Biomaterials and engineered microenvironments to control YAP/TAZ-dependent cell behavior (original) (raw)
. Author manuscript; available in PMC: 2020 Jan 30.
Published in final edited form as: Nat Mater. 2018 Oct 29;17(12):1063–1075. doi: 10.1038/s41563-018-0180-8
Abstract
Mechanical signals are increasingly recognized as overarching regulators of cell behavior, controlling stemness, organoid biology, tissue development and regeneration. Moreover, aberrant mechanotransduction is a driver of disease, including cancer, fibrosis and cardiovascular defects. A central question remains how cells compute a host of biomechanical signals into meaningful biological behaviors. Biomaterials and microfabrication technologies are essential to address this issue. Here we review a large body of evidence that connects diverse biomaterial-based systems to the functions of YAP/TAZ, two highly-related mechanosensitive transcriptional regulators. YAP/TAZ orchestrate the response to a suite of engineered microenviroments, emerging as a universal control system for cells in two and three dimensions, in static or dynamic fashions, over a range of elastic and viscoelastic stimuli, from solid to fluid states. This approach may guide the rational design of technological and material-based platforms with dramatically improved functionalities and inform the generation of new biomaterials for regenerative medicine applications.
Introduction
Most of our understanding of cell biology comes from investigations on few established cell lines cultured on glass or tissue culture plastic dishes. These culturing procedures in two dimensions (2D) dominate biomedical research because they are easy to adopt and highly reproducible. However, these unphysiologically rigid materials do not support the growth of primary cells (i.e., normal cells directly explanted from patients or animal models) and do not mimic the complexity of real cellular microenvironments1. Physical signals impinging on cell behavior can be static or dynamic - depending on tissue physiology (e.g., flows of blood or interstitial fluids, peristaltic movements, muscle contraction) – or can be dynamically altered during development, tissue repair or disease states (e.g., fibrosis, cancer, atherosclerosis). It is now clear that mechanical cues, long neglected in the intellectual landscape of cell biologists, are causal and fundamental in the control of cell fate decisions and, when aberrant, can be drivers of disease2–5. To tackle these issues, a new suite of biomaterial systems is being developed that more closely mimic the in vivo milieu of living tissues. Adoption of these systems is already advancing our basic understanding of the principles of tissue and organ function6,7. In addition, there is hope that specific biomaterials could be used for regenerative medicine applications.
Progress in this direction, however, cannot be uncoupled from a deeper appreciation of the means by which information on the tissue’s own composition, physical structure, shape and architecture is perceived by each of its constituent cells. These inputs are dictated by the cell's attachment to other cells and the landscape rigidity of the extracellular matrix (ECM) in which cells are embedded6. Physical forces then interplay with other elements of the cellular microenviroment, such as morphogen gradients and cell-cell signaling contacts, collectively impacting on cell fate decisions. Crucially, cells hard-wired within tissues must then compute mechanical and chemical signals into coherent biological responses, that invariably must converge on the control of gene expression by orchestrating transcription and epigenetics3.
In fact, the overarching role played by mechanical signals on cell shape and cytoskeletal organization, and, as such, on polarity, endocytosis, nuclear structure and organelle functions, strongly suggests that essentially every aspect of cell behavior may be affected by cellular mechanotransduction2,3,8–11. The use of biomaterials thus has the potential to revolutionize molecular and cell biology. That said, to "put biomaterials in every biomedical laboratory" the field has to overcome some technical challenges: biomaterial technologies are still artisanal, neither immediately accessible nor spreadable from lab to lab, blunting their exploitation by the biomedical research community at large. Also limiting is the lack of an overarching formal intellectual framework of how cells make sense of a host of biomechanical signals. Filling these gaps and knowledge barrier is essential to hold together the engineering and biomedical communities toward shared and defined goals, and prioritizing applicability of biomaterial platforms toward the most essential challenges.
We wrote this review to offer our readers a navigation guide on what is emerging as a cardinal principle of how cells and tissues mechanically sense biomaterials and transduce them into gene-expression programs, that is, by patterning the activity of two mechanosensitive transcription factors YAP/TAZ3,12–14.
A cell's perspective on material design
Cells in living tissues are exposed to myriad physical signals imposed by neighboring cells and the ECM. This includes ECM protein composition, stiffness and viscoelasticity, but also substrate topology (e.g. roughness, curvatures), and fibrosity15. A range of materials-based platforms has been designed to parse in vitro the complexity of the in vivo physical milieu by testing individual features of the cell's environment and their influence on cell behavior. At the same time, it is also clear that such reductionist approach has limits, as even the most defined material designed to study one individual feature will invariably indirectly affect other physicochemical parameters. For example, increasing crosslink densities in hydrogel networks increases stiffness but may also concomitantly change presentation of adhesive ligands, substrate porosity or viscoelastic properties16.
This raises the central question in biomaterial design of what should be prioritized in order to instruct a given cell type toward a desired phenotype (e.g., a specific differentiated, metabolic, proliferative, survival or other state). This brings back the focus on the cell and molecular determinants that drive cell behavior in response to mechanical cues. These cues collectively impact on individual cell geometry and polarity, as well as on architecture and molecular composition of the cell’s adhesion sites. All in all, this is integrated and transduced by the tension and fine organization of the cytoskeleton15, ultimately controlling gene expression through the activity of transcription factors. In this context YAP/TAZ activity can serve as molecular beacon of how the cell responds to its physical microenvironment (Box 1). Indeed, YAP/TAZ mechanotransduction is essential for a number of biological outcomes dictated by mechanical strains in vitro and vivo (reviewed in3). YAP/TAZ potently regulate organ size control, embryonic morphogenesis, and regeneration in the adult, as well as the onset of primary disease states, such as solid malignancies in a variety of organs, fibrosis and atherosclerosis. This is due to YAP/TAZ-driven gene expression programs, controlling cell proliferation, stemness, differentiation and metabolism (reviewed in3,14,17). Thus, biological outcomes associated to YAP/TAZ ON vs. OFF (or graded) activity states should be envisioned in the design of novel biomaterials toward specific biological applications.
Box 1. Tools to study YAP/TAZ mechanotransduction.
As outlined throughout the text, experimental manipulation of extracellular mechanical cues or of the cell’s own cytoskeletal organization and contractility potently affect YAP/TAZ nuclear functions. As resource to the reader, here we outline some of the most relevant readouts to study YAP/TAZ activity, and of the genetic or pharmacologic approaches to functionally assess the YAP/TAZ mechanotransduction pathway.
i. nucleo/cytoplasmic shuttling: YAP and TAZ proteins are found both in the cytoplasm and in the nucleus, where they interact with their DNA binding partners, i.e., members of the TEAD family of transcription factors, in order to regulate gene transcription14. YAP/TAZ subcellular localization, measured in terms of nuclear-to-cytoplasmic ratio by immunofluorescence staining, is a rapid and quantitatively reliable proxy used to assess YAP/TAZ modulation by mechanical and cytoskeletal cues12,13,41,92.
ii. expression of YAP/TAZ endogenous target genes: YAP/TAZ activity can be monitored by measuring the expression levels of validated direct endogenous target genes by quantitative real-time PCR. Of note, recent evidence on the genome-wide chromatin occupancy of YAP/TAZ-TEAD complexes is rapidly expanding the package of testable endogenous direct target genes17. iii. luciferase reporter assays: The most widely used synthetic luciferase reporters to monitor YAP/TAZ-dependent transcriptional activity is the 8XGTIIC reporter, composed of multimerized TEAD binding sites12,13. The main advantage of this or other synthetic reporters is that these integrate the net transcriptional activity of YAP/TAZ, independently of the search and validation of endogenous targets in a given cell type.
Experimental manipulation of mechanical cues. Different drugs are available to inhibit the F-actin cytoskeleton and have been successfully used to mechanically modulate YAP/TAZ activity12,13. This includes blebbistatin (inhibitor of the molecular motor myosin II), ML-7 (inhibitor of myosin light chain kinase, an upstream regulator of myosin activity), Y-27639 and C3 toxin (inhibitors of the Rho/ROCK signaling pathway), SMIFH2 (inhibitor of formin-homology 2 domains) and Latrunculin (inhibitor of the process of actin polymerization). Additionally, the discovery that actin capping/severing proteins (CapZ, Cofilin and Gelsolin in mammalian cells) as relevant YAP/TAZ regulators3,12 provided relevant tools to modulate the F-actin cytoskeleton in order to boost YAP/TAZ activation, even in otherwise mechanical inhibitory environments.
Engineering individual cell behavior
Engineering cell size
Micropatterning technologies (Box 2) have offered some of the first and seminal insights in mechanobiology. Micropatterned adhesive "islands" coated with ECM molecules (e.g., fibronectin) allow restricting the adhesive area and shape to which individual cells can attach in 2D. On small islands, cell spreading is reduced, narrowing the distance between focal adhesions, as such inducing a more relaxed cytoskeletal state typified by thicker cortical actin and reduced stress fibers. These substrates have been used to reveal the mechanical control of epidermal stemness vs. differentiation, endothelial proliferation vs. death, and mesenchymal stem cells (MSCs) differentiation10,18–20 (Fig.1). It is now clear that all what these apparently disparate outcomes have in common is the regulation and function of YAP/TAZ3,12,13,21. Cell spreading invariably increases YAP/TAZ nuclear accumulation and transcriptional activation, whereas confinement leads to YAP/TAZ turn off. For example, a lowYAP/TAZ state associated to small substrates causes death in endothelial cells, but fosters adipogenesis in MSCs at the expense of bone-like (osteogenic) differentiation13. Crucially, experimentally raising YAP or TAZ levels on cells plated on small islands readily rescues endothelial cell proliferation and induces osteogenic differentiation of MSCs. These results provided the first demonstration that YAP/TAZ are downstream readers and mediators of mechanotransduction13.
Box 2. Synthetic substrates for 2D cell cultures.
Microprinting
ECM proteins are microprinted on glass separated by nonadhesive regions to obtain confinement of single or multiple cells on adhesive islands of different sizes and shapes. The non-adhesive areas are typically fabricated on gold-coated substrates by poly(dimethylsiloxane) (PDMS) elastomeric stamps that release or induce the formation of protein-resistant polymer brushes, or by polymerizing non-adhesive precursors through a photomask93,94. The cell repellent pattern is then placed in a solution of ECM-like proteins (e.g. fibronectin, collagen I, laminin, etc), to transform the remaining areas into adhesive patterns by protein absorption.
