Variable Expression of S100 Protein in Sinonasal Malignant Mucosal Melanoma: A Potential Diagnostic Pitfall (original) (raw)
Abstract
Sinonasal malignant mucosal melanoma (SNM) is a rare, aggressive malignancy. The diagnosis of SNM is often quite challenging due to anatomical limitations, frequent lack of pigmentation, variable histologic appearances, and aberrant differentiation (e.g., positivity for cytokeratin, desmin, or neuroendocrine markers). S100 protein is routinely used as a standard screening marker for SNM, but it may lack optimal sensitivity. Our objective was to study the extent of immunohistochemical expression of S100 protein in SNM, and determine its diagnostic value by comparing it to a newer melanoma marker, SOX10. Twenty-three cases of sinonasal MMM were retrieved from the archival files of the Department of Pathology at UT Southwestern Medical Center. The patients included 14 men and 9 women, and ranged from 36 to 90 years (mean 64.9 years). Sections from blocks of formalin-fixed, paraffin-embedded tissue were used for immunohistochemical analysis with S100 protein and SOX10. The extent and intensity of immunostaining was recorded, and H-score was calculated. For a subset of negative or focally positive cases, S100 protein was repeated at a high-volume reference laboratory. S100 protein immunoexpression was quite variable in the SNM cases, with H-scores ranging from 0 to 300 (mean 123). While 11 of 23 cases exhibited strong and diffuse staining (H-score > 100) as expected for melanoma, 7 were weak and/or focal (H-score 1–100), and 5 were completely S100 protein-negative. For 10 cases, the negative or focal results were confirmed by reference laboratory staining. In contrast, all 23 SNM cases were diffusely and strongly positive for SOX10 (H-scores 210–300, mean 296). Our study demonstrated that S100 protein immunoexpression is extremely variable in SNM. Weak or even absent S100 protein staining is not uncommon in SNM, and should not dissuade pathologists from that diagnosis. Our data demonstrates that S100 protein is insufficiently sensitive to be used as a screening marker for SNM, but that SOX10 is consistently and robustly positive, and should therefore replace S100 protein for that purpose. Indeed, for any high-grade sinonasal tumor, pathologists must have a low threshold for utilizing additional markers to exclude the possibility of SNM.
Keywords: Malignant mucosal melanoma, S100 protein, SOX10, Sinonasal tract, Small round blue cell tumor
Introduction
Sinonasal melanoma (SNM) of the head and neck is a rare, aggressive malignancy that accounts for 0.5–2% of all melanomas and about 4% sinonasal malignancies [1–3]. SNM is usually diagnosed at advanced stage due to anatomical limitations, non-specific complaints and variable histological appearances. SNM originates predominantly from melanocytes of the mucosal surfaces lining the nasal cavity, accounting for approximately 80% cases, while the remaining 20% arise from the paranasal sinuses [4]. These anatomical locations and quality-of-life constraints make it very difficult to completely excise the tumor and obtain adequate margins [5]. Accordingly, despite advances in treatment, SNM is still associated with poor prognosis, a high risk of local recurrence (31–85%) and distant metastasis (25–50%), and a 5-year survival rate of 20–30% [1, 5, 6].
Pathologic diagnosis of SNM can be difficult due to marked cytological and architectural diversity mimicking other malignancies and limited quantity of the specimen. Multiple cell morphologies have been described for SNM including epithelioid, spindle, pleomorphic, rhabdoid, plasmacytoid and small cell. SNM is a great imitator and can mimic any other type of malignancy arising from sinonasal area, especially those belonging to the classification of “small round blue cell tumor,” such as NK/T cell lymphoma, plasmacytoma, squamous cell carcinoma, NUT carcinoma, sinonasal undifferentiated carcinoma, olfactory neuroblastoma, Ewing sarcoma, rhabdomyosarcoma, and neuroendocrine carcinoma, among others [7, 8]. SNM can also show aberrant differentiation either by histology (e.g., cartilaginous or skeletal muscle differentiation) or by immunohistochemistry, with occasional positivity for cytokeratin, desmin, and neuroendocrine markers. While clear-cut melanin pigmentation is helpful in supporting a SNM diagnosis, many cases have minimal or no pigmentation. Melanocytic immunohistochemical markers are also useful, but none of them has sufficient sensitivity or specificity by itself to support a final diagnosis. For all of these reasons, the diagnosis of SNM runs the risk of being delayed or even misdiagnosed.
