silvatica are indicated.
| Species | LSU | ITS | EF1-α | IGS |
|---|---|---|---|---|
| P. silvatica (P24)* | ref. | ref. | ref. | ref. |
| P. medullosa (P11)* | 1 | 5 | 4 | 8 |
| P. medullosa (P37) | 1 | 6 | 4 | n.a. |
| P. medullosa (P17) | 1 | 4 | n.a. | n.a. |
| P. medullosa (P20) | 1 | 6 | n.a. | n.a. |
| P. atrobrunnea (P32) | ref. | ref. | ref. | ref. |
| P. atrobrunnea (P36) | 0 | 0 | 5 | 0 |
| P. atrobrunnea (P54) | 0 | 0 | 1 | 0 |
Our LSU sequence of P. silvatica (HF678215) is slightly different from that already published in GenBank for the same isolate (AY129383) by Nugent & Saville (2004). This discrepancy is very likely caused by sloppy editing of the AY129383 sequence since our data is 100 % identical with the unpublished sequences obtained at DAOM (kindly sent by Scott A. Redhead along with the isolate). With regard to the indicated polyphyly in P. weraroa (Fig. 4c), this should be taken with care since this result is based just on GenBank data. According to our experience in our P. weraroa collections (P34 and one unpublished), sequencing of LSU often fails and generates poor data; further investigation of this species is needed.
The problem of Psilocybe atrobrunnea
Agaricus atrobrunneus Lasch, Linnaea 3: 423. 1828: Fr., Syst. Mycol. 3, Index: 8. 1832 (basion.). MycoBank 461744
≡ Psilocybe atrobrunnea (Lasch: Fr.) Gillet, Hyménomycètes: 586. 1878. MycoBank 198092.
Holotype. To our best knowledge, there is neither an original collection nor illustration. Guzmán (1983) did not report any holotype and according to our recent communication with curators of public herbaria (B, L, MSTR, MW, PAV, WRSL) supposed to contain Lasch’s collections (Stafleu & Cowan 1979) and others (BREM, FR, GFW, GOET, HAL, JE, M, REG, TUB, WI), no type specimens of Agaricus atrobrunneus exist.
Neotype. Singer’s (1986: 571) statement “P. atrobrunnea (Lasch) Gillet (P. dichroa Karst.; P. fuscofulva Peck; P. turficola Favre – synonymy according to A.H. Smith), the type (sensu Lasch) which I recollected has raphanaceous odor and slightly hexagonal sublentiform spores (LE).” is here considered as an effective neotypification: even if Singer’s designation is not a standard one, it matches all requirements for an effective choice according to the current International Code of Nomenclature for Algae, Fungi, and Plants of Melbourne (McNeill et al. 2012, hereinafter ‘Code’). From Singer’s statement we interpret that Singer collected again a collection of P. atrobrunnea in the original sense of Lasch, and he deposited it in LE. We have consulted with other mycologists and nomenclaturists regarding Singer’s statement, and they let us know that:
- a.
Lasch’s type (or a duplicate) of A. atrobrunneus could be deposited at LE, and therefore Singer examined the type and not a collection collected by him; - b.
Singer collected his collection in the same place where Lasch’s type was collected and therefore the word ‘type’ is referred to the original collection and not to Singer’s one; - c.
Singer’s word ‘recollected’ could mean ‘remembered’ and not ‘collected again’, and therefore again Singer’s sentence referred to the original type; or - d.
Singer’s neotypification was a not effective one as he didn’t mention a collecting number.
So, we must elucidate all above points before considering designation of a neotype. Our first logical step was to request to the curator of Komarov Botanical Institute (LE) information about any Singer’s collection of P. atrobrunnea. Fortunately, according to the curator Olga Morozova (2012, pers. com.), Singer deposited a collection of P. atrobrunnea in LE, which is now lost in the post or destroyed by suspicious customs agents when it was sent to Mexico for examination. Interestingly, Morozova provided us with the whole label data as follows: “Psilocybe atrobrunnea (Lasch: Fr.) Gillet, Russia, Leningrad region, Sovkhozy (st. Neva), in Pineto, inter muscos, IX-1936, coll. et det. R. Singer (LE 11847)”, and she pointed out to us that surely Singer (1986) had in mind this lost collection since no other collection of this taxon collected by Singer has been located at LE. On the other hand, Mueller (1995: 146, right column, second paragraph) in a paper honouring Singer’s work wrote the following statement: “Rolf consistently made it his practice to deposit type material in the institution where he was working”. In fact, the type of Janauaria amazonica also typified by Singer in 1986 (page 495, footnote) was deposited at F (Chicago). Thus, we also contacted Robert Lücking, curator at F, where Singer worked in 1986, and he informed us that “we do not have any collection under that name [_P. atrobrunnea_], neither type or non-type”.
