Acceleration of diabetic wound healing using a novel protease–anti-protease combination therapy (original) (raw)
Significance
Chronic wounds in diabetic patients are a devastating complication of diabetes that can lead to amputations or even death. Our work in db/db mice shows that matrix metalloproteinase (MMP)-9 contributes to delayed or impaired wound healing and that MMP-8 is involved in repairing the wound. A combination of a selective inhibitor of MMP-9 (a small molecule) and exogenously applied active recombinant MMP-8 (an enzyme) accelerates diabetic wound healing in mice.
Keywords: diabetic wound healing, MMP-8, MMP-9, inhibition, ND-336
Abstract
Nonhealing chronic wounds are major complications of diabetes resulting in >70,000 annual lower-limb amputations in the United States alone. The reasons the diabetic wound is recalcitrant to healing are not fully understood, and there are limited therapeutic agents that could accelerate or facilitate its repair. We previously identified two active forms of matrix metalloproteinases (MMPs), MMP-8 and MMP-9, in the wounds of db/db mice. We argued that the former might play a role in the body’s response to wound healing and that the latter is the pathological consequence of the disease with detrimental effects. Here we demonstrate that the use of compound ND-336, a novel highly selective inhibitor of gelatinases (MMP-2 and MMP-9) and MMP-14, accelerates diabetic wound healing by lowering inflammation and by enhancing angiogenesis and re-epithelialization of the wound, thereby reversing the pathological condition. The detrimental role of MMP-9 in the pathology of diabetic wounds was confirmed further by the study of diabetic MMP-9–knockout mice, which exhibited wounds more prone to healing. Furthermore, topical administration of active recombinant MMP-8 also accelerated diabetic wound healing as a consequence of complete re-epithelialization, diminished inflammation, and enhanced angiogenesis. The combined topical application of ND-336 (a small molecule) and the active recombinant MMP-8 (an enzyme) enhanced healing even more, in a strategy that holds considerable promise in healing of diabetic wounds.
Diabetes affects 340 million people in the world, including 29.1 million individuals in the United States (1). A complication in diabetic patients is the inability of wounds to heal, which resulted in 73,000 lower-limb amputations in the United States in 2010 (1). The standard treatment for diabetic foot ulcers includes debridement of the wound, treatment of infection with antibiotics, and reducing or eliminating weight pressure from the lower extremities (2). There is a paucity of pharmacological therapeutics that accelerate wound healing. Although becaplermin (PDGF) is approved for use in diabetic neuropathic ulcers, malignancies have been reported, and an increased risk of mortality was observed in patients treated with becaplermin (3).
In diabetic patients, high blood sugar triggers prolonged chronic inflammation, with concomitant elevated levels of matrix metalloproteinases (MMPs). The detrimental effect of MMPs in the diseased tissue has been attributed to the rapid turnover of potential growth factors, receptors, and the newly formed extracellular matrix, which are essential for wound healing (4). Hence, wound healing is impaired and delayed in diabetic patients. However, this process is not well understood, and the actual instigator MMPs are not known.
MMPs are a family of zinc-dependent endopeptidases that are capable of degrading extracellular matrix components and are involved in tissue remodeling and restructuring (5). MMPs are expressed as zymogens or pro-MMPs. Activation by proteolytic removal of the N-terminal prodomain is required for their catalytic functions. Active forms of MMPs are highly regulated by binding of tissue inhibitors of metalloproteinases (TIMPs). MMPs are presumed to play various roles in regulating inflammatory and repair processes (6) as well as in wound healing (7).
We recently reported on the identification and quantification of active MMP-8 and MMP-9 in a mouse model of diabetic wound healing by the use of an inhibitor-tethered resin that binds exclusively to active MMPs, in conjunction with proteomics analyses (8). Because MMP-9 was observed to be up-regulated only in diabetic wounds, whereas MMP-8 was found in both diabetic and nondiabetic wounds, we hypothesized that MMP-8 is beneficial and that MMP-9 is detrimental in diabetic wound healing. We now report that the use of either a novel and highly selective MMP-9 inhibitor of our design (ND-336, compound 1) or the application of the active recombinant MMP-8 accelerates wound healing in db/db mice. We further confirm the detrimental effect of MMP-9 on diabetic wound healing by the use of MMP-9–knockout mice. Finally, we document that the combination of a selective MMP-9 inhibitor (a small molecule) plus the active recombinant MMP-8 (an enzyme) accelerated wound healing even further in db/db mice. This combination is a potential pharmacological treatment for diabetic wound healing and holds great promise for recourse in this devastating disease.
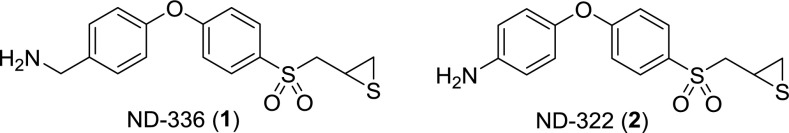
Scheme 1.
Structures of compounds 1 and 2.
Results and Discussion
Synthesis and MMP Inhibition Profile of ND-336.
There are 23 MMPs in humans (5), and their catalytic domains are very similar in structure; thus, the design of inhibitors that are selective for a particular MMP is extremely challenging. In fact, most inhibitors of MMPs are zinc chelators, which broadly inhibit many or all MMPs (9) and the related ADAMs (a disintegrin and metalloproteinase). Over the past several years we have produced a library of thiirane inhibitors for MMPs (10–12), which allowed the identification of specific inhibitors for selective inhibition of enzymes involved in various MMP-mediated diseases. The thiiranes undergo a reaction catalyzed by the target MMP, resulting in opening of the thiirane ring and generation of the thiolate, which is a tight-binding inhibitor (13). When we identified active MMP-8 and MMP-9 in the diabetic wounds and hypothesized that MMP-9 plays a detrimental role in the disease, the central criterion for selectivity of a suitable inhibitor became its ability to differentiate between MMP-8 and MMP-9, because the latter had to be inhibited in the presence of active MMP-8. We now report on the discovery of compound 1 (as shown in Fig. S1), which meets the requirement for potent inhibition of MMP-9 and lack thereof for MMP-8.
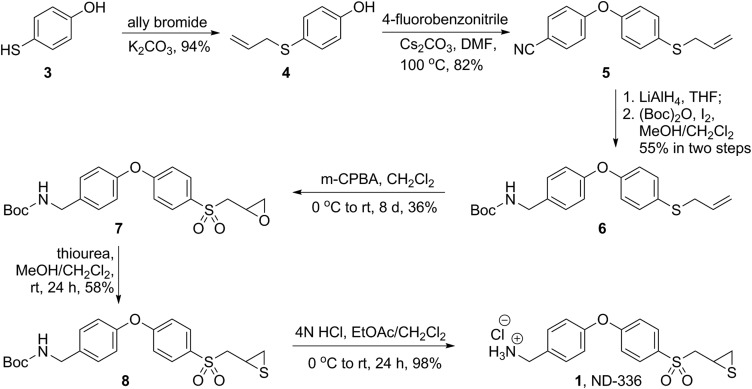
Fig. S1.
Synthesis of ND-336.
The binding constants for ND-336 with seven representative MMPs and two related ADAMs are given in Table 1. ND-336 inhibits MMP-2, MMP-9, and MMP-14 in a slow-binding mechanism, with inhibition constant (_K_i) values of 85 ± 1 nM, 150 ± 10 nM, and 120 ± 10 nM, respectively. Because MMP-2 and MMP-14 are absent in the diabetic wound (8), the inhibitor essentially targets MMP-9 in this microenvironment. The residence times (the time the drug remains bound to the target; calculated as 1/_k_off) (14) of ND-336 are 23.4 ± 0.2 min for MMP-2, 47.4 ± 4.4 min for MMP-9, and 12.6 ± 0.3 min for MMP-14. For comparison, the residence times of the endogenous protein inhibitors TIMPs are shorter: 6.9 min for MMP-2-TIMP1, 10.4 min for MMP-2-TIMP2, 7.9 min for MMP-9-TIMP1, and 6.7 min for MMP-9-TIMP2 (15). That is, ND-336 is better at inhibiting MMP-2 and MMP-9 than are the TIMPs that have evolved for this purpose. ND-336 exhibits marginal to no inhibition of MMP-1, MMP-3, MMP-7, ADAM9, and ADAM10, and it poorly inhibits MMP-8 in a linear noncompetitive manner (_K_i = 7,700 ± 100 nM). Combined with the 50-fold lower _K_i for MMP-9, the exceptional residence time of ND-336 contributes substantially to its selectivity. The residence time is an important contributor to the effective inhibition of MMP-9, in contrast to the linear noncompetitive inhibition of MMP-8 by ND-336, with a very short residence time and poorer dissociation constant.
Table 1.
