Efficacy and Safety of Lampalizumab for Geographic Atrophy Due to Age-Related Macular Degeneration: Chroma and Spectri Phase 3 Randomized Clinical Trials (original) (raw)
Abstract
Importance
Geographic atrophy (GA) secondary to age-related macular degeneration is a leading cause of visual disability in older individuals. A phase 2 trial suggested that lampalizumab, a selective complement factor D inhibitor, reduced the rate of GA enlargement, warranting phase 3 trials.
Objective
To assess the safety and efficacy of lampalizumab vs sham procedure on enlargement of GA.
Design, Setting, and Participants
Two identically designed phase 3 double-masked, randomized, sham-controlled clinical trials, Chroma and Spectri, enrolled participants from August 28, 2014, to October 6, 2016, at 275 sites in 23 countries. Participants were aged 50 years or older, with bilateral GA and no prior or active choroidal neovascularization in either eye and GA lesions in the study eye measuring 2.54 to 17.78 mm2 with diffuse or banded fundus autofluorescence patterns.
Interventions
Participants were randomized 2:1:2:1 to receive 10 mg of intravitreous lampalizumab every 4 weeks, sham procedure every 4 weeks, 10 mg of lampalizumab every 6 weeks, or sham procedure every 6 weeks, through 96 weeks.
Main Outcomes and Measures
Safety and efficacy assessed as mean change from baseline in GA lesion area at week 48 from centrally read fundus autofluorescence images of the lampalizumab arms vs pooled sham arms, in the intent-to-treat population and by complement factor I–profile genetic biomarker.
Results
A total of 906 participants (553 women and 353 men; mean [SD] age, 78.1 [8.1] years) were enrolled in Chroma and 975 participants (578 women and 397 men; mean [SD] age, 77.9 [8.1] years) were enrolled in Spectri; 1733 of the 1881 participants (92.1%) completed the studies through 48 weeks. The adjusted mean increases in GA lesion area from baseline at week 48 were 1.93 to 2.09 mm2 across all groups in both studies. Differences in adjusted mean change in GA lesion area (lampalizumab minus sham) were −0.02 mm2 (95% CI, −0.21 to 0.16 mm2; P = .80) for lampalizumab every 4 weeks in Chroma, 0.16 mm2 (95% CI, 0.00-0.31 mm2; P = .048) for lampalizumab every 4 weeks in Spectri, 0.05 mm2 (95% CI, −0.13 to 0.24 mm2; P = .59) for lampalizumab every 6 weeks in Chroma, and 0.09 mm2 (95% CI, −0.07 to 0.24 mm2; P = .27) for lampalizumab every 6 weeks in Spectri. No benefit of lampalizumab was observed across prespecified subgroups, including by complement factor I–profile biomarker. Endophthalmitis occurred after 5 of 12 447 injections (0.04%) or in 5 of 1252 treated participants (0.4%) through week 48.
Conclusions and Relevance
In Chroma and Spectri, the largest studies of GA conducted to date, lampalizumab did not reduce GA enlargement vs sham during 48 weeks of treatment. Results highlight the substantial and consistent enlargement of GA, at a mean of approximately 2 mm2 per year.
Trial Registration
ClinicalTrials.gov Identifier: NCT02247479 and NCT02247531
Two randomized clinical trials assess the safety and efficacy of lampalizumab intravitreal injection vs sham procedure in the treatment of enlargement of geographic atrophy secondary to age-related macular degeneration in older adults.
Key Points
Question
Does lampalizumab, a selective complement factor D inhibitor, reduce enlargement of lesions from geographic atrophy secondary to age-related macular degeneration?
Findings
In 2 phase 3 randomized clinical trials (906 Chroma participants and 975 Spectri participants), no meaningful differences in the primary end point of mean change from baseline in geographic atrophy lesion area at week 48 were identified among eyes receiving 10-mg lampalizumab intravitreal injections either every 4 weeks or every 6 weeks vs sham.
Meaning
These phase 3 trials showed that lampalizumab was ineffective as a treatment of geographic atrophy secondary to age-related macular degeneration.
Introduction
Geographic atrophy (GA), an advanced form of age-related macular degeneration (AMD), is a leading cause of visual disability in elderly individuals,1,2,3 with prevalence increasing substantially among those older than 75 years of age.2,3 No approved treatment slows or halts the progression of GA, or reverses the associated loss of macular tissue. In contrast, neovascular AMD, the other form of advanced AMD, is often treated successfully with intravitreous anti–vascular endothelial growth factor (anti-VEGF) medications.4,5,6 Similarly, the Age-Related Eye Disease Study7 and the Age-Related Eye Disease Study 28 reported that dietary supplements reduce the risk of developing advanced neovascular AMD but have no apparent effect on GA.
Occurrence and enlargement of GA lesions can result in substantial visual disability.9,10,11 Because lesions typically first appear outside the fovea,11,12,13 testing of best-corrected visual acuity (BCVA) may inadequately assess functional impairment in individuals with preserved foveal function despite loss of pericentral macula.14 Other measures, including low-luminance visual acuity, reading speed, fundus-controlled microperimetry, and patient-reported outcomes, might assess impairment of visual function in patients with GA,15,16 but these measures were not extensively used in earlier GA trials.
Although the pathophysiology of GA is incompletely understood, dysregulation of the complement cascade, a component of the innate immune system,17,18 has been implicated in AMD19,20 and in GA specifically.21 Overall, genetic factors are estimated to account for 71% to 80% of the risk of advanced AMD,22,23 and common genetic variants near CFH, CFI, C3, and C2/CFB, which act in the alternative complement pathway, may account for 57% of known disease risk variants.20
Given this genetic link, complement factor D was selected as a therapeutic target because it is the rate-limiting enzyme of the alternative complement pathway and is present in comparatively low abundance.24,25,26 Lampalizumab is an antigen-binding fragment of a humanized monoclonal antibody that is directed against, and inhibits, complement factor D.27,28 In a phase 2 trial, monthly intravitreous lampalizumab, 10 mg (n = 42), reduced the mean enlargement of GA lesion area from baseline to 18 months by 20% (80% CI, 4%-37%; P = .12) vs sham (n = 40).29 In an exploratory subgroup analysis of carriers of the complement factor I (CFI) risk allele, monthly lampalizumab reduced the enlargement of GA by 44% vs sham.29 No benefit was observed with lampalizumab treatment every 8 weeks.
