Too much of a good thing: regulated depletion of c-di-AMP in the bacterial cytoplasm (original) (raw)
. Author manuscript; available in PMC: 2017 Apr 1.
Published in final edited form as: Curr Opin Microbiol. 2016 Jan 7;30:22–29. doi: 10.1016/j.mib.2015.12.007
INTRODUCTION
The nucleotide 3’,5’- cyclic di-adenosine monophosphate (c-di-AMP) is a broadly conserved second messenger among the bacterial and archaeal domains, with many c-di-AMP synthesizing organisms being prominent human pathogens and of environmental importance. Whereas many of these species require cdi-AMP for growth and survival, elevated c-di-AMP levels result in aberrant physiology and attenuated virulence during infection. Given the expanding number of organisms recognized to synthesize c-di-AMP and the importance it plays in the diverse environments bacteria encounter, the molecular mechanisms pertaining to nucleotide metabolism and signal transduction are of increasing interest and focus.
All second messenger signaling systems have three fundamental requirements, a mechanism to (i) produce the signal, (ii) respond to changing levels of the signal, and (iii) remove the signal once adaptation to the stimulus has been executed. In vivo, c-di-AMP is synthesized by the diadenylate cyclase activity of DisA_N domain-containing proteins (Pfam PF02457), which are present in more than 11,000 bacterial and archaeal species. A wide array of protein and RNA targets have been implicated in signal transduction, linking c-di-AMP regulation to central metabolism, potassium transport, transcriptional control, and DNA damage responses, among other physiological processes [1]. The broad biological functions of these receptors are consistent with the seemingly pleotropic role of c-di-AMP in microbial physiology. For signal removal, bacteria encode at least two major classes of c-di-AMP phosphodiesterases. Hydrolytic activity of these enzymes is subject to posttranslational regulation mediated by environmental and metabolic cues within the bacterial cell. Additionally, we hypothesize that multidrug resistance transporters of the major facilitator superfamily (MFS), which can actively secrete c-di-AMP, also participate in controlling cytoplasmic c-di-AMP levels (Fig. 1). This review aims to summarize the major mechanisms for c-di-AMP hydrolysis and secretion, as well as its role in bacterial physiology and host-microbe interactions.
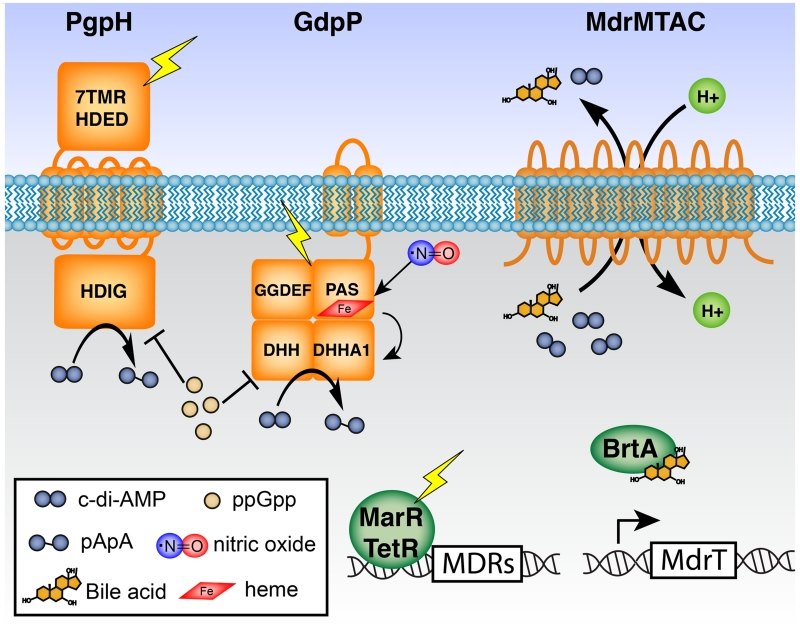
Figure 1.
Protein components of c-di-AMP depletion in the bacterial cytoplasm. PgpH and GdpP have sensory domains for integration of environmental and intracellular signals into enzymatic hydrolysis of c-di-AMP. DhhP (not shown) has a DHH-DHHA1 catalytic domain, but without accessory domains. Both PgpH and GdpP activities are inhibited by ppGpp. Heme-bound GdpP is activated by nitric oxide. Expression of MDRs is regulated by transcriptional factors of the MarR and TetR families. Small molecules such as bile acids influence DNA-binding activity of the TetR-like repressor BrtA and lead to transcriptional induction of MDRs. Enhanced MDR expression results in elevated secretion of bile acids and c-di-AMP.
1. THE DHH-DHHA1 DOMAIN PHOSPHODIESTERASES
The first characterized c-di-AMP phosphodiesterase is Bacillus subtilis GdpP, which has two transmembrane helices, a PAS (Per-Arnt-Sim) domain, a degenerate GGDEF domain, and the catalytic domains DHH and DHHA1 [2]. More recently, stand-alone DHH-DHHA1 domain proteins have also been reported to degrade c-di-AMP in certain bacterial species. Following the Borrelia burgdorferi homolog gene symbol, here we name these stand-alone proteins DhhP [3].
