Mesenchymal Stem Cells Overexpressing Interleukin-10 Promote Neuroprotection in Experimental Acute Ischemic Stroke (original) (raw)
Abstract
Interleukin (IL)-10 is a contributing factor to neuroprotection of mesenchymal stem cell (MSC) transplantation after ischemic stroke. Our aim was to increase therapeutic effects by combining MSCs and ex vivo IL-10 gene transfer with an adeno-associated virus (AAV) vector using a rat transient middle cerebral artery occlusion (MCAO) model. Sprague-Dawley rats underwent 90 min MCAO followed by intravenous administration of MSCs alone or IL-10 gene-transferred MSCs (MSC/IL-10) at 0 or 3 hr after ischemia reperfusion. Infarct lesions, neurological deficits, and immunological analyses were performed within 7 days after MCAO. 0-hr transplantation of MSCs alone and MSC/IL-10 significantly reduced infarct volumes and improved motor function. Conversely, 3-hr transplantation of MSC/IL-10, but not MSCs alone, significantly reduced infarct volumes (p < 0.01) and improved motor function (p < 0.01) compared with vehicle groups at 72 hr and 7 days after MCAO. Immunological analysis showed that MSC/IL-10 transplantation significantly inhibits microglial activation and pro-inflammatory cytokine expression compared with MSCs alone. Moreover, overexpressing IL-10 suppressed neuronal degeneration and improved survival of engrafted MSCs in the ischemic hemisphere. These results suggest that overexpressing IL-10 enhances the neuroprotective effects of MSC transplantation by anti-inflammatory modulation and thereby supports neuronal survival during the acute ischemic phase.
Keywords: adeno-associated virus, focal cerebral ischemia, gene transfer, intravenous transplantation, interleukin-10, mesenchymal stem cell, neuroprotection
Introduction
Ischemic stroke carries a high mortality and is a leading cause of severe neurological disability. Curative therapeutic options are limited and include intravenous recombinant tissue plasminogen activator and endovascular intervention, whereas cellular therapy has been actively investigated as a new potential treatment for neurological disorders, including ischemic stroke.1, 2, 3, 4
A number of pre-clinical and clinical studies have shown that bone marrow mesenchymal stem cell (MSC) transplantation is neuroprotective and improves motor function after cerebral ischemia.5, 6, 7, 8 In recent MSC-based cell therapy studies for cerebrovascular and cardiovascular diseases, most researchers have emphasized the paracrine action of MSCs rather than their differentiation potential within injured areas.7, 9 Administered MSCs preferentially migrate into injured brain tissue and can produce therapeutic effects via secretion of growth factors and cytokines, such as brain derived neurotrophic factor (BDNF), nerve growth factor (NGF), vascular endothelial growth factor (VEGF), and interleukin (IL)-6.7, 10, 11, 12, 13 In addition, MSCs enhance neuroprotection against cerebral ischemia by upregulating IL-10 expression in peri-ischemic lesion through macrophages, dendritic cells, and peripheral blood mononuclear cells.14, 15, 16 IL-10 is a pleiotropic anti-inflammatory cytokine mainly produced by type-2 helper T cells, which in turn regulate inflammatory reactions. Specifically, IL-10 inhibits macrophage activation, T cell proliferation, and pro-inflammatory cytokine release, including production of interferon-γ (IFN-γ), IL-2, and tumor necrosis factor alpha (TNF-α) from type-1 helper T cells.16, 17
Therapeutic effects of IL-10 administration in experimental stroke models have been reported.18, 19, 20, 21, 22 IL-10 plays a neuroprotective and vasculoprotective role in the cerebrovascular disorder by attenuating pro-inflammatory signals and upregulating anti-apoptotic proteins, such as B cell lymphoma 2 (Bcl-2) and Bcl-extra large (Bcl-xL).23, 24 Therefore, we genetically modified MSCs to secrete abundant IL-10 and hypothesized that they may improve MSC-based cell therapy for cerebral ischemia.
In the present study, we investigated the therapeutic benefit of adeno-associated virus (AAV)-mediated IL-10 overexpression in MSCs transplanted during the acute phase of ischemic stroke.
Results
IL-10 Production by IL-10 Gene-Transferred MSCs In Vitro
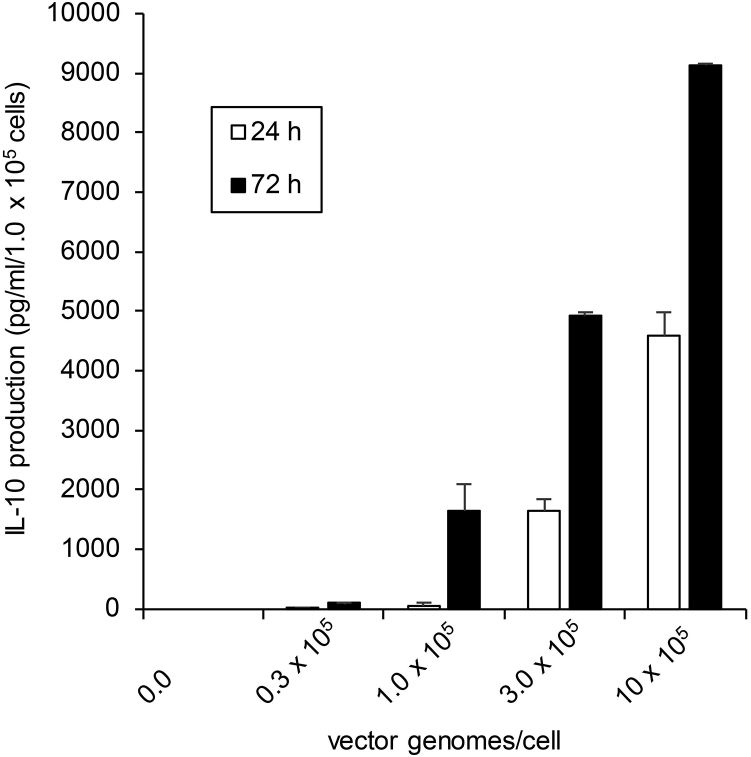
IL-10 concentrations in MSC culture supernatant were measured by ELISA. Nontransfected MSCs did not secrete IL-10 protein at either 24 or 72 hr. After transfection with 1 × 105 vector genomes (vg)/cell, MSCs produced IL-10 protein in the supernatant: 59.6 ± 44.5 pg/mL at 24 hr and 1,631.7 ± 450.8 pg/mL at 72 hr (Figure 1).
Figure 1.
ELISA Quantification of IL-10 In Vitro
IL-10 concentrations were determined in 24 or 72 hr culture supernatants of IL-10 gene-transferred MSCs. 1 × 105 MSCs were transfected with dsAAV1-CAG-rat IL-10 vg at 0, 0.3, 1, 3, and 10 × 105 per cell. Data are presented as mean ± SD. n = 3 for each group.