Now, we unambiguously can state that:
- a.
the type of Agaricus atrobrunneus Lasch was not deposited at LE; - b.
Singer’s collection is not a topotype, as Leningrad is more than 1 000 km far from ‘Marchiae Brandenburgicae’ where Lasch collected the original specimen of A. atrobrunneus; and - c.
Singer’s word ‘recollected’ can only mean collected again, as it was confirmed that Singer’s collection from Leningrad area was extant in LE.
Concerning if Singer’s neotypification was or was not an effective one, despite his statement being very brief, he gave us much information as:
- a.
he used the word ‘type’ as prescribed by the Art. 7.10 of the Code; - b.
he pointed out that his sense is Lasch’s original one (‘sensu Lasch’), and not in the sense of whatever synonym that he accepted; - c.
he mentioned that he was the collector (‘I recollected’) and in the label of the specimen at LE we can read “coll. & det. R. Singer”; - d.
he stated that his specimen has ‘raphanaceous odor’, a character mentioned by Lasch (“sapor odorque fere Rafani”); - e.
he cited a herbarium (‘LE’) in which his specimen was deposited (but lost), and we have verified that this is true.
Taking into consideration that the information provided by Singer meets all requirements for an effective publication of a neotype, we consider that Singer’s neotypification effective.
Some colleagues have emphasized that Singer did not mention a collection number or enough data to allow a precise identification of the type. They claim that in Art. 7.10 the second condition “if the type element is clearly indicated by direct citation including the term ‘type’ (typus) or an equivalent”, direct citation refers to a collecting number. However, this interpretation is not possible since:
- i.
there is no comma after direct citation and therefore the condition only can be referred to the use of the term ‘type’ or an equivalent; and since - ii.
many designations of neotypes, lectotypes, and epitypes are based on old illustrations in which no indication to a collecting number are available.
Also the definition of a neotype (Art. 9.6) states that it can be a specimen or a illustration selected to serve as nomenclatural type, but there is no mention that a precise collecting number must be provided. In this case, Singer also met this article as he used a specimen, which was deposited in LE; Singer probably did not provide more collection data because the specimen had been collected 50 years prior. We have also noted that Singer’s typifications based on taxa not described by him were more informal than those based on new taxa, as for example the typification of Psalliota sect. Bivelares Kauffman and Psalliota sect. Univelares Kauffman (Singer 1975).
So, our first reaction was to designate a new neotype for A. atrobrunneus, but once we studied the Code we realized that this was not possible because no specific provision to replace a lost neotype exists in the Code (as it exists in the Zoological Code; Art. 75.4.1)! We asked W. Greuter for advice, who along with N. Turland and J. McNeill agreed that the Code does not foresee the possibility that a neotype might get lost or be destroyed. Fortunately, as pointed out by W. Greuter, the Code (Art. 9.8) provides, however, designation of an epitype in case that a neotype “cannot be critically identified for purposes of the precise application of the name to a taxon”. Therefore, as a lost specimen cannot be used, all three of them proposed us to designate an epitype as an interim solution.
Nevertheless, it must be stressed that the combination of characteristics provided by Singer (“slightly hexagonal sublentiform spores”) does not match that observed in collections of P. atrobrunnea in the widely accepted concept of Guzmán (1983), Borovička (2006), and Noordeloos (2011): both American and European collections are characterized by subellipsoid basidiospores. Therefore, as the name A. atrobrunneus was published without any illustration and lacking important microscopic characters necessary to precisely identify species in the genus Psilocybe, and the fact that this name has been interpreted in two different ways in modern literature, we consider P. atrobrunnea a dubious and ambiguous name.
