Thermotaxis, circadian rhythms, and TRP channels in Drosophila (original) (raw)
Abstract
The fruit fly Drosophila melanogaster is a poikilothermic organism that must detect and respond to both fine and coarse changes in environmental temperature in order maintain optimal body temperature, synchronize behavior to daily temperature fluctuations, and to avoid potentially injurious environmental hazards. Members of the Transient Receptor Potential (TRP) family of cation channels are well known for their activation by changes in temperature and their essential roles in sensory transduction in both invertebrates and vertebrates. The Drosophila genome encodes 13 TRP channels, and several of these have key sensory transduction and modulatory functions in allowing larval and adult flies to make fine temperature discriminations to attain optimal body temperature, detect and avoid large environmental temperature fluctuations, and make rapid escape responses to acutely noxious stimuli. Drosophila use multiple, redundant signaling pathways and neural circuits to execute these behaviors in response to both increases and decreases in temperature of varying magnitudes and time scales. A plethora of powerful molecular and genetic tools and the fly's simple, well-characterized nervous system have given Drosophila neurobiologists a powerful platform to study the cellular and molecular mechanisms of TRP channel function and how these mechanisms are conserved in vertebrates, as well as how these channels function within sensorimotor circuits to generate both simple and complex thermosensory behaviors.
Keywords: circadian rhythms, Drosophila melanogaster, nociception, thermosensation, thermotaxis, TRPA1, TRP channels
Abbreviations: A1, 1st Antennal Segment; A2, 2nd Antennal Segment; A3, 3rd Antennal Segment; AC, Anterior Cell; AL, Antennal Lobe; AR, Arista; Clk, Clock protein; Cry, Cryptochrome; Cyc, Cycle protein; Dbt, Double Time protein; DN1, DN2, DN3, Dorsal Neuron group 1, 2, 3; GFP, Green Fluorescent Protein; GPCR, G Protein-Coupled Receptor; lLNv, Ventral Lateral Neuron, large cell body; LN, Lateral Neuron; LNd, Dorsal Lateral Neuron; LNv, Ventral Lateral Neuron; LPN, Lateral Posterior Neuron; mdIV, Multidendritic Neuron, class IV; NEL, Nocifensive Escape Locomotion; PAP, Proximal Antennal Protocerebrum; PDF, Pigment Dispersing Factor; Per, Period protein; PKD1, Polycistic Kidney Disease 1; PLC, Phospholipase C; RNAi, RNA interference; SAC, Sacculus; sLNv, Ventral Lateral Neuron, small cell body; SLPR, Superior Lateral Protocerebrum; SOG, Suboesophageal Ganglion; thermoTRP, thermosensitive TRP channel; Tim, Timeless protein; TRP, Transient Receptor Potential; TRPA, Transient Receptor Potential, group A (ankyrin repeat); TRPC, Transient Receptor Potential, group C (canonical); TRPL, TRP-Like; TRPM, Transient Receptor Potential, group M (melastatin); TRPP, Transient Receptor Potential, group P (polycystic); TRPV, Transient Receptor Potential, group V (vanilloid); VFP, Venus Fluorescent Protein
Introduction
As is the case for all animals, the fruit fly Drosophila melanogaster must detect and respond to multiple modalities of sensory stimuli in its environment. As a poikilothermic organism, it is especially important that the fly is able to detect and respond appropriately to thermal fluctuations in its environment during both its larval and adult stages in order to maintain an optimal body temperature for growth and development (18°C to 24°C for laboratory wild-type animals). The role of temperature in shaping Drosophila biology have been well-characterized in developmental studies of lab-strain animals1,2 as well as in analyses of latitudinal clines in a variety of morphometric traits observed in wild populations.3-5 In laboratory studies of behavioral responses to temperature, thermal fluctuations may include relatively modest changes in temperature that the fly navigates to maintain optimal body temperature and to synchronize its behavior to cyclical changes in temperature that accompany the daily cycle of light and dark. Larger temperature fluctuations may be detected to engage behavioral strategies that allow animals to escape non-optimal temperatures that would negatively impact the long-term health of the animal or cause acute tissue damage.
The Transient Receptor Potential (TRP) family of ion channels consists of a diverse group of cation-selective ion channels that are conserved across the animal kingdom and which serve a variety of physiological functions.6 TRP channels are particularly known for their essential and diverse functions in multiple modalities of sensory transduction and signaling.7,8 The canonical TRP channel encoded by the Drosophila trp gene was identified for its role in phototransduction in the fly retina.9-12 The original trp mutants were identified based on their visual impairment under intense lighting conditions.11 These mutants and the trp gene itself were later named based on the mutant's transient receptor potential phenotype.12 Electroretinogram recordings from trp mutants show a rapid return to baseline during intense, prolonged light stimulation, unlike wild-type electroretinograms, which display a sustained component during prolonged stimulation.11 Molecular cloning and characterization of the trp gene indicated that it encodes a transmembrane protein, leading to the hypothesis that the TRP protein forms an ion channel.9,10 Subsequent electrophysiological experiments in vivo and in heterologous cells would reveal that TRP forms a Ca2+-permeable ion channel.13-15
Other TRP channels have been characterized for roles in chemosensation16 and the detection of diverse mechanical stimuli, including external mechanical force,17,18 osmotic deformation,19 and sound pressure-waves.20 Several vertebrate and invertebrate members of the TRP family are known for their roles in transducing changes in environmental temperature, and a subset of these channels are directly gated by changes in temperature as part of their sensory function- these channels are known as thermoTRPs.21,22 Non-thermoTRPs that function in the transduction of thermal stimuli likely function downstream of a primary temperature sensor, either in a primary transduction step or in a modulatory role. During the course of this review, we will consider both thermoTRPs and non-thermoTRPs, their roles in shaping temperature-driven behaviors in Drosophila, and their cellular and molecular mechanisms of function.
Temperature-Sensing TRP Channels in Drosophila Melanogaster
Channels in the TRP family are divided into groups based upon their amino acid sequences, with members of the same group often sharing similar functions and properties.23,24 The Drosophila genome contains genes encoding 13 TRP channels.25 In vertebrates, the TRPA, TRPM, and TRPV groups all contain thermoTRP channels that function in sensory transduction of high and low temperatures.26-31 The Drosophila genome contains genes encoding channels that are classified into each of these groups, but to date, only members of TRPA group have been demonstrated to act as thermoTRPs in insects.32-34 Drosophila TRPA channels thus have been the most widely studied for their roles in temperature-sensing behavior in Drosophila. However, members of the Drosophila TRPV, TRPC, and TRPP groups also play important roles in temperature sensing that may be independent of direct temperature activation (Table 1).35-37
Table 1.
Drosophila TRP channels with thermosensory functions
| Channel | Group | Activation mechanisms | Thermosensory function |
|---|---|---|---|
| dTRPA1 | TRPA | Reactive electrophiles48 | Detection of temperature fluctuations |
| Heat (>26°C for A isoform; within the comfortable range72,80 | |||
| >34°C for D isoform)34,50,51 | Thermotaxis response to elevated temperature44 | ||
| Gαq-PLC signaling46,49,72 | Noxious heat avoidance50,150 | ||
| Synchronization of circadian rhythms to temperature cycles141,142 | |||
| Painless | TRPA | Heat32 | Noxious heat avoidance18 |
| Pyrexia | TRPA | Heat33 | Synchronization of circadian rhythms to temperature cycles60 |
| Noxious heat resistance33 | |||
| TRP | TRPC | Gαq-PLC signaling66 | Thermotaxis responses to cool temperatures36 |
| Polyunsaturated fatty acids155 | |||
| Mechanical force156 | |||
| TRPL | TRPC | Gαq-PLC signaling67 | Thermotaxis responses to cool temperatures36 |
| Polyunsaturated fatty acids155 | |||
| Mechanical force156 | |||
| Inactive | TRPV | Osmotic stimulation82 | Thermotaxis responses to cool temperatures35 |
| Brivido-1 | TRPP (non-channel) | — | Thermotaxis responses to cool temperatures37 |
| Brivido-2 | TRPP (non-channel) | — | Thermotaxis responses to cool temperatures37 |
| Brivido-3 | TRPP (non-channel) | — | Thermotaxis responses to cool temperatures37 |
The Drosophila TRPA1 channel
The vertebrate TRPA1 channel is expressed in nociceptor neurons as well a variety of peripheral tissues and is well-known for its functions in nociception, inflammation, and chemosensation.26,38-41 The TRPA1 channel is activated by reactive chemicals (such as allyl isothiocyanate),38 poly-unsaturated fatty acids,42 and downstream of G protein signaling.39 While the human TRPA1 channel is not activated directly by changes in temperature, TRPA1 channels from other mammals may be activated by cold stimuli and act in nociceptors to detect noxious cold stimuli.26 Interestingly, the TRPA1 channels from some reptile species may be activated by heat. These notably include snakes that possess pit organs (e.g. vipers, pythons, and boas), in which the heat-activated TRPA1 functions as part of the transduction mechanism for the detection of infrared and thermal stimuli.43 The Drosophila TRPA1 channel (dTRPA1) is 32% identical and 54% similar to its mammalian ortholog by amino acid identity and is activated by elevated temperature as well as in response to reactive chemicals and downstream of intracellular signaling pathways.44-47 The dTRPA1 channel is expressed in a multiple classes of peripheral sensory neurons48-50 as well as several groups of central neurons,44,47 all of which may act as heat sensors for temperature-driven behaviors.
An important feature of the dTrpA1 gene is its use of multiple transcription start sites and mutually exclusive alternative splicing at the 12th and 13th exons to produce at least 4 dTRPA1 isoforms with differing expression patterns and heat-activation properties (Fig. 1A).46,50,51 The canonical dTrpA1-A isoform uses a downstream transcription start site and is spliced to include the 12th exon, while the dTrpA1-B isoform uses the same start site, but is spliced to include the 13th exon.46 GAL4 reporter transgenes that use promoter sequence upstream of the A/B start site drive expression of GFP in a relatively small number of neurons in the brain as well as in peripheral neurons in the antennae and labellum.44,46-49 The dTrpA1-C isoform uses an upstream transcriptional start site and is spliced to include the 13th exon, while the dTrpA1-D isoform uses the same start site, but is spliced to include exon 12.50,51 GAL4 reporter transgenes using promoter sequences upstream of the C/D start site drive expression of GFP in the Class IV multidendritic neurons (mdIVs) that act as larval nociceptors, as well as in the labellum and the neuroendocrine cells of the corpus cardiacum.50,51
Figure 1.
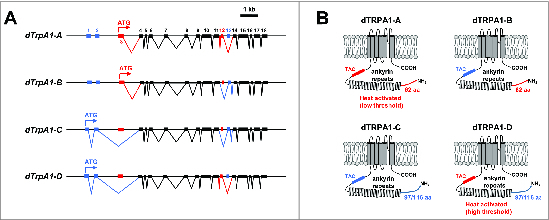
Schematic representation of dTrpA1 isoforms. (A) Gene structures of known dTrpA1 isoforms. Exons are numbered sequentially starting with the most 5′ exon and irrespective of isoform. The “A” isoform uses a translation start site in exon 3 and is spliced to include exon 12. The “B” isoform uses a translation start site in exon 3 and is spliced to include exon 13. The “C” isoform uses a translation start site in exon 1 and is spliced to include exon 13. The “D” isoform uses a translation start site in exon 1 and is spliced to include exon 12. (B) Protein structures of dTRPA1 protein isoforms. N-terminal regions encoded by exons 1 and 2 are drawn in blue, while N-terminal regions encoded by exon 3 are drawn in red. The TAC region between the ankyrin repeats and first transmembrane domain encoded by exon 12 is drawn in red, while the TRP ankyrin cap (TAC) region encoded by exon 13 is drawn in blue.