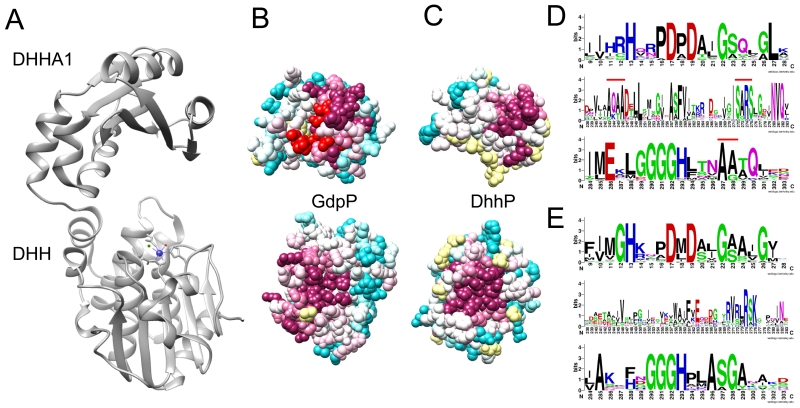
The catalytic domain of both DhhP and GdpP homologs belongs to the DHH-DHHA1 family (Pfam PF02272) that also includes RecJ exonuclease and NrnA oligoribonuclease. A Phyre2 structural model of the GdpP DHH-DHHA1 domain shows remarkable resemblance to the NrnA crystal structures and the DhhP structure of M. smegmatis (Fig. 2A) [4,5]. Among these proteins, the amino-terminal DHH subunit and the carboxy-terminal DHHA1 subunit are connected by a flexible loop to form a cleft. Based on similarity with the NrnA structures (PDB ID: 3W5W), GdpP and DhhP likely coordinate two metal ions, and the putative metal binding site is formed at the base of the DHH subunit by highly conserved His and Asp residues contained within the DHH motif. Within the DHH domain, GdpP and DhhP proteins show significant sequence conservation among their respective homologs and between each other (Fig 2B-E), consistent with the importance of this domain in catalytic activity. By contrast, these proteins are more divergent in the DHHA1 subunit, which we predict to provide substrate specificity (Fig. 2D-E, sequence logos row 2). In silico docking of c-di-AMP in the GdpP model predicts that the ligand interacts with both subunits (not shown). One nucleotide interacts with the base of the DHH subunit near the metal binding site, and the other nucleotide docks on the inter-domain face of the DHHA1 subunit, via a patch of conserved residues on the β-sheet, and helix α-10 (colored red in Fig. 2B and marked by a red bar in Fig. 2D). In the DhhP sequences, the corresponding residues show less conservation (Fig. 2E), perhaps explaining the seemingly distinct substrate specificity reported for these two protein families.
Figure 2.
Structural and sequence comparison of GdpP and DhhP catalytic domains. A – Crystal structure of the M. smegmatis DhhP (MSMEG_2630, PDB ID: 4LS9), also characterized as an NrnA oligoribonuclease. A metal ion is shown as a blue ball. A structural model of the GdpP DHH-DHHA1 domain, generated by the Phyre2 server, shows essentially the same fold, and is well aligned with NrnA. B and C – Comparison of conserved amino acids within the putative substrate recognition surface of the DHHA1 domain (top) and the metal binding site within the DHH domain (bottom). Similarly conserved residues are shown in magenta. GdpP also exhibits an additional conserved patch, shown in red, predicted for c-di-AMP binding by the Swissdock server. D - Sequence logos illustrating conserved residues of the GdpP DHH domain (top row) and DHHA1 domain (second and third rows), with conserved residues predicted for c-di-AMP binding labeled by a red bar. Residue 9 corresponds to Ile-341 in the B. subtilis GdpP sequence, residue 242 corresponds to Ile-574. E – Similar to D, but for DhhP homologs.
GdpP homologs are mostly present in the Firmicutes and Tenericutes phyla, with characterized examples in B. subtilis, S. aureus, L. monocytogenes, and Streptococcus sp [2,6-8]. GdpP exhibits exquisite specificity for cyclic dinucleotides [2,7]. Among various substrates tested, GdpP only degrades c-di-AMP and c-di-GMP, generating 5’-pApA and 5’-pGpG as products. Furthermore, GdpP exhibits a much higher KM for c-di-GMP than for c-di-AMP (over 250-fold), indicating that c-di-AMP is the preferred physiological substrate. Hydrolysis activity is dependent on Mn2+ ion and is optimal under alkaline conditions at pH 8.5 – 9.0, which is thought to be consistent with a two-metal ion mechanism,,perhaps similar to that of PgpH as discussed below.
Unlike the relatively restricted taxonomic distribution of GdpP, DhhP homologs are present in almost all cdi-AMP synthesizing species. Thus far, these proteins have been characterized in S. pneumoniae, B. burgdorferi, M. tuberculosis, and M. smegmatis [3,7,9,10]. Among those species, only S. pneumoniae also encodes a GdpP homolog, whereas others encode only DhhP. Similar to GdpP, DhhP prefers Mn2+ and activity is optimal under alkaline conditions. However, DhhP is more reminiscent of NrnA for their promiscuous substrate specificity. Most characterized DhhP proteins degrade both c-di-AMP and 5’-pApA into AMP, as well as c-di-GMP and 5’-pGpG into GMP. Nevertheless, in M. smegmatis, the catalytic efficiency (kcat/kM) is approximately 200-fold higher for c-di-AMP than for c-di-GMP, indicating that c-di-AMP is the much preferred substrate [10]. Additionally, at least the M. tuberculosis homolog has also been characterized as an NrnA with exonuclease activity on short single stranded nucleic acids, as well as phosphatase activity on pAp [5].