Currently, S100 protein is routinely used as a screening marker for melanoma including SNM, but in our experience, it may lack optimal sensitivity. Sry-related HMG-BOX gene 10 (SOX10) is a nuclear transcription factor that plays an important role in melanocytic development and has shown promise as a sensitive marker of primary cutaneous melanoma as well as nodal metastases [9–11]. Our objective was to study the immunohistochemical expression of S100 protein in SNM, and determine its diagnostic value by comparing it to SOX10.
Materials and Methods
Cases
With institutional IRB approval (STU 112017-073), 23 consecutive cases of SNM were retrieved from the archival files of the Department of Pathology, UT Southwestern Medical Center from 2005 to 2019. Consult cases were excluded. Basic demographic and clinical information was recorded. All cases had been diagnosed as primary SNM and were re-reviewed by an expert head and neck pathologist (JAB) to confirm the diagnosis.
Immunohistochemistry
Blocks of formalin-fixed, paraffin-embedded tissue were retrieved. Serial sections (5 µm-thickness) were used for S100 protein (4C4.9; Ventana; prediluted) and SOX10 (SP267, Ventana; prediluted) immunohistochemical analysis using standard autostaining protocols on a Ventana Benchmark XT autostainer (Ventana Medical Systems, Inc, Tucson, AZ). Deparaffinization and antigen retrieval (iVIEW detection system; Ventana) were carried out as an automated program of the Ventana autostainer. On-slide positive control tissue of a cutaneous melanoma known to be positive for S100 protein and SOX10 was included on each slide. For a subset of cases, S100 protein immunohistochemistry was repeated at a high-volume reference laboratory (ProPath, Dallas, Texas).
For SOX10 only nuclear staining was recorded, while for S100 protein, nuclear plus cytoplasmic staining was regarded as positive. The extent (% tumor cells) and the intensity (0, 1+, 2+, 3+) of staining were recorded, and an H-score was calculated by multiplying the two values.
Results
The results are summarized in Table 1. Twenty-three SNMs were identified from the archival files of the Department of Pathology, UT Southwestern Medical Center. They occurred in 14 men and 9 women ranging in age from 36 to 90 years of age, with a mean of 64.9 years. Seventeen (74%) involved the nasal cavity, 7 (31.8%) involved a paranasal sinus (4 maxillary, 2 ethmoid, 1 sphenoid), and 5 (22%) invaded the skull base. The histology of the SNMs was widely variable, with small cell, epithelioid, plasmacytoid, and spindle cell morphology encountered. All cases exhibited high-grade histologic features, with marked pleomorphism, high mitotic rates, and necrosis that was often extensive. Melanin pigment was seen in 13 cases (focal in 11, diffuse in 2), while 10 SNMs were amelanotic.
Table 1.