According to Smith (fide Singer 1986) and Guzmán (1983), the American species P. fuscofulva Peck, Bull. New York State Mus. Nat. Hist. 1, 2: 7. 1887 (MycoBank 222041) was considered synonymous with P. atrobrunnea s.auct., non Singer (1986). Both protologue, macroscopic appearance of the exsiccatae, and the microcharacters (shape and size of basidiospores and cheilocystidia) observed in the holotype (NYS 1310, studied by the authors) match well our fungus and we have therefore decided to adopt the name P. fuscofulva for both American and European collections representing the taxon previously known in the literature (except for Singer) as P. atrobrunnea or P. turficola; no designation of an epitype is necessary at this time for P. fuscofulva.
TYPE DESIGNATIONS
Psathyra silvatica Peck, Rep. State Bot. New York State Mus. 42: 116. 1889. (basion.). MycoBank 179953
≡ Psilocybe silvatica (Peck) Singer & A.H. Sm., Mycologia 50: 277.1958. MycoBank 304494.
Holotype. NYS 2788 (USA; leg. H. Peck).
Epitype (designated here, MBT177347) that supports the holotype cited above: (specimen) CANADA, Newfoundland, Gros Morne National Park, Berry Head Pond, gregarious on fir debris, 21 Sept. 1983, leg. & det. Scott A. Redhead, DAOM 187848. EMBL-Bank: HF678215, HF912360, HF912343, HF912366, HF912367.
An interpretative type is necessary for a precise application of the name for American collections.
Naucoria medullosa Bres., Fungi Trident. 2: 53. 1898. (basion.). MycoBank 188182
≡ Psilocybe medullosa (Bres.) Borov., Mykol. Sborn. 84, 4: 114. 2007. MycoBank 532147.
Holotype. S F-16066 (Italy; leg. G. Bresadola).
Epitype (designated here, MBT177348) that supports the holotype cited above: (specimen) CZECH REPUBLIC, Silesia, Bruntál district, Krnov-Ježník, gregarious on wood chips and spruce needles (Picea) in underbrush of Urtica and Rubus, 13 Oct. 2007, leg. J. Borovička, J. Kuba & F. Kuba, PRM 909630. The epitype collection comes directly from the same site as the sequenced specimen P11 (PRM 909584; EMBL-Bank: HF678212, HF912353, HF912339, HF912364, HF912365). The epitype collection is depicted in Noordeloos (2011) on p. 559 (bottom figure), p. 560 (bottom figure) and p. 561 (top figure).
Despite the fact that the protologue and the microcharacters of the holotype enable morphological identification of European collections, our results indicate that the American P. silvatica is a morphologically similar, but molecularly distinct species. Therefore, an interpretative type is necessary for a precise application of the name.
Acknowledgments
We are very grateful to colleagues and curators who kindly provided information or collections from their herbaria for microscopic study and/or molecular analysis: Vladimír Antonín (BRNM), Joseph F. Ammirati (WTU), Marc Appelhans (GOET), Reinhardt Berndt (ZT), Uwe Braun (HAL), Lenka Edrová (PRM), Marek Halama (WRSL), Genevieve Lewis-Gentry (FH), Karen Hansen (S), Jan Holec (PRM), Timm Karish (MNVD), Regina Kuhnert (IB), Karl-Henrik Larsson (O), Renée Lebeuf (Canada), Olivia Lee (UBC), Lorinda Leonardi (NYS), Robert Lücking (F), Pierre-Arthur Moreau (LIP), Olga Morozova (LE), Franz Oberwinkler (TUB), Peter Poschlod and Josef Simmel (REG), Jone Rakšeniene (WI), Scott A. Redhead (DAOM), Elena Savino (PAV), Alexey Seregin (MW), Harrie J.M. Sipman (B), Eric Smith (USA), Susanne Starke (GFW), Leiff Stridvall (Sweden), Bernd Tenbergen (MSTR), Dagmar Triebel (M), Michael Wallace (New Zealand), Petra Wolgas (BREM), Georg Zizka (FR), and Hans-Joachim Zündorf (JE). We also thank Werner Greuter (Switzerland) for invaluable advice on nomenclature concerning Psilocybe atrobrunnea and Alan Rockefeller (USA) who helped improve the overall language of the manuscript. Last but not least, thorough review of our manuscript by Brandon Matheny and an anonymous referee is greatly appreciated. This research was supported by the Long-term Development Projects RVO67985831, RVO61388971, and RVO60077344.