Due to the broad taxonomic distribution and weak sequence conservation within the DHHA1 domain of DhhP proteins, it is difficult to predict homologs with c-di-AMP phosphodiesterase activity, and whether their activity plays a direct role in c-di-AMP hydrolysis within certain bacterial species. Indeed, DHH-DHHA1 family members are widely found in taxonomic groups that do not synthesize c-di-AMP, such as α-, β-, and γ-proteobacteria. Additionally, DhhP may control c-di-AMP levels indirectly, by hydrolyzing 5’-pApA. The related second messenger c-di-GMP is degraded into 5’-pGpG by EAL domain phosphodiesterases, and into GMP by HD-GYP phosphodiesterases. The loss of P. aeruginosa oligoribonuclease Orn, which is functionally homologous to NrnA/DhhP, results in elevation of c-di-GMP levels, which one might predict is a consequence of direct hydrolysis of the nucleotide [11,12]. However, Orn actually degrades 5’-pGpG, which feedback inhibits the c-di-GMP phosphodiesterase activity by EAL domain proteins [11,12]. Because DhhP proteins are capable of hydrolyzing 5’-pApA, it may only be important to complete c-di-AMP hydrolysis in some organisms. For instance, dhhP deletion in L. monocytogenes does not affect c-di-AMP levels but significantly increases 5’-pApA accumulation (Huynh and Woodward, unpublished data). Thus, in defining a role for DhhP proteins as direct c-di-AMP phosphodiesterases, studies must include thorough kinetic characterization of substrate specificity to further support in vivo changes in c-di-AMP levels mediated by such proteins. Finally, although DhhP is the only DHH-DHHA1 domain protein in M. smegmatis, the dhhP mutant exhibits c-di-AMP levels indistinguishable from WT, indicating the presence of at least another c-di-AMP phosphodiesterase with a different catalytic domain than DHH-DHHA1 [10].
2. THE HD-DOMAIN PHOSPHODIESTERASES
PgpH was identified in L. monocytogenes as a second class of c-di-AMP specific phosphodiesterase that acts via the catalytic HD domain [13]. PgpH is a member of the HD superfamily, named after the His-Asp motif that coordinate metal ions required for activity [14]. PgpH homologs, which comprise a seven transmembrane domain and a cytoplasmic HD domain, also belong to the 7TM-7TMR_HD family (Pfam PF07698) of bacterial receptors.
PgpH homologs are present in most c-di-AMP synthesizing phyla, except for Deferribacteres, Tenericutes, and Actinobacteria. Although the majority of PgpH-harboring species are Firmicutes, PgpH is absent in the Staphylococcaceae family. Interestingly, PgpH is also present in more than 90% of DisA_N containing Cyanobacteria, Bacteroidetes, Fusobacteria, and δ-Proteobacteria.
Similar to GdpP, PgpH also exhibits an almost exquisite specificity for c-di-AMP. Among various nucleotides tested, only 5’-pApA and c-di-GMP appear to bind at much lower affinity than c-di-AMP. In vitro, the PgpH HD domain hydrolyzes c-di-AMP into 5’-pApA, and has no activity on 5’-pApA. Although PgpH also weakly hydrolyzes c-di-GMP into 5’-pGpG, c-di-AMP is the much preferred substrate, as is the case with GdpP. When purified without supplemented metal, PgpH incorporates mostly Fe2+ and Mn2+ ions. Although the identity of the bound metals does not affect ligand binding, hydrolysis activity is dependent on Mn2+ ions. The HD active site is required for both c-di-AMP binding and catalytic activity [13].
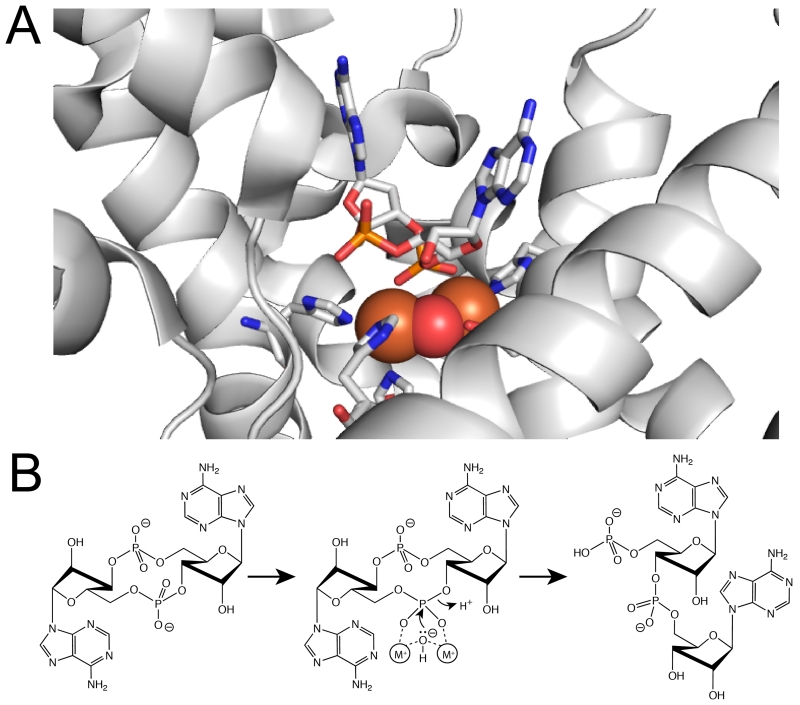
The crystal structure of the PgpH HD domain reveals both the c-di-AMP binding mode and catalytic mechanism ([13] and Fig. 3). In the active site, two metal ions are coordinated by several conserved His and Asp residues, including those of the HD motif. The c-di-AMP binding site is partially exposed. One nucleotide is coordinated by metal ions, as well as three Ala residues, and is solvent exposed. The other nucleotide is positioned in a pocket formed by Ile, Val, and Phe residues that would sterically clash with a guanine base, perhaps explaining c-di-AMP as the preferred substrate to c-di-GMP. The active site also harbors a bridging water molecule between two metal ions, and is positioned directly below the 5’-phosphate group of one nucleotide. Upon activation by metal ions, this nucleophilic water attacks the phosphorous atom, generating the 3’-hydroxyl of the other nucleotide as the leaving group. The side chain ammonium ion of a highly conserved Lys in the active site protonates the oxyanion to complete the reaction, generating 5’-pApA as the product, as shown in vitro (Fig. 3). Although the catalytic mechanism of GdpP and DhhP proteins are not defined, the presence of a bi-nuclear catalytic metal center suggests it might be similar to that of PgpH.