Clinical, histologic, and immunophenotypic findings of sinonasal malignant mucosal melanomas
| Case | Age | Sex | Site | Melanin pigment | UTSW S100 protein (H-score) | Reference Lab S100 protein (H-score) | SOX10 (H-score) |
|---|---|---|---|---|---|---|---|
| 1 | 74 | F | Nasal cavity | Focal | 10 | 10 | 300 |
| 2 | 67 | M | Nasal cavity, skull base | Diffuse | 100 | N/A | 300 |
| 3 | 60 | M | Nasal cavity | Focal | 160 | N/A | 300 |
| 4 | 61 | F | Nasal cavity | None | 300 | N/A | 300 |
| 5 | 68 | M | Nasal cavity | None | 2 | 0 | 300 |
| 6 | 80 | F | Nasal cavity | None | 300 | N/A | 300 |
| 7 | 81 | F | Maxillary sinus | None | 0 | 80 | 300 |
| 8 | 82 | M | Nasal cavity, skull base | Focal | 0 | 0 | 300 |
| 9 | 74 | M | Nasal cavity, skull base | Focal | 0 | 1 | 300 |
| 10 | 42 | M | Maxillary sinus | Diffuse | 300 | N/A | 300 |
| 11 | 48 | M | Nasal cavity | None | 40 | 30 | 300 |
| 12 | 67 | M | Nasal cavity | Focal | 300 | N/A | 300 |
| 13 | 56 | M | Nasal cavity | Focal | 30 | 30 | 300 |
| 14 | 90 | M | Nasal cavity | Focal | 160 | N/A | 300 |
| 15 | 84 | F | Ethmoid sinus, skull base | Focal | 2 | 0 | 300 |
| 16 | 45 | M | Maxillary sinus | Focal | 200 | N/A | 300 |
| 17 | 64 | F | Nasal cavity, skull base | None | 300 | N/A | 300 |
| 18 | 51 | F | Nasal cavity | None | 0 | 0 | 300 |
| 19 | 82 | F | Sphenoid sinus | None | 300 | N/A | 300 |
| 20 | 36 | F | Maxillary sinus | None | 140 | N/A | 300 |
| 21 | 58 | M | Nasal cavity, ethmoid sinus | Focal | 140 | N/A | 300 |
| 22 | 80 | M | Nasal cavity | None | 0 | 0 | 300 |
| 23 | 44 | M | Nasal cavity | Focal | 40 | N/A | 210 |
S100 protein immunoexpression was quite variable in the SNM cases. H-scores ranged from 0 to 300 (mean 123). Eleven of 23 cases exhibited strong and diffuse staining, defined as an H-score > 100, as expected for melanoma (Fig. 1). However, 7 cases exhibited weak and/or focal (H-score 1–100) S100 protein immunostaining (Fig. 2), and 5 were completely negative (Fig. 3). For 10 cases with negative and/or focal S100 protein staining, results were confirmed by reference laboratory staining. In 9 of 10 of these cases, the staining results at the reference laboratory were nearly identical. The remaining case, an SNM that was completely S100 protein-negative at UT Southwestern, showed 80% staining at 1+ intensity for an H-score of 80.
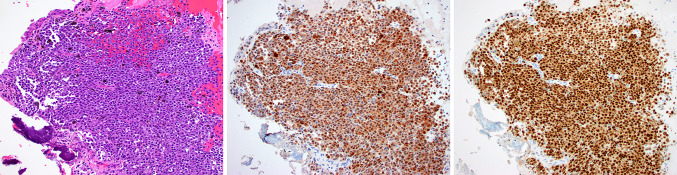
Fig. 1.
Case 6 was a sinonasal melanoma with melanin pigment (a). This example was diffusely and strongly positive for both S100 protein (b) and SOX10 (c), as expected for melanoma
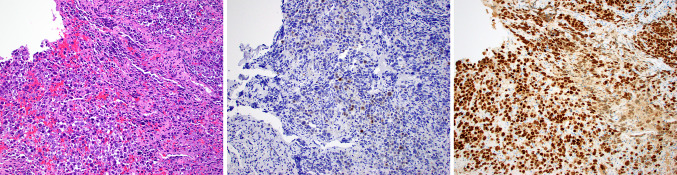
Fig. 2.
Case 13 was an amelanotic small round cell tumor (a) that was only focally and weakly positive for S100 protein (b), but strongly and diffusely positive for SOX10 (c)
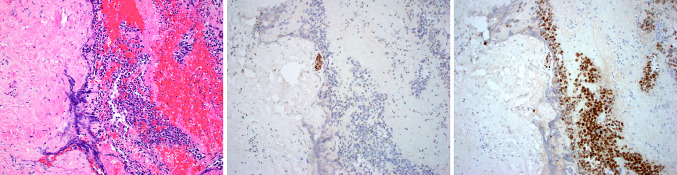
Fig. 3.