Infarct Volumes
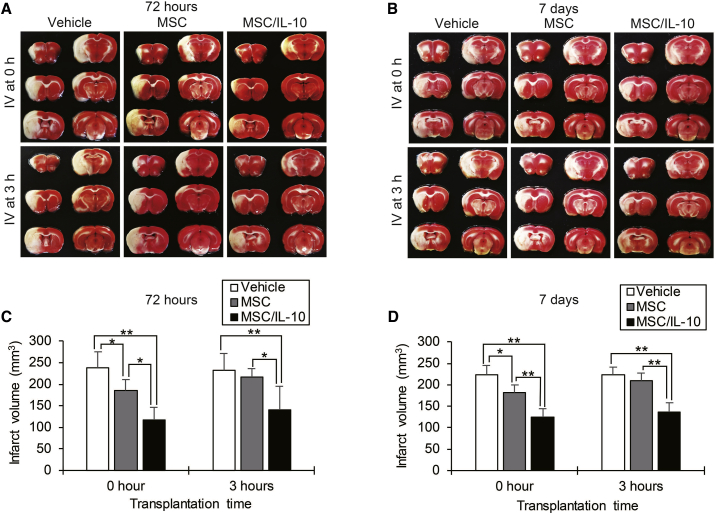
There were a total of four experimental groups: MSC-0 hr, transplanted MSCs alone immediately after reperfusion; MSC-3 hr, transplanted MSCs alone at 3 hr after reperfusion; MSC/IL-10-0 hr, transplanted MSC/IL-10 immediately after reperfusion; and MSC/IL-10-3 hr, transplanted MSC/IL-10 at 3 hr after reperfusion. Infarct volumes (mm3) were assessed in 2,3,5-tripenyltetrazolium chloride (TTC)-stained sections at 72 hr or 7 days after middle cerebral artery occlusion (MCAO) (Figures 2A and 2B). At 72 hr after MCAO, infarct volumes in both MSC-0 hr (185.1 ± 26.3, p < 0.05) and MSC/IL-10-0 hr (117.3 ± 29.5, p < 0.01) were markedly smaller than vehicle (238.1 ± 37.1). In contrast, MSC/IL-10-3 hr (140.8 ± 54.1, p < 0.01), but not MSC-3 hr (217.0 ± 18.6), showed a 39% reduction in infarct volume compared with vehicle (232.4 ± 38.9) (Figure 2C). At 7 days after MCAO, infarct volumes in both MSC-0 hr (180.8 ± 17.8, p < 0.05) and MSC/IL-10-0 hr (124.7 ± 19.1, p < 0.01) were also smaller than in vehicle (223.2 ± 22.3). In contrast, MSC/IL-10-3 hr (137.2 ± 22.4, p < 0.01), but not MSC-3 hr (210.2 ± 17.7), displayed a significant 38% reduction in infarct volume compared with vehicle (222.7 ± 19.5) (Figure 2D).
Figure 2.
Reduction of Infarct Volumes as Detected by TTC Staining
(A and B) Representative photographs showing TTC staining at 72 hr (A) and 7 days (B) after MCAO in vehicle, MSC, or MSC/IL-10-transplanted reperfusion groups at 0 or 3 hr. (C and D) Quantitative analysis of infarct volumes in each group at 72 hr (C) and 7 days (D) after MCAO. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 5 for each group.
Neurological Outcome
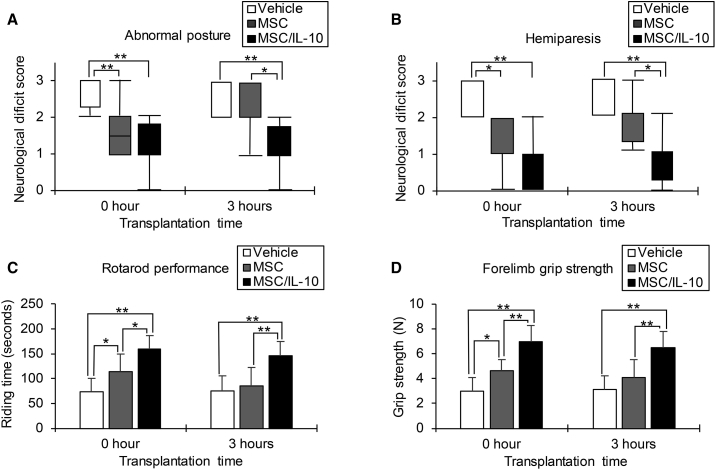
Neurological scores and motor function at 7 days after MCAO are shown (Figure 3). Both MSC-0 hr and MSC/IL-10-0 hr showed significant improvements in posture score (MSC-0 hr, p < 0.01; MSC/IL-10-0 hr, p < 0.01) and palsy score (MSC-0 hr, p < 0.05; MSC/IL-10-0 hr, p < 0.01) compared with vehicle. Motor function was also ameliorated in both MSC-0 hr and MSC/IL-10-0 hr compared with vehicle, as assessed by rotarod performance (MSC-0 hr, p < 0.05; MSC/IL-10-0 hr, p < 0.01) and forelimb grip strength (MSC-0 hr, p < 0.05; MSC/IL-10-0 hr, p < 0.01). However, MSC/IL-10-3 hr, but not MSC-3 hr, showed significant improvements in posture score (p < 0.01) and hemiparesis score (p < 0.01) compared with vehicle. MSC/IL-10-3 hr, but not MSC-3 hr, exhibited remarkable recovery in rotarod performance (p < 0.01) and forelimb grip strength (p < 0.01) compared with vehicle.
Figure 3.
Improvement of Neurological Score and Motor Function
(A and B) Abnormal posture (A) and hemiparesis (B) assessment at 7 days after MCAO in vehicle, MSC, or MSC/IL-10-transplanted reperfusion groups at 0 or 3 hr. Box plots indicate the median and interquartile range, and whiskers indicate the maximum and minimum values (*p < 0.05, **p < 0.01). n = 8 for each group. (C and D) Rotarod performance (C) and forelimb grip strength (D) assessment at 7 days after MCAO in vehicle, MSC, or MSC/IL-10-transplanted reperfusion groups at 0 or 3 hours. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 8 for each group.
Microglial Activation
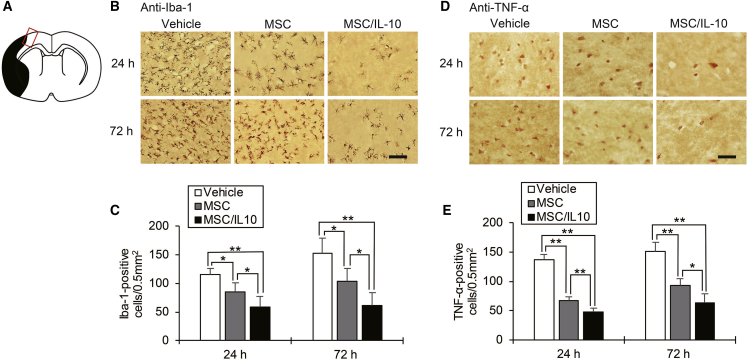
Immunohistochemistry staining of ionized calcium binding adaptor molecule 1 (Iba-1) as a marker of microglial activation in the cortical ischemic boundary zone (IBZ) is shown in Figure 4B. Significantly fewer Iba-1-positive microglia were detected in both MSC-0 hr and MSC/IL-10-0 hr compared with vehicle at 24 hr (MSC-0 hr, p < 0.05; MSC/IL-10-0 hr, p < 0.01) and 72 hr (MSC-0 hr, p < 0.05; MSC/IL-10-0 hr, p < 0.01) after MCAO. Furthermore, the number of Iba-1-positive microglia in MSC/IL-10-0 hr was markedly lower than in MSC-0 hr at 24 hr (p < 0.05) and 72 hr (p < 0.05) after MCAO (Figure 4C).