Inhibition profile of ND-336
| Enzyme | Inhibition type | _k_on, s−1M−1 | _k_off, 103s−1 | _K_i |
|---|---|---|---|---|
| MMP-1* | 4% inhibition @ 100 µM | |||
| MMP-2 | Slow-binding | 8,380 ± 110 | 0.712 ± 0.006 | 85 ± 1 nM† |
| MMP-3* | 23% inhibition @ 100 µM | |||
| MMP-7 | 1% inhibition @ 100 µM | |||
| MMP-8* | Linear noncompetitive | 7,700 ± 100 nM | ||
| MMP-9* | Slow-binding | 2,360 ± 100 | 0.352 ± 0.033 | 150 ± 10 nM† |
| MMP-14* | Slow-binding | 10,800 ± 400 | 1.33 ± 0.03 | 120 ± 10 nM† |
| ADAM9 | 31% inhibition @ 100 µM | |||
| ADAM10 | 14% inhibition @ 100 µM |
Inhibition of MMP-9 with ND-336 in Diabetic Wound Healing.
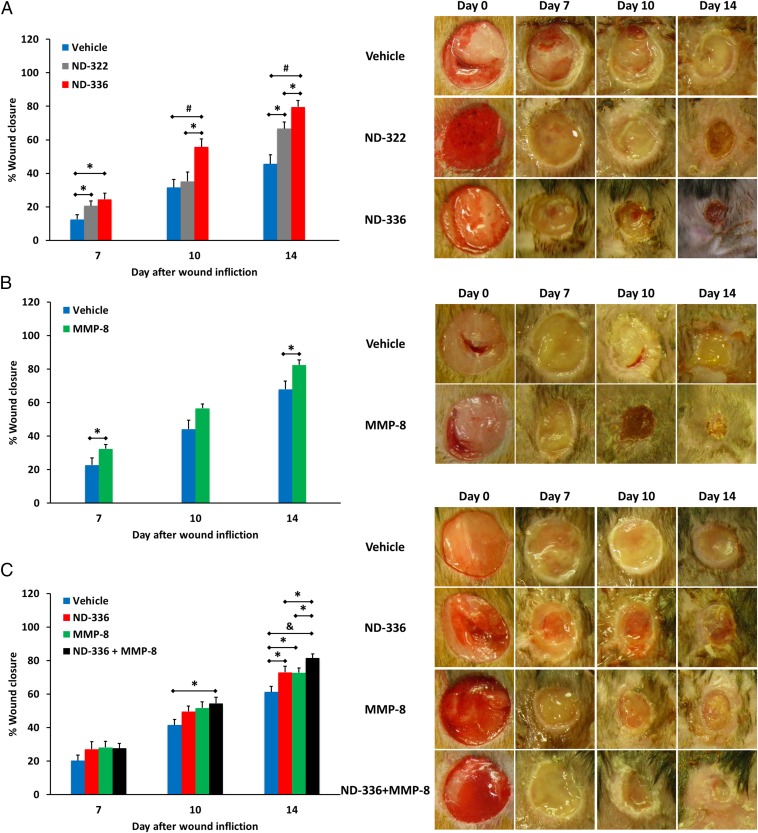
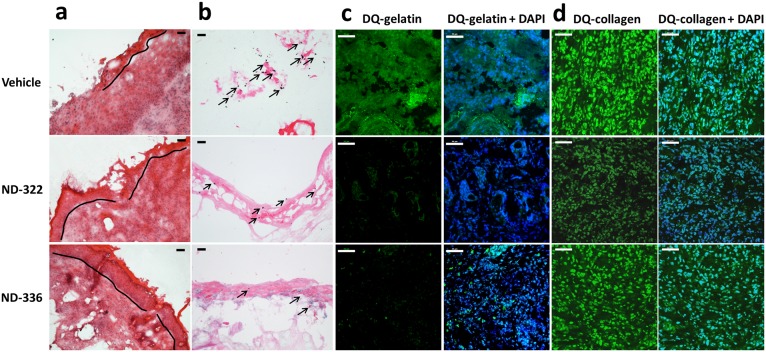
ND-336 was evaluated in a mouse model of diabetic wound healing. Excisional wounds were made on the dorsal thorax of db/db mice, and the wounds were treated topically with vehicle, ND-336, or ND-322 (compound 2), which was used as a positive control. ND-322 inhibits MMP-9 as a slow-binding inhibitor with a _K_i of 870 ± 110 nM and inhibits MMP-8 as a linear noncompetitive inhibitor with a _K_i of 2,600 ± 400 nM, with a threefold selectivity for MMP-9 over MMP-8 (10). Wounds treated with ND-336 healed 1.2- to 1.6-fold faster than those treated with ND-322 and twofold faster than those treated with vehicle (Fig. 1_A_). We attribute the superior efficacy of ND-336 over ND-322 to ND-336’s more selective inhibition of MMP-9 than of MMP-8. Because human wounds heal by re-epithelialization, we evaluated the wounds with H&E staining to visualize the epithelium. Treatment with ND-336 resulted in almost complete re-epithelialization compared with partial re-epithelialization in the ND-322– and vehicle-treated groups (Fig. S2_A_). Because MMP-9 activity is associated with the induction of apoptosis (16), we evaluated the wounds by the TUNEL, which detects DNA fragmentation resulting from apoptotic cells. As shown in Fig. S2_B_, numerous apoptotic cells were found in the vehicle-treated group, but apoptosis was significantly decreased in the ND-322– and ND-336–treated groups. In situ zymography detects MMP activity in vivo using the fluorogenic substrates DQ-gelatin for gelatinase (MMP-2 and MMP-9) activity and DQ-collagen for collagenase (MMP-1, MMP-8, and MMP-13) activity. Because only active MMP-8 and MMP-9 were identified by our proteomics analyses in db/db wounds (8), the gelatinase activity observed by in situ zymography corresponds to MMP-9 activity, and the collagenase activity is reflective of MMP-8 activity. Treatment with ND-336 significantly decreased MMP-9 activity (Fig. S2_C_, Bottom Left), but MMP-8 activity was not affected (Fig. S2_D_, Bottom Left), as expected from the kinetic profile of ND-336 (Table 1). In contrast, treatment with ND-322 significantly decreased MMP-9 activity (Fig. S2_C_, Middle Left); however, it also decreased MMP-8 activity (Fig. S2_D_, Middle Left). Merged images stained with DAPI (blue) indicated comparable numbers of nuclei in the wound tissues treated with vehicle, ND-322, and ND-336 (right panels in Fig. S2 C and D).
Fig. 1.
Effect of MMP-9 inhibition, topical treatment with exogenously added MMP-8, and combined MMP-9 inhibition and exogenous MMP-8 on diabetic wound healing. A single 8-mm wound was made on the dorsal thorax of db/db mice. *P < 0.05, #P < 0.01, &P < 0.001 indicate statistically significant differences in wound closure between the indicated groups. Statistical significance was evaluated by the two-tailed Mann–Whitney u test. (A) Wound healing after treatment with ND-336 (0.1 mg per wound per day), ND-322 (0.1 mg per wound per day) as a positive control, or vehicle. Data are shown as mean ± SEM (n = 8 mice per group on days 7, 10, and 14). (B) Wound healing after exogenously added MMP-8 (1 µg per wound per day). Data are shown as mean ± SEM (n = 20, 9, and 9 mice on days 7, 10, and 14, respectively, for the vehicle group; n = 20, 10, and 10 mice on days 7, 10, and 14, respectively, for the MMP-8 group). (C) Wound healing after treatment with combined ND-336 (0.05 mg per wound per day) and MMP-8 (1 µg per wound per day). Data are shown as mean ± SEM; n = 13, 14, 12, and 12 for the groups treated with vehicle, ND-336, MMP-8, and ND-336 + MMP-8, respectively.
Fig. S2.
Effect of MMP-9 inhibition on diabetic wound healing. A single 8-mm wound was made on the dorsal thorax. Wounds were treated with ND-336 (0.1 mg per wound per day), ND-322 (0.1 mg per wound per day) as a positive control, or vehicle. H&E staining, TUNEL, and in situ zymography with DQ-gelatin and DQ-collagen were performed (n = 3 mice per group). (A) H&E staining on day 14. Re-epithelialization is indicated by the black line. Pictures were taken with a 10× lens. (Scale bars, 50 µm.) (B) TUNEL images of wounds on day 14. Arrows point to representative TUNEL+ (apoptotic) cells. Pictures were taken with a 10× lens. (Scale bars, 50 µm.) (C) In situ zymography with gelatinase fluorogenic substrate DQ-gelatin (Left, green), and merged with nuclear DNA staining by DAPI (Right, blue). Pictures were taken with a 40× lens. (Scale bars, 50 µm.) (D) In situ zymography with collagenase fluorogenic substrate DQ-collagen (Left, green) and merged with nuclear DNA staining by DAPI (Right, blue). Pictures were taken with a 40× lens. (Scale bars, 50 µm.)