Figure 3.
A- The c-di-AMP binding pocket in the HD domain active site of PgpH. His and Asp residues coordinate two metal ions (orange spheres). The phosphate of c-di-AMP coordinates these metal ions and hydrolysis occurs via a nucleophilic water molecule (red sphere) that is bridging between the metal ions. B- HD-domain catalytic mechanism for the phosphodiesterase reaction. Activated hydroxide group from the bridging water attacks the scissile phosphorous atom, with a 3’-hydroxyl as the leaving group, generating the linear product 5’-pApA. DHH-DHHA1 domains likely exhibit bi-nuclear active sites with a similar catalytic mechanism.
3. SENSORY AND REGULATORY ROLES OF C-DI-AMP PHOSPHODIESTERASES
Environmental cues can be integrated into a signaling network by affecting activity of signal metabolizing proteins, thereby controlling downstream phenotypic output. In addition to the catalytic domains, many cyclic di-nucleotide phosphodiesterases also encompass sensory domains likely involved in coordinating environmental and intracellular signals. For L. monocytogenes, which harbors both GdpP and PgpH, these proteins appear to exhibit dominant activities within distinct environments. Whereas GdpP is mostly active in host cells, PgpH is mostly active in broth culture [13]. This observation suggests that these phosphodiesterases are responsive to distinct regulatory signals. The domain architectures of both GdpP and PgpH both encompass sensory components that could foreseeably facilitate these roles (Fig. 1).
For GdpP, the catalytic DHH-DHHA1 domain is associated with a PAS and GGDEF domain [2]. The PAS domain of B. subtilis and G. thermodenitrificans have been found to bind heme despite the lack of characteristic heme-binding amino acids [15]. Structural analysis suggests an unusual porphyrin-binding mode in which heme can be partially inserted in a hydrophobic pocket and further accommodated by local conformational changes near the dimer interface [16]. When reconstituted with heme in vitro, the holo-protein exhibits substantially lower catalytic efficiency than apo-protein, indicating that heme is inhibitory for DHH-DHHA1 catalytic activity. Furthermore, the ferrous heme iron also coordinates NO, which stimulates catalytic activity [15].
The regulation of DHH-DHHA1 catalytic activity suggests a role for GdpP in sensing heme and/or NO signals (Fig. 1). Both of these molecules are important during bacterial infection, as host-derived heme can serve as an important source of iron, and host cells generate NO via inducible nitric oxide synthase (iNOS) upon bacterial invasion. This would be consistent with the observation that the L. monocytogenes homolog is more active in host cells than in broth growth. In addition, the S. suis gdpP mutant has reduced hemolytic activity on erythrocytes [17], and the L. lactis gdpP mutant is highly sensitive to heme stress [16]. Beyond heme, PAS domains have been implicated as allosteric receptors of a broad array of small molecule signals. Therefore, it is likely that regulation of c-di-AMP hydrolysis by GdpP proteins may have diverged from heme to a variety of other stimuli commensurate with the environmental conditions met by a particular organism.
Although no regulatory signals have been identified for PgpH, this protein has seven transmembrane helices reminiscent of G protein-coupled receptors, which act as small molecule sensors in eukaryotes. Furthermore, many PgpH homologs have a large extracellular domain that presumably acts as a sensor for detecting environmental signals, with cell wall components as one possibility. Indeed, c-di-AMP has been linked to peptidoglycan properties and lipoteichoic acid (LTA) stress in S. aureus [18]. In L. monocytogenes, the disruption of LTA synthesis resulted in hyper-induction of type I IFN response, similar to the phenotype conferred by elevated c-di-AMP accumulation [19].
