Microfoam Sclerotherapy for Varicose Veins: a Retrospective Analysis of a Modified Technique (original) (raw)
Abstract
Varicose veins (VVs) are generally characterized by their elongated, twisted, bulging, superficial appearance on the lower extremities and usually present with acute or chronic venous diseases. Despite diagnostic and surgical advances in the management of VV, patients suffer from post-therapeutic complications and recurrence. We present findings from a retrospective study of a modified treatment modality in patients with varicose veins who attended St. Thomas Institute of Research on Venous Diseases, Changanassery, Kerala. The hospital caters to patients from India and outside. Out of 14,707 patients treated from 1997 till May 2013, 6,350 patients from January to March 2011 were selected for the study from the routine clinical practice (mid-segment) to facilitate follow-up. They were categorized according to Clinical Etiologic Anatomic Pathophysiologic (CEAP) clinical classification system. Baseline data were accrued using a questionnaire. Patients were treated by microfoam sclerotherapy giving a maximum importance to smaller veins, depicting a modified technique of microfoam sclerotherapy (MMFST). This is based on the significance of microscopic venous valves (MVVs) in the development of chronic venous disease (CVD). Follow-up was according to a predefined schedule, and improvements and complications were recorded. A positive family history of VVs was reported in 85.23 % of patients. Half the study population belonged to CEAP clinical class IV. There were no significant complications in patients throughout the 2 to 6 years of follow-up. Recurrence was rarely reported during follow-up and was corrected by repeating the procedure as required during follow-up. MMFST is an innovation in the treatment of VVs based on new principles, with the potential to control and revert the symptoms of CVD, with minimal complications.
Keywords: Chemical bombing, Complications, Microfoam, Modified sclerotherapy, Recurrence, Varicose veins, Microscopic venous valves
Introduction
The history of venous surgery starts from 3500 BC, when the Egyptians described the disease as “serpentine windings” on the leg. The commendation about the treatment for varicose vein (VV) was that “there is no medical treatment; if anyone attempts to do any surgery, he will make the patient’s head fall down”. Afterwards, in 430 BC, it was Hippocrates who by seeing the beauty of veins resembling grapes described the disease as VV. According to him, no medical or surgical treatment could do any good for the patient, but probably, applying a searing iron on the vein might cure the disease. Various treatment modalities were tried in subsequent centuries, but the disease remains an unsolved enigma. Trendelenburg’s operation (1882) and the stripping of vein are still the gold standard [1, 2]. The crude method of stripping has been replaced by various newer modifications like laser, radio frequency, or sclerotherapy. The latter got evolved without changing the basic principle of surgical intervention, which is almost two centuries old. None of the current techniques bring forth successful outcomes for the patients with latter stages of chronic venous disease (CVD), except ablation of large veins. The treatment of tiny veins by use of laser or sclerotherapy is mainly considered for cosmetic purpose.
Against this background, with an experience of successfully treating more than 14,707 patients using sclerotherapy, a modification in microfoam sclerotherapy (MFST) based on a newer outlook and principle was tried from 2007. A midstream of 6,350 patients from January 2007 to March 2011 was analyzed in detail for evaluation of effectiveness of the method. These patients were followed up till May 2013 at St. Thomas Institute of Research on Venous Disease, Changanassery, Kerala. The technique is referred to as modified MFST or “chemical bombing.”