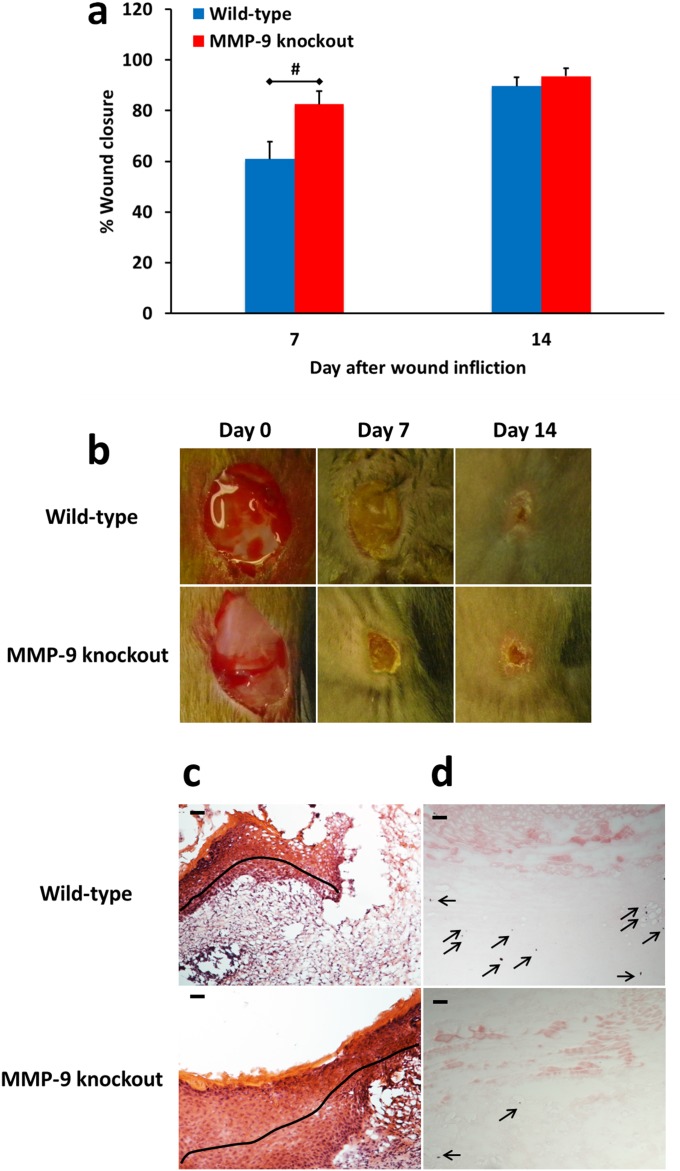
Ablation of MMP-9 in Diabetic Wound Healing.
We induced diabetes in MMP-9–knockout mice to confirm the detrimental role of MMP-9 in diabetic wound healing. We administered streptozotocin, which destroys insulin-producing beta cells in the pancreas by necrosis (17) and used wild-type mice treated with streptozotocin as the control group. As shown in Fig. S3, the wounds of streptozotocin-treated MMP-9–knockout mice healed faster than those of streptozotocin-treated wild-type mice and resulted in complete re-epithelialization and in diminished apoptosis. This study confirmed that MMP-9 is involved in the pathology of diabetic wounds. Because enhanced expression of MMP-8 has been reported in MMP-9–knockout mice (18), the acceleration of wound healing that we observe in diabetic MMP-9–knockout mice could be explained by the up-regulation of MMP-8 and the absence of MMP-9. Our findings in diabetic MMP-9–knockout mice differ from those of Kyriakides et al. (19), who suggested that MMP-9 is required for normal progression of wound closure because MMP-9 gene ablation in nondiabetic mice led to delayed wound healing caused by compromised re-epithelialization, attenuated keratinocyte wound migration, and reduced clearance of fibrin clots. However, inflammation and angiogenesis in wounds of nondiabetic MMP-9–knockout mice were similar to those in control mice (19). Others have found that skin inflammation is alleviated in MMP-9–knockout mice (20) and that inhibition of MMP-9 with leptomycin B suppressed inflammation in UVB-irradiated murine skin (21), consistent with our findings.
Fig. S3.
Effect of MMP-9 gene ablation on diabetic wound healing. Diabetes was induced in wild-type and MMP-9–knockout mice by treatment with streptozotocin (150 mg/kg i.p.) and confirmed by a fasting blood glucose of >300 mg/dL. Excisional 8-mm wounds were inflicted 2 wk later. (A) Wound healing in wild-type and MMP-9–knockout streptozotocin-induced diabetic mice. Data are shown as mean ± SEM (n = 13 and 7 mice per group on days 7 and 14, respectively; total, 26 mice). #P < 0.01 indicates statistically significant differences in wound healing between the two indicated groups using the two-tailed Mann–Whitney u test. (B) Representative wound images (all to the same scale) on days 0, 7, and 14. (C) H&E staining for representative wounds in wild-type and MMP-9–knockout streptozotocin-induced diabetic mice (n = 3 mice per group per time point). Re-epithelialization is indicated by the black line. Pictures were taken with a 10× lens. (Scale bars, 50 µm.) (D) TUNEL images of representative wounds on day 7 (n = 3 mice per group per time point). Arrows point to representative TUNEL+ (apoptotic) cells. Pictures were taken with a 10× lens. (Scale bars, 50 µm.)
Effect of MMP-8 in Diabetic Wound Healing.
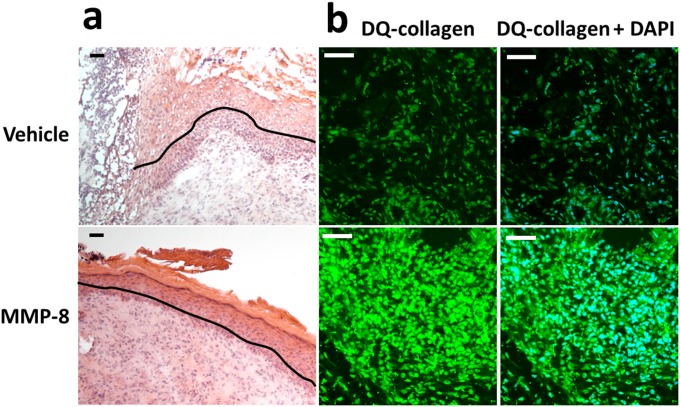
To test the hypothesis that MMP-8 contributes to the repair in diabetic wound healing, we evaluated the effect of topical application of the protease MMP-8 to wounds of db/db mice. We cloned the gene and expressed and purified active murine recombinant MMP-8 (SI Materials and Methods). The active recombinant MMP-8 was applied topically to the wounds at 10-fold the level found in the wounds (8). MMP-8 accelerated wound healing at this level in db/db mice, with statistical differences observed on days 7 and 14 (Fig. 1_B_), and resulted in complete re-epithelialization (Fig. S4_A_). Increased MMP-8 activity in the MMP-8–treated db/db mice was confirmed by in situ zymography (Fig. S4_B_). This study indicated that MMP-8 plays a beneficial repair role in diabetic wound healing. Our findings are in agreement with those of Gutiérrez-Fernández et al. (22), who found that nondiabetic mice deficient in MMP-8 have delayed wound healing. Interestingly, MMP-8–knockout mice have significantly increased levels of MMP-9 (22), because of compensatory expression, which contributes to delayed wound healing.
Fig. S4.
Topical treatment with exogenously added MMP-8 accelerates wound healing in db/db mice. A single 8-mm punch biopsy lesion on the dorsal thorax was administered to mice. Wounds were treated with MMP-8 (1 µg per wound per day; n = 20 mice) or vehicle (reaction buffer, n = 20 mice). H&E staining and in situ zymography with DQ-collagen were performed with on day 14 after wound infliction (n = 3 mice per group). (A) H&E staining for representative wounds treated with vehicle or MMP-8. Re-epithelialization is indicated by the black line. Pictures were taken with a 10× lens. (Scale bars, 50 µm.) (B) In situ zymography with collagenase fluorogenic substrate DQ-collagen (Left, green) and merged with nuclear DNA staining by DAPI (Right, blue). Pictures were taken with a 40× lens. (Scale bars, 50 µm.)
Effect of the Combination of MMP-9 Inhibitor and Exogenously Added Active Recombinant MMP-8.
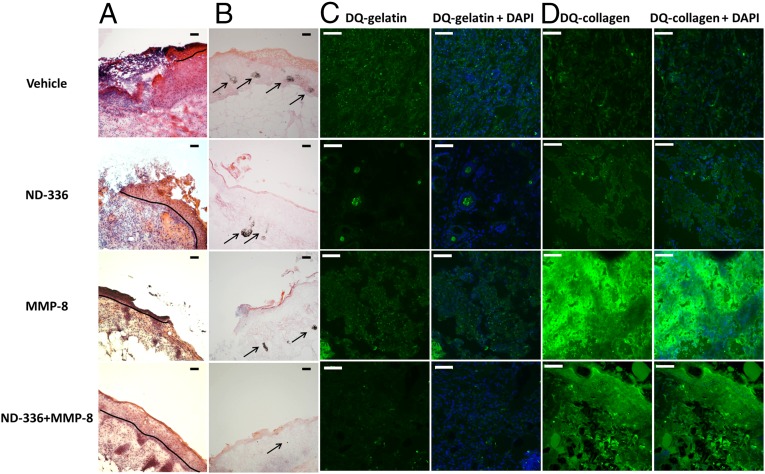
Because either inhibition of MMP-9 by ND-336 or topically applied exogenously added active recombinant MMP-8 alone accelerated wound healing, we investigated the effect of the combination therapy. We first determined that the application of 0.05 mg ND-336 per wound per day was the lowest dose that accelerated wound healing in db/db wounds (Fig. S5). We also determined that 1 µg of MMP-8 per wound per day was the dose that most effectively accelerated wound repair (Fig. S6). As seen in Fig. 1_C_, the combined treatment not only showed significant acceleration of wound healing compared with the vehicle group on both days 10 and 14, but also showed significantly faster healing on day 14 than when a single agent was used in the treatment. Histological assessment of the wounds revealed that the combination of the MMP-9 inhibitor and MMP-8 resulted in more complete re-epithelialization than seen with either of the two agents alone or with vehicle (Fig. 2_A_) and in substantial reduction in apoptotic cells relative to the other three groups (Fig. 2_B_). In situ zymography with DQ-gelatin showed inhibition of MMP-9 in the groups treated with ND-336 and with the combination of ND-336 and MMP-8 (Fig. 2_C_, Left). In situ zymography with DQ-collagen indicated the presence of MMP-8 in the vehicle- and ND-336–treated groups, but significantly increased MMP-8 activity was found in the groups treated with MMP-8 and with the combination of ND-336 and MMP-8 (Fig. 2_D_, Left).