Figure 4.
Immunohistochemical Staining for Iba-1 and TNF-α in the Cortical Ischemic Boundary Zone
(A) The red-lined square on the brain map shows the cortical ischemic boundary zone. The black area represents the ischemic lesion. (B) Immunohistochemical examination of Iba-1 at 24 and 72 hr after MCAO. Scale bar, 100 μm. (C) Number of immunopositive cells. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 5 for each group. (D) Immunohistochemical examination of TNF-α at 24 and 72 hr after MCAO. Scale bar, 40 μm. (E) Number of immunopositive cells. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 5 for each group.
Pro-inflammatory Cytokine Levels
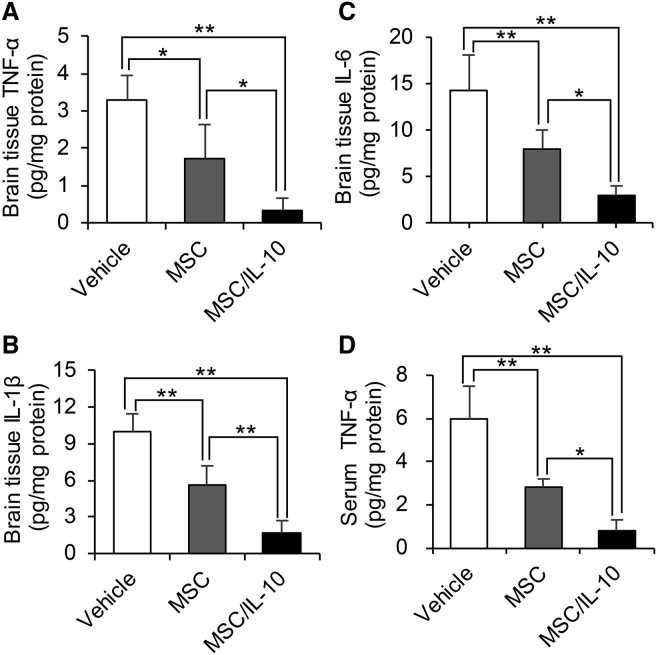
Immunohistochemistry staining of TNF-α in the cortical IBZ is shown in Figure 4D. TNF-α-positive cells in both MSC-0 hr and MSC/IL-10-0 hr were significantly fewer than in vehicle at 24 hr (MSC-0 hr, p < 0.01; MSC/IL-10-0 hr, p < 0.01) and 72 hr (MSC-0 hr, p < 0.01; MSC/IL-10-0 hr, p < 0.01) after MCAO. Furthermore, TNF-α-positive cells in MSC/IL-10-0 hr were fewer than in MSC-0 hr at 24 hr (p < 0.01) and 72 hr (p < 0.05) after MCAO (Figure 4E). TNF-α, IL-1β, and IL-6 levels were also analyzed in brain tissue by ELISA. MSC/IL-10-0 hr markedly suppressed brain tissue TNF-α, IL-1β, and IL-6 levels compared with vehicle (all p < 0.01 versus vehicle) and MSC-0 hr (p < 0.05, p < 0.01, and p < 0.05 versus vehicle, respectively) (Figures 5A–5C). Additionally, MSC/IL-10-0 hr significantly suppressed serum TNF-α levels compared with vehicle (p < 0.01) and MSC-0 hr (p < 0.05) (Figure 5D).
Figure 5.
Effect of MSC Transplantation on ELISA expression of TNF-α, IL-1β, and IL-6 in Brain Tissue Extracts or Serum
(A–C) Quantification of TNF-α (A), IL-1β (B), and IL-6 (C) expression in ischemic hemisphere extracts at 72 hr after MCAO. (D) Quantification of TNF-α expression in serum at 72 hr after MCAO. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 4 for each group.
Ischemia-Induced Neuronal Damage
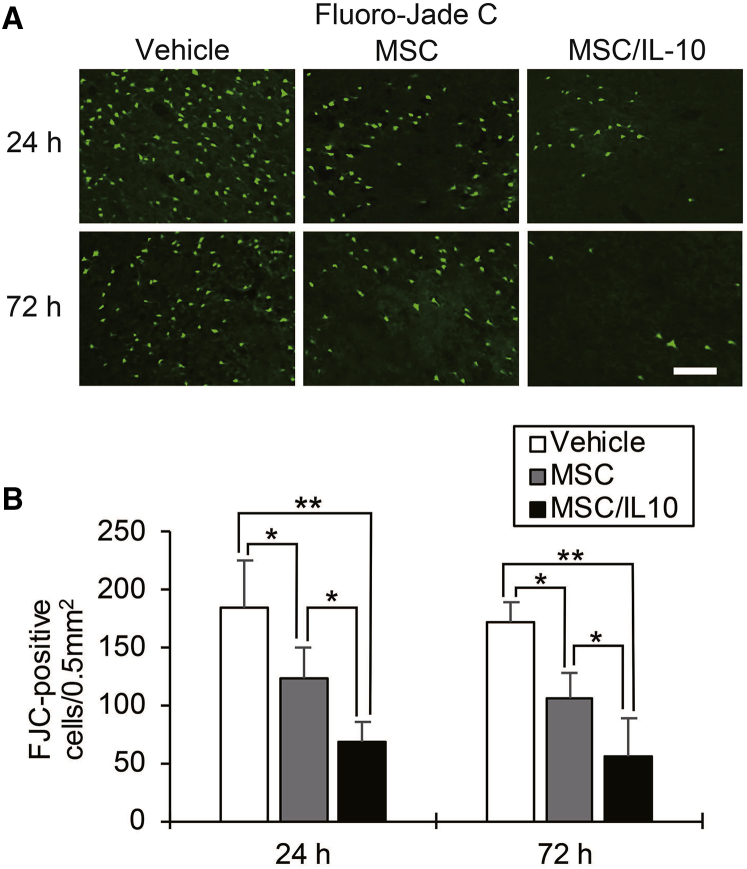
Fluoro-Jade C (FJC) staining of neuronal degeneration in the cortical IBZ is shown in Figure 6A. Significantly fewer FJC-positive cells were detected in both MSC-0 hr and MSC/IL-10-0 hr than in vehicle at 24 hr (MSC-0 hr, p < 0.05; MSC/IL-10-0 hr, p < 0.01) and 72 hr (MSC-0 hr, p < 0.01; MSC/IL-10-0 hr, p < 0.01) after MCAO. Furthermore, the number of FJC-positive cells in MSC/IL-10-0 hr was markedly lower than in MSC-0 hr at 24 hr (p < 0.05) and 72 hr (p < 0.05) after MCAO (Figure 6B).
Figure 6.