Method
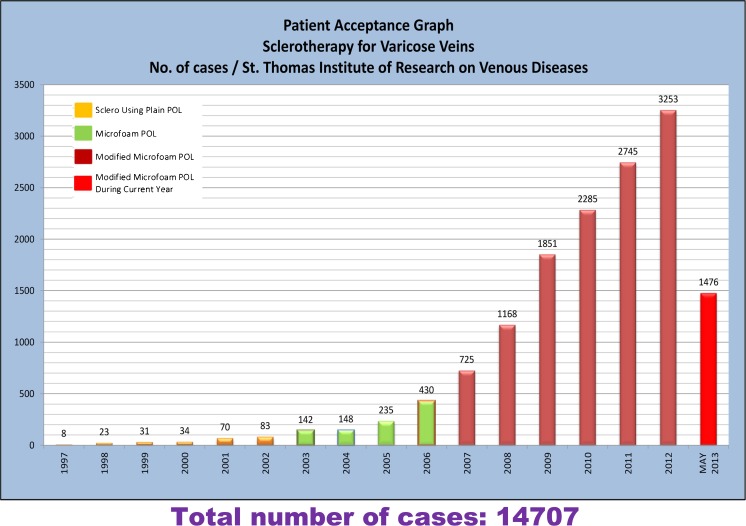
Patients aged between 15 and 90 years presenting with symptoms of VV at the St. Thomas Institute of Research on Venous Disease, Changanassery, Kerala, between January 2007 and March 2011 were included. Patients from India and outside are routinely referred to our hospital. This is a specialized center that has catered 14,707 patients till May 2013 (Fig. 1). The study was approved by the institutional human ethics committee.
Fig. 1.
Patient acceptance graph showing the number of varicose vein patients treated till date
Inclusion Criteria
Treatment naïve as well as patients with recurrent VV belonging to clinical etiology anatomy pathology (CEAP) class I to VI [3] were included in the study. Data for demographics, occupation, and lifestyle were collected from every patient using a predefined format. Written informed consent was obtained from all subjects. The types of veins involved and related clinical signs and complications were recorded. A specific, bespoke questionnaire in English was used to accrue the information. Patients were clinically examined as per CEAP classification and assessed by a single trained surgeon prior to and after treatment, for follow-up.
Diagnosis
Color duplex ultrasound scans (Doppler Machine model: Philips HD15 Pure Wave Ultrasound System and Philips Envisor, with a high frequency linear probe of 3–12 MHz, wide band) were performed by experienced ultrasonologists in all cases with repeated assessments for reflux in both supine and erect positions. The duplex was performed only prior to the procedure to ascertain the patency of deep veins and to distinguish between primary and secondary VV.
Classification of Clinical Manifestations of CVD
Clinical manifestations of CVD were classified according to CEAP clinical classification [3]. The anatomical distribution and the type of VV on the leg were determined as per the following criteria: T1, tributaries [T1/A minor tributaries (<1 mm), T1/B major tributaries (1 to <3 mm)]; T2, (trunkal A, early; B, fully developed).
Procedure
Preparation
Before sclerotherapy, all general and systemic investigations, including HBsAg, HIV, VDRL, HbA1C, ECG, and color Doppler study of the venous system of lower limb up to the level of umbilicus, were routinely done. The proposed leg was washed with soap and then shaved and cleaned from the inguinal region to the foot.
Materials and Technique
A set of four 5-ml plastic syringes, a three-way connector, a set of 26-G hypodermic needles, water for injection, and 2 ml × two ampoules of polidocanol (POL) (30 mg per ml) were used. Distilled water (3 ml) was added to 0.2 ml Asklerol™ (6 mg of POL). This mixture was agitated with 2 ml of air to prepare microfoam by Tessari’s technique [4]. Hence, the concentration of the prepared sclerosant was 0.2 % (2 mg/ml). The maximum quantity of POL that was used for a patient was 120 mg. The volume of sclerosant administered to each patient varied as per the discretion of the surgeon. The maximum dose of POL administered in our technique was 120 mg, which is lower than the conventional 140 to 180 mg (2 mg/kg for a 70–80-kg patient) [5]. Moreover, the maximum concentration of POL used in the present technique was 0.2 %, which is below the conventional concentration (0.5 to 1 %). This concentration gives an excellent sclerosant effect even for the largest veins.
Our view of increasing dilution is supported in the technology report of the American Society of Dermatologic Surgery: “The advantage of foam is that the sclerosing power of the solution is increased to 2-fold to 3-fold, while decreasing the toxicity four fold” (quote [6]). In addition, the use of lower quantity of drug with higher dilution not only reduces the complications but also helps to sclerose larger areas of vein distribution [7].
Patients undergoing the modified MFST do not require anesthesia. The procedure is carried out in a well-equipped theater with monitoring facility, as apprehension and slight pain may cause neurogenic shock.