Case 8 was an example of sinonasal melanoma that consisted of undifferentiated appearing small round cells with abundant hemorrhage (a). S100 protein was completely negative. Note the small nerve that served as a positive internal control (b). SOX10 immunostaining, on the other hand, was strong and diffuse in both the tumor and the small nerve (c)
All 23 SNMs were diffusely and strongly positive for SOX10 (H-scores 270–300, mean 296) (Figs. 1, 2, and 3). Only one SNM had an H-score that was not 300; this case demonstrated zones of aberrant cartilage-like differentiation that showed patchy but strong SOX10 (and S100 protein) expression. Other areas of the tumor demonstrated a more classic small cell appearance that was diffusely SOX10-positive, for an approximate overall extent of 70% (Fig. 4).
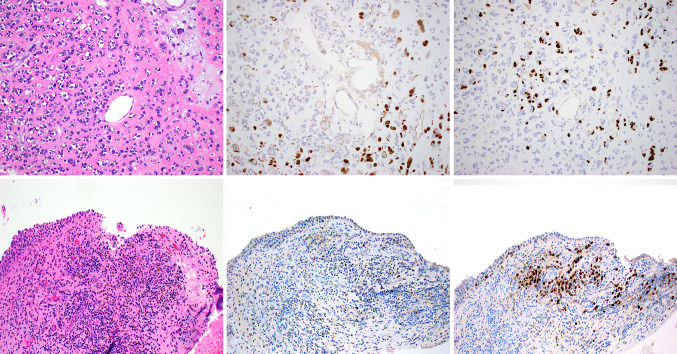
Fig. 4.
Case 23 was an unusual sinonasal melanoma with areas showing pink matrix-like material, resembling osteoblastic or chondroblastic differentiation (a). These portions of the tumor were focally and strongly positive for both S100 protein (b) and SOX10 (c). Other areas of the tumor were composed of small round cells difficult to separate from lymphocytes. Melanin pigment was noted in these areas (d). These areas showed very limited S100 protein staining (e), but were strongly positive for SOX10 (f)
Discussion
Sinonasal tract pathology is fraught with difficulty, and is often regarded as one of the most challenging areas in head and neck pathology. Reasons for this difficulty include the rarity of sinonasal neoplasms in general, the limited amount of tissue often received due to anatomical constraints, and frequent tissue artifact (e.g., frozen or crush artifact). Moreover, there is an increasingly long list of newly described entities such that staying up-to-date on sinonasal tumor classification becomes very problematic [12]. The “undifferentiated” or “small round blue cell” sinonasal tumor is particularly known for diagnostic pitfalls, as dozens of different tumor types with different prognoses and treatment strategies can have near-identical histologic appearances [7, 8, 13]. Immunohistochemistry is mandatory to resolve this differential diagnosis, and in some cases, molecular techniques are also needed.