Fluoro-Jade C Staining of Neuronal Degeneration in the Cortical Ischemic Boundary Zone
(A) Immunohistochemical examination of Fluoro-Jade C at 24 and 72 hr after MCAO. Scale bar, 100 μm. (B) Number of immunopositive cells. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 5 for each group.
IL-10 Expression in the Ischemic Hemisphere and Serum
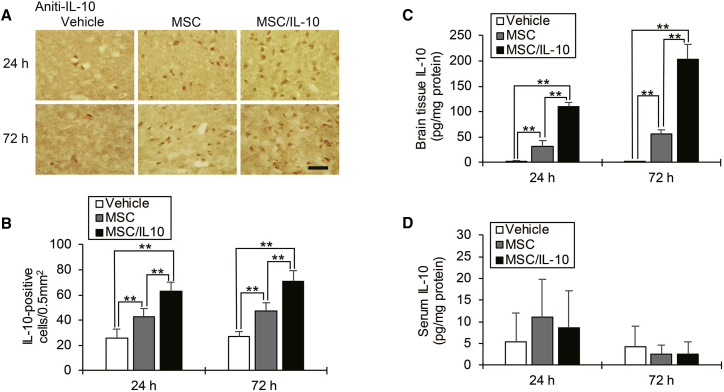
Immunohistochemistry staining of IL-10 in the cortical IBZ is shown in Figure 7A. The number of IL-10-positive cells in both MSC-0 hr and MSC/IL-10-0 hr was significantly more than in vehicle at 24 hr (MSC-0 hr, p < 0.01; MSC/IL-10-0 hr, p < 0.01) and 72 hr (MSC-0 hr, p < 0.01; MSC/IL-10-0 hr, p < 0.01) after MCAO. Furthermore, significantly more IL-10-positive cells were detected in MSC/IL-10-0 hr than in MSC-0 hr at 24 hr (p < 0.01) and 72 hr (p < 0.01) after MCAO (Figure 7B). Again, brain tissue IL-10 levels were analyzed by ELISA at 24 and 72 hr after MCAO. In both MSC-0 hr and MSC/IL-10-0 hr, IL-10 levels were markedly increased compared with vehicle at 24 hr (MSC-0 hr, p < 0.01; MSC/IL-10-0 hr, p < 0.01) and 72 hr (MSC-0 hr, p < 0.01; MSC/IL-10-0 hr, p < 0.01) after MCAO. Furthermore, brain tissue IL-10 levels in MSC/IL-10-0 hr were also higher than in MSC-0 hr at 24 hr (p < 0.01) and 72 hr (p < 0.01) after MCAO (Figure 7C). However, ELISA showed no statistical difference in serum IL-10 levels among groups (Figure 7D).
Figure 7.
IL-10 Expression in the Ischemic Hemisphere and Serum
(A) Immunohistochemical examination of IL-10 at 24 and 72 hr after MCAO. Scale bar, 100 μm. (B) Number of immunopositive cells. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 5 for each group. (C) ELISA quantification of IL-10 expression in the brain at 24 and 72 hr after MCAO. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 4 for each group. (D) ELISA quantification of IL-10 expression in serum at 24 and 72 hr after MCAO. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 4 for each group.
Tracking, Biodistribution, and Quantification of Engrafted MSCs
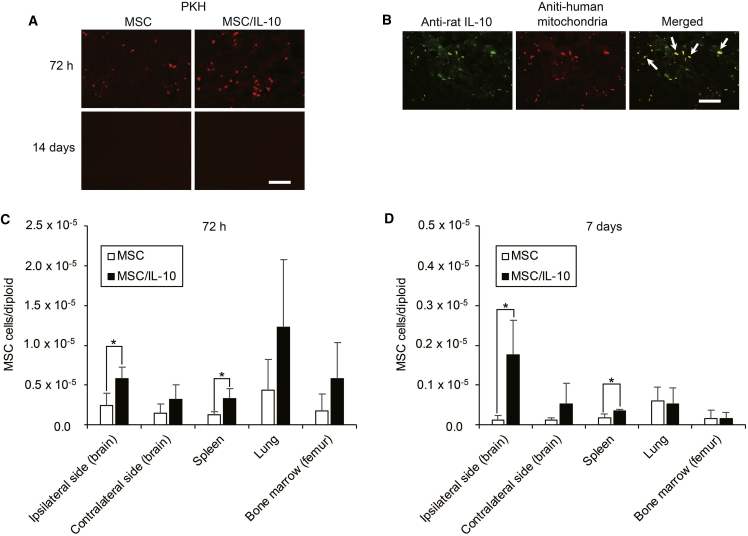
Engrafted MSCs were detected in the ischemic hemisphere by PKH26 labeling and double immunofluorescence analysis. PKH26-labeled cells were detected in both MSC-0 hr and MSC/IL-10-0 hr at 72 hr, whereas none were detected in either group at 14 days after MCAO (Figure 8A). Merged images showed rat IL-10 expressing MSCs in MSC/IL-10-0 hr at 72 hr after MCAO (Figure 8B). Biodistribution and quantification of engrafted MSCs using real-time PCR with human-specific Alu sequences is shown in Figures 8C and 8D. Significantly more human cells were detected in MSC/IL-10-0 hr than in MSC-0 hr at 72 hr and 7 days after MCAO in the ipsilateral hemisphere (72 hr, p < 0.05; 7 days, p < 0.05) and spleen (72 hr, p < 0.05; 7 days, p < 0.05).
Figure 8.
Tracking, Biodistribution, and Quantification of Engrafted MSCs
(A) Tracking of engrafted MSCs and MSC/IL-10 in the ischemic hemisphere by PKH26 labeling at 72 hr and 14 days after MCAO. Fluorescent microscopy detected red fluorescent engrafted cells. Scale bar, 100 μm. (B) Immunofluorescence staining of rat IL-10 (green) and human mitochondria (red) in the ischemic hemisphere of MSC/IL-10-transplanted rats at 72 hr after MCAO. Arrows in the merged image show rat IL-10 expression of engrafted MSC/IL-10. Scale bar, 100 μm. (C and D) Biodistribution and quantification of engrafted MSCs and MSC/IL-10 at 72 hr (C) and 7 days (D) after MCAO using Alu-based real-time PCR. Data are presented as mean ± SD (*p < 0.05). n = 4 for each group.