To test phase 2 observations, we conducted 2 identically designed phase 3 randomized clinical trials, Chroma and Spectri, to assess the efficacy and safety of 10 mg of lampalizumab administered by intravitreal injection every 4 or 6 weeks vs sham treatment. These studies also prospectively investigated the prognostic and predictive diagnostic hypothesis of the CFI profile genetic biomarker. The 48-week primary outcome of these trials is presented herein.
Methods
The Chroma (trial protocol and statistical analysis plan are available in Supplement 1) and Spectri (trial protocol and statistical analysis plan are available in Supplement 2) studies were identically designed, phase 3 double-masked, multicenter, randomized, sham injection–controlled clinical trials at 131 (Chroma) and 144 (Spectri) sites in 23 countries. The studies adhered to the tenets of the Declaration of Helsinki30 and were conducted in accordance with the International Conference on Harmonisation E6 Guidelines for Good Clinical Practice31 and with applicable local, state, and federal laws. All sites received institutional review board or ethics committee approval before study initiation (eAppendix 1 in Supplement 3). Participants provided written informed consent. An independent data monitoring committee provided ongoing oversight. Key aspects of the study design are described herein and in eAppendix 2 in Supplement 3.
Study Population
Eligible participants (eTable 1 in Supplement 3) were aged 50 years or older with bilateral GA secondary to AMD and no evidence of active or prior choroidal neovascularization (CNV) nor previous treatment for CNV in either eye. Key study eye inclusion criteria were a total GA lesion size from 2.54 to 17.78 mm2 (1-7 disc areas) measured on blue-light fundus autofluorescence, as confirmed by the reading center; perilesional banded or diffuse autofluorescence patterns; and an Early Treatment Diabetic Retinopathy Study (ETDRS) BCVA letter score of 49 or more (Snellen equivalent, 20/100 or better). Geographic atrophy lesions could be multifocal or unifocal, but at least 1 lesion had to be 1.27 mm2 or larger (≥0.5 disc areas). In study eyes with a BCVA letter score of 79 or more (Snellen equivalent, 20/25 or better), at least 1 lesion was required within 250 μm of the foveal center. One eye was selected as the study eye. If both eyes were eligible, the eye with the poorer visual function as determined by the investigator and the patient was selected, followed by the eye with the larger GA lesion. Participants were also evaluated at screening for CFI-profile genetic biomarker status (eTable 2 in Supplement 3).
Randomization
Participants were randomly assigned 2:1:2:1 to receive 10 mg of lampalizumab every 4 weeks, sham procedure every 4 weeks, 10 mg of lampalizumab every 6 weeks, and sham procedure every 6 weeks, via an interactive voice and web response system. In the sham groups, the eye was prepped in a manner similar to lampalizumab groups to preserve masking, including subconjunctival anesthesia. However, instead of an actual intravitreal injection, only the hub of a syringe was placed against the planned injection site. For randomization, a permuted block design was used, and participants were stratified by CFI-profile biomarker status, baseline BCVA ETDRS chart Snellen equivalent (20/50 or better vs worse than 20/50), sex, and eligibility for microperimetry. Participant numbers were capped by CFI-profile biomarker status to achieve a 3:2 ratio for CFI-positive to CFI-negative participants. Sham arms were pooled for analysis, resulting in a 1:1:1 ratio for lampalizumab every 4 weeks, lampalizumab every 6 weeks, and sham.
Study Treatment and Assessments
Treatment was administered to the study eye at randomization (day 1) and every 4 or 6 weeks (±5 days) thereafter through 44 weeks for groups receiving treatment every 4 weeks or 42 weeks for groups receiving treatment every 6 weeks, before week 48 primary efficacy assessments, continuing through 90 or 92 weeks per study design. Safety and ocular assessments, including BCVA, were performed at day 8 and at each subsequent visit on the same day as treatment. Verbatim descriptions of adverse events (AEs) were coded using Medical Dictionary for Regulatory Activities, version 20.0.32 Fundus images of both eyes at screening and specified visits were evaluated at the Doheny Image Reading Center (Los Angeles, California). Autofluorescence pattern eligibility was determined by the GRADE Reading Center (Bonn, Germany). Additional visual function assessments were performed as scheduled.
Outcomes
The primary efficacy outcome was mean change in GA lesion area from baseline to week 48 measured by fundus autofluorescence, graded at the reading center. Secondary efficacy outcomes assessing visual function were exploratory at week 48, with formal statistical testing planned at week 96. Safety outcomes were assessed through a summary of ocular and nonocular AEs, deaths, results of serial electrocardiograms (selected participants), incidence of antidrug antibodies, and ocular assessments.
Statistical Analysis
For each study, a sample size of 188 CFI-positive participants per lampalizumab treatment arm and 94 CFI-positive participants per sham arm provided greater than 95% power to detect a difference in change in GA lesion area assuming a population difference of 1.45 mm2 (approximately 40% reduction relative to sham control) and an SD of 2.51 in the CFI-positive population. A sample size of 124 CFI-negative participants per lampalizumab treatment arm and 62 CFI-negative participants per sham arm provided 80% power to detect a difference assuming a population difference of 0.66 mm2 (approximately 40% reduction relative to control) and an SD of 1.68 in the CFI-negative population (eTable 3 in Supplement 3). Calculations were based on 2-sided t tests at the α = .0495 level with the assumption of a 15% dropout rate by week 48.
The primary efficacy analysis for comparison between each lampalizumab arm and the pooled sham arms was performed on the intent-to-treat population (all randomized participants) using a mixed effects model repeated-measures model based on available data to week 48, with no imputation for missing data. Change-from-baseline analysis excluded participants without a baseline measurement or at least 1 postbaseline measurement. The primary analysis adjusted for baseline GA lesion area, subfoveal vs nonsubfoveal location, and multifocal vs nonmultifocal configuration; CFI-profile biomarker status; BCVA (better than vs worse than 20/50 Snellen equivalent); and sex. Preplanned subgroup analyses by CFI-profile biomarker were performed similarly, except with the model fit separately for each biomarker group and without biomarker status as a covariate. Hypothesis testing was performed at a 2-sided α = .0496 level to account for a 0.0001 nominal penalty for each of 4 planned independent data monitoring committee unmasked data reviews occurring before the primary analysis.