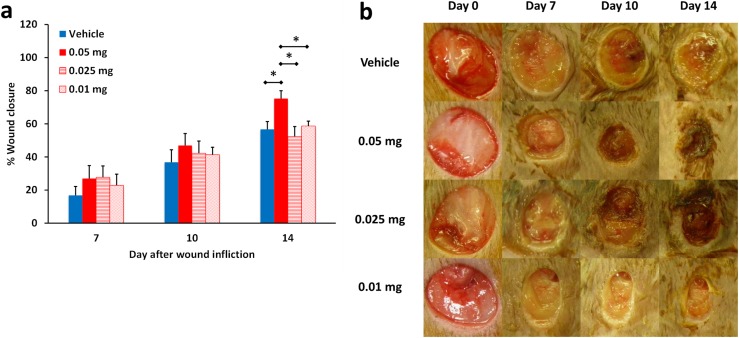
Fig. S5.
Dose–response of ND-336 in diabetic wound healing. (A) Wound healing in db/db mice treated with ND-336 at 0.05, 0.025, and 0.01 mg per wound per day. Data are shown as mean ± SEM (n = 7, 6, 7, and 7 mice, respectively, for vehicle, 0.05-mg, 0.025-mg, and 0.01-mg treatments; total, 27 mice). *P < 0.05 indicates statistically significant differences in wound healing by the two-tailed Mann–Whitney u test. (B) Representative wound images (all to the same scale) on days 0, 7, 10, and 14.
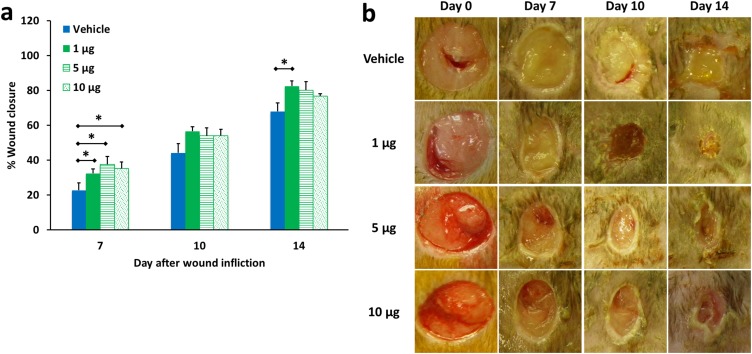
Fig. S6.
Dose–response of MMP-8 in diabetic wound healing. (A) Wound healing in db/db mice treated with MMP-8 at 1, 5, and 10 µg per wound per day. Data are shown as mean ± SEM (n = 20, 9, and 9 mice on days 7, 10, and 14, respectively, for the vehicle-treated group; n = 20, 10, and 10 mice on days 7, 10, and 14, respectively, for the group treated with 1 µg MMP-8 per wound per day; n = 10, 10, and 10 mice on days 7, 10, and 14, respectively, for the group treated with 5 µg MMP-8 per wound per day; n = 10, 10, and 9 mice on days 7, 10, and 14, respectively, for the group treated with 10 µg MMP-8 per wound per day). *P < 0.05 indicates statistically significant differences in wound healing using the two-tailed Mann–Whitney u test. (B) Representative wound images (all to the same scale) on days 0, 7, 10, and 14.
Fig. 2.
Effect of MMP-9 inhibition and exogenous MMP-8 on diabetic wound healing. Mice received a single 8-mm excisional wound on the dorsal thorax. Wounds were treated with vehicle, ND-336 (0.05 mg per wound per day), MMP-8 (1 µg per wound per day), or ND-336 (0.05 mg per wound per day) plus MMP-8 (1 µg per wound per day). H&E staining, TUNEL, and in situ zymography with DQ-gelatin and DQ-collagen were performed on day 14 (n = 3 mice per group). (A) H&E staining for representative wounds on day 14. Re-epithelialization is indicated by the black line. Pictures were taken with a 10× lens. (Scale bars, 50 µm.) (B) TUNEL images of representative wounds on day 14. Arrows point to representative TUNEL+ (apoptotic) cells. Pictures were taken with a 10× lens. (Scale bars, 50 µm.) (C) In situ zymography with gelatinase fluorogenic substrate DQ-gelatin (Left, green) and merged with nuclear DNA staining by DAPI (Right, blue). Pictures were taken with a 40× lens. (Scale bars, 50 µm.) (D) In situ zymography with collagenase fluorogenic substrate DQ-collagen (Left, green) and merged with nuclear DNA staining by DAPI (Right, blue). Pictures were taken with a 40× lens. (Scale bars, 50 µm.)
MMP-9 Inhibition and Exogenously Added MMP-8 Decrease Inflammation and Enhance Angiogenesis.
Inflammation is necessary for normal wound healing. However, increased or prolonged inflammation has been shown to delay wound healing in nondiabetic mice (22). IL-6, a proinflammatory cytokine (23), plays a crucial role in the inflammatory response in wound repair (24). IL-6–deficient mice display impaired wound healing that is reversed with the administration of IL-6 (25). Delayed wound healing in IL-6–knockout mice was accompanied by attenuated leukocyte infiltration, re-epithelialization, angiogenesis, and collagen accumulation (26). TGF-β1 is a cytokine that elicits recruitment of inflammatory cells during wound healing (27). TGF-β1 is up-regulated during wound healing, suggesting that it regulates wound repair (28). Immunodeficient TGF-β1–knockout mice show delayed wound healing, with accompanying delays in the inflammatory, proliferation, and maturation phases of wound healing (27). TGF-β induces pro-MMP-9 in human skin (29), and TGF-β1 stimulates the production of MMP-9 in human corneal epithelial cells (30) and in human keratinocytes (31). Enhanced TGF-β1 signaling accelerates re-epithelialization (32). These studies suggest that IL-6 and TGF-β1 play important roles in wound healing. Therefore we measured the concentrations of IL-6 and TGF-β1 by ELISA in wounds of db/db mice.
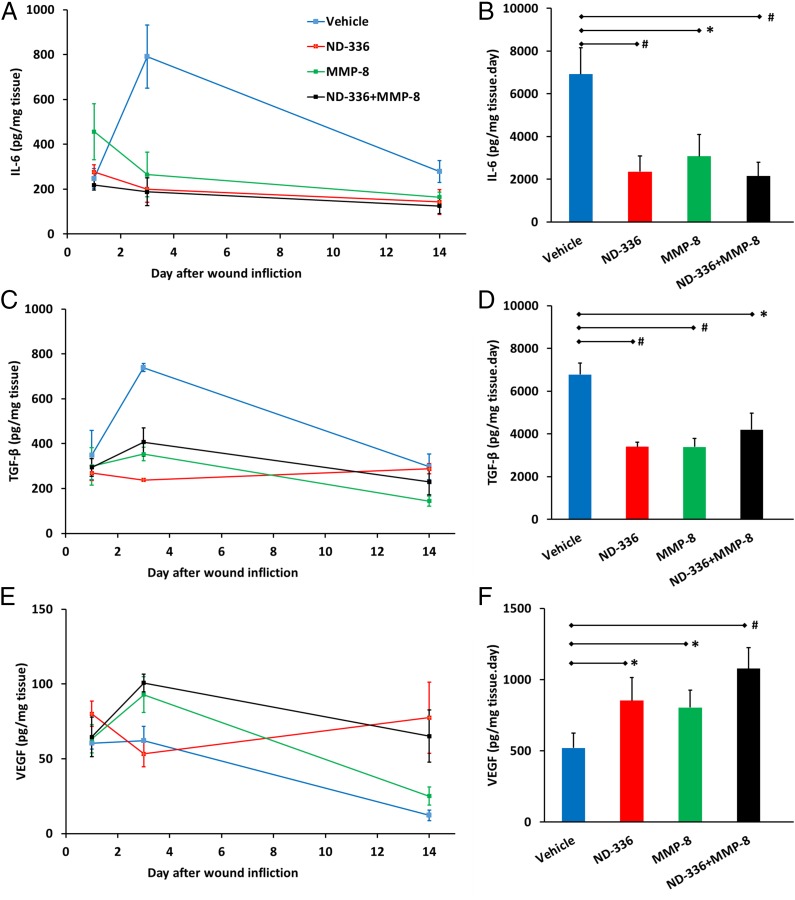
In vehicle-treated db/db mice, IL-6 was elevated throughout the course of the study (Fig. 3_A_). Treatment with either ND-336 or MMP-8 significantly reduced IL-6, and the combination of ND-336 and MMP-8 decreased IL-6 more than either agent alone (Fig. 3 A and B). Likewise, treatment with ND-336, MMP-8, or combined ND-336 and MMP-8 significantly reduced the levels of TGF-β1 (Fig. 3 C and D).