Discussion
Here, we show that overexpressing IL-10 enhances the neuroprotective effect of MSCs and extends the therapeutic time window of MSC transplantation after transient focal ischemia. Cerebral ischemia evokes a strong inflammatory response that is characterized by rapid microglial activation, production of pro-inflammatory mediators, and infiltration of inflammatory cells into injured brain tissue.25 These reactions play a pivotal role in infarct progression and edema formation in the acute phase of ischemia.26 IL-10, a key anti-inflammatory cytokine in MSC neuroprotection, suppresses expression of various pro-inflammatory cytokines, such as IFN-γ, IL-1β, IL-2, IL-6, and TNF-α, in the inflammatory environment.16, 17, 27 In addition, in vitro cell culture studies have shown that IL-10 reduces apoptosis of primary cortical neurons and neurites after oxygen-glucose deprivation through the upregulation of signal transducer and activator of transcription 3 (STAT-3) and phosphatidylinositol 3-kinase (PI3K)/AKT pathways.28, 29 These pathways mediate neuronal survival and neurite growth by modulating expression of the antiapoptotic proteins Bcl-2 and Bcl-xL.23, 30, 31 Here, as expected, IL-10-overexpressing MSCs significantly reduced infarct volume and enhanced motor functional recovery at 72 hr and 7 days after MCAO. Further, ELISA showed that MSC/IL-10 transplantation after ischemic stroke led to higher IL-10 levels and lower pro-inflammatory cytokine (TNF-α, IL-1β, and IL-6) levels in ischemic brain tissue. Moreover, MSC/IL-10 transplantation elevated IL-10 levels in the ischemic hemisphere without affecting serum IL-10 levels, thereby likely decreasing the risk of systemic IL-10-induced adverse reactions, such as anemia, thrombocytopenia, and immunosuppression.22, 32 MSC/IL-10 transplantation also significantly reduced neuronal damage in the cortical IBZ.
We performed heterologous transplantation of human MSCs into the rat tail vein. Intravenous administration of MSCs is minimally invasive and an effective and safe procedure for stroke patients.33, 34, 35 Recent studies have shown that engrafted human MSCs survive in damaged CNS tissue for a few weeks and exert neuroprotective and immunomodulatory effects in human/rat xenotransplantation models without immunosuppressive drugs.36, 37 We detected intravenously transplanted MSC/IL-10 and non-genetically modified MSCs in the ischemic hemisphere at 72 hr, which disappeared at 14 days after MCAO. Furthermore, we investigated biodistribution and the relative number of engrafted MSCs and MSC/IL-10 using human-specific Alu-based real-time PCR as an established method to detect human DNA in mixed samples from other species.38 Compared with non-genetically modified MSCs, higher numbers of engrafted MSC/IL-10 were detected in the ischemic hemisphere and spleen at 72 hr and 7 days after MCAO. These results indicate that overexpressing IL-10 may improve engrafted MSC survival in the injured brain and spleen. The spleen is known to be associated with systemic inflammatory stroke responses via pro-inflammatory cytokines and chemokine production by splenic lymphocytes, macrophages, and neutrophils.39 Here, ELISA showed that MSC/IL-10 transplantation suppressed serum TNF-α levels as a marker of systemic inflammation. Thus, our results suggest that systemic delivery of MSC/IL-10 may enhance the systemic anti-inflammatory effects of MSCs by greater accumulation, not only in the ischemic hemisphere, but also in the spleen.
We used the AAV vector to transfer the rat IL-10 gene into human MSCs. The AAV vector is derived from a non-pathogenic human virus belonging to the parvovirus family and can transduce non-dividing cells and achieve long-term gene expression in vivo, with minimal immune responses.40 Despite its most frequent use for gene therapy, the AAV vector has the potential risk of cytotoxicity and viral DNA integration into the host genome.41, 42 Although administration of an AAV vector encoding rat IL-10 is reportedly neuroprotective against cerebral ischemia, neutralizing AAV antibodies are found in 20%–40% of adult humans and some animal species.22, 43 In the presence of neutralizing AAV antibodies, more than double the amount of the AAV vector is needed. Random integration of AAV DNA into the host genome occurs at a very low frequency, but gene therapy using a large amount of AAV vector may cause integration of AAV DNA into the host genome. Risk of AAV DNA integration might be avoided by transferring the AAV vector into MSCs instead of direct AAV vector administration.
We found that IL-10 gene transfer extended the ischemic therapeutic time window of MSC transplantation from 0 to 3 hr after ischemia reperfusion. In contrast, recent studies using rat models reported that MSC administration after more than 24 hr of ischemia reperfusion improved motor function recovery in the subacute phase of ischemic stroke.5, 6, 7, 10 Further studies are needed to investigate the therapeutic time window of MSC/IL-10 transplantation after cerebral ischemia and evaluate neurological deficits at the subacute and chronic stages of stroke.
In conclusion, our study suggests that IL-10 gene transfer is desirable to enhance the neuroprotective effects of MSC transplantation during the acute phase of ischemic stroke by suppression of the neuroinflammatory reaction and promotion of host neuronal survival. Combined therapy of AAV-induced IL-10 gene expression and MSC transplantation may be safer than direct IL-10 gene transfer with the AAV vector and be clinically applied for stroke therapy in the future.
Materials and Methods
Plasmid and Vector Construction
The recombinant double-strand AAV vector plasmid pdsAAV-CBA-EGFP was a kind gift from Arun Srivastava (University of Florida College of Medicine). pdsAAV-CAG-EGFP was generated by replacing the chicken beta-actin (CBA) promoter region of pdsAAV-CBA-EGFP with the cytomegalovirus immediate-early enhancer/chicken β-actin hybrid (CAG) promoter region, as previously described.44 Using the In-Fusion HD Cloning Kit (TakaRa Bio), the EGFP region of pdsAAV-CAG-EGFP was replaced with rat IL-10 to generate pdsAAV-CAG-IL-10: rat IL-10 driven by the CAG promoter. Rat IL-10 was PCR cloned from rat splenocyte cDNA, as previously described.24 Recombinant dsAAV vectors (dsAAV1-CAG-IL-10) were generated from cultured serum-free medium using polyethylenimine-based triple transfections of HEK293 cells.45 AAV vector titer was determined by real-time PCR using a 7500 Fast Real-Time Instrument (Applied Biosystems) with rat IL-10 gene primers: forward, 5′-TAAGCTCCAAGACAAAGGGTG-3′; and reverse, 5′-GTCCTCCAGTCCAGTAGATG-3′.
Cell Preparation
Human bone-marrow-derived MSCs were manufactured by JCR Pharmaceuticals. MSCs were cultured at 37°C in 98% humidity and 5% CO2. The culture medium was DMEM (Gibco-BRL) supplemented with 10% fetal bovine serum (FBS) (Gibco-BRL), 1% antibiotic-antimyocotic (Wako), and 50 μg/mL ascorbic acid 2-phosphate (Wako).
Ex Vivo Gene Delivery to MSCs
Second to fourth passage cultured MSCs were placed in each well of a six-well dish (1 × 106 cells). Cells were infected with a viral suspension of 1 × 105 vg/cell, and incubated in FBS-free medium 24 hr before transplantation.
Transient MCAO Model
All experimental procedures were performed according to guidelines approved by the Nippon Medical School Animal Ethics Committee. Male Sprague-Dawley rats (8 weeks; 250–300 g) purchased from Sankyo Labo Service were used. Rats were fasted overnight before and after surgery and had free access to tap water. Anesthesia was initially induced with 5% halothane, and then maintained with 1% halothane in a mixture of 70% N2O and 30% O2 under spontaneous breathing. A polyethylene PE-50 catheter was inserted into the tail artery to monitor the arterial blood gas condition, blood glucose, and mean arterial blood pressure. Levels were measured immediately before, during, and after ischemia. Rats were subjected to 90 min MCAO using an intraluminal suture technique.46 Briefly, the left common, internal carotid, and external carotid arteries were carefully exposed through a midline incision. The common and external carotid arteries were double ligated using 4-0 silk sutures. Focal cerebral ischemia was induced by insertion of a silicone rubber-coated 4-0 nylon thread through the left internal carotid artery for 90 min. Reperfusion was achieved by withdrawing the thread. Temporal muscle temperature was maintained at 37°C ± 0.5°C during the procedure.