In addition, both GdpP and PgpH activities are inhibited by ppGpp (Fig. 1). For GdpP, ppGpp is a competitive inhibitor of c-di-AMP [2]. By contrast, for PgpH, ppGpp does not compete with c-di-AMP for active site binding, although hydrolysis activity is inhibited in a concentration-dependent manner. Since neither GdpP nor PgpH hydrolyzes ppGpp, this alarmone appears to be a regulatory molecule in c-di-AMP hydrolysis. Conversely, c-di-AMP also affects ppGpp levels, further indicating the reciprocal relationship between stringent response and c-di-AMP signaling. Surprisingly, both low and high c-di-AMP levels lead to an increase in ppGpp synthesis. The L. monocytogenes pgpH and S. aureus gdpP mutants, as well as the L. monocytogenes dacA mutant, all accumulate high ppGpp levels [8,20,21]. These contradictory observations could perhaps be explained by the critical role of c-di-AMP in central metabolism. Altered c-di-AMP levels cause a metabolic imbalance, possibly leading to an alert signal for stringent response [22].
Despite structural similarities between c-di-AMP and c-di-GMP, it is unclear whether there is cross-talk between these two signaling networks. Several bacterial species, with the examples of Bacillus, Clostridium, Listeria, Mycobacterium, and Streptomyces sp., produce both molecules [23]. At the molecular level, all characterized receptor proteins for c-di-AMP are specific for this nucleotide, with minimal binding to c-di-GMP [22,24]. Additionally, although c-di-AMP phosphodiesterases can degrade c-di-GMP in vitro, it does not appear to be a physiological substrate. Nevertheless, both signaling pathways may share some common input signals. For instance, many c-di-GMP phosphodiesterase enzymes are associated with PAS domains, which have also been shown to respond to heme and oxygen, just as shown for GdpP [25]. Furthermore, in L. monocytogenes, the accumulation of c-di-AMP and c-di-GMP both diminishes virulence, albeit through different mechanisms [13,26]. Interestingly, c-di-AMP accumulation also contributes to biofilm formation in S. mutans, reminiscent of c-di-GMP regulation [27]. Thus, further studies into possible interactions between c-di-AMP and c-di-GMP signaling networks will expand our knowledge in these pathways.
4. PHYSIOLOGICAL LESSONS LEARNED FROM C-DI-AMP PHOSPHODIESTERASE MUTANTS
Whereas early work focused on the essentiality of c-di-AMP, an increasing number of recent studies have reported the toxicity of c-di-AMP accumulation. The various phenotypes of phosphodiesterase mutants reflect the multi-faceted roles of c-di-AMP in bacterial physiology. These mutants exhibit moderate to severe growth defects, such as the case of B. burgdorferi [3]. As demonstrated for the dacA mutant, phosphodiesterase mutants also exhibit altered cell wall metabolism and cell division. The S. aureus and B. subtilis mutants are more resistant to cell wall antibiotics, partially due to increased peptidoglycan cross-linking [18,28]. Many species display aberrant cell morphology, such as altered cell length and chain formation, which can be partially rescued by magnesium supplementation [3,18,29]. Additionally, phosphodiesterase mutants are severely defective for osmotic stress tolerance, due to the inhibition of c-di-AMP on potassium transport [18,24,30]. All pathogenic species are diminished for virulence at high c-di-AMP levels, with hyper-induction of the host type I interferon response in some cases [3,7,13,17,31-33]. Interestingly, in Mycobacterium sp., for which c-di-AMP is not essential, high c-di-AMP levels readily alter bacterial physiology and greatly attenuate virulence [32]. In bacterial phosphodiesterase mutants, c-di-AMP protein targets are presumably in the ligand-bound state. Thus, a thorough investigation of their phenotypes is likely to provide mechanistic insight into the c-di-AMP interaction network.
For c-di-GMP signaling, 5’-pGpG has been shown to be involved in biofilm formation and the orn mutant produces more biofilm, reminiscent of the S. aureus and S. suis gdpP mutants [17,18]. Although no physiological roles have been documented for 5’-pApA, the observation that some c-di-AMP phosphodiesterases only hydrolyze the nucleotide to 5’-pApA and not fully to AMP may reflect a specific function for this nucleotide. Thus, upon c-di-AMP accumulation, the degradation product 5’-pApA may also increase in levels, especially in the absence of efficient DhhP/NrnA activity. Similarly, under c-di-AMP deplete conditions, 5’-pApA levels may significantly drop. Are the physiological defects of phosphodiesterase mutants a consequence of c-di-AMP elevation or are they attributable to elevated 5’-pApA? Along this same reasoning, is c-di-AMP actually essential or is it the loss of the breakdown product 5’-pApA? To date, we are unaware of any studies to directly discriminate between the effects of altered cdi-AMP and 5’-pApA. A detailed characterization of 5’-pApA binding proteins through proteomic or ORFeome screening methods may ultimately resolve these questions.