REFERENCES
- Agurell S, Nilsson JLG. 1968. Biosynthesis of psilocybin. Part II. Incorporation of labelled tryptamine derivatives. Acta Chemica Scandinavica 22: 1210–1218. [DOI] [PubMed] [Google Scholar]
- Antonín V, Dvořák D. 2010. New, rare and lesser-known macromycetes in Moravia (Czech Republic) IX. Acta Musei Moraviae, Scientiae biologicae 95: 143–162. [Google Scholar]
- Antonín V, Sedlák P, Tomšovský M. 2013. Taxonomy and phylogeny of European Gymnopus subsection Levipedes (Basidiomycota, Omphalotaceae). Persoonia 31: 79–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonín V, Tomšovský M, Sedlák P, et al. 2009. Morphological and molecular characterization of the Armillaria cepistipes – A. gallica complex in the Czech Republic and Slovakia. Mycological Progress 8: 259–271. [Google Scholar]
- Aoki T, O’Donnell K, Homma Y, et al. 2003. Sudden-death syndrome of soybean is caused by two morphologically and phylogenetically distinct species within the Fusarium solani species complex – F. virguliforme in North America and F. tucumaniae in South America. Mycologia 95: 660–684. [PubMed] [Google Scholar]
- Beug M, Bigwood J. 1982. Psilocybin and psilocin levels in twenty species from seven genera of wild mushrooms in the Pacific Northwest (U.S.A.). Journal of Ethnopharmacology 5: 271–278. [DOI] [PubMed] [Google Scholar]
- Bobbink R, Hornung M, Roelofs JGM. 1998. The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. Journal of Ecology 86: 717–738. [Google Scholar]
- Borovička J. 2006. Psilocybe atrobrunnea (Lasch) Gillet in the Czech Republic. Mykologický Sborník 83: 3–9. [In Czech with English summary.] [Google Scholar]
- Borovička J. 2007. Psilocybe medullosa (Bres.) Borovička, comb. nova. Mykologický Sborník 84: 114. [Google Scholar]
- Borovička J. 2011. Psilocybe silvatica, mysterious species of the European mycoflora. Mykologický Sborník 88: 4–11. [In Czech with English summary.] [Google Scholar]
- Borovička J, Noordeloos ME, Gryndler M, et al. 2011. Molecular phylogeny of Psilocybe cyanescens complex in Europe, with reference to the position of the secotioid Weraroa novae-zelandiae. Mycological Progress 10: 149–155. [Google Scholar]
- Borovička J, Rockefeller A, Werner PG. 2012. Psilocybe allenii – a new bluing species from the Pacific Coast, USA. Czech Mycology 64: 181–195. [Google Scholar]
- Bresadola G. 1898. Fungi tridentini novi vel nondum delineati, descripti, et iconibus ilustrati, t. II. Zippel, Italy. [Google Scholar]
- Favre J. 1939. Champignons rares ou peu connus des Hautsmairais jurassiens, III. Bulletin trimestriel de la Société mycologique de France 35: 196–219. [Google Scholar]
- Fries EM. 1815. Observationes Mycologicae 1. Officina Berlingiana, Lund. [Google Scholar]
- Fries EM. 1821. Systema Mycologicum 1. Gerhardi Bonnier, Havniae. [Google Scholar]
- Gerhardt E. 1996. Taxonomic revision of the genera Panaeolus and Panaeolina (Fungi, Agaricales, Coprinaceae). E. Schweizerbart’sche Verlagsbuchhandlung (Nägele u. Obermiller), Germany. [In German.] [Google Scholar]
- Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- Guzmán G. 1983. The genus Psilocybe. Nova Hedwigia, Beiheft 74, Liechtenstein. [Google Scholar]
- Guzmán G. 1995. Supplement to the monograph of the genus Psilocybe. In: Petrini O, Horak E. (eds), Taxonomic monographs of Agaricales. Bibliotheca Mycologica; 159: 91–141. [Google Scholar]