Studies have demonstrated that the dTRPA1-A channel is a bona fide thermoTRP with a threshold of ~25°C,34 but this is not a property shared by all channel isoforms.50,51 When expressed in Drosophila S2R+ cells along with the genetically encoded calcium sensor GCaMP, the dTRPA1-A isoform mediates calcium transients in response to elevated temperature of around 26°C, while the dTRPA1-D isoform mediated calcium transients with a higher threshold (~36°C).50 The dTRPA1-B and dTRPA1-C isoforms did not mediate detectable heat-induced calcium transients, but all 4 isoforms produced robust calcium transients in response to allyl isothiocynate (a potent TRPA1 activator).50 These results are consistent with results obtained from electrophysiological records in Xenopus oocytes showing that dTRPA1-A is a more temperature-sensitive channel than dTRPA1-D (Q10 = 130 vs. 7.5).51
These studies of dTRPA1 isoforms expressed in heterologous expression systems indicate that the 37 amino acid residues encoded by exon 12 and the 36 amino acid residues encoded by exon 13 may be essential determinants of temperature activation of the channel, as both splice forms containing exon 12 (i.e. A and D) encode temperature-sensitive channels, while those encoded by splice forms containing exon 13 (i.e., B and C) are not.50 The amino acids encoded by exons 12 and 13 are contained in the intracellular N-terminus of the protein immediately preceding the first transmembrane domain (Fig. 1B), and thus an important role for these residues is generally consistent with studies of chimeric and point-mutant vertebrate TRPA1 channels that indicate an important role for the N-terminal region of the channel in conferring temperature sensitivity.52,53 A role for the extreme N-terminus of the dTRPA1 protein in determining temperature sensitivity is also suggested by the differing Q10 values observed for the dTRPA1-A and dTRPA1-D isoforms in electrophysiological experiments, as well as by mutagenesis studies showing that elimination of basic residues in dTRPA1-D N-terminus can increase the temperature sensitivity of the channel.51 Interestingly and conversely, recent studies of human TRPA1 have indicated that the N-terminal intracellular region is dispensable for activation of the channel by temperature.54 These conflicting observations may be reconciled by a recent thermodynamic model of temperature gating that suggests that the temperature sensitivity of temperature-gated channels arises from differences in heat capacity between open and closed conformations and that these heat capacity differences could arise from the solvent exposure of distributed hydrophobic residues, eliminating the need for a dedicated temperature sensor domain.55 This model has been validated by a recent mutagenesis study in which temperature sensitivity was conferred on normally temperature-insensitive voltage-gated potassium channels by altering the polarity of residues that become solvent-exposed during voltage gating.56 These developments suggest that the N-terminal intracellular regions of TRPA1 channels might play an allosteric role in controlling or modulating temperature sensitivity, but are not themselves a temperature sensor domain.
It is important to note that consensus has not yet been reached on nomenclature for the differing isoforms of the dTrpA1 mRNA and the dTRPA1 channel subunit. The nomenclature used in this review was introduced upon the discovery that the dTrpA1-A and dTrpA1-B isoforms are generated by alternative splicing46 and then extended upon the identification of the dTrpA1-C and dTrpA1-D mRNA variants.50 Contemporaneous studies that identified the dTrpA1-D mRNA named the subunit encoded by this mRNA as dTRPA1(A), while naming the canonical subunit (i.e. the dTRPA1-A subunit described in this review) dTRPA1(B), based on the relative positions of the start codons used by these isoforms.51 It is also important to note that 2 different 5′ ends have been cloned for the dTrpA1-C/D mRNAs, one that encodes a 97 amino acid N-terminus50 and another that encodes a 116 amino acid N-terminus via a slightly upstream start site51 (Fig. 1B). It is unclear whether both start sites cloned in these isoforms are actually used in vivo, and thus the 97 amino acid N-terminus may simply be a truncated form of the 116 amino acid N-terminus.
Other Drosophila TRPA channels
The Drosophila genome contains genes encoding 3 additional TRPA channels that do not have mammalian orthologs (Painless, Pyrexia, and Waterwitch).18,33,57 Electrophysiological studies have demonstrated that the Painless channel is a thermoTRP with an activation threshold of ∼42°C when expressed in HEK293 cells.32 GAL4 reporters for the Painless gene drive GFP expression in a large number of central neurons and peripheral sensory neurons, including the multidendritic neurons that tile the larval body wall and in chemosensory neurons of the labellum.18,45 Like the dTrpA1 gene, the Painless gene makes use of multiple transcription start sites to produce at least 3 different channel isoforms with differing intracellular N-terminal regions (Fig. 2).58 Only the long isoform has been characterized electrophysiologically,45 but behavioral evidence (described below) suggests that these isoforms may have differing roles in transducing information about elevated temperature.58
Figure 2.
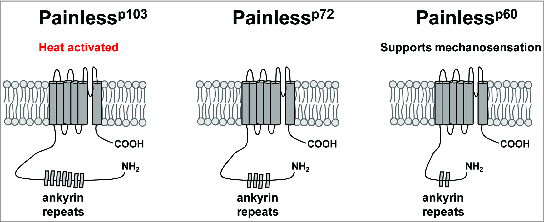
Schematic representation of Painless protein isoforms. The Painlessp103, Painlessp72, and Painlessp60 protein isoforms are schematized with varying numbers of ankyrin repeats in their N-terminal intracellular regions.
Like Painless and dTRPA1, the Pyrexia channel is a thermoTRP that is expressed in a wide variety of sensory tissues.33,59 When expressed in Xenopus oocytes or HEK293 cells, Pyrexia mediates heat-activated currents with a threshold of ∼40°C.33 GAL4 reporters that use promoter sequences upstream of the pyrexia start codon drive GFP expression in a wide variety of sensory neurons and supporting cells,33 including an unidentified population of neurons in the second and third antennal segments and in the cap cells that support chordotonal neurons in Johnston's Organ and the femur of adult flies.59-61 Flies lacking Pyrexia function were initially characterized for their intolerance to thermal stress, as 60% of pyrexia mutants were found to become paralyzed following 3 minutes of exposure to a 40°C environment, as compared to less than 10% of wild-type controls.33 Roles for Pyrexia in temperature sensing and circadian entrainment to temperature cycles have since been discovered and will be described in detail below.60,61 The final Drosophila TRPA channel, Waterwitch, is not known to be activated by changes in temperature or to have any role in behavioral responses to thermal stimuli, instead playing an important role in hygrosensation and behavioral responses to changes in humidity.57
Non-TRPA group TRP channels
In vertebrates, the TRPV and TRPM groups also contain characterized thermoTRP channels,30,31,62,63 but temperature-dependent activation of the Drosophila members of these groups have not been observed. Mammalian members of the TRPV sub-family of ion channels have been well-characterized for their activation by elevated temperatures and their roles in the sensory transduction of thermal stimuli. The TRPV1 channel, like TRPA1, is well-known for its central role in nociceptive signaling and is activated by elevated temperatures and by the chemical capsaicin.30 The mammalian TRPV2, TRPV3, and TRPV4 channels are also heat-activated.27-29,62 The Drosophila TRPV channels, Inactive and Nanchung, are not known to be directly activated by changes in temperature. However, Inactive may play an indirect role in the detection of cool temperatures that will be discussed below.35 The mammalian TRPM8 channel has a well-described role in detection of cool temperatures and is activated by the ligand menthol.31,63 However, the Drosophila TRPM is not activated by cool temperatures and does not currently have a described sensory function.
Drosophila also possess TRP channels from the TRPC and TRPP groups that have established roles in mediating temperature-sensitive behavior,36,37 although it remains to be determined whether TRP channels from either of these groups are bona fide thermoTRPs in Drosophila. The TRP and TRPL channels are both Drosophila members of the TRPC family who have well-described roles in phototransduction and are activated downstream of a Rhodopsin-Gαq-phospholipase Cβ signaling cascade.9,10,64-68 However, both of these channels also have roles in behavioral responses to cool stimuli that will be described below.36
The Drosophila Brivido-1, Brivido-2, and Brivido-3 proteins share sequence homology with the TRPP group of mammalian TRP channels, specifically the PKD1 and PKD1-Like proteins.37 The mammalian PKD1 protein contains 11 transmembrane domains and regulates the function of TRPP2 channel subunits, but is not known to form ion channels on its own.69,70 Similar to PKD1, the Brivido proteins contain between 8 and 10 transmembrane segments and are not known to form ion channels independently. Despite this, the Brivido proteins function in behavioral responses to cool stimuli and cold-activation of peripheral sensory neurons (as described below).37 It is possible that the Brivido proteins function similarly to the mammalian PKD1 protein and regulate the function of TRPP ion channels. However, there is no described sensory function for TRPP ion channel subunits in mammals or invertebrates- so the cellular and molecular mechanism for Brivido proteins’ functions in cool-detection remains unclear.
Thermotaxis and Behavioral Responses to Changes in Environmental Temperature
In the laboratory, Drosophila are commonly reared at temperatures ranging from 15°C to 25°C, with 24°C being the preferred temperature of adult flies71 and 18°C being the preferred temperature of 3rd instar larvae.44,72,73 Flies are capable of navigating to optimal temperatures within this “comfortable” range and also capable of avoiding non-optimal temperatures that fall above or below of this optimal range. This temperature-driven navigation is known as thermotaxis, and behavioral studies of thermotaxis behavior in both larvae and adult flies have made significant contributions to understanding the molecular, cellular, and circuit-level mechanisms that underlie temperature sensing. Assays of thermotaxis behavior are generally performed using an arena that can be heated or cooled to produce a temperature gradient that can then be divided into a warm and cool halves or into zones (Fig. 3A and B).44,71,74 Populations of animals are then placed in the arena and allowed to distribute via thermotactic locomotion for a set period of time. The distribution of animals across regions of differing temperature may then be used to calculate an avoidance index to determine the proportion of animals that avoid a warm or cool stimulus (Fig. 3C)73,74 or to create a histogram showing the distribution of animals across a temperature gradient.71 Multiple molecular and cellular mechanisms contribute to differing aspects of these behaviors, such as sensing small temperature fluctuations within the comfortable range versus large temperature variations above or below the preferred temperature.
Figure 3.
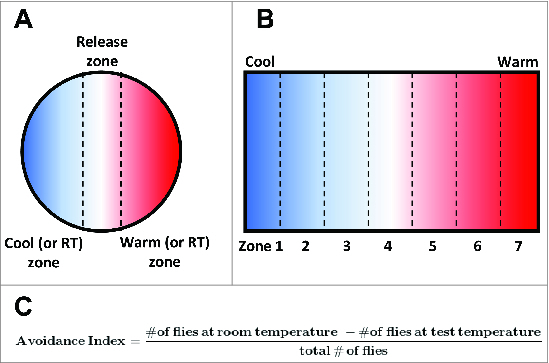
Paradigms for assaying thermosensory behavior in Drosophila larvae and adults. (A) Schematic of a circular assay chamber in which flies or larvae must choose between warm and cool (or room temperature and test temperature) regions. The distribution of individuals between these regions can then be used to calculate and avoidance (or preference) index. (B) Schematic of an assay chamber that can be used to test the distribution of individuals across a temperature gradient. The chamber is heated (or cooled) at one end to produce a temperature gradient and divided into zones. The proportional distribution of flies or larvae across these zones can then be calculated. (C) A standard equation used to generate an avoidance index of flies tested in an experimental chamber that requires them to navigate to either room temperature or a test temperature.
Warmth sensors in the central nervous system
Both larvae and adult flies engage in thermotaxis behavior to escape temperatures higher than 25°C.44,71 These behaviors require the dTRPA1 channel and likely use the innate temperature sensitivity of the channel as a direct temperature sensor, as the temperature threshold for channel activation in heterologous cells is equivalent to the temperature threshold for activation of temperature-sensing neurons and for the behavior in vivo.34,44,47
Drosophila larvae lacking dTRPA1 function show a significantly reduced preference for cooler temperatures when tested in an assay that forces them to choose between the warmer (i.e., less comfortable) and cooler (i.e. more comfortable) portions of a temperature gradient.44 It should be noted that in this experiment, the entire thermal gradient (27°C–41°C) was above the threshold for dTRPA1 activation, and thus these results are consistent with dTRPA1 acting as a direct temperature sensor. The site of action of dTRPA1 for these experiments has not been positively identified, but it is likely that dTRPA1 functions in a small number of central neurons, as opposed to peripheral neurons. Expression of tetanus toxin under the control of a dTrpA1-A/B-Gal4 driver that drives expression in a small number of dTRPA1-expressing neurons in the brain and in the neuroendocrine cells of the corpus cardiacum was sufficient to reduce heat avoidance similar to the effect observed in a dTrpA1 mutant.44
Similar to the effect observed in Drosophila larvae, adult flies lacking dTRPA1 function also show defective avoidance of warmer temperatures.47 When allowed to passively distribute on a temperature gradient ranging from 18°C to 32°C, flies lacking dTRPA1 function displayed increased accumulation within the 28°C to 32°C range as compared to wild-type controls.47 As in larvae, dTRPA1 is expressed in a small number of central neurons in the adult fly brain (Fig. 4).47 These dTRPA1-positive neurons (as detected by anti-dTRPA1 antisera) have been grouped into 3 groups based on position in the brain: the lateral cell, ventral cell, and anterior cell (AC) clusters. The AC neurons are likely to be the principle central thermosensors for adult warmth avoidance. First, restoration of dTRPA1 expression to the AC neurons (but not the lateral cell or ventral cell neurons) of a dTrpA1 mutant is sufficient to restore wild-type heat-avoidance behavior.47 Second, tissue-specific knockdown of dTrpA1 transcript in the AC neurons using _dTrpA1_-specific RNAi and an AC-expressed dTrpA1-Gal4 driver caused loss of heat avoidance similar to that observed in a dTrpA1 mutant.47 Thus, dTRPA1 function in the AC neurons is both necessary and sufficient for wild-type thermal preference in adult flies. Finally, measurements of heat-induced calcium transients in the AC neurons using GCaMP show that the cells are activated by heat with a threshold of ∼27°C.47 This threshold is similar to the threshold for activation of heterologously expressed dTRPA1,34 and indeed, the heat-induced transients are absent from the AC neurons in dTrpA1 mutants.