To assess robustness of the primary efficacy results, additional analyses included the growth slope of the GA lesion area over 48 weeks, the change from baseline in the square root of the GA lesion area at week 48, and the percentage change from baseline in the GA lesion area at week 48. Exploratory analyses by prespecified clinical subgroup were performed using mixed effects model repeated-measures analysis similar to the primary efficacy analysis, excluding baseline covariates not relevant for the particular subgroup. Safety analyses were performed on the population that received 1 or more doses of lampalizumab or sham, grouped according to actual treatment received regardless of assignment. Analyses were performed using SAS, version 9.4 (SAS Institute), separately by study and based on pooled data from Chroma and Spectri, which included an additional covariate adjustment for study, as appropriate.
Results
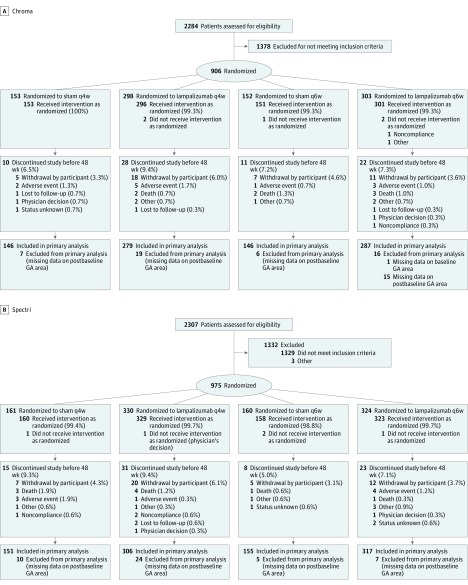
Between August 28, 2014, and October 6, 2016, 906 Chroma participants and 975 Spectri participants were randomized to receive sham every 4 weeks (153 Chroma participants; 161 Spectri participants), lampalizumab every 4 weeks (298 Chroma participants; 330 Spectri participants), sham every 6 weeks (152 Chroma participants; 160 Spectri participants), or lampalizumab every 6 weeks (303 Chroma participants; 324 Spectri participants) (Figure 1). The baseline demographic characteristics of the participants (Table 1 and eTables 4 and 5 in Supplement 3) were well balanced across treatment groups (mean [SD] age of 78.0 [8.1] years, 1131 [60.1%] female, and 1827 [97.1%] white). The mean baseline GA lesion area was between 7.55 and 8.50 mm2 across treatment groups. The mean baseline BCVA letter score was between 65 and 66 (approximate Snellen equivalent, 20/50) in each group.
Figure 1. CONSORT Flow Diagram for Chroma and Spectri Randomized Clinical Trials.
GA indicates geographic atrophy; q4w, every 4 weeks; and q6w, every 6 weeks.
Table 1. Pooled Demographic and Baseline Characteristics of Chroma and Spectri Participants.
| Characteristic | Sham | Lampalizumab, 10 mg | All (N = 1881) | |||
|---|---|---|---|---|---|---|
| q4w (n = 314) | q6w (n = 312) | Pooled (n = 626) | q4w (n = 628) | q6w (n = 627) | ||
| Demographics | ||||||
| Age, y | ||||||
| Mean (SD) | 78.1 (8.1) | 78.0 (7.9) | 78.0 (8.0) | 77.4 (7.9) | 78.5 (8.3) | 78.0 (8.1) |
| Median (range) | 78 (51-96) | 78 (51-95) | 78 (51-96) | 78 (50-95) | 80 (53-97) | 79 (50-97) |
| Female sex, No. (%) | 187 (59.6) | 190 (60.9) | 377 (60.2) | 379 (60.4) | 375 (59.8) | 1131 (60.1) |
| White race, No. (%)a | 306 (97.5) | 302 (96.8) | 608 (97.1) | 608 (96.8) | 611 (97.4) | 1827 (97.1) |
| Tobacco use, No. (%) | ||||||
| Never | 153 (48.7) | 136 (43.6) | 289 (46.2) | 293 (46.7) | 290 (46.3) | 872 (46.4) |
| Previous | 147 (46.8) | 155 (49.7) | 302 (48.2) | 295 (47.0) | 295 (47.0) | 892 (47.4) |
| Current | 14 (4.5) | 21 (6.7) | 35 (5.6) | 40 (6.4) | 42 (6.7) | 117 (6.2) |
| Study eye baseline characteristics | ||||||
| GA area,b mm2 | ||||||
| Mean (SD) | 7.557 (3.884) | 7.942 (4.025) | 7.749 (3.956) | 8.119 (3.904) | 8.314 (4.249) | 8.061 (4.044) |
| Median (range) | 6.460 (1.58-17.56) | 7.020 (2.61-30.56) | 6.695 (1.58-30.56) | 7.325 (2.54-17.74) | 7.485 (2.29-22.19) | 7.205 (1.58-30.56) |
| GA lesion contiguity, No. (%)b | ||||||
| Multifocal | 238 (75.8) | 253 (81.1) | 491 (78.4) | 496 (79.0) | 477 (76.2) | 1464 (77.9) |
| Nonmultifocal | 76 (24.2) | 59 (18.9) | 135 (21.6) | 132 (21.0) | 149 (23.8) | 416 (22.1) |
| GA lesion location, No. (%)b | ||||||
| Subfoveal | 172 (54.8) | 166 (53.2) | 338 (54.0) | 329 (52.4) | 320 (51.1) | 987 (52.5) |
| Nonsubfoveal | 142 (45.2) | 146 (46.8) | 288 (46.0) | 299 (47.6) | 306 (48.9) | 893 (47.5) |
| Hyperautofluorescence pattern, No. (%) | ||||||
| Banded | 12 (3.8) | 11 (3.5) | 23 (3.7) | 22 (3.5) | 35 (5.6) | 80 (4.3) |
| Diffuse | 301 (95.9) | 301 (96.5) | 602 (96.2) | 605 (96.3) | 591 (94.3) | 1798 (95.6) |
| Not applicable | 1 (0.3) | 0 | 1 (0.2) | 1 (0.2) | 1 (0.2) | 3 (0.2) |
| BCVA, mean (SD) letter scorec | 66.4 (10.0) | 65.6 (9.6) | 66.0 (9.8) | 66.1 (9.8) | 66.0 (9.9) | 66.0 (9.9) |
| <64 (worse than 20/50) | 115 (37.0) | 118 (38.2) | 233 (37.6) | 253 (40.5) | 247 (39.7) | 733 (39.3) |
| ≥64 (20/50 or better) | 196 (63.0) | 191 (61.8) | 387 (62.4) | 372 (59.5) | 375 (60.3) | 1134 (60.7) |
| LLVA, mean (SD) letter scored | 36.6 (16.0) | 36.2 (16.3) | 36.4 (16.1) | 36.5 (17.6) | 36.0 (16.7) | 36.3 (16.8) |
| Low-luminance deficit (BCVA − LLVA), mean (SD) letter scoree | 29.8 (16.1) | 29.3 (15.9) | 29.6 (16.0) | 29.6 (16.3) | 30.1 (15.7) | 29.7 (16.0) |
A total of 1733 of 1881 participants (92.1%) in Chroma and Spectri completed the first 48 weeks of the study, during which across treatment arms more than 76% of participants receiving treatment every 4 weeks received at least 12 injections (13 possible) and more than 85% of participants receiving treatment every 6 weeks received at least 8 injections (9 possible) (eAppendix 3 in Supplement 3).