- Guzmán G, Kroeger P, RamírezGuillén F, et al. 2008. Psilocybe (Basidiomycotina, Agaricales, Strophariaceae) in Canada, with a special review of species from British Columbia. Mycotaxon; 106: 179–193. [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hausknecht A, Singer R. 1986. A new species of Psilocybe (Agaricales). Plant Systematics and Evolution 151: 295–301. [Google Scholar]
- Høiland K. 1978. The genus Psilocybe in Norway. Norwegian Journal of Botany 25: 111–122. [Google Scholar]
- Horak E. 2005. European agarics and boleti. Elsevier, Germany. [In German.] [Google Scholar]
- Horita A, Weber LJ. 1961. The enzymic dephosphorylation and oxidation of psilocybin and psilocin by mammalian tissue homogenates. Biochemical Pharmacology 7: 47–54. [DOI] [PubMed] [Google Scholar]
- Kallifatidis B, Borovička J, Stránská J, et al. 2014. Fluorescent random amplified microsatellites (F-RAMS) analysis of mushrooms as a forensic investigative tool. Forensic Science International: Genetics 9: 25–32. [DOI] [PubMed] [Google Scholar]
- Keller T, Schneider A, Regenscheit P, et al. 1999. Analysis of psilocybin and psilocin in Psilocybe subcubensis Guzmán by ion mobility spectrometry and gas chromatography-mass spectrometry. Forensic Science International 99: 93–105. [DOI] [PubMed] [Google Scholar]
- Knudsen H, Vesterholt J. (eds). 2008. Funga Nordica: Agaricoid, boletoid and cyphelloid genera. Nordsvamp, Denmark. [Google Scholar]
- Kosentka P, Sprague SL, Ryberg M, et al. 2013. Evolution of the toxins muscarine and psilocybin in a family of mushroom-forming fungi. PLoS ONE 8: e64646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasch WG. 1828. Enumeratio Agaricorum Marchiae Brandenburgicae, nondum in floris nostratibus nominatorum, cum observationibus in cognitos et novorum descriptionibus. Linnaea 3: 378–430. [Google Scholar]
- Lassmann T, Sonnhammer EL. 2005. Kalign – an accurate and fast multiple sequence alignment algorithm. BMC Bioinformatics 6: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AY, Smith AH, Paul AG. 1965. Production of psilocybin in Psilocybe baeocystis saprophytic culture. Journal of Pharmaceutical Sciences 54: 1576–1579. [DOI] [PubMed] [Google Scholar]
- Levine WG. 1967. Formation of blue oxidation product from psilocybin. Nature 215: 1292. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kawahara N, Yokoyama K, et al. 2006. Phylogenetic relationship of psychoactive fungi based on rRNA gene for a large subunit and their identification using the TaqMan assay (II). Forensic Science International 163: 51–58. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Yokoyama K, Makino Y, et al. 2003. Phylogenetic relationship of psychoactive fungi based on the rRNA gene for a large subunit and their identification using the TaqMan assay. Chemical and Pharmaceutical Bulletin 51: 710–714. [DOI] [PubMed] [Google Scholar]
- McNeill J, Barrie FR, Buck WR, et al. 2012. International Code of Nomenclature for Algae, Fungi, and Plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. Regnum Vegetabile 154. Koeltz Scientific Books, Germany: http://www.iapt-taxon.org/nomen/main.php. [Google Scholar]
- Moncalvo JM, Vilgalys R, Redhead SA, et al. 2002. One hundred and seventeen clades of euagarics. Molecular Phylogenetics and Evolution 23: 357–400. [DOI] [PubMed] [Google Scholar]
- Moser M, Jülich W. 1995. Farbatlas der Basidiomyceten, 13. Lieferung. Stuttgart. [Google Scholar]
- Mueller GM. 1995. Rolf Singer, 1906-1994. Mycologia; 87: 144–147. [Google Scholar]
- Noordeloos ME. 1999. Genus Psilocybe. In: Bas C, Kuyper TW, Noordeloos ME, et al. (eds), Flora agaricina neerlandica, vol. IV: 28–79. Balkema, Netherlands. [Google Scholar]