Figure 4.
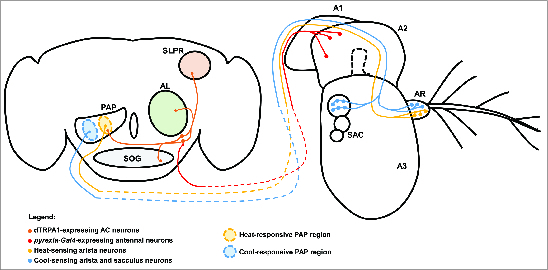
Central and peripheral neural mechanisms for detecting changes in temperature. The adult Drosophila brain and antenna are schematized here, with colored circles representing neuronal cell bodies, colored lines representing axonal connections, and colored diamonds representing presynaptiC-terminals. Heat-responsive and cool-responsive regions of the proximal antennal protocerebrum (PAP) are schematized as filled ovals with dashed outlines. Heat-sensing neurons of the aristae (AR) connected to the third antennal segment (A3) send projections to the brain that synapse in the heat-sensitive region of the PAP. Cool-sensing neurons of the aristae and sacculus (SAC) send projections to the cool-responsive region of the PAP. Putative _pyrexia-Gal4_-expressing neurons in the second antennal segment (A2) project to the brain and synapse on the dTRPA1-expressing AC neurons (it is important to note that the exact location and identity of these neurons are unknown). The AC neurons project to the heat-responsive region of the PAP, as well as to the suboesophageal ganglion (SOG), the superior lateral protocerebrum (SLPR), and the antennal lobe (AL). Also depicted is the first antennal segment (A1).
While the AC neurons are highly likely to be central temperature sensors for thermotaxis behavior, the complete neural circuit that underlies their behavioral function is unknown. Projections from the AC neurons innervate the olfactory lobe (Fig. 4), the site of first-order synapses between olfactory sensory neurons and olfactory projection neurons.47 These results suggest the possibility of multimodal integration of thermal stimuli with chemosensory signals within the olfactory lobe. The AC additionally project to the suboesophageal ganglion,47 the superior lateral protocerebrum,47 and the proximal antennal protocerebrum (PAP).37 The possible significance of these projections is unknown, but projections from the AC neurons to the PAP overlap with projections from warmth-sensing neurons in the aristae (as described below) to the PAP,37 suggesting that the PAP may function to integrate information from multiple warmth sensors (Fig. 4). Additionally, the AC neurons receive inputs from peripheral warmth-sensing neurons in the second antennal segment (as described below), suggesting that the AC neurons themselves may be a site of integration for cell-intrinsic and cell-extrinsic temperature signals.61
Warmth sensors in the antennae
The antennae of many insect species are multimodal sensory structures.75,76 As such, the antennae of Drosophila have long been considered potential sites of action for thermosensory neurons that initiate thermotaxis behavior. Flies in which the third antennal segment and aristae have been bilaterally ablated have been shown to distribute broadly across a 18°C to 31.5°C temperature gradient, as compared to un-ablated controls or flies in which only the aristae had been removed, which accumulated with a peak at ∼24°C.71 Furthermore, mutations in the spineless gene, which produce severe defects in antennal development, produce similar defects in thermotaxis behavior.71,74 Together these results suggested a role for the third antennal segment, better known for its role in olfaction, in responses to both hot and cool stimuli. The role of the third antennal segment in behavioral responses to heat has been rendered unclear, however, by subsequent studies that indicate a more specific role for the third antennal segment in thermotaxis responses to uncomfortably cool temperatures, but not uncomfortably warm temperatures.47
Despite the unclear role for antennal temperature sensors in the generation of behavioral responses to elevated temperature, it is clear that the antennae contain at least 2 independent heat sensors. The first of these temperature sensors is thought to be located in the second antennal segment and is dependent on the function of the Pyrexia TRPA channel, suggesting that the sensor is comprised of as-yet unidentified _pyrexia_-expressing neurons in that structure (Fig. 4).61 Within the second antennal segment, the pyrexia-Gal4 reporter is most obviously expressed by the cap cells that support the chordotonal neurons in Johnston's Organ,59 a structure that is well-known for its role in detecting and generating behavioral responses to gravitational forces and auditory stimuli.77,78 However, unidentified _pyrexia-Gal4_-expressing neurons send projections to the AC neurons, which act as central temperature sensors, and appear to influence their electrical activity.61 Wild-type AC neurons expressing GCaMP display a 2-peaked calcium response during a temperature-ramp protocol, with the first peak occurring at ∼25°C and the second at ∼27°C.61 The first peak occurs near the threshold for dTRPA1 activation and is significantly decreased in dTrpA1 mutants. The second peak is absent in animals in which the second antennal segments have been removed and in pyrexia mutants, consistent with a model in which _pyrexia_-expressing neurons in the second antennal segment send information about environmental temperature to the AC neurons.61 The functional significance of temperature sensing in _pyrexia_-expressing antennal neurons is unclear, as pyrexia mutants have no defect in thermotaxis,61 but these results do demonstrate that multiple heat sensors function in the Drosophila nervous system and may be integrated, in this case, in the AC neurons (Fig. 4).
While the AC neurons and thermosensory neurons of the second antennal segment appear to generate physiological and behavioral responses to temperatures warmer than those preferred by wild-type Drosophila, the antennae also contain neurons that generate responses to small temperature fluctuations within the comfortable range (18°C–24°C). Two-photon calcium imaging of the Drosophila antennae has revealed 6 ciliated sensory neurons in the aristae that are temperature sensitive.37 These neurons can be divided into 2 populations: 3 neurons that respond specifically to cooling stimuli and are inhibited by heat (to be discussed below) and 3 neurons that are stimulated by heat and inhibited by cooling stimuli. Remarkably, the calcium responses in both of these populations of neurons are activated by temperature fluctuations as low as ∼0.5°C.37 The projections of the warmth-sensing and cool-sensing neurons of the aristae terminate in distinct regions of the PAP, and the projections of the warmth-sensing neurons overlap in the PAP with those of the AC neurons, suggesting that the PAP may be thermotopically organized and act as an integration region for thermosensory information (Fig. 4).37
Further studies of the warmth-sensing neurons in the Drosophila aristae have identified novel molecular mechanisms for the detection of thermal stimuli as well as provided important insights into the organization of Drosophila thermosensory behavior. Blockade of synaptic transmission in the warmth-sensing neurons of the aristae by cell-specific expression of the tetanus toxin light chain produces a significant defect in rapid avoidance of warm temperatures.37,79 This behavior is distinct from thermotaxis responses that occur over a longer time scale, as neither dTRPA1 nor AC neuron function is required for this rapid avoidance.79 These results suggest that Drosophila thermotaxis and thermosensory behaviors may be broken down into simpler component behaviors that require differing sensory neurons, neural circuits in the brain, and molecular temperature sensors.
While the central circuits that allow different types of thermosensory neurons to contribute to different aspects of thermosensory behavior remain to be elucidated, it is certainly clear that these circuits make use of differing molecular mechanisms. While thermotaxis by adult flies over long time scales along a shallow temperature gradient requires dTRPA1 function in the AC neurons (as described above),47 more rapid thermotaxis behaviors mediated by the warmth-sensing neurons of the aristae require the gustatory receptor, GR28B(D).79 Loss of GR28B(D) function results in a loss of rapid thermal avoidance behavior, and expression of GR28B(D) is sufficient to confer thermal sensitivity upon a wide variety of tissues.79 These results suggest that GR28B(D) is a bona fide thermosensor, although the cellular and molecular mechanisms for its function remain unknown.
Warmth sensors in other tissues
When tested for temperature preference along a thermal gradient (see Fig. 3) Drosophila larvae are capable not only of avoiding uncomfortable low (<18°C) and high (>24°C) temperatures, but also of navigating to an optimal temperature (18°C) within a comfortable range (18°C–24°C).72,80 The heat-activated dTRPA1 channel is required for this thermal preference behavior, but the ability of larvae to make fine thermal discriminations below the described temperature threshold for dTRPA1 activation (∼25°C) suggests that TRPA1 is not functioning as direct temperature sensor for this behavior.72 However, the simultaneous discovery that, in addition to a requirement for dTRPA1, the Gαq subunit, Gαq, and the phospholipase C enzyme, NorpA, are also required for thermal discrimination suggested that dTRPA1 could function downstream of a G protein-coupled receptor (GPCR) signaling cascade.72
This hypothesis was confirmed by the identification of the Rh1 rhodopsin, NinaE, as essential for larval thermal preference within the comfortable range.80 The role of NinaE was found not to be light-dependent, and the thermal preference function of NinaE occurs not in Bolwig's Organ cells that function as larval photoreceptors, but in some other unidentified cells that express both TRPA1 and NinaE.80 Epistasis analysis supported the existence of a NinaE-Gαq-NorpA-dTRPA1 signaling cascade, defining a novel signaling mechanism for temperature discrimation.80 Questions remain to be elucidated about this thermosensory mechanism. For instance, how does NinaE function as a thermosensor? The instrinsic thermal sensitivity is far too low and too slow to mediate the behaviors described above, suggesting the possibility that an accessory factor is required.80,81 Additionally, the site of action of the signaling cascade is unknown. dTRPA1 is not known to be expressed in the Bolwig's Organs, the traditional site of NinaE action. Single-cell RT-PCR analysis of cultured larval cells reveals that cells co-expressing NinaE and dTRPA1 do exist, but their identities are currently unknown.80
Cool sensors in the peripheral nervous system
In order to avoid tissue damage and maintain an optimal growth rate, Drosophila larvae and adult flies must also avoid sub-optimally cool temperatures. When released in the middle of a cool temperature gradient (∼15°C–21°C), Drosophila larvae show strong preference for movement toward the warmer portion of the gradient and away from the cooler portion.36 This cool-avoidance behavior requires the function of the TRP and TRPL channels,36 which have been historically characterized for their roles in phototransduction and which are not known to be directly temperature sensitive.10,36,64 A similar cool-avoidance paradigm likewise reveals a role for the TRPV channel, Inactive, in avoiding uncomfortably cool temperatures.35 Like TRP and TRPL, Inactive is not known to be a temperature-activated channel, having more well-described roles in auditory transduction.82
Despite the well-described roles for TRP and TRPL in phototransduction in the larval photoreceptor neurons, their roles in cool temperature avoidance are distinct from their roles in phototransduction at a cellular and molecular level. Elimination of the larval photoreceptor neurons of the Bolwig Organ by cell-specific overexpression of the pro-apoptotic protein Hid or mutation of the transcription factor GLASS eliminates phototaxis responses without altering responses to uncomfortably cool stimuli.36 Thus TRP and TRPL must function in different sets of neurons to fulfill their roles in thermotaxis and phototaxis. While the site of action of TRP and TRPL has not been formally demonstrated, the neurons of the terminal organ are an appealing candidate site. The terminal organ neurons show robust responses to cold stimuli, as measured with the genetically encoded calcium sensor, Cameleon, and extracellular electrophysiological recording.73 Furthermore, elimination of synaptic vesicle release in these neurons via cell-specific expression of the tetanus toxin light chain produces cool-avoidance defects similar to those observed in trp and trpl mutants.73 The molecular mechanism for TRP and TRPL functions in phototransduction and thermotransduction are also distinct from each other. Larvae that are mutant for NorpA, the phospholipase C enzyme essential for activating TRP and TRPL in phototransduction, show significant phototaxis defects, but no defects in avoiding the cool (<18°C) half of a thermotaxis arena.36 These findings, which show a TRP-/TRPL-dependent, NorpA-independent mechanism for cool avoidance, stand in contrast to experiments discussed above, which demonstrate that NorpA, TRPA1, and NinaE function are required for larvae to avoid mild elevated temperatures (19°C–24°C).72,80 Clearly, distinct molecular mechanisms must exist for larval behavioral responses to warm and cool temperatures.
Adult flies also avoid uncomfortably cool stimuli using a mechanism that is cellularly and molecularly distinct from that used for warmth avoidance. A group of 3 ciliated sensory neurons in the aristae of the Drosophila antenna, as well as a cluster of neurons in the sacculus of the third antennal segment, have been shown to respond to cooling stimuli with calcium transients, as measured with GCaMP.37 The cool sensors for these neurons require the TRPP family subunits Brivido-1, Brivido-2, and Brivido-3. A GAL4 reporter for brivido-1 drives GFP expression in these cool-responsive neurons, as well as a small population of non-temperature-sensitive neurons in the third antennal segment.37 RNAi-knockdown of either brivido-1 or brivido-2 results in defective cooling-sensitive calcium responses, while knockdown of any of the 3 channels results in defective behavioral avoidance of cool temperatures.37 As described above, the Brivido subunits most closely resemble human PKD1 proteins, which do not form ion channels, but instead regulate the function of TRPP2 channels.69,70 Thus it is unlikely that Brivido homomers or heteromers form cooling-activated ion channels on their own. However, it is possible that Brivido subunits instead support the function of some as-yet unidentified cooling-activated ion channel.