After the Spectri primary analysis in September 2017, lampalizumab treatment was suspended for both studies at the sponsor’s recommendation with the agreement of the chair of the independent data monitoring committee because the apparent lack of efficacy did not warrant continued intravitreal injections.
Efficacy of Lampalizumab Treatment
GA Enlargement
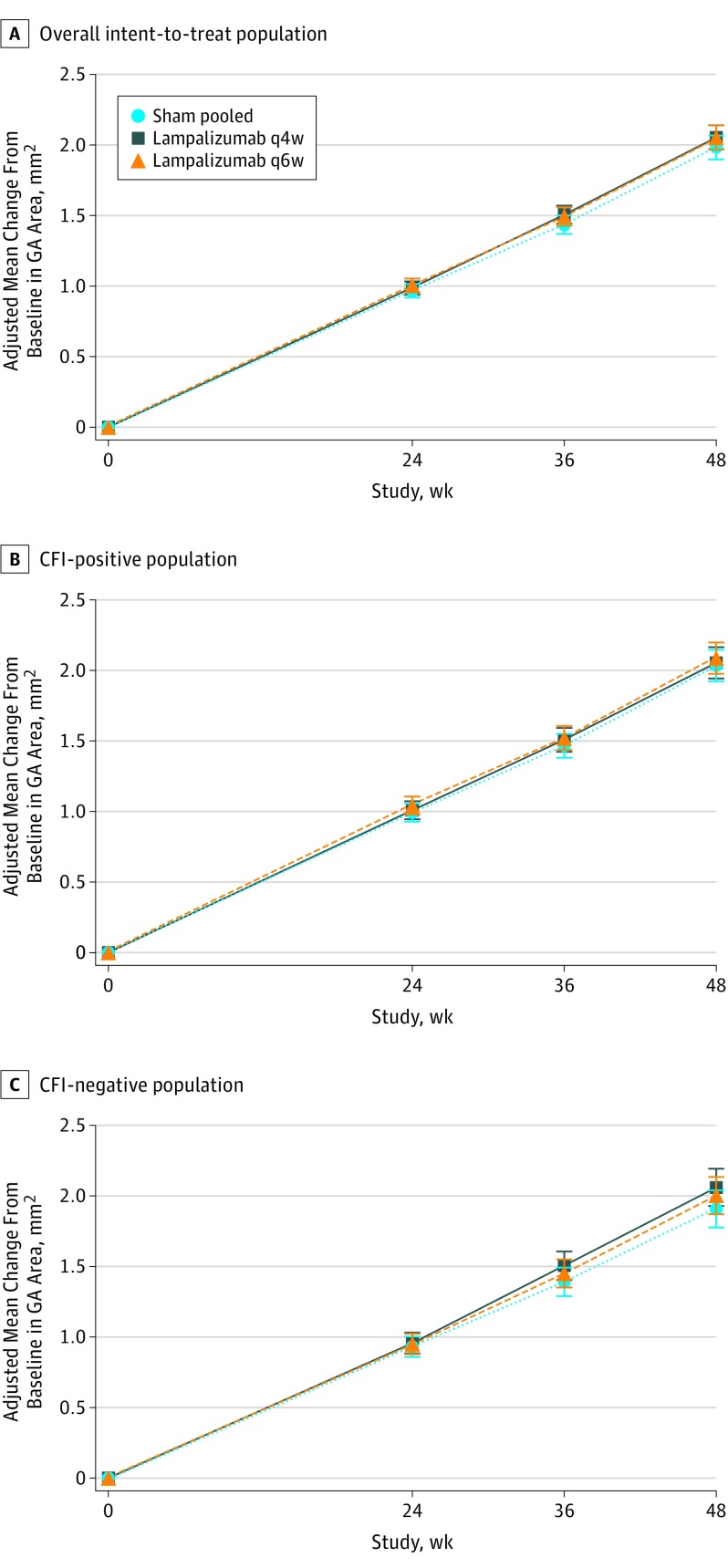
At week 48, the adjusted mean increase in GA lesion area from baseline was 1.93 to 2.09 mm2 across all groups in both studies (Table 2, Figure 2A, and eFigure 1A-B in Supplement 3). The differences in the adjusted mean change of the GA lesion area (lampalizumab minus sham) were −0.02 mm2 (95% CI, −0.21 to 0.16 mm2; P = .80) for lampalizumab every 4 weeks in Chroma, 0.16 mm2 (95% CI, 0.00-0.31 mm2; P = .048 favoring sham) for lampalizumab every 4 weeks in Spectri, 0.05 mm2 (95% CI, −0.13 to 0.24 mm2; P = .59) for lampalizumab every 6 weeks in Chroma, and 0.09 mm2 (95% CI, −0.07 to 0.24 mm2; P = .27) for lampalizumab every 6 weeks in Spectri. Similarly, no benefit of lampalizumab over sham was observed in robustness assessments for the primary efficacy result (Table 2 and eTables 6 and 7 in Supplement 3). Furthermore, no benefit of lampalizumab over sham was observed for either CFI-profile biomarker subgroup (Figure 2 and eFigure 1 and eTable 8 in Supplement 3). Because baseline characteristics, follow-up, treatment adherence, and primary outcomes were similar in Chroma and Spectri, subsequent results report pooled data, with unpooled results in Supplement 3.