Hydrogels
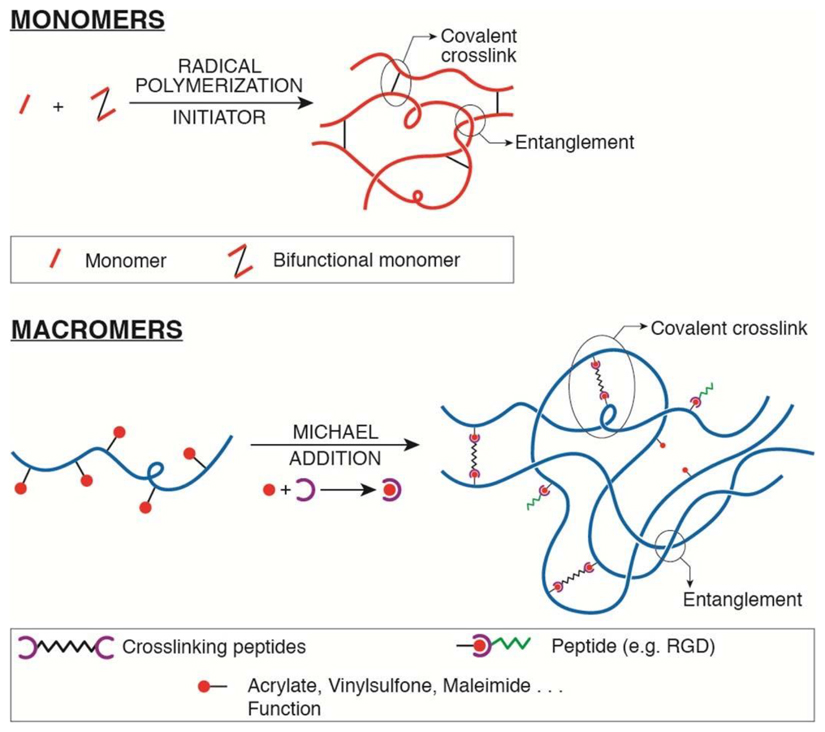
Among synthetic hydrogels, the most currently used are poly(acrylamide), PAA, and poly(ethylene glycol), PEG; natural polymer hydrogels commonly used are type I collagen, fibrin, hyaluronic acid (HA) and alginate, often modified with polymerizable functions to control their mechanical properties and biofunctionalization. Generally, hydrogel networks are produced by different polymerization and crosslinking reactions (free-radical or Michael addition66, eventually UV assisted) starting from synthetic monomers or macromers, or from modified natural polymers, to produce 3D polymeric meshes hold together by covalent bonds and physical entanglements. Mechanical properties are tuned by changing polymer density and/or crosslinker concentration. The tunability of mechanical properties, together with an extremely high water content, render the physical properties of in vitro hydrogels comparable to those of ECM in vivo. Detailed protocols for generation of PAA 2D hydrogels with tunable rigidity have been reported95. Proper adhesive sites must be also present. Natural hydrogels, such as collagen and fibrin, provide several integrin-binding sites, the most established being arginine-glycine-aspartic acid (RGD) sequence. Conversely, synthetic hydrogels must be additioned with specific ECM-like proteins
Micro- and nano- pillars arrays
Elastomeric pillar arrays have been largely used to decouple substrate rigidity from adhesive properties16,54. Typically, micro- and nano-pillars are fabricated by hardening PDMS on a Silicon mold. Micrometric pillars have been shown to induce myosin contractions around the individual pillar96–99, while nanometric pillars (diameter <500nm) mimic a continuous soft substrate similarly to hydrogels, regardless of the amount of ligands available to cells32. However, rigid nanopillars built in SU8 allow unprecedented subcellular spatial resolution of traction forces30.
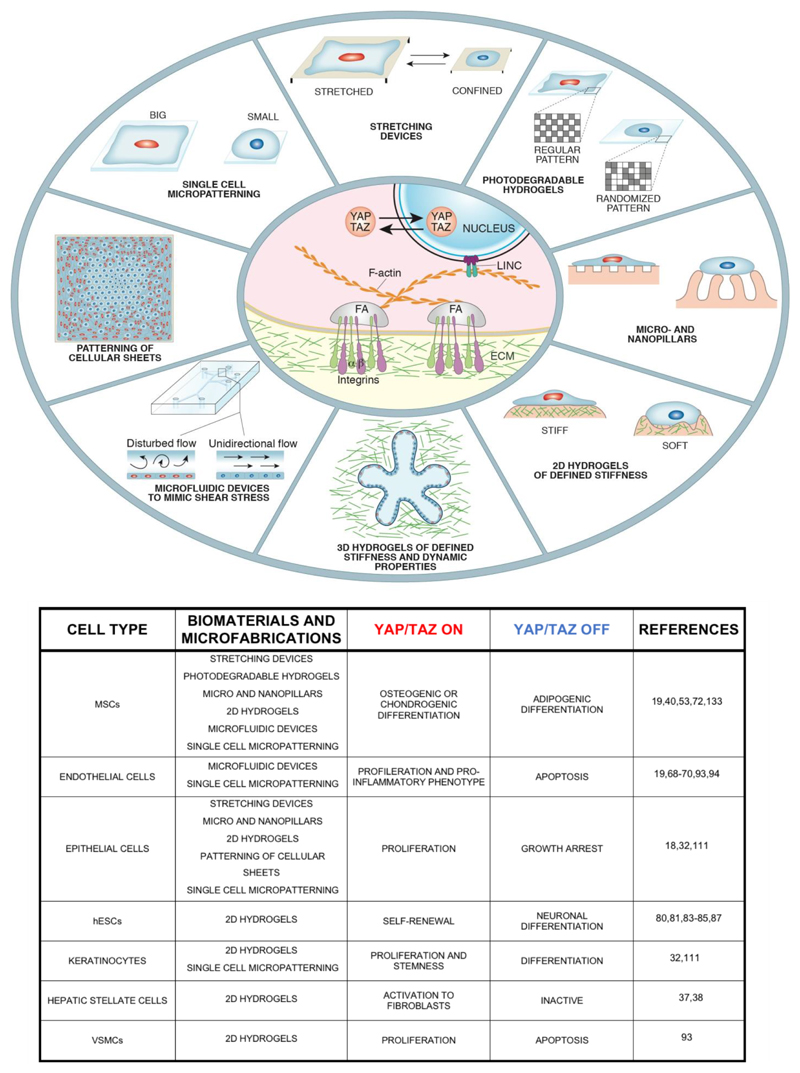
Figure 1. Microfabrication and biomaterial designs, their biological effects and YAP/TAZ regulation.
Top panel: Schematic representation of engineered substrates adopted to study the effect of physical and mechanical manipulations on cells. Different stimuli and experimental conditions converge on YAP/TAZ regulation (Activation in Red, Attenuation in Blue). Central oval: the basics of YAP/TAZ mechanotransduction, as their nuclear localization and activity rely on the mechanic coupling of the cell to the ECM through integrin and associated changes in F-actin organization (FA, Focal Adhesions). Bottom panel: table summarizing the biological consequences of biomaterial-based regulation of various cell types broadly adopted in the biomaterial community, and the causality of YAP/TAZ activity levels for these responses.
Dosing substrate stiffness
Another influential set of experiments has been the use of PAA (Poly-Acrylamide) hydrogels of defined rigidities (Box 3). This revealed that MSCs are mechanical "chameleons", adopting fates driven by the rigidity of the corresponding tissue in vivo (i.e., turning into adipocytes at fat-like rigidities, neurons at brain-like rigidities, muscle at intermediate and bone at highest stiffness)8. In these assays, substrate stiffness ultimately affects cell spreading and ability to develop tensional forces by integrin clustering at adhesion sites in a "catch-bond" model of ECM-integrin-F-actin association, whereby lifetime of adhesive spots increases with tension generated by traction over progressively more rigid materials3. This molecular mechanism feeds on YAP/TAZ activity and, in so doing, the cell fates instructed over a gradient of ECM rigidities are ultimately driven by dosing YAP/TAZ mechanotransduction3,13 (Fig.1).
Box 3. Introducing dynamic changes in substrate properties.
Two main types of material-based systems have been developed to change mechanical cues on 2D and 3D substrates: stretching devices and photosensitive biomaterials. Briefly, stretching devices are based on a straightforward procedure to strain a PDMS flexible 2D membrane coated with collagen or fibronectin on which epithelial cells are plated. Biaxial strain is imposed by inflating a chamber below PDMS to increase the chamber volume and stretch the overlaying membrane for a certain time12, or in a cyclic manner32. Alternatively, the strain is created by positioning a pillar beneath the center of the membrane and applying a vacuum pressure to deform the membrane around the pillar31.
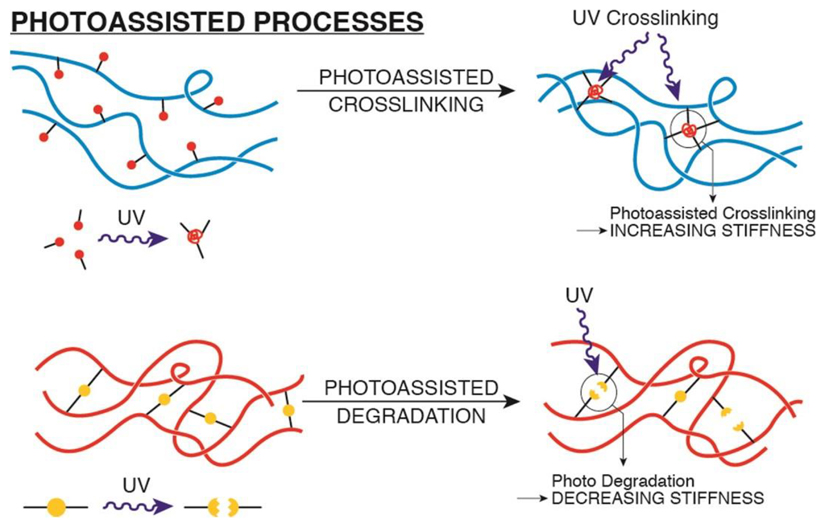
Photosensitive biomaterials are based on UV mediated crosslinking (to increase stiffness) and photo- degradation (to decrease stiffness) of hydrogel networks that already contain encapsulated cells, and offer precise, quantitative and spatiotemporal control of hydrogel mechanical properties. A simple method to dynamically stiffen a hydrogel has been developed using a methacrylated polymer, such as HA22,100,101. A methacrylated polymer functionalized with thiol-containing RGD peptides, through Michael addition, is ideally suited for dynamic crosslinking as it avoids toxicity associated to residual unbound monomers. Of note, HA is a natural polymer with its own cell surface receptor, whose mechanical and signaling properties remain undefined.