5. POSSIBLE ROLES FOR C-DI-AMP SECRETION
L. monocytogenes secretes c-di-AMP through the multidrug efflux pumps MdrM, MdrT, MdrA, and MdrC (MDR’s), of the MFS superfamily [34]. During broth growth, the roles of secreted c-di-AMP remain unclear, though extracellular c-di-AMP enhances vancomycin resistance, suggesting a putative role for extracellular c-di-AMP in bacterial stress responses [35]. During growth in the host cytosol, secreted c-di-AMP induces a host type I interferon transcriptional response [34]. Why might a pathogen secrete an inflammatory molecule at the risk of immune detection? We hypothesize that coupling c-di-AMP secretion to MDR activity provides a phosphodiesterase-independent mechanism to reduce nucleotide levels in the bacterial cytoplasm in the face of a variety of environmental stresses. In addition to c-di-AMP secretion, MdrT, and to a lesser extent, MrdM, are also efflux pumps for bile acids [36]. Bile acids cause de-repression of mdrT and mdrM expression through the transcription factor BrtA, resulting in increased transporter expression and bile efflux, likely concurrent with c-di-AMP secretion. Since bile-induced transcriptomes significantly overlap with those of the major virulence regulator PrfA and stress sigma factor σB, c-di-AMP may also serve as an intracellular stress reporter, and c-di-AMP secretion is perhaps coupled with certain stress responses. Although c-di-AMP secretion has been best characterized in L. monocytogenes, MFS transporters are widely conserved among bacteria and the capacity to secrete c-di-AMP is likely to extend well beyond this one organism. For instance, among c-di-AMP synthesizing species, MdrT homologs are present in Bacillus, Clostridium, Streptococcus, Enterococcus, Lactococcus, and Mycobacterium sp. Furthermore, c-di-AMP may also be secreted through some other secretion systems. Indeed, recent evidence suggests that both M. tuberculosis and C. trachomatis may secrete c-di-AMP during infection [33,37]. Further studies in other species will be valuable in understanding the role of c-di-AMP export as a mechanism of regulating signal transduction to affect bacterial responses in the face of environmental stress.
CONCLUSIONS
Like other second messengers c-di-AMP levels must be controlled to prevent toxic accumulation. Removal of c-di-AMP is achieved by hydrolytic activity of specific phosphodiesterases, and perhaps also by secretory activities of MDR’s. These depletion strategies appear to be regulated by environmental and intracellular signals, and integrated with other stress response pathways. These observations, together with the various phenotypes of phosphodiesterase mutants, open many questions for future studies. Because the role of DhhP in c-di-AMP hydrolysis is uncertain in some species, there may still be other unidentified families of c-di-AMP phosphodiesterases. It is also intriguing that, although c-di-AMP is widespread, many of the identified protein-binding partners are not of a single domain family and the conservation of receptors appears to be relatively poor even among related organisms of the same phylum. This distribution may reflect unique physiological roles of the nucleotide among the many c-di-AMP producing organisms. Furthermore, many questions remain to be unveiled in the context of cross-talk with other signaling pathways, such as those mediated by c-di-GMP and ppGpp, which share certain similarities or common physiological phenotypes to that of c-di-AMP. Fundamental studies of c-di-AMP signaling have also revealed significant therapeutic promise. The decreased virulence associated with elevated c-di-AMP suggests that regulation of hydrolysis or secretion may hold promise for therapeutic intervention of bacterial infections. Furthermore, the potent immune-stimulatory activity of c-di-AMP and other cyclic di-nucleotides show promise as vaccine adjuvants for the prevention infectious and malignant diseases. Since the discovery of c-di-AMP seven years ago, it is certainly clear that tremendous progress has been made in our understanding of this novel second messenger. Nevertheless, the biological effects and molecular mechanisms of action pertaining to this pleitropic mediator of bacterial physiology, pathogenesis, and host immunity are far from complete and many exciting times surely await this blossoming field.
HIGHLIGHTS.
- C-di-AMP is an essential second messenger but its accumulation is toxic to bacterial physiology and pathogenesis
- Two mechanisms for c-di-AMP hydrolysis that have been reported, and secretion of the nucleotide is another likely mechanism for controlling c-di-AMP levels.
- GdpP and DhhP degrade c-di-AMP by the catalytic DHH-DHHA1 domain and contains a PAS domain, which binds small signal molecules such as heme and/or nitric oxide.
- PgpH degrades c-di-AMP by the catalytic HD domain and contains an extracellular domain, as well as seven transmembrane helices, likely for signal detection.
- Multidrug resistance transporters secrete c-di-AMP from the cytoplasm. C-di-AMP secretion may be coupled with stress responses.