Table 2. Change in GA Area From Baseline at Week 48 in Chroma and Spectri Pooled Intent-to-Treat Populationa.
| Measure | Sham | Lampalizumab, 10 mg | |
|---|---|---|---|
| Pooled (n = 598) | q4w (n = 596) | q6w (n = 603) | |
| Change from baseline in GA area at 48 wk, mm2 | |||
| Adjusted mean (SE) | 1.984 (0.043) | 2.055 (0.043) | 2.054 (0.043) |
| Difference in means (vs sham pooled) | 0.071 | 0.070 | |
| 95% CI | –0.049 to 0.191 | –0.050 to 0.190 | |
| Relative reduction, % | –3.6 | –3.5 | |
| P value | .25 | .25 | |
| Rate of change in GA area (growth slope) from baseline to 48 wk, mm2/365.25 db | |||
| Adjusted mean slope (SE) | 1.998 (0.045) | 2.076 (0.045) | 2.085 (0.045) |
| Difference in slopes (vs sham pooled) | 0.078 | 0.086 | |
| 95% CI | –0.048 to 0.204 | –0.039 to 0.212 | |
| Relative reduction, % | –3.9 | –4.3 | |
| P value | .22 | .18 | |
| Change from baseline in square root of GA area at 48 wk, mm | |||
| Adjusted mean (SE) | 0.342 (0.007) | 0.349 (0.007) | 0.352 (0.007) |
| Difference in means (vs sham pooled) | 0.006 | 0.010 | |
| 95% CI | –0.013 to 0.026 | –0.009 to 0.029 | |
| Relative reduction, % | –1.8 | –2.9 | |
| P value | .53 | .32 | |
| % Change from baseline in GA area at 48 wk | |||
| Adjusted mean (SE) | 30.032 (0.856) | 29.546 (0.859) | 30.815 (0.853) |
| Difference in means (vs sham pooled) | –0.486 | 0.783 | |
| 95% CI | –2.864 to 1.891 | –1.586 to 3.153 | |
| Relative reduction, % | 1.6 | –2.6 | |
| P value | .69 | .52 |
Figure 2. Adjusted Mean Change From Baseline in Geographic Atrophy (GA) Area Over Time From Baseline to 48 Weeks in Chroma and Spectri Pooled as Measured on Fundus Autofluorescence Imaging.
A, Overall intent-to-treat population. B, Complement factor I (CFI)–positive population. C, CFI-negative population. The mixed effects model repeated-measures analysis was adjusted for baseline GA area, baseline GA lesion location, baseline GA lesion contiguity, baseline best-corrected visual acuity category, sex, biomarker status (overall population only), and study. Error bars indicate 95% CIs. q4w Indicates every 4 weeks; q6w, every 6 weeks.
GA Enlargement by Clinical Subgroup
No consistent benefit of lampalizumab over sham was observed for any subgroup (eFigures 2-4 in Supplement 3).
Best-Corrected Visual Acuity
Best-corrected visual acuity declined from baseline to week 48 in all arms of both studies (eTable 9 and eFigure 5 in Supplement 3), with an adjusted mean BCVA letter score change of −4.9 (95% CI, −5.8 to −4.0) for sham treatment, −4.1 (95% CI, −5.0 to −3.2) for lampalizumab every 4 weeks, and −4.9 (95% CI, −5.8 to −3.9) for lampalizumab every 6 weeks.
Safety of Lampalizumab Treatment
No new ocular or nonocular safety signals beyond what would be anticipated with intravitreal injections were observed with lampalizumab through week 48 (eTables 10-19 in Supplement 3). The percentage of participants with ocular AEs and serious AEs (SAEs) were higher with lampalizumab compared with sham treatment, in alignment with expectations for intravitreal injections. Overall, 2.7% (17 of 619) of participants receiving the sham treatment, 6.2% (39 of 626) of participants receiving lampalizumab every 4 weeks, and 6.1% (38 of 626) of participants receiving lampalizumab every 6 weeks experienced 1 or more ocular SAEs.
Increases in intraocular pressure (IOP) were of interest because lampalizumab was injected as 0.1 mL, twice the volume of most intravitreal injections of anti-VEGF. Incidences of any IOP of 30 mm Hg or higher after injection, regardless of whether the events were considered SAEs, were reported in 0.3% (2 of 618) of participants receiving the sham treatment, 8.3% (52 of 625) of participants receiving lampalizumab every 4 weeks, and 5.6% (35 of 626) of participants receiving lampalizumab every 6 weeks. Increases in IOP considered to be SAEs were reported in 0.2% (1 of 619) of participants receiving the sham treatment, 3.2% (20 of 626) of the participants receiving lampalizumab every 4 weeks, and 2.6% (16 of 626) of participants receiving lampalizumab every 6 weeks. The mean preinjection IOP remained constant from baseline to week 48 across all arms (eTable 18 and eFigure 6 in Supplement 3). Per investigator discretion, 3.1% (39 of 1252) of participants receiving lampalizumab also received paracentesis in the study eye owing to AEs of increased IOP or transient vision loss (5.6 procedures per 1000 injections).
Endophthalmitis occurred after 5 of 12 447 injections (0.4 events per 1000 injections [0.04%]) or in 5 of 1252 treated participants (0.4%) through week 48. Neovascular AMD was observed after randomization in 1.1% (7 of 619) of study eyes in the group receiving the sham treatment, 1.9% (12 of 626) of study eyes in the group receiving lampalizumab every 4 weeks, 1.9% (12 of 626) of study eyes in the group receiving lampalizumab every 6 weeks, 1.3% (8 of 619) of fellow eyes in the group receiving the sham treatment, 1.6% (10 of 626) of fellow eyes in the group receiving lampalizumab every 4 weeks, and 1.8% (11 of 626) of fellow eyes in the group receiving lampalizumab every 6 weeks, with no events of bilateral neovascular AMD (eTable 19 in Supplement 3).
Nonocular SAEs were reported in 16.6% (103 of 619) of participants in the group receiving the sham treatment, including 7 deaths; 19.2% (120 of 626) of participants in the group receiving lampalizumab every 4 weeks, including 7 deaths; and 13.9% (87 of 626) of participants in the group receiving lampalizumab every 6 weeks, including 5 deaths.