Sinonasal malignant mucosal melanoma (SNM) is a rare malignancy that is important to correctly diagnose because it carries a poor prognosis (5-year survival of 20–30%) and therefore requires aggressive, multimodality therapy [14, 15]. SNM may be difficult to recognize, however, because it frequently exhibits a small round blue cell appearance, and can occasionally show aberrant differentiation by immunohistochemistry (e.g., staining for epithelial, mesenchymal, or neuroendocrine markers) or even light microscopy (e.g., overt cartilaginous features) [16, 17]. S100 protein is frequently employed as a screening marker for melanoma in any anatomic location because it has been repeatedly shown to be highly sensitive [7, 8, 13, 18]. It has also been documented, however, that some melanomas may be only weakly/focally positive, or even negative for S100 protein [6, 15, 19]. It has been our anecdotal experience that S100 protein expression in SNM is often underwhelming and occasionally lacking, leading pathologists to prematurely dismiss melanoma as a diagnostic possibility. This study confirmed those impressions, with S100 protein H-scores ranging from 0 to 300 (mean 123). While 18 of 23 (78%) were positive to some degree, only 11 of 23 cases exhibited the strong and diffuse staining (defined as H-score > 100) that is traditionally expected for melanoma. Moreover, 5 (22%) SNM were completely negative for S100 protein, an alarmingly high number when considering that many pathologists use this immunostain alone as screen to rule out SNM when confronted with a high-grade sinonasal malignancy. We did consider the possibility that S100 protein immunohistochemistry at our institution was faulty or overly weak, but all cases were run with an on-slide positive control that demonstrated robust staining. Moreover, most cases had normal tissues (e.g., nerves, seromucinous glands) that served as internal positive controls (Fig. 3). In addition, 10 weak or negative SNM cases were sent for confirmatory S100 protein testing at a high-volume reference laboratory which showed results that were almost completely concordant. Only one case that was negative at our institution showed weak staining (H-score 80) at the reference laboratory. There was no consultation bias (i.e., cases that were sent for second opinion because S100 protein results were unexpected) because consult cases were excluded. Finally, it is our unpublished experience that outside slides sent for our review often show similarly weak S100 protein results in SNM.
SOX10 is a relatively recently introduced nuclear marker of melanocytic and schwannian differentiation. There have been numerous studies showing that SOX10 has comparably high sensitivity to S100 protein in melanomas in general, but only one study, to our knowledge, examined its expression in SNM specifically [19]. We found that SOX10 was consistently expressed in a diffuse and strong manner in SNM. All 23 cases were 3+ in intensity, and only 1 case, an unusual SNM that exhibited aberrant cartilage-like differentiation, demonstrated less than 100% tumor staining.
Taken together, our findings indicate that SOX10 demonstrates superior sensitivity to S100 protein for the diagnosis of SNM. Our findings confirm those by Liu, et al. which showed similarly variable S100 protein and robust SOX10 staining results [19]. Practically speaking, these findings illustrate that S100 protein cannot function alone as an effective screening tool to rule out SNM when dealing with an undifferentiated-appearing tumor of the sinonasal tract. Not only is a significant subset completely S100 protein-negative, an even larger group shows weak and patchy staining that the unwary pathologist may not expect in an SNM and mistakenly dismiss as nonspecific. For SNM, SOX10 is much more consistently positive when compared to S100 protein, and its staining is much more extensive and intense in SNM. As a result, SOX10 is better suited than S100 protein as a standalone marker to rule out the possibility of SNM. It is not clear if these findings are unique to SNM or whether it extends to melanomas of other mucosal sites. Although studies specifically addressing this question are few, published rates of S100 protein positivity for melanomas of the oral cavity, conjunctiva, and vagina have ranged from 83 to 97%, with most studies noting a small subset with focal or weak staining [20–23].
It must be emphasized that neither S100 protein nor SOX10 are very specific for SNM, as both markers are consistently positive in myoepithelial neoplasms and nerve sheath tumors, among others. Accordingly, S100 protein and SOX10 staining results must be interpreted in light of histologic and other immunohistochemical findings. Most SNM demonstrate large, eosinophilic, so-called “cherry red” nucleoli, and some will demonstrate melanin pigmentation and pagetoid tumor growth in the overlying sinonasal epithelium. HMB-45 and Melan-A are melanocytic immunostains that are not sufficiently sensitive to utilize as screening markers, but they are far more specific than S100 protein and SOX10 and thus can help support a diagnosis of SNM. Carcinomas that contain myoepithelial cells are not only positive for S100 protein and SOX10, but are also strongly positive for cytokeratins and other myoepithelial markers such as smooth muscle actin, p40, p63, calponin, or GFAP. Finally, malignant peripheral nerve sheath tumor, particularly the epithelioid variant, can be difficult to distinguish from melanoma. Fortunately, epithelioid malignant peripheral nerve sheath tumor is consistently negative for HMB-45 and Melan-A, and frequently shows loss of H3K27me3 and/or SMARCB1 expression [24–28].