Patient is made to sit on the operating table with legs hanging down freely. The leg is cleaned with surgical spirit, and the foot is placed on a sterile towel kept on the lap of the surgeon. The surgeon reviews the size and distribution of veins over the foot up to the upper thigh, which enables the surgeon to decide the areas for the administration of the sclerosant. There is no necessity to change the dilution depending on the size of the vein.
The conventional technique of sclerotherapy [5] was modified. After the sclerosant is administered, the sclerotherapy is performed mainly into the smallest tributaries, from the lowermost part of the lower limb from the base of the toes, ankle, and the lower part of the leg and worked up towards larger veins higher up. During the procedure, administration of the sclerosant very close to the perforators and saphenofemoral/saphenopopliteal (SF/SP) junctions is avoided. The sclerosant was infused slowly into the tributaries of <3 mm, but in larger veins, the injection was forceful to create further foaming with blood inside the vessel.
If large veins are involved in the thigh, the infusion is initiated there, in order to occlude the vein. This will help to prevent sclerosant escaping into the deep vein and avoid the risk of deep vein thrombosis (DVT). After withdrawal of needle from the vein, the punctured site is kept pressed with gauze. The retrograde flow of sclerosant is facilitated by compressing the vein with a finger kept proximally. Retrograde massaging helps the sclerosant escape into smallest tributaries. Once the procedure is completed, a compression bandage is applied starting from the foot, avoiding the toes, towards the thigh to help the veins remain collapsed and stuck together. The bandage need not be applied for more than 3 or 4 weeks. The foot is kept raised above the level of the umbilicus, throughout day time, with the knee fully extended during the post-procedural period, to facilitate gravitational flow, particularly if the patient is having swelling of lower leg and foot. The extent of hardening of the veins is noted during the review as this is indicative of successful sclerotherapy.
There is absolutely no fear of air embolism with this procedure. To be fatal, 100 ml/s of air has to be injected [8]. In this procedure, <50 ml of air is injected in 10 min.
Follow-up
Since the basic pathology of VV is the same for all classes, a uniform follow-up schedule was worked out for review.
- Monthly review × 3 months
- Quarterly × 1 year
- Half yearly × 2 years
- Once a year × 2/3 years
The immediate post-procedural sequel such as intravenous thrombosis and thrombophlebitis in sclerosed veins may produce some amount of pain, induration, and discomfort. Skipped veins, recanalization, or neovascularization were identified and treated preferably after 3 months. This helps to evaluate improvement after therapy. Improvement was assessed by progress in healing of wound, return of normal color of skin, absence of edema, and symptomatic relief of discomfort in the leg.
Statistical Analysis
Information from answered questionnaires and medical records were entered into Stata 11 software package, Stata Corp., USA. Continuous variable differences were analyzed using Student’s t test, and categorical data were analyzed using chi-square test. Fisher’s exact test was used when data were below a minimum frequency or when the value was nil.
Results
A total of 6,350 patients with VV and CVD were evaluated in this study. There were more female patients than males (2,930 men and 3,420 women). Majority of the patients were between 30 and 60 years of age (mean ± SD, 49.4 ± 13 years). The usual ratio of men/women in clinical practice is 1:2, but we found this to be around 45:55 in our study.
The frequency of disease symptoms and clinical manifestations in all patients are mentioned in Tables 1 and 2, respectively.
Table 1.
Presenting complaints as per legs in patients with chronic venous diseases
| Patient details | Male = 2,930 | Female = 3,420 | Total = 6,350 | P value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Right/left leg involvement | 856 (29.23) | 958 (28.00) | 1,814 (28.57) | |
| Both legs involvement | 2,072 (70.77) | 2,462 (72.00) | 4,536 (71.43) | 0.276 |
| Common complaints | ||||
| Pain | 2,023 (69.09) | 2,476 (72.36) | 4,499 (70.85) | 0.004 |
| Hemorrhage | 330 (11.27) | 367 (10.72) | 697 (10.98) | 0.488 |
| Thrombophlebitis | 35 (1.20) | 49 (1.43) | 84 (1.32) | 0.411 |
| Cellulitis | 183 (6.25) | 260 (7.60) | 443 (6.98) | 0.036 |
| Lower limb edema | 1,128 (38.52) | 1,371 (40.06) | 2,499 (39.35) | 0.211 |
| Itching | 1,200 (40.98) | 1,462 (42.72) | 2,662 (41.92) | 0.161 |
| Pigmentation | 2,054 (70.15) | 2,406 (70.31) | 4,460 (70.24) | 0.89 |
| Ulceration | 895 (30.57) | 940 (27.47) | 1,835 (28.90) | 0.007 |
Table 2.