UV-mediated hydrogel degradation has been developed based on PEG polymeric hydrogels containing photodegradable groups (such as nitrobenzene derivatives), that undergo network depolymerization27,102–104. The main advantage of these systems is that substrate elasticity can be spatially patterned through mask-assisted light-patterning. Similar approaches have been also exploited for 2 photon-assisted patterning in 3D.
More recently, time-dependent stiffening through photo-activated modifications of hydrogels (Box 4) allowed to recapitulate the temporal changes of ECM rigidity occurring during some diseases. For example, liver fibrosis is now envisioned as a contributing factor in the loss of liver regenerative properties observed in cirrhotic patients, and for emergence of liver cancer. The primary source of such stiff-ECM are myofibroblasts derived from transdifferentiation of hepatic stellate cells. This conversion is YAP/TAZ-dependent and can be mimicked by stiffening the substrate material, leading to YAP/TAZ activation and fibrosis22–24.
Box 4. Synthetic substrates for 3D cell and organoid cultures.
Biomaterials for 3D cultures must have some distinctive requirements: i) possibility to control stiffness around a physiological range; ii) short and non-toxic gelification reactions; iii) presence of degradable or labile (i.e., ionic) crosslinks allowing physical network remodeling by breaking reversible bonds through applied forces (e.g., alginate gels); iv) cell adhesiveness. Several natural or synthetic hydrogels have been developed with these features105–110. Here we list some of the more commonly adopted.
Methacrylated HA hydrogels are functionalized with both methacrylate and maleimide groups, and crosslinked by protease-degradable oligopeptides using Michael-type reactions between maleimides and thiols present in the peptides. Then UV photopolymerization is used to crosslink the hydrogel after cell encapsulation. By carefully selecting the reaction conditions (type and amount of photoinitiator, UV dose and wavelength) cell toxicity can be minimized. HA hydrogels are also used to enable independent co-presentation of different adhesive motif (e.g. N-cadherin and RGD)88.
Alginate hydrogels can be coupled with RGD peptides (carbodiimide chemistry) and low molecular weight PEG spacers, to produce hydrogels with viscoelastic behaviour39. Even though alginate is non-degradable by mammalian cells, stress relaxation of the substrate, enabling traction forces by cells, is still assured by weak, ionic bonds between alginate chains, that can be easily changed by cellular forces.
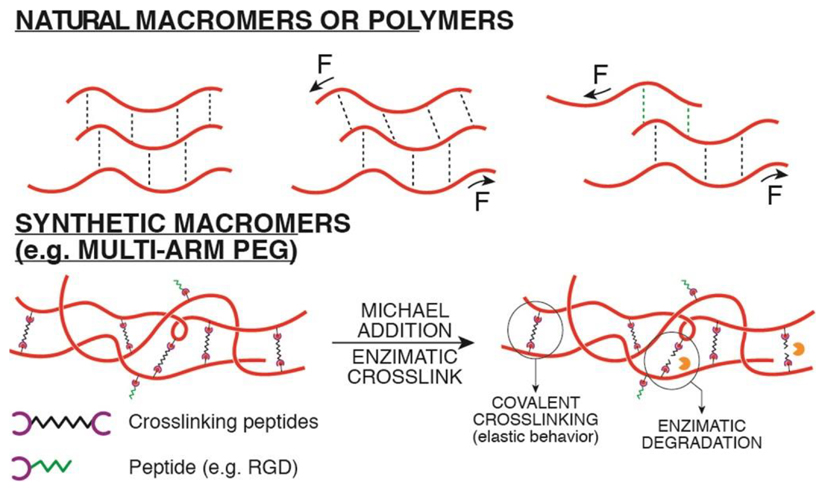
Among synthetic hydrogels, PEG based hydrogels represent the most flexible and biocompatible synthetic material that can be used for 3D cell culture systems: many precursors are commercially available in the form of variously functionalized macromers111. These can be modified with a number of different functional groups giving rise to different polymerization reactions for hydrogel crosslinking with proteasesensitive and adhesive peptides. Multi-arm PEGs act as building blocks allowing for a particularly precise control of crosslinking degree and bio-functionalization. Both chemical, via Michael addition, and enzymatic crosslinking methods have been adopted7,112. Enzymatic crosslinking mediated by the transglutaminase Factor XIIIa offers a cell-friendly, gentle crosslinking reaction113.
Controlling substrate topology
Recent progress in material sciences has enabled the exploration of physical parameters other than stiffness, such as fine topological features of the adhesive substrate. For example, plating MSCs on hydroxyapatite discs of defined surface roughness revealed that optimal osteogenic differentiation is attained within a fine roughness range in which oriented F-actin arrangement is promoted and peak levels of YAP/TAZ are achieved25. In addition, topological features can be engineered by lithography or electrospinning. Indeed, controlling orientation of MSCs with respect to the orientation of adhesive fibers instructs MSC differentiation toward a chondrogenic fate, again in a YAP/TAZ-dependent manner26. Photopatterning approaches on hydrogels (Box 4) also allow modulating the fine spatial pattern and organization of adhesive spots of different elastic moduli. Intriguingly, MSCs experience high YAP/TAZ activity on regularly patterned stiff and soft spots, but display YAP/TAZ inhibition when the same spots are presented to cells in a randomly spaced organization. This reveals that cells do not “average” rigidity sensing and that substrate rigidity is not relevant per se, but only to the extent to which it can instruct specific cytoskeletal organizations that either activate or repress YAP/TAZ activity27 (Fig.1).
Raising stiffness in standard PAA hydrogels invariably influences material porosity and, in turn, the length of the fiber segment between two adjacent anchoring points, defined as tethering. It is plausible that, depending on the experimental conditions28,29, tethering may also contribute to YAP/TAZ regulation since it can regulate differentiation of MSCs and epidermal progenitors, that, as detailed above and below, are YAP/TAZ driven processes. That said, stiffness ostensibly plays a dominant role, as rigid PAA substrates coated with cell-adhesive but non-fibrous (and therefore non-tetherable) RGD peptides can still sustain YAP/TAZ-driven programs28.
Insights on rigidity sensing from nanopillars
Advanced microfabrication methods were recently adopted to culture cells over an array of "nano"-pillars (Box 2), offering topological cues at an unprecedented spatial resolution. Coupling this treatment with YAP/TAZ read-outs allowed dissecting the relative contribution of two different rigidity sensing machineries: one located at the cell's edge, mediated by sarcomeric-like contractions between adjoining integrin adhesive sites pinching the substrate, and one located at less dynamic adhesive spots in close proximity to the nucleus. Only the latter is instrumental for YAP/TAZ activation mediated by coupling integrinβ1-bound ECM with LINC-bound nucleoskeleton through F-actin bundles30 (Fig.1).
Patterning cellular sheets in 2D
Microfabrication has been also used to study emergent properties of multicellular sheets, and in particular how forces emerging at the tissue-level can impart positional information and thus pattern the collective behavior of otherwise naive cells. When endothelial or mammary epithelial cells are cultured on large ECM islands (300 μm diameter), proliferation is initially even throughout the island but, as cell crowding increases, it becomes spatially patterned: cells at the center of the island undergo growth arrest by "contact-inhibition" while cells at the borders and corners keep on proliferating. The latter are in fact those experiencing highest tractional stress, as measured by micromechanical force sensors arrays, and highest mechanical restructuring of the cytoskeleton through Rho activation11. Such mechanical patterning of multicellular sheets translates into patterns of YAP/TAZ activity: inhibited at the center and kept high at the borders, where they are instrumental for the localized proliferation12 (Fig.1). Notably, in spite of its historical naming, "contact-inhibition" of proliferation is largely a consequence of mechanical cell confinement to a small projected area due to cell crowding, and ensuing cytoskeletal relaxation not dissimilar to confining individual cells into small adhesive areas12. Although ECM islands with edges and corners do not exist in vivo, real tissues have well defined architectural forms - pits, folds and branching points - that may generate "hot spots" of tensional stress due to localized cell distortion. This notion has been validated by seeding cells on PDMS-based stretching devices (Box 3) revealing that spatial control of cellular strain is causal for YAP/TAZ activation and cell proliferation12,31 (Fig.1). Repeated mechanical strains can also trigger YAP/TAZ activation at rigidity levels that would be below threshold under static conditions, as shown by cycling cell stretching over soft pillar arrays32.
Engineering 3D environments
In spite of the groundbreaking discoveries made possible by 2D microfabrication, cells in physiological conditions are embedded within a complex 3D environment, rich of multiple ECM components, other cell types and soluble growth factors, in which even simple monostratified epithelia in fact assume complex 3D topological landscapes. Since cell behavior in 3D may be sometimes difficult to extrapolate from what known in 2D cultures, we are witnessing to an exploding interests in biomaterials able to support encapsulated 3D cultures and, in the long run, to mimic natural niches with combined control of mechanical and soluble signals7.
Classic work with mammary epithelial cells on reconstituted basement membranes revealed that switching culturing conditions from rigid 2D coated dishes to 3D hydrogels dramatically changes cell behavior: notably, in 3D, cells turn off tumor-like behaviors typical of 2D cultures, and instead acquire a growth-arrested state, gain apico-basal polarity and self-organize into overtly normal, mammary acinar-like structures33. A second landmark discovery has been the demonstration of the primary role of ECM stiffness in the 2D-3D transition. Clearly, in hindsight, extracellular rigidity dramatically drops from 2D plastic to 3D all-around ECM; yet, the formal evidence that stiffness and mechanotransduction were involved in these cell fate changes came after experimental raising the 3D basement membrane stiffness through progressive addition of fibrillar collagen. This led to the reappearance of malignant behaviors of oncogenically-primed epithelial cells even when these were cultured in 3D2. Importantly, what ultimately drives these phenotypic switches is the relative ON vs. OFF state of YAP/TAZ activity that accompanies tumor vs. normal-like phenotypic states: experimental inactivation of YAP/TAZ prevents the gain of aggressive cellular properties induced by increased ECM rigidity, while YAP or TAZ overexpression is sufficient to phenocopy the effect of ECM stiffening in otherwise benign cells12.