Regulation of Drosophila Circadian Rhythms by Temperature
Drososphila adults and larvae use thermotaxis to avoid non-optimal temperatures in their environment. There is also substantial adaptive value in coordinating daily patterns of behavior to avoid being active during times of non-optimal temperature, such as hot middays or cold nights. Circadian clocks are biological oscillators that allow organisms to synchronize their physiology and behavior to the 24-hour cycles of light and dark and concomitant temperature variation that result from the rotation of the Earth on its axis. The central components of a cellular circadian clock are the molecular oscillations of clock genes and their products, which are altered in their abundance, subcellular localization, and phosphorylation state over a 24-hour cycle.83,84 These molecular oscillations occur in peripheral tissues as well as in so-called “pacemaker neurons” in the central nervous system that integrate environmental information in order to synchronize the circadian clock to those stimuli.85-87
Molecular and cellular components of the Drosophila circadian clock
The cyclical accumulation and subcellular translocation of the Period (Per) and Timeless (Tim) proteins are perhaps the most fully understood molecular oscillations in Drosophila pacemaker cells and are often considered to be the centerpiece of the circadian clock.83,84,88 Briefly, the helix-loop-helix transcription factors, clock (Clk) and cycle (Cyc), form a heterodimer that binds the per and tim promoter regions to activate transcription, leading to accumulation of per and tim mRNAs.89-91 Tim interacts with Per to form a heterodimer, a configuration that blocks the phosphorylation and proteasomal degradation of Per.92-94 However, Tim is quickly degraded in the presence of light, preventing either protein from accumulating during the light phase of the light-dark cycle.95-98 During the dark phase of the light-dark cycle, however, Per and Tim accumulate in the cytoplasm and eventually translocate into the nucleus in complex with the Double-time (Dbt) kinase.99,100 In the nucleus, Per, Tim, and Dbt bind to the Clk-Cyc dimer and allows for Dbt-dependent phosphorylation of Clk.100,101 Phosphorylation inhibits binding of the Clk-Cyc dimer to DNA, thus preventing further per and tim transcription (and presumably transcription of other cycling genes).100 This negative feedback loop is the molecular basis for the cyclic accumulation of per and tim gene products.
One major feature of cells that contain circadian clocks is their ability to sustain molecular oscillations in the absence of outside input from the animal's environment or from other cells. As such, when flies are housed in constant darkness, the rhythmic accumulation of Per and Tim proteins described above will continue to occur in cells containing a circadian oscillator with a period of around 24 hours.102,103 Likewise, flies housed under these conditions will continue to exhibit circadian behavior patterns with a similar period. However, a second essential feature of cells that contain circadian clocks is their ability to synchronize their molecular oscillations between each other and entrain them to cyclical environmental stimuli or Zeitgebers (‘time givers’) such as light and temperature.84,104 Synchronization of the circadian clock to light stimuli is largely dependent on the blue-light photoreceptor, Cryptochrome (Cry).105-107 When Cry is activated by light, it binds to Tim and causes its light-dependent degradation (as part of the negative feedback loop described in the preceding paragraph).108,109
The central circadian clocks in adult Drosophila are the so-called “pacemaker neurons” in the central nervous system.85,86 There are roughly 150 pacemaker neurons in the adult Drosophila brain, and these can be divided into 3 major groups based on their anatomical positions (Fig. 5).87 These are the dorsal neurons (DNs), the lateral neurons (LNs), and the lateral posterior neurons (LPNs). The DN and LN groups can be further subdivided based on neuronal position and morphology. Thus the DNs are divided into the DN1, DN2, and DN3 subgroups based on their positions within the brain, while the LNs are divided into a dorsal LNd group along with small and large ventral LNv groups (sLNvs and lLNvs) (Fig. 5).
Figure 5.
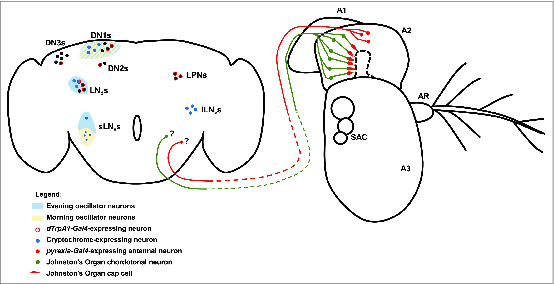
Central and peripheral neural mechanisms for circadian entrainment to temperature. The adult Drosophila brain and antenna are schematized here, with colored circles representing neuronal cell bodies, colored lines representing axonal connections, and colored diamonds representing presynaptiC-terminals. Shaded areas are used to represent morning and evening oscillators in the circadian circuit. Unidentified _pyrexia-Gal4_-expressing neurons of the second antennal segment (A2) project to the brain and potentially provide input to central circadian pacemakers via unknown synapses. Chordotonal neurons in Johnston's Organ of the second antennal segment and also in the body (not shown) are associated with Pyrexia-expressing cap cells and provide input to the central circadian pacemakers via unknown synapses. The morning oscillator (cream shading) is comprised of 4 of the small ventral lateral neurons (sLNvs) and a subset of the first group of dorsal neurons (DN1s). The evening oscillator is comprised of the fifth sLNv neuron, the dorsal lateral neurons (LNds), and a subset of the DN1s. Cryptochrome functions as the central nervous system photoreceptor of the circadian system and is expressed in the sLNvs, the large ventral lateral neurons (lLNvs), a subset of the LNds, and a subset of the DN1s. dTRPA1 functions as a component of a central nervous system temperature sensor for the circadian system. dTrpA1-Gal4 is expressed in the lateral posterior neurons (LPNs) as well as subsets of the sLNvs, the LNds, the DN1s, the second group of dorsal neurons (DN2s), and the third group of dorsal neurons (DN3s).
While pacemaker neurons of all subgroups are capable of sustaining clock gene oscillations, they also differ within and between subgroups in their connectivity, gene expression profiles, and function. For example, only the LNv neurons express pigment dispersing factor (PDF), a major neuropeptide output of circadian system that functions to synchronize the individual circadian clocks.110,111 Pacemaker neurons can also be categorized by their roles in generating or supporting the morning or evening peaks in Drosophila locomotor behavior, which anticipate the daily onset of light and dark respectively.112 Four of the sLNvs are responsible for the morning peak and are considered to be the master oscillators of the Drosophila circadian system, as they alone are sufficient to sustain circadian locomotor rhythms under constant darkness (Fig. 5).113-116 The fifth sLNv and the LNds are responsible for supporting the evening activity peak (Fig. 5).113,114 The DN1s may actually support either the morning or evening peak of activity in an ambient temperature dependent manner.117 Additionally, not all pacemaker neurons share intrinsic light sensitivity, as only subsets of the the LNvs, LNds, and DN1s express Cry, while the photoreceptor is not present at all in the LPNs, DN2s, or DN3s (Fig. 5).105,118,119
Circadian entrainment and phase shifting in response to thermal stimuli
Light is the most thoroughly characterized Zeitgeber in Drosophila, producing robust entrainment of molecular and behavioral rhythms. However, cyclical changes in temperature have also long been known for their powerful ability to shift and entrain behavioral rhythms in Drosophila.104,120,121 It is likely that in a poikilothermic organism such Drosophila, one of the major selective advantages of entraining behavior to a light-dark cycle is that behavior will also be synchronized with concomitant cycles of environmental temperature, allowing the fly to avoid cool nights and hot middays. Given the importance of temperature as an environmental factor influencing Drosophila development and fitness, it is not surprising that environmental temperature may impinge upon the circadian system in multiple different ways. In addition to the Zeitgeber function mentioned above, ambient temperature may play a modulatory role in pacemaker neuron function to alter the precise relationship between light-dark cycles and behavior. Exposure to non-physiological or noxious temperatures may cause phase shifts in the circadian rhythm, likely due to disruption of the molecular interactions between clock components.
An essential feature of the circadian clock is temperature compensation. Although the clock may be entrained or modulated by temperatures within a physiological range, the period length of the molecular and behavioral rhythms themselves are relatively temperature insensitive in wild-type animals.122 For example, in constant darkness, behavioral rhythms are neither sped up by high temperatures nor slowed down by cool temperatures. However, non-physiological temperatures may produce phase shifts or disrupt clock function by acting directly on the clock's molecular components and their interactions. Brief, noxious heat pulses (∼37°C) have been demonstrated to cause dramatic decreases in Per and Tim protein abundance and phase shifts in locomotor activity rhythms.123,124 This phenomenon is not entirely understood, but likely involves disruption of the Per-Tim complex via a Cry-dependent mechanism.125 It is important to note that this mechanism by which non-physiological temperatures impinge on the circadian clock is likely independent from the mechanisms by which temperatures in a physiological range can entrain behavior or modulate entrainment. Temperatures within a physiological range depend, at least in part, upon cellular temperature sensors similar to those used for thermotaxis behavior.
While daily temperature oscillations of relatively low magnitude (<5°C) within a physiological range have long been known to be sufficient to synchronize circadian locomotor rhythms,120 the neural and molecular substrates of this entrainment have remained slightly more elusive. Temperature cycles are sufficient to entrain circadian behavior under constant light or constant dark conditions (i.e., in the absence of light Zeitgebers), and this behavioral entrainment is reflected in the cycling of clock genes in all or almost all pacemaker neurons in the brain.126,127 However, experiments in which flies are exposed to simultaneous light and temperature cycles that are out of phase with each other demonstrate that the that clock gene cycling in the DN and LPN neuron groups tends to remain in phase with temperature cycles, while clock gene cycling in the LNs tends to remain in phase with light cycles.128 These results and those described below suggest the presence of independent temperature- and light-entrainable oscillators in the brain. The hypothesis that the DN and LPN pacemaker neuron groups function as the temperature cycle-sensitive oscillators of the circadian system is strengthened by ablation studies that target the PDF-expressing pacemaker neurons, a manipulation that eliminates the LNv groups, but spares the DN and LPN groups. When the PDF neurons are ablated, animals retain a weak ability to entrain to temperature cycles, and clock gene expression continues to cycle in the LPN and DN groups, indicating that these groups are sufficient to synchronize circadian rhythms of behavior to temperature cycles.126,129 Interestingly, Drosophila adults also display a circadian rhythm in temperature preference, showing a stronger preference for warmer temperatures during the late portion of the light phase than during the dark phase.130 This circadian rhythm is dependent on the DN2 neurons, which also control entrainment to environmental temperature rhythms in larvae.130,131
The potential presence of separate light- and temperature-entrainable oscillators in the fly brain raises interesting questions about how light and temperature information are integrated in the circadian system. For instance, how are light-entrained rhythms modulated by temperature and vice versa? In studies that use a paradigm in which flies are exposed to simultaneous light and temperature cycles that are 12 hours out of phase with each other, clock gene oscillation largely depended on Cry expression. Cyclic expression of Tim in Cry-expressing neurons tended to remain in phase with the light-dark cycle, while expression of Tim in Cry-negative cells stayed in phase with the temperature cycles.132 Interestingly, when this experiment was performed in mutants lacking Cry, all pacemaker neurons showed cyclical Tim expression in phase with temperature cycles.132 Consistent results were obtained in behavioral experiments in which behavior largely entrained to the light-dark cycle when light and temperature cycles were out of phase, but readily entrained to the temperature cycle when the Cry-expressing neurons were ablated.132 Subsequent experiments have shown that the DN and LNd groups of pacemaker neurons are capable of supporting behavioral entrainment to temperature cycles under constant darkness conditions, but not under constant light conditions.133 However, in a cry mutant background, the DN and LNd were sufficient to support behavioral entrainment to temperature cycles during constant light conditions.133 Together these experiments support a model in which most pacemaker neurons can entrain to temperature cycles, but Cry antagonizes this entrainment in a light-dependent manner in a subset of them. As such, flies will generally entrain to light-dark cycles instead of temperature cycles when the 2 are out of phase, but will readily entrain to temperature cycles in the absence of Cry function.