Fig. 3.
MMP-9 inhibition and/or exogenous MMP-8 result in decreased inflammation and increased angiogenesis. Data represent the mean ± SD (n = 3 mice per group per time point; total, 36 mice). *P < 0.05 and #P < 0.01 indicate statistically significant differences between the indicated groups. Statistical significance was evaluated by the Student’s t test using a two-tail distribution and unequal variance. (A) Concentrations of IL-6 as a function of time after wound infliction. (B) The AUC for IL-6 showed that IL-6 levels were reduced significantly upon treatment with ND-336, MMP-8, or combined ND-336 and MMP-8. (C) Concentrations of TGF-β1 as a function of time after wound infliction. (D) The AUC for TGF-β1 showed that TGF-β1 levels were reduced significantly upon treatment with ND-336, MMP-8, or combined ND-336 and MMP-8. (E) Concentrations of VEGF as a function of time after wound infliction. (F) AUC for VEGF showed that VEGF levels were increased significantly upon treatment with ND-336, MMP-8, or combined ND-336 and MMP-8.
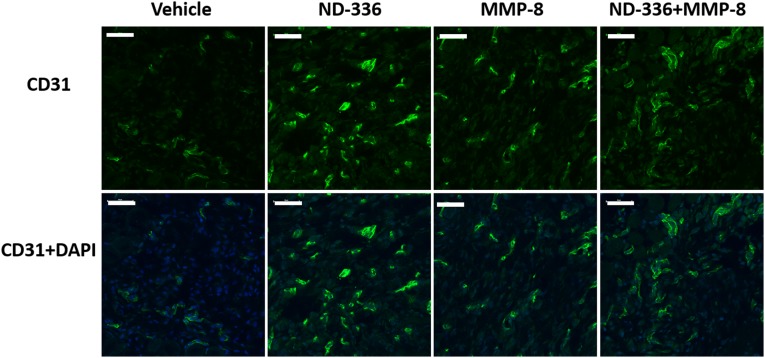
Angiogenesis is essential for wound healing (33), facilitating the removal of debris and the development of granulation tissue that help wound closure. CD31 is found on the surface of endothelial cells and is widely used as a marker for angiogenesis. Using anti-CD31 antibodies, we found increased angiogenesis in the groups treated with ND-336, MMP-8, or combined ND-336 plus MMP-8 (Fig. S7). We used a second angiogenesis marker, VEGF, which enhances vascular permeability, promoting the formation of new blood vessels (34). The levels of VEGF were determined by ELISA as a function of time after wound infliction. Treatment with ND-336, MMP-8, or combined ND-336 and MMP-8 increased VEGF concentrations in the wounds compared with vehicle treatment (Fig. 3 E and F). Our results are in agreement with the increased VEGF levels seen in human wound fluid (35) and in epidermal keratinocytes (36).
Fig. S7.
MMP-9 inhibition, exogenous MMP-8 treatment, and combined MMP-9 inhibition and exogenous MMP-8 increase angiogenesis as measured by anti-CD31. Tissues were collected 14 d after wound infliction (n = 3 mice per group).
In summary, we have shown that the novel MMP-9 inhibitor ND-336 accelerates diabetic wound healing by decreasing inflammation and by enhancing angiogenesis and re-epithelialization of the wound, thus reversing the pathological condition. Topical administration of active recombinant MMP-8 accelerated diabetic wound healing, resulting in complete re-epithelialization, diminished inflammation, and enhanced angiogenesis. The combination of a selective MMP-9 inhibitor with added MMP-8 was the best strategy to accelerate diabetic wound healing and holds promise for the treatment of diabetic wounds.
Materials and Methods
Synthesis of ND-336.
Detailed procedures for the synthesis of ND-336 are given in SI Materials and Methods.
Synthesis of ND-322.
ND-322 was synthesized as previously reported (10).
Enzyme Inhibition Studies.
Human recombinant active MMP-2 and MMP-7 and the catalytic domains of MMP-3 and MMP-14/MT1-MMP were purchased from EMD Chemicals, Inc.; human recombinant catalytic domains of MMP-1, MMP-8, and MMP-9 were purchased from Enzo Life Sciences, Inc.; human recombinant active ADAM9 and ADAM10 were purchased from R&D Systems. Fluorogenic substrates (7-methoxycoumarin-4-yl)acetyl (Mac)-Pro-Leu-Gly-Leu-[Nβ-(2,4-dinitrophenyl)-L-2,3-diaminopropionyl](Dap)(Dnp)-Ala-Arg-NH2 (for MMP-2, MMP-7, MMP-9, and MMP-14) and Mac-Arg-Pro-Lys-Pro-Val-Glu-norvaline (Nva)-Trp-Arg-Lys(Dnp)-NH2 (for MMP-3) were purchased from Peptides International; Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 (for MMP-1, MMP-8, and ADAM10) and Mac-Pro-Leu-Ala-Gln-Ala-Val-Dpa-Arg-Ser-Ser-Ser-Arg-NH2 (for ADAM9) were purchased from R&D Systems. The _K_m values for MMP-2, MMP-9, and MMP-14 were the same as previously reported by Gooyit et al. (37). Inhibitor stock solutions (10 mM) were prepared freshly in DMSO before enzyme inhibition assays. We followed the methodology for enzyme inhibition studies as reported previously by Page-McCaw, et al. (38). Enzyme inhibition studies were carried out using a Cary Eclipse fluorescence spectrophotometer (Varian). Compound 1 was stable in the buffers used in the kinetic assays.
Animals.
Female diabetic db/db mice (BKS.Cg-Dock7 m +/+ Lepr db/J, 8 wk old) were purchased from the Jackson Laboratory and were fed 5001 Laboratory Rodent Diet (LabDiet) and water ad libitum. Mice were housed in polycarbonate shoebox cages with hardwood bedding at 72 ± 2 °F and 12-h/12-h light/dark cycles. All procedures involving vertebrate animals were approved by the Institutional Animal Care and Use Committee at the University of Notre Dame.
Excisional Diabetic Wound Model.
The dorsal area of the mice was shaved, and a single excisional wound 8 mm in diameter was made on the dorsal thorax with a biopsy punch (Miltex) while the animals were under isofluorane anesthesia. Wounds were covered with Tegaderm dressing (3M Company). Topical treatment was started the next day.
Wound Measurements.
Mice were anesthetized with isofluorane, and wounds were photographed with an Olympus SP-800 UZ camera mounted on a tripod at a fixed distance; a ruler was included in the digital photo. Wound areas were calculated using NIH ImageJ software (version 1.48) and expressed as percent change in wound area relative to day 0.
ND-336 Diabetic Wound-Healing Study.
For the ND-336 diabetic wound-healing study, db/db mice were divided into three groups (n = 8 mice per group, total 24 mice): vehicle-treated (50 µL of 20% DMSO, 80% propylene glycol per wound per day), ND-322–treated (50 µL of a 2-mg/mL solution of ND-322 in 20% DMSO/80% propylene glycol, equivalent to 0.1 mg per wound per day), and ND-336–treated (50 µL of a 2 mg/mL solution of ND-336 in 20% DMSO/80% propylene glycol, equivalent to 0.1 mg per wound per day). Wound measurements were taken on days 0, 7, 10, and 14. Animals were killed on day 14, and wounds were embedded in optimal cutting temperature (OCT) compound, cryosectioned, and analyzed by H&E staining, TUNEL, and in situ zymography.
Exogenous MMP-8 Study.
Female diabetic db/db mice (BKS.Cg-Dock7m +/+ Leprdb/J, 8 wk old, 38 ± 3 g, n = 40) were used for the exogenous MMP-8 study. Wounds were inflicted as described, and on the next day the wounds were treated topically with MMP-8 (50 μL of 20 μg/mL MMP-8 in reaction buffer) or vehicle (50 μL reaction buffer) once each day for 14 d. The reaction buffer consisted of 50 mM Tris (pH 7.5), 10 mM CaCl2, 150 mM NaCl, and 0.05% (wt/vol) Brij-35. Digital photographs of the wounds were taken on days 0, 7, 10, and 14 while animals were under isoflurane anesthesia. On days 7 and 14, 20 mice (n = 10 per group) were killed. The wounds were excised, embedded in OCT compound, and cryosectioned for histological evaluation and in situ zymography.
Combined ND-336 and MMP-8 Study.