A potential caveat of the above experiments and all other set-ups using natural ECMs, such as basement membranes or collagen I, is the difficulty to discriminate between stiffness alone and other cues - such as density of adhesive sites, fibrosity, elasticity. Natural ECM also complicates the dissection of how cells respond to mechanical stimuli by modifying the mechanics of their surroundings. Indeed, in response to increased stiffness cells can secrete proteases that modify adhesion, local ECM topology and other physical properties34.
The use of fully synthetic 3D hydrogels (Box 4) allowed a deeper understanding of how cells tune their behavior by negotiating with distinct mechanical features of their environment. This brought to the discovery that, in order to sustain YAP/TAZ mechanotransduction - and, in so doing activate essentially all of the known biological effects of biomaterials - stiffness is only one of the required features, and only within an optimal range. Another, and possibly even more central attribute, is indeed the possibility to dynamically restructure the cell's environment. For example, maintenance of elevated YAP/TAZ activity levels occurs in synthetic hydrogels when cells are allowed to remodel their substrate through matrix-metalloproteinase (MMPs). In contrast, covalentlycrosslinked hydrogels not amenable to cell-mediated remodeling impose to cells insurmountable spatial constraints that forces cell rounding irrespectively of ECM rigidity, leading to YAP/TAZ inhibition34.
Crucially, the interpretation of these results is well grounded in genetics, as skeletal SCs mutant for MMP14 are unable to preserve high-YAP/TAZ levels and fail osteogenic differentiation both in vivo, in transgenic mice, and ex-vivo, in collagen-based hydrogels35. Of note, MMP14-mutant cells display completely normal behavior in 2D, revealing that dynamic mechanical interactions of cells with their 3D niches is vital for SC fate determination in vivo. Reinforcing the role of YAP/TAZ as pivots of these events, lack of ECM remodeling in MMP-mutant cells becomes dispensable when YAP/TAZ levels are artificially sustained35.
Material degradation allows cells to exert traction forces by triggering integrin clustering in a manner not dissimilar to what freely permitted in 2D environments; as such, just like too much of a good thing can be detrimental, synthetic hydrogels that are either overly stiff or in which crosslinking density exceed what cells can degrade prevent YAP/TAZ activation and oppose osteogenic fates in MSCs34–36. From a physical standpoint, it was recently proposed that pericellular proteolytic degradation of covalently cross-linked hydrogels locally converts an elastic matrix into a visco-elastic fluid37 (Fig.2). This is relevant, as natural matrices, such as collagens or basement membranes, are not elastic but in fact viscoelastic38,39. In line with this notion, an alginate-based hydrogel (Box 4) of appropriate initial bulk stiffness is sufficient to sustain YAP/TAZ mechanotransduction without formal need of proteolytic degradation because it allows mechanical remodeling through stress-relaxation that involves only rupture of ionic-bonds with subsequent hydrogel flow39 (Fig.2).
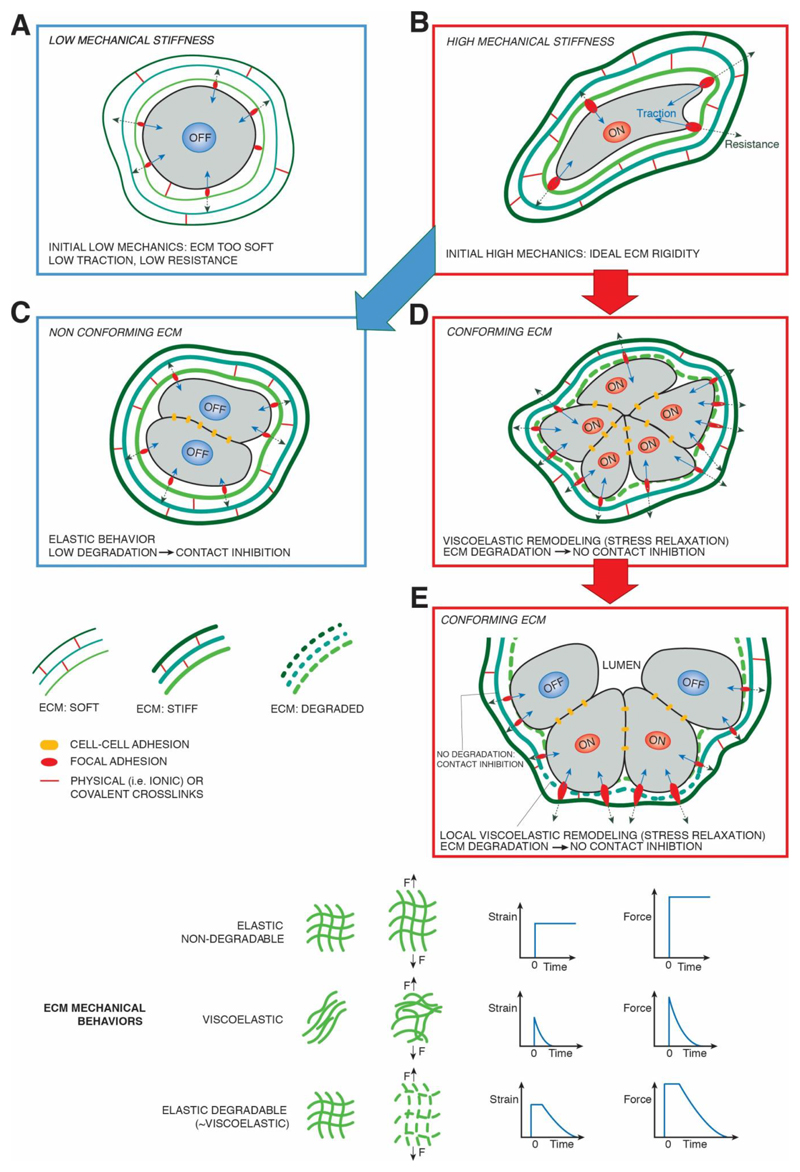
Figure 2. Dynamic preservation of stem cells in organoid outgrowths requires "conforming" properties of biomaterials.
Schematic representation of a single stem cell, seeded in 3D hydrogels, either natural or synthetic. A) low substrate stiffness causes reduced ECM resistance (dotted arrows) leading to poor cellular traction forces (blue arrows), YAP/TAZ inhibition (OFF) and loss of stemness and of proliferative potential. B) "high" ECM rigidities, whose ideal value depends on the specific cell type, sustain YAP/TAZ activation (ON). This is mediated by stronger resistance of the ECM and build-up of sufficient traction forces by actomyosin contractility, in turn leading to maturation of FA, F-actin remodeling and cellular strain. However, in absence of "conforming" properties of the ECM (C), such as on substrates retaining elastic properties and that cannot be pericellularly degraded, cell division leads to progressive cell confinement, increased cell-cell adhesion (similar to 2D contact inhibition) and reduced traction forces. Note that the ECM remains ideally stiff as in (B). This leads to YAP/TAZ turn off, and loss of stemness and proliferative potential. D) YAP/TAZ driven stemness and proliferation are preserved as long as the material can conform to the proliferative outgrowth of the organoids thanks to biomaterials endowed with viscoelastic properties due either to stress relaxation potential and/or degradation by spontaneous hydrolysis or by MMPs. E) A conforming ECM is permissive for self-organization of the ever expanding organoid, as local patterns of high and low ECM viscolasticity are translated in patterns of YAP/TAZ activity: only cells displaying high mechanical stress retain stemness (YAP/TAZ ON) whereas those experiencing lower traction, contact inhibition and/or reduced MMP expression undergo differentiation permitted by YAP/TAZ turn OFF. The bottom part of the figures outlines the various types of ECM and their associated mechanical behavior.
Conforming properties of synthetic biomaterials
The recent introduction of organoid technologies, allowing the growth of self-renewing and self-organizing mini-organs starting from primary somatic stem cells, have expanded the possibility to study normal and diseased tissues ex-vivo, with promise to cure disease through gene-therapy in autologous stem cells, or to biobank human tissues in order to test therapies in unprecedented personalized medicine approaches40. Strikingly, YAP/TAZ are essential for the outgrowth of organoids in multiple tissues, serving as stemness-endowing transcription factors41,42. For example, YAP/TAZ sustain the selfrenewal and expandability over multiple passaging of intestinal, mammary, pancreatic and airway organoids41–43. In these systems, YAP/TAZ inactivation leads to demise of the organoid culture, indicating either their requirement at sustaining the survival of organoids' stem cells, or, as recently suggested, the need of YAP/TAZ for a constant regeneration of stem cells from non-stem cells during organoid passaging (i.e., dissociation to single cells and regrowth of secondary organoids)41,42, a step potentially involving a transient activation of fetal-like gene-expression programs43. Further work is required to discriminate between these possibilities. However, organoids culture protocols are still addicted to Matrigel, a reconstituted basement membrane of undefined and variable composition; this impedes some of the clinical applications of organoids, raising interest on the development of synthetic designer matrices - i.e. chemically and physically defined hydrogels - as ideal supporting environments for organoid cultures7. Each organoid represents the outgrowth of a single stem cell in a medium saturated of growth factors, and this introduces a new dynamic variable in these 3D systems: the increasing of organoid size over time due to cell proliferation. Recent findings revealed that even in these complex experimental settings cell fate decisions are dictated by material stiffness and degradability (Fig.2). First, at the onset of organoid growth from individual enmeshed cells, an optimal stiffness level must be provided to sustain YAP/TAZ and, in so doing, stemness and proliferation7. Then, as cells become progressively confined within the expanding organoid, contact inhibition blunts YAP/TAZ activity, leading to loss of stem cells and eventually to organoid demise. At this step, matrix relaxation and degradability become crucial in order for the organoid to undergo self-organization, namely, to reach a tissue-like configuration. In intestinal organoids, this occurs by localizing points of high mechanical stress at the tip of fingerlike protrusions (i.e., crypt bottoms), where YAP/TAZ are activated to preserve stem cells, while the rest of the organoid undergoes differentiation7 (Fig.2).