Intravenous Transplantation of MSCs
A PE-50 catheter was inserted into the tail vein during ischemia. MSCs (1 × 106 in 1 mL of PBS), MSC/IL-10 (1 × 106 in 1 mL of PBS), or vehicle (1 mL of PBS) was intravenously injected through the catheter at 0 or 3 hr after ischemia reperfusion. No immunosuppressive drugs were used.
Experimental Groups
There were four experimental groups: MSC-0 hr, transplanted MSCs alone immediately after reperfusion; MSC-3 hr, transplanted MSCs alone at 3 hr after reperfusion; MSC/IL-10-0 hr, transplanted MSC/IL-10 immediately after reperfusion; and MSC/IL-10-3 hr, transplanted MSC/IL-10 at 3 hr after reperfusion.
Measurement of Infarct Volumes
To measure infarct volumes, rats were decapitated at 72 hr or 7 days after MCAO (n = 5 for each group). Brains were cut into 2-mm-thick coronal sections and stained with TTC (Wako). Infarct areas were separately determined using ImageJ (NIH). Infarct volumes were calculated by adding total infarct areas.
Behavioral Tests
Neurological symptoms were assessed at 7 days after MCAO by an observer blinded to the study protocol. Hemiparesis and abnormal posture were evaluated using the previously described scoring system.47 The right hind limb of each rat was gently extended with a round-tipped forceps, and the flexor response was scored as 0, normal; 1, slight deficit; 2, moderate deficit; or 3, severe deficit. For assessment posture, rats were suspended by the tail, and forelimb flexion and body twisting was scored as 0, normal; 1, slight twisting; 2, marked twisting; or 3, marked twisting and forelimb flexion. The rotarod test was performed using a rotarod device (Model 7750; Ugo Basile) to examine locomotor coordination ability. Rotating speed of the rod was started at 4 rpm and accelerated to 40 rpm over 5 min with an increase in speed every 30 s. The average reading (in seconds) of three successive trials was obtained for each rat. Preoperative rats that repeatedly fell were placed back onto the rod until they could balance for 150 s. Forelimb grip strength was determined using a grip strength meter (Chatillon DFIS-10; Ametek). The average reading (in newtons) of three successive trials was obtained for each rat.
Immunohistochemistry
At 24 or 72 hr after MCAO, rats were deeply anesthetized and transcardially perfused with heparinized saline followed by 4% paraformaldehyde in PBS (n = 5 for each group). Brains were rapidly removed and coronal cryosections (20 μm) were cut. Briefly, sample sections were incubated with 10% goat serum in PBS to block nonspecific reactions, followed by overnight incubation at 4°C with rabbit polyclonal antibodies against Iba-1 (1:500, Wako), rabbit polyclonal antibody against TNF-α (1:100, Abbiotec), or rabbit polyclonal antibody against IL-10 (1:100, Bioss). Next, sections were processed with biotinylated goat anti-polyvalent (Thermo Fisher Scientific) at room temperature for 1 hr, followed by avidin-biotin-peroxidase complex (Vector Laboratories) for 30 min. Labeled secondary antibodies were visualized using diaminobenzidine. Each process was followed by several brief PBS washes. Immunopositive cells in the cortical IBZ were counted in three randomly chosen square fields (0.5 mm2). To determine the effect of each treatment upon neuronal degeneration, FJC staining was performed according to manufacturer protocols using a commercially available kit (FJC Ready-to-Dilute Staining Kit for Identifying Degenerating Neurons; Biosensis). Positively stained cells in 0.5 mm2 regions in the IBZ adjacent to the ischemic core were counted using a microscope (Eclipse E500W; Nikon) by an investigator blinded to the experimental groups.
ELISA
Concentration of IL-10 in brain tissue extracts from the ischemic hemisphere, serum, and culture supernatant were measured using an ELISA kit (R&D Systems) according to the manufacturer’s instructions. Concentrations of TNF-α, IL-1β, and IL-6 in brain tissue extracts or serum were also measured by ELISA kits (R&D Systems). Colorimetric absorbance was recorded at a wavelength of 450 nm.
PKH26 Labeling and Immunofluorescent Staining
A PKH26 red fluorescent cell linker kit (Sigma-Aldrich) was used to detect engrafted MSCs and MSC/IL-10. Rats injected with PKH26-labeled MSCs or MSC/IL-10 were decapitated at 72 hr or 14 days after MCAO. Frozen brains were cut into 20-μm cryostat sections. For identification of IL-10 secreting MSC/IL-10, brain sections (20 μm) from MSC/IL-10-transplanted rats at 72 hr after MCAO were incubated overnight at 4°C with rabbit polyclonal antibody against IL-10 (1:50) and mouse polyclonal antibody against human mitochondria (1:800, Abcam). Alexa 488 goat anti-rabbit (1:250) or Alexa 568 goat anti-mouse (1:250) (Invitrogen-Life Technologies) was used as the secondary antibody. Sections were protected from light and incubated with secondary antibodies for 1 hr at room temperature. Sections of PKH26-labeled or immunofluorescent-stained samples were mounted using Vectashield (mounting medium for fluorescence) with DAPI (Vector Laboratories). Fluorescent images were captured using a microscope at 200 × magnification (Eclipse E500W; Nikon).
Alu-Based Real-Time PCR
Biodistribution and relative amount of engrafted MSCs were determined using Alu-based real-time PCR. Genomic DNA was prepared from the brain, spleen, lungs, and femoral bone marrow of MSC-transplanted rats using the QIAamp DNA mini kit (QIAGEN). To quantify the amount of human DNA from engrafted MSCs, real-time PCR was performed using human-specific Alu sequence primers: forward, 5′-GTCAGGAGATCGAGACCATCCC-3′; and reverse, 5′-TCCTGCCTCAGCCTCCCAAG-3′. Real-time PCR was performed in 1-μL volumes containing 0.5 μM each primer, 25 μL of SYBER premix Ex Taq (2 ×) (Takara Bio), and 5-μL sample DNA (final 20 ng/μL) using an ABI 7500 System (Applied Biosystems). The PCR protocol consisted of 2 min of denaturation at 95°C, followed by 40 cycles of 95°C for 15 s, 68°C for 30 s, and 72°C for 30 s. To determine the diploid chromosome count of each sample, a housekeeping gene was used, rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The primers for rat GAPDH were forward, 5′-CAACGACCCCTTCATTGACC-3′; and reverse, 5′-GAAGACGCCAGTAGACTCCA-3′. In each sample, the average cell number of engrafted MSCs per diploid was determined.