ACKNOWLEDGEMENTS
Research in the Woodward laboratory is funded by NIH Grants AI116669 and AI108698, the Life Sciences Discovery Fund 11875805, the University of Washington Royalty Research Foundation, and a Biomedical Scholarship from the Pew Charitable Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Corrigan RM, Grundling A. Cyclic di-AMP: another second messenger enters the fray. Nat Rev Microbiol. 2013;11:513–524. doi: 10.1038/nrmicro3069. [DOI] [PubMed] [Google Scholar]
- 2.Rao F, See RY, Zhang D, Toh DC, Ji Q, Liang ZX. YybT is a signaling protein that contains a cyclic dinucleotide phosphodiesterase domain and a GGDEF domain with ATPase activity. J Biol Chem. 2010;285:473–482. doi: 10.1074/jbc.M109.040238.** Of outstanding interest: The first and most extensive biochemical analysis of GdpP phosphodiesterase
- 3.Ye M, Zhang JJ, Fang X, Lawlis GB, Troxell B, Zhou Y, Gomelsky M, Lou Y, Yang XF. DhhP, a c-di-AMP phosphodiesterase of Borrelia burgdorferi, is essential for cell growth and virulence. Infect Immun. 2014 doi: 10.1128/IAI.00030-14.* Of special interest: A demonstration that toxic c-di-AMP levels prevent bacterial growth and confer pleotropic phenotypes
- 4.Uemura Y, Nakagawa N, Wakamatsu T, Kim K, Montelione GT, Hunt JF, Kuramitsu S, Masui R. Crystal structure of the ligand-binding form of nanoRNase from Bacteroides fragilis, a member of the DHH/DHHA1 phosphoesterase family of proteins. FEBS Lett. 2013;587:2669–2674. doi: 10.1016/j.febslet.2013.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivastav R, Kumar D, Grover A, Singh A, Manjasetty BA, Sharma R, Taneja B. Unique subunit packing in mycobacterial nanoRNase leads to alternate substrate recognitions in DHH phosphodiesterases. Nucleic Acids Res. 2014;42:7894–7910. doi: 10.1093/nar/gku425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witte CE, Whiteley AT, Burke TP, Sauer JD, Portnoy DA, Woodward JJ. Cyclic di-AMP is critical for Listeria monocytogenes growth, cell wall homeostasis, and establishment of infection. MBio. 2013;4 doi: 10.1128/mBio.00282-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai Y, Yang J, Eisele LE, Underwood AJ, Koestler BJ, Waters CM, Metzger DW, Bai G. Two DHH subfamily 1 proteins in Streptococcus pneumoniae possess cyclic di-AMP phosphodiesterase activity and affect bacterial growth and virulence. J Bacteriol. 2013;195:5123–5132. doi: 10.1128/JB.00769-13.*Of special interest: First report of a DhhP-type phosphodiesterase
- 8.Corrigan RM, Bowman L, Willis AR, Kaever V, Grundling A. Cross-talk between two nucleotide-signaling pathways in Staphylococcus aureus. J Biol Chem. 2015;290:5826–5839. doi: 10.1074/jbc.M114.598300.*Of special interest: A report on the connection between c-di-AMP signaling and stringent response, as demonstrated in the case of c-di-AMP accumulation
- 9.Manikandan K, Sabareesh V, Singh N, Saigal K, Mechold U, Sinha KM. Two-Step Synthesis and Hydrolysis of Cyclic di-AMP in Mycobacterium tuberculosis. PLoS One. 2014;9:e86096. doi: 10.1371/journal.pone.0086096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Q, Luo Y, Zheng C, Yin K, Ali MK, Li X, He J. Functional analysis of a c-di-AMP-specific phosphodiesterase MsPDE from Mycobacterium smegmatis. Int J Biol Sci. 2015;11:813–824. doi: 10.7150/ijbs.11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen D, Mechold U, Nevenzal H, Yarmiyhu Y, Randall TE, Bay DC, Rich JD, Parsek MR, Kaever V, Harrison JJ, et al. Oligoribonuclease is a central feature of cyclic diguanylate signaling in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2015;112:11359–11364. doi: 10.1073/pnas.1421450112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orr MW, Donaldson GP, Severin GB, Wang J, Sintim HO, Waters CM, Lee VT. Oligoribonuclease is the primary degradative enzyme for pGpG in Pseudomonas aeruginosa that is required for cyclic-di-GMP turnover. Proc Natl Acad Sci U S A. 2015;112:E5048–5057. doi: 10.1073/pnas.1507245112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huynh TN, Luo S, Pensinger D, Sauer JD, Tong L, Woodward JJ. An HD-domain phosphodiesterase mediates cooperative hydrolysis of c-di-AMP to affect bacterial growth and virulence. Proc Natl Acad Sci U S A. 2015;112:E747–756. doi: 10.1073/pnas.1416485112.**Of outstanding interest: The first identification of PgpH, with extensive biochemical, structural, and genetic analysis of this phosphodiesterase
- 14.Aravind L, Koonin EV. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci. 1998;23:469–472. doi: 10.1016/s0968-0004(98)01293-6. [DOI] [PubMed] [Google Scholar]