We thus introduce here the unifying concept of "conforming properties" of biomaterials for proliferating multicellular aggregates: the need to allow cells to find their own "sweet spot" of pericellular degradability that allows cells to constantly perceive an "ideal" stiffness (i.e., proliferating and stem promoting) while accommodating increased tissue size, but without paying the price of insufficient mechanical traction due to contact inhibition, insufficient or excessive ECM remodeling and degradation (Fig.2). In keeping with this concept, we propose that current organoid technologies may benefit from biomaterials designed to sustain YAP/TAZ activity over time as this would lead to preservation of their stemness and proliferative attributes. Synthetic material systems endowed with conforming potential, and currently used to support organoid expansion, are multi-arm PEG that can be hydrolytically and/or proteolytically locally degraded by the expanding organoid7. Yet, these systems are hardly amenable to passaging, require addition of fibrillar ECM proteins for optimal performance and are technically challenging to set up7. These limitations may be overcome by biomaterials intrinsically endowed with dynamic properties granted by their viscoelastic nature. This is the case of ECM-functionalized alginate hydrogels, in which stress relaxation is allowed by the sliding of polymeric chains crosslinked by weak ionic bonds (Box 4); these biomaterials can be thus easily and dynamically restructured by the cells without need of MMPs and may be used to achieve optimal conforming properties to improve organoid culture in the future. Natural polymers, such as HA gels, are frequently used to support 3D cell culture, but these are typically elastic and non-fibrillar limiting their conforming potential and thus their applicability to organoid cultures. However, HA can be variably chemically modified to change its physical forms, including viscolasticity44, or combined in interpenetrating networks with collagen I, as such capturing both the viscoelasticity and fibrillarity of natural ECM45. Moreover, conforming properties might be experimentally achieved by incorporation of photodegradable moieties as chemical crosslinkers (Box 3).
Mimicking shear stress in vitro
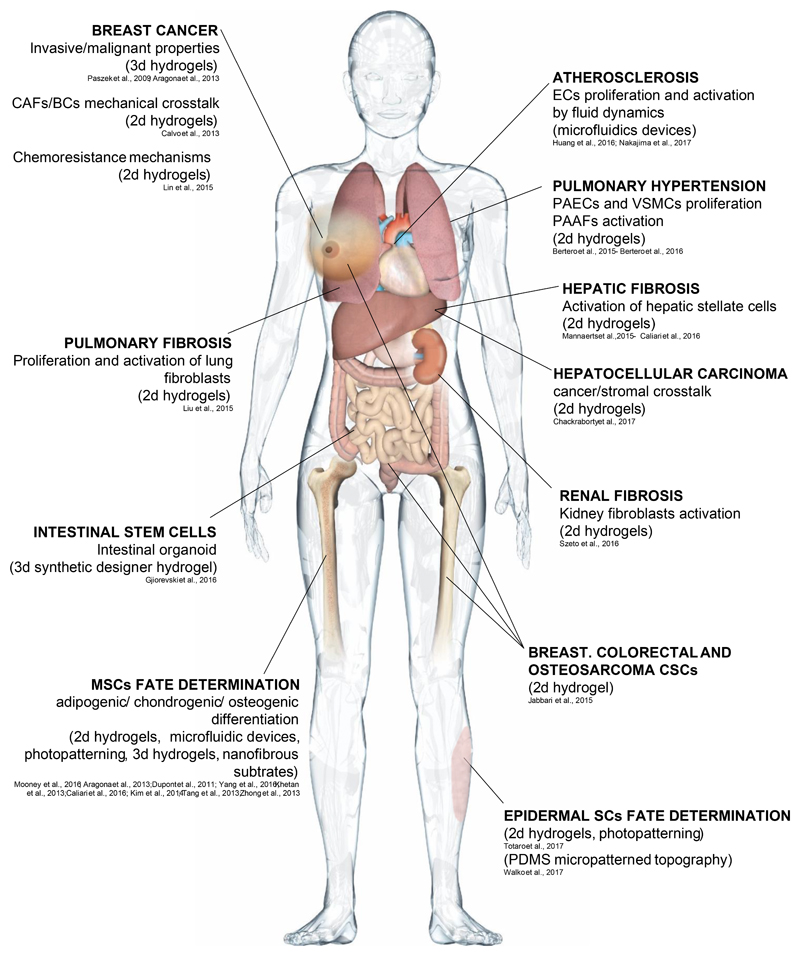
Mechanical forces associated to liquid flows are another class of crucial physical cues that can instruct cell behavior in physiological and pathological conditions. A point in case is the cardiovascular system, in which altered blood flow represents a prime causative factor for the development of atherosclerotic plaques3. Blood flow can be simulated by microfluidic devices that employ computer controlled systems to mimic in vitro the fluid shear stresses experienced by endothelial cells in vivo. Culturing of human endothelial cells (ECs) in microfluidic chambers under an oscillatory flow of the culture media mimics atheroprone-like turbolent flow conditions, as opposed to atheroprotective unidirectional fluid stream. Oscillatory flow specifically activates YAP/TAZ in ECs and this fosters a pro-inflammatory response that comprises induction of proliferation of ECs, secretion of inflammatory cytokines and exposure of monocyte-adhesion molecules46. Mechanistically, oscillatory flow has been shown to induce Integrin-β3 conformational changes46, resulting in deposition of thick cortical F-actin bundles, that promote YAP/TAZ nuclear accumulation. Importantly, these in vitro experiments have been validated in vivo, confirming the role of YAP/TAZ mechanotransduction as main culprit of atherosclerosis in mouse models (Fig.3). Thus, the design of biomaterial-based systems can be inspired by our knowledge of in vivo systems but, in turn, can reveal unexpected interpretations on classic physiopathological phenomena that have so far escaped a molecular explanation of their underlying causes.
Figure 3. Biomaterials mimicking altered microenvironments in human diseases.
Each arrow indicates a specific human disease or stem cell behavior, the biomaterialbased system used to investigate it and the biological read-outs.
Microfluidic devices have been also employed to study the effect of shear stress on fate determination of human MSCs, that in the bone marrow are in fact continuously subjected to interstitial fluid flow47. Indeed, interstitial levels of shear stress conveyed by a PDMS microfluidic system have been shown to promote hMSCs osteogenesis through TAZ nuclear accumulation48. In a further sophistication, fluid shear forces have been coupled with topological cues, by culturing hMSCs on microfluidic nanofibrous scaffolds formed by electrospinning, phenocopying collagen fiber deposition. This revealed that a specific angle of flow, perpendicular to fiber orientation, promotes fibrochondrogenesis of hMSCs through YAP/TAZ activation26. Intriguingly, this correlated with flow-induced nuclear deformation, that is recently emerging as an exciting area of investigation at the verge between YAP/TAZ mechanotransduction and gene expression3,30.
Engineering stem cells with biomaterials
Engineered microenvironments are offering mechanistic insights into how the ECM and physical forces regulate the behavior of stem cells, revealing how these control some of their cardinal features, such as self-renewal, proliferation and differentiation potentials. This ideally connects with the biology of YAP/TAZ, essential for organ growth, cell plasticity and stem cell regeneration. The convergence of biomaterials and YAP/TAZ fields opens unexpected avenues for biomaterials to orchestrate stem cells fates in artificial niches.
Biomaterials to orchestrate epidermal fates
The skin is a paradigmatic mechanosensitive tissue, as it needs to constantly adapt to changes in body shape during development and adulthood; this tissue must also rapidly respond to wounds interrupting its mechanical continuum and triggering transient increase of tissue stiffness and local cell deformation. These changes are perceived as mechanical stretching by the basal layer of the epidermis that contains progenitor cells49. YAP and TAZ have been found to be key factors that foster the self-renewal and proliferation of basal epidermal stem cells, during development and wound healing50,51. The recent application of biomimetic substrate scaffolds to the culture of primary human keratinocytes has been crucial for the molecular characterization of the mechanotransduction pathways that govern epidermal stem cells fate. When plated on PA hydrogels at near to physiological stiffness (> 4KPa), or allowed to stretch over large fibronectin adhesive patterns, primary keratinocytes experience high YAP/TAZ activity and this maintains stemness properties. Conversely, progenitors undergo differentiation into mature keratinocytes when plated on softer substrate or when confined to small fibronectin adhesive areas, where YAP/TAZ are inhibited21 (Fig.3). These mechanical challenges phenocopy the reduced ECM adhesion and loss of YAP/TAZ experienced when epidermal cells move suprabasally to undergo differentiation to generate a stratified epithelium in vivo.
Interestingly, biomaterials allowed to uncover an unexpected mechanism by which mechanical forces can orchestrate the spatial organization and fate of the whole epidermis, and offering insights that received genetic validation in transgenic mice21. Notably, this is mediated by a crosstalk between YAP/TAZ and the Notch pathway, by which mechanical regulation of YAP/TAZ reverberates into a mechanical regulation of Notch signaling, at once preserving stemness in the basal layer and favoring differentiation as keratinocytes moves suprabasally21.
Epidermal SCs might be governed not only by bulk stiffness parameters but also by topological cues. Indeed, recent evidence obtained by culturing primary human keratinocytes on a PDMS-based biomimetic platform that mimics the topography of the epidermal-dermal interface observed in vivo52, showed that YAP/TAZ are preferentially localized in the nuclei of Integrinβ1-positive stem cell clusters, localized at the tips of the protrusions in reconstituted epidermal rete ridges52. Intriguingly, the topological cues that dictate such YAP/TAZ-dependent preservation of somatic stem cells at points of highest curvature is closely reminiscent of that one observed in 3D PEG-based hydrogels used in intestinal stem cells organoid cultures and discussed above7.
Mechanically defined biomaterials for human pluripotent stem cells
The use of synthetic biomaterials of defined stiffness in human pluripotent stem cells (hESCs or hiPSCs) cultures has led to the appreciation that mechanical signals are pervasive cues that influence cell fate even in these contexts. Indeed, hESCs are profoundly influenced by substrate rigidity: when cultured on stiff (60KPa) collagencoated PAA hydrogels, YAP/TAZ are nuclearly localized and essential for the selfrenewal of hESCs3,53. In contrast, culturing hESCs on compliant biocompatible substrates, such as soft micropost arrays cause YAP/TAZ cytoplasmic sequestration, favoring neuronal differentiation53–55. Neuroepithelial differentiation also occurs when YAP/TAZ levels are lowered in hESCs cultured at high cell density3, a condition assimilated to the previously described “contact inhibition” phenomena whereby cells are progressively boxed into progressively smaller adhesive areas. The exploration of diverse engineered substrates for the culture of pluripotent stem cells revealed that, similarly to substrate stiffness, other physical parameters can influence pluripotent cell fate through YAP/TAZ, as in the case of anisotropic grated nanopatterns that induce neuronal differentiation through YAP/TAZ inhibition56. How hESCs and iPSCs might similarly interpret compliant substrates and anisotropic patterns remains to be elucidated.