Patients with chronic venous diseases classified into different (CEAP) clinical classifications based on age
| Total patients’ class | Left leg | Right leg | ||||||
|---|---|---|---|---|---|---|---|---|
| <40 (%) | 40–59 (%) | 60+ (%) | Total (%) | <40 (%) | 40–59 (%) | 60+ (%) | Total (%) | |
| 0 | 0.08 | 0.00 | 0.00 | 0.02 | 0.08 | 0.09 | 0.07 | 0.09 |
| 1 | 22.58 | 16.34 | 14.36 | 17.28 | 20.76 | 15.46 | 15.08 | 16.55 |
| 2 | 3.53 | 2.68 | 2.12 | 2.74 | 4.48 | 2.87 | 2.00 | 3.02 |
| 3 | 50.61 | 51.64 | 53.99 | 51.97 | 53.32 | 55.74 | 55.58 | 55.16 |
| 4 | 15.13 | 18.54 | 19.34 | 17.97 | 14.66 | 16.44 | 18.40 | 16.50 |
| 5 | 7.83 | 10.76 | 10.18 | 9.97 | 6.71 | 9.34 | 8.87 | 8.64 |
| 6 | 0.23 | 0.03 | 0.00 | 0.07 | 0.00 | 0.06 | 0.00 | 0.03 |
The patients presented various clinical symptoms of VV. Pain and pigmentation was reported by 26 % of patients, while itching and edema were evident in 15 and 14 % of patients, respectively. Bleeding, thrombophlebitis, and cellulitis were reported by 4, 1, and 2 % of patients, respectively.
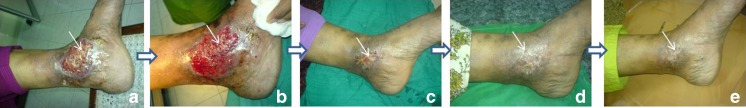
The patients were followed up during personal review or over phone, as per the predefined schedule to evaluate the effectiveness of sclerosis. Out of 6,350 patients treated, 5,397 were reviewed in post-procedural follow-up. Majority of patients (63 %) did not present any discernable adverse reactions and maintained an asymptomatic status throughout the follow-up period. Recurrence was observed in 12 % of patients. Pigmentation along the veins and pain was reported by 8 and 7 % of patients, respectively. Symptoms like bleb formation, pigmentation, and itching over the sclerosed vein were reported in 2 and 8 % of patients. Itching alone was reported in 4 % of patients, while 2 % of patients reported bleb formation and cellulitis. Minor symptoms like thrombophlebitis and retention were observed in 1 % of patients. No serious complications were observed or reported in any patient throughout the follow-up period. Minor complications like blister formation at injection site was observed, which was treated. Gradual disappearance of the VV and its associated lesions were visually evident to the treating surgeon, indicating a clinical efficacy of the technique. Figure 2a represents the limb-bearing conspicuous lesions before sclerotherapy. Figure 2b shows the limb just after modified MFST. Figure 2c–e represents gradual amelioration of lesions on subsequent days following sclerotherapy. Staining and itching over the line of vein were occasional findings. Local hirsutism was not uncommon. Allergic or anaphylactic reaction to POL was not detected in any of the patients. Skin test showed pseudoreaction and was ignored.