Ambient temperature within a physiological range may also modulate entrainment of the circadian clock in a non-Zeitgeber fashion. For instance, flies entrained to a light-dark cycle and housed at low temperatures in the physiological range (18°C) display a phase-advanced evening peak of activity, while those housed at a high physiological temperature (29°C) display a phase-delayed activity peak in the evening.134 The molecular basis for this shift depends at least in part on regulated splicing of the 3′ end of the per mRNA.134 The splicing event is not restricted at low temperatures and short day length, but inhibited synergistically by high temperature and long day length.135,136 Thus Per can accumulate more quickly and advance the circadian clock under cool and short-day conditions. Interestingly, this splicing event is also highly dependent on NorpA function outside of the visual system.135,136 Another example of this temperature modulation of circadian entrainment has been observed specifically in the DN1 neurons.117 The DN1s are sufficient to support morning and evening peaks of behavior when entrained to a light dark cycle, but these peaks are differentially sensitive to ambient temperature- the morning peak is suppressed at low ambient temperatures, while the evening peak is suppressed by high temperature.117 These types of adaptations, in which ambient temperature has a modulatory effect on entrainment, may allow flies to adapt to seasonal changes in day length, advancing their evening peak of activity to avoid cold winter nights and delaying their evening peak to avoid hot summer afternoons.104,134,135
Peripheral temperature sensors and the circadian system
Drosophila are capable of entraining to relatively low-amplitude temperature rhythms that exist well within the temperature compensation range of the molecular clock and which do not appear to impinge directly on its cycling.120 Thus identifying the temperature sensor or sensors that provide input to the circadian system is an important goal for understanding temperature entrainment. In some tissues, the molecular clock appears to detect temperature oscillations using a cell-autonomous mechanism.137 Molecular oscillations of clock gene activity can be assayed by measuring luciferase activity in tissue cultured from transgenic flies expressing luciferase under the control of per regulatory sequences. In these assays, cycles of luciferase activity in cultured peripheral tissues can be entrained to environmental temperature cycles, suggesting the presence of a tissue-intrinsic temperature sensor in the circadian clock of many types of circadian tissues.137 However, the pacemaker neurons in the central nervous system do not appear capable of entraining to temperature oscillations in this ex vivo system, suggesting that peripheral input is required.104,138
The role of the peripheral nervous system, specifically the chordotonal organs, in providing information about temperature cycles to the central circadian oscillator was clarified by the discovery of mutants for the nocte gene, which display strong defects in their ability to synchronize locomotor rhythms to cyclical changes in temperature and in their ability to phase shift appropriately in response to temperature pulses.137 The nocte locus encodes a large glutamine-rich protein that is broadly expressed in the adult fly, notably in the peripheral nervous system, as determined by GAL4 reporter-driven GFP expression. Expression of RNAi targeting nocte in the chordotonal organ neurons, external sensory organ neurons, and a small population of brain neurons produced the same defect in temperature entrainment as that observed in the nocte mutant.137 The chordotonal organs of nocte mutants were further shown to have structural defects and mislocalization of structural proteins, and mutants for genes encoding additional structural components also show defects in circadian temperature entrainment.137 These pieces of evidence combined point to the chordotonal organs as the peripheral temperature sensors that provide information to the pacemaker neurons for temperature entrainment (Fig. 5). This is consistent with behavioral experiments demonstrating a role for the larval chordotonal neurons in behavioral avoidance of cool temperatures and also with calcium imaging experiments demonstrating that the chordotonal organ neurons show calcium transients in response to relatively small temperature deflections within the comfortable range.35,73
The TRPA channel, Pyrexia, is a candidate component of the temperature sensor of the chordotonal organs for their role in circadian entrainment to temperature cycles. GAL4 reporters using pyrexia promoter sequences are expressed in the cap cells of the adult chordotonal organs and in a putative population of second antennal segment neurons that synapse on the AC neurons in the brain.33,59,61 Mutants lacking Pyrexia function show defects in synchronizing their behavior to temperature cycles, and these defects are specific to low temperatures, as pyrexia mutants are unable to entrain to 16°C–20°C temperature cycles, but are unaffected in their ability to entrain to cycles that include higher temperatures.60 This result is consistent with a model in which multiple peripheral and central temperature sensors provide input to the pacemaker neurons of the brain to allow accurate circadian entrainment over a broad range of temperatures (Fig. 5).
The central targets of the chordotonal organ neurons and _pyrexia_-expressing neurons in the circadian system of the brain are unknown, and thus the circuit-level mechanism for the function of multiple circadian temperature sensors remains to be elucidated. It is also important to note that Pyrexia's function in circadian entrainment seems to occur at temperatures that are much lower than those that have been demonstrated to activate Pyrexia in heterologous cells (<20°C vs. >40°C),33 suggesting that Pyrexia alone may not sense temperature directly, but instead may be a component of a heat-sensitive complex or signaling pathway. Additionally, if Pyrexia functions in the cap cells of the chordotonal organs to support entrainment to temperature cycles, it is unclear how this non-neural site of action would produce signals that are then transmitted to central circadian oscillators. The cap cells of the chordotonal organs are tightly coupled to chordotonal neurons via extracellular matrix and play an essential role in neural morphogenesis.139,140 Thus it is possible that Pyrexia function in the cap cells impinges on chordotonal neuron function or development.
Central temperature sensors and the circadian system
In addition to an extrinsic temperature sensor located in peripheral sensory neurons, some clock neurons also use a cell-intrinsic temperature sensor to synchronize their circadian clock with environmental temperature cycles. A subset of pacemaker neurons express a GAL4 reporter for dTrpA1 (Fig. 5).141,142 These _dTrpA1-Gal4_-expressing cells include the LPNs, the non-cryptochrome-expressing LNds, and small subset of DN neurons (Fig. 5). dTrpA1 mutants display a mild, but statistically significant, defect in behavioral entrainment to warm-cool temperature cycles as well as defects in rhythmic Per protein accumulation under these conditions.141 Interestingly, when the _cry_-expressing pacemaker neurons are ablated in a dTrpA1 mutant background, entrainment of locomotor behavior to temperature cycles is lost entirely.141 This suggests a potential model in which 2 independent temperature sensors provide partially redundant temperature information to different populations of clock neurons for entrainment of behavior to temperature cycles. One temperature sensor appears to be contained within the _dTrpA1_-positive, _cry_-negative clock neurons (i.e. the LPNs and LNds), while a second temperature sensor appears to act through the _cry_-positive pacemaker neurons. It is possible that these neurons are the targets of temperature information from the chordotonal organs or other extrinsic temperature sensors.
While dTRPA1-expressing clock cells appear to comprise a temperature sensor for circadian entrainment, the temperature-activation properties of dTRPA1 do not appear to be required for this feature. First, dTrpA1 mutants are defective entraining to temperature cycles that are below the characterized temperature activation threshold of the dTRPA1 channel (16°C–25°C), suggesting that the channel is not directly sensing the environmental temperature change.141 Second, the temperature entrainment defect of the dTrpA1 mutant can be rescued by expression of the dTRPA1-B isoform,141 which does not respond to elevated temperature when expressed in S2R+ cells.50 This information suggests that dTRPA1 may actually function downstream of some other primary temperature sensor. While the identity of this temperature sensor and the mechanism of dTRPA1 activation in the transmission of temperature information is unknown, it is reasonable to hypothesize the presence of a Gαq-PLC signaling cascade that acts upstream of dTRPA1, as such a mechanism has been identified for the transmission of thermal information for thermotaxis80 and mutants for the Drosophila PLC, NorpA, also show defects in entrainment to temperature cycles.137,143
Escape Responses to Noxious Temperatures
In addition to navigating toward optimal body temperatures and avoiding those higher or lower than the optimal range, Drosophila larvae and adults also execute rapid escape responses to temperatures that have the potential to cause acute tissue damage. The best understood example of this behavior is the nocifensive escape locomotion (NEL) performed by Drosophila larvae in response to noxious high temperature,18 as well as in response to noxious mechanical stimulation144 and short-wavelength light.145 During NEL, larvae halt their normal peristaltic locomotion and execute a series of lateral rolls around their long body axis. The principle sensory neurons that detect noxious stimuli to evoke NEL are the Class IV multidendritic neurons (mdIV), which extend elaborate dendritic arborizations that tile the larval body wall.146 While the ethological relevance of this behavior is not fully understood, it is suggested that NEL may help Drosophila larvae avoid parasitization following ovipositor penetration by parasitoid wasps.146,147
Painless mediates larval responses to noxious heat
The first identified nociception-defective mutants were those in the Painless gene, which encodes a TRPA channel that is not directly orthologous to the vertebrate TRPA1 channel (Table 1), but does have orthologs in other insect species. Mutant larvae lacking functional Painless channels show significant defects in NEL responses to thermal stimuli above 39°C as well as to harsh mechanical stimuli.18 When expressed in HEK293 cells, the Painless channel mediates a calcium-dependent, heat-activated current with a threshold of ∼42°C in the presence of intracellular calcium.32 This temperature threshold is similar to the threshold observed for the stimulation of NEL as well as for multidendritic neuron action potential firing (∼39°C), as observed by extracellular recording.18 These results raise the intriguing possibility that Painless is the primary noxious heat sensor in the mdIV neurons for nociception behavior, although this hypothesis has not yet been directly tested.
As with the Drosophila TrpA1 gene, the Painless gene produces multiple transcription units via alternative splicing and the use of alternative transcription start sites.58 The longest isoform (Painlessp103) possesses a 468 amino acid intracellular N-terminal domain that contains 8 ankyrin repeats, while the shorter Painlessp72 and Painlessp60 isoforms possess 183 amino acid and 83 amino acid N-terminal domains respectively (Fig. 2).58 These multiple isoforms appear to have important consequences for nociceptor function, as the Painlessp103 and Painlessp60 isoforms show distinct subcellular localization patterns and roles in thermal and mechanical nociception. When tagged with a C-terminal Venus Fluorescent Protein (VFP), the Painlessp103 isoform localizes to the cell bodies, axons, and dendrites of larval multidendritic neurons and is sufficient to rescue the thermal nociception defect, but not the mechanical nociception defect, of a painless mutant. Conversely, the VFP-tagged Painlessp60 isoform localizes exclusively to the cell bodies of multidendritic neurons and rescues the mechanical nociception defects, but not the thermal nociception defects, of a painless mutant.58 These results support an important role for mRNA processing of ion channel genes in shaping sensory neuron sensitivity, but important questions remain. For instance, the expression patterns and relative expression levels of each Painless isoform are not known. Additionally, while Painlessp103 is a bona fide temperature-activated channel,32 the intrinsic properties of the other isoforms are unknown. Finally, the contributions of differences in channel subcellular localization to function remain to be elucidated.
Specific isoforms of dTPRA1 determine the sensitivity of larval nociceptors
In vertebrates, the TRPA1 channel plays a central role in nociceptive signaling, with imputed roles in chemical, mechanical, and cold nociception.39,148 It is also an essential mediator of chronic pain following tissue damage and inflammation.41,149 Like its mammalian ortholog, dTRPA1 plays a similarly central role in nociceptive signaling. Mutant larvae lacking dTRPA1 function show defects in NEL responses to both noxious thermal and noxious mechanical stimuli.50,150 Expression of dTrpA1-C/D-Gal4 is restricted almost exclusively to the mdIV neurons in the peripheral nervous system, and expression of the dTRPA1-C cDNA in the mdIV neurons of dTrpA1 mutant larvae is sufficient to rescue the nociception defective phenotype, indicating that dTRPA1 normally functions in the nociceptor neurons.50
While a role for dTRPA1 in thermal nociception is obvious based on analysis of mutant phenotypes, the exact role of the dTRPA1 channel in transducing/transmitting thermal stimuli is less clear. Of the 4 dTRPA1 isoforms described above, only the –C and –D isoforms appear to be expressed in the mdIV neurons, based on analysis of GAL4 reporters.50 dTRPA1-C is able to restore heat-responsive NEL to a dTrpA1 mutant when expressed in the mdIV neurons, but does not mediate heat-activated calcium influx when expressed heterologously in S2R+ cells.50 The fact that the non-heat-activated dTRPA1-C isoform is sufficient for behavioral responses to noxious heat suggests that the role of dTRPA1 in thermal nociception is not that of a primary sensory transducer. Instead, dTRPA1 may act as an amplifier for thermal stimuli transduced by other heat-activated receptors such as Painless, Gr28B(D), or NinaE. This downstream signaling role of dTRPA1 would be consistent with activation of the vertebrate TRPA1 channel downstream of calcium influx and GPCR signaling and the role of dTRPA1 in larval thermotaxis behavior at 18°C.39,72,151
The role of RNA processing in regulating thermal nociception also remains to be elucidated. Based on the analysis of GAL4 reporter expression, both dTRPA1-C and dTRPA1-D are potentially expressed in the mdIV neurons, but have differing thermal-activation properties.50 In theory, the alternative splicing decision that chooses between dTRPA1-C and –D expression could have a large effect on the thermal sensitivity of the mdIV neurons. The temperature threshold for activation of dTRPA1-D is lower than the temperature threshold for NEL. Thus it is tempting to hypothesize that an increase in dTRPA1-D expression via regulated alternative splicing underlies the decrease in NEL temperature threshold observed following UV-induced damage to the larval epidermis.152,153
Painless and dTRPA1 are required for behavioral responses to noxious heat in adult flies
Adult Drosophila must also avoid noxious thermal stimuli. This behavior is measured by assaying flies’ ability to avoid entering and being paralyzed by the noxious (46°C) portion of a heated chamber.150,154 A genome-wide RNAi screen for genes required for this behavioral avoidance identified both Painless and dTrpA1, as RNAi knockdown of either gene results in a reduced ability to avoid the noxious portion of the chamber (a result that was subsequently confirmed in genetic mutants).150 The sensory neurons and neuronal circuitry underlying the avoidance of noxious heat in adult flies remains incompletely understood. Inhibition of synaptic transmission in _Painless_-expressing neurons results in complete loss of heat avoidance, confirming the importance of _Painless_-expressing cells.150 Refinement of the inhibition of synaptic transmission reveals that the mushroom bodies do not appear to have a role in the behavior, while lesion experiments suggest that the antennae and labella may play minor roles in noxious heat avoidance.150 A possible role for the adult multidendritic neurons has not been described.