In summary, our study demonstrated that S100 protein immunoexpression is extremely variable and inconsistent in SNM. As a result, weak or even absent S100 protein staining should not dissuade pathologists from an SNM diagnosis, particularly in the face of suspicious histologic findings such as “cherry red” nucleoli. S100 protein is not sensitive enough to be used as a standalone screening marker for SNM. In contrast, SOX10 is consistently robustly positive in SNM, and should therefore be used with, or in place of, S100 protein for ruling out melanoma when dealing with a high-grade, poorly differentiated malignant sinonasal neoplasm.
Funding
This study was funded by the Jane B. and Edwin P. Jenevein M.D. Endowment for Pathology at UT Southwestern Medical Center.
Compliance with Ethical Standards
Conflict of interest
All authors declare that he/she has no conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (IRB 112017-073), which did not require informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moreno MA, Roberts DB, Kupferman ME, et al. Mucosal melanoma of the nose and paranasal sinuses, a contemporary experience from the M.D. Anderson Cancer Center. Cancer. 2010;116:2215–2223. doi: 10.1002/cncr.24976. [DOI] [PubMed] [Google Scholar]
- 2.Gal TJ, Silver N, Huang B. Demographics and treatment trends in sinonasal mucosal melanoma. Laryngoscope. 2011;121:2026–2033. doi: 10.1002/lary.21925. [DOI] [PubMed] [Google Scholar]
- 3.Francisco AL, Furlan MV, Peresi PM, et al. Head and neck mucosal melanoma: clinicopathological analysis of 51 cases treated in a single cancer centre and review of the literature. Int J Oral Maxillofac Surg. 2016;45:135–140. doi: 10.1016/j.ijom.2015.08.987. [DOI] [PubMed] [Google Scholar]
- 4.Clifton N, Harrison L, Bradley PJ, et al. Malignant melanoma of nasal cavity and paranasal sinuses: report of 24 patients and literature review. J Laryngol Otol. 2011;125:479–485. doi: 10.1017/S0022215110002720. [DOI] [PubMed] [Google Scholar]
- 5.Samstein RM, Carvajal RD, Postow MA, et al. Localized sinonasal mucosal melanoma: outcomes and associations with stage, radiotherapy, and positron emission tomography response. Head Neck. 2016;38:1310–1317. doi: 10.1002/hed.24435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad ML, Busam KJ, Patel SG, et al. Clinicopathologic differences in malignant melanoma arising in oral squamous and sinonasal respiratory mucosa of the upper aerodigestive tract. Arch Pathol Lab Med. 2003;127:997–1002. doi: 10.5858/2003-127-997-CDIMMA. [DOI] [PubMed] [Google Scholar]
- 7.Bridge JA, Bowen JM, Smith RB. The small round blue cell tumors of the sinonasal area. Head Neck Pathol. 2010;4:84–93. doi: 10.1007/s12105-009-0158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rooper LM, Bishop JA. Sinonasal small round blue cell tumors: an immunohistochemical approach. Surg Pathol Clin. 2017;10:103–123. doi: 10.1016/j.path.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Miettinen M, McCue PA, Sarlomo-Rikala M, et al. Sox10—a marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: a systematic analysis of 5134 tumors. Am J Surg Pathol. 2015;39:826–835. doi: 10.1097/PAS.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol. 2008;32:1291–1298. doi: 10.1097/PAS.0b013e3181658c14. [DOI] [PubMed] [Google Scholar]
- 11.Karamchandani JR, Nielsen TO, van de Rijn M, et al. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol. 2012;20:445–450. doi: 10.1097/PAI.0b013e318244ff4b. [DOI] [PubMed] [Google Scholar]
- 12.Bishop JA. Newly Described Tumor Entities in Sinonasal Tract Pathology. Head Neck Pathol. 2016;10:23–31. doi: 10.1007/s12105-016-0688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iezzoni JC, Mills SE. "Undifferentiated" small round cell tumors of the sinonasal tract: differential diagnosis update. Am J Clin Pathol. 2005;124(Suppl):S110–121. doi: 10.1309/59RBT2RK6LQE4YHB. [DOI] [PubMed] [Google Scholar]