In the combined ND-336 and MMP-8 study, female db/db mice (n = 51) were divided into four groups: vehicle -treated [50 µL of 10% DMSO/10% propylene glycol/80% saline per wound per day dosed in the morning and 50 µL of reaction buffer 50 mM Tris (pH 7.5), 10 mM CaCl2, 150 mM NaCl, and 0.05% (wt/vol) Brij-35) per wound per day dosed in the afternoon], ND-336–treated [50 µL of 1 mg/mL ND-336 (equivalent to 0.05 mg per wound per day) in 10% DMSO/10% propylene glycol/80% saline dosed in the morning and 50 µL of reaction buffer dosed in the afternoon], MMP-8–treated [50 µL of 10% DMSO/10% propylene glycol/80% saline per wound per day dosed in the morning and 50 µL of 20 µg/mL of MMP-8 in reaction buffer (equivalent to 1 μg per wound per day) dosed in the afternoon], and combined ND-336– and MMP-8–treated (0.05 mg of ND-336 in 50 µL of 10% DMSO/10% propylene glycol/80% saline per wound per day dosed in the morning and 1 µg of MMP-8 in 50 µL of reaction buffer per wound per day dosed in the afternoon). Mice were killed on days 7, 10, and 14, and the excised wounds were embedded in OCT compound and cryosectioned for histological evaluation and in situ zymography.
Measurement of IL-6, TGF-β1, and VEGF by ELISA.
Wound tissues (n = 3 mice per group) were harvested and were frozen immediately in liquid nitrogen on days 1, 3, and 14. The extracted tissues were homogenized in cold lysis buffer containing EDTA-free protease inhibitor mixture (Pierce). The lysates were analyzed for protein concentration by the Bradford protein assay (Bio-Rad). The levels of IL-6, TGF-β1, and VEGF in the lysates were determined by ELISA, following the manufacturer’s protocol (Abcam). The cytokine levels for each mouse sample were expressed in picograms per milligram of tissue. The area under the curve (AUC) was calculated by the linear trapezoid rule using GraphPad Prism 5 for Windows Version 5.01 (GraphPad Software, Inc.). AUC is reported as mean ± SD.
Statistical Analyses.
Data were analyzed for statistical significance using the two-tailed Mann–Whitney u test (GraphPad Prism 5). Inflammation and angiogenesis markers were analyzed for statistical significance by the Student _t_-test (Excel) using a two-tail distribution and unequal variance because the Mann–Whitney u test will always give a P value greater than 0.05 regardless of the groups differences when the total sample size is seven or less.
SI Materials and Methods
Synthesis of ND-336.
The synthesis of ND-336 is shown in the scheme in Scheme 1 in the main text. As shown in Fig. S1, the reaction of 4-mercaptophenol (compound 3) with allyl bromide (39) produced compound 4, which was allowed to react with 4-fluorobenzonitrile to afford diphenyl ether, compound 5, in good yields (94% and 82% for the first and second steps, respectively). Subsequent reduction of the nitrile with LiAlH4, followed by _t_-butoxycarbonyl (Boc)-protection of the resultant amine yielded compound 6, which was oxidized to the corresponding oxirane, compound 7. The reaction of compound 7 with thiourea produced the Boc-protected thiirane, compound 8. After the final acid Boc-deprotection, the desired ND-336 was in hand as the HCl salt.
Chemistry.
All reactions were performed under nitrogen atmosphere, unless otherwise noted. 1H and 13C NMR spectra were recorded on Varian INOVA-500 or Varian UnityPlus 300 spectrometer (Varian Inc.) or Bruker AVANCE III HD 500 or Bruker AVANCE III HD 400 (Bruker Corporation). TLC silica gel 60 F254 aluminum sheets (EMD Millipore Corporation) were used for TLC. Flash chromatography was performed with an automated chromatograph system, Combiflash RF 200i UV/Vis (Teledyne Isco). High-resolution mass spectra were obtained by electrospray ionization (ESI) using a BrukermicrOTOF/Q2 mass spectrometer (Bruker Daltonik). Purity of the prepared compounds was in general >95%, as confirmed by ultra performance liquid chromatography (UPLC). Conditions are detailed in the UPLC section below. We prepared 4-(allylthio)phenol (compound 4) as previously described (39, 40).
4-(4-(Allylthio)phenoxy)benzonitrile (Fig. S1, Compound 5).
A mixture of compound 4 (1.45 g, 8.72 mmol), 4-fluorobenzonitrile (1.01 g, 8.38 mmol), and Cs2CO3 (4.26 g, 13.1 mmol) in dimethylformamide (50 mL) was heated at 100 °C for 3.5 h. After the addition of saturated aqueous LiBr (250 mL), the mixture was extracted with hexanes/EtOAc (9:1). The combined organic layers were washed with water and brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The resultant residue was purified by silica gel chromatography (hexanes/EtOAc, 97:3) to give compound 5 (1.84 g, 82%) as an oil. 1H NMR (300 MHz, CDCl3) δ 7.77–7.52 (m, 2H), 7.49–7.30 (m, 2H), 7.14–6.82 (m, 4H), 5.87 (ddt, J = 16.9, 10.0, 6.9 Hz, 1H), 5.29–4.93 (m, 2H), 3.53 (dt, J = 6.9, 1.1 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 161.6, 153.9, 134.4, 133.7, 132.7, 132.4, 121.0, 119.0, 118.2, 118.1, 106.3, 38.2. high-resolution mass spectrometry (HRMS) (ESI+, m/z): calculated (calcd) for C16H14NO [M + H]+, 268.0791; found, 268.0799.
_t_-Butyl 4-(4-(allylthio)phenoxy)benzylcarbamate (Fig. S1, Compound 6).
A solution of compound 5 (4.98 g, 18.63 mmol) in THF (78 mL) was added dropwise to LiAlH4 (2.12 g, 55.89 mmol) in tetrahydrofuran (78 mL) at 0 °C over a period of 30 min. The ice bath was removed, and the reaction mixture was stirred at room temperature for 1.5 h, at which point the TLC showed the reaction to be complete. The solution was cooled again in ice water and quenched carefully with the dropwise addition of 2.4 mL water, 2.4 mL 15% aqueous NaOH, and 7.2 mL water. The solution was warmed gradually to room temperature and stirred for 30 min, filtered through a celite pad, extracted with diethyl ether and EtOAc. The combined organic layer was washed with water and brine, and the solution was dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure to give the crude primary amine, which was used directly in the next step.
To a mixture of amine (4.3 g, 15.84 mmol) and (Boc)2O (5.2 g, 23.77 mmol) in MeOH/CH2Cl2 (3:2, 150 mL), a catalytic amount of iodine (402 mg, 1.58 mmol, 10 mol%) was added. After the reaction mixture was stirred for 24 h at room temperature, the solvent was evaporated in vacuo, and EtOAc was added. The solution was washed with 5% aqueous Na2S2O3 and saturated NaHCO3 and was dried over anhydrous Na2SO4. The solvent was evaporated in vacuo, and the residue was purified by silica gel chromatography (hexanes/EtOAc, 95:5) to afford compound 6 (3.21 g, 55% in two steps). 1H NMR (300 MHz, CDCl3) δ 7.38–7.32 (m, 2H), 7.31–7.25 (m, 2H), 6.99–6.90 (m, 4H), 6.17–6.09 (m, 1H), 5.08–5.03 (m, 2H), 4.85 (s, b, 1H), 4.29 (d, J = 3.0 Hz, 2H), 3.49–3.47 (m, 2H), 1.46 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 156.8, 156.4, 156.1, 134.0, 133.2, 131.4, 131.2, 129.2, 127.2, 119.3, 117.7, 79.7, 44.4, 38.8, 28.6. HRMS (ESI+, m/z): calcd for C21H25NNaO3S [M + Na]+, 394.1447; found, 394.1472.
_t_-Butyl 4-(4-((oxiran-2-ylmethyl)sulfonyl)phenoxy)benzylcarbamate (Fig. S1, Compound 7).
Meta-chloroperbenzoic acid (_m_-compu) (2.03 g, 11.8 mmol) was added in batches to a solution of compound 6 (0.88 g, 2.36 mmol) in CH2Cl2 (8 mL) immersed in an ice-water bath. After completion of the addition, the ice-water bath was removed, and the solution was stirred at room temperature for 3 d. Another batch of _m_-CPBA (1.02 g, 5.89 mmol) was added, and the mixture was stirred at room temperature for an additional 5 d. The suspension was filtered, and the filtrate was diluted with CH2Cl2 and washed with 10% aqueous sodium thiosulfate, followed by saturated NaHCO3 and brine. The organic layer was dried over anhydrous Na2SO4, the suspension was filtered, and the solution was concentrated in vacuo. The product was purified by silica gel chromatography (hexanes/EtOAc, 2:1–1:1) to yield compound 7 (0.36 g, 36%). 1H NMR (400 MHz, CDCl3) δ 7.91–7.65 (m, 2H), 7.27–7.25 (m, 2H), 7.06–6.95 (m, 4H), 5.22 (s, b, 1H), 4.24 (d, J = 5.1 Hz, 2H), 3.44–3.02 (m, 3H), 2.72 (dd, J = 8.0, 2.0 Hz, 1H), 2.39 (dd, J = 4.8, 2.0 Hz, 1H), 1.39 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 163.0, 154.1, 136.6, 130.7, 129.5, 123.2, 120.7, 118.0, 117.8, 79.7, 59.8, 46.04, 45.99, 44.1, 28.6. HRMS (ESI+, m/z): calcd for C21H25NNaO6S [M + Na]+, 442.1295; found, 442.1292.