Acknowledgments
I would like to thank Dr. Dan Tracey at Duke University and the Department of Biology at Appalachian State University for their support during the research and writing of this review.
About the Author
Andrew Bellemer is an assistant professor in the Department of Biology at Appalachian State University. He completed his postdoctoral training under Dr. Dan Tracey in the Department of Anesthesiology at Duke University, where he studied the role of dTRPA1 in thermal nociception. He is continuing his research in thermal nociception, investigating the cellular and molecular mechanisms that tune sensory neuron function. 
References
- 1.Powsner L. The effects of temperature on the durations of the developmental stages of Drosophila melanogaster. Physiol Zool 1935; 8:474–520. [Google Scholar]
- 2.Delcour J, Lints FA. Environmental and genetic variations of wing size, cell size and cell division rate, inDrosophila melanogaster. Genetica 1966; 37:543-56; http://dx.doi.org/ 10.1007/BF01547152 [DOI] [PubMed] [Google Scholar]
- 3.James AC, Azevedo R, Partridge L. Cellular basis and developmental timing in a size cline of Drosophila melanogaster. Genetics 1995; 140:659-66; PMID:7498744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James AC, Azevedo RB, Partridge L. Genetic and environmental responses to temperature of Drosophila melanogaster from a latitudinal cline. Genetics 1997; 146:881-90; PMID:9215894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuntz SG, Eisen MB. Drosophila embryogenesis scales uniformly across temperature in developmentally diverse species. PLoS Genet 2014; 10:e1004293; PMID:24762628; http://dx.doi.org/ 10.1371/journal.pgen.1004293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatachalam K, Montell C. TRP channels. Ann Rev Bioch 2007; 76:387-417; PMID:17579562; http://dx.doi.org/ 10.1146/annurev.biochem.75.103004.142819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Julius D. TRP channels and pain. Ann Rev Cell Dev Biol 2013; 29:355-84; PMID:24099085; http://dx.doi.org/ 10.1146/annurev-cellbio-101011-155833 [DOI] [PubMed] [Google Scholar]
- 8.Damann N, Voets T, Nilius B. TRPs in our senses. Curr Biol 2008; 18:R880-9; PMID:18812089; http://dx.doi.org/ 10.1016/j.cub.2008.07.063 [DOI] [PubMed] [Google Scholar]
- 9.Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science 1985; 230:1040-3; http://dx.doi.org/ 10.1126/science.3933112 [DOI] [PubMed] [Google Scholar]
- 10.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron 1989; 2:1313-23; PMID:2516726; http://dx.doi.org/ 10.1016/0896-6273(89)90069-X [DOI] [PubMed] [Google Scholar]
- 11.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature 1969; 224:285-7; PMID:5344615; http://dx.doi.org/ 10.1038/224285a0 [DOI] [PubMed] [Google Scholar]
- 12.Minke B, Wu C-F, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature 1975; 258:84-7; PMID:810728; http://dx.doi.org/ 10.1038/258084a0 [DOI] [PubMed] [Google Scholar]
- 13.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 1992; 8:643-51; PMID:1314617; http://dx.doi.org/ 10.1016/0896-6273(92)90086-S [DOI] [PubMed] [Google Scholar]
- 14.Vaca L, Sinkins WG, Hu Y, Kunze DL, Schilling WP. Activation of recombinant trp by thapsigargin in Sf9 insect cells. Am J Physiol 1994; 267:C1501-5; PMID:7977711 [DOI] [PubMed] [Google Scholar]
- 15.Xu XZ, Li HS, Guggino WB, Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell 1997; 89:1155-64; PMID:9215637; http://dx.doi.org/ 10.1016/S0092-8674(00)80302-5 [DOI] [PubMed] [Google Scholar]
- 16.Bandell M, Macpherson LJ, Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol 2007; 17:490-7; PMID:17706410; http://dx.doi.org/ 10.1016/j.conb.2007.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science 2000; 287:2229-34; http://dx.doi.org/ 10.1126/science.287.5461.2229 [DOI] [PubMed] [Google Scholar]
- 18.Tracey WD Jr., Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell 2003; 113:261-73; PMID:12705873; http://dx.doi.org/ 10.1016/S0092-8674(03)00272-1 [DOI] [PubMed] [Google Scholar]
- 19.Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Šali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor–related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000; 103:525-35; PMID:11081638; http://dx.doi.org/ 10.1016/S0092-8674(00)00143-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Chung YD, Park DY, Choi S, Shin DW, Soh H, Lee HW, Son W, Yim J, Park CS, et al.. A TRPV family ion channel required for hearing in Drosophila. Nature 2003; 424:81-4; PMID:12819662; http://dx.doi.org/ 10.1038/nature01733 [DOI] [PubMed] [Google Scholar]
- 21.Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci 2003; 4:529-39; PMID:12838328; http://dx.doi.org/ 10.1038/nrn1141 [DOI] [PubMed] [Google Scholar]
- 22.Latorre R, Vargas G, Orta G, Brauchi S. Frontiers in neuroscience voltage and temperature gating of thermoTRP channels In: Liedtke WB, Heller S, eds. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL: ): CRC Press Taylor & Francis Group, LLC, 2007. [Google Scholar]
- 23.Montell C. The TRP superfamily of cation channels. Sci STKE 2005; 2005:re3; PMID:15728426 [DOI] [PubMed] [Google Scholar]
- 24.Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, et al.. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell 2002; 9:229-31; PMID:11864597; http://dx.doi.org/ 10.1016/S1097-2765(02)00448-3 [DOI] [PubMed] [Google Scholar]
- 25.Fowler MA, Montell C. Drosophila TRP channels and animal behavior. Life Sci 2013; 92:394-403; PMID:22877650; http://dx.doi.org/ 10.1016/j.lfs.2012.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al.. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003; 112:819-29; PMID:12654248; http://dx.doi.org/ 10.1016/S0092-8674(03)00158-2 [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, et al.. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002; 418:181-6; PMID:12077604; http://dx.doi.org/ 10.1038/nature00882 [DOI] [PubMed] [Google Scholar]
- 28.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, et al.. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002; 418:186-90; PMID:12077606; http://dx.doi.org/ 10.1038/nature00894 [DOI] [PubMed] [Google Scholar]
- 29.Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci 2002; 22:6408-14; PMID:12151520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389:816-24; PMID:9349813; http://dx.doi.org/ 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- 31.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002; 416:52-8; PMID:11882888; http://dx.doi.org/ 10.1038/nature719 [DOI] [PubMed] [Google Scholar]
- 32.Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. Drosophila painless is a Ca2+-requiring channel activated by noxious heat. J Neurosci 2008; 28:9929-38; PMID:18829951; http://dx.doi.org/ 10.1523/JNEUROSCI.2757-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y, Lee Y, Lee J, Bang S, Hyun S, Kang J, Hong ST, Bae E, Kaang BK, Kim J. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet 2005; 37:305-10; PMID:15731759; http://dx.doi.org/ 10.1038/ng1513 [DOI] [PubMed] [Google Scholar]
- 34.Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature 2003; 423:822-3; PMID:12815418; http://dx.doi.org/ 10.1038/423822a [DOI] [PubMed] [Google Scholar]
- 35.Kwon Y, Shen WL, Shim HS, Montell C. Fine thermotactic discrimination between the optimal and slightly cooler temperatures via a TRPV channel in chordotonal neurons. J Neurosci 2010; 30:10465-71; PMID:20685989; http://dx.doi.org/ 10.1523/JNEUROSCI.1631-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenzweig M, Kang K, Garrity PA. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc Natl Acad Sci U S A 2008; 105:14668-73; PMID:18787131; http://dx.doi.org/ 10.1073/pnas.0805041105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallio M, Ofstad TA, Macpherson LJ, Wang JW, Zuker CS. The coding of temperature in the Drosophila brain. Cell 2011; 144:614-24; PMID:21335241; http://dx.doi.org/ 10.1016/j.cell.2011.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004; 427:260-5; PMID:14712238; http://dx.doi.org/ 10.1038/nature02282 [DOI] [PubMed] [Google Scholar]
- 39.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004; 41:849-57; PMID:15046718; http://dx.doi.org/ 10.1016/S0896-6273(04)00150-3 [DOI] [PubMed] [Google Scholar]
- 40.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A 2005; 102:12248-52; PMID:16103371; http://dx.doi.org/ 10.1073/pnas.0505356102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest 2005; 115:2393-401; PMID:16110328; http://dx.doi.org/ 10.1172/JCI25437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motter AL, Ahern GP. TRPA1 is a polyunsaturated fatty acid sensor in mammals. PloS One 2012; 7:e38439; PMID:22723860; http://dx.doi.org/ 10.1371/journal.pone.0038439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sanchez EE, Perez JC, Weissman JS, Julius D. Molecular basis of infrared detection by snakes. Nature 2010; 464:1006-11; PMID:20228791; http://dx.doi.org/ 10.1038/nature08943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev 2005; 19:419-24; PMID:15681611; http://dx.doi.org/ 10.1101/gad.1278205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Anzi B, Tracey WD Jr., Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol 2006; 16:1034-40; PMID:16647259; http://dx.doi.org/ 10.1016/j.cub.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 46.Kwon Y, Kim SH, Ronderos DS, Lee Y, Akitake B, Woodward OM, Guggino WB, Smith DP, Montell C. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr Biol 2010; 20:1672-8; PMID:20797863; http://dx.doi.org/ 10.1016/j.cub.2010.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature 2008; 454:217-20; PMID:18548007; http://dx.doi.org/ 10.1038/nature07001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 2010; 464:597-600; PMID:20237474; http://dx.doi.org/ 10.1038/nature08848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim SH, Lee Y, Akitake B, Woodward OM, Guggino WB, Montell C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc Natl Acad Sci U S A 2010; 107:8440-5; PMID:20404155; http://dx.doi.org/ 10.1073/pnas.1001425107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong L, Bellemer A, Yan H, Ken H, Jessica R, Hwang RY, Pitt GS, Tracey WD. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP Channel. Cell Rep 2012; 1:43-55; PMID:22347718; http://dx.doi.org/ 10.1016/j.celrep.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang K, Panzano VC, Chang EC, Ni L, Dainis AM, Jenkins AM, Regna K, Muskavitch MA, Garrity PA. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature 2012; 481:76-80; http://dx.doi.org/ 10.1038/nature10715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cordero-Morales JF, Gracheva EO, Julius D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc Natl Acad Sci U S A 2011; 108:E1184-91; PMID:21930928; http://dx.doi.org/ 10.1073/pnas.1114124108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jabba S, Goyal R, Sosa-Pagan JO, Moldenhauer H, Wu J, Kalmeta B, Bandell M, Latorre R, Patapoutian A, Grandl J. Directionality of temperature activation in mouse TRPA1 ion channel can be inverted by single-point mutations in ankyrin repeat six. Neuron 2014; 82:1017-31; PMID:24814535; http://dx.doi.org/ 10.1016/j.neuron.2014.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moparthi L, Survery S, Kreir M, Simonsen C, Kjellbom P, Hogestatt ED, Johanson U, Zygmunt PM. Human TRPA1 is intrinsically cold- and chemosensitive with and without its N-terminal ankyrin repeat domain. Proc Natl Acad Sci U S A 2014; 111:16901-6; PMID:25389312; http://dx.doi.org/ 10.1073/pnas.1412689111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clapham DE, Miller C. A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels. Proc Natl Acad Sci U S A 2011; 108:19492-7; PMID:22109551; http://dx.doi.org/ 10.1073/pnas.1117485108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chowdhury S, Jarecki BW, Chanda B. A molecular framework for temperature-dependent gating of ion channels. Cell 2014; 158:1148-58; PMID:25156949; http://dx.doi.org/ 10.1016/j.cell.2014.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu L, Li Y, Wang R, Yin C, Dong Q, Hing H, Kim C, Welsh MJ. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature 2007; 450:294-8; PMID:17994098; http://dx.doi.org/ 10.1038/nature06223 [DOI] [PubMed] [Google Scholar]