- 14.Mochel MC, Duncan LM, Piris A, et al. Primary mucosal melanoma of the sinonasal tract: a clinicopathologic and immunohistochemical study of thirty-two cases. Head & Neck Pathology. 2015;9:236–243. doi: 10.1007/s12105-014-0570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson LD, Wieneke JA, Miettinen M. Sinonasal tract and nasopharyngeal melanomas: a clinicopathologic study of 115 cases with a proposed staging system. Am J Surg Pathol. 2003;27:594–611. doi: 10.1097/00000478-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Wenig BM, Dulguerov P, Kapadia SB, et al. et al. Neuroectodermal tumours. In: Barnes L, Eveson JW, Reichart P, et al.et al., editors. World Health Organization classification of tumours: pathology and genetics of head and neck tumors. Lyon: IARC Press; 2005. pp. 65–75. [Google Scholar]
- 17.Smith SM, Schmitt AC, Carrau RL, et al. Primary sinonasal mucosal melanoma with aberrant diffuse and strong desmin reactivity: a potential diagnostic pitfall! Head Neck Pathol. 2015;9:165–171. doi: 10.1007/s12105-014-0553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franchi A. An update on sinonasal round cell undifferentiated tumors. Head Neck Pathol. 2016;10:75–84. doi: 10.1007/s12105-016-0695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu HG, Kong MX, Yao Q, et al. Expression of Sox10 and c-kit in sinonasal mucosal melanomas arising in the Chinese population. Head Neck Pathol. 2012;6:401–408. doi: 10.1007/s12105-012-0375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu CH, Chen HH, Liu CM, et al. HMB-45 may be a more sensitive maker than S-100 or Melan-A for immunohistochemical diagnosis of primary oral and nasal mucosal melanomas. J Oral Pathol Med. 2005;34:540–545. doi: 10.1111/j.1600-0714.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 21.Prasad ML, Jungbluth AA, Iversen K, et al. Expression of melanocytic differentiation markers in malignant melanomas of the oral and sinonasal mucosa. Am J Surg Pathol. 2001;25:782–787. doi: 10.1097/00000478-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Heegaard S, Jensen OA, Prause JU. Immunohistochemical diagnosis of malignant melanoma of the conjunctiva and uvea: comparison of the novel antibody against melan-A with S100 protein and HMB-45. Melanoma Res. 2000;10:350–354. doi: 10.1097/00008390-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Gupta D, Malpica A, Deavers MT, et al. Vaginal melanoma: a clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 2002;26:1450–1457. doi: 10.1097/00000478-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Pekmezci M, Cuevas-Ocampo AK, Perry A, et al. Significance of H3K27me3 loss in the diagnosis of malignant peripheral nerve sheath tumors. Mod Pathol. 2017;29:4–13. doi: 10.1038/modpathol.2017.97. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer IM, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4–13. doi: 10.1038/modpathol.2015.134. [DOI] [PubMed] [Google Scholar]
- 26.Folpe AL. Selected topics in the pathology of epithelioid soft tissue tumors. Mod Pathol. 2014;27(Suppl 1):S64–S79. doi: 10.1038/modpathol.2013.175. [DOI] [PubMed] [Google Scholar]
- 27.Jo VY, Fletcher CD. Epithelioid malignant peripheral nerve sheath tumor: clinicopathologic analysis of 63 cases. Am J Surg Pathol. 2015;39:673–682. doi: 10.1097/PAS.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 28.Schaefer IM, Dong F, Garcia EP, et al. Recurrent SMARCB1 inactivation in epithelioid malignant peripheral nerve sheath tumors. Am J Surg Pathol. 2019;43:835–843. doi: 10.1097/PAS.0000000000001242. [DOI] [PMC free article] [PubMed] [Google Scholar]