- Noordeloos ME. 2011. Fungi Europaei, Volume 13: Strophariaceae s.l. Edizioni Candusso, Italy. [Google Scholar]
- Norvell L. 2010. Report of the Nomenclature Committee for Fungi: 15. Taxon 59: 291–293. [Google Scholar]
- Nugent KG, Saville BJ. 2004. Forensic analysis of hallucinogenic fungi: a DNA-based approach. Forensic Science International 140: 147–157. [DOI] [PubMed] [Google Scholar]
- Peck CH. 1889. Report of botanist. Annual report on the New York State Museum of Natural History 42: 99–144. [Google Scholar]
- Pornpakakul S, Suwancharoen S, Petsom A, et al. 2009. A new sesquiterpenoid metabolite from Psilocybe samuiensis. Journal of Asian Natural Products Research 11: 12–17. [DOI] [PubMed] [Google Scholar]
- Ramírez-Cruz V, Guzmán G, Villalobos-Arámbula AR, et al. 2013. Phylogenetic inference and trait evolution of the psychedelic mushroom genus Psilocybe sensu lato (Agaricales). Botany 91: 573–591. [Google Scholar]
- Redhead SA, Moncalvo JM, Vilgalys R, et al. 2007. Proposal to conserve the name Psilocybe (Basidiomycota) with a conserved type. Taxon 56: 255–257. [Google Scholar]
- Repke D, Leslie D, Guzmán G. 1977. Baeocystin in Psilocybe, Conocybe and Panaeolus. Lloydia 40: 566–578. [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Mark P van der, et al. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. 1948. Diagnosis fungorum novorum agaricalium. Sydowia 2: 26–42. [Google Scholar]
- Singer R. 1975. The Agaricales in modern taxonomy, 3rd ed. Koeltz Scientific Books, Germany. [Google Scholar]
- Singer R. 1986. The Agaricales in modern taxonomy, 4th ed. Koeltz Scientific Books, Germany. [Google Scholar]
- Singer R, Smith AH. 1958. Mycological investigations on Teonanácatl, the Mexican hallucinogenic mushrooms, II: A taxonomic monograph of Psilocybe, section Caerulescentes. Mycologia; 50: 262–303. [Google Scholar]
- Smith AH. 1940. New and unusual agarics from Michigan II. Papers of the Michigan Academy of Science, Arts and Letters, Part 1: Botany and Forestry 26: 61–68. [Google Scholar]
- Stafleu FA, Cowan RS. 1979. Taxonomic literature II. Ed. 2. Regnum Vegetabile 98. [Google Scholar]
- Stamets P. 1996. Psilocybin mushrooms of the world. Ten Speed Press, USA. [Google Scholar]
- Stijve T. 1985. The genus Panaeolus – a chemical exploration. Coolia 28: 81–89. [In Dutch with English summary.] [Google Scholar]
- Stijve T, Klán J, Kuyper TW. 1985. Occurrence of psilocybin and baeocystin in the genus Inocybe (Fr.) Fr. Persoonia 12: 469–473. [Google Scholar]
- Stříbrný J, Borovička J, Sokol M. 2003. Content of psilocybin and psilocin in some species of macromycetes. Soudní Lékařství 48: 45–49. [In Czech with English summary.] [PubMed] [Google Scholar]
- Stridvall L, Stridvall A. 1990. Phaeogalera medullosa (Bresadola) Moser – a nomenclatoric discussion. Jordstjärnan 11: 8–17. [In Swedish with English summary]. [Google Scholar]
- Swofford DL. 2000. PAUP*: Phylogenetic analysis using parsimony (*and other methods). Version 4b10. Sinauer Associates, Sunderland, USA. [Google Scholar]
- Thiers B. 2012. [continuously updated]. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium; http://sweetgum.nybg.org/ih/. [Google Scholar]
- Tomšovský M, Vampola P, Sedlák P, et al. 2010. Delimitation of central and northern European species of the Phellinus igniarius group (Basidiomycota, Hymenochaetales) based on analysis of ITS and translation elongation factor 1 alpha DNA sequences. Mycological Progress 9: 431–445. [Google Scholar]