Fig. 2.
a–e Pre- and post-procedural images of varicose veins representing gradual healing of the lesions. a The limb bearing conspicuous lesions before the sclerotherapy. b The limb just after sclerotherapy. c–e Gradual amelioration of the lesions on subsequent days following sclerotherapy. The affected area has been indicated by an arrow
Discussion
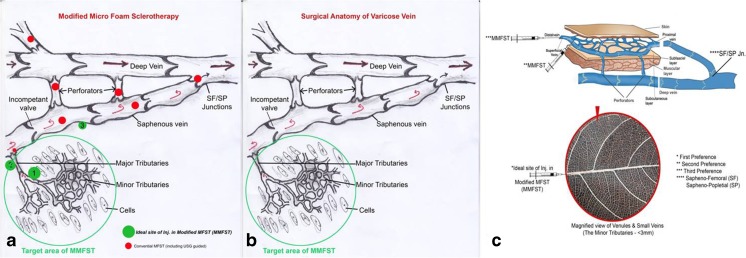
Our technique of infusion of sclerosant into the small networking tributaries is based on an entirely different concept based on microscopic venous valves (MVVs). All recent reports support our view [9, 10]. The conventional principle of sclerotherapy involves slow and steady injection of sclerosant into the large veins near the junctions and perforators and into the tributaries [7] (Fig. 3a). Our technique was a modified sclerotherapy. This modification has distinct advantages over the conventional technique (Fig. 3b, c).
Fig. 3.
a Surgical anatomy of varicose veins, b modified microfoam sclerotherapy, and c the preference hierarchy of the veins for the modified microfoam sclerotherapy
Firstly, the risk of DVT is minimized as the tributaries, and SF/SP junctions are avoided. The blood cannot flow from a low-pressure gradient deep vein to a higher pressure gradient, in the superficial vein owing to physical laws. Hence, it was not logical to ablate the saphenofemoral or saphenopopliteal junction.
Secondly, there is significantly lower probability of an embolus transported to a deep vein like femoral or popliteal vein. The thrombus formed in the smaller veins and venules has a lesser chance of transforming to a mobile embolus, as the flow rate of blood in the tributaries is sluggish. The injection of the sclerosant into the small networking tributaries could be termed as chemical bombing or modified MFST, which initiates a localized chemical inflammation, culminating in hardening of the veins. This phenomenon assures a minimal risk of venous complications as the junctions (SF/SP and perforators) are spared.
Thirdly, it ensures the restoration of hemodynamic flow of blood in the smaller veins. Microscopic venous valves have been reported to exist even in veins and venules located in human digits [9]. They have been associated with two physiological roles. MVVs prevent blood reflux in small sized veins and restrict flow from postcapillary venules back into the capillary bed [9] and prevent the reflux and skin changes due to the progression of venous insufficiency [10]. It has been reported that failure of MVV in small superficial veins is a key to the skin changes of venous insufficiency [10]. The MVVs are tissue protectant valves, and the other valves in the larger veins are unidirectional. MVVs are arranged in a very close proximity towards the lowermost part of the body when compared to the face, where their number and proximity get reduced. The unidirectional valves control the onward flow, and the number gets reduced as the size of vein gets increased and in close proximity to the heart. There are no valves above the level of umbilicus. Abnormalities in the venous endothelium and smooth muscle cells lead to vein wall dilatation with secondary valvular incompetence [11]. These MVVs counteract venous hypertension caused by valvular failure of larger veins. At the microcirculatory level, they prevent reflux from postcapillary venules into the capillary bed and into arteriovenous anastomosis (AVAs) [8]. It could be hypothesized that the clinical efficacy of our technique, involving the infusion of sclerosant into smaller veins (diameter of <3 mm), may be attributed to the presence of MVVs in the microcirculatory bed constituting the venous distribution system of legs. Moreover, the concentration of the sclerosant can be reduced remarkably (to 0.2 %), larger area can be covered in one sitting, and the procedure ensures optimal safety. This modified sclerotherapy involving infusion of sclerosant into the miniscule superficial veins for CVD is a de novo surgical innovation, which has not been published elsewhere in the world.
Another distinct advantage of the technique is the minimal concentration and volume of the sclerosant used. High concentration and volume of sclerosant have been associated with an increased risk of side effects [12]. The modified sclerotherapeutic technique can be performed successfully at a low dose (up to 120 mg) and volume of sclerosant (up to 0.2 %), ensuring a better safety profile as compared to the conventional technique.