- 58.Hwang RY, Stearns NA, Tracey WD. The ankyrin repeat domain of the TRPA protein painless is important for thermal nociception but not mechanical nociception. PloS One 2012; 7:e30090; PMID:22295071; http://dx.doi.org/ 10.1371/journal.pone.0030090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Y, Liu L, Ben-Shahar Y, Jacobs JS, Eberl DF, Welsh MJ. TRPA channels distinguish gravity sensing from hearing in Johnston's organ. Proc Natl Acad Sci U S A 2009; 106:13606-11; PMID:19666538; http://dx.doi.org/ 10.1073/pnas.0906377106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolfgang W, Simoni A, Gentile C, Stanewsky R. The Pyrexia transient receptor potential channel mediates circadian clock synchronization to low temperature cycles in Drosophila melanogaster. Proc Biol Sci 2013; 280:20130959; PMID:23926145; http://dx.doi.org/ 10.1098/rspb.2013.0959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang X, Platt MD, Lagnese CM, Leslie JR, Hamada FN. Temperature integration at the AC thermosensory neurons in Drosophila. J Neurosc 2013; 33:894-901; PMID:23325228; http://dx.doi.org/ 10.1523/JNEUROSCI.1894-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999; 398:436-41; PMID:10201375; http://dx.doi.org/ 10.1038/18906 [DOI] [PubMed] [Google Scholar]
- 63.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, et al.. A TRP channel that senses cold stimuli and menthol. Cell 2002; 108:705-15; PMID:11893340; http://dx.doi.org/ 10.1016/S0092-8674(02)00652-9 [DOI] [PubMed] [Google Scholar]
- 64.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell 1996; 85:651-9; PMID:8646774; http://dx.doi.org/ 10.1016/S0092-8674(00)81232-5 [DOI] [PubMed] [Google Scholar]
- 65.Leung HT, Geng C, Pak WL. Phenotypes of trpl mutants and interactions between the transient receptor potential (TRP) and TRP-like channels in Drosophila. J Neurosci 2000; 20:6797-803; PMID:10995823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell 1988; 54:723-33; PMID:2457447; http://dx.doi.org/ 10.1016/S0092-8674(88)80017-5 [DOI] [PubMed] [Google Scholar]
- 67.Obukhov AG, Harteneck C, Zobel A, Harhammer R, Kalkbrenner F, Leopoldt D, Lückhoff A, Nürnberg B, Schultz G. Direct activation of trpl cation channels by G alpha11 subunits. EMBO J 1996; 15:5833-8; PMID:8918461 [PMC free article] [PubMed] [Google Scholar]
- 68.Hu Y, Schilling WP. Receptor-mediated activation of recombinant Trpl expressed in Sf9 insect cells. Biochem J 1995; 305 (Pt 2):605-11; PMID:7832780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsiokas L. Function and regulation of TRPP2 at the plasma membrane. Am J Physiol Renal Physiol 2009; 297:F1-9; PMID:19244406; http://dx.doi.org/ 10.1152/ajprenal.90277.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson PD. Polycystin: new aspects of structure, function, and regulation. J Am Soc Nephrol 2001; 12:834-45; PMID:11274246 [DOI] [PubMed] [Google Scholar]
- 71.Sayeed O, Benzer S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc Natl Acad Sci U S A 1996; 93:6079-84; PMID:8650222; http://dx.doi.org/ 10.1073/pnas.93.12.6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci 2008; 11:871-3; PMID:18660806; http://dx.doi.org/ 10.1038/nn.2170 [DOI] [PubMed] [Google Scholar]
- 73.Liu L, Yermolaieva O, Johnson WA, Abboud FM, Welsh MJ. Identification and function of thermosensory neurons in Drosophila larvae. Nat Neurosci 2003; 6:267-73; PMID:12563263; http://dx.doi.org/ 10.1038/nn1009 [DOI] [PubMed] [Google Scholar]
- 74.Zars T. Two thermosensors in Drosophila have different behavioral functions. J Comp Physiol A 2001; 187:235-42; PMID:11401203; http://dx.doi.org/ 10.1007/s003590100194 [DOI] [PubMed] [Google Scholar]
- 75.Altner H, Tichy H, Altner I. Lamellated outer dendritic segments of a sensory cell within a poreless thermo- and hygroreceptive sensillum of the insect Carausius morosus. Cell Tissue Res 1978; 191:287-304; PMID:679268; http://dx.doi.org/ 10.1007/BF00222425 [DOI] [PubMed] [Google Scholar]
- 76.Altner H, Routil C, Loftus R. The structure of bimodal chemo-, thermo-, and hygroreceptive sensilla on the antenna of Locusta migratoria. Cell Tissue Res 1981; 215:289-308; PMID:7214477 [DOI] [PubMed] [Google Scholar]
- 77.Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci 2000; 20:5981-8; PMID:10934246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Gopfert MC, Ito K. The neural basis of Drosophila gravity-sensing and hearing. Nature 2009; 458:165-71; PMID:19279630; http://dx.doi.org/ 10.1038/nature07810 [DOI] [PubMed] [Google Scholar]
- 79.Ni L, Bronk P, Chang EC, Lowell AM, Flam JO, Panzano VC, Theobald DL, Griffith LC, Garrity PA. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature 2013; 500:580-4; PMID:23925112; http://dx.doi.org/ 10.1038/nature12390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen WL, Kwon Y, Adegbola AA, Luo J, Chess A, Montell C. Function of rhodopsin in temperature discrimination in Drosophila. Science 2011; 331:1333-6; http://dx.doi.org/ 10.1126/science.1198904 [DOI] [PubMed] [Google Scholar]
- 81.Hardie RC, Martin F, Cochrane GW, Juusola M, Georgiev P, Raghu P. Molecular basis of amplification in Drosophila phototransduction: roles for G protein, phospholipase C, and diacylglycerol kinase. Neuron 2002; 36:689-701; PMID:12441057; http://dx.doi.org/ 10.1016/S0896-6273(02)01048-6 [DOI] [PubMed] [Google Scholar]
- 82.Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang BK, et al.. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci 2004; 24:9059-66; PMID:15483124; http://dx.doi.org/ 10.1523/JNEUROSCI.1645-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harmer SL, Panda S, Kay SA. Molecular bases of circadian rhythms. Ann Rev Cell Dev Biol 2001; 17:215-53; PMID:11687489; http://dx.doi.org/ 10.1146/annurev.cellbio.17.1.215 [DOI] [PubMed] [Google Scholar]
- 84.Peschel N, Helfrich-Forster C. Setting the clock–by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett 2011; 585:1435-42; PMID:21354415; http://dx.doi.org/ 10.1016/j.febslet.2011.02.028 [DOI] [PubMed] [Google Scholar]
- 85.Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol 2008; 18:R84-93; PMID:18211849; http://dx.doi.org/ 10.1016/j.cub.2007.11.061 [DOI] [PubMed] [Google Scholar]
- 86.Hardin PE. The circadian timekeeping system of Drosophila. Curr Biol 2005; 15:R714-22; PMID:16139204; http://dx.doi.org/ 10.1016/j.cub.2005.08.019 [DOI] [PubMed] [Google Scholar]
- 87.Helfrich-Forster C. Neurobiology of the fruit fly's circadian clock. Genes Brain Behav 2005; 4:65-76; PMID:15720403; http://dx.doi.org/ 10.1111/j.1601-183X.2004.00092.x [DOI] [PubMed] [Google Scholar]
- 88.Hardin PE, Hall JC, Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 1990; 343:536-40; PMID:2105471; http://dx.doi.org/ 10.1038/343536a0 [DOI] [PubMed] [Google Scholar]
- 89.Allada R, White NE, So WV, Hall JC, Rosbash M. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell 1998; 93:791-804; PMID:9630223; http://dx.doi.org/ 10.1016/S0092-8674(00)81440-3 [DOI] [PubMed] [Google Scholar]
- 90.Darlington TK, Wager-Smith K, Ceriani MF, Staknis D, Gekakis N, Steeves TD, Weitz CJ, Takahashi JS, Kay SA. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science 1998; 280:1599-603; http://dx.doi.org/ 10.1126/science.280.5369.1599 [DOI] [PubMed] [Google Scholar]
- 91.Rutila JE, Suri V, Le M, So WV, Rosbash M, Hall JC. CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell 1998; 93:805-14; PMID:9630224; http://dx.doi.org/ 10.1016/S0092-8674(00)81441-5 [DOI] [PubMed] [Google Scholar]
- 92.Price JL, Blau J, Rothenfluh A, Abodeely M, Kloss B, Young MW. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 1998; 94:83-95; PMID:9674430; http://dx.doi.org/ 10.1016/S0092-8674(00)81224-6 [DOI] [PubMed] [Google Scholar]
- 93.Gekakis N, Saez L, Delahaye-Brown AM, Myers MP, Sehgal A, Young MW, Weitz CJ. Isolation of timeless by PER protein interaction: defective interaction between timeless protein and long-period mutant PERL. Science 1995; 270:811-5; http://dx.doi.org/ 10.1126/science.270.5237.811 [DOI] [PubMed] [Google Scholar]
- 94.Grima B, Lamouroux A, Chelot E, Papin C, Limbourg-Bouchon B, Rouyer F. The F-box protein slimb controls the levels of clock proteins period and timeless. Nature 2002; 420:178-82; PMID:12432393; http://dx.doi.org/ 10.1038/nature01122 [DOI] [PubMed] [Google Scholar]
- 95.Hunter-Ensor M, Ousley A, Sehgal A. Regulation of the Drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell 1996; 84:677-85; PMID:8625406; http://dx.doi.org/ 10.1016/S0092-8674(00)81046-6 [DOI] [PubMed] [Google Scholar]
- 96.Myers MP, Wager-Smith K, Rothenfluh-Hilfiker A, Young MW. Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science 1996; 271:1736-40; http://dx.doi.org/ 10.1126/science.271.5256.1736 [DOI] [PubMed] [Google Scholar]
- 97.Lee C, Parikh V, Itsukaichi T, Bae K, Edery I. Resetting the Drosophila clock by photic regulation of PER and a PER-TIM complex. Science 1996; 271:1740-4; http://dx.doi.org/ 10.1126/science.271.5256.1740 [DOI] [PubMed] [Google Scholar]
- 98.Zeng H, Qian Z, Myers MP, Rosbash M. A light-entrainment mechanism for the Drosophila circadian clock. Nature 1996; 380:129-35; PMID:8600384; http://dx.doi.org/ 10.1038/380129a0 [DOI] [PubMed] [Google Scholar]
- 99.Curtin KD, Huang ZJ, Rosbash M. Temporally regulated nuclear entry of the Drosophila period protein contributes to the circadian clock. Neuron 1995; 14:365-72; PMID:7857645; http://dx.doi.org/ 10.1016/0896-6273(95)90292-9 [DOI] [PubMed] [Google Scholar]
- 100.Yu W, Zheng H, Houl JH, Dauwalder B, Hardin PE. PER-dependent rhythms in CLK phosphorylation and E-box binding regulate circadian transcription. Genes Dev 2006; 20:723-33; PMID:16543224; http://dx.doi.org/ 10.1101/gad.1404406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee C, Bae K, Edery I. PER and TIM inhibit the DNA binding activity of a Drosophila CLOCK-CYC/dBMAL1 heterodimer without disrupting formation of the heterodimer: a basis for circadian transcription. Mol Cell Biol 1999; 19:5316-25; PMID:10409723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zerr DM, Hall JC, Rosbash M, Siwicki KK. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci 1990; 10:2749-62; PMID:2117644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sehgal A, Rothenfluh-Hilfiker A, Hunter-Ensor M, Chen Y, Myers MP, Young MW. Rhythmic expression of timeless: a basis for promoting circadian cycles in period gene autoregulation. Science 1995; 270:808-10; http://dx.doi.org/ 10.1126/science.270.5237.808 [DOI] [PubMed] [Google Scholar]
- 104.Glaser FT, Stanewsky R. Synchronization of the Drosophila circadian clock by temperature cycles. Cold Spring Harb Sym Quant Biol 2007; 72:233-42; PMID:18419280; http://dx.doi.org/ 10.1101/sqb.2007.72.046 [DOI] [PubMed] [Google Scholar]
- 105.Egan ES, Franklin TM, Hilderbrand-Chae MJ, McNeil GP, Roberts MA, Schroeder AJ, Zhang X, Jackson FR. An extraretinally expressed insect cryptochrome with similarity to the blue light photoreceptors of mammals and plants. J Neurosci 1999; 19:3665-73; PMID:10233998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 1998; 95:681-92; PMID:9845370; http://dx.doi.org/ 10.1016/S0092-8674(00)81638-4 [DOI] [PubMed] [Google Scholar]
- 107.Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 1998; 95:669-79; PMID:9845369; http://dx.doi.org/ 10.1016/S0092-8674(00)81637-2 [DOI] [PubMed] [Google Scholar]