Statistical Analysis
Data represent mean ± SD. One-way ANOVA followed by the post hoc Tukey honest significant difference (HSD) test was used for comparisons of infarct volumes, rotarod test balance time, forelimb grip strength, immunohistochemical cell counts, and ELISA data. To compare neurological scores, the Wilcoxon rank-sum test was used. Student’s t test was performed to examine real-time PCR data. Differences were considered significant at p < 0.05. JMP9 software (SAS Institute) was used for all statistical analysis.
Author Contributions
M.N. performed most of the experiments, wrote the manuscript, and contributed to the study concept and design. C.N. and T.O. conceived the experiments and wrote the paper. K.S. and A.N.-T. conceived and performed the experiments. M.U., Y.N.-K., S.S., Y.N., K.I., T.H., and K.K. interpreted the data and supervised the project. All authors read and approved the manuscript.
Conflicts of Interest
K.I. and T.H. receive compensation as employees of JCR Pharmaceuticals Co., Ltd. The authors have no other conflicts of interest to declare.
Acknowledgments
This work was supported by grants from JCR Pharmaceuticals Co., Ltd. (Hyogo, Japan) and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (26293328). We thank Arun Srivastava (University of Florida College of Medicine) for providing pdsAAV-CBA-EGFP and Akihiro Umezawa (National Research Institute for Child Health and Development, Tokyo) for technical advice on the design of human-specific Alu primers.
Contributor Information
Chikako Nito, Email: cnito@nms.ac.jp.
Takashi Okada, Email: t-okada@nms.ac.jp.
References
- 1.Skolarus L.E., Meurer W.J., Shanmugasundaram K., Adelman E.E., Scott P.A., Burke J.F. Marked regional variation in acute stroke treatment among Medicare beneficiaries. Stroke. 2015;46:1890–1896. doi: 10.1161/STROKEAHA.115.009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asadi H., Dowling R., Yan B., Wong S., Mitchell P. Advances in endovascular treatment of acute ischaemic stroke. Intern. Med. J. 2015;45:798–805. doi: 10.1111/imj.12652. [DOI] [PubMed] [Google Scholar]
- 3.Yoo J., Kim H.S., Hwang D.Y. Stem cells as promising therapeutic options for neurological disorders. J. Cell. Biochem. 2013;114:743–753. doi: 10.1002/jcb.24427. [DOI] [PubMed] [Google Scholar]
- 4.Liu X., Ye R., Yan T., Yu S.P., Wei L., Xu G., Fan X., Jiang Y., Stetler R.A., Liu G. Cell based therapies for ischemic stroke: from basic science to bedside. Prog. Neurobiol. 2014;115:92–115. doi: 10.1016/j.pneurobio.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vu Q., Xie K., Eckert M., Zhao W., Cramer S.C. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82:1277–1286. doi: 10.1212/WNL.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosseini S.M., Farahmandnia M., Razi Z., Delavarifar S., Shakibajahromi B. 12 hours after cerebral ischemia is the optimal time for bone marrow mesenchymal stem cell transplantation. Neural Regen. Res. 2015;10:904–908. doi: 10.4103/1673-5374.158354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyoshima A., Yasuhara T., Kameda M., Morimoto J., Takeuchi H., Wang F., Sasaki T., Sasada S., Shinko A., Wakamori T. Intra-arterial transplantation of allogeneic mesenchymal stem cells mounts neuroprotective effects in a transient ischemic stroke model in rats: analyses of therapeutic time window and its mechanisms. PLoS One. 2015;10:e0127302. doi: 10.1371/journal.pone.0127302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bang O.Y. Clinical trials of adult stem cell therapy in patients with ischemic stroke. J. Clin. Neurol. 2016;12:14–20. doi: 10.3988/jcn.2016.12.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martire A., Bedada F.B., Uchida S., Pöling J., Krüger M., Warnecke H., Richter M., Kubin T., Herold S., Braun T. Mesenchymal stem cells attenuate inflammatory processes in the heart and lung via inhibition of TNF signaling. Basic Res. Cardiol. 2016;111:54. doi: 10.1007/s00395-016-0573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao M.Z., Nonoguchi N., Ikeda N., Watanabe T., Furutama D., Miyazawa D., Funakoshi H., Kajimoto Y., Nakamura T., Dezawa M. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J. Cereb. Blood Flow Metab. 2006;26:1176–1188. doi: 10.1038/sj.jcbfm.9600273. [DOI] [PubMed] [Google Scholar]
- 11.Kurozumi K., Nakamura K., Tamiya T., Kawano Y., Ishii K., Kobune M., Hirai S., Uchida H., Sasaki K., Ito Y. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol. Ther. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Li Y., Chen J., Chen X.G., Wang L., Gautam S.C., Xu Y.X., Katakowski M., Zhang L.J., Lu M., Janakiraman N. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y., He M., Zhou X., Liu J., Hou N., Bin T., Zhang Y., Li T., Chen J. Endogenous IL-6 of mesenchymal stem cell improves behavioral outcome of hypoxic-ischemic brain damage neonatal rats by supressing apoptosis in astrocyte. Sci. Rep. 2016;6:18587. doi: 10.1038/srep18587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Zhu H., Liu Y., Li Q., Lu S., Feng M., Xu Y., Huang L., Ma C., An Y. Human mesenchymal stem cell transplantation protects against cerebral ischemic injury and upregulates interleukin-10 expression in Macacafascicularis. Brain Res. 2010;1334:65–72. doi: 10.1016/j.brainres.2010.03.080. [DOI] [PubMed] [Google Scholar]
- 15.Liu N., Chen R., Du H., Wang J., Zhang Y., Wen J. Expression of IL-10 and TNF-α in rats with cerebral infarction after transplantation with mesenchymal stem cells. Cell. Mol. Immunol. 2009;6:207–213. doi: 10.1038/cmi.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kyurkchiev D., Bochev I., Ivanova-Todorova E., Mourdjeva M., Oreshkova T., Belemezova K., Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells. 2014;6:552–570. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard M., O’Garra A., Ishida H., de Waal Malefyt R., de Vries J. Biological properties of interleukin 10. J. Clin. Immunol. 1992;12:239–247. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- 18.de Bilbao F., Arsenijevic D., Moll T., Garcia-Gabay I., Vallet P., Langhans W., Giannakopoulos P. In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. J. Neurochem. 2009;110:12–22. doi: 10.1111/j.1471-4159.2009.06098.x. [DOI] [PubMed] [Google Scholar]
- 19.Ooboshi H., Ibayashi S., Shichita T., Kumai Y., Takada J., Ago T., Arakawa S., Sugimori H., Kamouchi M., Kitazono T. Postischemic gene transfer of interleukin-10 protects against both focal and global brain ischemia. Circulation. 2005;111:913–919. doi: 10.1161/01.CIR.0000155622.68580.DC. [DOI] [PubMed] [Google Scholar]