Mouse and human ES cells (or iPSCs of any origin) are equivalent to embryonic pluripotent cells but of embryos at different developmental stages, that are indeed known to require very different media composition to preserve pluripotency and induce differentiation57. Consistently, mouse and human ESCs display different mechanical requirements58, as, oppositely to hESCs, YAP/TAZ are not required for the self-renewal of mESCs, but instead favor their differentiation41. Classic Yamanaka-type of reprogramming with OSKM transcription factors has indeed proven more efficient when mouse fibroblasts are cultured in a 3D PEG-based soft hydrogel59, associated to reduced YAP/TAZ activity. In line, reprograming of mouse somatic cells into iPSCs in a 3D environment reaches maximal efficiency when the PEG-based substrate comprises a dynamic component provided by MMP-cleavable peptide bridges59. Thus, a mechanically plastic environment is integral for imbuing fate plasticity, and should be thus carefully considered along with the biological response of each individual cell type to YAP/TAZ activation or inhibition in the design of three-dimensional biomimetic matrices.
Modelling 2D and 3D microenvironments to study diseases
Misregulated mechanical signaling from a structurally altered microenvironment, or from aberrant cellular mechanosensing properties, are emerging as potent drivers in a number of diseases, ranging from vascular and fibrotic maladies, to tumor emergence and invasion2–5,60,61 (Fig.3). These aberrant microenvironments can be accurately recapitulated ex vivo by biomaterial design. This is key for understanding disease pathogenesis and for the development of patient-specific applications.
YAP/TAZ misregulation is a main effector of pathogenesis mediated by changes in the physical properties of the microenvironment, as extensively reviewed elsewhere3. In addition to the atherosclerosis models detailed above in the context of fluid flow46, pulmonary hypertension (PH) is another deathly vascular disease that has long been associated to mechanical alterations of the ECM62. The disease pathogenesis entails aberrant proliferation of pulmonary endothelial (PAECs) and vascular smooth muscle cells (VSMCs), and promotion of pro-fibrotic responses in pulmonary artery adventitial fibrobalsts (PAAFs). Culturing these three vascular cell types on soft vs. stiff collagencoated PAA hydrogels showed that a stiff microenvironment is remarkably sufficient to trigger YAP/TAZ activation, and, in so doing, to foster PAECs and VSMCs aberrant proliferation, as well as to promote collagen production and crosslinking in PAAFs63. In these environments, cells also undergo metabolic rewiring towards aerobic glycolysis64, also typical of PH65.
Fibrotic diseases, characterized by abnormal remodeling of ECM components by activated fibroblasts (myofibroblasts), are a major cause of death worldwide and a greatly unmet medical need. Biomaterials mimicking these aberrant microenvironments are key for understanding the molecular mechanisms of these disorders. In fact, YAP/TAZ mechanotransduction is emerging as key player in fibrotic diseases. In addition to liver fibrosis (see above)22,23, YAP/TAZ abnormal activation also underlines renal fibrosis. Kidney-derived fibroblasts experience high YAP/TAZ activity when cultured on fibronectin-coated stiff PAA or silicon hydrogels, as opposed to more compliant substrates, and stiffness-induced YAP/TAZ nuclear accumulation promotes TGF-β signaling by YAP-mediated nuclear retention of Smad2/33. Of note, high YAP/TAZ activity also promotes proliferation and secretion of ECM proteins in lung fibroblasts cultured on collagen-coated stiff hydrogels66, indicating that YAP/TAZ are involved in positive feedbacks that lock pathological mechanotransduction in a self-sustaining loop. Collectively these findings open to the fascinating perspective that targeting YAP/TAZ mechanotransduction may be therapeutically effective for the treatment of fibrosis in diverse organs.
An aberrantly stiff mechanical environment has long been associated to the development of diverse solid cancers, suggesting that altered mechanotransduction could contribute to tumorigenesis2,3,67. YAP and TAZ are pervasively activated in the majority of solid tumors, where they foster formation of cancer stem cells and are associated to malignant properties, including metastatic capacity and chemoresistance (reviewed in17). YAP/TAZ are thus prime candidates to transduce aberrant mechanical cues into malignant behaviors. Consistently, aberrant mechanoactivation of YAP/TAZ mediates the effect of rigidity-induced cell proliferation and invasiveness in epithelial cells primed by oncogenic mutations2,12. Moreover, non-adhesive PEG-based 3D hydrogels of different stiffness revealed that an optimal hydrogel stiffness exists for preservation of CSCs in various tumor types, in correspondence to maximal YAP/TAZ activation levels68.
Challenging cancer cells and cancer associated fibroblasts (CAFs) with varying stiffness also revealed that YAP/TAZ mechanotransduction serves as a self-reinforcing loop that locks cancer cells into a mechanically hyperactivated state69. YAP/TAZ are activated in normal fibroblasts plated on top of stiff collagen-based hydrogels, converting these cells into CAFs, which in turn secrete ECM proteins that re-fuel YAP/TAZ mechanoactivation in CAFs, breast cancer cells and possibly all other cell types of the tumor microenvironment69. Similarly, hepatocellular carcinoma (HCC)-derived cells, when plated on stiff PA hydrogels, overexpress the proteoglycan Agrin, which is an ECM component that in fact sustains YAP/TAZ mechanotransduction to foster cell growth70.
Crucially, YAP/TAZ mechanotransduction might also be implicated in the emergence of chemoresistance mechanisms, such as in the case of Lapatinib resistant HER2-positive breast cancer cells, for which chemoresistance can be overcome through YAP/TAZ inhibition by seeding cells on soft PAA hydrogels71. YAP/TAZ mechanotransduction was also found to mediate resistance to the BRAF inhibitor Vemurafenib in melanoma cell lines3,17,72. Thus, targeting mechanical activation of YAP/TAZ, either in the extracellular space or at the level of intracellular mechanosensing, might constitute a promising target for the design of combinatorial therapies.
A YAP/TAZ compass to inform on biomaterial design
By laying at the interface between biomaterials and molecular biology, knowledge on YAP/TAZ regulation has the potential to guide the rational design of material-based platforms. By mimicking the ideal YAP/TAZ-stimulating stiffness of healing tissues, or of body fluids secreted during tissue repair, biomaterials can boost wound healing or reactivate tissue-renewal and even endow rejuvenating properties wreaked havoc by diseases or aging. For example, orally administered hydrogels mimicking the rigidity of the intestinal mucin gels required for intestinal repair73,74 might be tailored to transiently induce maximal YAP/TAZ activation and, as such, boost YAP/TAZ-dependent intestinal regenerative potential. Edible hydrogels (alginate or chitosan as example) are already in use in humans food supplements, and could be adapted to this purpose. Standard of care for wound closure is the "split thickness" skin grafting, involving transplantation of dermis and the whole epidermis. Yet, this procedure is invasive and associated to donorsite morbidity75. Grafting biodegradable scaffolds with mechanical and topological features phenocopying, or in fact enhancing, those of dermal ridges (see above) might serve as "superniches" for YAP/TAZ mechanotransduction21. This would boost epithelialization from the wound margin76, overcoming the need of transplantation. Next, conforming properties of biomaterials should be tailored to modulate YAP/TAZ activation in self-organizing 3D minitissues, to dynamically shape "tissue origami” by incorporation of photodegradable or photoactivatable moieties (Box 3). These artificial 3D tissues may mimic natural developmental processes, sustain production of specific stem cells or, at an extreme speculative end, represent synthetic "meta-tissues" endowed with ad hoc attributes never found in the adult body; the latter may recapitulate the regenerative potency of YAP-expressing cells of fetal stages, as recently implied43, or even of features of tissues found in other species, that indeed retain broader YAP-driven regenerative capacities77. Conversely, biomaterials limiting YAP/TAZ mechanotransduction may be delivered at the tumor resection margins to overcome local relapse2. Intriguingly, examples of such materials already exist in nature, such as the high-molecular weight, elastic HA responsible of the remarkable long-living and cancerrefractory traits of the naked mole rat78,79.
Although in this review we emphasized the role of mechanical signal as key regulators of YAP/TAZ for somatic stem cells, organoid biology and regenerating tissues43,77, it is clear that other inputs, such as growth factors, morphogen signals and cell-to-cell contact, do feed on YAP/TAZ activity converging with and refining the effects of mechanical signals12. Prominent examples of such inputs are Wnt and GPCR-binding ligands41,79; or the interplay between YAP/TAZ and Notch signaling, by which information on structural and architectural complexity of living tissues are imbued to individual cells and transmitted to neighboring cells for fine-grained local cell decisions79. Incorporation of these soluble cues will be critical to generate groundbreaking biomaterials phenocopying the detailed spatiotemporal control of cell behavior imparted by living tissue niches, at all scales and over large distances, instructing that a cell - and not its neighbor - respond to those signals, that a group of cells morph into a given shape while respecting tissue boundaries, or to recapitulate ex vivo why that tumor cell gains metastatic properties, but not most of its genetic clones.
Outlook
The convergence of biomaterial science and YAP/TAZ mechanotransduction opens unprecedented opportunities for biomaterial-based therapies; for developmental, stem and tissue biology; and for the rational design of new materials with dramatically improved functionalities. For example, patterns of physical forces may be adopted in the next generation of biomaterials for organoid cultures allowing to regulate YAP/TAZ at will in order to faithfully reproduce normal tissues. Current organoid technologies are also limited by the fact that these epithelial cultures lack the stromal cell composition normally found in healthy or diseased tissues80, although some exciting pioneering work in this direction has been reported by using mESCs-derived organoids81. For example, biomaterials able to sustain the formation of YAP-activated CAFs enmeshed within tumor cells should be invaluable to understand in vitro the emergence of cells resistant to targeted chemotherapy69.