Discussion
To our knowledge, Chroma and Spectri were the largest, most comprehensive studies of GA conducted to date. In the primary analysis, lampalizumab did not reduce the enlargement of GA lesions from baseline at week 48 vs sham. Furthermore, no benefit of lampalizumab was suggested by the results of robustness assessments or subgroup analyses, including by CFI-profile biomarker. No new safety signals were observed with lampalizumab treatment, and incidences of endophthalmitis, increase in IOP, or other injection-related SAEs were low and consistent with those observed in studies of anti-VEGF.5,6,33
The Chroma and Spectri trials provide the largest cohorts to date of patients with bilateral GA and no CNV in either eye, with detailed documentation of anatomical and functional outcomes. The rates of progression of GA in Chroma and Spectri (approximately 2 mm2 per year on average) were within the range of previous studies (approximately 0.53-2.6 mm2 per year),15 with differences across studies likely attributable to inclusion criteria reflected in the characteristics of each study cohort. In Chroma and Spectri, eligibility criteria included factors associated with faster GA progression, such as bilateral GA and banded or diffuse perilesional fundus autofluorescence patterns.15 Consistent with prior studies,15 Chroma and Spectri subgroup analyses demonstrated that larger baseline GA lesion area, multifocal configurations, and nonfoveal GA lesions are associated with faster rates of progression. This large data set, from 2 multicenter global trials conducted in 23 countries, is likely generalizable to the broader population of patients with GA who would meet the eligibility criteria of these trials and could serve as an important normative database for future studies and provide further insights into the natural history of GA.
The Chroma and Spectri cohorts experienced a notable decline in visual function, with a mean BCVA letter score loss of approximately 5 letters in 48 weeks. This finding underscores the potential burden of vision loss from GA.
The safety outcomes presented here can inform future trials through at least 1 year. Intravitreal injection volumes of 0.1 mL were associated with low rates of increased posttreatment IOP SAEs and no change in mean pretreatment IOP during 48 weeks, suggesting that this volume may be given safely within a trial setting. Also, Chroma and Spectri documented that new CNV in patients with bilateral GA occurred in less than 2% of study or fellow eyes. This finding is consistent with observational studies, which reported conversion rates of 2% at 2 years and 11% at 4 years in patients with bilateral GA and no baseline CNV,34 and a conversion rate of 1.5% by 1 to 2 years in studies in which most patients had bilateral GA.35 In contrast, for patients with CNV in 1 eye and GA in the other, much higher rates of CNV in the eye with GA have been reported (18% at 2 years34 and 34%-49% at 4-5 years34,36), similar to conversion rates for eyes with large drusen or focal hyperpigmentations.36,37 Thus, future GA trials must consider the effect of including participants with any history of CNV in either eye because its presence may confound the accurate measurement of the enlargement of GA lesions and affect visual function assessments.
The primary rationales for exploring complement inhibition in GA were the strong genetic linkage and the feasibility of clinical trials evaluating the enlargement of GA lesions. To date, 6 molecules that act as complement pathway inhibitors have entered clinical trials for GA, including APL-2 (target, C3), which met its primary end point in a phase 2 trial38; CLG-561 (target, properdin), currently in a phase 2 trial39; and avacincaptad pegol (target, C5),40 currently in a phase 2b trial. Two other C5 inhibitors, one given systemically41 and the other intravitreally,42 were not effective in phase 2 trials. Taken together with the Chroma and Spectri results, it remains unclear whether the complement cascade is an appropriate intraocular therapeutic target for GA, at least through the alternative pathway via complement factor D or downstream in the cascade via C5. Geographic atrophy therapeutics investigating targets outside the complement cascade are also in development.
Although the CFI-profile biomarker was thought to be associated with faster progression of GA based on the Mahalo phase 2 trial of lampalizumab,29 the much larger prospective analysis of Chroma and of Spectri does not support CFI-profile status as a genetic biomarker for progression of GA. This finding is consistent with other studies performed after the initiation of Chroma and Spectri, which also reported no association between CFI risk alleles and the rate of GA progression.43,44,45 Although it is still not clear why such results were observed in Mahalo, in light of the results from Chroma and Spectri, one may hypothesize that they may have been related to a small sample size and may have occurred by chance.
Strengths and Limitations
There are several strengths and limitations of these studies that could affect the interpretation of the results. The randomization of a large cohort; duplication of results across 2 identically designed, multicenter, double-masked, randomized clinical trials; and good follow-up and adherence to the protocol make it less likely that confounding or bias affected these topline results. However, the results apply only to 48 weeks of treatment and may not apply to all cases of GA. Based on the inclusion and exclusion criteria of these trials, they may not apply to patients with smaller or larger lesions, unilateral GA, autofluorescence patterns other than banded or diffuse, eyes with current or prior CNV, GA from causes other than AMD, or earlier disease stages.
Conclusions
In 2 identically designed phase 3 trials, lampalizumab, a selective complement factor D inhibitor, did not reduce the enlargement of GA lesions vs sham. The results highlight both the potential burden of vision loss facing patients with bilateral GA and the substantial retinal tissue loss that occurs during 48 weeks. Further analysis of Chroma and Spectri, including genotype-phenotype correlations enabled by whole-genome sequencing, may yield additional insights into AMD pathophysiology and support future clinical trials.
Supplement 1.
Trial Protocol for Chroma
Supplement 2.
Trial Protocol for Spectri
Supplement 3.