- 20.Liesz A., Bauer A., Hoheisel J.D., Veltkamp R. Intracerebral interleukin-10 injection modulates post-ischemic neuroinflammation: an experimental microarray study. Neurosci. Lett. 2014;579:18–23. doi: 10.1016/j.neulet.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Bodhankar S., Chen Y., Vandenbark A.A., Murphy S.J., Offner H. IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke. Metab. Brain Dis. 2013;28:375–386. doi: 10.1007/s11011-013-9413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang Q.J., Jiang M., Wang X.H., Le L.L., Xiang M., Sun N., Meng D., Chen S.F. Pre-existing interleukin 10 in cerebral arteries attenuates subsequent brain injury caused by ischemia/reperfusion. IUBMB Life. 2015;67:710–719. doi: 10.1002/iub.1429. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Z., Peng X., Insolera R., Fink D.J., Mata M. Interleukin-10 provides direct trophic support to neurons. J. Neurochem. 2009;110:1617–1627. doi: 10.1111/j.1471-4159.2009.06263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nomoto T., Okada T., Shimazaki K., Yoshioka T., Nonaka-Sarukawa M., Ito T., Takeuchi K., Katsura K.I., Mizukami H., Kume A. Systemic delivery of IL-10 by an AAV vector prevents vascular remodeling and end-organ damage in stroke-prone spontaneously hypertensive rat. Gene Ther. 2009;16:383–391. doi: 10.1038/gt.2008.151. [DOI] [PubMed] [Google Scholar]
- 25.Jin R., Yang G., Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinig T.J., Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr. Opin. Neurol. 2009;22:294–301. doi: 10.1097/wco.0b013e32832b4db3. [DOI] [PubMed] [Google Scholar]
- 27.Shichita T., Ago T., Kamouchi M., Kitazono T., Yoshimura A., Ooboshi H. Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. J. Neurochem. 2012;123(Suppl 2):29–38. doi: 10.1111/j.1471-4159.2012.07941.x. [DOI] [PubMed] [Google Scholar]
- 28.Sharma S., Yang B., Xi X., Grotta J.C., Aronowski J., Savitz S.I. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res. 2011;1373:189–194. doi: 10.1016/j.brainres.2010.11.096. [DOI] [PubMed] [Google Scholar]
- 29.Lin L., Chen H., Zhang Y., Lin W., Liu Y., Li T., Zeng Y., Chen J., Du H., Chen R. IL-10 protects neurites in oxygen-glucose-deprived cortical neurons through the PI3K/Akt pathway. PLoS One. 2015;10:e0136959. doi: 10.1371/journal.pone.0136959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isele N.B., Lee H.S., Landshamer S., Straube A., Padovan C.S., Plesnila N., Culmsee C. Bone marrow stromal cells mediate protection through stimulation of PI3-K/Akt and MAPK signaling in neurons. Neurochem. Int. 2007;50:243–250. doi: 10.1016/j.neuint.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Read D.E., Gorman A.M. Involvement of Akt in neurite outgrowth. Cell. Mol. Life Sci. 2009;66:2975–2984. doi: 10.1007/s00018-009-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worthmann H., Tryc A.B., Dirks M., Schuppner R., Brand K., Klawonn F., Lichtinghagen R., Weissenborn K. Lipopolysaccharide binding protein, interleukin-10, interleukin-6 and C-reactive protein blood levels in acute ischemic stroke patients with post-stroke infection. J. Neuroinflammation. 2015;12:13. doi: 10.1186/s12974-014-0231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honmou O., Houkin K., Matsunaga T., Niitsu Y., Ishiai S., Onodera R., Waxman S.G., Kocsis J.D. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J.S., Hong J.M., Moon G.J., Lee P.H., Ahn Y.H., Bang O.Y., STARTING collaborators A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 35.Wang L.Q., Lin Z.Z., Zhang H.X., Shao B., Xiao L., Jiang H.G., Zhuge Q.C., Xie L.K., Wang B., Su D.M. Timing and dose regimens of marrow mesenchymal stem cell transplantation affect the outcomes and neuroinflammatory response after ischemic stroke. CNS Neurosci. Ther. 2014;20:317–326. doi: 10.1111/cns.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribeiro T.B., Duarte A.S., Longhini A.L., Pradella F., Farias A.S., Luzo A.C., Oliveira A.L., Olalla Saad S.T. Neuroprotection and immunomodulation by xenografted human mesenchymal stem cells following spinal cord ventral root avulsion. Sci. Rep. 2015;5:16167. doi: 10.1038/srep16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J.A., Kim B.I., Jo C.H., Choi C.W., Kim E.K., Kim H.S., Yoon K.S., Choi J.H. Mesenchymal stem-cell transplantation for hypoxic-ischemic brain injury in neonatal rat model. Pediatr. Res. 2010;67:42–46. doi: 10.1203/PDR.0b013e3181bf594b. [DOI] [PubMed] [Google Scholar]
- 38.Nicklas J.A., Buel E. Development of an Alu-based, real-time PCR method for quantitation of human DNA in forensic samples. J. Forensic Sci. 2003;48:936–944. [PubMed] [Google Scholar]
- 39.Liu Z.J., Chen C., Li F.W., Shen J.M., Yang Y.Y., Leak R.K., Ji X.M., Du H.S., Hu X.M. Splenic responses in ischemic stroke: new insights into stroke pathology. CNS Neurosci. Ther. 2015;21:320–326. doi: 10.1111/cns.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daya S., Berns K.I. Gene therapy using adeno-associated virus vectors. Clin. Microbiol. Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howard D.B., Powers K., Wang Y., Harvey B.K. Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology. 2008;372:24–34. doi: 10.1016/j.virol.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schultz B.R., Chamberlain J.S. Recombinant adeno-associated virus transduction and integration. Mol. Ther. 2008;16:1189–1199. doi: 10.1038/mt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rapti K., Louis-Jeune V., Kohlbrenner E., Ishikawa K., Ladage D., Zolotukhin S., Hajjar R.J., Weber T. Neutralizing antibodies against AAV serotypes 1, 2, 6, and 9 in sera of commonly used animal models. Mol. Ther. 2012;20:73–83. doi: 10.1038/mt.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noro T., Miyake K., Suzuki-Miyake N., Igarashi T., Uchida E., Misawa T., Yamazaki Y., Shimada T. Adeno-associated viral vector-mediated expression of endostatin inhibits tumor growth and metastasis in an orthotropic pancreatic cancer model in hamsters. Cancer Res. 2004;64:7486–7490. doi: 10.1158/0008-5472.CAN-03-1296. [DOI] [PubMed] [Google Scholar]
- 45.Tomono T., Hirai Y., Okada H., Adachi K., Ishii A., Shimada T., Onodera M., Tamaoka A., Okada T. Ultracentrifugation-free chromatography-mediated large-scale purification of recombinant adeno-associated virus serotype 1 (rAAV1) Mol. Ther. Methods Clin. Dev. 2016;3:15058. doi: 10.1038/mtm.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nito C., Ueda M., Inaba T., Katsura K., Katayama Y. FK506 ameliorates oxidative damage and protects rat brain following transient focal cerebral ischemia. Neurol. Res. 2011;33:881–889. doi: 10.1179/1743132811Y.0000000019. [DOI] [PubMed] [Google Scholar]
- 47.Yonemori F., Yamaguchi T., Yamada H., Tamura A. Evaluation of a motor deficit after chronic focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 1998;18:1099–1106. doi: 10.1097/00004647-199810000-00006. [DOI] [PubMed] [Google Scholar]