_t_-Butyl 4-(4-((thiiran-2-ylmethyl)sulfonyl)phenoxy) benzylcarbamate (Fig. S1, Compound 8).
Thiourea (55.3 mg, 0.73 mmol) was added to a solution of compound 7 (138.4 mg, 0.33 mmol) in MeOH/CH2Cl2 (1:1, 3 mL), and the resulting mixture was stirred at room temperature for 24 h. The solvent was removed under reduced pressure, and the residue was partitioned between CH2Cl2 and water. The organic layer was washed with water and brine, dried over anhydrous Na2SO4, and filtered. Evaporation of the solvent gave the crude product, which was purified by silica gel chromatography (83.4 mg, 58%). 1H NMR (300 MHz, CDCl3) δ 7.85 (d, J = 8.7 Hz, 2H), 7.33 (d, J = 8.7 Hz, 2H), 7.13–6.97 (m, 4H), 4.95 (s, b, 1H), 4.33 (d, J = 5.9 Hz, 2H), 3.51 (dd, J = 14.1, 5.7 Hz, 1H), 3.17 (dd, J = 14.1, 7.8 Hz, 1H), 3.11–2.98 (m, 1H), 2.53 (dd, J = 6.3, 1.6 Hz, 1H), 2.15 (dd, J = 5.1, 1.6 Hz, 1H), 1.46 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 163.1, 156.0, 154.0, 136.3, 132.1, 130.8, 129.4, 120.7, 117.8, 79.8, 62.8, 44.1, 28.5, 26.2, 24.4. HRMS (ESI+, m/z): calcd for C21H25NNaO5S2 [M + Na]+, 458.1066; found, 458.1089.
(4-(4-((Thiiran-2-ylmethyl)sulfonyl)phenoxy)phenyl)methanamine HCl salt (Compound 1).
HCl (0.7 mL, 4 N in 1,4-dioxane) was added to a solution of thiirane (compound 8) (61.0 mg, 0.14 mmol) in CH2Cl2/EtOAc (1:1, 4 mL). After stirring at room temperature for 24 h, the mixture was concentrated under reduced pressure. The resulting crude compound was triturated with diethyl ether, and the product was obtained by filtration (51.0 mg, 98%). 1H NMR (300 MHz, CD3OD) δ 7.93 (d, J = 8.6 Hz, 2H), 7.58 (d, J = 8.6 Hz, 2H), 7.19 (d, d, J = 8.4 Hz, 4H), 4.16 (s, 2H), 3.57–3.43 (m, 2H), 3.11–2.99 (m, 1H), 2.52 (dd, J = 6.3, 1.4 Hz, 1H), 2.14 (dd, J = 5.1, 1.4 Hz, 1H). 13C NMR (75 MHz, CD3OD) δ 162.5, 156.2, 133.0, 131.3, 131.1, 130.0, 120.7, 118.2, 62.0, 42.6, 25.8, 23.0. HRMS (ESI+, m/z): calcd for C16H18NO3S2 [M + H]+, 336.0723; found, 336.0709. The purity of ND-336 was >95%, as determined by UPLC with UV detection.
Determination of Water Solubility.
A saturated aqueous solution of ND-336 was prepared, and the solution was filtered. A 100-fold dilution of the filtrate was analyzed by UPLC with UV detection (see conditions below) using peak area and linear regression parameters calculated from a calibration curve. The calibration curve was prepared using known concentrations of ND-336 in acetonitrile. The assay was linear from 0.5 to 100 µg/mL with an _R_2 value of 0.999. The water solubility of ND-336 is 4.90 ± 0.06 mg/mL
UPLC.
A Waters Acquity UPLC System (Waters Corporation) equipped with a binary solvent manager, an autosampler, a column heater, and a photodiode array detector was used to test the purity and water solubility of ND-336. An Acquity UPLC HSS C18 column (1.8 μm, 2.1 × 100 mm; Waters Corporation) was used. The mobile phase consisted of water (A) and acetonitrile (B) elution at 0.5 mL/min with 85% A, 15% B for 2 min, followed by a 5-min linear gradient to 5% A, 95% B, then held at 5% A, 95% B for 2 min, followed by a 1-min linear gradient back to 85% A, 15% B and then held at 85% A, 15% B for 1 min. ND-336 was detected by UV at 245 nM. The retention time was 4.33 min.
MMP-9–Knockout Study.
Female MMP-9–knockout mice [B6.FVB(Cg)-Mmp9 tm1Tvu/J, 8 wk old, 19 ± 2 g, n = 14] and wild-type mice (C57BLKS/6J, 8 wk old, 19 ± 2 g, n = 14, with the same background as the MMP-9–knockout mice) were used. The mice were acclimated to the study room for 1 wk before commencement of the study. Diabetes was induced by i.p. injection of streptozotocin (Sigma-Aldrich) at 150 mg/kg. Streptozotocin was dissolved in 100 mM sodium citrate buffer (pH 4.5) and administered within 15 min after preparation. After streptozotocin treatment, the mice were housed in disposable cages and given 10% sucrose water to drink for 2 d. The fasting blood glucose levels were determined 2 d after streptozotocin treatment. Animals with blood glucose greater than 300 mg/dL were considered diabetic (41). Animals with blood glucose less than 300 mg/dL received a second dose of streptozotocin 1 wk later. The average blood glucose level of all the animals was determined to be 465 ± 113 mg/dL. Wounds were inflicted as described, and digital photographs were taken on days 0, 7, and 14. Mice were killed on days 7 and 14 (n = 7 mice per group), and the wounds were excised, embedded in OCT compound, and cryosectioned for histological evaluation.
Cloning and Purification of Mouse MMP-8 and Effect of MMP-8 on Diabetic Wound Healing.
The gene for the catalytic domain (304–852 bp) without the prodomain of MMP-8 was optimized for expression in Escherichia coli and was synthesized by GenScript with unique NdeI and XhoI restriction sites flanking the gene at the 5′ and 3′ termini, respectively. The gene was cloned into vector pET28a. E. coli DH5α was transformed by this construct. The recombinant MMP-8 was expressed in E. coli BL21 (DE3) and purified using a previously published method (42), with the induction by 0.5 mM isopropyl β-d-1-thiogalactopyranoside at 20 °C. The purity of the protein was determined to be ≥95% by SDS/PAGE. The enzyme concentration was evaluated spectrophotometrically using the extinction coefficient predicted by ProtParam (41) (Δε280 = 1,9681.6 M/cm). Aliquots of the concentrated protein were stored in 50 mM Tris (pH 7.5), 5 mM CaCl2, 300 mM NaCl, 20 µM ZnCl2, 0.5% (wt/vol) Brij-35, 30% glycerol at −80 °C.
Dose–Response Study with ND-336.
We had shown that ND-336 at a dose of 0.1 mg per wound per day accelerated diabetic wound healing. To find the lowest dose of ND-336 that was efficacious in diabetic wound healing, a dose–response study was conducted at 0.05, 0.025, and 0.01 mg of ND-336 per wound per day. For this study, db/db mice were divided into four treatment groups: vehicle (50 µL of 20% DMSO/80% propylene glycol; n = 7 mice), 0.05 ND-336 mg per wound per day (50 µL of 1 mg/mL ND-336 in 20% DMSO/80% propylene glycol; n = 6 mice), 0.025 mg ND-336 per wound per day (50 µL of 0.5 mg/mL ND-336 in 20% DMSO/80% propylene glycol; n = 7 mice), 0.01 mg ND-336 per wound per day (50 µL of 0.2 mg/mL ND-336 in 20% DMSO/80% propylene glycol; n = 7 mice).
As shown in Fig. S5, on day 14 the group treated with 0.05 mg ND-336 per wound per day exhibited significantly better healing than the groups treated with vehicle, 0.025 mg ND-336 per wound per day, or 0.01 mg ND-336 per wound per day. All subsequent studies were performed at the 0.05 mg ND-336 per wound per day level.
Dose–Response Study with MMP-8.
We had determined that MMP-8 applied topically at a dose of 1 µg of MMP-8 per wound per day accelerated wound healing in db/db mice. We next investigated the effect of higher dose levels of MMP-8 on wound healing. MMP-8 was applied topically to wounds of db/db mice at 1, 5, and 10 µg per wound per day, corresponding, respectively, to 10-, 50-, and 100-fold concentrations of active MMP-8 found in wounds. Mice were divided into four treatment groups and were treated on days 7, 10, and 14 with vehicle [50 µL of reaction buffer (50 mM Tris, pH 7.5), 10 mM CaCl2, 150 mM NaCl, and 0.05% (wt/vol) Brij-35; n = 20, 9, and 9 mice on days 7, 10, and 14, respectively], with 1 µg MMP-8 per wound per day (50 µL of 20 μg/mL MMP-8 in reaction buffer; n = 20, 10, and 10 mice on days 7, 10, and 14, respectively), 5 µg MMP-8 per wound per day (50 µL of 100 μg/mL MMP-8 in reaction buffer; n = 10, 10, and 10 mice on days 7, 10, and 14, respectively), or 10 µg MMP-8 per wound per day (50 µL of 200 μg/mL MMP-8 in reaction buffer; n = 10, 10, and 9 mice on days 7, 10, and 14, respectively).