eAppendix 1. Institutional Review Boards and Ethics Committees
eAppendix 2. Methods
eAppendix 3. Results
eTable 1. Full Eligibility Criteria for Chroma and Spectri
eTable 2. Complement Factor I (CFI)-Profile Biomarker Status Definition
eTable 3. Power and Minimum Detectable Difference for the Primary End Point for Each of Chroma and Spectri
eTable 4. Participant Demographics and Baseline Characteristics in Chroma
eTable 5. Participant Demographics and Baseline Characteristics in Spectri
eTable 6. Week 48 Outcomes in Chroma, Intent-to-Treat Population
eTable 7. Week 48 Outcomes in Chroma, Intent-to-Treat Population
eTable 8. Geographic Atrophy Area Change from Baseline to Week 48 by CFI-Profile Biomarker Status, for Chroma and Spectri, Pooled and Separate
eTable 9. Best-Corrected Visual Acuity Change From Baseline at Week 48
eTable 10. Adverse Event Overview, Ocular and Non-Ocular, First 48 Weeks, Chroma and Spectri Pooled
eTable 11. Ocular Adverse Events in the Study Eye Occurring in ≥1% of Participants in any Treatment Group, First 48 Weeks, Chroma and Spectri Pooled
eTable 12. Non-Ocular Adverse Events Occurring in ≥1% of Participants in any Treatment Group, First 48 Weeks, Chroma and Spectri Pooled
eTable 13. Serious Ocular Adverse Events in the Study Eye, First 48 Weeks, Chroma and Spectri Pooled
eTable 14. Non-Ocular Serious Adverse Events (SAEs) by MedDRA System Organ Class and Non-Ocular SAEs Occurring in ≥0.5% of Participants in any Treatment Group, First 48 Weeks, Chroma and Spectri Pooled
eTable 15. Adverse Events Leading to Death During the First 48 Weeks, Chroma and Spectri Pooled
eTable 16. Adverse Events Leading to Treatment Discontinuation, First 48 Weeks, Chroma and Spectri Pooled
eTable 17. Ocular and Non-Ocular Adverse Events of Special Interest, First 48 Weeks, Chroma and Spectri Pooled
eTable 18. Increased Intraocular Pressure in the Study Eye and Fellow Eye, First 48 Weeks, Chroma and Spectri Pooled
eTable 19. Neovascular Age-Related Macular Degeneration in the Study and Fellow Eyes, First 48 Weeks, Chroma and Spectri Pooled
eFigure 1. Adjusted Mean Geographic Atrophy Area Change From Baseline to Week 48
eFigure 2. Adjusted Mean Change in Geographic Atrophy (GA) Area From Baseline to Week 48 in the Study Eye by Clinical Subgroup, Chroma and Spectri Pooled
eFigure 3. Adjusted Mean Change in GA Area From Baseline to Week 48 in the Study Eye by Clinical Subgroup, Lampalizumab q4w Vs Sham
eFigure 4. Adjusted Mean Change in GA Area From Baseline to Week 48 in the Study Eye by Clinical Subgroup, Lampalizumab q6w Vs Sham
eFigure 5. Mean Change in Best-Corrected Visual Acuity Over Time From Baseline to Week 48
eFigure 6. Mean (A) Pre-Dose and (B) Post-Dose Intraocular Pressure (IOP) in mm Hg Over Time in the Study Eye, First 48 Weeks, Chroma and Spectri Pooled
References
- 1.Flaxman SR, Bourne RRA, Resnikoff S, et al. ; Vision Loss Expert Group of the Global Burden of Disease Study . Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221-e1234. [DOI] [PubMed] [Google Scholar]
- 2.Rudnicka AR, Kapetanakis VV, Jarrar Z, et al. Incidence of late-stage age-related macular degeneration in American whites: systematic review and meta-analysis. Am J Ophthalmol. 2015;160(1):85-93.e3. [DOI] [PubMed] [Google Scholar]
- 3.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-e116. [DOI] [PubMed] [Google Scholar]
- 4.Brown DM, Kaiser PK, Michels M, et al. ; ANCHOR Study Group . Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-1444. [DOI] [PubMed] [Google Scholar]
- 5.Heier JS, Brown DM, Chong V, et al. ; VIEW 1 and VIEW 2 Study Groups . Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. [DOI] [PubMed] [Google Scholar]
- 7.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Age-Related Eye Disease Study 2 Research Group Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005-2015. [DOI] [PubMed] [Google Scholar]
- 9.Brown JC, Goldstein JE, Chan TL, Massof R, Ramulu P; Low Vision Research Network Study Group . Characterizing functional complaints in patients seeking outpatient low-vision services in the United States. Ophthalmology. 2014;121(8):1655-62.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunness JS, Rubin GS, Applegate CA, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104(10):1677-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sunness JS, Rubin GS, Zuckerbrod A, Applegate CA. Foveal-sparing scotomas in advanced dry age-related macular degeneration. J Vis Impair Blind. 2008;102(10):600-610. [PMC free article] [PubMed] [Google Scholar]
- 12.Klein R, Meuer SM, Knudtson MD, Klein BE. The epidemiology of progression of pure geographic atrophy: the Beaver Dam Eye Study. Am J Ophthalmol. 2008;146(5):692-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindblad AS, Lloyd PC, Clemons TE, et al. ; Age-Related Eye Disease Study Research Group . Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127(9):1168-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunness JS. Reading newsprint but not headlines: pitfalls in measuring visual acuity and color vision in patients with bullseye maculopathy and other macular scotomas. Retin Cases Brief Rep. 2008;2(1):83-84. [DOI] [PubMed] [Google Scholar]
- 15.Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology. 2018;125(3):369-390. [DOI] [PubMed] [Google Scholar]
- 16.Sadda SR, Chakravarthy U, Birch DG, Staurenghi G, Henry EC, Brittain C. Clinical endpoints for the study of geographic atrophy secondary to age-related macular degeneration. Retina. 2016;36(10):1806-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walport MJ. Complement: second of two parts. N Engl J Med. 2001;344(15):1140-1144. [DOI] [PubMed] [Google Scholar]
- 19.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat Rev Immunol. 2013;13(6):438-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, Henry EC, Brittain C. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37(5):819-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seddon JM, Cote J, Page WF, Aggen SH, Neale MC. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol. 2005;123(3):321-327. [DOI] [PubMed] [Google Scholar]
- 23.Seddon JM, Silver RE, Kwong M, Rosner B. Risk prediction for progression of macular degeneration: 10 common and rare genetic variants, demographic, environmental, and macular covariates. Invest Ophthalmol Vis Sci. 2015;56(4):2192-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesavre PH, Müller-Eberhard HJ. Mechanism of action of factor D of the alternative complement pathway. J Exp Med. 1978;148(6):1498-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volanakis JE, Narayana SV. Complement factor D, a novel serine protease. Protein Sci. 1996;5(4):553-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volanakis JE, Barnum SR, Giddens M, Galla JH. Renal filtration and catabolism of complement protein D. N Engl J Med. 1985;312(7):395-399. [DOI] [PubMed] [Google Scholar]
- 27.Katschke KJ Jr, Wu P, Ganesan R, et al. Inhibiting alternative pathway complement activation by targeting the factor D exosite. J Biol Chem. 2012;287(16):12886-12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loyet KM, Good J, Davancaze T, et al. Complement inhibition in cynomolgus monkeys by anti-factor D antigen-binding fragment for the treatment of an advanced form of dry age-related macular degeneration. J Pharmacol Exp Ther. 2014;351(3):527-537. [DOI] [PubMed] [Google Scholar]
- 29.Yaspan BL, Williams DF, Holz FG, et al. ; MAHALO Study Investigators . Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med. 2017;9(395):eaaf1443. doi: 10.1126/scitranslmed.aaf1443 [DOI] [PubMed] [Google Scholar]
- 30.World Medical Association WMA Declaration of Helsinki—ethical principles for medical research involving human subjects. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed October 25, 2017.
- 31.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline: guideline for good clinical practice E6(R1). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Published June 10, 1996. Accessed April 12, 2018.
- 32.Medical Dictionary for Regulatory Activities, version 20.0. https://www.meddra.org. Published March 2017. Accessed April 12, 2018.