- 108.Lin FJ, Song W, Meyer-Bernstein E, Naidoo N, Sehgal A. Photic signaling by cryptochrome in the Drosophila circadian system. Mol Cell Biol 2001; 21:7287-94; PMID:11585911; http://dx.doi.org/ 10.1128/MCB.21.21.7287-7294.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ceriani MF, Darlington TK, Staknis D, Mas P, Petti AA, Weitz CJ, Kay SA. Light-dependent sequestration of TIMELESS by CRYPTOCHROME. Science 1999; 285:553-6; http://dx.doi.org/ 10.1126/science.285.5427.553 [DOI] [PubMed] [Google Scholar]
- 110.Helfrich-Forster C, Homberg U. Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J Comp Neurol 1993; 337:177-90; PMID:8276996; http://dx.doi.org/ 10.1002/cne.903370202 [DOI] [PubMed] [Google Scholar]
- 111.Helfrich-Forster C. Development of pigment-dispersing hormone-immunoreactive neurons in the nervous system of Drosophila melanogaster. J Comp Neurol 1997; 380:335-54; PMID:9087517; http://dx.doi.org/ 10.1002/(SICI)1096-9861(19970414)380:3%3c335::AID-CNE4%3e3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- 112.Engelmann W, Mack J. Different oscillators control the circadian rhythm of eclosion and activity inDrosophila. J Comp Physiol 1978; 127:229-37; http://dx.doi.org/ 10.1007/BF01350113 [DOI] [Google Scholar]
- 113.Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 2004; 431:862-8; PMID:15483615; http://dx.doi.org/ 10.1038/nature02926 [DOI] [PubMed] [Google Scholar]
- 114.Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 2004; 431:869-73; PMID:15483616; http://dx.doi.org/ 10.1038/nature02935 [DOI] [PubMed] [Google Scholar]
- 115.Rieger D, Shafer OT, Tomioka K, Helfrich-Forster C. Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J Neurosci 2006; 26:2531-43; PMID:16510731; http://dx.doi.org/ 10.1523/JNEUROSCI.1234-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rieger D, Wulbeck C, Rouyer F, Helfrich-Forster C. Period gene expression in four neurons is sufficient for rhythmic activity of Drosophila melanogaster under dim light conditions. J Biol Rhythms 2009; 24:271-82; PMID:19625729; http://dx.doi.org/ 10.1177/0748730409338508 [DOI] [PubMed] [Google Scholar]
- 117.Zhang Y, Liu Y, Bilodeau-Wentworth D, Hardin PE, Emery P. Light and temperature control the contribution of specific DN1 neurons to Drosophila circadian behavior. Curr Biol 2010; 20:600-5; PMID:20362449; http://dx.doi.org/ 10.1016/j.cub.2010.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Benito J, Houl JH, Roman GW, Hardin PE. The blue-light photoreceptor CRYPTOCHROME is expressed in a subset of circadian oscillator neurons in the Drosophila CNS. J Biol Rhythms 2008; 23:296-307; PMID:18663237; http://dx.doi.org/ 10.1177/0748730408318588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Emery P, Stanewsky R, Helfrich-Forster C, Emery-Le M, Hall JC, Rosbash M. Drosophila CRY is a deep brain circadian photoreceptor. Neuron 2000; 26:493-504; PMID:10839367; http://dx.doi.org/ 10.1016/S0896-6273(00)81181-2 [DOI] [PubMed] [Google Scholar]
- 120.Wheeler DA, Hamblen-Coyle MJ, Dushay MS, Hall JC. Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J Biol Rhythms 1993; 8:67-94; PMID:8490212; http://dx.doi.org/ 10.1177/074873049300800106 [DOI] [PubMed] [Google Scholar]
- 121.Matsumoto A, Matsumoto N, Harui Y, Sakamoto M, Tomioka K. Light and temperature cooperate to regulate the circadian locomotor rhythm of wild type and period mutants of Drosophila melanogaster. J Insect Physiol 1998; 44:587-96; PMID:12769941; http://dx.doi.org/ 10.1016/S0022-1910(98)00046-8 [DOI] [PubMed] [Google Scholar]
- 122.Konopka RJ, Pittendrigh C, Orr D. Reciprocal behaviour associated with altered homeostasis and photosensitivity of Drosophila clock mutants. J Neurogenet 1989; 6:1-10; PMID:2506319; http://dx.doi.org/ 10.3109/01677068909107096 [DOI] [PubMed] [Google Scholar]
- 123.Edery I, Rutila JE, Rosbash M. Phase shifting of the circadian clock by induction of the Drosophila period protein. Science 1994; 263:237-40; http://dx.doi.org/ 10.1126/science.8284676 [DOI] [PubMed] [Google Scholar]
- 124.Sidote D, Majercak J, Parikh V, Edery I. Differential effects of light and heat on the Drosophila circadian clock proteins PER and TIM. Mol Cell Biol 1998; 18:2004-13; PMID:9528772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaushik R, Nawathean P, Busza A, Murad A, Emery P, Rosbash M. PER-TIM interactions with the photoreceptor cryptochrome mediate circadian temperature responses in Drosophila. PLoS Biol 2007; 5:e146; PMID:17535111; http://dx.doi.org/ 10.1371/journal.pbio.0050146 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Yoshii T, Heshiki Y, Ibuki-Ishibashi T, Matsumoto A, Tanimura T, Tomioka K. Temperature cycles drive Drosophila circadian oscillation in constant light that otherwise induces behavioural arrhythmicity. Eur J Neurosci 2005; 22:1176-84; PMID:16176360; http://dx.doi.org/ 10.1111/j.1460-9568.2005.04295.x [DOI] [PubMed] [Google Scholar]
- 127.Yoshii T, Vanin S, Costa R, Helfrich-Forster C. Synergic entrainment of Drosophila's circadian clock by light and temperature. J Biol Rhythms 2009; 24:452-64; PMID:19926805; http://dx.doi.org/ 10.1177/0748730409348551 [DOI] [PubMed] [Google Scholar]
- 128.Miyasako Y, Umezaki Y, Tomioka K. Separate sets of cerebral clock neurons are responsible for light and temperature entrainment of Drosophila circadian locomotor rhythms. J Biol Rhythms 2007; 22:115-26; PMID:17440213; http://dx.doi.org/ 10.1177/0748730407299344 [DOI] [PubMed] [Google Scholar]
- 129.Busza A, Murad A, Emery P. Interactions between circadian neurons control temperature synchronization of Drosophila behavior. J Neurosci 2007; 27:10722-33; PMID:17913906; http://dx.doi.org/ 10.1523/JNEUROSCI.2479-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaneko H, Head LM, Ling J, Tang X, Liu Y, Hardin PE, Emery P, Hamada FN. Circadian rhythm of temperature preference and its neural control in Drosophila. Curr Biol 2012; 22:1851-7; PMID:22981774; http://dx.doi.org/ 10.1016/j.cub.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Picot M, Klarsfeld A, Chelot E, Malpel S, Rouyer F. A role for blind DN2 clock neurons in temperature entrainment of the Drosophila larval brain. J Neurosci 2009; 29:8312-20; PMID:19571122; http://dx.doi.org/ 10.1523/JNEUROSCI.0279-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoshii T, Hermann C, Helfrich-Forster C. Cryptochrome-positive and -negative clock neurons in Drosophila entrain differentially to light and temperature. J Biol Rhythms 2010; 25:387-98; PMID:21135155; http://dx.doi.org/ 10.1177/0748730410381962 [DOI] [PubMed] [Google Scholar]
- 133.Gentile C, Sehadova H, Simoni A, Chen C, Stanewsky R. Cryptochrome antagonizes synchronization of Drosophila's circadian clock to temperature cycles. Curr Biol 2013; 23:185-95; PMID:23333312; http://dx.doi.org/ 10.1016/j.cub.2012.12.023 [DOI] [PubMed] [Google Scholar]
- 134.Majercak J, Sidote D, Hardin PE, Edery I. How a circadian clock adapts to seasonal decreases in temperature and day length. Neuron 1999; 24:219-30; PMID:10677039; http://dx.doi.org/ 10.1016/S0896-6273(00)80834-X [DOI] [PubMed] [Google Scholar]
- 135.Collins BH, Rosato E, Kyriacou CP. Seasonal behavior in Drosophila melanogaster requires the photoreceptors, the circadian clock, and phospholipase C. Proc Natl Acad Sci U S A 2004; 101:1945-50; PMID:14766972; http://dx.doi.org/ 10.1073/pnas.0308240100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Majercak J, Chen WF, Edery I. Splicing of the period gene 3'-terminal intron is regulated by light, circadian clock factors, and phospholipase C. Mol Cell Biol 2004; 24:3359-72; PMID:15060157; http://dx.doi.org/ 10.1128/MCB.24.8.3359-3372.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Glaser FT, Stanewsky R. Temperature synchronization of the Drosophila circadian clock. Curr Biol 2005; 15:1352-63; PMID:16085487; http://dx.doi.org/ 10.1016/j.cub.2005.06.056 [DOI] [PubMed] [Google Scholar]
- 138.Sehadova H, Glaser FT, Gentile C, Simoni A, Giesecke A, Albert JT, Stanewsky R. Temperature entrainment of Drosophila's circadian clock involves the gene nocte and signaling from peripheral sensory tissues to the brain. Neuron 2009; 64:251-66; PMID:19874792; http://dx.doi.org/ 10.1016/j.neuron.2009.08.026 [DOI] [PubMed] [Google Scholar]
- 139.Mrkusich EM, Osman ZB, Bates KE, Marchingo JM, Duman-Scheel M, Whitington PM. Netrin-guided accessory cell morphogenesis dictates the dendrite orientation and migration of a Drosophila sensory neuron. Development 2010; 137:2227-35; PMID:20530550; http://dx.doi.org/ 10.1242/dev.047795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Carlson SD, Hilgers SL, Juang JL. Ultrastructure and blood-nerve barrier of chordotonal organs in the Drosophila embryo. J Neurocytol 1997; 26:377-88; PMID:9278867; http://dx.doi.org/ 10.1023/A:1018564904170 [DOI] [PubMed] [Google Scholar]
- 141.Lee Y, Montell C. Drosophila TRPA1 functions in temperature control of circadian rhythm in pacemaker neurons. J Neurosci 2013; 33:6716-25; PMID:23595730; http://dx.doi.org/ 10.1523/JNEUROSCI.4237-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee Y. Contribution of Drosophila TRPA1-expressing neurons to circadian locomotor activity patterns. PloS One 2013; 8:e85189; PMID:24367706; http://dx.doi.org/ 10.1371/journal.pone.0085189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ito C, Goto SG, Tomioka K, Numata H. Temperature entrainment of the circadian cuticle deposition rhythm in Drosophila melanogaster. J Biol Rhythms 2011; 26:14-23; PMID:21252362; http://dx.doi.org/ 10.1177/0748730410391640 [DOI] [PubMed] [Google Scholar]
- 144.Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr Biol 2010; 20:429-34; PMID:20171104; http://dx.doi.org/ 10.1016/j.cub.2009.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 2010; 468:921-6; PMID:21068723; http://dx.doi.org/ 10.1038/nature09576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol 2007; 17:2105-16; PMID:18060782; http://dx.doi.org/ 10.1016/j.cub.2007.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Robertson JL, Tsubouchi A, Tracey WD. Larval defense against attack from parasitoid wasps requires nociceptive neurons. PloS One 2013; 8:e78704; PMID:24205297; http://dx.doi.org/ 10.1371/journal.pone.0078704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006; 50:277-89; PMID:16630838; http://dx.doi.org/ 10.1016/j.neuron.2006.03.042 [DOI] [PubMed] [Google Scholar]
- 149.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006; 124:1269-82; PMID:16564016; http://dx.doi.org/ 10.1016/j.cell.2006.02.023 [DOI] [PubMed] [Google Scholar]
- 150.Neely GG, Keene AC, Duchek P, Chang EC, Wang QP, Aksoy YA, Rosenzweig M, Costigan M, Woolf CJ, Garrity PA, et al.. TrpA1 regulates thermal nociception in Drosophila. PloS One 2011; 6:e24343; PMID:21909389; http://dx.doi.org/ 10.1371/journal.pone.0024343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 2007; 10:277-9; PMID:17259981; http://dx.doi.org/ 10.1038/nn1843 [DOI] [PubMed] [Google Scholar]
- 152.Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol 2009; 19:799-806; PMID:19375319; http://dx.doi.org/ 10.1016/j.cub.2009.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Babcock DT, Shi S, Jo J, Shaw M, Gutstein HB, Galko MJ. Hedgehog signaling regulates nociceptive sensitization. Curr Biol 2011; 21:1525-33; PMID:21906949; http://dx.doi.org/ 10.1016/j.cub.2011.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Neely GG, Hess A, Costigan M, Keene AC, Goulas S, Langeslag M, Griffin RS, Belfer I, Dai F, Smith SB, et al.. A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell 2010; 143:628-38; PMID:21074052; http://dx.doi.org/ 10.1016/j.cell.2010.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature 1999; 397:255-9; PMID:9930700; http://dx.doi.org/ 10.1038/16703 [DOI] [PubMed] [Google Scholar]
- 156.Hardie RC, Franze K. Photomechanical responses in Drosophila photoreceptors. Science 2012; 338:260-3; http://dx.doi.org/ 10.1126/science.1222376 [DOI] [PubMed] [Google Scholar]