Another area in which we envision a profound impact of studies at the interface between materials science and YAP/TAZ mechanotransduction is the study of embryonic development. Organogenesis indeed involves the stepwise elaboration of tissue structures and their harmonious interlocking, such that each step relies on the shapes and topology of tissues created in the preceding step. Printing of “DNA-Velcro” adhesive domains has recently enabled to robustly command the pattern of tensional forces within multicellular clusters of fibroblasts, driving the folding of overlaying epithelial cells into macroscopic 3D architectures82. In principle, the folding trajectories of these “living origami” could be in fact obtained by substituting fibroblasts with “soft robots”, i.e., biomaterials with programmable, tunable properties. The pattern of YAP/TAZ mechanotransduction, or expression of YAP/TAZ synthetic reporters, may thus be used to inform such biologically-inspired tissue-engineering approaches. Moreover, cell specification at any specific location in development is dictated by gene-expression regulated by the anisotropies in the ECM and neighboring tissues; in turn, differential gene expression sets the stage for the next round of morphogenesis, until the organ reaches its final size and overall architecture. YAP/TAZ are essential for organ size control and ECM proteins constitute a large fraction of YAP/TAZ target genes3; this suggests that YAP/TAZ may serve as mediators of serial rounds of gene-expression and morphogenesis. Consistent with this view, rhythmic mechanical activation of YAP/TAZ has been recently identified as the engine of the “segmentation clock”, underlying the formation of the somites - precursors of muscle, dermis and skeletal tissues83. This model predicts that oscillating waves of stretching and compaction of entire fields of cells pattern YAP/TAZ to orchestrate the formation of new tissue boundaries and impart positional information, for example by linking mechanotransduction with the expression of Hox genes.
Cells constantly remodel their ECM according to the mechanical strains they receive in a process called “dynamic reciprocity”84. Intriguingly, the use of photodegradable hydrogels was instrumental to show that the cell retains memory of its mechanical history85. Persistence of YAP/TAZ activity has been associated to such memory events, suggesting that these factors may operate as epigenetic determinants85. These observations remind how mechanotransduction and YAP/TAZ signaling remains a field in its infancy and that uncovering additional layers of regulation may provide additional routes of exploitation for biomaterials and engineering. Indeed, while the majority of studies to date reporting mechanotransduction through YAP/TAZ have uncovered changes in localization of these factors, activation of YAP/TAZ target genes goes beyond their nuclear accumulation, and must entail the function of other transcription factors86 and epigenetic regulators, not to mention crosstalk with other signaling pathways17. Moreover, other transcription factors have been recently reported to be under control of cell mechanics in specific cellular and experimental contexts2,87 and, likely, others will be discovered in the near future. Knowledge on these regulations would further expand the repertoire and possibilities of biologically inspired materials.
The role of specific cellular features for YAP/TAZ mechanotransduction, such as presence of curvatures, apico-basal polarization and co-presence of cell-cell and cellECM adhesiveness88, remains largely unexplored areas of investigation. Indeed, understanding how cell shape and physical attributes instruct cell fates must be investigated, ultimately, at the level of individual cells and subcellular structures. Only very recently tools have been reported to create artificial micro-niches by means of miniaturized 3D protein patterns, topographical features and structured hydrogels tailored to host and shape one or few cells89.
Finally, major emphasis in mechanotransduction has been placed on the F-actin cytoskeleton, its tension and resistance exerted by the ECM. Yet, the cell has its own springs represented by the organization of microtubules, intermediate filaments and the nuclear lamina. How these cytoskeletal structures respond to biomaterials in 3D cellular contexts, and, in turn, affect YAP/TAZ mechanotransduction, is another potentially exciting area, also incited by the recent discovery that YAP/TAZ nuclear entry is influenced by the stretching of nuclear pores3. Considering the role of lamins in aging syndromes90, of intermediate filaments in a host of human genetic diseases91 and of microtubules as targets of chemotherapy4, we anticipate that exploration of biomaterials through robust molecular read-outs and effectors will provide fresh insights into a broad number of open biomedical questions.
Acknowledgements
The authors thank all members of the S.P. laboratory for discussion. This work is supported by AIRC Special Program Molecular Clinical Oncology ‘5 per mille’, by an AIRC PI-Grant to S.P., and by Epigenetics Flagship project CNR-MIUR grants to S.P. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 670126-DENOVOSTEM).
Footnotes
Author Contributions
All authors contributed equally to all aspects of the article (researching data for article, substantial contribution to discussion of content, writing, review/editing of manuscript before submission).
Competing interests statement
The authors declare no competing interests.
References
- 1.Schwartz MA, Chen CS. Cell biology. Deconstructing dimensionality. Science. 2013;339:402–404. doi: 10.1126/science.1233814. [DOI] [PubMed] [Google Scholar]
- 2.Northey JJ, Przybyla L, Weaver VM. Tissue Force Programs Cell Fate and Tumor Aggression. Cancer Discov. 2017;7:1224–1237. doi: 10.1158/21598290.CD-16-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nature reviews Molecular cell biology. 2017;18:758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nature reviews Molecular cell biology. 2009;10:63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nature reviews Molecular cell biology. 2017;18:728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gjorevski N, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539:560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 8.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 9.Huang S, Ingber DE. Cell tension, matrix mechanics, and cancer development. Cancer Cell. 2005;8:175–176. doi: 10.1016/j.ccr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 10.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 11.Nelson CM, et al. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci U S A. 2005;102:11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aragona M, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154 doi: 10.1016/j.cell.2013.07.042. 10471059. [DOI] [PubMed] [Google Scholar]
- 13.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474 doi: 10.1038/nature10137. 179183. [DOI] [PubMed] [Google Scholar]
- 14.Totaro A, Panciera T, Piccolo S. YAP/TAZ upstream signals and downstream responses. Nature cell biology. 2018;20:888–899. doi: 10.1038/s41556-018-0142-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyckmans J, Boudou T, Yu X, Chen CS. A hitchhiker's guide to mechanobiology. Developmental cell. 2011;21:35–47. doi: 10.1016/j.devcel.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu J, et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7:733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 19.Watt FM, Jordan PW, O'Neill CH. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc Natl Acad Sci U S A. 1988;85:5576–5580. doi: 10.1073/pnas.85.15.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connelly JT, et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nature cell biology. 2010;12:711–718. doi: 10.1038/ncb2074. [DOI] [PubMed] [Google Scholar]
- 21.Totaro A, et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat Commun. 2017;8 doi: 10.1038/ncomms15206. 15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caliari SR, et al. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Scientific reports. 2016;6 doi: 10.1038/srep21387. 21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannaerts I, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. Journal of hepatology. 2015;63:679–688. doi: 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, et al. Hepatocyte TAZ/WWTR1 Promotes Inflammation and Fibrosis in Nonalcoholic Steatohepatitis. Cell Metab. 2016;24:848–862. doi: 10.1016/j.cmet.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang W, et al. Surface topography of hydroxyapatite promotes osteogenic differentiation of human bone marrow mesenchymal stem cells. Materials science & engineering C, Materials for biological applications. 2016;60:45–53. doi: 10.1016/j.msec.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Zhong W, Zhang W, Wang S, Qin J. Regulation of fibrochondrogenesis of mesenchymal stem cells in an integrated microfluidic platform embedded with biomimetic nanofibrous scaffolds. PloS one. 2013;8:e61283. doi: 10.1371/journal.pone.0061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang C, et al. Spatially patterned matrix elasticity directs stem cell fate. Proc Natl Acad Sci U S A. 2016;113:E4439–4445. doi: 10.1073/pnas.1609731113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen JH, et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nature materials. 2014;13:979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trappmann B, et al. Extracellular-matrix tethering regulates stem-cell fate. Nature materials. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 30.Shiu JY, Aires L, Lin Z, Vogel V. Nanopillar force measurements reveal actin-cap-mediated YAP mechanotransduction. Nature cell biology. 2018;20 doi: 10.1038/s41556-017-0030-y. 262271. [DOI] [PubMed] [Google Scholar]
- 31.Benham-Pyle BW, Pruitt BL, Nelson WJ. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science. 2015;348:1024–1027. doi: 10.1126/science.aaa4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui Y, et al. Cyclic stretching of soft substrates induces spreading and growth. Nat Commun. 2015;6 doi: 10.1038/ncomms7333. 6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caliari SR, Vega SL, Kwon M, Soulas EM, Burdick JA. Dimensionality and spreading influence MSC YAP/TAZ signaling in hydrogel environments. Biomaterials. 2016;103:314–323. doi: 10.1016/j.biomaterials.2016.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Y, et al. MT1-MMP-dependent control of skeletal stem cell commitment via a beta1-integrin/YAP/TAZ signaling axis. Developmental cell. 2013;25:402–416. doi: 10.1016/j.devcel.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huebsch N, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature materials. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultz KM, Kyburz KA, Anseth KS. Measuring dynamic cell-material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc Natl Acad Sci U S A. 2015;112:E3757–3764. doi: 10.1073/pnas.1511304112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khetan S, et al. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nature materials. 2013;12:458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaudhuri O, et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nature materials. 2016;15:326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartfeld S, Clevers H. Stem cell-derived organoids and their application for medical research and patient treatment. Journal of molecular medicine. 2017 doi: 10.1007/s00109-017-1531-7. [DOI] [PubMed] [Google Scholar]
- 41.Azzolin L, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Panciera T, et al. Induction of Expandable Tissue-Specific Stem/Progenitor Cells through Transient Expression of YAP/TAZ. Cell stem cell. 2016;19:725–737. doi: 10.1016/j.stem.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yui S, et al. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell stem cell. 2018;22:35–49 e37. doi: 10.1016/j.stem.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23:H41–56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lou J, Stowers R, Nam S, Xia Y, Chaudhuri O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials. 2018;154:213–222. doi: 10.1016/j.biomaterials.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature. 2016 doi: 10.1038/nature20602. [DOI] [PubMed] [Google Scholar]
- 47.Yourek G, McCormick SM, Mao JJ, Reilly GC. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen Med. 2010;5:713–724. doi: 10.2217/rme.10.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim KM, et al. Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation. PloS one. 2014;9:e92427. doi: 10.1371/journal.pone.0092427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watt FM. Mammalian skin cell biology: at the interface between laboratory and clinic. Science. 2014;346:937–940. doi: 10.1126/science.1253734. [DOI] [PubMed] [Google Scholar]