- 33.Busbee BG, Ho AC, Brown DM, et al. ; HARBOR Study Group . Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046-1056. [DOI] [PubMed] [Google Scholar]
- 34.Sunness JS, Gonzalez-Baron J, Bressler NM, Hawkins B, Applegate CA. The development of choroidal neovascularization in eyes with the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106(5):910-919. [DOI] [PubMed] [Google Scholar]
- 35.Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HP, Schmitz-Valckenberg S; FAM-Study Group . Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143(3):463-472. [DOI] [PubMed] [Google Scholar]
- 36.Macular Photocoagulation Study Group Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol. 1997;115(6):741-747. [DOI] [PubMed] [Google Scholar]
- 37.Solomon SD, Jefferys JL, Hawkins BS, Bressler NM, Bressler SB; Submacular Surgery Trials Research Group . Risk factors for second eye progression to advanced age-related macular degeneration: SST report No. 21 Submacular Surgery Trials Research Group. Retina. 2009;29(8):1080-1090. [DOI] [PubMed] [Google Scholar]
- 38.Apellis Pharmaceuticals. Apellis Pharmaceuticals announces that APL-2 met its primary endpoint in a phase 2 study in patients with geographic atrophy, an advanced form of age-related macular degeneration. http://apellis.com/pdfs/Press%20Release%20FILLY%2012%20Month%20Results%20FINAL%20FINAL%20170823.pdf. Published August 24, 2017. Accessed February 16, 2018.
- 39.ClinicalTrials.gov. CLG561 proof-of-concept study as a monotherapy and in combination with LFG316 in subjects with geographic atrophy (GA). https://clinicaltrials.gov/ct2/show/NCT02515942. Accessed December 6, 2017.
- 40.Ophthotech. Ophthotech provides update on Zimura complement programs for treatment of eye diseases. http://investors.ophthotech.com/news-releases/news-release-details/ophthotech-provides-update-zimurar-complement-programs-treatment. Published September 19, 2017. Accessed February 16, 2018.
- 41.Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: the COMPLETE study. Ophthalmology. 2014;121(3):693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zamiri P. Complement C5 inhibition for AMD. Paper presented at: Angiogenesis; February 6, 2016; Miami, FL. [Google Scholar]
- 43.Wurzelmann JI, Lopez FJ, Fries M, et al. SNPs associated with complement factor I do not predict 4-month lesion growth rate in geographic atrophy [ARVO abstract]. Invest Ophthalmol Vis Sci. 2015;56(7):2850. [Google Scholar]
- 44.Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al. Association between growth of geographic atrophy and the complement factor I locus. Ophthalmic Surg Lasers Imaging Retina. 2015;46(7):772-774. [DOI] [PubMed] [Google Scholar]
- 45.Grassmann F, Fleckenstein M, Chew EY, et al. Clinical and genetic factors associated with progression of geographic atrophy lesions in age-related macular degeneration. PLoS One. 2015;10(5):e0126636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1.
Trial Protocol for Chroma
Supplement 2.
Trial Protocol for Spectri
Supplement 3.
eAppendix 1. Institutional Review Boards and Ethics Committees
eAppendix 2. Methods
eAppendix 3. Results
eTable 1. Full Eligibility Criteria for Chroma and Spectri
eTable 2. Complement Factor I (CFI)-Profile Biomarker Status Definition
eTable 3. Power and Minimum Detectable Difference for the Primary End Point for Each of Chroma and Spectri
eTable 4. Participant Demographics and Baseline Characteristics in Chroma
eTable 5. Participant Demographics and Baseline Characteristics in Spectri
eTable 6. Week 48 Outcomes in Chroma, Intent-to-Treat Population
eTable 7. Week 48 Outcomes in Chroma, Intent-to-Treat Population
eTable 8. Geographic Atrophy Area Change from Baseline to Week 48 by CFI-Profile Biomarker Status, for Chroma and Spectri, Pooled and Separate
eTable 9. Best-Corrected Visual Acuity Change From Baseline at Week 48
eTable 10. Adverse Event Overview, Ocular and Non-Ocular, First 48 Weeks, Chroma and Spectri Pooled
eTable 11. Ocular Adverse Events in the Study Eye Occurring in ≥1% of Participants in any Treatment Group, First 48 Weeks, Chroma and Spectri Pooled
eTable 12. Non-Ocular Adverse Events Occurring in ≥1% of Participants in any Treatment Group, First 48 Weeks, Chroma and Spectri Pooled
eTable 13. Serious Ocular Adverse Events in the Study Eye, First 48 Weeks, Chroma and Spectri Pooled
eTable 14. Non-Ocular Serious Adverse Events (SAEs) by MedDRA System Organ Class and Non-Ocular SAEs Occurring in ≥0.5% of Participants in any Treatment Group, First 48 Weeks, Chroma and Spectri Pooled
eTable 15. Adverse Events Leading to Death During the First 48 Weeks, Chroma and Spectri Pooled
eTable 16. Adverse Events Leading to Treatment Discontinuation, First 48 Weeks, Chroma and Spectri Pooled
eTable 17. Ocular and Non-Ocular Adverse Events of Special Interest, First 48 Weeks, Chroma and Spectri Pooled
eTable 18. Increased Intraocular Pressure in the Study Eye and Fellow Eye, First 48 Weeks, Chroma and Spectri Pooled
eTable 19. Neovascular Age-Related Macular Degeneration in the Study and Fellow Eyes, First 48 Weeks, Chroma and Spectri Pooled
eFigure 1. Adjusted Mean Geographic Atrophy Area Change From Baseline to Week 48
eFigure 2. Adjusted Mean Change in Geographic Atrophy (GA) Area From Baseline to Week 48 in the Study Eye by Clinical Subgroup, Chroma and Spectri Pooled
eFigure 3. Adjusted Mean Change in GA Area From Baseline to Week 48 in the Study Eye by Clinical Subgroup, Lampalizumab q4w Vs Sham
eFigure 4. Adjusted Mean Change in GA Area From Baseline to Week 48 in the Study Eye by Clinical Subgroup, Lampalizumab q6w Vs Sham
eFigure 5. Mean Change in Best-Corrected Visual Acuity Over Time From Baseline to Week 48
eFigure 6. Mean (A) Pre-Dose and (B) Post-Dose Intraocular Pressure (IOP) in mm Hg Over Time in the Study Eye, First 48 Weeks, Chroma and Spectri Pooled