- 15.Rao F, Ji Q, Soehano I, Liang ZX. Unusual heme-binding PAS domain from YybT family proteins. J Bacteriol. 2011;193:1543–1551. doi: 10.1128/JB.01364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan E, Rao F, Pasunooti S, Pham TH, Soehano I, Turner MS, Liew CW, Lescar J, Pervushin K, Liang ZX. Solution structure of the PAS domain of a thermophilic YybT protein homolog reveals a potential ligand-binding site. J Biol Chem. 2013;288:11949–11959. doi: 10.1074/jbc.M112.437764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du B, Ji W, An H, Shi Y, Huang Q, Cheng Y, Fu Q, Wang H, Yan Y, Sun J. Functional analysis of c-di-AMP phosphodiesterase, GdpP, in Streptococcus suis serotype 2. Microbiol Res. 2014;169:749–758. doi: 10.1016/j.micres.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Grundling A. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 2011;7:e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tadmor K, Pozniak Y, Burg Golani T, Lobel L, Brenner M, Sigal N, Herskovits AA. Listeria monocytogenes MDR transporters are involved in LTA synthesis and triggering of innate immunity during infection. Front Cell Infect Microbiol. 2014;4:16. doi: 10.3389/fcimb.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Bayles DO, Mason TM, Wilkinson BJ. A cold-sensitive Listeria monocytogenes mutant has a transposon insertion in a gene encoding a putative membrane protein and shows altered (p)ppGpp levels. Appl Environ Microbiol. 2006;72:3955–3959. doi: 10.1128/AEM.02607-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whiteley AT, Pollock AJ, Portnoy DA. The PAMP c-di-AMP is essential for Listeria monocytogenes growth in tich but not minimal media due to a toxic Increase in (p)ppGpp. Cell Host Microbe. 2015;17:788–798. doi: 10.1016/j.chom.2015.05.006.*Of special interest: A report on the connection between c-di-AMP signaling and stringent response, as demonstrated in the case of c-di-AMP depletion
- 22.Sureka K, Choi PH, Precit M, Delince M, Pensinger DA, Huynh TN, Jurado AR, Goo YA, Sadilek M, Iavarone AT, et al. The cyclic dinucleotide c-di-AMP is an allosteric regulator of metabolic enzyme function. Cell. 2014;158:1389–1401. doi: 10.1016/j.cell.2014.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corrigan RM, Campeotto I, Jeganathan T, Roelofs KG, Lee VT, Grundling A. Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proc Natl Acad Sci U S A. 2013;110:9084–9089. doi: 10.1073/pnas.1300595110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang AL, Tuckerman JR, Gonzalez G, Mayer R, Weinhouse H, Volman G, Amikam D, Benziman M, Gilles-Gonzalez MA. Phosphodiesterase A1, a regulator of cellulose synthesis in Acetobacter xylinum, is a heme-based sensor. Biochemistry. 2001;40:3420–3426. doi: 10.1021/bi0100236. [DOI] [PubMed] [Google Scholar]
- 26.Chen LH, Koseoglu VK, Guvener ZT, Myers-Morales T, Reed JM, D’Orazio SE, Miller KW, Gomelsky M. Cyclic di-GMP-dependent signaling pathways in the pathogenic Firmicute Listeria monocytogenes. PLoS Pathog. 2014;10:e1004301. doi: 10.1371/journal.ppat.1004301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng X, Zhang Y, Bai G, Zhou X, Wu H. Cyclic di-AMP mediates biofilm formation. Mol Microbiol. 2015 doi: 10.1111/mmi.13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo Y, Helmann JD. Analysis of the role of Bacillus subtilis sigma(M) in beta-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol Microbiol. 2012;83:623–639. doi: 10.1111/j.1365-2958.2011.07953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehne FM, Gunka K, Eilers H, Herzberg C, Kaever V, Stulke J. Cyclic di-AMP homeostasis in Bacillus subtilis: both lack and high level accumulation of the nucleotide are detrimental for cell growth. J Biol Chem. 2013;288:2004–2017. doi: 10.1074/jbc.M112.395491.* Of special interest: The first report to show that high c-di-AMP levels have negative impacts upon bacterial physiology.
- 30.Smith WM, Pham TH, Lei L, Dou J, Soomro AH, Beatson SA, Dykes GA, Turner MS. Heat resistance and salt hypersensitivity in Lactococcus lactis due to spontaneous mutation of llmg_1816 (gdpP) induced by high-temperature growth. Appl Environ Microbiol. 2012;78:7753–7759. doi: 10.1128/AEM.02316-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho KH, Kang SO. Streptococcus pyogenes c-di-AMP phosphodiesterase, GdpP, influences SpeB processing and virulence. PLoS One. 2013;8:e69425. doi: 10.1371/journal.pone.0069425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Bai Y, Zhang Y, Gabrielle VD, Jin L, Bai G. Deletion of the cyclic di-AMP phosphodiesterase gene (cnpB) in Mycobacterium tuberculosis leads to reduced virulence in a mouse model of infection. Mol Microbiol. 2014;93:65–79. doi: 10.1111/mmi.12641.*Of special interest: A demonstration that high c-di-AMP levels attenuate M. tuberculosis virulence, with some evidence for c-di-AMP secretion by this bacterium
- 33.Dey B, Dey RJ, Cheung LS, Pokkali S, Guo H, Lee JH, Bishai WR. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat Med. 2015;21:401–406. doi: 10.1038/nm.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan Zeevi M, Shafir NS, Shaham S, Friedman S, Sigal N, Nir Paz R, Boneca IG, Herskovits AA. Listeria monocytogenes multidrug resistance transporters and cyclic di-AMP, which contribute to type I interferon induction, play a role in cell wall stress. J Bacteriol. 2013;195:5250–5261. doi: 10.1128/JB.00794-13.*Of special interest: The first paper to systematically characterize MDRs responsible for c-di-AMP secretion and the first to implicate extracellular c-di-AMP function.
- 36.Quillin SJ, Schwartz KT, Leber JH. The novel Listeria monocytogenes bile sensor BrtA controls expression of the cholic acid efflux pump MdrT. Mol Microbiol. 2011;81:129–142. doi: 10.1111/j.1365-2958.2011.07683.x. [DOI] [PubMed] [Google Scholar]
- 37.Barker JR, Koestler BJ, Carpenter VK, Burdette DL, Waters CM, Vance RE, Valdivia RH. STING-dependent recognition of cyclic di-AMP mediates type I interferon responses during Chlamydia trachomatis infection. MBio. 2013;4:e00018–00013. doi: 10.1128/mBio.00018-13. [DOI] [PMC free article] [PubMed] [Google Scholar]