Fig. S6 shows that treatment with 1 µg MMP-8 per wound per day accelerated wound healing most effectively. All subsequent studies were conducted using 1 µg of MMP-8 per wound per day.
Histological Evaluation, Apoptosis Detection, and in Situ Zymography.
Wounds (n = 3 mice per group per day) were embedded in OCT compound (Tissue-Tek; Sakura Finetek) and were cryosectioned at 12-µm thickness for H&E staining and at 8-µm thickness for in situ zymography. Re-epithelialization was assessed on a fluorescent microscope (Nikon Eclipse 90_i_; Nikon Instruments, Inc.). Apoptotic cells in wound sections were assessed using a modified TUNEL assay kit, following the manufacturer's instructions (Trevigen, Inc.). For in situ zymography, unfixed cryostat sections of wound tissues were incubated in reaction buffer (50 mM Tris-buffered saline, pH 7.6) containing DQ-gelatin or DQ-collagen (Molecular Probes, Inc.) at 37 °C for 6 h. After fixation in 4% paraformaldehyde in PBS, cells were counterstained with DAPI, and the images were visualized by fluorescence microscopy. Immunofluorescent detection of vascular density was performed by staining the tissues with Alexa Fluor 488 anti-mouse CD31 (BioLegend) followed by staining the nuclei with DAPI. The images for in situ zymography and vascular density were obtained by confocal microscopy.
Acknowledgments
We thank Sarah Chapman for the preparation of wound tissue sections, H&E staining, and TUNEL and Hualiang Pi for cloning and purification of MMP-8. This work was supported by American Diabetes Association Pathway to Stop Diabetes Grant 1-15-ACN-06 and Neilsen Foundation Grant 282987. M. Gooyit was a Ruth L. Kirschstein National Research Service Award Fellow of the Chemistry-Biochemistry-Biology Interface Program at the University of Notre Dame, supported by Training Grant GM075762 from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Centers for Disease Control and Prevenion 2014. National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States. (Centers for Disease Control and Prevention, Atlanta, GA)
- 2.Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther. 2012;3(1):4. doi: 10.1007/s13300-012-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziyadeh N, Fife D, Walker AM, Wilkinson GS, Seeger JD. A matched cohort study of the risk of cancer in users of becaplermin. Adv Skin Wound Care. 2011;24(1):31–39. doi: 10.1097/01.ASW.0000392922.30229.b3. [DOI] [PubMed] [Google Scholar]
- 4.Wilgus TA. Growth Factor-Extracellular Matrix Interactions Regulate Wound Repair. Adv Wound Care (New Rochelle) 2012;1(6):249–254. doi: 10.1089/wound.2011.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19(1):34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravanti L, Kähäri VM. Matrix metalloproteinases in wound repair (review) Int J Mol Med. 2000;6(4):391–407. [PubMed] [Google Scholar]
- 8.Gooyit M, et al. A chemical biological strategy to facilitate diabetic wound healing. ACS Chem Biol. 2014;9(1):105–110. doi: 10.1021/cb4005468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Chang M, Hansen CN, Basso DM, Noble-Haeusslein LJ. Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury. Neurotherapeutics. 2011;8(2):206–220. doi: 10.1007/s13311-011-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gooyit M, et al. Selective water-soluble gelatinase inhibitor prodrugs. J Med Chem. 2011;54(19):6676–6690. doi: 10.1021/jm200566e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee M, et al. Structure-activity relationship for thiirane-based gelatinase inhibitors. ACS Med Chem Lett. 2012;3(6):490–495. doi: 10.1021/ml300050b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Testero SA, et al. Sulfonate-containing thiiranes as selective gelatinase inhibitors. ACS Med Chem Lett. 2011;2(2):177–181. doi: 10.1021/ml100254e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes C, et al. Active site ring-opening of a thiirane moiety and picomolar inhibition of gelatinases. Chem Biol Drug Des. 2009;74(6):527–534. doi: 10.1111/j.1747-0285.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Copeland RA, Pompliano DL, Meek TD. Drug-target residence time and its implications for lead optimization. Nat Rev Drug Discov. 2006;5(9):730–739. doi: 10.1038/nrd2082. [DOI] [PubMed] [Google Scholar]
- 15.Olson MW, Gervasi DC, Mobashery S, Fridman R. Kinetic analysis of the binding of human matrix metalloproteinase-2 and -9 to tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2. J Biol Chem. 1997;272(47):29975–29983. doi: 10.1074/jbc.272.47.29975. [DOI] [PubMed] [Google Scholar]
- 16.Gu Z, et al. S-nitrosylation of matrix metalloproteinases: Signaling pathway to neuronal cell death. Science. 2002;297(5584):1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 17.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6):537–546. [PubMed] [Google Scholar]
- 18.Tang J, et al. MMP-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J Cereb Blood Flow Metab. 2004;24(10):1133–1145. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- 19.Kyriakides TR, et al. 2009. Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biology: Journal of the International Society for Matrix Biology 28(2):65–73.
- 20.Purwar R, Kraus M, Werfel T, Wittmann M. Modulation of keratinocyte-derived MMP-9 by IL-13: A possible role for the pathogenesis of epidermal inflammation. J Invest Dermatol. 2008;128(1):59–66. doi: 10.1038/sj.jid.5700940. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi T, Shinkai H. Leptomycin B reduces matrix metalloproteinase-9 expression and suppresses cutaneous inflammation. J Invest Dermatol. 2005;124(2):331–337. doi: 10.1111/j.0022-202X.2004.23595.x. [DOI] [PubMed] [Google Scholar]
- 22.Gutiérrez-Fernández A, et al. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8) FASEB J. 2007;21(10):2580–2591. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasukawa H, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4(6):551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 24.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: Molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 25.Gallucci RM, et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14(15):2525–2531. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- 26.Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73(6):713–721. doi: 10.1189/jlb.0802397. [DOI] [PubMed] [Google Scholar]
- 27.Crowe MJ, Doetschman T, Greenhalgh DG. Delayed wound healing in immunodeficient TGF-beta 1 knockout mice. J Invest Dermatol. 2000;115(1):3–11. doi: 10.1046/j.1523-1747.2000.00010.x. [DOI] [PubMed] [Google Scholar]
- 28.Amendt C, Mann A, Schirmacher P, Blessing M. Resistance of keratinocytes to TGFbeta-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J Cell Sci. 2002;115(Pt 10):2189–2198. doi: 10.1242/jcs.115.10.2189. [DOI] [PubMed] [Google Scholar]
- 29.Han YP, Tuan TL, Hughes M, Wu H, Garner WL. Transforming growth factor-beta - and tumor necrosis factor-alpha -mediated induction and proteolytic activation of MMP-9 in human skin. J Biol Chem. 2001;276(25):22341–22350. doi: 10.1074/jbc.M010839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HS, Shang T, Chen Z, Pflugfelder SC, Li DQ. TGF-beta1 stimulates production of gelatinase (MMP-9), collagenases (MMP-1, -13) and stromelysins (MMP-3, -10, -11) by human corneal epithelial cells. Exp Eye Res. 2004;79(2):263–274. doi: 10.1016/j.exer.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Salo T, Lyons JG, Rahemtulla F, Birkedal-Hansen H, Larjava H. Transforming growth factor-beta 1 up-regulates type IV collagenase expression in cultured human keratinocytes. J Biol Chem. 1991;266(18):11436–11441. [PubMed] [Google Scholar]
- 32.Reynolds LE, et al. Accelerated re-epithelialization in beta3-integrin-deficient- mice is associated with enhanced TGF-beta1 signaling. Nat Med. 2005;11(2):167–174. doi: 10.1038/nm1165. [DOI] [PubMed] [Google Scholar]
- 33.Tonnesen MG, Feng X, Clark RA. 2000. Angiogenesis in wound healing. J Invest Dermatol 5(1):40–46.
- 34.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16(5):558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nissen NN, et al. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152(6):1445–1452. [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 37.Gooyit M, et al. O-phenyl carbamate and phenyl urea thiiranes as selective matrix metalloproteinase-2 inhibitors that cross the blood-brain barrier. J Med Chem. 2013;56(20):8139–8150. doi: 10.1021/jm401217d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikejiri M, et al. Potent mechanism-based inhibitors for matrix metalloproteinases. J Biol Chem. 2005;280(40):33992–34002. doi: 10.1074/jbc.M504303200. [DOI] [PubMed] [Google Scholar]
- 40.Goux C, Lhoste P, Sinou D. Palladium(0)- Catalyzed Alkylation of Thiols. Tetrahedron. 1994;50(34):10321–10330. [Google Scholar]
- 41.Gasteiger E, et al. Protein identification and analysis tools on the ExPASy server. In: Walker JM, editor. The Proteomics Protocols Handbook. Humana Press; Totowa, NJ: 2005. pp. 571–607. [Google Scholar]
- 42.Botos I, et al. Structure of recombinant mouse collagenase-3 (MMP-13) J Mol Biol. 1999;292(4):837–844. doi: 10.1006/jmbi.1999.3068. [DOI] [PubMed] [Google Scholar]