- 50.Lee MJ, Byun MR, Furutani-Seiki M, Hong JH, Jung HS. YAP and TAZ regulate skin wound healing. J Invest Dermatol. 2014;134:518–525. doi: 10.1038/jid.2013.339. [DOI] [PubMed] [Google Scholar]
- 51.Schlegelmilch K, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walko G, et al. A genome-wide screen identifies YAP/WBP2 interplay conferring growth advantage on human epidermal stem cells. Nat Commun. 2017;8 doi: 10.1038/ncomms14744. 14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musah S, et al. Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc Natl Acad Sci U S A. 2014;111:13805–13810. doi: 10.1073/pnas.1415330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Y, et al. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nature materials. 2014;13 doi: 10.1038/nmat3945. 599604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Price AJ, Huang EY, Sebastiano V, Dunn AR. A semi-interpenetrating network of polyacrylamide and recombinant basement membrane allows pluripotent cell culture in a soft, ligand-rich microenvironment. Biomaterials. 2017;121:179–192. doi: 10.1016/j.biomaterials.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Ankam S, Lim CK, Yim EK. Actomyosin contractility plays a role in MAP2 expression during nanotopography-directed neuronal differentiation of human embryonic stem cells. Biomaterials. 2015;47:20–28. doi: 10.1016/j.biomaterials.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Martello G, Smith A. The nature of embryonic stem cells. Annu Rev Cell Dev Biol. 2014;30:647–675. doi: 10.1146/annurev-cellbio-100913-013116. [DOI] [PubMed] [Google Scholar]
- 58.Chowdhury F, et al. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. PloS one. 2010;5:e15655. doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caiazzo M, et al. Defined three-dimensional microenvironments boost induction of pluripotency. Nature materials. 2016;15:344–352. doi: 10.1038/nmat4536. [DOI] [PubMed] [Google Scholar]
- 60.Mammoto A, Ingber DE. Cytoskeletal control of growth and cell fate switching. Curr Opin Cell Biol. 2009;21:864–870. doi: 10.1016/j.ceb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci. 2011;124:1195–1205. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lammers S, et al. Mechanics and function of the pulmonary vasculature: implications for pulmonary vascular disease and right ventricular function. Comprehensive Physiology. 2012;2:295–319. doi: 10.1002/cphy.c100070. [DOI] [PubMed] [Google Scholar]
- 63.Bertero T, et al. Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell reports. 2015;13:1016–1032. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bertero T, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. The Journal of clinical investigation. 2016;126:3313–3335. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cottrill KA, Chan SY. Metabolic dysfunction in pulmonary hypertension: the expanding relevance of the Warburg effect. Eur J Clin Invest. 2013;43:855–865. doi: 10.1111/eci.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu F, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308:L344–357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 68.Jabbari E, Sarvestani SK, Daneshian L, Moeinzadeh S. Optimum 3D Matrix Stiffness for Maintenance of Cancer Stem Cells Is Dependent on Tissue Origin of Cancer Cells. PloS one. 2015;10:e0132377. doi: 10.1371/journal.pone.0132377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calvo F, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nature cell biology. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chakraborty S, et al. Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell reports. 2017;18:2464–2479. doi: 10.1016/j.celrep.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 71.Lin CH, et al. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Mol Biol Cell. 2015;26:3946–3953. doi: 10.1091/mbc.E15-07-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirata E, et al. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell. 2015;27:574–588. doi: 10.1016/j.ccell.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Das S, et al. Mice deficient in Muc4 are resistant to experimental colitis and colitis-associated colorectal cancer. Oncogene. 2016;35:2645–2654. doi: 10.1038/onc.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luu Y, et al. Human intestinal MUC17 mucin augments intestinal cell restitution and enhances healing of experimental colitis. Int J Biochem Cell Biol. 2010;42:996–1006. doi: 10.1016/j.biocel.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kanapathy M, et al. Epidermal grafting for wound healing: a review on the harvesting systems, the ultrastructure of the graft and the mechanism of wound healing. Int Wound J. 2017;14:16–23. doi: 10.1111/iwj.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elbediwy A, et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143:1674–1687. doi: 10.1242/dev.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mateus R, et al. Control of tissue growth by Yap relies on cell density and Factin in zebrafish fin regeneration. Development. 2015;142:2752–2763. doi: 10.1242/dev.119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seluanov A, Gladyshev VN, Vijg J, Gorbunova V. Mechanisms of cancer resistance in long-lived mammals. Nature reviews Cancer. 2018;18:433–441. doi: 10.1038/s41568-018-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Totaro A, Castellan M, Di Biagio D, Piccolo S. Crosstalk between YAP/TAZ and Notch Signaling. Trends Cell Biol. 2018;28:560–573. doi: 10.1016/j.tcb.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nature cell biology. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 81.McCauley HA, Wells JM. Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish. Development. 2017;144:958–962. doi: 10.1242/dev.140731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hughes AJ, et al. Engineered Tissue Folding by Mechanical Compaction of the Mesenchyme. Developmental cell. 2018;44:165–178 e166. doi: 10.1016/j.devcel.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hubaud A, Regev I, Mahadevan L, Pourquie O. Excitable Dynamics and Yap-Dependent Mechanical Cues Drive the Segmentation Clock. Cell. 2017;171:668–682 e611. doi: 10.1016/j.cell.2017.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–176. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nature materials. 2014;13:645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zanconato F, et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nature cell biology. 2015;17 doi: 10.1038/ncb3216. 12181227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Posern G, Treisman R. Actin' together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 88.Cosgrove BD, et al. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nature materials. 2016;15:1297–1306. doi: 10.1038/nmat4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Q, et al. Extracellular matrix scaffolding guides lumen elongation by inducing anisotropic intercellular mechanical tension. Nature cell biology. 2016;18:311–318. doi: 10.1038/ncb3310. [DOI] [PubMed] [Google Scholar]
- 90.Dorado B, Andres V. A-type lamins and cardiovascular disease in premature aging syndromes. Curr Opin Cell Biol. 2017;46:17–25. doi: 10.1016/j.ceb.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 91.Lowery J, Kuczmarski ER, Herrmann H, Goldman RD. Intermediate Filaments Play a Pivotal Role in Regulating Cell Architecture and Function. J Biol Chem. 2015;290:17145–17153. doi: 10.1074/jbc.R115.640359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138 doi: 10.1242/dev.070987. 39073914. [DOI] [PubMed] [Google Scholar]
- 93.Tseng Q, et al. A new micropatterning method of soft substrates reveals that different tumorigenic signals can promote or reduce cell contraction levels. Lab Chip. 2011;11:2231–2240. doi: 10.1039/c0lc00641f. [DOI] [PubMed] [Google Scholar]
- 94.Thery M. Micropatterning as a tool to decipher cell morphogenesis and functions. J Cell Sci. 2010;123:4201–4213. doi: 10.1242/jcs.075150. [DOI] [PubMed] [Google Scholar]
- 95.Tse JR, Engler AJ. Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol. 2010;Chapter 10 doi: 10.1002/0471143030.cb1016s47. Unit 10 16. [DOI] [PubMed] [Google Scholar]
- 96.Ghassemi S, et al. Fabrication of elastomer Pillar Arrays with Modulated Stiffness for Cellular Force Measurements. J Vac Sci Technol B Microelectron Nanometer Struct Process Meas Phenom. 2008;26:2549–2553. doi: 10.1116/1.3013424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghassemi S, et al. Cells test substrate rigidity by local contractions on submicrometer pillars. Proc Natl Acad Sci U S A. 2012;109:5328–5333. doi: 10.1073/pnas.1119886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meacci G, et al. alpha-Actinin links extracellular matrix rigidity-sensing contractile units with periodic cell-edge retractions. Mol Biol Cell. 2016;27 doi: 10.1091/mbc.E16-02-0107. 34713479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moe AA, et al. Microarray with micro- and nano-topographies enables identification of the optimal topography for directing the differentiation of primary murine neural progenitor cells. Small. 2012;8:3050–3061. doi: 10.1002/smll.201200490. [DOI] [PubMed] [Google Scholar]
- 100.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and longterm cellular responses to dynamic mechanics. Nat Commun. 2012;3 doi: 10.1038/ncomms1792. 792. [DOI] [PubMed] [Google Scholar]
- 101.Ondeck MG, Engler AJ. Mechanical Characterization of a Dynamic and Tunable Methacrylated Hyaluronic Acid Hydrogel. J Biomech Eng. 2016;138 doi: 10.1115/1.4032429. 021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ma H, Killaars AR, DelRio FW, Yang C, Anseth KS. Myofibroblastic activation of valvular interstitial cells is modulated by spatial variations in matrix elasticity and its organization. Biomaterials. 2017;131:131–144. doi: 10.1016/j.biomaterials.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang H, Haeger SM, Kloxin AM, Leinwand LA, Anseth KS. Redirecting valvular myofibroblasts into dormant fibroblasts through lightmediated reduction in substrate modulus. PloS one. 2012;7:e39969. doi: 10.1371/journal.pone.0039969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang H, Tibbitt MW, Langer SJ, Leinwand LA, Anseth KS. Hydrogels preserve native phenotypes of valvular fibroblasts through an elasticityregulated PI3K/AKT pathway. Proc Natl Acad Sci U S A. 2013;110:19336–19341. doi: 10.1073/pnas.1306369110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.DeForest CA, Anseth KS. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat Chem. 2011;3:925–931. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DeForest CA, Anseth KS. Advances in bioactive hydrogels to probe and direct cell fate. Annu Rev Chem Biomol Eng. 2012;3:421–444. doi: 10.1146/annurevchembioeng-062011-080945. [DOI] [PubMed] [Google Scholar]
- 107.Foster AA, et al. Protein-engineered hydrogels enhance the survival of induced pluripotent stem cell-derived endothelial cells for treatment of peripheral arterial disease. Biomater Sci. 2018;6:614–622. doi: 10.1039/c7bm00883j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kharkar PM, Kloxin AM, Kiick KL. Dually degradable click hydrogels for controlled degradation and protein release. J Mater Chem B. 2014;2:5511–5521. doi: 10.1039/c4tb00496e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun F, Zhang WB, Mahdavi A, Arnold FH, Tirrell DA. Synthesis of bioactive protein hydrogels by genetically encoded SpyTag-SpyCatcher chemistry. Proc Natl Acad Sci U S A. 2014;111:11269–11274. doi: 10.1073/pnas.1401291111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vazquez-Portalati NN, Kilmer CE, Panitch A, Liu JC. Characterization of Collagen Type I and II Blended Hydrogels for Articular Cartilage Tissue Engineering. Biomacromolecules. 2016;17:3145–3152. doi: 10.1021/acs.biomac.6b00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Azagarsamy MA, Anseth KS. Bioorthogonal Click Chemistry: An Indispensable Tool to Create Multifaceted Cell Culture Scaffolds. ACS Macro Lett. 2013;2:5–9. doi: 10.1021/mz300585q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cruz-Acuna R, et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nature cell biology. 2017;19:1326–1335. doi: 10.1038/ncb3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ehrbar M, et al. Biomolecular hydrogels formed and degraded via sitespecific enzymatic reactions. Biomacromolecules. 2007;8:3000–3007. doi: 10.1021/bm070228f. [DOI] [PubMed] [Google Scholar]