It was observed that during post-procedural period, the patients had no significant complications such as DVT and other comorbid vascular disorders. Minimal recurrence was observed in those patients who had a very large number of small veins and in whom the dosage limit did not permit sclerosing all veins. The underlying reason could be neovascularization, neocanalization, or skipping.
Conclusion
The modification of MFST is a potential innovation to ameliorate clinical symptoms of VV without significant complications. It has very low incidences of recurrence, which can be completely brought under control if properly followed up by repeated sclerotherapy. The procedure is more physiological, and the success of the procedure is attributed to the distal location of the sclerotherapeutic intervention, avoiding the major junctions and perforators whereby venous flow is routed from the tissue level to the deep vein. No costly equipments or materials are required for the procedure and, hence, may be useful for a wider patient pool.
Acknowledgments
We would like to thank Pinaki Ghosh, Sunita Nair, and Richa Goyal Capita India Pvt. Ltd., Mumbai, for providing editorial support to the manuscript. The authors are thankful to The Director, St Thomas Hospital, Changanassery, and BioQuest Solutions Pvt. Ltd., Bangalore, for guidance during review of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.van den Bremer J, Moll FL. Historical overview of varicose vein surgery. Ann Vasc Surg. 2010;24:426–432. doi: 10.1016/j.avsg.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 2.Royle J, Somjen GM. Varicose veins: Hippocrates to Jerry Moore. ANZ J Surg. 2007;77:1120–1127. doi: 10.1111/j.1445-2197.2007.04331.x. [DOI] [PubMed] [Google Scholar]
- 3.Eklof B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248–1252. doi: 10.1016/j.jvs.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Tessari L, Cavezzi A, Frullini A. Preliminary experience with new sclerosing foam in the treatment of varicose veins. Dermatol Surg. 2001;27:58–60. [PubMed] [Google Scholar]
- 5.Khunger N, Sacchidanand S. Standard guidelines for care: sclerotherapy in dermatology. Indian J Dermatol Venereol Leprol. 2011;77:222–231. doi: 10.4103/0378-6323.77478. [DOI] [PubMed] [Google Scholar]
- 6.American Society for Dermatologic Surgery. http://www.asds.net/search.aspx?searchtext=advantage%20of%20foam%20is%20that%20the%20sclerosing%20power%20of%20the%20solution%20is. Accessed 27 Nov 2013
- 7.Wollman JC. Sclerosant foams: stabilities, physical properties and rheological behaviour. Phlebologie. 2010;39:208–217. [Google Scholar]
- 8.Mirski MA, Lele AV, Fitzsimmons L, Toung TJ. Diagnosis and treatment of vascular air embolism. Anesthesiology. 2007;106:164–177. doi: 10.1097/00000542-200701000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Caggiati A, Phillips M, Lametschwandtner A, Allegra C. Valves in small veins and venules. Eur J Vasc Endovasc Surg. 2006;32:447–5. doi: 10.1016/j.ejvs.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Vincent JR, Jones GT, Hill GB, van Rij AM. Failure of microvenous valves in small superficial veins is a key to the skin changes of venous insufficiency. Am J Vasc Surg. 2011;54(6 Suppl):62S–69S. doi: 10.1016/j.jvs.2011.06.085. [DOI] [PubMed] [Google Scholar]
- 11.Golledge J, Quigley FG. Pathogenesis of varicose veins. Eur J Vasc Endovasc Surg. 2003;25:319–324. doi: 10.1053/ejvs.2002.1843. [DOI] [PubMed] [Google Scholar]
- 12.Theivacumar NS, Darwood R, Gough MJ. Neovascularisation and recurrence 2 years after varicose vein treatment for sapheno-femoral and great saphenous vein reflux: a comparison of surgery and endovenous laser ablation. Eur J Vasc Endovasc Surg. 2009;38:203–207. doi: 10.1016/j.ejvs.2009.03.031. [DOI] [PubMed] [Google Scholar]